Abstract
Current therapeutic treatments improving the impaired transportation of oxygen in acute respiratory distress syndrome (ARDS) have been found to be relevant and beneficial for the therapeutic treatment of COVID-19 patients suffering from severe respiratory complications. Hence, we report the preclinical and the preliminary results of the Phase I/II clinical trial of LEAF-4L6715, a liposomal nanocarrier encapsulating the kosmotropic agent trans-crocetin (TC), which, once injected, enhance the oxygenation of vascular tissue and therefore has the potential to improve the clinical outcomes of ARDS and COVID-19 in severely impacted patients. We demonstrated that the liposomal formulation enabled to increase from 30 min to 48 h the reoxygenation properties of free TCs in vitro in endothelial cells, but also to improve the half-life of TC by 6-fold in healthy mice. Furthermore, we identified 25 mg/kg as the maximum tolerated dose in mice. This determined concentration led to the validation of the therapeutic efficacy of LEAF-4 L6715 in a sepsis mouse model. Finally, we report the preliminary outcomes of an open-label multicenter Phase I/II clinical trial (EudraCT 2020–001393-30; NCT04378920), which was aimed to define the appropriate schedule and dosage of LEAF-4L6715 and to confirm its tolerability profile and preliminary clinical activity in COVID-19 patients treated in intensive care unit.
Keywords: Clinical trial, COVID-19, Drug delivery, Nanomedicine, Sepsis
Graphical abstract
1. Introduction
Patients with severe COVID-19 complications frequently present with acute respiratory distress syndrome (ARDS), which is characterized by tissue/organ hypoxia and hypoperfusion that ultimately results in multisystemic organ failure and, in some cases, death [1,2]. The physiopathology of ARDS includes pulmonary edema due to alveolar injury followed by an inflammatory/infectious process, as observed in SARS-CoV-2 infection, leading to acute hypoxemia with bilateral pulmonary infiltrates [3,4]. Thus, widening of the interstitial space between the alveolus and the blood vessel is a hallmark of ARDS [5]. This interstitial space is a key driver of hypoxemia, resulting in a low ratio of partial arterial pressure of oxygen (PaO2) to fraction of inspired oxygen (FiO2) (PaO2/FiO2) that is characteristic of impaired diffusion [6]. In severe cases, patients require mechanical ventilation as the standard supportive care. While ventilatory support and vasopressors are routinely used for the management of patients with severe ARDS associated with COVID-19 and sepsis, they have significant limitations, such as barotrauma, superadded infections and prolonged deconditioning requiring extensive rehabilitation [7]. Therefore, the development of treatments that could overcome these limitations by shortening the time the patient spends on mechanical ventilation and/or improving the outcomes from complications of ARDS is needed.
Although increasing the amount of oxygen levels in the blood and hypoxic tissues is an appealing solution, especially at the microcirculatory level, this task remains challenging to approach. Various compounds have been developed to attempt to enhance the oxygenation level and have been assessed in clinical settings; these compounds include hemoglobin substitutes (derived from human or bovine hemoglobin and perfluorocarbon (PFC) compounds) [8,9] as well as numerous modified hemoglobin-related products (e.g., Baxter HemAssist®, Hemopure®, and Northfield PolyHeme), which have been tested in hemorrhagic shock and in cardiac surgery with extracorporeal circulation. However, due to the observation of numerous adverse events (AEs) (renal failure, hypertensive crisis, cardiac infarction, death) resulting from drug exposure, clinical trials involving the abovementioned compounds were interrupted a decade ago [10].
The natural product crocetin, particularly the pure all-trans isomer trans-crocetin (TC), attracts increasing attention for its ability to enhance systemic oxygen diffusion along a diffusion gradient in water and plasma based on its ability to alter the structure of water in plasma, causing additional hydrogen bonds to form among the water molecules [11,12]. This induced kosmotropic effect can lead to the enhanced diffusion of small molecules such as oxygen in the medium containing the TC molecules. This approach has been demonstrated to improve therapeutic treatment of ARDS in animal models [13,14]. The therapeutic activity of TC can be explained through a quadruple effect: i) enhancing systemic blood oxygen levels, ii) enhancing oxygen diffusion, iii) repairing damaged endothelium, and iv) inhibiting inflammatory signaling pathways [13]. Nonetheless, the use of TC is limited by their poor solubility and relatively short half-life (~30 min), properties that hamper their therapeutic effects and their clinical development [14].
In the present study, we report LEAF-4L6715, a novel liposomal nanoparticle encapsulating TC, that can be injected intravenously (iv) and might improve the abovementioned limitation. LEAF-4L6715 is a stable liposomal formulation that facilitates the sustained release of TC to increase its half-life in the blood and ultimately enhancing the reoxygenation of hypoxic tissues (Fig. 1A). This formulation was originally designed to be used in hypoxic conditions such as sepsis, malaria and tumor models. However, in the context of the COVID-19 pandemic, we redirected the development of LEAF-4L6715 based on the observation that most patients with severe COVID-19 complications had evidence of ARDS and severe underlying hypoxic conditions [1,3]. Specifically, our goal was to provide a clinical treatment that could be rapidly implemented for patients with severe COVID-19 complications and that has the potential to both save patients' lives and help with the logistics challenges of the European healthcare systems that are currently strained due to the insufficient number of critical care beds and mechanical ventilators available in intensive care units (ICUs). Since preclinical studies have shown that TC could mitigate hypoxic effects of ARDS in animal models [12], we hypothesized that LEAF-4L6715 could be used to treat hypoxia in patients with ARDS induced by COVID-19 and ultimately reduce the duration of mechanical ventilator use. Here, we aimed to validate the properties of LEAF-4L6715 i) in vitro compared to free TC, ii) in vivo to determine the toxicity profile and maximum tolerated dose (MTD) in healthy mice as well as the reoxygenation efficacy in a mouse model of sepsis, and iii) to validate the clinical pharmacokinetic (PK) properties, safety and potential activity in an open-label multicenter Phase I/II clinical trial (EudraCT 2020–001393-30; NCT04378920) in COVID-19 patients treated by mechanical ventilation in ICU.
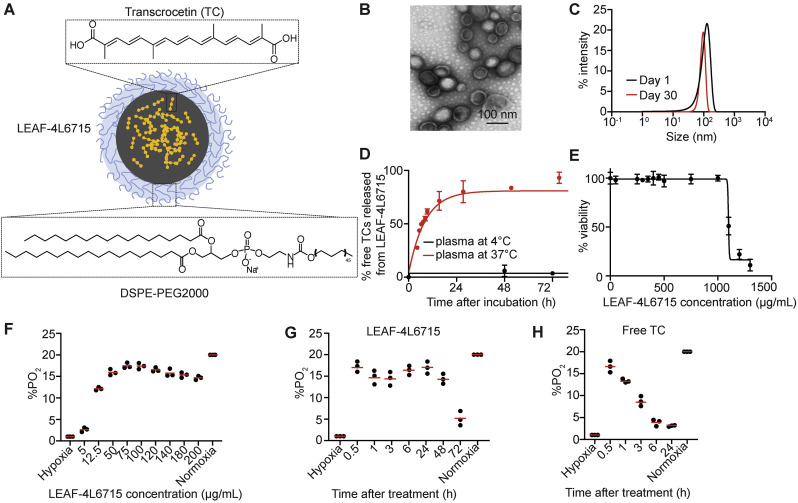
Fig. 1.
