Abstract
Origanum elongatum L. is an endemic aromatic and medicinal plant. This work reports previous studies on O. elongatum concerning its taxonomy, botanical description, geographical distribution, bioactive compounds, toxicology, and biological effects. Chemical analyses showed that O. elongatum contains different chemical compounds, in particular volatile compounds. Pharmacological investigations showed that volatile compounds and extracts from O. elongatum exhibit different pharmacological properties, such as antibacterial, antifungal, antiviral, antioxidant, vasodilator, corrosion inhibitor, and hepatoprotective effects. Moreover, toxicological reports revealed the safety of this species. The pharmacological effects of O. elongatum could be correlated with the main compounds, which exhibit different pharmacological properties with numerous mechanism insights.
1. Introduction
Origanum elongatum (Bonnet) Emberger et Maire is an endemic aromatic and medicinal species of Morocco. It is a medicinal plant of the Lamiaceae family, a perennial herb of the Origanum genus. It is distributed in the wild species and is limited to the northeast (NE) of Morocco and extends from the Middle Atlas to the Rif Mountains ranges, mainly at high altitude on the mountains (the mountain of Tazekka and the mountain of Bouyablane).
Phytochemical investigations showed that O. elongatum contains several classes of bioactive compounds, including terpenoids, flavonoids, oxygenated compounds, hydrocarbon compounds, and phenolic compounds [1–8]. The main volatile compounds of this species are carvacrol, thymol, linalool, and limonene. Chemical analysis showed that the chemical composition is different between several published studies depending on the plant part and the collection site.
Pharmacological reports showed that extracts and essential oils (EOs) of O. elongatum exhibited various biological activities such as antibacterial [3, 4, 6, 9–11], antifungal [3, 12], antiparasitic [5, 13–16], antiviral [6], antioxidant [1], vasodilator [10, 17], corrosion inhibitor [18], and hepatoprotective effects [19]. These biological effects are certainly due to the chemical composition of O. elongatum, in particular the main compounds such as carvacrol, linalool, and thymol. Indeed, literature reports revealed that these compounds possess several pharmacological effects. Moreover, the toxicological investigations showed that O. elongatum caused a slight change in behavior with loss of appetite and temporary sedation without any change in pathophysiological and neurological activity and LD50 was greater than 3000 mg/kg [19].
This review is designed to explore all previous studies on O. elongatum L. in terms of taxonomy, botanical description, geographic distribution, ethnobotanical prospecting, and toxicology and all the investigations on the biological activities of the different parts of this plant, and we will summarize the list of all phytochemicals isolated and identified from the extracts or EOs of this plant. This work aims to provide a scientific basis for further studies and the development of medicinal agents from O. elongatum.
2. Research Methodology
The collection of data about Origanum elongatum concerned its botanical description, taxonomy, destruction, phytochemistry, and biological properties. Numerous databases such as Google Scholar, Web of Science, Scopus, ScienceDirect, SpringerLink, Wiley Online, SciFinder, and PubMed were consulted to collect publications about O. elongatum. The collected data have covered all years. The collected articles were organized in tables, analyzed, and highlighted in this review according to each field. The chemical compounds of O. elongatum were PubChem database and their chemical structures were drawn using ChemDraw Pro 8.0 software.
3. Results and Discussion
3.1. Botanical Description
Origanum elongatum (Bonnet) Emberger et Maire is an endemic species of Morocco. It is a woody perennial, which stems up to 90 cm. Its stems are erect, light or dark brown (purplish) and at the bases hirsute (hairs c. 1.5 mm long), otherwise, glabrous, and often glaucous leaves. Branches of the first order are present, in the upper 1/3−1/2 of the stems, up to 15 pairs per stem, 4 cm long; branches of the second order sometimes present; those of the third order seldom so. All branches entirely or for the greater part consist of spikes. Leaves up to 30 pairs per stem are shortly petiolate in the lower part to subsessile in the upper part (petioles up to 5 mm long), ovate or oval, margins entire, tops obtuse, 10 mm long, 8 mm wide, somewhat leathery, light green or purplish, often glaucous, glabrescent (pilose to glabrous; hairs c. 1.2 mm long), sessile glands up to 1600 per cm2. Spikes are very loose and tenuous, 40 mm long, c. 3 mm wide. Bracts are 10 pairs per spike, ± lanceolate, tops acute, 3 mm long, 1 mm wide, glabrous or pilosellous, green, often glaucous. Flowers are subsessile. Calyces are 3.5 mm long, outside glabrous or pilosellous; teeth 1 mm long. Corollas are 6 mm long, pink, outside pilosellous; upper lips divided, for c. 1/5, into 2, c. 0.3 mm long lobes; lower lips divided, for c. 3/5, into 3, somewhat unequal, 1 mm long lobes. Staminal filaments are up to 2 and 3.5 mm long. Styles are up to 8 mm long [20].
