Abstract
Supplemental Digital Content is available in the text.
For individuals with a diagnosis of a chronic myeloproliferative neoplasm (MPN), many changes have occurred since the onset of the SARS-CoV-2 pandemic. Many patients within the United Kingdom had to “shield” for prolonged periods and experienced a major shift in MPN-directed healthcare. Increasingly, we are understanding potential clinical implications of SARS-CoV-2 natural infection and generated immune responses within the MPN community.1–4 Comprehensive analyses of COVID-19 vaccine-mediated immune responses in MPN patients requires clarification.
In the United Kingdom, prioritization for COVID-19 vaccination was based upon revised Joint Committee on Vaccination and Immunisation (JCVI) recommendations and patients with “blood cancer” were eligible to receive COVID-19 vaccination based on diagnosis alone from January 2021. MPN patient community opinions and experiences regarding vaccination is of major interest to aid future vaccination planning. We hereby report on a large, cross-sectional, electronic survey of 964 individuals with a MPN concerning pivotal aspects of COVID-19 vaccination such as vaccine uptake, vaccine-related side effects (SEs), effects on MPN-related symptom burden, and attitudes towards perceived risks.
Respondents were invited to take part via directed email, website article, and social media platforms coordinated from the MPN Voice charity on March 10, 2021, with a period of 21 days for survey completion. All respondent data were collected anonymously, with analysis presented in aggregate.
There were 1218 individual respondents, 964 (79.1%) of whom completed all questions. Analysis focused on these individuals to avoid potential result skewing. Patient demographics, characteristics, and treatments are summarized in Table 1.
Table 1.
Respondent Demographics, Disease Categorization, Treatment, and Vaccine Received.
| Demographic | Respondents (%) |
|---|---|
| Total responses | 1218 |
| Complete responses | 964 |
| Gender | |
| Male | 254 (26.4) |
| Female | 707 (73.4) |
| Trans male | 1 (0.1) |
| Trans female | 0 (0.0) |
| Other | 1 (0.1) |
| Age | |
| 16–24 | 2 (0.2) |
| 25–35 | 25 (2.6) |
| 36–45 | 104 (10.8) |
| 46–59 | 299 (31.0) |
| 60–69 | 322 (33.4) |
| 70–75 | 136 (14.1) |
| 76 or older | 76 (7.9) |
| Ethnicity | |
| White—English, Welsh, Scottish, Northern Irish, or British | 816 (84.7) |
| White—Irish | 38 (4.0) |
| White—any other | 71 (7.3) |
| Mixed or multiple ethnic groups—White and Black African | 2 (0.2) |
| Mixed or multiple ethnic groups—White and Asian | 2 (0.2) |
| Mixed or multiple ethnic groups—any other | 2 (0.2) |
| Asian or Asian British—Indian | 3 (0.3) |
| Asian or Asian British—Pakistani | 1 (0.1) |
| Asian or Asian British—Chinese | 2 (0.2) |
| Asian or Asian British—any other | 7 (0.7) |
| Black, African, Caribbean, or Black British—African | 4 (0.4) |
| Black, African, Caribbean, or Black British—Caribbean | 6 (0.6) |
| Black, African, Caribbean, or Black British—any other | 1 (0.1) |
| Other ethnic group—any other | 6 (0.6) |
| Prefer not to answer | 2 (0.2) |
| Country | |
| England | 764 (79.2) |
| Scotland | 68 (7.0) |
| Wales | 33 (3.4) |
| Northern Ireland | 12 (1.2) |
| Republic of Ireland | 16 (1.7) |
| Other | 54 (5.6) |
| Prefer not to answer | 17 (1.8) |
| Diagnosis | |
| Polycythaemia vera (PV) | 270 (28.0) |
| Essential thrombocythemia (ET) | 548 (56.9) |
| Primary myelofibrosis (MF) | 42 (4.4) |
| Post-ET myelofibrosis | 39 (4.0) |
| Post-PV myelofibrosis | 29 (3.0) |
| Prefibrotic myelofibrosis | 7 (0.7) |
| MPN—unclassified | 16 (1.7) |
| Other | 13 (1.4) |
| Current treatment | |
| Observation | 134 (15.2) |
| Venesection | 111 (12.6) |
| Hydroxycarbamide | 437 (49.7) |
| Interferon | 23 (2.6) |
| Pegylated interferon A | 101 (11.5) |
| Anagrelide | 26 (3.0) |
| Ruxolitinib | 83 (4.4) |
| Busulfan | 2 (0.2) |
| Other | 103 (11.7) |
| Vaccine received | |
| AstraZeneca (ChAdOx1-S [recombinant]) | 457 (51.8) |
| Pfizer/BioNTech (BNT162b2) | 414 (46.9) |
| Moderna (mRNA-1273) | 10 (1.1) |
| Johnson & Johnson (JNJ-78436735) | 1 (0.1) |
Respondents were asked to clarify if they had confirmatory evidence of prior COVID-19 infection. A total of 927 (96.1%) respondents reported no previous confirmed COVID-19 infection via either formal COVID-19 respiratory polymerase chain reaction testing or antigen-based lateral flow test. In total, only 81 patients (8.7%) reported belief of previous COVID-19 infection based on symptoms only.
