Abstract
Introduction:
Social determinants of health (SDOH) account for 80% of modifiable factors in a population’s health. Addressing SDOH in a healthcare setting can improve care, patient experience, health outcomes, and decrease cost. Therefore, screening for SODH in the pediatric setting has become an essential and evidence-based component of pediatric preventative care. Multiple barriers exist for its implementation, particularly for trainees.
Methods:
Using resident-driven quality improvement (QI) methodology, we aimed to increase SDOH screening to >90% for 9 individual questions at newborn and 1-year well visits and completely screen for all 9 questions at more than 40% of visits. Parents were provided with a paper screening form upon arrival to be completed before visits. We performed tests of change to improve distribution, documentation, and quality of interventions.
Results:
The primary outcome of complete screening for all 9 questions increased from 24% to 43% at newborn visits and 28% to 83% at 1-year visits. Screenings that identified at least 1 need increased from 8% to 19%, with provider response to an identified need increasing from 20% to 40%. These metrics were the secondary outcome measures.
Conclusions:
The use of parent completed paper screening forms improved SDOH screening, documentation, and interventions by residents and faculty.
INTRODUCTION
Adverse social determinants of health (SDOH) are associated with significant behavioral, developmental, and learning problems in children.1 As such, screening for SDOH has become an essential and evidence-based component of pediatric preventative care, as stated in the fourth edition of the Bright Futures guidelines. These guidelines include specific recommendations on screening for these domains.2,3
Many barriers exist for successful SDOH screening implementation, such as time constraints during routine well-child care visits and provider discomfort in exploring these issues.4–6 These issues are particularly real for pediatric residents. They spend only a fraction of their primary care training and have limited curricula on addressing adverse SDOH in the clinical setting. These limitations make them less likely to screen patients or to have the ability to provide resources to those who screen positive.7,8 These barriers can be mitigated in primary care practices that have a multidisciplinary healthcare team, where social workers and behavioral health providers can help address needs identified by screening.
Screening tools and methods vary, but self-completed forms have been shown to elicit private information more effectively than face-to-face screening by physicians.1,9,10 This method has been trialed for screening for intimate partner violence. It allows for improved screening and referrals for community resources11 while not significantly increasing the length of visits for residents or faculty providers.
Many institutions seek ways to address the need to screen for SDOH while considering the barriers faced in primary care practice. Still, few studies have gone beyond screening to look at referrals and outcomes.7,8,12 At our practice, at baseline, screening for individual questions occurred between 50% and 80% of the time, and for all questions, less than 25% of the time. Based on this known screening gap locally and nationally, especially in the minority, publically insured populations,4–6 we identified improving screening for SDOH as the practice quality improvement (QI) initiative for the 2015–2016 year. There is limited published data on initiatives to increase screening by residents caring for high-risk populations, despite their lower screening rates than attendings.
Thus, this resident-led QI project aimed to increase SDOH screening rates to 90% or higher (goal rate) for nine individual questions and thoroughly screen for all 9 questions at more than 40% of all newborns and one-year well-child visits by April 2016.
METHODS
Context
Setting
This resident-led QI initiative took place at an urban academic primary care, community-based, hospital-affiliated resident/faculty practice in Northern Manhattan, New York City. The practice is part of New York-Presbyterian’s Ambulatory Care Network, an affiliate of Columbia University Irving Medical Center. The average pediatric visit volume per year is approximately 10,000, and the practice serves over 850 children between 0 and 2 years of age and almost 5,000 children in total. The majority (71%) of the patient population in the neighborhood is Hispanic, and 48% of the patient population are immigrants, two-thirds from the Dominican Republic. More than one-quarter of the families in this neighborhood have household incomes below the federal poverty level.
