Background:
Anterior vertebral body tethering (AVBT) is a growth-modulation technique theorized to correct adolescent idiopathic scoliosis (AIS) without the postoperative stiffness imposed by posterior spinal fusion. However, data are limited to small series examining short-term outcomes. To assess AVBT’s potential as a viable alternative to posterior spinal fusion (PSF), a comprehensive comparison is warranted. The purpose of this meta-analysis was to compare postoperative outcomes between patients with AIS undergoing PSF and AVBT. Our primary objective was to compare complication and reoperation rates at available follow-up times. Secondary objectives included comparing mid-term Scoliosis Research Society (SRS)-22 scores, and coronal and sagittal-plane Cobb angle corrections.
Methods:
We performed a systematic review of outcome studies following AVBT and/or PSF procedures. The inclusion criteria included the following: AVBT and/or PSF procedures; Lenke 1 or 2 curves; an age of 10 to 18 years for >90% of the patient population; <10% non-AIS scoliosis etiology; and follow-up of ≥1 year. A single-arm, random-effects meta-analysis was performed. Deformity corrections, complication and reoperation rates, and postoperative SRS-22 scores were recorded.
Results:
Ten AVBT studies (211 patients) and 14 PSF studies (1,069 patients) were included. The mean follow-up durations were similar for both groups. Pooled complication rates were 26% for AVBT versus 2% for PSF, and reoperation rates were 14.1% for AVBT versus 0.6% for PSF with nonoverlapping confidence intervals (CIs). The pooled reoperation rate among studies with follow-up times of ≥36 months was 24.7% in AVBT versus 1.8% in PSF. Deformity correction, clinical outcomes, and mid-term SRS-22 scores were similar.
Conclusions:
Our study showed greater rates of complications and reoperations with AVBT compared with PSF. Reoperation rates were significantly greater in AVBT studies with longer follow-up (≥36 months). Deformity correction, clinical outcomes, and mid-term SRS-22 scores were similar. While a potential fusionless treatment for AIS merits excitement, clinicians should consider AVBT with caution. Future long-term randomized prospective studies are needed.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Adolescent idiopathic scoliosis (AIS) is the most common spinal deformity treated by pediatric orthopaedic surgeons1. The current gold standard is posterior spinal fusion (PSF) with pedicle screw fixation, which has shown to be a robust technique for correcting scoliotic deformities2. PSF has been associated with a low complication rate and good long-term outcomes3-5. However, concern exists regarding stiffness imparted by PSF along with long-term effects of adjacent-segment disease, but recent studies suggest that stiffness may not be as substantial of a concern as previously thought6. Moreover, despite good outcomes and a low complication rate, PSF is perceived to be accompanied by perioperative or long-term complications: infection, adding-on, pulmonary and neurological injury, and disc degeneration7-9. As such, interest in non-fusion solutions for AIS correction persists.
In August 2019, anterior vertebral body tethering (AVBT) was granted humanitarian device exemption status by the U.S. Food and Drug Administration (FDA) for skeletally immature patients (Risser score of ≤2 or Sanders score of ≤5) with curves between 30° and 65°, although more recent literature has suggested 45° to 65°10. AVBT harnesses the Hueter-Volkmann principle to guide growth and correct the deformity, and while growth-modulation techniques are not new, earlier strategies, such as vertebral stapling, have demonstrated challenges such as implant loosening10. In AVBT, compressive forces applied to the convexity of the deformity by a polyethylene tether allow the patient’s growth to realign the spine. This concept has been applied to growth plates across the physis of the tibia and femur to correct deformities. AVBT extrapolates this concept to spinal deformity correction11 and has shown promise in animal models12,13.
Hitherto, most clinical studies of AVBT have been single-surgeon or institutional case series with varying results. To assess AVBT’s potential as an alternative to PSF, a comprehensive comparison is warranted. To our knowledge, apart from a recent retrospective cohort analysis of 49 patients, which indicated greater residual deformity, complications, and revisions for AVBT compared with PSF at 5 years14, no study has investigated differences in early and mid-term outcomes between patients undergoing AVBT and PSF.
The purpose of this systematic review and meta-analysis was to compare outcomes of patients with AIS undergoing PSF and AVBT. Our primary objective was to compare complication and reoperation rates. Secondary objectives included a comparison of Scoliosis Research Society (SRS)-22 scores and coronal and sagittal-plane Cobb angle measurements.
Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Literature Search
A systematic review of the literature was conducted for outcome studies following AVBT and/or PSF procedures. Reports from annual meetings of the SRS and in PubMed MEDLINE, Scopus, and Embase were included in the search (see Appendix Supplementary Table 1). The references of included articles were also screened for potential articles. All studies underwent review by 2 independent reviewers via Rayyan review software15. Our inclusion criteria were as follows: (1) human clinical outcomes study, (2) follow-up of ≥2 years, (3) selective thoracic fusion or Lenke 1 or 2 curves, (4) patient age of 10 to 18 years, (5) AIS, (6) primary surgical procedures, and (7) at least 1 study arm with AVBT or PSF procedures. Case reports were excluded. Studies investigating anterior vertebral body stapling, Harrington rods, and anterior fusion were excluded.
On initial review, the AVBT studies meeting these criteria were too few to conduct a satisfactory meta-analysis. Therefore, AVBT and PSF studies reporting clinical outcomes with ≥1 year of follow-up were further reviewed, and studies with patients younger than 10 years or those with a non-AIS scoliosis etiology were included if they comprised <10% of the patient population. Additionally, studies including patients with non-selective thoracic fusion or non-Lenke 1 or 2 curves were reviewed for patient-level data. If available, outcomes were recalculated without these patients and included.