At survey closure, 882 (91.5%) respondents reported COVID-19 vaccination; AstraZeneca (ChAdOx1-S [recombinant]) (n = 457; 51.8%), Pfizer/BioNTech (BNT162b2) (n = 414; 46.9%), Moderna (mRNA-1273) (n = 10; 1.1%), and Johnson & Johnson (JNJ-78436735) (n = 1; 0.1%). The majority (n = 825, 93.5%) had received their first dose only, as expected given the current UK Department of Health and Social Care’s policy on delaying second dose administration by 12 weeks. In total, 82 respondents (8.5%) reported they had not received COVID-19 vaccination by survey closure. Within this group, 29 were currently booked for and awaiting to receive their first dose. Overall, 53 respondents reported that they were not currently booked for vaccination: 14 had been offered and 39 had not been offered. One explanation for not being offered COVID-19 vaccination that the authors were aware of related to several anecdotal reports from patients that their primary care provider (PCP) had not coded their MPN as a blood cancer, potentially reflecting an education gap. As such, patients may not have been identified within the appropriate JCVI category.
Of the 882 respondents receiving a COVID-19 vaccine, 147 (16.7%) reported concerns regarding COVID-19 vaccination, all providing a reason. Reasons were grouped into themes, with respondents often giving more than one concern. Main concerns related to risk of SEs and potential serious reactions (30.6%). There were recurring concerns about vaccine efficacy in MPN patients (15.4%), how vaccination may interfere with the underlying MPN condition (14.3%), particularly focused on potential for disease alteration or progression due to “mRNA technology,” and potential interaction with MPN-directed therapies (10.2%). Other concerns related to emerging reports at time of survey completion of potential risk of thrombotic events associated with COVID-19 vaccination (9.5%), with several respondents sign posting the AstraZeneca vaccine. Concerns also included the “unknown long-term risks of COVID-19 vaccines” (6.8%) and safety of new vaccines in general (6.1%). It was of interest to see that only 5.4% of respondents reported concerns relating to the United Kingdom’s delayed second dose strategy given concerns amongst the healthcare community, and the widespread media coverage, of the unknown efficacy or safety of this policy. The authors expected a higher proportion of concern, in line with our clinical experiences, from patients on this issue.
When asked whether respondents felt they received sufficient information about COVID-19 vaccination, 78 (8.8%) answered no. Free text responses were offered by 55 respondents. Main concerns related to a desire for more information regarding how vaccinations potentially interact with MPNs (54.5%). Generally, respondents highlighted a lack of information around safety and efficacy of COVID-19 vaccines in patients with “MPN and blood cancer.” A small proportion (10.9%) reported they received information on COVID-19 vaccination in leaflet form only after vaccination, often reporting no information being provided as to which vaccine they would receive or opportunity for information prevaccination. Less common themes related to information on exact timing of second dose, potential interactions with current MPN medications, long-term SEs, and general efficacy.
With regards to COVID-19 vaccine preference, 731 (82.9%) of the 882 vaccinated respondents reported no overall vaccine preference. Preference for a certain vaccine was given by 138 respondents, stating a preference for Pfizer 65.2%, AstraZeneca 30.4%, Moderna 2.9%, and 1.4% other, which likely reflects the vaccines licensed in the United Kingdom at the time of survey. Respondents often gave more than one reason for their preference. Preference for Pfizer vaccine-related mainly to perceived “greater efficacy in protection” (35.5%), lesser SE profile (13.0%), and more widely available information (5.1%). Preference for AstraZeneca vaccine related mainly due to concerns around potential serious reactions or anaphylaxis with the Pfizer vaccine (5.8%), “better protection,” particularly relating to delayed second dosing strategy (5.8%), and the fact it was “made within the United Kingdom” (5.8%). Some respondents reported preference for a Pfizer vaccine as they felt the technology was more advanced, whereas other respondents preferred AstraZeneca vaccine as the method of vaccine production was “more traditional” and “better trusted.”