LEAF-4L6715 is an efficient blood reoxygenation molecule. (A) Schematic representation of LEAF-4L6715, a liposomal nanoparticle encapsulating TC. (B) Transmission electron microscopy of LEAF-4L6715. (C) DLS measurements of LEAF-4L6715 at day 1 and day 30 after treatment confirmed its stability over time at 4 °C. (D) Measurements of TC release from LEAF-4L6715 in the plasma demonstrated sustained release over time, with a plateau 30 h after incubation at 37 °C. No release was observed at 4 °C. (E) Cell viability assays (CellTiter-Glo) performed 72 h after treatment of HUVECs with various concentrations of LEAF-4L6715. (F) Flow cytometric analysis of HUVECs incubated with 2 μg/mL free TC over time. The %PO2 was assessed based on the readout of the BioTracker 520 green hypoxia dye. (G) Quantification of %PO2 based on various concentration of LEAF-4L6715 after 24 h of treatment in hypoxic conditions. A plateau is observed from 20 μg/mL. (H) Quantification of %PO2 based on the flow cytometric data of HUVECs incubated with 20 μg/mL LEAF-4L6715 over time and (I) with free trans-crocetine (TC) incubated at 20 μg/mL. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Results and discussion
2.1. A long-term in situ reoxygenation drug product
LEAF-4L6715 is a spherical lipidic bilayer nanoparticle measuring approx. 95 nm size, as determined by transmission electron microscopy (Fig. 1B), and 105 nm measured by dynamic light scattering (DLS) (Fig. 1C). DSPE-PEG are at the surface of the lipidic nanoparticle to increase the bioavailability and PK properties of the drug product. The polydispersity index (PDI) (~0.03) delineated the uniformity of the size distribution and confirmed that the liposomes were stable for up to 1 month in PBS (Fig. 1C, Fig. S2). The loading capacity of the liposome was determined by high-performance liquid chromatography (HPLC) and suggested an encapsulation of approx. 115.3 g/mol of TC (drug/phospholipid ratio) (Fig. S1). The liposomal formulation controlled the release kinetics of the encapsulated molecules; when mixed with plasma at 4 °C, less than 4% of TCs was released from the liposome over 3 days confirming the stability of the lipidic envelop, while at 37 °C, a slow and controlled release of TC was observed for up to 24 h, reaching a plateau of 75% release after 36 h (Fig. 1D).
We first sought to evaluate in vitro, the reoxygenation properties of LEAF-4L6715 in endothelial cells (HUVECs). The toxicity of LEAF-4L6715 in HUVECs was determined after 72 h of treatment by viability assays (CellTiter-Glo, Promega). No toxicity was observed for concentrations up to 1 mg/mL, with a half maximal inhibitory concentration (IC50) of 1136 ± 21 μg/mL (Fig. 1E, Fig. S3). Then, we incubated the HUVECs in hypoxic conditions (1% PO2) for 24 h before treatment with either free TC, various concentrations of LEAF-4L6715 (Fig. 1F-H), or empty liposomes (Fig. S4). As expected, the cells under continuous hypoxic conditions treated with free TC demonstrated a rapid reoxygenation effect at 30 min posttreatment (up to 15% PO2 with 5 μg/mL TC) by flow cytometry with a rapid decrease in the free TC efficacy over time (back to 5% PO2 at 3 h after treatment) (Fig. 1H-I, Fig. S4). For the cells under continuous hypoxic conditions treated with LEAF-4L6715 (Fig. 1H), we observed the same increase in oxygen levels as observed with the free TC treatment but with a longer prolonged oxygenation effect. The rapid oxygenation effect of LEAF-4L6715 is likely attributable to a biphasic release of TC from liposomes that includes a quick (approx. 25% in the first 6 h) initial release of part of the payload followed by a more sustained release of TC from the liposomes. Importantly, these data also defined the range of concentrations of injected LEAF-4L6715 that induce an efficient reoxygenation effect: between 75 μg/mL and 120 μg/mL (Fig. 1F).
2.2. A safe platform for in vivo proof-of-concept evaluation
Prior to evaluating the therapeutic activity of LEAF-4L6715 in ARDS conditions, we determined its PK properties and toxicity in healthy mice. The study was designed to compare the PK characteristics of LEAF-4L6715 and its counterpart free TC after one single iv injection at equal doses of 2.5 mg/kg (Fig. 2A). As expected by the use of liposomes as nanocarriers, the half-life of LEAF-4L6715 increased the TC concentration in the blood by approximately 6-fold (3.718 vs 0.619 h), while its exposure (AUC) increased by approximately 12-fold (32.4 vs 2.7 h*μg/mL) compared to the half-life and the exposure observed with free TC (Table S1).
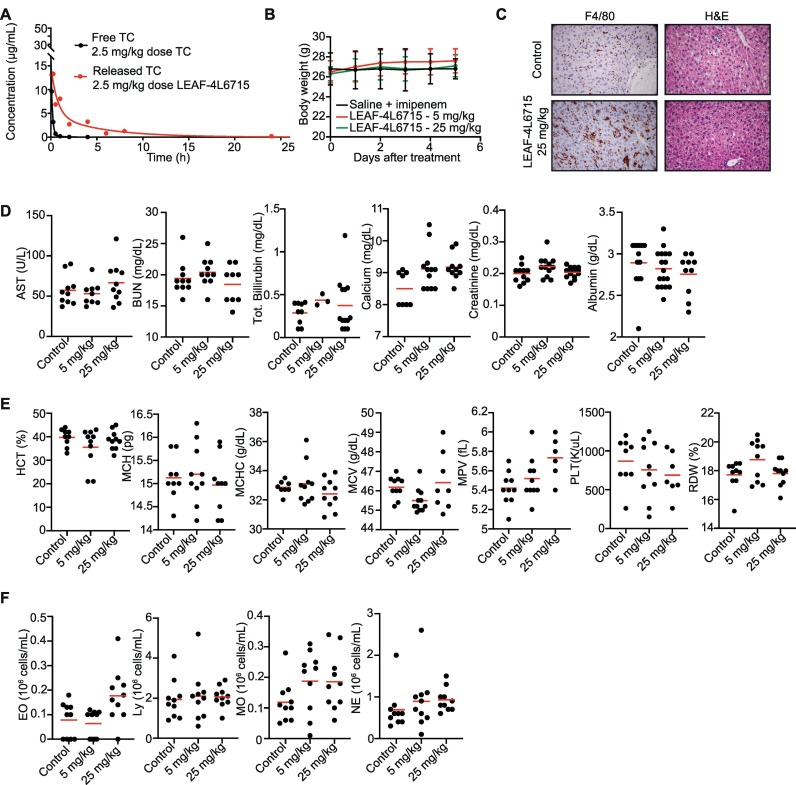
Fig. 2.