Data Extraction
When reported, rates of complications and reoperation were extracted from each study. In some instances, the total number of complications was reported in lieu of the number of patients affected by complications. In these instances, we used the minimum possible number of patients with complications to calculate our complication rate, ensuring that complication rates would not be overestimated. Complications and reoperations were only recorded as 0 if reported as such. Otherwise, we assumed that complications were not recorded and patients from those studies were not included in the respective analyses. Additional data variables are illustrated in Tables I and II.
TABLE I.
Characteristics of Included Studies*
| Characteristic | AVBT | PSF | ||
| Studies | Patients | Studies | Patients | |
| Total | 10 | 211 | 14 | 1,069 |
| Study design | ||||
| Case series | 9 (90) | 188 (89.1) | 1 (7.1) | 21 (2.0) |
| Retrospective cohort | 1 (10) | 23 (10.9) | 11 (78.6) | 943 (88.2) |
| Prospective cohort | 0 (0) | 0 (0) | 1 (7.1) | 64 (6.0) |
| RCT | 0 (0) | 0 (0) | 1 (7.1) | 41 (3.8) |
| Scoliosis etiology | ||||
| Idiopathic | 9 (90) | 209 (99.0) | 14 (100) | 1,069 (100) |
| Syndromic | 1 (10) | 2 (1.0) | 0 (0) | 0 (0) |
| Surgical technique† | ||||
| Open | 2 (20) | 37 (17.5) | 14 (100) | 1,069 (100) |
| Thoracoscopic | 8 (80) | 121 (57.3) | 0 (0) | 0 (0) |
| Not reported | 1 (10) | 53 (25.2) | 0 (0) | 0 (0) |
| Follow-up | ||||
| First erect | 8 (80) | |||
| Through 24 mo | 7 (80) | 159 (75.4) | 14 (100) | 1,057 (98.9) |
| Through 36 mo | 4 (40) | 105 (49.8) | 8 (57.1) | 730 (68.3) |
| Through 48 mo | 2 (20) | 58 (27.5) | 3 (21.4) | 494 (46.2) |
AVBT = anterior vertebral body tethering, and PSF = posterior spinal fusion. The values are given as the number, with the percentage in parentheses.
One study included patients with both open and thoracoscopic procedures.
TABLE II.
Additional Characteristics of Included Studies*
| Characteristic | AVBT | PSF |
| Sample size, median (IQR) | 20.0 (11.5-28.5) | 51.5 (32.3-112.5) |
| Publication year, median (IQR) | 2018 (2017-2019) | 2016.5 (2013-2017) |
| Female (no. [%]) | 140 (66.4) | 445 (41.6) |
| Age (yr) | 12.4 | 14.2 |
| Follow-up (mo) | 33.7 | 46.9 |
| Preop. Risser score | 0.4 | 1.4 |
| Preop. Sanders score | 3.1 | 3.7 |
| Preop. flexibility (°) | 44.6 | 39.2 |
| Vertebrae fused/tethered (no.) | 7.3 | 10.2 |
AVBT = anterior vertebral body tethering, PSF = posterior spinal fusion, and IQR = interquartile range. Frequency weighted averages are reported unless otherwise specified.
Imputation
Studies that did not provide means for a particular outcome or patient-level data to recalculate the means were not used for the respective analysis. To provide the most conservative estimates for curve measurements, worst-case imputation was used when averages for curve measurements were provided but standard deviations (SDs) were not. Worst-case imputation involved imputing missing SDs on the basis of the largest SD provided by other studies within the same subgroup. Worst-case-scenario imputation has been recognized as among the most conservative approaches for dealing with missing data in meta-analyses16.
Statistical Analysis
Because most AVBT studies are case series, a single-arm meta-analysis was performed. Study characteristics were weighted on the sample size. Pooled means of primary and secondary outcomes were calculated using a random-effects model with a restricted maximum-likelihood (REML) approach. Compared with the traditional maximum-likelihood approach, REML is protective against underestimating standard errors and producing overconservative results17,18. To account for the skewed complication rate, we calculated the pooled effect size by taking the logarithm of the complication rate plus 1. Pooled means and standard errors were used to calculate effect sizes and 95% confidence intervals (CIs) for secondary outcomes. If an outcome of interest was only reported by 1 study, it was excluded from analysis. A random-effects model was used to account for the predicted heterogeneity among studies. Heterogeneity was assessed using I2. Publication bias was assessed using funnel plots and an Egger test. Significance for primary outcome comparisons was defined as nonoverlapping CIs. Calculations were performed using Stata 14.2 (StataCorp).
Source of Funding
There was no external source of funding.
Results
Studies and Patients
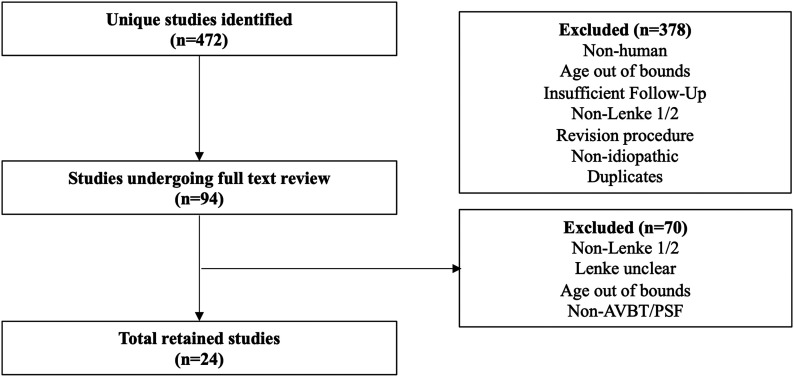
Twenty-four studies19-42 (38 arms) met the inclusion criteria (Fig. 1) and were used for meta-analysis (Tables I and II). Ten studies (n = 211) investigated AVBT outcomes, with an average follow-up of 33.7 months (range, 14.4 to 49.5 months). Fourteen studies (n = 1,069) investigated PSF outcomes, with average follow-up of 46.9 months (range, 21.2 to 86.4 months). There were 10 case series, 12 retrospective cohort studies, 1 prospective cohort study, and 1 randomized controlled trial (RCT). Mean preoperative Risser scores were 0.4 and 1.4 for AVBT and PSF patients, respectively (Table II). The average number of vertebrae fused or tethered was 7.3 for patients who underwent AVBT and 10.2 for those with PSF. The number and percentage of studies and patients with data reported for specific preoperative and postoperative measures can be found in Supplementary Table 2 (see Appendix).