QI Curriculum
All pediatric residents receive training in QI methodology via a residency QI curriculum. The curriculum requires yearly longitudinal QI projects, led by the pediatric resident teams while on their ambulatory block rotation. It is embedded in their continuity clinic practice. During the residents’ month-long ambulatory rotation, each resident leads the QI project and then hands off to the residents on the following block, allowing for a yearlong longitudinal project where all faculty and residents participate. This improvement team consisted of seven pediatric faculty and 19 pediatric residents working in collaboration with nurses, medical assistants, registration staff, social work, mental health providers, and practice leadership.
Screening Questions
Questions focused on SDOH were drawn from the American Academy of Pediatrics and The Joint Commission guidelines. Before the start of this study, these questions were already part of the well-child visit note template. Nine social and environmental risk factors, including smoke exposure, domestic violence, smoke and carbon monoxide detectors, family changes, weapons in the home, window guards, tuberculosis, and lead exposure, were designated for routine screening in our practice and were asked by the physician during the patient visit and documented as part of the note in the medical record template. We chose these SDOH to align with Bright Futures guidelines (family changes, domestic violence, smoke exposure, smoke and carbon monoxide detectors, and window guards), AAP (weapons), Joint Commission guidelines (smoke exposure), and Department of Health guidelines (lead exposure), and CDC (tuberculosis screening).13,14
QI Interventions
At the study’s onset, we designed the key driver diagram to identify the key stakeholders, possible interventions, and barriers (Fig. 1). Primary drivers included provider and patient barriers to workflows for screening, reporting, and documentation. Based on the key drivers involved and the literature supporting self-report screeners, parents received a literacy-level appropriate paper prescreening form with all nine questions, available in English or Spanish, at registration before the provider encounter (Fig. 2). Prescreening forms were 2-sided, one with screening questions and the other included local resources for patients who screened positively. Patients were given the screening tool at the time of registration and asked to complete it as part of their care. Medical assistants assisted in the completion of the form for those patients who struggled with literacy. Beyond stressing that questions were being asked to optimize care, patients were allowed to leave the form blank. If patients left the screening form blank, the physician seeing them asked the SDOH questions in person during the visit. Physicians were responsible for inputting the screening responses into the EMR, whether from the screener or the in-person intake. The physician gave a list of resources to all patients with a positive screen. If needed, the patients were referred to social work.
Fig. 1.
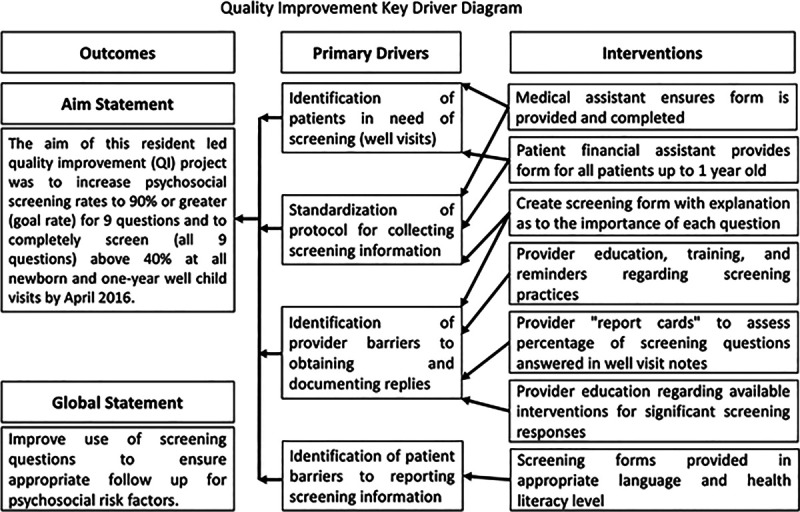
Quality improvement key driver diagram.
Fig. 2.