Pharmacokinetics and toxicity assessments of LEAF-4L6715. (A) PKs of free TC and LEAF-4L6715 in healthy BALB/c mice. (B) Posttreatment body weight measurements for 6 days after a single injection of either saline + imipenem, 5 mg/kg LEAF-4L6715, or 25 mg/kg LEAF-4L6715. (C) H&E and F4/80 (Kupffer cells) staining of the liver at day 5 after treatment with saline and imipenem or with 25 mg/kg LEAF-4L6715. (D) Basic metabolic profiles (n = 10 per group), (E) complete blood counts, and (F) white blood cell differential counts assessed 5 days after intravenous injection of saline and imipenem and 5 mg/kg or 25 mg/kg LEAF-4L6715. Two-way ANOVA was performed to compare the significant differences between cohorts of mice.
The toxicity of the LEAF-4L6715 molecule has been studied in healthy mouse models at incremental doses ranging from 5 to 25 mg/kg (Fig. 2). The objective of this study was to evaluate the toxicity of LEAF-4L6715 compared to saline. Forty male mice aged 9 to 10 weeks were treated for 5 days with different daily doses of LEAF-4L6715 and monitored for AEs. This group was compared to a control group treated with saline and the antibiotic imipenem aimed to control infection in the sepsis mouse model used in the therapeutic study. For doses up to 25 mg/kg, we did not observe any sign of toxicity, and the body weight remained stable after treatment (Fig. 2B). Importantly, although F4/80 staining highlighted an important quantity of LEAF-4L6715 molecules accumulating in the liver, as shown by the increased amount of positive staining of macrophages/Kupffer cells (Fig. 2C, Fig. S5) as expected with the use of liposomal nanocarriers. The hematoxylin and eosin (H&E)-stained slides of the liver confirmed the absence of macroscopic changes 5 days after treatment at the highest tested concentrations (25 mg/kg) (Fig. 2C). In addition, we did not observe modifications of the basic metabolic profiles (Fig. 2D), complete blood counts (Fig. 2E), or white blood cell differentiation (Fig. 2F) at any of the tested concentrations. This is consistent with the observations made in the control groups.
2.3. Preclinical therapeutic efficacy in a mouse model of ARDS-related sepsis
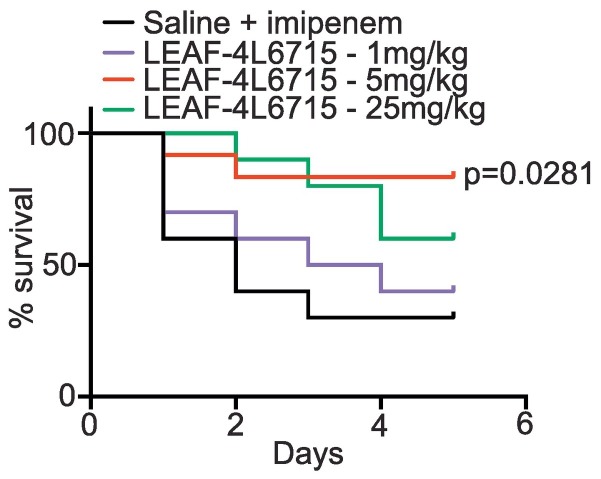
The first step in our study to validate the therapeutic properties of LEAF-4L6715 consisted of creating a cecal ligation and puncture (CLP)-induced mouse model of sepsis. In each group, ten male mice aged 8 to 10 weeks were treated once a day for 5 days. The mice in the control group received a daily administration of 0.9% saline (0.3 mL) via intraperitoneal (ip) injection in conjunction with 12.5 mg/kg imipenem twice a day. The other mice were iv treated with either 1 mg/kg, 5 mg/kg, or 25 mg/kg LEAF-4L6715 with a drug-to-lipid ratio of 80 mg/mol lipid (D/L 80) once per day in addition with 12.5 mg/kg imipenem twice a day (Fig. 3 ). Only the group treated with 5 mg/kg LEAF-4L6715 demonstrated significant therapeutic improvements compared to the control group (P = 0.0281, log rank), with 80% survival at 5 days after CLP vs. 30% for the control group. The absence of improved therapeutic efficacy (P = 0.652, log-rank) for the group that was injected with the lowest concentration of LEAF-4L6715 (1 mg/kg) can likely be explained by the fact that the dose injected was subtherapeutic. Data from the group that was treated with a 25 mg/kg LEAF-4L6715 concentration showed 60% survival at 5 days after CLP, but the short duration of this study (5 days), and the limited size of the cohort did not allow us to demonstrate a significant difference in terms of survival in comparison to the control group (P = 0.0699, log-rank).
Fig. 3.
Therapeutic efficacy of LEAF-4L6715 in a mouse model of sepsis. The Kaplan-Meier curves represent the therapeutic efficacy of LEAF-4L6715 in mice that underwent CLP. Log-rank tests were performed to compare the survival of the treated mice to that of the control group.
As a result, although the data set was limited, 25 mg/kg was considered the no observed AE level (NOAEL). Hence, our in vivo findings confirmed the efficacy of the treatment through the reduction in the mortality rate in the group treated with the intermediate dose level compared to the control groups.