3.2. Taxonomy and Geographic Distribution
Origanum is derived from two Greek words, “oros” which means mountain and “ganos” which means shine; this word would mean “ornament of the mountains” [21]. The appearance of the French term was in the 13th century, European (Origanum sp.) and Mexican (Lippia sp.) “oregano.” The name “Oregano” is commonly used around the world to define a spicy aroma and flavor [22].
The genus Origanum belongs to the Lamiaceae family and the Nepetoideae subfamily. The taxonomic point of view was completely revised by Dr. JH Ietswaart in 1980 [20]. In this work, Ietswaart divided the genus into 3 groups, 10 sections, grouping in total 38 species (one with 6 subspecies and another with 3 varieties), and 17 hybrids. This classification was based on the morphological characters of the plant (length of the stem, number of branches, and shape of the leaves) [20].
The flora of Morocco includes five taxa of Origanum, of which two (O. compactum and O. vulgare subsp. virens) are considered to be Ibero-Moroccan taxa and the other three taxa (O. elongatum, O. grosii, and O. fontqueri) are endemic in Morocco [2]. Due to their very similar morphologies, these three Moroccan steno-endemics are subject to taxonomic confusion. According to some databases [23], O. grosii and O. × fontqueri are considered to be synonyms of O. elongatum. However, Ietswaart [20] described O. grosii and O. elongatum as two different Origanum species according to their morphological characters, the length of the stems, ears, and leaves of O. Elongatum being larger than those of O. Grosii, but with O. grosii having a longer and wider bract than O. elongatum, while the hybrid, O. × fontqueri, is not described in the Ietswaart classification [20]. On the other hand, O. elongatum and O. grosii show obvious morphological differences from O. compactum (section Prolaticorolla Ietsw.) and from O. vulgare subsp. virens (section Origanum L.).
Origanum elongatum (Bonnet) Emb. & Maire belongs to the Elongatispica section (Section 7) of group C [20]. It is recognized in Morocco by its common Arabic name “Zaatar.” The geographical distribution of the wild species is limited to the NE of Morocco and extends from the Middle Atlas to the Rif Mountains ranges, mainly at high altitude on the mountains (the mountain of Tazekka and the mountain of Bouyablane) [20].
3.3. Ecological Factors
The wild species of O. elongatum grows at altitudes between 400 and 1500 m [24]; it abounds in open forests, rockeries, and mountain matorrals, on siliceous substrates and deep and well-drained soils. It is characterized by a fairly significant bioclimatic plasticity ranging from semiarid to per humid. The most favorable vegetation stages for this species are the thermo-Mediterranean and the meso-Mediterranean [25]. These oregano flowers from June to October [20] are known for their white inflorescence attached to vertical stems [24]. The abundance of their inflorescences, their lightness, and the sequence of flowering provide an ornamental interest to this species [20]. O. elongatum grows readily in temperate continental climates and grows rapidly, but with limited development in size. However, harvesting is possible in the first year but with a low yield of dry matter [7]. The germination of O. elongatum seeds is extremely affected by abiotic factors, such as temperature, salinity stress, and pH. Thus, a temperature of 20°C, a pH of 6, and a salinity of 1 g/L constitute the optimal conditions for germination of this species [26].
