Abstract
Candida albicans is a prevalent human fungal pathogen. Rapid genomic change, due to aneuploidy, is a common mechanism that facilitates survival from multiple types of stresses including the few classes of available antifungal drugs. The stress survival of aneuploids occurs despite the fitness costs attributed to most aneuploids growing under idealized lab conditions. Systematic study of the aneuploid state in C. albicans has been hindered by the lack of a comprehensive collection of aneuploid strains. Here, we describe a collection of diploid C. albicans aneuploid strains, each carrying one extra copy of each chromosome, all from the same genetic background. We tested the fitness of this collection under several physiological conditions including shifts in pH, low glucose, oxidative stress, temperature, high osmolarity, membrane stress, and cell wall stress. We found that most aneuploids, under most conditions, were less fit than their euploid parent, yet there were specific conditions under which specific aneuploid isolates provided a fitness benefit relative to the euploid parent strain. Importantly, this fitness benefit was attributable to the change in the copy number of specific chromosomes. Thus, C. albicans can tolerate aneuploidy of each chromosome and some aneuploids confer improved growth under conditions that the yeast encounters in its host niches.
Keywords: Candida albicans, aneuploidy, fitness costs, fitness benefits, antifungal drugs, trisomy; tetrasomy, monosomy
Introduction
Candida albicans is the most prevalent human fungal pathogen (Pfaller et al. 2019). Aneuploidy, an unbalanced genomic state in which whole or segmental chromosomes, are missing or supernumerary, is often detected in yeasts and can be important for adaptation to stress conditions (reviewed in Tsai and Nelliat 2019). The first reported aneuploid karyotype in C. albicans that promoted stress adaptation was chromosome 5 monosomy (Chr5x1), which facilitated growth on l-sorbose as the sole carbon source (Janbon et al. 1998). In addition to whole chromosome aneuploidies, segmental aneuploidies alone can confer beneficial phenotypes under stress conditions. A classic example is isochromosome 5 L (i(5L)), which was acquired recurrently in clinical azole-resistant C. albicans isolates (Selmecki et al. 2006). i(5L) confers azole resistance because it provides two additional copies of two genes: ERG11, which encodes lanosterol 14-alpha-demethylase, an enzyme critical for ergosterol biosynthesis and the target of azole drugs, and TAC1, which encodes a positive regulator of efflux pump gene expression (Selmecki et al. 2008). Aneuploidy also can affect responses to other antifungal drugs. For example, chromosome 2 trisomy (Chr2x3) and Chr5x1 promote adaptation to caspofungin (Yang et al. 2017, 2019).
In C. albicans, certain chromosome changes recurrently appeared under specific drug selection conditions (Ford et al. 2015; Selmecki et al. 2008; Yang et al. 2013, 2017, 2019), while others did not. Karyotype-specific properties also appear in Saccharomyces cerevisiae: aneuploid strains with similar karyotypes have similar patterns with respect to their ability to grow across a range of different conditions (Pavelka et al. 2010). Different aneuploidies clearly give rise to different phenotypes, presumably because of specific genes that are present in altered dosage on the aneuploid chromosome (Selmecki et al. 2008; Sionov et al. 2010; Will et al. 2010). Furthermore, some aneuploidies yield multiple phenotypes that are attributable to different sets of gene(s) on the same aneuploid chromosome (Yang et al. 2019).
Some phenotypes may be associated with the aneuploid state per se. In S. cerevisiae, many aneuploid strains are sensitive to conditions that interfere with protein synthesis and protein folding, which require more energy (Torres et al. 2007). Furthermore, aneuploids exhibit general hypo-osmotic-like stress and endocytic defects and a dependence on the Art-Rsp5 pathway, due to proteome imbalance (Tsai et al. 2019). The ability to distinguish between phenotypes that are chromosome-specific and those that are general properties of aneuploidy ideally requires examination of all possible aneuploids. Thus, a systematic collection of C. albicans aneuploid strains would provide an important tool for distinguishing chromosome-specific phenotypes from general aneuploid properties.