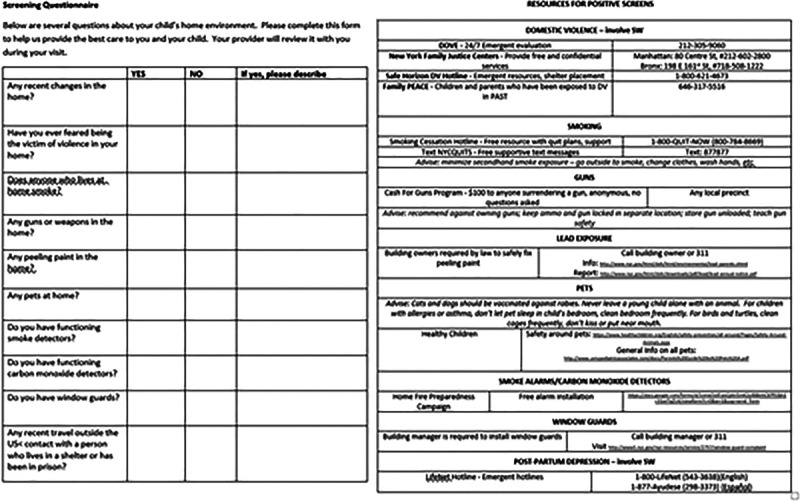
Screening form (front) with resource information (back).
We performed Plan-Do-Study-Act (PDSA) cycles to improve the distribution of forms, documentation of answers, and quality of interventions (Table 1). The first PDSA cycle focused on creating and distributing the prescreening form, including the flow from distribution to documentation and the workflows for positive screening results. The second PDSA cycle utilized interdisciplinary medical home meetings to remind all team members about the new workflows and solicit feedback. The third cycle aimed to improve provider engagement by posting run charts in provider spaces to document progress. The fourth cycle is further aimed at provider engagement by sending targeted report cards to all providers to review their documentation and screening rates. PDSA cycles targeted all care team members. Monthly emails were sent to all, updating on the progress of the project. Following the project’s implementation, sustainability was measured at multiple time points over the subsequent 3 months to ensure that this new system was maintained in our system, including through the transition period of the new academic year in July.
Table 1.
Project PDSA Cycles
| PDSA Cycle | Cycle | Cycle Logistics |
|---|---|---|
| 1 | Prescreening form | • The form replicated the already existing questions in the medical record template |
| • This form was given to parents by the registration staff for completion before the medical visit | ||
| • Medical assistants were charged with ensuring that forms were completed and available for the providers before the patient encounter | ||
| • Providers were responsible for inputting the results of the screening into the electronic medical record | ||
| • Workflows for positive screens were created and information documented on the screening forms for easy communication to families | ||
| 2 | Medical home meeting reminders | • At the weekly medical home meeting, where all staff are present, reminders were made to the registration staff and medical assistants to assist with distribution and completion of screening forms |
| 3 | Run charts posted | • To engage providers, reminders were posted in the work rooms to complete documentation in the medical record |
| • Run charts were posted in provider and staff spaces to document progress | ||
| 4 | Report cards | • Targeted report cards were sent to providers based on chart review of their patient records |
| • Percent of their patients with documented screening was sent to providers, along with an average for the practice |
Measurement
The primary outcome measure studied was the rate of complete screening for all SDOH questions. The process measure of how many of individual screening questions were documented was also tracked. Secondary outcome measures were positive screen rate and positive screen with intervention rate.
A balancing measure of cycle time, the time from patient arrival to time leaving the office, was unable to be tracked in our current electronic medical record.
Data Collection
We extracted rates of provider documentation of the 9 screening questions at all newborn and 1-year visits from the electronic medical record via chart review. Data were collected to determine (1) documentation of the screening questions; (2) if the screening section was completed in its entirety, partially or not at all; (3) how many of the screening questions had a positive response, and (4) if screened positive, were any interventions documented. We collected data throughout the project, from September 2015 to May 2016, and continued collecting data through August 2016 to assess sustainability. We collected baseline data from December 2014 through August 2015 via randomly selected charts for 1-year-old patients seen in the months before the project’s initiation. We reviewed the data from their newborn visit as well for baseline newborn data. For study purposes, newborn and 1-year visits were the focus due to the higher frequency of required visits at these ages, thus allowing for PDSA cycle changes to be tracked more easily. Also, there are higher risks of adverse health events in early childhood related to SDOH.15
Provider comfort in screening for SDOH was also assessed via an anonymous retrospective pre-post survey. On a 5-point Likert scale, providers were asked how the QI project had impacted their comfort in screening for SDOH and counseling families regarding resources (5: much more comfortable, 1: not comfortable).