3.4. Phytochemistry
The phytochemical analysis of O. elongatum extracts and EOs revealed the presence of a set of compounds, which are summarized in Table 1. Terpenoids were among the chemical classes dominating in O. elongatum EOs (Figure 1).
Table 1.
Chemical composition of extracts and essential oils of O. elongatum.
| Country | Part | Extracts/essential oils | Compounds groups | Compounds | References |
|---|---|---|---|---|---|
| Morocco | Leaves | Ethyl acetate and methanol extracts | Total polyphenols | — | [17] |
| Flavonoids | — | ||||
| Morocco | Leaves and flowering tops | Essential oil | Terpenoids | α-Thujene, β-myrcene, p-cymene, γ-terpinene, linalool, terpinene-4-ol, thymol, carvacrol, β-caryophyllene, β-bisabolene, and caryophyllene oxide | [1] |
| Morocco | Aerial parts | Essential oil | Terpenoids | Carvacrol, thymol, p-cymene, α-terpinene, limonene, thymoquinone, and thymohydroquinone | [2] |
| Morocco | Aerial parts | Essential oil | Terpenoids | Carvacrol, γ-terpinene, p-cymene, α-phellandrene, caryophyllene, 3-carene, and α-pinene | [3] |
| Morocco | Aerial parts | Essential oil | Terpenoids | Thymol, γ-terpinene, and p-cymene | [4] |
| Morocco | Aerial parts | Essential oil | Terpenoids | Carvacrol, thymol, γ-terpinene, and p-cymene | [5] |
| Morocco | Leaves and flowering tops | Essential oil | Terpenoids | Carvacrol, thymol, γ-terpinene, and p-cymene | [6] |
| Morocco | Seeds | Essential oil | Terpenoids | Carvacrol, thymol, γ-terpinene, p-cymene, and linalool | [8] |
| Morocco | Seeds | Essential oil | Terpenoids | Carvacrol, p-cymene, γ-terpinene, and linalool | [7] |
Figure 1.
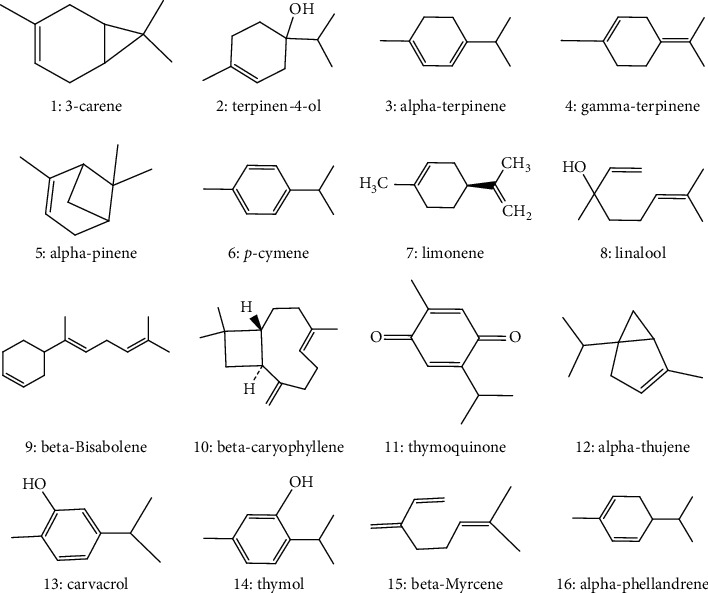
Chemical structures of terpenoids identified in O. elongatum EOs.
The methanol extract and the ethyl acetate extract from the leaves of O. elongatum are rich in phenolic compounds. The total phenol content of these extracts is 153.22 ± 2.67 mg GAE/g of methanol extract and 130 ± 3.0567 mg GAE/g of ethyl acetate extract. However, the flavonoid content is 4.83 ± 0.72 mgEQ/g of ethyl acetate extract and 5.02 ± 0.26 mgEQ/g of methanol extract [17].
The analysis by gas chromatography of O. elongatum EO from Morocco shows that it consists of 11 compounds, of which 3 are in the majority; carvacrol (60.42%), p-cymene (13.9%), and γ-terpinene (9.4%) [1].