In S. cerevisiae, two collections of disomic haploid strains were isolated using genetic manipulations (Pavelka et al. 2010; Torres et al. 2007). Because C. albicans does not appear to undergo meiosis, different approaches to obtain C. albicans aneuploids are required. This includes using the parasexual mating cycle, in which diploid cells of opposite mating type first mate to form tetraploid cells and then undergo concerted chromosome loss to return to the diploid or near-diploid state (Bennett and Johnson 2003), which often results in trisomic progeny, despite the lack of meiosis.
Given that many aneuploid chromosome combinations have been observed in C. albicans, it has long been suggested that C. albicans is more tolerant of aneuploidy than S. cerevisiae (Bouchonville et al. 2009). Aneuploidy clearly affects cell fitness differently under different growth conditions. In lab experiments in rich medium, S. cerevisiae disomic derivatives of haploids have fitness defects while trisomic derivatives of diploid isolates, as well as wild strains often exhibit lower fitness costs (Filteau et al. 2015; Hose et al. 2020; Pavelka et al. 2010; Peter et al. 2018; Torres et al. 2007; Will et al. 2010). Here we used a collection of C. albicans aneuploids to test this hypothesis for different trisomic homologs of each chromosome. We found that, in standard lab medium, all trisomic C. albicans strains were less fit than the diploid parent, and tetrasomic isolates as well as monosomy resulted in much lower fitness levels. Yet, specific aneuploidies conferred more rapid growth or higher biomass accumulation under some physiological stress conditions. This highlights the utility of this collection of strains, which has the potential to identify a broad range of phenotypes that may be altered by chromosome-specific and/or general features of aneuploidy.
Materials and methods
Identifying aneuploid isolates
As described previously (Yang et al. 2019), C. albicans reference strain SC5314 was streaked from −80°C glycerol stocks to YPD agar and incubated at 37°C for 24 h. Several randomly chosen colonies were suspended in distilled water, cell density was determined using a Cellometer (Nexcelom), and approximately 1.0 × 106 cells were spread onto YPD plates supplemented with drugs at concentrations that inhibited growth of the vast majority of SC5314 cells (Yang et al. 2017, 2019; Yang et al., manuscript in preparation). The plates were incubated at 30°C (fluconazole) or 37°C (other drugs) for 3 days. Rare colonies that appeared (usually at a frequency of 10−3 to 10−4) were randomly chosen and saved in −80°C glycerol stocks. Strains representing different karyotypes are listed in Supplementary Table S1.
Colony instability assay
As described previously (Yang et al. 2019), aneuploid strains were streaked from −80°C freezer to YPD agar and incubated at 37°C for 36 h. One small colony was randomly chosen and suspended in distilled water. Cells were diluted with distilled water and approximately 200 cells were spread on an YPD plate and incubated at 37°C for 36 h. One small (S) colony and one large (L) colony were randomly chosen for further studies.
Fitness assay
As described previously (Rosenberg et al. 2018), approximately 1 × 103 cells/ml were suspended in the medium specified in figure legends and 150 µl was transferred to 96-well plates. Optical density (OD595nm) was measured every 15 min for 36 h at 37°C using a Tecan plate reader (Infinite F200 PRO; Tecan, Switzerland).
Spot assay
As described previously (Yang et al. 2019), strains were streaked onto YPD agar, incubated at 37°C for 36 h (aneuploid strains) or 24 h (parent) and several colonies were chosen randomly and suspended in distilled water. Cell densities were adjusted to 1 × 107 cells/ml and 3 µl of 10-fold serial dilutions was spotted on YPD agar plates supplemented with the compounds described in figure legends. The plates were incubated at 37°C for 3 days and then photographed.
Assay of sensitivity to zymolyase
As described previously (Yang et al. 2013), cells were suspended in 10 mM Tris-HCl (pH 7.5) containing 50 µg/ml of zymolyase 20 T (U.S. Biological, Swampscott, MA, USA). Cell density was adjusted to OD600 of 0.5, incubated at 37°C for 4 h and the decrease in optical density was monitored at 1 h time intervals.