3. Clinical study
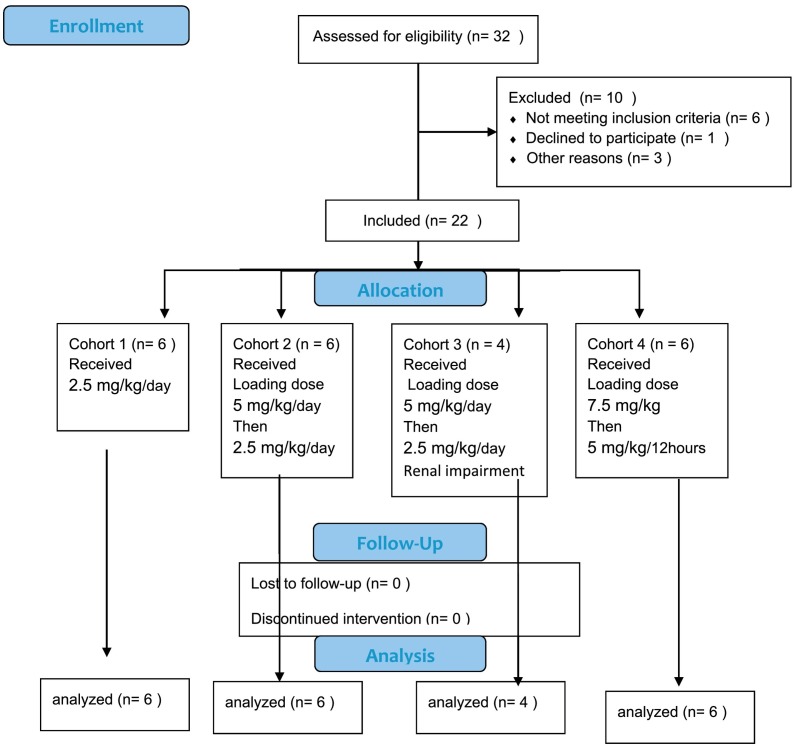
LEAF-4L6715 was fast-tracked in a multicenter open-label Phase I/II clinical trial (NCT04378920; EUDRA2020–001393-30) because of the urgent need to provide a solution to ICUs that were facing difficulties in keeping up with the volume of COVID-19 patients with severe complications. A total of 12 patients have been enrolled in the Phase I/II trial. The patients' characteristics are listed in Tables 1 and Table 2 . Patients enrolled in this study were, on average, treated in the ICU for a period of 12 days before initiation of treatment with LEAF-4L6715. The main goal of this clinical trial was to determine the optimal injection protocol of LEAF-4L6715 in patients, to determine its safety and PK profile, and to confirm a trend in therapeutic efficacy. A specific therapeutic efficacy trial will be performed through a future phase III clinical trial.
Table 1.
Treatment protocol and patient characteristics.
| Cohort 1 | Cohort 2 | All | ||
|---|---|---|---|---|
| Provided Consent | 6 | 6 | 12 | |
| Enrolled/Treated | 6 | 6 | 12 | |
| Completed study treatment | 6 | 6 | 12 | |
| Dose | 2.5 mg/kg | Day 1: 5 mg/kg loading dose | ||
| Day 2 onwards: 2.5 mg/kg | ||||
| Schedule | Daily | Daily | ||
| Renal function | Normal | Normal | ||
| Patient Characteristics | ||||
| Age | Years (range) | 66.0 (56, 75) | 66.5 (34, 81) | 66.0 (34, 81) |
| BMI | kg/m2 (range) | 30.6 (24, 39) | 28.7 (24, 36) | 29.7 (24, 39) |
| Height | cm (range) | 169 (152, 175) | 178 (160, 189) | 173 (152, 189) |
| Gender | ||||
| Female (%) | 2 (33.3) | 2 (33.3) | 4 (33.3) | |
| Male (%) | 4 (66.7) | 4 (66.7) | 8 (66.7) | |
| Weight | kg (range) | 84.0 (70, 112) | 82.0 (77, 118) | 83.0 (70, 118) |
| Coexisting medical conditions | ||||
| Hypertension | 4 | 3 | 7 | |
| Obesity (BMI >35) | 3 | 1 | 4 | |
| Type 2 diabetes | 0 | 1 | 1 | |
| Cancer | 0 | 0 | 0 | |
| Immunodepression | 1 | 0 | 1 |
Table 2.
Enrollment characteristics of patients included in the study.
| History of hospitalization | Cohort 1 | Cohort 2 | All | |
|---|---|---|---|---|
| Hospitalized in ICU | 6 | 6 | 12 | |
| Number of days between hospitalization and admission to intensive care | Day | 2.3 ± 2.1 | 2.7 ± 3.4 | 2.5 ± 2.7 |
| Number of days between first appearance of symptoms and admission to intensive care | Day | 6.3 ± 4.2 | 10.3 ± 8.1 | 8.3 ± 6.2 |
| Number of days between hospitalization and first intubation | Day | 2.5 ± 2.4 | 2.2 ± 1.9 | 2.3 ± 2.2 |
| Number of days between admission to intensive care and first day of treatment | Day | 8 ± 4.7 | 10.7 ± 6.7 | 9.3 ± 5.7 |
| Number of days between admission to intensive care and first day of treatment | Day | 8 ± 4.7 | 10.7 ± 6.7 | 9.3 ± 5.7 |
| Viral disease characteristics | ||||
| COVID-19 nasopharyngeal smear result | 4 | 5 | 9 | |
| COVID-19 results in tracheal aspirations | 6 | 6 | 12 | |
| Severity of COVID lesions on initial scan | ||||
| 0: No lesions | 0 | |||
| 1: 1–25% (slight) | 0 | |||
| 2: 26–50% (moderate) | 3 | 2 | 5 | |
| 3: 51–75% (severe) | 3 | 3 | ||
| 4: 76–100% (critical) | 1 | 1 | ||
| Not available | 2 | 1 | 3 |
3.1. PK profile for dosing regimen optimization
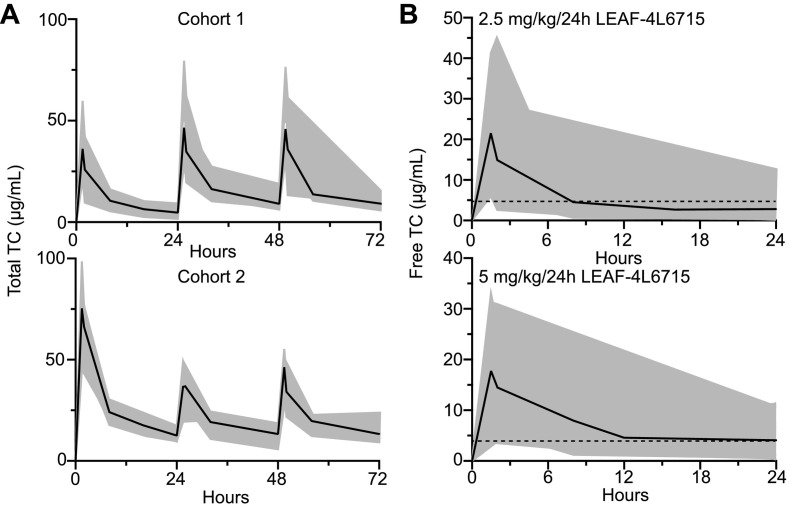
The patients were treated into successive cohorts driven by the PK results observed (Fig. 4 ). The 6 patients in the first cohort (cohort 1) received a total of 30 doses of 2.5 mg/kg LEAF-4L6715 daily through a 90-min iv infusion which is one-tenth of the presumed NOAEL of 25 mg/kg. After each injection, we did not observe any treatment-related AEs. The preliminary PK data of the total drug indicated that at least three days of treatment with a daily injection of LEAF-4L6715 would be required to reach a steady state, suggesting the need to inject a loading dose of LEAF-4L6715 to reach the minimum level of free drugs for efficient reoxygenation fixed at 0.4 μg/mL in the blood [16] (Fig. 5A and Table 3 ).
Fig. 4.