In the same country (Morocco), 28 compounds were identified in O. elongatum EO, including carvacrol, thymol, and p-cymene, constituting the majority compounds. Additionally, limonene, thymoquinone, and thymohydroquinone have been reported in some EOs [2].
The main compounds identified in O. elongatum EO are carvacrol (63.06%), γ-terpinene (15.99%), p-cymene (9.51%), and other compounds, with appreciable percentages such as α-phellandrene, caryophyllene, and α-pinene [3].
On the other hand, a previous study [4] showed that the chemical profile of O. elongatum EO shows the predominance of oxygenated compounds (65.14%), followed by hydrocarbon compounds (28.02%), knowing that thymol is the major compound with 63.44%. These results confirm the findings of [5], which showed that the main constituents found in the aerial parts of O. elongatum EO are carvacrol (67.34%), γ-terpinene (3.29%), p-cymene (3.62%), and thymol (1.79%). However, in 2013, Moussaoui et al. [6] showed that the main constituents identified in the O. elongatum EO are carvacrol (40.12%), thymol (14.24%), p-cymene (16.19%), and γ-terpinene (13.48%).
Chromatographic analysis of the seeds of O. elongatum EO revealed the richness of the chemical composition predominated by carvacrol with a percentage of 79.2%, followed by γ-terpinene (3.7%), p-cymene (5.2%), and linalool (2.4%) [7, 8].
3.5. Pharmacological Studies
EOs and extracts from O. elongatum showed different pharmacological properties such as antibacterial, antiparasitic, anticancer, and antioxidant effects (Figure 2). In the following part, all of these biological activities will be discussed.
Figure 2.
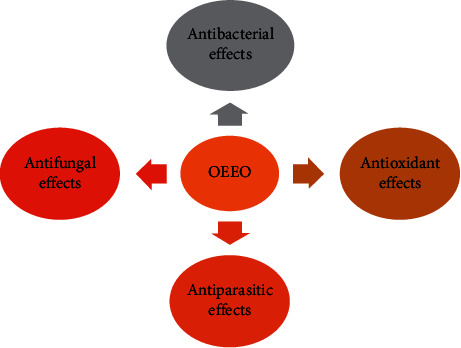
Pharmacological properties of O. elongatum.
3.6. Antibacterial Activity
Several studies showed the antibacterial efficacy of different EOs or extracts from different O. elongatum parts [3, 4, 6, 9–11]. Table 2 summarizes all the studies evaluating the antimicrobial activity of O. elongatum extracts and EOs.
Table 2.
Antibacterial effects of O. elongatum.
| Use part | Extracts | Used method | Tested strains | Key results | References |
|---|---|---|---|---|---|
| Leaves | Ethanolic extract | Method of diffusion in solid medium Macromethod of dilution in liquid medium |
P | — | [1] |
| P3 | Ф = 10 ± 0.8 mm | ||||
| P65 | Ф = 11 ± 0.8 mm | ||||
| P381 | Ф = 8.5 ± 0.4 mm | ||||
| P2 | Ф = 9 ± 0.8 mm | ||||
| P5 | Ф = 8.33 ± 0.8 mm | ||||
| Leaves | Aqueous extract | P | Ф = 11.66 ± 0.47 mm | ||
| P3 | Ф = 11 ± 0.95 mm | ||||
| P65 | Ф = 11.83 ± 0.23 mm | ||||
| P381 | Ф = 9.33 ± 1.24 mm | ||||
| P2 | Ф = 10 ± 0 mm | ||||
| P5 | Ф = 9.5 ± 0.4 mm | ||||
|
| |||||
| Aerial parts | Essential oil | Agar diffusion methods, broth microdilution assay | Staphylococcus aureus | Ф = 35.67 ± 0.66 mm | [3] |
| MIC = 0.03% | |||||
| MMC = 0.13% | |||||
| Escherichia coli | Ф = 26.33 ± 1.66 mm | ||||
| MIC = 0.03% | |||||
| MMC = 0.03% | |||||
| Pseudomonas aeruginosa | Ф = 9.33 ± 0.66 mm | ||||
| MIC = 0.5% | |||||