Fig. 1.
Flow diagram of our search algorithm showing the total number of studies reviewed and included and the reasons for exclusion.
Complications and Reoperation Rates
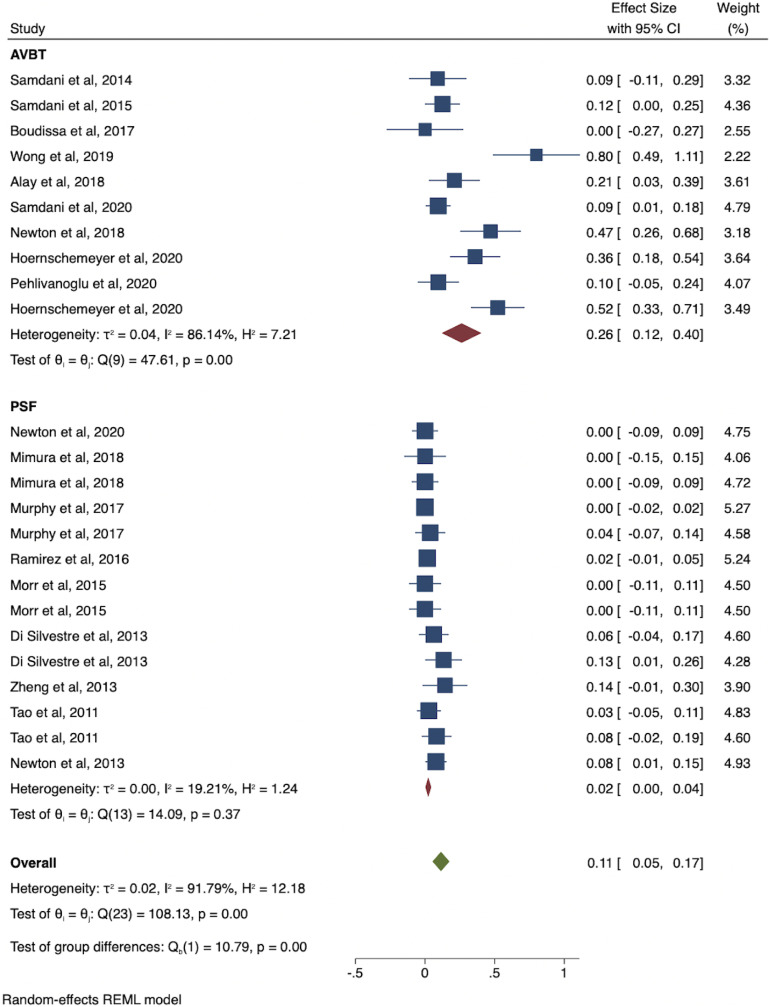
Complication data were available for all patients with AVBT (n = 211) and 57% of those with PSF (n = 610). The pooled complication rate was 26% (95% CI: 12% to 40%) for AVBT studies and 2% (95% CI: 0% to 4%) for PSF studies (Table III). Meta-analysis indicated nonoverlapping CIs between the 2 groups (Fig. 2). The most common complications for AVBT were tether breakage (n = 17; 7.5%), overcorrection (n = 17; 7.5%), and pulmonary complications (n = 11; 4.8%) (Table IV). The pooled complication rate excluding tether breakages for the AVBT group was 17.4% (95% CI: 8.0% to 26.7%). The most common complications for PSF were neurological complications (n = 6; 0.5%), screw pullout/loosening (n = 6; 0.5%), and infection (n = 4; 0.3%). Of studies with follow-up of <36 months, the pooled complication rate for AVBT was 11.8% (CI: 4.4% to 18.6%) and the reoperation rate was 2.9% (95% CI: 0% to 8.4%), and the pooled complication rate for PSF was 1.0% (95% CI: 0% to 2.4%) and the reoperation rate was 1.3% (0% to 1.7%). Of studies with follow-up of ≥36 months, the pooled complication rate for AVBT was 25.2% (95% CI: 19.1% to 31.7%) and the reoperation rate was 24.7% (95% CI: 10.7% to 38.7%), and the pooled complication rate for PSF was 2.9% (95% CI: 0.5% to 5.3%) and the reoperation rate was 1.8% (95% CI: 0% to 5.4%). There was heterogeneity among all studies reporting complication rates (I2 = 91.8%) and within the AVBT group (I2 = 86.1%). Pooled reoperation rates for AVBT and PSF were 14.1% (95% CI: 5.6% to 22.6%) and 0.6% (95% CI: 0.0% to 2.3%). The most common reason for reoperation following AVBT was overcorrection (n = 17; 7.7%), and the most common reason following PSF was adding-on (n = 2; 0.3%) (Table V). The pooled AVBT-to-PSF conversion rate was 1.4% (CI: 0% to 4.5%). All conversions were due to deformity progression despite tethering.
Fig. 2.
Forest plot of pooled complication rates between AVBT and PSF studies.
TABLE III.