CONSORT flow diagram.
Fig. 5.
Pharmacokinetic profiling and dosage optimization of LEAF-4L6715 in 18 COVID-19 patients. (A) PK profile of total TC from LEAF-4L6715 over a 3-day period with a daily injection of 2.5 mg/kg in cohort 1 (n = 6) and an initial loading dose of 5 mg/kg followed by a daily injection of 2.5 mg/kg LEAF-4L6715 in cohort 2 (n = 6). (B) PK profile of free TC over a 24 h period for 2.5 mg/kg and 5 mg/kg injections of LEAF-4L6715.
Table 3.
Pharmacokinetic properties of LEAF-4L6715 in 12 patients with COVID-19. (Top) Total drug concentration in the blood, (bottom) released trans-crocetin (free drug) in the blood.
| Cohort | N | Kel24h | R24h | T1/2 (h) | Ctrough (μg/mL) | Cmax (μg/mL) | AUC0-t (h*μg/mL) | Vz (L) | Cl (L/h) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total drug | 1 | 6 | 0.08 | 0.92 | 9.44 | 4.70 | 36.13 | 263.78 | 7.78 | 0.08 |
| 2 | 6 | 0.07 | 0.88 | 10.35 | 12.51 | 75.37 | 635.46 | 7.50 | 0.62 | |
| Free drug | 1 | 6 | 0.11 | 0.62 | 7.53 | 2.38 | 18.68 | 132.20 | 15.66 | 1.93 |
| 2 | 6 | 0.09 | 0.83 | 8.43 | 4.88 | 47.42 | 311.59 | 13.52 | 1.51 |
Hence, the 6 patients in the second cohort (cohort 2) were subsequently treated with 38 doses with a loading dose of 5 mg/kg LEAF-4L6715 on day 1 followed by an injection of 2.5 mg/kg daily. The targeted therapeutic range of the TC released from the liposomes (0.4 to 49 μg/mL [15]) was achieved from day 1 due to the loading dose, while preserving the same PK parameters as compared with the findings from cohort 1 (Table 3).
We did not observe any safety concerns in cohort 2. The injection of the maintenance dose of 2.5 mg/kg/day of LEAF-4L6715 from day 2 onwards caused the free drug concentration in cohort 2 to fall below the threshold of activity at 12 h for half of the patients (median 0.58 μg/mL, ranging from 0 to 1.44 μg/mL). The preliminary PKs as of day 1 indicated that the free drug concentrations in cohorts 1 and 2 were at their lowest at 16 h and 24 h (ranging between 0.3 and 5.2 μg/mL) (Fig. 5B). Hence, based on a bicompartmental PK model, we determined that LEAF-4L6715 should be injected in patients every 12 h to maintain a sustained concentration of TC in the blood.
Based on the results observed, it was decided to modify the treatment procedure so that it would include a first injection of a loading dose that would be increased to 7.5 mg/kg, followed by the injection of a dose of 5 mg/kg every 12 h thereafter. These doses and schedule were planned for the treatment of the patients enrolled in the next cohort.
3.2. Clinical safety of LEAF-4L6715
Two serious AEs were reported in the study. All four were determined to be unrelated to treatment with LEAF-4L6715 and were determined to be consistent with complications generally observed with the underlying disease. In addition, we observed transaminase, renal and electrolyte abnormalities, as well as a few hematologic (anemia) and coagulopathy [thrombocytopenia and increased international normalized ratio (INR)] abnormalities that we did not consider to be AEs related to the studied drug, as all three were expected to be complications due to the underlying condition. Finally, we observed a few elevations in bilirubin measurement (grades 1 and 2). At this day, over this preliminary assessment, we did not report any other AEs likely to be related to the administration of LEAF-4L6715.
3.3. Clinical efficacy of LEAF-4L6715
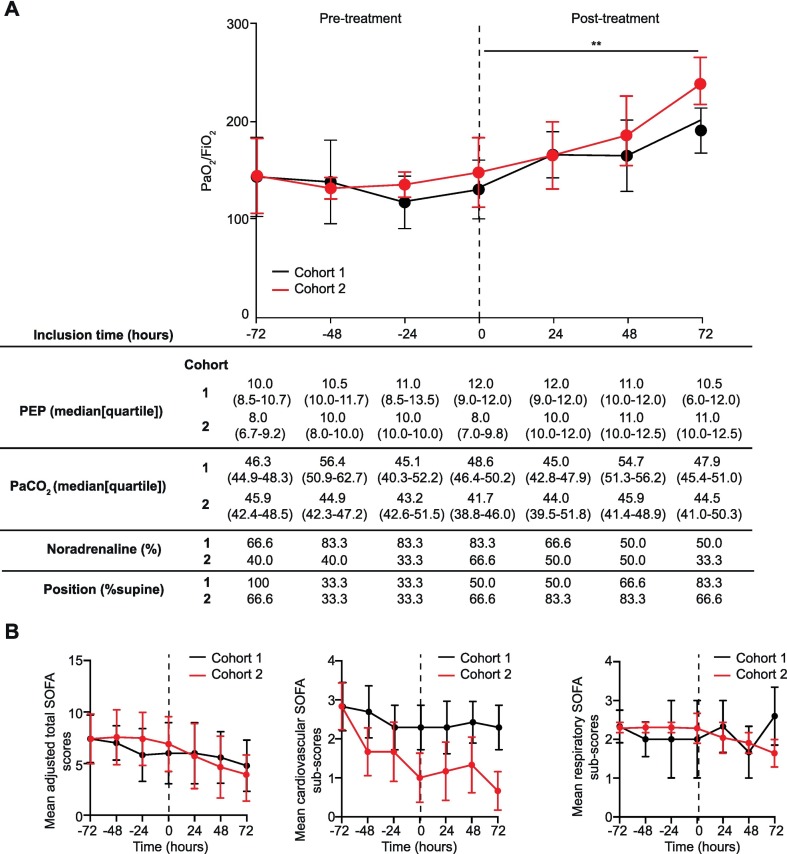
The primary efficacy criterion was an improvement in 25% or more in the ratio of partial arterial pressure of oxygen (O2) to inspired fraction of O2 (PaO2/FiO2 ratio) in patients with ARDS under artificial respiratory support. Overall, 45% of the patients had an increase of >25% during the first 24 h of treatment with LEAF-4L6715. All patients in cohort 2, as well as 50% of patients in cohort 1, had a > 25% improvement in their PaO2/FiO2 ratio at any time during the treatment period (Fig. 6A). Of interest, the PaO2/FiO2 ratio increased over time with treatment by LEAF-4L6715, accelerating on day 2 after treatment (Fig. 6A). The number of patients requiring noradrenaline in cohort 1 and 2 decreased from 9 patients (75%) at baseline to 5 patients (42%) by day 3 of treatment with LEAF-4L6715. Overall, in cohort 1 and 2, after administration of the treatment with LEAF-4L6715, we observed a trend in which the mean total sequential organ failure assessment (SOFA) scores decreased by more than 2 points from baseline to day 8 (Fig. 6B, Fig. S7). At the 28 day assessment, 11 out of 12 patients (92%) were alive. The findings of this ongoing study, although promising, are preliminary and demonstrate encouraging trends of activity. The objective of the presented clinical study remained to identify the optimal dose and schedule for clinical use of LEAF-4L6715. After this early assessment, an increase of the administered dose and a shortened interval between two administrations are being studied in ongoing additional cohorts. Moreover, after the identification of the recommended dosage and schedule, there will be a need to demonstrate the efficacy of LEAF-4L6715 in a large, randomized Phase III clinical trial of patients with ARDS due to COVID-19.