When asked whether respondents trusted their health care provider (HCP) to honestly inform on risks and benefits of vaccination, 885 respondents gave an opinion, of which 788 (89.0%) reported trusting their HCP. However, 97 respondents reported mistrust of their HCP. The main reasoning given was that HCPs do not yet know enough about COVID-19 vaccines (34.0%), HCPs did not explain the risks or benefits (30.0%), HCPs do not have up to date information on MPN or vaccines in patients with MPN (12.0%) and a lack of trust in their PCP (10.0%), predominantly due to a lack of understanding of MPN. One potential explanation for this could be that large-scale vaccination centers were developed in the United Kingdom and members of the public were trained to administer COVID-19 vaccines. As such, vaccinators may not have been aware of MPNs, or have in-depth knowledge of the conditions, to provide individualized specialist opinions on COVID-19 vaccination in MPN, and patients would likely assume all vaccinators were a HCP.
Regarding objective assessment of COVID-19 vaccination effects on MPN symptom burden, 141 (16%) of the 882 vaccinated respondents reported exacerbation or worsening of baseline MPN symptoms using MPN-10 score.5 A total of 124 respondents completed both the pre- and postvaccination MPN-10 assessment. The median MPN-10 score prevaccination was 28 (range, 3–69), postvaccination 41 (range, 6–90) and overall cumulative change +8 (range, 0–40), P < 0.0001 (Figure 1). On average, fatigue showed the largest increase in symptom burden from MPN-10 score across all 3 disease groups (PV +2.83, ET +2.66, and MF +2.00).
Figure 1.
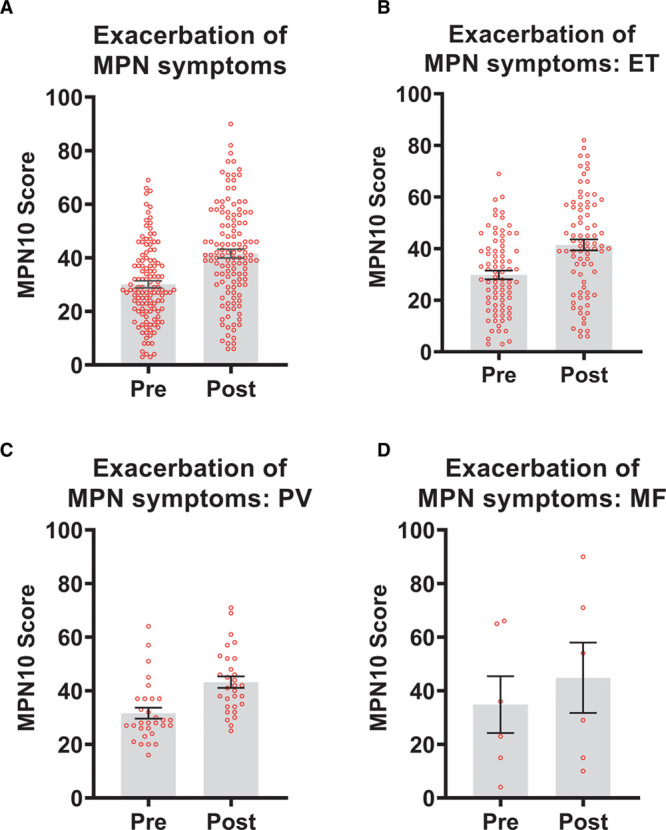
Exacerbation of myeloproliferative neoplasm (MPN) symptoms based on MPN-10 score pre and post COVID-19 vaccination. (A) Dot-boxplot of paired median MPN-10 scores for overall respondents (n = 124); median score pre 28 vs median score post 41 (P < 0.0001). Dot-boxplots for pre- and postvaccination scores. (B) Essential thrombocythaemia (ET) cohort (n = 80); median scores pre 28 vs. post 41 (P < 0.0001). (C) Polycythaemia vera (PV) cohort, n = 30; median scores pre 28 vs post 41 (P < 0.0001). (D) Primary myelofibrosis (MF) cohort n = 6, median pre scores 23 vs post 29 (P = 0.42). Wilcoxon signed-rank test was used analysis of paired nonparametric data. Statistical analyses were conducted on IBM SPSS Statistics version 27. Statistical significance is set at P < 0.05. GraphPad Prism version 9 used to generate graphs.