Aggregate Postoperative Outcomes in AVBT and PSF Studies*
| Outcome | Pooled Mean (95% Confidence Interval) | |
| AVBT | PSF | |
| Complication rate (%) | 26.0 (12.0-40.0) | 2.0 (0.0-4.0) |
| <36 mo | 11.8 (4.4-18.6) | 1.0 (0.0-2.4) |
| ≥36 mo | 25.2 (19.1-31.7) | 2.9 (0.5-5.3) |
| Reoperation rate (%) | 14.1 (5.6-22.6) | 0.6 (0.0-2.3) |
| <36 mo | 2.9 (0.0-8.4) | 1.3 (0.0-1.7) |
| ≥36 mo | 24.7 (10.7-38.7) | 1.8 (0-5.4) |
| Conversion to PSF (%) | 1.4 (0-4.5) | Not applicable |
| Main thoracic curve (°) | ||
| Preop. | 46.0 (42.3-50.0) | 53.3 (52.8-53.9) |
| First erect | 24.9 (20.1-29.8) | 16.6 (12.8-20.3) |
| 12 to <24 mo | 24.6 (17.8-31.4) | 13.3 (8.7-17.8) |
| ≥24 to <36 mo | 21.5 (8.3-34.7) | 21.9 (17.4-26.4) |
| ≥36 mo | 22.5 (14.1-30.9) | 22.7 (19.6-25.8) |
| Compensatory lumbar curve (°) | ||
| Preop. | 28.7 (25.6-32.0) | 30.9 (29.2-32.5) |
| First erect | 19.3 (16.6-22.4) | 9.9 (8.1-11.7) |
| 12 to <24 mo | 16.5 (11.2-21.7) | Insufficient data |
| ≥24 to <36 mo | 13.2 (8.4-18.0) | 10.7 (8.0-13.5) |
| ≥36 mo | 18.0 (3.5-32.5) | 15.2 (13.3-17.1) |
| Thoracic kyphosis (°) | ||
| Preop. | 24.3 (17.8-30.8) | 23.0 (20.7-25.2) |
| First erect | 22.1 (16.5-27.7) | 31.0 (27.9-33.3) |
| 12 to <24 mo | 25.0 (13.4-36.6) | Insufficient data |
| ≥24 to <36 mo | 23.0 (19.6-26.4) | 17.9 (15.1-20.7) |
| ≥36 mo | 22.5 (12.0-33.0) | 24.5 (21.9-27.1) |
| Lumbar lordosis (°) | ||
| Preop. | 52.0 (46.2-57.9) | 47.2 (28.1-66.3) |
| First erect | 46.5 (40.1-52.8) | Insufficient data |
| 12 to <24 mo | 56.0 (47.2-64.9) | Insufficient data |
| ≥24 to <36 mo | 52.7 (48.6-56.8) | 46.3 (42.3-50.3) |
| ≥36 mo | 55.1 (51.3-58.8) | 46.1 (25.0-67.1) |
| Thoracic rotation (°) | ||
| Preop. | 13.7 (12.1-15.2) | 15.4 (12.4-18.4) |
| First erect | 10.0 (8.7-11.2) | 6.0 (4.5-7.5) |
| 12 to <24 mo | 8.1 (5.9-10.3) | Insufficient data |
| ≥24 to <36 mo | 6.9 (4.8-8.9) | 8.07 (5.0-11.1) |
| ≥36 mo | 8.4 (1.0-15.7) | 13.0 (3.3-22.6) |
| Postop. SRS-22 self-image | 4.27 (4.0-4.56) | 4.23 (4.07-4.40) |
| Postop. SRS-22 total | 4.36 (4.06-4.65) | 4.30 (4.17-4.43) |
AVBT = anterior vertebral body tethering, and PSF = posterior spinal fusion. The complication rate is reported as the minimum possible number of patients with at least 1 complication. Nonoverlapping confidence intervals for the primary outcomes of interest are highlighted in bold.
TABLE IV.
Characteristics of Complications*
| Complication | AVBT | PSF |
| Tether breakage | 17 (7.5) | — |
| Overcorrection | 17 (7.5) | 2 (0.15) |
| Pulmonary† | 11 (4.8) | 1 (0.08) |
| Neurological | 2 (0.88) | 6 (0.46) |
| Infection | 1 (0.44) | 4 (0.31) |
| Adding-on | 7 (3.2) | 2 (0.30) |
| Screw pullout/loosening | 1 (0.44) | 6 (0.46) |
| Other‡ | 11 (4.8) | 6 (0.46) |
| Overall | 67 (26.0) | 27 (2.0) |
The values are given as the number, with the percentage in parentheses. Percentages were calculated by finding the pooled average of the complication of interest/total number of patients in studies reporting complication data. AVBT = anterior vertebral body tethering, and PSF = posterior spinal fusion.
Pulmonary complications included atelectasis, pleural effusion, pulmonary edema, and pneumothorax.
Indicates complications mild in nature, reported as “other” or with a frequency of 1 across all studies.
TABLE V.
Reasons for Reoperation*
| Reason | AVBT | PSF |
| Tether breakage/pedicle screw loosening | 7 (3.2) | 1 (0.15) |
| Overcorrection | 17 (7.7) | 0 (0) |
| Adding-on | 7 (3.2) | 2 (0.30) |
| Other† | 0 (0) | 1 (0.15) |
| Overall | 31 (14.1) | 4 (0.6) |
The values are given as the number, with the percentage in parentheses. Percentages were calculated by finding the pooled average of the number of reoperations/total number of patients in studies reporting reoperation data. AVBT = anterior vertebral body tethering, and PSF = posterior spinal fusion.
Rib hump deformity.
Coronal-Plane Angles
SDs were imputed for 12 PSF cohorts. No imputations for AVBT were necessary. Baseline Cobb angles for the groups were disparate (AVBT: 46.0°; PSF: 55.3°). Cobb angles in studies reporting ≥36 months of follow-up were similar (AVBT: 22.5°; PSF: 22.7°). Preoperative (AVBT: 28.7°; PSF: 30.9°) and final (AVBT: 18.0°; PSF: 15.2°) lumbar curves in studies reporting ≥36 months of follow-up were similar.
