Abstract
S. Ray and A. Reddy recently anticipated the implication of circadian rhythm in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the causative agent of the coronavirus disease (Covid-19). In addition to its key role in the regulation of biological functions, the circadian rhythm has been suggested as a regulator of viral infections. Specifically, the time of day of infection was found critical for illness progression, as has been reported for influenza, respiratory syncytial and parainfluenza type 3 viruses. We analyzed circadian rhythm implication in SARS-CoV-2 virus infection of isolated human monocytes, key actor cells in Covid-19 disease, from healthy subjects. The circadian gene expression of BMAL1 and CLOCK genes was investigated with q-RTPCR. Monocytes were infected with SARS-CoV-2 virus strain and viral infection was investigated by One-Step qRT-PCR and immunofluorescence. Interleukin (IL)-6, IL-1β and IL-10 levels were also measured in supernatants of infected monocytes. Using Cosinor analysis, we showed that BMAL1 and CLOCK transcripts exhibited circadian rhythm in monocytes with an acrophase and a bathyphase at Circadian Time (CT)6 and CT17. After 48 h, the amount of SARS-CoV-2 virus increased in the monocyte infected at CT6 compared to CT17. The high virus amount at CT6 was associated with significant increased release in IL-6, IL-1β and IL-10 compared to CT17. Our results suggest that time day of SARS-CoV-2 infection affects viral infection and host immune response. They support consideration of circadian rhythm in SARS-CoV-2 disease progression and we propose circadian rhythm as a novel target for managing viral progression.
Keywords: Covid-19, Circadian rhythm, Monocytes, Inflammatory cytokine
1. Introduction
The implication of circadian rhythm (CR) in pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has been recently anticipated [1]. The CR regulates physiological processes in living organisms with a period of 24 h [2]. Rhythmicity depends on central and peripheral oscillators whose activity relies on two main feedback loops managed by a clock genes cascade under the regulation of the main clock gene BMAL1 [2]. The host susceptibility to microorganism is likely under control of biological clocks [3]. The time of day of infection is critical for illness progression as reported for influenza, respiratory syncytial and parainfluenza type 3 viruses [[4], [5], [6]]. We previously reported that CR is a key actor at the interface between infection susceptibility, clinical presentation and prognosis of infection [3,7].
There are some evidence that enable to anticipate the role of CR in SARS-CoV-2 infection. The absence of Bmal1 has an impact on intracellular replication of coronaviruses, especially vesicular trafficking, endoplasmic reticulum and protein biosynthesis [8]. Knock-out of BMAL1 markedly decreases the replication of several viruses such as Dengue or Zika [9]. Finally, among key proteins involved in SARS-CoV-2 interaction with the host recently published [10], it has been identified 30% of them being associated with circadian pathway [1]. Clearly, the evidence of an implication of CR in SARS-CoV-2 infection of human cells are lacking. In this study, we wondered if SARS-CoV-2 infection and cytokine production by human monocytes, innate immune cells affected by Covid-19, were regulated by CR.