Fig. 6.
LEAF-4L6715 efficiently improved the oxygenation of COVID-19 patients in cohort 1 and 2 (n = 6/group). (A) Validation of the major endpoint criteria of the study, including PaO2/FiO2 ratio, PEP, PaCO2, noradrenaline status, and position of the patients 3 days before and 3 days after the first injection of LEAF-4L6715. (B) Mean adjusted SOFA score along with cardiovascular and respiratory SOFA subscores. All data are presented as the mean ± standard error of the mean. The adjusted SOFA score excludes the liver biology to avoid the interference with the AE related to the LEAF-4L6715 exposure. Wilcoxon tests were performed to compare the different increase of PaO2/FiO2 within the same group and the SOFA scores evolutions. **p-value<0.01.
4. Conclusion
In conditions such as COVID-19 and sepsis, ARDS leading to systemic oxygen deprivation or hypoxia has been identified as the primary cause of death, especially in severe cases. For patients suffering from these conditions, hypoxia has been linked to a sequence of self-enhancing poor prognostic factors for clinical outcome. Treatment options for patients with severe COVID-19 are currently limited to mechanical ventilation and supportive care, with poor survival outcomes.
In vitro and preclinical therapeutic results demonstrated the reoxygenation activity of TC, and the optimization of its exposure by encapsulating into a liposomal nanocarrier with the LEAF-4L6715 formulation. These preclinical data enabled us to receive fast-track approval for a clinical study to evaluate this novel liposomal formulation. The adaptive design of the dose escalation study provided an opportunity to explore doses and schedules to attain an optimal therapeutic effect.
The patients enrolled in the study were hospitalized in the ICU for an average of 12 days prior to treatment. A total of 45% of patients met the primary endpoint of activity with a > 25% improvement in the PaO2/FiO2 ratio at 24 h despite a suboptimal exposure of the treatment [16]. Additional markers of improvement in respiratory function during treatment included a general decrease in the FiO2 requirement, a slight decrease in PaCO2, a decrease in aggressive ventilation, a decrease in the use of sedatives and paralytic agents, and a decrease in the number of patients requiring placement in a prone position. Furthermore, the observed improvement in the total SOFA score and subscores indicated that treatment with LEAF-4L6715 may improve microcirculation and thus perfusion of other organs, thereby enhancing multiorgan function in addition to the benefits observed for the respiratory system. Together, these early observations suggest the potential clinical benefit of LEAF-4 L6715 in treating patients with COVID-19 and, more broadly, in other situations of high unmet medical need with a similar underlying pathophysiology, such as sepsis, where multiorgan dysfunction is an important contributor to morbidity and mortality.
In the first 12 patients enrolled in this study, we have not identified any AEs related to the clinical use of LEAF-4L6715. In conclusion, the overall risk/benefit profile of LEAF-4L6715 for the treatment of patients with ARDS due to COVID-19 requiring mechanical ventilation appears to be encouraging knowing the suboptimal exposure considering the PK results. The findings of this ongoing study, although promising, are very preliminary and limited by the small sample size. An increase of the administered dose and a shortened interval between two administrations are being studied in ongoing additional cohorts. Moreover, after the identification of the recommended dosage and schedule there will be a need to specify the efficacy to assess LEAF-4L6715 in a large, randomized Phase III clinical trial of patients with ARDS due to COVID-19.
5. Materials and methods
5.1. Transcrocetin synthesis
TC salt was manufactured by Chemspec-API (New Jersey, US) under cGMP through a multi-step total synthesis approach. The chemical structure of TSC (trans sodium crocetinate) was confirmed by 1H NMR, high resolution mass spectroscopy (HRMS), and elemental analysis. The purity of TC salt was analyzed by HPLC with a specification no less than 98%.
5.2. LEAF-4L6715 manufacturing
LEAF-4L6715 was prepared by the active loading of free TC into liposome through the calcium gradient. Briefly, multilamellar vesicles (MLVs) were formed by hydration of ethanolic lipids in calcium acetate solution. The MLVs were passed through high pressure extruder over a stack of polycarbonate membranes at elevated temperature to form small unilamellar vesicles (SUVs). The external calcium acetate was removed by tangential flow filtration (TFF) to generate the calcium gradient. Free TC solution was mixed with the diafiltered liposome, and loaded into the liposome through heat exchanger. The TC loaded liposomes were purified by buffer exchange with histidine buffered saline (pH 6.5) through TFF. The bulk drug product was diluted to the target concentration, sterile filtered, and stored at 2–8 °C. LEAF-4L6715 drug products used in clinical trial were manufactured in cGMP environment according to the procedures defined in the batch record, and released after meeting the specifications.
5.3. Drug release quantification
The TC release profile from LEAF-4L6715 was estimated by quantifying the absorbance of TC at 390 nm in a plate reader (SpectraMax ID, Molecular Device). Initially, we have constructed a standard calibration curve of free TC at various concentration and quantified the amount of total TC present in LEAF-4L6715 by treatment of DMSO for 15 min which confirms the release of total TC from the nano formulation (Fig. S1). Next, we have incubated LEAF-4L6715 with isolated human plasma at a concentration of 75 μg/mL at 4 °C and 37 °C. After incubation of the LEAF-4L6715 formulation for various time points, the total mixture is centrifuged to obtain free TC in the supernatant which is then used for TC quantification. We represented the amount of TC obtained at each time points as a percentage of the total amount of TC present in LEAF-4L6715.
5.4. In vitro hypoxia quantification
HUVECs (passages 3 and 4) were plated at ~80% confluence for 24 h under hypoxic conditions (1% O2, 37 °C). For quantification of the hypoxia levels, cells were then incubated for 1 h with 1.5 μM BioTracker 520 Green Hypoxia Dye (Sigma-Aldrich) before being washed and stored in the hypoxia chamber for 1 h. Finally, the cells were treated with either PBS or LEAF-4L6715 at various concentrations and times. Hypoxia levels were quantified with a BD Accuri™ C6 cell analyzer with a direct readout of the BioTracker probe. Hypoxic conditions were maintained during the totality of the study for the hypoxia study groups. The results were analyzed with FlowJo software version 10 (TreeStar).