Sagittal Alignment and Thoracic Rotation
Preoperative thoracic kyphosis (AVBT: 24.3°; PSF: 23.0°), lumbar lordosis (AVBT: 52.0°; PSF: 47.2°), and thoracic rotation (AVBT: 13.7°; PSF: 15.4°) angles were similar. Both groups displayed similar findings for all 3 measurements at ≥36 months of follow-up (Table III).
SRS-22 Scores
Postoperative SRS-22 scores were reported in 2 AVBT studies and 7 PSF studies. There was no significant difference found between AVBT and PSF for the postoperative SRS-22 self-image (4.27 versus 4.23) or total scores (4.36 versus 4.3).
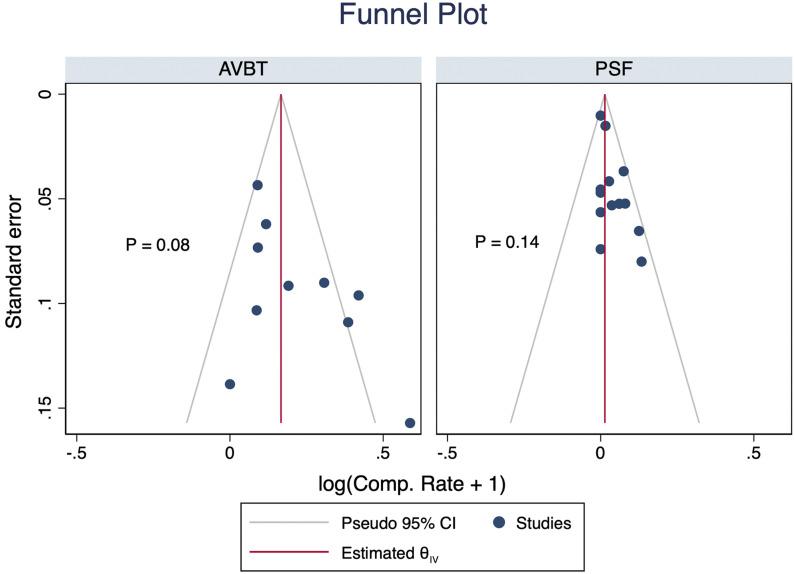
Publication Bias
No significant publication bias was detected (Fig. 3).
Fig. 3.
Funnel plot for detection of publication bias.
Discussion
Since the Harrington instrumentation era, spine surgeons have sought a fusionless solution to AIS, for which AVBT has recently emerged. Animal models have been promising, and the technique has demonstrated favorable perioperative outcomes compared with PSF, including decreased operative time, blood loss, length of stay, and return-to-school time13,14,42. However, whether AVBT is noninferior to spinal fusion with regard to mid-term postoperative outcomes remains unclear. Additionally, it is unclear whether AVBT actually modulates growth longitudinally or preserves motion, and which patients stand to benefit by this technique. Before it can be considered a treatment of choice, AVBT must be compared with PSF so clinicians can properly counsel families. This study is the first, to our knowledge, to comprehensively report on aggregate complication and reoperation data following AVBT for the treatment of patients with predominantly thoracic curves.
We found that patients receiving AVBT had higher complication rates compared with those with PSF. While it is difficult to compare these 2 groups statistically, the CIs of the groups were nonoverlapping (PSF, 0% to 4%; AVBT, 12% to 40%). The most common complication affecting AVBT patients was tether breakage (7.5%). The durability of the polyethylene tether was previously questioned by Newton et al., who found that 52% of patients had a suspected or broken tether compromising curve correction at 5-year follow-up14. If breakage occurs prior to skeletal maturity, patients may risk continued progression and subsequent revision. Moreover, a lack of long-term studies regarding the effect of particulate debris on lung tissue or the thoracic cage from a severed polyethylene cable may result in complications yet to be identified43,44. If one accepts AVBT tether breakage as an expected outcome, the complication rate for AVBT was 17.4% (95% CI: 8.0% to 26.7%), which still had a CI that did not overlap with the upper bound of the PSF group. Moreover, we compared complication and reoperation rates between studies with follow-up times of <36 months with those with follow-up times of ≥36 months. AVBT studies with follow-up of ≥36 months reported reoperation and complication rates with CIs higher than those with shorter follow-up times, indicating that AVBT outcomes may deteriorate with time, suggesting that the minimum follow-up in clinical studies on AVBT should be ≥36 months rather than the traditional 2 years.
Overcorrection was the second most common AVBT complication (7.5%) and the most common reason for reoperation. While growth potential is thought to drive progressive correction in AVBT, it also poses a risk of overcorrection. Wong et al. felt that this was a greater risk in patients with an open triradiate cartilage; however, the exact risk factor or method for mitigating this risk is unclear22. Because the expected growth curve in the spine is less well understood than that in the lower extremity, growth must be closely monitored. As predictive modeling of vertebral body growth improves, surgeons may be able to more closely identify the “window of opportunity” to modulate coronal-plane deformity and minimize this complication. Additionally, we found that 16% of all AVBT complications were pulmonary-related, which substantiates previous speculation that AVBT’s anterior thoracoscopic approach may be associated with pulmonary disturbances14. Despite increased complications, AVBT is still in its infancy. Surgeon experience and limited knowledge of optimal operative techniques and tether properties may impact results. Previous authors have noted decreases in operative time with increased experience19. This is highlighted by our range of complication-rate data (0% to 80%). PSF with pedicle screws has been used for AIS for >25 years, and substantial research has been directed at decreasing the surgical complication profile of intervention for AIS5. Ten-year follow-up data on selective PSF procedures showed exceptionally durable outcomes and a low revision rate44.