Next-generation sequencing was performed as described in Liu et al. (2018) with slight modifications. Briefly, cells were grown on YPD plates at 37°C at a density of approximately 300 colonies per plate. When colonies showed different sizes on the plates, only small colonies were collected. Colonies were dispersed in 1 ml distilled water and collected by centrifugation in a microfuge for 1 min. Genomic DNA was extracted using the phenol–chloroform method (Selmecki et al. 2015). The genomic DNA library was prepared by BGI (Wuhan, China) according to their standard preparation protocol. Briefly, genomic DNA (1 µg) was randomly fragmented with a Covaris LE220. An Agencourt AMPure XP-Medium kit was used to select, end repair, and 3′ adenylate fragments with average size of 300–400 bp, and to ligate adaptors to the ends of the 3′ adenylated fragment. The products were amplified by PCR and purified. The purified double-stranded PCR products were heat denatured and circularized with a splint oligo sequence. The single-strand circular DNA (ssCirDNA) was formatted as the final library and qualified by QC. The final qualified libraries were sequenced by BGISEQ-500. ssCir DNA molecules formed a DNA nanoball (DNB) containing more than 300 copies through rolling-cycle replication. The DNBs were loaded into a patterned nano array by using high-density DNA nano chip technology and were sequenced on the BGISEQ-500 platform using BGISEQ-500 high-throughput sequencing kit (PE100). Finally, pair-end 100 bp reads were obtained by combinational probe–anchor synthesis.
Variant calling
De novo variant detection was conducted using the Genome Analysis Toolkit (Mutect, v2.2-25) (Cibulskis et al. 2013). Called variants were annotated using SnpEff (V4.3) (Cingolani et al. 2012) using the SC5314 reference genome fasta and gene feature file (A21-s02-m09-r08). Parental variants were removed, and all remaining variants were verified visually using the Integrative Genomics Viewer (IGV, v2.8.2) (Robinson et al. 2017).
Statistical analysis
Significance of differences between parental and aneuploid growth curves was performed using a two-tailed paired t-test using GraphPad Prism (version 5.01).
Data availability
The genome sequence data are available at via ArrayExpress at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-9739. Supplemental material is available at figshare: https://doi.org/10.25386/genetics.14349062.
Results and discussion
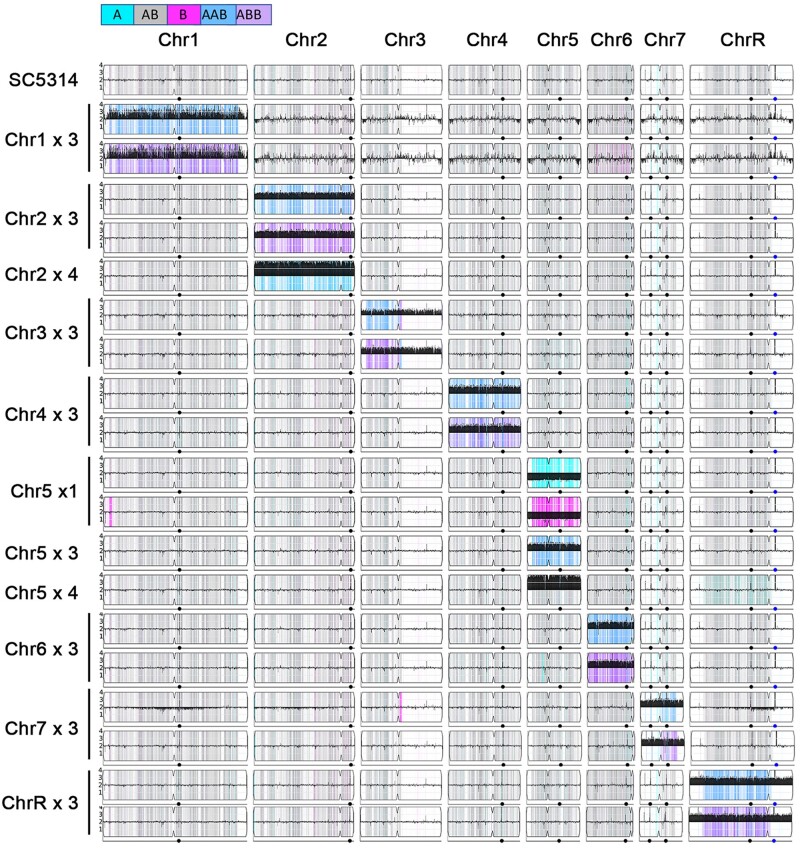
We used SC5314, a diploid laboratory strain with eight pairs of highly heterozygous chromosomes and an established haplotype (Legrand et al. 2008; Muzzey et al. 2013; van het Hoog et al. 2007). Over the years, we identified aneuploid isolates using a wide range of different selection pressures such as antifungal drug caspofungin and chemotherapeutic drug hydroxyurea (Yang et al. 2017, 2019). In addition, we also screened for mutants able to survive from azoles (fluconazole, ketoconazole, miconazole), another two members of echinocandins (micafungin, anidulafungin), flucytosine, amphotericin B, sphingolipid pathway inhibitors (myriocin and aureobasidin A), endoplasmic reticulum stress inducers (tunicamycin and brefeldin A), and other chemotherapeutic drugs such as fluorouracil (Yang et al., manuscript in preparation). Combining these collections allowed us to collect many aneuploid isolates, all derived from lab strain SC5314. The collection includes trisomic isolates with extra copies of each homolog of each chromosome (AAB and ABB) except Chr5 (AAB only), isolates with chromosomes 2 or 5 tetrasomy and chromosome 5 monosomy (Figure 1 and Supplementary Table S1).