| MMC = 0.5% | |||||
|
| |||||
| Leaves | Ethanolic extract | Agar-well diffusion assay | Escherichia coli | Ф = 30.33 ± 2.51 mm | [11] |
|
| |||||
| Leaves | Essential oil | Disk-diffusion assay Microtitration method |
Salmonella CECT 915 | Ф = 34.33 ± 4.04 mm | [6] |
| MIC = 0.0625% | |||||
| MBC = 0.0625% | |||||
| Salmonella S64 | Ф = 28.17 ± 1.61 mm | ||||
| MIC = 0.0625% | |||||
| MBC = 0.125% | |||||
| E. coli O157 : H7 CECT4267 | Ф = 19.67 ± 1.15 mm | ||||
| MIC = 0.25% | |||||
| MBC = 0.5% | |||||
| L. monocytogenes CECT4031 | Ф = 34.00 ± 0.00 mm | ||||
| MIC = 0.125% | |||||
| MBC = 0.0625% | |||||
| L. monocytogenes L23 | Ф = 31.00 ± 3.46 mm | ||||
| MIC = 0.5% | |||||
| MBC = 0.5% | |||||
|
| |||||
| Flowering tops | Essential oil | Disk-diffusion assay Microtitration method |
Salmonella CECT 915 | Ф = 31.50 ± 2.78 mm | [6] |
| MIC = 0.0625 | |||||
| MBC = 0.125% | |||||
| Salmonella S64 | Ф = 28.17 ± 1.61 mm | ||||
| MIC = 0.0625% | |||||
| MBC = 0.125% | |||||
| E. coli O157 : H7 CECT4267 | Ф = 18.00 ± 0.00 mm | ||||
| MIC = 0.25% | |||||
| MBC = 0.5% | |||||
| L. monocytogenes CECT4031 | Ф = 29.00 ± 1.73 mm | ||||
| MIC = 0.125% | |||||
| MBC = 0.25% | |||||
| L. monocytogenes L23 | Ф = 31.00 ± 3.46 mm | ||||
| MIC = 0.5% | |||||
| MBC = 0.5% | |||||
|
| |||||
| Flowering tops | Essential oil | Total mesophilic aerobic flora (FMAT) | Significant effect on microbial growth | [10] | |
|
| |||||
| Aerial parts | Essential oil | Agar-well diffusion method Microdilution assay |
E. coli ATCC 25922 | Ф = 21.33 ± 0.57 mm | [4] |
| MIC = 0.5% | |||||
| MBC < 1% | |||||
| E. coli K12 | Ф = 16.00 ± 1.00 mm | ||||
| MIC = 0.25% | |||||
| MBC = 0.5% | |||||
| B. subtilis DCM 6633 | Ф = 24.66 ± 1.52 mm | ||||
| MIC = 0.5% | |||||
| MBC = 0.5% | |||||
| S. aureus ATCC 25923 | Ф = 27.00 ± 1.73 mm | ||||
| MIC = 0.125% | |||||
| MBC = 0.125% | |||||
|
| |||||
| Aerial parts | Dissolved volatile fraction | Agar-well diffusion method Microdilution assay |
E. coli ATCC 25922 | Ф = 28.33 ± 0.57 mm | [4] |
| MIC = 0.125% | |||||
| MBC = 0.25% | |||||
| E. coli K12 | Ф = 17.00 ± 1.73 mm | ||||
| MIC = 0.125% | |||||
| MBC = 0.125% | |||||
| B. subtilis DCM 6633 | Ф = 41.00 ± 2.64 mm | ||||
| MIC = 0.0625% | |||||
| MBC = 0.0625% | |||||
| S. aureus ATCC 25923 | Ф = 30.00 ± 2.00 mm | ||||
| MIC = 0.0312% | |||||
| MBC = 0.0312% | |||||
Bouharb et al. [9] evaluated in vitro the antibacterial activity of two extracts (aqueous and ethanolic) of O. elongatum leaves, from the Zerhoun region (central Morocco), on the growth of six strains of Pseudomonas aeruginosa (P, P3, P65, P381, P2, and P5), using the agar diffusion method and the broth macrodilution method of the active extract. The screening test revealed that O elongatum aqueous extract was more active than the ethanolic extract, with zones of inhibition ranging from 9.33 to 11.83 and 8.33 to 11 mm, respectively. Douhri et al. [19] also studied the antibacterial activity in vitro of ethanolic extracts of O. elongatum