We also investigated coronal and sagittal deformity correction at various postoperative times. Preoperative main thoracic curves for the AVBT and PSF cohorts had nonoverlapping CIs, suggesting potential baseline differences in patient populations. The most recently published AVBT indications include Risser 0 or 1 or Sanders 3 or 4 patients with primary thoracic curves between 45° and 65°10. While indications are evolving, AVBT is generally thought to require “growth remaining” to harness growth modulation. Thus, it is unsurprising that patients who undergo AVBT are younger with smaller curves. Interestingly, there were no differences between the groups in any measurements at the postoperative follow-up periods of ≥24 to <36 months and ≥36 months. While there appeared to be coronal-deformity correction at first-erect measurements, there was no significant difference between those made at first-erect and those at ≥36 months in the AVBT group. Given AVBT’s theorized growth-correction principle, one would expect continual reduction over time. Our results, along with those of recent studies examining long-term postoperative changes18, suggest otherwise. The reason for this may lie in age differences found in AVBT studies. Wong et al. suggested that tensioning of the tether should be related to curve size and growth remaining22. A younger patient is likely to have more correction over time than a skeletally mature patient. As such, tension could be adjusted accordingly. The ideal patient for AVBT remains unclear but would likely include a skeletally younger patient with a curve approaching the traditional surgical range for PSF.
Finally, our study found that postoperative SRS-22 self-image and total scores were similar between the groups. However, only 36% of patients undergoing AVBT and 57.6% undergoing PSF had recorded SRS-22 outcomes; thus, readers should cautiously interpret these results. Future research efforts in this area are necessary.
This meta-analysis had limitations. First, nearly all of the AVBT articles were case series, limiting our study design to a single-arm meta-analysis. Thus, the data in this study stem from heterogenous patient populations, and the potential for confounding abounds. Next, the lack of Cobb-angle-correction SDs precluded accurate estimations of the variability of correction rates. Third, SDs for curve measurements were frequently unreported in the PSF group, which necessitated imputation. Fourth, we only assumed 0 complications and reoperations for studies that explicitly reported so. Therefore, it is possible that some patients who underwent PSF had unreported complications. However, our complication rate is similar to that of a previous meta-analysis investigating PSF outcomes of patients with AIS45. The lack of patient-level data prevented us from comparing complication rates by surgical approach (open versus thoracoscopic). Lastly, our revised inclusion criteria, applied symmetrically to both AVBT and PSF studies, resulted in the inclusion of 2 patients with syndromic etiologies and 4 patients who were 9 years of age. However, these patients comprised <3% of our total population, and excluding all other patients in these studies who otherwise met inclusion criteria would have led to a loss of 40% of our AVBT population. As such, to increase our generalizability and not introduce bias by exclusion of these studies, we accepted slightly more variance with regard to these criteria.
While the potential for a fusionless treatment for AIS has generated excitement, clinicians should approach this new technology with caution. Our study showed greater rates of complications and reoperations with AVBT compared with PSF, with similar correction and clinical outcome scores obtained at each follow-up. Among AVBT studies, reoperations and complications were greater at longer follow-up. As the majority of current AVBT studies have <3 years of follow-up14,44, it seems prudent to recommend a prospective double-armed cohort study designed to compare the outcomes following the 2 treatment strategies over a minimum of 3, but preferably 5, years. Ideally, this would be a randomized trial, but we recognize that patient recruitment could be a potential issue. Additionally, studies of long-term adjacent-segment disease with both treatment strategies and motion of the treated and untreated segments would be helpful for counseling patients. For now, physicians should counsel patients about the higher complication and reoperation rates compared with the gold-standard treatment and employ a shared decision-making model when considering AVBT.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJSOA/A287).
Footnotes
Investigation performed at the Perelman School of Medicine, University of Pennsylvania, and Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A286).
Max Shin, BA, and Gabriel R. Arguelles, BA, contributed equally to this manuscript.
References
- 1.Choudhry MN, Ahmad Z, Verma R. Adolescent idiopathic scoliosis. Open Orthop J. 2016. May 30;10:143-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghandhari H, Ameri E, Nikouei F, Haji Agha Bozorgi, Majdi S, Salehpour M. Long-term outcome of posterior spinal fusion for the correction of adolescent idiopathic scoliosis. Scoliosis Spinal Disord. 2018. August 2;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lonstein JE. Selective thoracic fusion for adolescent idiopathic scoliosis: long-term radiographic and functional outcomes. Spine Deform. 2018. Nov-Dec;6(6):669-75. [DOI] [PubMed] [Google Scholar]