2. Results and discussion
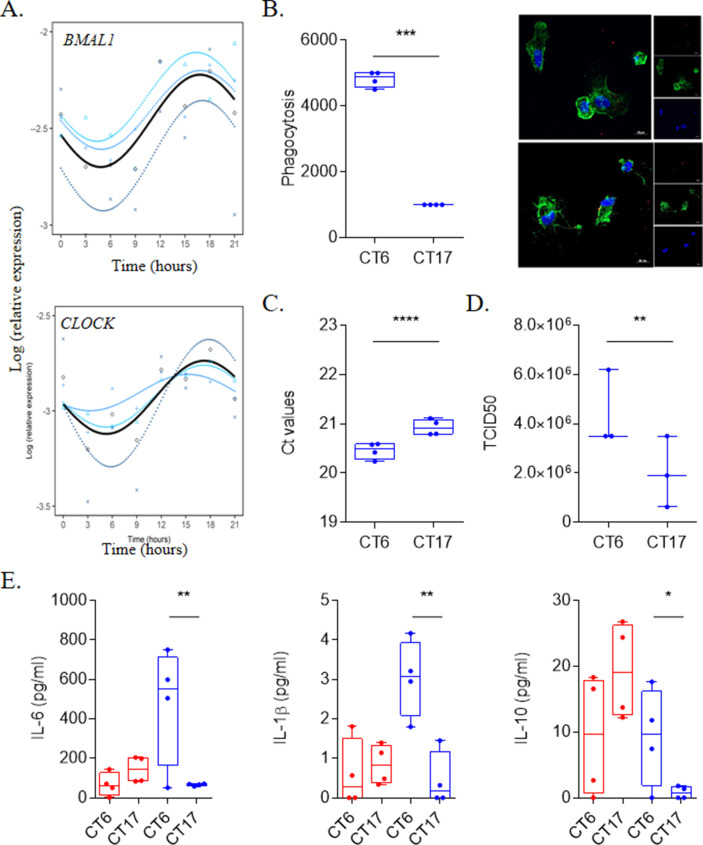
We first investigated circadian oscillations of resting monocytes. Every 3 h during 24 h, total RNA was extracted and expression of BMAL1 and CLOCK genes was investigated in unstimulated monocytes. Expression of investigated genes exhibited CR in monocytes with an acrophase (peak of the rhythm) and a bathyphase (trough of the rhythm) at Circadian Time (CT)6 and CT17, respectively (Fig. 1 A, Table 1 ). These two time points represent the beginning of the active and the resting periods in humans [11]. To assess the involvement of CR in infection of monocytes with SARS-CoV-2, we incubated monocytes with SARS-CoV-2 at the bathyphase (CT6) or at the acrophase (CT17) during 48 h. As illustrated in Fig. 1B, viral uptake by monocytes was higher at CT6 than at CT17. Additionally, we showed that the amounts of both SARS-CoV-2 RNA virus (Fig. 1C) and titer (Fig. 1D) were higher in supernatants of monocytes infected at CT6 than at CT17 (Fig. 1B). Our data showed for the first time that entry and multiplication of SARS-CoV-2 in human monocytes varies with the time of day. This finding is reminiscent of what has been previously reported with herpes and influenza virus in murine models of infection [6]. It is noteworthy that CRs are different in rodents and humans, thus limitating extrapolations to understand pathogenesis of SARS-CoV-2 infection.
Fig. 1.
SARS-CoV-2 infection is link to circadian rhythm. (A) Circadian rhythm of BMAL1 and CLOCK genes in monocytes using Cosinor model from 4 different donors. (B) Virus load (n = 4) and titers (n = 3) at CT6 and CT17 time from experiments that were repeated 3 times using at least 3 different donors. (C) Representative pictures and phagocytosis activity of monocytes (F-actin in green and nucleus in blue) infected by SARS-CoV-2 virus (red). Results are expressed as the mean ± SEM of 4 donors with 3 independent experiments. (D) Level of IL-6, IL-1β and IL-10 of unstimulated (red) and infected cells at CT6 and CT17. Results are expressed as the mean of 4 donors from 3 independent experiments. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Rhythmic parameters (mesor, amplitude and acrophase) for BMAL1 and CLOCK genes.