5.5. Preclinical studies
5.5.1. Mouse model
Female C57BL/6 mice ordered from Envigo Laboratories were acclimated to the housing conditions and handled in accordance with Animal Use Protocol (AUP) number TP-05. The animals were acclimated for approximately 1 week prior to the start of the study. Only animals that were deemed healthy were included in this study. All procedures carried out in this experiment were conducted in compliance with the laws, regulations, and guidelines of the National Institutes of Health and with the approval of the TransPharm Animal Care and Use Committee.
5.5.2. CLP procedure
On day−1, mice were anesthetized with isoflurane and brought to a surgical plane. The lower quadrants of the abdomen were shaved using an electric trimmer. On day 0, mice were anesthetized with isoflurane and brought to a surgical plane. The shaved area was disinfected with three alternating scrubs of chlorhexidine surgical scrub and 70% isopropanol. An abdominal longitudinal skin midline incision was made with iris scissors without penetrating the peritoneal cavity. After the initial incision, small scissors were used to extend the incision 1.5–2 cm to access the peritoneal cavity. The midline white fascia of the abdominal musculature was identified and dissected for intermuscular incision and incision of the fascia and peritoneal layers. The cecum was exteriorized using blunt anatomical forceps, leaving the remainder of the small and large intestines within the peritoneal cavity and avoiding breach or damage to the mesenteric blood vessels. The cecum was ligated with a sterile 9.5 mm stainless steel surgical clip below the ileocecal valve at the designated position (approximately 70% of the cecum was ligated). Care was taken not to occlude the bowel. Before cecal perforation, the cecal contents were gently pushed toward the distal cecum. The cecum was then perforated using a 16-gauge needle for severe grade sepsis. A single through-and-through puncture midway between the ligation and the tip of the cecum in a mesenteric-to-antimesenteric direction was performed. After the needle was removed, the cecum was returned to the abdominal cavity without spreading feces from the cecum onto the abdominal wall wound margins, and a small droplet of feces was extruded from both the mesenteric and antimesenteric penetration holes. Droplet size was kept as consistent as possible. The peritoneum, fasciae, and abdominal musculature were closed by applying simple running sutures (4–0 PDS or chromic gut surgical sutures), and the skin incision was closed with 9 mm autoclips or surgical glue.
5.6. Clinical study design and methods
The clinical study was designed to determine a safe and optimal dosing regimen for LEAF-4L6715 based on the clinical results and PK profiles (NCT04378920; EUDRACT2020–001393-30). The targeted therapeutic range of the free TC released from the liposomes should be contained between 0.4 and 49 μg/mL [16]. The safety was assessed based on NCBI-NIH criteria. To validate the preliminary efficacy of LEAF-4L6715, an improvement in the PaO2/FiO2 ratio by 25% or more at 24 h was targeted as primary efficacy criterion. The secondary efficacy criteria included evolution over time of P/F ratio, partial pressure of carbon dioxide (PaCO2), body position under the mechanical ventilation (prone versus supine), positive expiratory pressure value used in mechanical ventilation, cardiovascular supportive drug (noradrenaline) administration, survival. This study was designed to be adaptive and accommodate dose and schedule changes as needed when factors such as safety, efficacy or PKs evolved. An independent data monitoring committee (IDMC) was designed to monitor all data and to assess all decisions addressing the conduct of the study by the steering committee. We initially selected a 2.5 mg/kg/day dose of the LEAF-4L6715 molecule for the first administration in patients, which is one-tenth of the presumed NOAEL of 25 mg/kg identified in preclinical development (Fig. 2C). This first dosage sounds in line with the knowledge that free TCs have been safely administered in humans at doses up to 2 mg/kg/day (18).
Signed informed consent from each patient or his or her legal representative was obtained before any procedures were begun. In order to be included in this study, patients had to be older than 18 years old, have acute respiratory distress syndrome with a PaO2/FiO2 ratio of less than 200 and be under artificial ventilation support with a life expectancy of at least 24 h. Patients were required to have normal liver function as defined by ALT, AST and alkaline phosphate levels measuring less than 3 times the upper limit of the normal value for the institution, a platelet count above >100,000 cells/mm3, hemoglobin >8 g/dL and an absolute neutrophil count of >1000 cells/mm3. Any patient enrolled in another therapeutic clinical trial with the same endpoint was excluded, as well as pregnant or breastfeeding patients and patients who had hemoglobinopathy or a known hypersensitivity to crocetins, LEAF-4L6715 or any of its excipients. Patients receiving extracorporeal membrane oxygenation were also excluded. The French agency of medicine (Agence National de Sécurité du medicament et des produits de santé; ANSM) approved the use of LEAF-4L6715 in clinical study in April 11th 2020 (registration number MEDAECNAT-2020-03-00066). The first version of the protocol was approved by the ethical committee of CPP Sud Méditerannée III in April 8th 2020, under application number 2020.04.08_20.03.27.77425.
5.7. PK study
Blood samples were collected from subjects to determine the plasma concentration of total drug and Free TC prior to infusion and then at 1.5 (end of infusion), 2, 4, 8, 12, 24 h after the start of infusion each day over 5 days. The concentrations of total LEAF-4L6715 and free TC were both measured in each sample which were prepared before Liquid Chromatography – Mass Spectrometry (LC-MS) measurement, with the same approach using an automated robotic station (Beckman) under ISO 9001 compliance. In a first step, 12.5 μL of each sample was transferred into 96 microplates (Greiner ref. 650,201) and diluted 1/20 with human plasma. Calibration, for standard curve (0.5, 1, 5, 10, 20, 30 μM) and quality control samples were added in empty wells. For Total Drug determination, 100 μL of 1/20 diluted samples was transferred to a new plate (Greiner ref. 780,201), and 250 μL of acetonitrile (containing 10 μM Ibuprofen) was added. After mixing and centrifugation, measurement was performed using LC-MS. For Free TC determination, 100 μL of 1/20 diluted samples was transferred to a new plate (Greiner ref. 650,201), where 100 μL of D-buffer (3%, NH4OH, 0.33 N NaOH) was added. Then 100 μL of this mix was distributed onto Oasis HLB 96-well plate (Waters ref. 186,000,128) already pre-activated with 2 × 500 μL Methanol followed by 1 mL L-buffer (1% formic acid). After 3 washes with 500 μL of L-buffer and 1 wash with 500 μL of H2O, an elution was performed using 200 μL of F-buffer (90% Methanol, 3% NH4OH) into another 96 well plate (Waters ref. 186,005,837). From this eluted fraction 150 μL was then transferred to a new plate (Greiner 780,201), where control was previously added [Ibuprofen (Sigma ref. I4883) 10 μM]. After evaporation (vacuum centrifuge at 50 °C). the pellet was solubilized with 200 μL of acetonitrile 50% in H2O followed by vortexing and centrifugation (3 min, 3500G) before LC-MS analysis. Samples were analyzed using an Ultra Hight Performance Liquid Chromatography (UHPLC) coupled to a triple quadrupole Shimadzu LC-MS 8030 mass spectrometer, with an electrospray ionisation (ESI) source in the negative mode. Calibration and quality control samples were analyzed in the same series of injections for all fractions. The LC-MS analyses were performed on a C18 Kinetex column (50 mm × 2.1 mm, 2.6 μm, 100 A) as a stationary phase and mobile phase composition was 0.05% formic acid in water (A) and acetonitrile (B), The following gradient was applied for the elution (0 min: 95% A, 5% B; 1.20–1.4 min: 5% B, 95% B; 1.42–2.8 min: 95% A, 5% B), at 0.5 mL/min as flow rate. The temperature of column was kept at 40 °C. Injection volume was 1 μL. The mass spectrometer conditions were as follows: temperature of the block heater was maintained at 400 °C and the one of the desolvation line at 250 °C. 4500 V were used for the interface voltage. The nitrogen nebulizing gas flow was set at 2 L/min and the drying gas flow at 15 mL/min. The collision gas used was argon at 230 kPa. Multiple reaction monitoring (MRM) mode was used for quantitation. Ibuprofen was used as an internal standard, its MRM transition was 204.9 to161.15. MRM transition was 321.0 to 283.0, 239.15 for crocetin. The maximal and minimal concentration (Cmax and Ctrough) were obtained from the measure of concentration at 1.5 h and the last concentration measured, respectively. The area under the concentration-time curve from zero to the last quantifiable concentration (AUC0-t) was calculated using the linear trapezoidal rule, using actual elapsed time values. Others PK parameters were calculated by a non-compartmental analysis (NCA) performed with Pkanalix2020, Monolix suite (Lixoft®).