leaves. The results showed a very important antimicrobial effect against Escherichia coli (30.33 ± 2.51 mm). Moreover, El Harsal and colleagues evaluated the antimicrobial activity of the volatile fractions extracted from the hydrosol (DVF) and of EOs from O. elongatum aerial parts growing in northern Morocco against four bacterial strains; E. coli ATCC 25922, E. coli K12, S. aureus ATCC 25923, and B. subtilis DCM 6633. They found that the antibacterial effect of DVF was significantly higher than that of total EO. The DVF was active against all the studied bacteria; the strongest effect was observed on B. subtilis DCM 6633 with a large inhibition zone (41.0 ± 2.6 mm). Also, the total EO of O. elongatum was highly active against S. aureus ATCC 25923, B. subtilis DCM 6633, and E. coli ATCC 25922, with inhibition zones ranging from 21.3 to 24.6 mm, while a moderate effect was observed against E. coli K12 [4].
In another study, the O. elongatum EO extracted from flowering tops and that extracted from leaves were tested against five microorganisms (Salmonella S64, Salmonella CECT 915, Listeria monocytogenes CECT4031, L. monocytogenes L23, and E. coli O157 : H7 CECT4267), using the disk-diffusion assay and the microtitration assays. Additionally, the EOs showed the highest activities against the microorganisms tested, in particular against Salmonella and L. monocytogenes with zones of inhibition varying between 21.67 ± 0.58 mm and 34.33 ± 4.04 mm. The moderate activity was recorded against E. coli, with zones of inhibition from 14.33 ± 2.52 to 19.67 ± 1.15 mm [6]. Furthermore, a concentration of 0.06% of O. elongatum EO, extracted from flowering tops, showed a significant increase in the growth of total mesophilic aerobic flora (FMAT) [10]. In addition, the antibacterial activity of the O. elongatum EO (aerial part flowering) was tested against Staphylococcus aureus, P. aeruginosa, and E. coli. Therefore, an important inhibitory activity against all the strains tested was observed, with an inhibition diameter between 9.33 and 35.67 mm and high efficacy against E. coli and S. aureus [3].
3.7. Antifungal Activity
O. elongatum is one of the plants with antifungal properties [3, 12]. Indeed, several studies have evaluated these properties in O. elongatum EOs (Table 3). The antifungal activity of the essential oil of O. elongatum aerial parts was tested and evaluated by the microdilution method against three strains of fungi: Candida, Aspergillus, and Rhizopus [12]. Therefore, all Candida strains showed marked sensitivity to the essential oil. The Rhizopus strain was less sensitive, whereas for Aspergillus, this oil showed an effect only on tree strains. In another study, the antifungal activity of O. elongatum EO was evaluated by the agar plug diffusion method, which consequently showed promising results against Aspergillus brasiliensis (no measurable zone of inhibition) and Candida albicans (33.67 ± 0.33 mm) [3].
Table 3.
Antifungal activity of O. elongatum.