Figure 1.
Ymap karyotypes of C. albicans aneuploid collection. Representative karyotypes of singly trisomic, tetrasomic, and monosomic strains derived from parent strain SC5314 generated using Ymap (Abbey et al. 2014) as described in (Yang et al. 2019). Read depth was normalized to that of the diploid parent and is shown on the y-axis on a log2 scale converted to absolute copy numbers (1–4). Allelic ratios (A:B) are color-coded: gray, 1:1 (A/B); cyan, 1:0 (A or A/A); magenta, 0:1 (B or B/B); purple, 1:2 (A/B/B); blue, 2:1 (A/A/B); light blue, 3:1 (A/A/A/B)).
Aneuploid isolates were initially identified based upon three criteria for phenotypic variation: first, improved growth, relative to the parent, in the presence of (at least one) specific stress; second, instability of the acquired adaptive state when selection was removed, which was usually detected as the formation of a mixture of smaller (S) and larger (L) colonies on nonselective medium (Yang et al. 2019) (Figure 2A); and third, loss of the adaptive state from the L colonies taken from nonselective medium (Yang et al. 2019). Next-generation sequencing of colony progeny that fulfilled the three criteria revealed aneuploidy in most isolates. Furthermore, the L derivatives of the initially aneuploid isolates (those that had lost the adaptive state) were euploid, consistent with the idea that the aneuploid chromosome facilitated growth in the presence of the relevant drug and that loss of the aneuploidy, in the absence of selection, was accompanied by the loss of the adaptive phenotype (Yang et al. 2013, 2019).
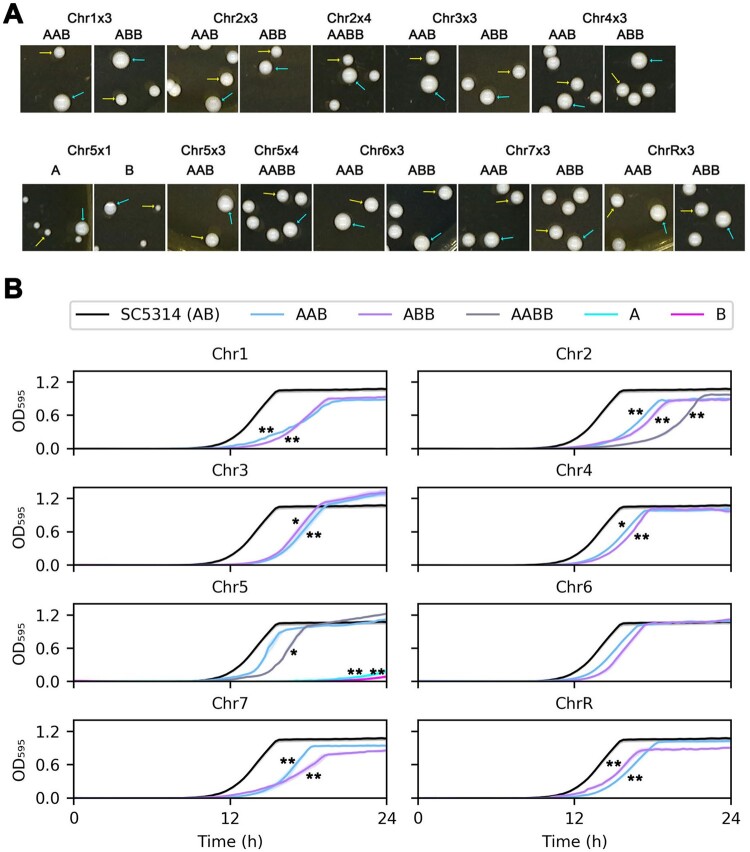
Figure 2.