- 4.Buckland AJ, Moon JY, Betz RR, Lonner BS, Newton PO, Shufflebarger HL, Errico TJ; Harms Study Group. Ponte osteotomies increase the risk of neuromonitoring alerts in adolescent idiopathic scoliosis correction surgery. Spine (Phila Pa 1976). 2019. February 1;44(3):E175-80. [DOI] [PubMed] [Google Scholar]
- 5.Kwan KYH, Koh HY, Blanke KM, Cheung KMC. Complications following surgery for adolescent idiopathic scoliosis over a 13-year period. Bone Joint J. 2020. April;102-B(4):519-23. [DOI] [PubMed] [Google Scholar]
- 6.Uehara M, Takahashi J, Ikegami S, Kuraishi S, Futatsugi T, Oba H, Takizawa T, Munakata R, Koseki M, Kato H. Correlation of lower instrumented vertebra with spinal mobility and health-related quality of life after posterior spinal fusion for adolescent idiopathic scoliosis. Clin Spine Surg. 2019. August;32(7):E326-9. [DOI] [PubMed] [Google Scholar]
- 7.Danielsson AJ, Romberg K, Nachemson AL. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis: a case-control study. Spine (Phila Pa 1976). 2006. February 1;31(3):275-83. [DOI] [PubMed] [Google Scholar]
- 8.Kepler CK, Meredith DS, Green DW, Widmann RF. Long-term outcomes after posterior spine fusion for adolescent idiopathic scoliosis. Curr Opin Pediatr. 2012. February;24(1):68-75. [DOI] [PubMed] [Google Scholar]
- 9.Green DW, Lawhorne TW, 3rd, Widmann RF, Kepler CK, Ahern C, Mintz DN, Rawlins BA, Burke SW, Boachie-Adjei O. Long-term magnetic resonance imaging follow-up demonstrates minimal transitional level lumbar disc degeneration after posterior spine fusion for adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2011. November 1;36(23):1948-54. [DOI] [PubMed] [Google Scholar]
- 10.Newton PO. Spinal growth tethering: indications and limits. Ann Transl Med. 2020. January;8(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott AC. Treatment of infantile Blount disease with lateral tension band plating. J Pediatr Orthop. 2012. Jan-Feb;32(1):29-34. [DOI] [PubMed] [Google Scholar]
- 12.Newton PO, Fricka KB, Lee SS, Farnsworth CL, Cox TG, Mahar AT. Asymmetrical flexible tethering of spine growth in an immature bovine model. Spine (Phila Pa 1976). 2002. April 1;27(7):689-93. [DOI] [PubMed] [Google Scholar]
- 13.Newton PO, Farnsworth CL, Upasani VV, Chambers RC, Varley E, Tsutsui S. Effects of intraoperative tensioning of an anterolateral spinal tether on spinal growth modulation in a porcine model. Spine (Phila Pa 1976). 2011. January 15;36(2):109-17. [DOI] [PubMed] [Google Scholar]
- 14.Newton PO, Bartley CE, Bastrom TP, Kluck DG, Saito W, Yaszay B. Anterior spinal growth modulation in skeletally immature patients with idiopathic scoliosis: a comparison with posterior spinal fusion at 2 to 5 years postoperatively. J Bone Joint Surg Am. 2020. May 6;102(9):769-77. [DOI] [PubMed] [Google Scholar]
- 15.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016. December 5;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clin Trials. 2008;5(3):225-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harville DA. Maximum likelihood approaches to variance component estimation and to related problems. J Am Stat Assoc. 1977. June;72(358):320-38. [Google Scholar]
- 18.Thompson WA., Jr. The problem of negative estimates of variance components. Ann Math Stat. 1962. March;33(1):273-89. [Google Scholar]
- 19.Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ, Betz RR. Anterior vertebral body tethering for idiopathic scoliosis: two-year results. Spine (Phila Pa 1976). 2014. September 15;39(20):1688-93. [DOI] [PubMed] [Google Scholar]
- 20.Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ, Betz RR. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J. 2015. July;24(7):1533-9. Epub 2014 Dec 16. [DOI] [PubMed] [Google Scholar]
- 21.Boudissa M, Eid A, Bourgeois E, Griffet J, Courvoisier A. Early outcomes of spinal growth tethering for idiopathic scoliosis with a novel device: a prospective study with 2 years of follow-up. Childs Nerv Syst. 2017. May;33(5):813-8. Epub 2017 Mar 21. [DOI] [PubMed] [Google Scholar]
- 22.Wong HK, Ruiz JNM, Newton PO, Gabriel Liu KP. Non-fusion surgical correction of thoracic idiopathic scoliosis using a novel, braided vertebral body tethering device: minimum follow-up of 4 years. JB JS Open Access. 2019. December 12;4(4):e0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilgor C, Cebeci B, Abul K, Lahut S, Ergene G, Senay S, Alanay A. Non-fusion thoracoscopic anterior vertebral body tethering for adolescent idiopathic scoliosis: preliminary results of a single European center. In: Scoliosis Research Society 53rd Annual Meeting & Course Final Program; 2018 October 10-13. Scoliosis Research Society; 2018. Abstract no. 35. p 199-200. [Google Scholar]
- 24.Samdani A, Pahys J, Ames R, Grewal H, Pelletier G, Betz R, Hwang S. Prospective follow-up of anterior vertebral body tethering for idiopathic scoliosis: interim results from an FDA IDE study. In: Abstracts from the 43rd Annual Meeting of The American Society of Pediatric Neurosurgeons. J Neurosurg Pediatr. 2020;25(3):50. [Google Scholar]
- 25.Newton PO, Kluck DG, Saito W, Yaszay B, Bartley CE, Bastrom TP. Anterior spinal growth tethering for skeletally immature patients with scoliosis: a retrospective look two to four years postoperatively. J Bone Joint Surg Am. 2018. October 3;100(19):1691-7. [DOI] [PubMed] [Google Scholar]