Analysis and Ethical Considerations
We analyzed the data quantitatively. The percent of screening completed was plotted overtime on run charts, and descriptive statistics were used. Rules for special cause variation were applied to the centerlines. The Columbia Institutional Review Board approved this study.
RESULTS
The primary outcome, screening for all questions, increased from an average of 25% to 58% (P < 0.001) at newborn visits and 30% to 56% (P = 0.008) at 1-year visits (Fig. 3). Following the paper screens’ implementation, rates increased to 45% in the newborns and 39% in the one-year-olds. Medical home reminders increased the rates to 56% (P = 0.009) and 30%. Provider reminders increased the rates to 60% in both groups (P = 0.002, P = 0.04, respectively), and following report cards, the rates reached 84% and 65% (P < 0.001, P = 0.005, respectively). The peak goal rate for newborns occurs following cycle four and then decreased during the sustainability phase, with the 1-year-old rates increasing (P = 0.05, P < 0.001, respectively). Following the implementation of the project, the centerline shifted.
Fig. 3.
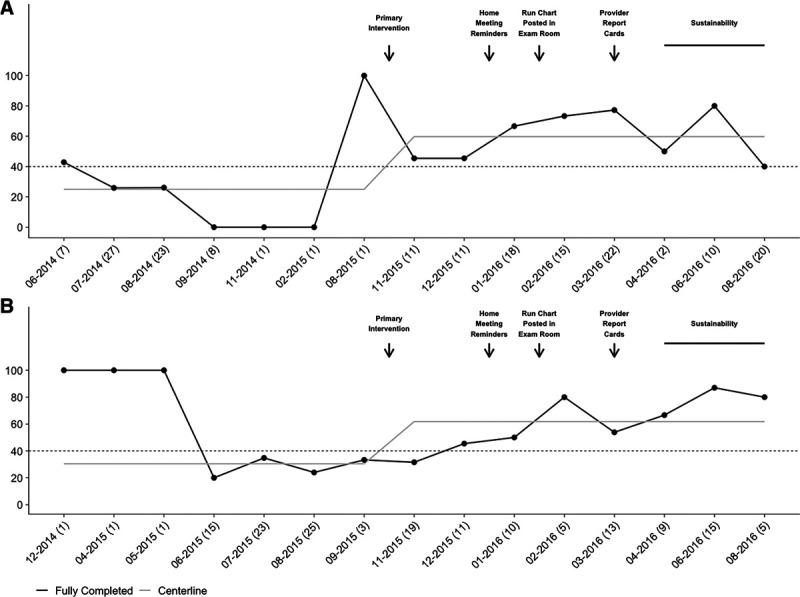
Run chart of completed SDOH screening documentation. Run chart: Newborn (A) and 1-year visits (B) at which documentation of all nine screening questions increased following the implementation of paper screening forms and subsequent PDSA cycles. The horizontal line indicates the goal rate of 40%.
At baseline, 2 individual questions at newborn visits and zero questions at 1-year visits were asked at goal rate. Seven individual questions at newborn visits and eight questions at 1-year visits were asked at the goal rate by the end of the project in May 2016.
Based on the results of provider surveys, at the end of the project, 93% of providers felt more comfortable or much more comfortable screening for SDOH and counseling families on needs.
In addition to increasing screening for SDOH, the secondary outcome tracked positive screens and positives-screens-with-intervention rates during the QI initiative (Fig. 4). Positive screening rates ranged from 16.6% to 33.9% after initiating the project, increasing from a baseline of 4% (P < 0.05 for all). Documented positive-screens-with-interventions increased from a baseline of 16% to a peak of 75%. The positive screen rate and the positive screens with documented intervention rate remained stable during the sustainability phase at 24% and 66%, respectively, demonstrating improvement beyond the study period.