Effects of aneuploidies on fitness in rich medium. In (A), approximately 200 colonies of each putative aneuploid strain were plated on YPD agar plates, incubated at 37°C for 36 h and then photographed. Yellow arrows indicate small (S) colonies. Cyan arrows indicate large (L) colonies. In (B), small colonies (S) for each isolate were chosen from YPD plates. Growth was monitored every 15 min at 37°C for 36 h. Data are represented as the mean ± SD of three biological replicates. * and ** indicate P-values <0.05 and P < 0.001, respectively, as determined by two-tailed tests.
Whole genome sequencing of the 19 isolates identified a total of 386 mutations compared to the SC5314 parent (Supplementary Table S2): 207 in coding regions and 179 mutations in the noncoding regions. Among the coding regions were 114 synonymous mutations, 90 missense mutations, 1 nonsense mutation, and 2 mutations in a noncoding transcript. Other than the Chr5x4 (AABB) strain, which had a heterozygous nonsense mutation of SEC7, a gene whose expression is repressed in caspofungin (Liu et al. 2005), none of the mutations affected genes with known effects on drug responses. The small number of SNPs, together with the reversion of the drug response in L colonies that reverted to euploidy, suggests that changes in drug responses were due to aneuploidies rather than solely to point mutations. However, we cannot rule out the possible contributions of some point mutations.
To test the fitness of the aneuploid collection, we first monitored growth dynamics in rich YPD broth. In general, different trisomic strains had different fitness costs, and the difference between large and small colony sizes seen for a given aneuploid strain (Figure 2A) was roughly proportional to the difference in growth rate seen for these colonies in growth curves (Figure 2B). Isolates monosomic for Chr5 also grew very slowly. Overall, most aneuploid strains (the S colonies) were less fit than their parent. Only trisomy of Chr5 and Chr6 did not incur obvious fitness losses in rich medium. Importantly, the L colonies, which were spontaneous homozygous diploid derivatives of the aneuploid S colonies formed large colonies and grew with kinetics like those of the parental strain (Supplementary Figure S1). While chromosome size (and the accompanying additional burden of extra DNA) might contribute to fitness loss, this cannot be the sole reason, given that trisomy of Chr7 (950 kb, the smallest chromosome) had a similar fitness cost to trisomy of Chr1 (3.2 MB, the largest chromosome). Of note, the measured fitness costs are probably underestimated, given that the return to the euploid (L colony) state occurred with a frequency of 10−2 to 10−3 and that these euploids had an increased growth relative to the aneuploids.
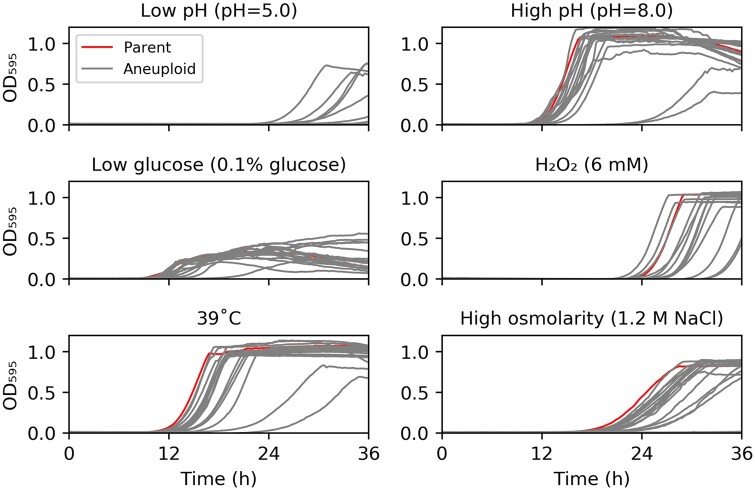
We also analyzed the fitness of the aneuploid collection under several stress conditions that reduce the growth of euploid strains including environmental perturbations, such as low (5.0) and high pH (8.0), nutrient limitation (0.1% glucose), oxidative stress (hydrogen peroxide, 6 mM), elevated temperature (39°C, as a proxy for febrile temperature), and high osmolarity (1.2 M NaCl) (Figure 3). We found a wide range of phenotypic variation across the aneuploid strains, and under particular stress conditions, particular aneuploidies conferred better fitness than the euploid parent. Chr7x3 (ABB) was better fit in the presence of H2O2, Chr3x3 (AAB and ABB), Chr6x3 (AAB), and Chr7x3 (ABB) were better fit at low pH. Under other stress conditions, the fitness difference between most trisomy strains and the euploid parent was also not as obvious as the difference when grown in the absence of stress. Here too, we think the fitness benefits of some aneuploid isolates under certain stress conditions likely are underestimates.