| Gene | Donors | Mesor | P-value | Amplitude | P-value | Acrophase | P-value |
|---|---|---|---|---|---|---|---|
| BMAL1 | 1 | −2.33 | <0.001 | 0.23 | 0.05 | 16.37 | 0.03 |
| 2 | −2.40 | <0.001 | 0.20 | 0.003 | 16.91 | <0.001 | |
| 3 | −2.64 | <0.001 | 0.28 | 0.008 | 17.12 | 0.02 | |
| CLOCK | 1 | −2.92 | <0.001 | 0.16 | <0.001 | 17.20 | <0.001 |
| 2 | −2.90 | <0.001 | 0.09 | 0.03 | 15.29 | 0.007 | |
| 3 | −2.96 | <0.001 | 0.33 | 0.03 | 17.83 | 0.001 |
Covid-19 disease is characterized by runaway immune system leading to a cytokine storm consisting of high circulating levels of cytokines including IL-6, IL-1β and IL-10 [12]. We wondered if the interaction of SARS-CoV-2 with monocytes affected cytokine production at two points of the CR. The amounts of IL-6, IL-1β and IL-10 were significantly increased at CT6 (Fig. 1E) when the amount of infection is highest. Hence, the interaction of SARS-CoV-2 with monocytes resulted in distinct cytokine pattern according to daytime.
We demonstrate here that the time day of SARS-CoV-2 infection determines consistently viral infection/replication and host immune response. It is likely that SARS-CoV-2 exploits clock pathway for its own gain. Our findings support consideration of CR in SARS-CoV-2 disease progression and suggest that CR represents a novel target for managing viral progression. This study also highlights the importance of the time of treatment administration to Covid-19 patients since CR was found regulating pharmacokinetics of several drugs [13]. Several treatments are proposed to prevent the occurrence of severe forms in Covid-19. They include passive immunization, cytokines, anti-cytokine antibody or corticoids [14]. All these candidates affect the immune response known to oscillate during the day and their administration according to CR of SARS-CoV-2. Finally, the well-documented CR disturbance in intensive care units [15] should be considered in the clinical and therapeutic management of patients with severe Covid-19.
3. Methods
3.1. Cells and virus
SARS-CoV-2 strain MI6 was cultured in Vero E6 cells (American type culture collection ATCC® CRL-1586™) in Minimum Essential Media (Life Technologies, Carlsbad, CA, USA) supplemented with 4% fetal bovine serum (FBS). The viral titer was evaluated using the TCID50 method corresponding to the amount of virus inducing a cytopathic effect in 50% of infected Vero E6 cells.
Blood from anonymous healthy donors (n = 4, 1 men (38 year) and 3 women (28, 34 and 39 year) were collected from leukopacks (convention n°7828, Etablissement Français du Sang, Marseille, France) at one point and peripheral blood mononuclear cells (PBMCs) were retrieved and frozen. At the beginning of the experiment, human monocytes were isolated from PBMCs of each donors following CD14 selection using MACS magnetic beads (Miltenyi Biotec, Bergisch, Germany) as previously described [16]. The purity of CD14 cells after selection was 98%. Monocytes (5.105 cells/well) were cultured in Roswell Park Memorial Institute medium-1640 (Life Technologies) containing 10% of FBS, 100 U/mL penicillin and 50 μg/mL streptomycin (Life Technologies).
After isolation, the cells were cultured for 12 h and then were infected with 50 μl virus suspension (0.1 multiplicity of infection (MOI)) at the bathyphase (CT6) and acrophase (CT17) for 48 h at 37 °C in the presence of 5% CO2.
3.2. Circadian gene expression
Isolated monocytes were cultured for 12 h and then the circadian rhythm was assessed from midnight every 3 h for 24 h. Total RNA was extracted using the RNA Mini Kit (Qiagen) and a quantitative Real-Time PCR was performed according to the manufacturer's instructions (MMLV Kit, Life Technologies and Smart SYBRGreen kit, Roche Applied Science). Circadian gene expression was investigated using specific primers targeting BMAL1 (5′-3′AAACCAACTTTTCTATCAGACGATGAA; 3′-5′ TCGGTCACATCCTACGACAAAC) and CLOCK ( 5′-3′ AAGTTAGGGCTGAAAGACGACG; 3′-5′ GAACTCCGAGAAGAGGCAGAAG) genes [7]. Results were normalized using ACTB gene (β-actin) (5′-3′ GGAAATCGTGCGTGACATTA; 3′-5′ AGGAAGGAAGGCTGGAAGAG) as it was previously observed that this housekeeping endogenous gene did not oscillate over time [17,18]. The results are expressed according to the appropriate formula: gene expression = Log (2−ΔCt) relative expression, with Ct (Cycle threshold), ΔCt = Ct target gene - Ct β-actin. The Cosinor analysis based on an extrapolation from measurements of a few points over 24 h was used to evaluate the CR of the clock genes BMAL1 and CLOCK.