Fig. 4.
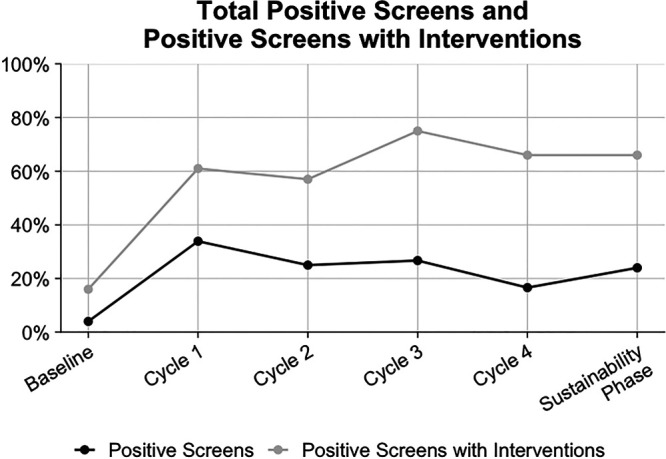
Run chart: Positive Screen results and positive screens with documented interventions.
DISCUSSION
This project led to increased screening rates, identification of positive screens, and appropriate referrals. On average, 58% of newborn and 56% of 1-year-old visits had the complete screening completed through the sustainability phase, a significant increase from baseline and surpassing our 40% goal, with screening rates at the end of the project as high as 86%. These results support the feasibility of screening for environmental and SDOH before the visit. Our results add to the QI literature around using SDOH screening tools in the outpatient setting and support resident-led longitudinal QI projects. The importance of this is paramount as growing literature has shown that increased screening for SDOH and subsequent referral improve health outcomes in adult and pediatric settings.11,16,17
The increase in positive screens and positive screens-with-intervention after using the screener is comparable to other studies demonstrating success in screener implementation.10,11,18 Some reasons for the significant increase in positive screens in this initiative include ease of the bilingual literacy-appropriate screener use before seeing the provider, ease of interpretation and data entry, and workflow integration. The immediate availability of the resources section attached to the screener contributed to the increase in screens-with-intervention. However, statistical significance was not met, likely in part due to the small number of patients.
Lack of training and lack of comfort in asking patients about social needs is a barrier to some.17,19 However, through didactic training and division level support for faculty, screening for SDOH has become ingrained in our practice. Data suggested that sustainability was better in the 1-year-old patients, likely because more newborns were cared for by new interns. This possibility underscores the need for continual reinforcement of prescreening workflows and related educational content to root this practice and overcome comfort barriers. Additionally, one large component of reported discomfort, lack of referral resources,5 has been addressed in our practice and incorporated into residency training.
This intervention builds on prior studies that sought to improve screening and referral rates, although this is one of the first to do so through a resident-led QI project. Colvin et al20 showed that a brief intervention could increase screening and referral for SDOH in the inpatient setting. Hassan et al21 utilized a web-based tool to identify health-related social concerns in adolescents/young adults and help them obtain resources. In another study, the immigrant status and limited English proficiency in a low-income population impacted referral utilization and follow-up.22 The integration of residents into the QI process and subsequent workflows is likely one reason for success. Some studies have shown residents may represent one of the primary drivers of low screening rates due to lack of training, comfort, and knowledge. Given that we found that most providers’ comfort increased following this project, the use of resident-led QI may help overcome these barriers. The fact that our practice is an accredited pediatric medical home, which includes integrated mental health services, a full-time social worker and weekly interdisciplinary rounds, helped residents to feel comfortable that we as a team could respond to needs addressed by the screening tool.