Figure 3.
Fitness under physiological stress conditions. SC5314 and the aneuploid collection were grown in YPD medium in 96-well plates under the following conditions: low pH (YEPD adjusted to pH 5.0), high pH (YPD with pH 8.0), low glucose (YPD with 0.1% glucose), oxidative stress (YPD supplemented with 6 mM H2O2), febrile temperature (39°C), and high osmolarity (YPD with 1.2 M NaCl). The plates were incubated at 37°C unless otherwise specified. Data represent the mean ± SD of three technical replicates.
To analyze fitness during exposure to cell membrane and cell wall stresses, we exposed the strains to sodium dodecyl sulfate (SDS) and caffeine. SDS is a detergent that disrupts cell membranes, activates stress responses including the cell wall integrity pathway, and restricts cell growth (Kono et al. 2012); caffeine elicits pleiotropic effects including inhibiting cell growth, reducing cell fitness, arresting the cell cycle, and ultimately leading to cell death (Kuranda et al. 2006) (Figure 4A). Interestingly, Chr6x3 (both AAB and ABB) grew better than the diploid parent strain on both SDS and caffeine. Given that Chr6x3 in an SC5314 derivative exhibited reduced virulence and increased commensal growth ability (Forche et al. 2019), we suggest that higher tolerance to cell membrane and cell wall stresses may underpin the improved ability of Chr6x3 trisomic isolates to colonize and reside in the host gastrointestinal tract.
Figure 4.
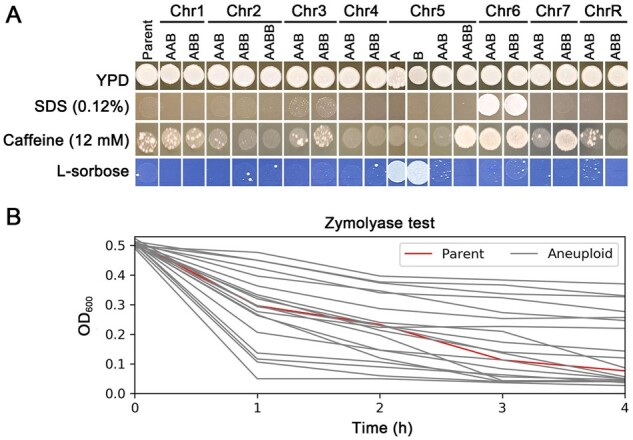
Effects of aneuploidies on membrane stress and cell wall stress. In (A), spot assays identify aneuploidies that confer increased and decreased fitness in different stresses. Comparison of parent and all trisomic, monosomic, and tetrasomic aneuploid strains for the ability to grow on SDS or caffeine, or sorbose (6.7 g/L yeast nitrogen base without amino acids, 2% l-sorbose, 2% agar). Each spot contained approximately 3.0×104 cells. Plates were incubated at 37°C for 3 days and then photographed. In (B), the parent (red line) and the aneuploid strains were suspended in 10 mM Tris-HCl (pH 7.5) containing 50 µg/ml of zymolyase. Cell suspensions were maintained at 37°C for 4 h. The optical density was measured at 1-h time intervals. Each data point was the mean ± SD of three technical replicates.