3.3. Viral RNA extraction and PCR
Viral RNA was extracted from the supernatant of infected cells using NucleoSpin® Viral RNA Isolation kit (Macherey-Nagel) and Covid-19 virus detection was performed using One-Step qRT-PCR SuperScript™ III Platinum™ (Life Technologies) targeting the gene E.
3.4. Immunofluorescence
Infected cells (5.105 cells/well) were fixed and incubated with phalloidin-555 and 4′,6-diamidino-2-phenylindole (DAPI) to labelled F-actin and nucleus respectively. SARS-CoV-2 virus was labelled using first an anti-SARS-CoV-2 antibody (Spike protein, Thermo Fisher) and then a secondary anti-rabbit Alexa 647 (Thermo Fisher).
Pictures were acquired using confocal microscopy (LSM 8000 Airyscan confocal microscope, ×63, oil objective) and the phagocytosis activity of monocytes was determined as the phagocytosis index according to the following formula (evaluating 100 cells for each donor): percentage of phagocytosis (((average number of infected cells x 100)/total number of counted cells) x average number of particles or viruses/cells).
3.5. Immunoassays
Levels of interleukin (IL)-6, IL-1β and IL-10 were measured in cell supernatants using an enzyme-linked immunosorbent assay technique (R&D systems). The sensitivity of the assays was (pg/ml) 15.4 for IL-6, 0.125 for IL-1β and 3.9 for IL-10.
3.6. Statistical analysis
Statistical analyses were performed with GraphPad Prism (7.0, La Jolla, CA) and R studio v3.4.0. Continuous variables were expressed as medians ± interquartile, and comparisons between two groups were made using the Mann-Whitney non-parametric test for unmatched data and the Student t-test for matched data.
For CR analysis, we used the Cosinor transformation to estimate for a given variable its variations over a 24-h period. CR parameters were investigated including the acrophase (time elapsed until a maximum activity) and its inverse the batyphase, the amplitude (half of the maximum variation of the considered rhythm) and the mesor (average gene expression). A significant CR is defined when the three circadian parameters are statistically significant. Statistical significance was defined as P ≤ 0.05.
Funding statement
Soraya Mezouar was first supported by the “Fondation pour la Recherche Médicale” postdoctoral fellowship (reference: SPF20151234951) and then by the “Fondation Méditerranée Infection”. This work was supported by the French Government under the “Investissements d'avenir” (investments for the future) program managed by the “Agence Nationale de la Recherche” (reference: 10-IAHU-03).
Disclosure statement
The authors declare no conflict of interest.