Our workflows were sustained beyond our intervention period. We believe this prescreening workflow can be spread to similar outpatient sites, as we have successfully spread this workflow to 3 additional primary care practices within our ambulatory network. The low-resource requirement of our screening mechanism can translate to other ambulatory settings. Other sites wishing to implement screening could implement a similar prescreening workflow that utilizes their site resources and improves implementation via feedback and cycles of change. Although many primary care practices may have migrated to electronic previsit screens since 2016, not all screenings are completed before the visit. Not all primary care practices have a prescreening workflow. Thus, provider participation may be required for screening during a visit. A paper prescreening QI intervention may provide a proof of concept for practices looking to migrate to electronic prescreening. Moreover, some screening questions require provider clinical input, such as screening for domestic violence in our workflow, thus making the provider role in SDOH workflows essential along with the larger care team. Finally, pediatric trainees must be engaged and aware of SDOH workflows and resources in their communities.
This study has several limitations. First, this QI project involved a single site in our ambulatory care network and may not be generalizable to other practices. Second, we used a paper screener for data collection and relied on staff to ensure form completion, which was then manually entered by the provider. It is, therefore, possible that some patient data were not captured, including from patient refusals. Third, we did not track cycle time and did not know if this intervention impacted overall cycle time as this was a prescreen. It is also possible that recognizing positive screens detracted from physician attention to other essential tasks during well-child care visits given the additional time required to address a positive screen. Some studies have reported that screening tools can identify unmet needs and the desire for assistance, increasing referrals for unmet social needs.11,23 This assessment is something to consider in future iterations of our screener. Additionally, although we sought to address limited English proficiency using a low-literacy-level tool in English and Spanish, this workflow could not be used for patients who spoke other languages. Finally, we did not look at resource linkage as an outcome as it was not a goal, nor can we say that the unmet needs identified were met. However, a follow-up to this intervention will help in the future as we assess the effectiveness of referrals.
Next Steps
Our screening tool incorporated several SDOH as well as environmental health risks. As a result of this screening process’s success, other high-value SDOH, including food insecurity and maternal depression, was integrated into the subsequent workflow and became part of the medical record template. The screener’s current iterations are being completed at all sites via tablet by the patient or caregiver to aid in the documentation, referrals, and follow-up, as has been previously demonstrated elsewhere.24 Other PDSA cycles to consider include tracking visit cycle time, prescreening via the medical record before visits, and providing the screener in additional languages common in our patient population.
CONCLUSIONS
Screening for SDOH is an essential but often missed part of well-child care. The use of parent completed paper screening forms increased screening, documentation, and interventions by residents and faculty. This successful resident-led QI initiative met ACGME and CLER guidelines for experiential learning in QI. Moreover, it has led to a task force working to spread psychosocial screening via validated questionnaires at all network primary care practices.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online June 23, 2021
Presented at the Pediatric Academic Societies Meeting, April 2017, San Francisco, CA.
J.E.M. is supported by the National Institutes of Health (TL1TR001875).
To cite: Friedman S, Caddle S, Motelow JE, Meyer D, Lane M. Improving Screening for Social Determinants of Health in a Pediatric Resident Clinic: A Quality Improvement Initiative. Pediatr Qual Saf 2021;6:e419.