Relative to the euploid parent, several of the isolates, including Chr7x3 ABB, had a fitness benefit on caffeine, while several other aneuploids, including Chr7x3 AAB, had a fitness cost on caffeine. This suggests that the aneuploidy affects growth in caffeine stress in an allele-specific manner. Alternatively, the papillated growth seen in the euploid parent on caffeine may be due to the appearance of new aneuploidies that arise on the caffeine-containing plate, perhaps because caffeine can induce aneuploidy through asymmetric cell divisions (Katsuki et al. 2008). In this alternative model, specific genes on different aneuploid chromosomes likely affect both the ability to withstand the growth-inhibiting effects of caffeine, as well as the susceptibility to caffeine-enhanced genome instability. As expected (Janbon et al. 1998), Chr5x1, and no other aneuploid isolates grew well on sorbose. Finally, we found a wide range of sensitivity to cell wall digestion with zymolyase, a cocktail of 1,3-β-glucanase and protease activities used to degrade yeast cell walls. Zymolyase digestion of cell walls results in cell lysis due to increased internal turgor pressure (Ovalle et al. 1999) (Figure 4B).
In conclusion, C. albicans can tolerate trisomy of each chromosome. In the absence of stress, aneuploid strains are less fit but can spontaneously revert to better fitness by reverting to euploidy. Under particular stress conditions, some aneuploid chromosomes confer better fitness than the euploid parent. This collection can be used to identify karyotype-specific properties, as well as commonalities shared by aneuploid strains, both of which provide potential antifungal drug targets. RNA-seq and proteome analysis of the collection isolates will facilitate the identification of druggable targets. Considering the limited arsenal of antifungal drugs, large scale screening for novel chemicals that can specifically eliminate aneuploid cells, or that have synergistic effects with current antifungal drugs, will extend available therapeutic options. The collection is also useful for investigating the evolvability of aneuploidy to ask whether aneuploidy drives genome instability and accelerates the appearance of new traits. We hope this collection will be used as a community resource for studying the effects of specific aneuploids and aneuploidy in general, and we will make it available upon request.
Acknowledgments
The authors thank Dr. Hung-Ji Tsai for critical reading of the manuscript. The authors apologize to authors whose work has not been cited due to limitations of space.
Funding
This project was supported by the National Natural Science Foundation of China (No. 82020108032 to Y-.Y.J., No. 81673478 and No. 81872910 to Y-.B.C.), Shanghai Key Basic Research Project (No. 19JC1414900 to Y-.B.C.), National Institute of Health (R01AI143689) and the Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Diseases (No. 1020388) to A.S., and the Israel Science foundation (#997/18 to J.B.).
Conflicts of interest
None declared.
Literature cited
- Bennett RJ, Johnson AD.. 2003. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 22:2505–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchonville K, Forche A, Tang KE, Selmecki A, Berman J.. 2009. Aneuploid chromosomes are highly unstable during DNA transformation of Candida albicans. Eukaryot Cell. 8:1554–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, et al. 2013. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 31:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang Le L, Coon M, Nguyen T, et al. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 6:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filteau M, Hamel V, Pouliot MC, Gagnon-Arsenault I, Dube AK, et al. 2015. Evolutionary rescue by compensatory mutations is constrained by genomic and environmental backgrounds. Mol Syst Biol. 11:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, Solis NV, Swidergall M, Thomas R, Guyer A, et al. 2019. Selection of Candida albicans trisomy during oropharyngeal infection results in a commensal-like phenotype. PLoS Genet. 15:e1008137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CB, Funt JM, Abbey D, Issi L, Guiducci C, et al. 2015. The evolution of drug resistance in clinical isolates of Candida albicans. Elife. 4:e00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose J, Escalante LE, Clowers KJ, Dutcher HA, Robinson D, et al. 2020. The genetic basis of aneuploidy tolerance in wild yeast. Elife. 9:e52063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon G, Sherman F, Rustchenko E.. 1998. Monosomy of a specific chromosome determines L-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc Natl Acad Sci U S A. 95:5150–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki Y, Nakada S, Yokoyama T, Imoto I, Inazawa J, et al. 2008. Caffeine yields aneuploidy through asymmetrical cell division caused by misalignment of chromosomes. Cancer Sci. 99:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K, Saeki Y, Yoshida S, Tanaka K, Pellman D.. 2012. Proteasomal degradation resolves competition between cell polarization and cellular wound healing. Cell. 150:151–164. [DOI] [PubMed] [Google Scholar]