In total, 874 respondents receiving COVID-19 vaccination answered questions regarding SEs (See Supplemental Digital Content, Table 2, http://links.lww.com/HS/A170). Respondents were presented with 6 multiple choice SE and duration ranges. The most frequent SE were: “sore arm” (80.5%), fatigue (57.7%), and headache (44.9%). The majority reported that SE lasted up to 36 hours only. In total, 162 respondents gave further information on SE, which were individually assessed. Categorization based on Common Terminology Criteria for Adverse Events (CTCAE)6 version 5 was undertaken. Individual respondents often reported several SE, each counted separately. Grouping based on CTCAE resulted in 38 additional different SE being reported, within 10 groups. The top 3 systems included general (21.1%), gastrointestinal (18.4%), and nervous system disorders (18.4%) (See Supplemental Digital Content, Figure 2, http://links.lww.com/HS/A170).
With regards to previous history of thrombotic events before COVID-19 vaccination, 954 respondents answered (See Supplemental Digital Content, Table 3, http://links.lww.com/HS/A170). Of this, 734 (76.9%) reported no prior history of thrombotic events. No respondents in this survey reported a thrombotic event following COVID-19 vaccination, which was particularly reassuring given emerging reports at the time of survey completion of thrombotic events associated with COVID-19 vaccination, alongside the inherent thrombotic risk associated with MPNs2,7 which was a particular concern expressed by patients in the author’s own clinical practice. However, we acknowledge that thrombotic events post COVID-19 vaccination are rare.
In total, 8 (0.8%) respondents declined offer of COVID-19 vaccination. Respondents were presented with 12 potential explanations. Concerns focused on SEs (n = 5), vaccine development happening too quickly (n = 4), safety of vaccines (n = 3), and perceived lack of efficacy due to MPN (n = 2). Two patients said a HCP had advised against vaccination and 1 patient reported they did not require vaccination due to previous infection. A total of 3 free-text responses were collected: concern that vaccination placed additional “stress on their immune system” following COVID-19 infection, advised not to receive a vaccine by a complementary and alternative medicine provider, and undecided and wanted to discuss with oncology provider first.
To our knowledge, this is the first survey of opinions and experiences of COVID-19 vaccination in patients with MPN. Reassuringly, there appears to be overall favorable uptake and attitudes towards current vaccination policies, with main concerns relating to potential SEs and undetermined efficacy in MPN patients. Common SE profiles in this survey were not dissimilar to other vaccines and appeared tolerable. There was a statistically significant increase in overall cohort symptom severity, based on MPN-10 score. However, it is important to recognize there is overlap between potential vaccine-related symptoms and MPN-related symptoms. Therefore, at present, it is difficult to draw definitive conclusions from these results. Future research, in addition to recording success of vaccination, should aim to focus on duration and severity of symptoms captured within the MPN-10 assessment following vaccination, SE profiles following second dose vaccination, as well as uptake of second dose vaccination given the concerns, albeit of low incidence, of thrombotic thrombocytopenic events following COVID-19 vaccination.
Disclosures
PS has received speaker fee from Celgene. SK has received research grant from Celgene and Novartis, and received speaker honorarium from Alexion. PH has received research funding from Bristol Myers Squibb and speaker fees from Incyte. HdL has received grants and speakers fees from Bristol Myers Squibb and Incyte; speakers fees from Novartis and Pfizer. DPM has received speaker fees and advisory boards from Novartis, Celgene, and Jazz Pharmaceuticals. CNH: Novartis; speaker fees from Novartis, Janssen, CTI, Celgene, and Medscape; advisory board: Incyte, CTI, Sierra Oncology, Novartis, Celgene, Roche, AOP pharma, and Geron. All the other authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Barbui T, Vannucchi AM, Alvarez-Larran A, et al. High mortality rate in COVID-19 patients with myeloproliferative neoplasms after abrupt withdrawal of ruxolitinib. Leukemia. 2021; 35:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbui T, De Stefano V, Alvarez-Larran A, et al. Among classic myeloproliferative neoplasms, essential thrombocythemia is associated with the greatest risk of venous thromboembolism during COVID-19. Blood Cancer J. 2021; 11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salisbury RA, Curto-Garcia N, O’Sullivan J, et al. Results of a national UK physician reported survey of COVID-19 infection in patients with a myeloproliferative neoplasm. Leukemia. 2021. February 12. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington P, Harrison CN, Dillon R, et al. Evidence of robust memory T-cell responses in patients with chronic myeloproliferative neoplasms following infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Br J Haematol. 2021; 193:692–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012; 30:4098–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health, U.S Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 5. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed April 01, 2021.
- 7.Hultcrantz M, Björkholm M, Dickman PW, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Ann Intern Med. 2018; 168:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