REFERENCES
- 1.Garg A, Butz AM, Dworkin PH, et al. Improving the management of family psychosocial problems at low-income children’s well-child care visits: the WE CARE project. Pediatrics. 2007; 120:547–558 [DOI] [PubMed] [Google Scholar]
- 2.Committee on Psychosocial Aspects of C. Family H. American Academy of Pediatrics. The new morbidity revisited: a renewed commitment to the psychosocial aspects of pediatric care. Committee on Psychosocial Aspects of Child and Family Health. Pediatrics. 2001; 108:1227–30 [DOI] [PubMed] [Google Scholar]
- 3.Hagan JF, Shaw JS, Duncan PM. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. American Academy of Pediatrics; 2017 [Google Scholar]
- 4.Kogan MD, Schuster MA, Yu SM, et al. Routine assessment of family and community health risks: parent views and what they receive. Pediatrics. 2004; 1136 suppl1934–1943 [PubMed] [Google Scholar]
- 5.Hochstein M, Sareen H, Olson L, et al. A comparison of barriers to the provision of developmental assessments and psychological screenings during pediatric health supervision. Pediatr Res. 2001; 49:17 [Google Scholar]
- 6.Olson AL, Kemper KJ, Kelleher KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of maternal depression. Pediatrics. 2002; 110:1169–1176 [DOI] [PubMed] [Google Scholar]
- 7.Patel M, Bathory E, Scholnick J, et al. Resident documentation of social determinants of health: effects of a teaching tool in the outpatient setting. Clin Pediatr (Phila). 2018; 57:451–456 [DOI] [PubMed] [Google Scholar]
- 8.Klein MD, Alcamo AM, Beck AF, et al. Can a video curriculum on the social determinants of health affect residents’ practice and families’ perceptions of care? Acad Pediatr. 2014; 14:159–166 [DOI] [PubMed] [Google Scholar]
- 9.MacMillan HL, Wathen CN, Jamieson E, et al. ; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006; 296:530–536 [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb L, Hessler D, Long D, et al. A randomized trial on screening for social determinants of health: the iScreen study. Pediatrics. 2014; 134:e1611–e1618 [DOI] [PubMed] [Google Scholar]
- 11.Garg A, Toy S, Tripodis Y, et al. Addressing social determinants of health at well child care visits: a cluster RCT. Pediatrics. 2015; 135:e296–e304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgenlander MA, Tyrrell H, Garfunkel LC, et al. Screening for social determinants of health in pediatric resident continuity clinic. Acad Pediatr. 2019; 19:868–874 [DOI] [PubMed] [Google Scholar]
- 13.Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017; 64:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health NYSDo. Guidelines for Health Care Providers for the Prevention, Identification, and Management of Lead Exposure in Children; 2019. Available at https://www.health.ny.gov/publications/2501.pdf. Accessed February 3, 2021
- 15.Wilensky GR, Satcher D. Don’T forget about the social determinants of health. Health Aff (Millwood). 2009; 28:w194–w198 [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb LM, Wing H, Adler NE. A systematic review of interventions on patients’ social and economic needs. Am J Prev Med. 2017; 53:719–729 [DOI] [PubMed] [Google Scholar]
- 17.Garg A, Cull W, Olson L, et al. Screening and referral for low-income families’ social determinants of health by US pediatricians. Acad Pediatr. 2019; 19:875–883 [DOI] [PubMed] [Google Scholar]
- 18.Berger-Jenkins E, Monk C, D’Onfro K, et al. Screening for both child behavior and social determinants of health in pediatric primary care. J Dev Behav Pediatr. 2019; 40:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schickedanz A, Hamity C, Rogers A, et al. Clinician experiences and attitudes regarding screening for social determinants of health in a large integrated health system. Med Care. 2019; 57suppl 6 suppl 2S197–S201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colvin JD, Bettenhausen JL, Anderson-Carpenter KD, et al. Multiple behavior change intervention to improve detection of unmet social needs and resulting resource referrals. Acad Pediatr. 2016; 16:168–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan A, Scherer EA, Pikcilingis A, et al. Improving social determinants of health: effectiveness of a web-based intervention. Am J Prev Med. 2015; 49:822–831 [DOI] [PubMed] [Google Scholar]
- 22.Uwemedimo OT, May H. Disparities in utilization of social determinants of health referrals among children in immigrant families. Front Pediatr. 2018; 6:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bottino CJ, Rhodes ET, Kreatsoulas C, et al. Food insecurity screening in pediatric primary care: can offering referrals help identify families in need? Acad Pediatr. 2017; 17:497–503 [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb LM, Tirozzi KJ, Manchanda R, et al. Moving electronic medical records upstream: incorporating social determinants of health. Am J Prev Med. 2015; 48:215–218 [DOI] [PubMed] [Google Scholar]