- 26.Hoernschemeyer DG, Boeyer ME, Robertson ME, Loftis CM, Worley JR, Tweedy NM, Gupta SU, Duren DL, Holzhauser CM, Ramachandran VM. Anterior vertebral body tethering for adolescent scoliosis with growth remaining: a retrospective review of 2 to 5-year postoperative results. J Bone Joint Surg Am. 2020. July 1;102(13):1169-76. [DOI] [PubMed] [Google Scholar]
- 27.Pehlivanoglu T, Oltulu I, Ofluoglu E, Sarioglu E, Altun G, Korkmaz M, Yildirim K, Aydogan M. Thoracoscopic vertebral body tethering for adolescent idiopathic scoliosis: a minimum of 2 years’ results of 21 patients. J Pediatr Orthop. 2020. Nov-Dec;40(10):575-80. [DOI] [PubMed] [Google Scholar]
- 28.Segal DN, Grabel ZJ, Konopka JA, Boissonneault AR, Yoon E, Bastrom TP, Flynn JM, Fletcher ND; Harms Study Group. Fusions ending at the thoracolumbar junction in adolescent idiopathic scoliosis: comparison of lower instrumented vertebrae. Spine Deform. 2020. April;8(2):205-11. Epub 2020 Feb 5. [DOI] [PubMed] [Google Scholar]
- 29.Mimura T, Takahashi J, Ikegami S, Kuraishi S, Shimizu M, Futatsugi T, Uehara M, Oba H, Koseki M, Kato H. Can surgery for adolescent idiopathic scoliosis of less than 50 degrees of main thoracic curve achieve good results? J Orthop Sci. 2018. January;23(1):14-9. Epub 2017 Sep 22. [DOI] [PubMed] [Google Scholar]
- 30.Di Silvestre M, Bakaloudis G, Ruosi C, Pipola V, Colella G, Greggi T, Ruffilli A, Vommaro F. Segmental vs non-segmental thoracic pedicle screws constructs in adolescent idiopathic scoliosis: is there any implant alloy effect? Eur Spine J. 2017. October;26(Suppl 4):533-8. Epub 2017 Mar 27. [DOI] [PubMed] [Google Scholar]
- 31.Murphy JS, Upasani VV, Yaszay B, Bastrom TP, Bartley CE, Samdani A, Lenke LG, Newton PO. Predictors of distal adding-on in thoracic major curves with AR lumbar modifiers. Spine (Phila Pa 1976). 2017. February 15;42(4):E211-8. [DOI] [PubMed] [Google Scholar]
- 32.Badve SA, Goodwin RC, Gurd D, Kuivila T, Kurra S, Lavelle WF. Uniplanar versus fixed pedicle screws in the correction of thoracic kyphosis in the treatment of adolescent idiopathic scoliosis (AIS). J Pediatr Orthop. 2017. December;37(8):e558-62. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez N, Valentin P, García-Cartagena M, Samalot S, Iriarte I. One-step (standard) versus two-step surgical approach in adolescent idiopathic scoliosis posterior spinal fusion: which is better? Eur J Orthop Surg Traumatol. 2016. July;26(5):441-6. Epub 2016 May 13. [DOI] [PubMed] [Google Scholar]
- 34.Morr S, Carrer A, Alvarez-García de Quesada LI, Rodriguez-Olaverri JC. Skipped versus consecutive pedicle screw constructs for correction of Lenke 1 curves. Eur Spine J. 2015. July;24(7):1473-80. Epub 2015 Jan 20. [DOI] [PubMed] [Google Scholar]
- 35.Di Silvestre M, Lolli F, Bakaloudis G, Maredi E, Vommaro F, Pastorelli F. Apical vertebral derotation in the posterior treatment of adolescent idiopathic scoliosis: myth or reality? Eur Spine J. 2013. February;22(2):313-23. Epub 2012 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng CK, Kan WS, Li P, Zhao ZG, Li K. Treatment for severe idiopathic upper thoracic scoliosis in adolescence. J Spinal Disord Tech. 2013. April;26(2):107-11. [DOI] [PubMed] [Google Scholar]
- 37.Tao F, Shi Z, Xie Y, Pan F, Wu Y, Zhang Y, Wang Z, Li M. Determination of lowest instrumented vertebra by the location of apical vertebra in Lenke type 1 adolescent idiopathic scoliosis. Int Orthop. 2011. April;35(4):561-7. Epub 2010 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mladenov KV, Vaeterlein C, Stuecker R. Selective posterior thoracic fusion by means of direct vertebral derotation in adolescent idiopathic scoliosis: effects on the sagittal alignment. Eur Spine J. 2011. July;20(7):1114-7. Epub 2011 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Işık M, Özdemir HM, Sakaoğulları A, Cengiz B, Aydoğan NH. The efficacy of in situ local autograft in adolescent idiopathic scoliosis surgery: a comparison of three different grafting methods. Turk J Med Sci. 2017. December 19;47(6):1728-35. [DOI] [PubMed] [Google Scholar]
- 40.Newton PO, Marks MC, Bastrom TP, Betz R, Clements D, Lonner B, Crawford A, Shufflebarger H, OʼBrien M, Yaszay B; Harms Study Group. Surgical treatment of Lenke 1 main thoracic idiopathic scoliosis: results of a prospective, multicenter study. Spine (Phila Pa 1976). 2013. February 15;38(4):328-38. [DOI] [PubMed] [Google Scholar]
- 41.Ergene G. Early-term postoperative thoracic outcomes of videothoracoscopic vertebral body tethering surgery. Turk Gogus Kalp Damar Cerrahisi Derg. 2019. October 23;27(4):526-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Graham CK, Robbins C, Caird MS, Farley FA. Elevated serum titanium levels in children with early onset scoliosis treated with growth-friendly instrumentation. J Pediatr Orthop. 2020. July;40(6):e420-3. [DOI] [PubMed] [Google Scholar]
- 43.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011. December 7;93(23):2164-71. [DOI] [PubMed] [Google Scholar]
- 44.Louer C, Jr, Yaszay B, Cross M, Bartley CE, Bastrom TP, Shah SA, Lonner B, Cahill PJ, Samdani A, Upasani VV, Newton PO. Ten-year outcomes of selective fusions for adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2019. May 1;101(9):761-70. [DOI] [PubMed] [Google Scholar]
- 45.Lykissas MG, Jain VV, Nathan ST, Pawar V, Eismann EA, Sturm PF, Crawford AH. Mid- to long-term outcomes in adolescent idiopathic scoliosis after instrumented posterior spinal fusion: a meta-analysis. Spine (Phila Pa 1976). 2013. January 15;38(2):E113-9. [DOI] [PubMed] [Google Scholar]