Funding
The Institut de Cancérologie Strasbourg Europe (ICANS), Strasbourg, France, is the sponsor of the study and covered all costs associated with the study. LEAF4Life provided LEAF-4L6715 for the study.
Authors' contributions
PMM, OC, PC, VC, MV, VS, MV, CN, VM, PG, PV, AD, and XP designed the study. The sponsor organized clinical data collection (AR, XD, VC, MV, AB, and VS). The PK study was carried out by PC, PG, ZX, MT, BG, and PV. Preclinical data were obtained by MCD, MB, NL, ND, JD, GK, ZX, MT, ZH, KK, BG, CN, VM, and AD.. Clinical data analyses were performed by MV and PC. Data were interpreted by PMM, OC, PC, CN, VM, AD, and XP. VM, AD, and XP wrote the manuscript. All authors revised the manuscript and approved its submission. The corresponding authors have all access to the data presented in the study and were responsible for submitting the manuscript.
Availability of data and materials
Data are available upon detailed and justified request to the sponsor of the study (secretariatdg@icans.eu). ANSM has previously reviewed all the presented data in this study and has full access to the data.
Declaration of Competing Interest
ND, JD, KG, HX, RH, KK, BG, CN, and VM are employees of LEAF4Life, Inc. XP is member of the scientific committee and a board member of LEAF4Life, Inc. The other authors declare no competing interests.
Acknowledgments
The authors would like to acknowledge ANSM (Agence Nationale de Sécurité du Médicament et des Produits de Santé) for its support toward the completion of the study. We are grateful to F. Daubeuf, R. Hany, C. Bourban, S. Ramanoudjame, S. Gioria and A. Obrecht (PCBIS, UMS3286) for technical help and automation of process.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jconrel.2021.06.033.
Appendix A. Supplementary data
Supplementary material
References
- 1.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N. Engl. J. Med. 2017;377:1904–1905. doi: 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan E., Beitler J.R., Brochard L., Calfee C.S., Ferguson N.D., Slutsky A.S., Brodie D. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir. Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuinness G., Zhan C., Rosenberg N., Azour L., Wickstrom M., Mason D.M., Thomas K.M., Moore W.H. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020;297:E252–E262. doi: 10.1148/radiol.2020202352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullon J., Giacoppe G., Clagett C., McCune D., Dillard T. Transfusions of polymerized bovine hemoglobin in a patient with severe autoimmune hemolytic anemia. N. Engl. J. Med. 2000;342:1638–1643. doi: 10.1056/NEJM200006013422204. [DOI] [PubMed] [Google Scholar]
- 9.Goodnough L.T., Brecher M.E., Kanter M.H., AuBuchon J.P. Transfusion medicine. First of two parts--blood transfusion. N. Engl. J. Med. 1999;340:438–447. doi: 10.1056/NEJM199902113400606. [DOI] [PubMed] [Google Scholar]
- 10.Tao Z., Ghoroghchian P.P. Microparticle, nanoparticle, and stem cell-based oxygen carriers as advanced blood substitutes. Trends Biotechnol. 2014;32:466–473. doi: 10.1016/j.tibtech.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Gainer J.L. Trans-sodium crocetinate for treating hypoxia/ischemia. Expert Opin. Investig. Drugs. 2008;17:917–924. doi: 10.1517/13543784.17.6.917. [DOI] [PubMed] [Google Scholar]
- 12.Gainer J.L., Stennett A.K., Murray R.J. The effect of trans sodium crocetinate (TSC) in a rat oleic acid model of acute lung injury. Pulm. Pharmacol. Ther. 2005;18:213–216. doi: 10.1016/j.pupt.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D., Qi B.Y., Zhu W.W., Huang X., Wang X.Z. Crocin alleviates lipopolysaccharide-induced acute respiratory distress syndrome by protecting against glycocalyx damage and suppressing inflammatory signaling pathways. Inflamm. Res. 2020;69:267–278. doi: 10.1007/s00011-019-01314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong K.H., Xie Y., Huang X., Kadota K., Yao X.S., Yu Y., Chen X., Lu A., Yang Z. Delivering crocetin across the blood-brain barrier by using gamma-Cyclodextrin to treat Alzheimer’s disease. Sci. Rep. 2020;10:3654. doi: 10.1038/s41598-020-60293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stennett A.K., Dempsey G.L., Gainer J.L. trans-Sodium crocetinate and diffusion enhancement. J. Phys. Chem. B. 2006;110:18078–18080. doi: 10.1021/jp064308+. [DOI] [PubMed] [Google Scholar]
- 16.Guerin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., Mercier E., Badet M., Mercat A., Baudin O., Clavel M., Chatellier D., Jaber S., Rosselli S., Mancebo J., Sirodot M., Hilbert G., Bengler C., Richecoeur J., Gainnier M., Bayle F., Bourdin G., Leray V., Girard R., Baboi L., Ayzac L., P. S. Group Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data are available upon detailed and justified request to the sponsor of the study (secretariatdg@icans.eu). ANSM has previously reviewed all the presented data in this study and has full access to the data.