Author contributions
A.B.D and L.G performed experiments. A.B.D, L.G, B.C and S.M analyzed the data. A.B.D, M.L, S.M and J.L.M supervised the work. A.B.D, S.M and J.L.M wrote the manuscript. All authors reviewed and approved the submitted manuscript. All authors reviewed the draft of the manuscript and provided intellectual input.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Ray S., Reddy A.B. COVID-19 management in light of the circadian clock. Nat. Rev. Mol. Cell Biol. 2020 doi: 10.1038/s41580-020-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tognini P., Thaiss C.A., Elinav E., Sassone-Corsi P. Circadian coordination of antimicrobial responses. Cell Host Microbe. 2017;22:185–192. doi: 10.1016/j.chom.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Diallo A.B., Coiffard B., Leone M., Mezouar S., Mege J.-L. For whom the clock ticks: clinical chronobiology for infectious diseases. Front. Immunol. 2020;11:1457. doi: 10.3389/fimmu.2020.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majumdar T., Dhar J., Patel S., Kondratov R., Barik S. Circadian transcription factor BMAL1 regulates innate immunity against select RNA viruses. Innate Immun. 2017;23:147–154. doi: 10.1177/1753425916681075. [DOI] [PubMed] [Google Scholar]
- 5.Edgar R.S., Stangherlin A., Nagy A.D., Nicoll M.P., Efstathiou S., O'Neill J.S., Reddy A.B. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc. Natl. Acad. Sci. U. S. A. 2016;113:10085–10090. doi: 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta S., Tang S.Y., Devine J.C., Anderson S.T., Nayak S., Zhang S.L., Valenzuela A., Fisher D.G., Grant G.R., López C.B., FitzGerald G.A. Circadian control of lung inflammation in influenza infection. Nat. Commun. 2019;10:4107. doi: 10.1038/s41467-019-11400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coiffard B., Diallo A.B., Culver A., Mezouar S., Hammad E., Vigne C., Nicolino-Brunet C., Dignat-George F., Baumstarck K., Boucekine M., Leone M., Mege J.-L. Circadian rhythm disruption and sepsis in severe trauma patients. Shock Augusta Ga. 2019;52:29–36. doi: 10.1097/SHK.0000000000001241. [DOI] [PubMed] [Google Scholar]
- 8.Fehr A.R., Perlman S. In: Coronaviruses. Maier H.J., Bickerton E., Britton P., editors. Springer New York; New York, NY: 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. [Google Scholar]
- 9.Zhuang X., Magri A., Hill M., Lai A.G., Kumar A., Rambhatla S.B., Donald C.L., Lopez-Clavijo A.F., Rudge S., Pinnick K., Chang W.H., Wing P.A.C., Brown R., Qin X., Simmonds P., Baumert T.F., Ray D., Loudon A., Balfe P., Wakelam M., Butterworth S., Kohl A., Jopling C.L., Zitzmann N., McKeating J.A. The circadian clock components BMAL1 and REV-ERBα regulate flavivirus replication. Nat. Commun. 2019;10:377. doi: 10.1038/s41467-019-08299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Hüttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.-P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu-Ozturk D., Wang H.-Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., García-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y., Tang Q., Chen G., Xie M., Yu S., Zhao J., Chen L. New insights into the circadian rhythm and its related diseases. Front. Physiol. 2019;10:682. doi: 10.3389/fphys.2019.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and immunotherapeutics. Signal Transduct Target Ther. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruben M.D., Smith D.F., FitzGerald G.A., Hogenesch J.B. Dosing time matters. Science. 2019;365:547–549. doi: 10.1126/science.aax7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saghazadeh A., Rezaei N. Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharm. 2020;84:106560. doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna H., van der Horst G.T.J., Reiss I., Martin D. Clinical chronobiology: a timely consideration in critical care medicine. Crit. Care. 2018;22:124. doi: 10.1186/s13054-018-2041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Azzouz E., Boumaza A., Mezouar S., Bardou M., Carlini F., Picard C., Raoult D., Mège J.-L., Desnues B. Tropheryma whipplei increases expression of human leukocyte antigen-G on monocytes to reduce tumor necrosis factor and promote bacterial replication. Gastroenterology. 2018;155:1553–1563. doi: 10.1053/j.gastro.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 17.Satou R., Shibukawa Y., Kimura M., Sugihara N. Light conditions affect rhythmic expression of aquaporin 5 and anoctamin 1 in rat submandibular glands. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser T.S., Poehn B., Szkiba D., Preussner M., Sedlazeck F.J., Zrim A., Neumann T., Nguyen L.-T., Betancourt A.J., Hummel T., Vogel H., Dorner S., Heyd F., von Haeseler A., Tessmar-Raible K. The genomic basis of circadian and circalunar timing adaptations in a midge. Nature. 2016;540:69–73. doi: 10.1038/nature20151. [DOI] [PMC free article] [PubMed] [Google Scholar]