- Kuranda K, Leberre V, Sokol S, Palamarczyk G, Francois J.. 2006. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol Microbiol. 61:1147–1166. [DOI] [PubMed] [Google Scholar]
- Legrand M, Forche A, Selmecki A, Chan C, Kirkpatrick DT, et al. 2008. Haplotype mapping of a diploid non-meiotic organism using existing and induced aneuploidies. PLoS Genet. 4:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Lee REB, Barker KS, Lee RE, Wei L, . et al. 2005. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob Agents Chemother. 49:2226–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Luo Z, Wang Y, Pham NT, Tuck L, . et al. 2018. Rapid pathway prototyping and engineering using in vitro and in vivo synthetic genome SCRaMbLE-in methods. Nat Commun. 9:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzey D, Schwartz K, Weissman JS, Sherlock G.. 2013. Assembly of a phased diploid Candida albicans genome facilitates allele-specific measurements and provides a simple model for repeat and indel structure. Genome Biol. 14:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovalle R, Spencer M, Thiwanont M, Lipke PN.. 1999. The spheroplast lysis assay for yeast in microtiter plate format. Appl Environ Microbiol. 65:3325–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelka N, Rancati G, Zhu J, Bradford WD, Saraf A, et al. 2010. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature. 468:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J, De Chiara M, Friedrich A, Yue JX, Pflieger D, et al. 2018. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature. 556:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN.. 2019. Twenty years of the SENTRY Antifungal Surveillance Program: results for Candida species from 1997-2016. Open Forum Infect Dis. 6:S79–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Wenger AM, Zehir A, Mesirov JP.. 2017. Variant review with the integrative genomics viewer. Cancer Res. 77:e31–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A, Ene IV, Bibi M, Zakin S, Segal ES, et al. 2018. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat Commun. 9:2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Forche A, Berman J.. 2006. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 313:367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J.. 2008. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 68:624–641. [DOI] [PubMed] [Google Scholar]
- Selmecki AM, Maruvka YE, Richmond PA, Guillet M, Shoresh N., et al. 2015. Polyploidy can drive rapid adaptation in yeast. Nature 519:349–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionov E, Lee H, Chang YC, Kwon-Chung KJ.. 2010. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 6:e1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, et al. 2007. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 317:916–924. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Nelliat A.. 2019. A double-edged sword: aneuploidy is a prevalent strategy in fungal adaptation. Genes (Basel). 10:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HJ, Nelliat AR, Choudhury MI, Kucharavy A, Bradford WD, et al. 2019. Hypo-osmotic-like stress underlies general cellular defects of aneuploidy. Nature. 570:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Het Hoog M, Rast TJ, Martchenko M, Grindle S, Dignard D, et al. 2007. Assembly of the Candida albicans genome into sixteen supercontigs aligned on the eight chromosomes. Genome Biol. 8:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will JL, Kim HS, Clarke J, Painter JC, Fay JC, et al. 2010. Incipient balancing selection through adaptive loss of aquaporins in natural Saccharomyces cerevisiae populations. PLoS Genet. 6:e1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Bijlani S, Jiang Y-y, Denga J, MacCallum D et al. Tolerance mechanisms dependent upon aneuploidies that confer cross tolerance to other antifungals. In preparation. [Google Scholar]
- Yang F, Kravets A, Bethlendy G, Welle S, Rustchenko E.. 2013. Chromosome 5 monosomy of Candida albicans controls susceptibility to various toxic agents, including major antifungals. Antimicrob Agents Chemother. 57:5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Teoh F, Tan ASM, Cao Y, Pavelka N, et al. 2019. Aneuploidy enables cross-adaptation to unrelated drugs. Mol Biol Evol. 36:1768–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Zhang L, Wakabayashi H, Myers J, Jiang Y, et al. 2017. Tolerance to caspofungin in Candida albicans is associated with at least three distinctive mechanisms that govern expression of FKS genes and cell wall remodeling. Antimicrob Agents Chemother. 61:e00071-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genome sequence data are available at via ArrayExpress at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-9739. Supplemental material is available at figshare: https://doi.org/10.25386/genetics.14349062.