| Use part | Extracts | Used method | Tested strains | Key results | References |
|---|---|---|---|---|---|
| Aerial parts | Essential oil | Agar plug diffusion method | Candida albicans | Ф = 33.67 ± 0.33 mm | [3] |
| Aspergillus brasiliensis | No measurable zone of inhibition | ||||
|
| |||||
| Aerial parts | Essential oil | Microdilution method | Candida | Sensitive to the essential oil | [12] |
| Aspergillus | Susceptible to the oil | ||||
| Rhizopus | Moderately susceptible to the oil | ||||
3.8. Antiparasitic Activity
Many species of the genus Origanum have shown antiparasitic activities [13–15]. Moreover, the antiparasitic effect of O. elongatum was reported by several investigators (Table 4) [5, 16]. In 2017, Ramzi and collaborators [5] tested the acaricidal activity of the EOs of O. elongatum leaves on the Varroa mite. Therefore, these plant-derived EOs showed certain effectiveness against Varroa. Besides, the antiparasitic effect of O. elongatum EO was evaluated in experimental animals (female Wistar rats) infected with 6 Anisakis larvae using the gastric catheter method [16]. This technique was also used to administer O. elongatum (46.9 mg/0.5 mL of olive oil). Consequently, an EO activity against larva L3 of Anisakis pegreffii was observed; moreover, significant alterations of the esophageal region and the cuticle were detected in a large number of recovered larvae.
Table 4.
Other activities of O. elongatum.
| Activities | Use part | Extracts | Experimental approach | Key results | References |
|---|---|---|---|---|---|
| Antiviral | Leaves | Essential oil | Cytopathogenic murine norovirus (MNV-1) RAW 264.7 cells |
0.37 log10TCID50/ml reductions | [6] |
| Flowering tops | Essential oil | Cytopathogenic murine norovirus (MNV-1) RAW 264.7 cells |
0.75 log10TCID50/ml reductions | [6] | |
|
| |||||
| Antiparasitic | Leaves | Essential oil | Colonies of Apis mellifera bees Efficacy against Varroa mite in beehives |
Significant increase in mite drop All Varroa mites died |
[5] |
| Leaves | Essential oil | Larva L3 of Anisakis pegreffii isolated from the host Scomber japonicas and Trachurus trachurus Female Wistar rats infected with 6 Anisakis larvae by gastric catheter Administration of O. elongatum (46.9 mg/0.5 mL of olive oil) |
Significant larvicidal activity Significant alterations in the esophageal region and cuticle detected in a large number of recovered larvae |
[16] | |
|
| |||||
| Antioxidant | Leaves | Essential oil | EC50 = 1.20 g of extract/g DPPH | [1] | |
|
| |||||
| Vasodilatory activity | Leaves | Methanol extract | Perfusion pressure (PP) of the mesenteric bed of the rat Synthesis inhibitor endothelial vasodilators factors Vasoconstrictor α-mimetic: phenylephrine (PHE) Difference between the blood pressure before injection and blood pressure after injection |
Vasodilatory activity (PP = 50 mmHg) | [17] |
|
| |||||
| Vasodilatory activity | Leaves | Ethyl acetate extract | Perfusion pressure (PP) of the mesenteric bed of the rat Synthesis inhibitor endothelial vasodilators factors Vasoconstrictor α-mimetic: phenylephrine (PHE) Difference between the blood pressure before injection and blood pressure after injection |
Vasodilatory activity (PP = 20 mmHg) | [17] |
|
| |||||
| Effect on pomegranate juice quality | Flowering tops | Essential oil | pH variation Determination of total sugars Growth of natural flora in the pomegranate juice |
Improved the juice conservation process while preserving the nutritional and organoleptic qualities | [10] |
|
| |||||
| Corrosion inhibition | Leaves and flowers | Methanol/chloroform extract | Corrosion current density (jcorr) Electrochemical measurements Electrochemical impedance spectroscopy (EIS) Mass loss method Adsorption isotherms |
Corrosion potential (ecorr) decreased from −399.446 mV/ESC to −365.607 mV/ESC Significant decrease in corrosion current (jcorr) Increased the charge transfer resistance (Rct) with increased OEE concentration Ability of OEE to act as a protective layer against corrosion on mild steel |
[18] |
|
| |||||
| Hepatoprotective effect against carbon tetrachloride (CCl4) | Leaves | Methanol extract | Single-dose intraperitoneal injection of carbon tetrachloride (CCl4) (0.6 ml/kg) induced hepatotoxicity in rats Rats treated orally by gavage four doses of 250, 500, 1000, and 2000 mg/kg body weight |
Significant (P < 0.0001) decrease in serum aminotransferase levels and canalicular enzyme ALP reduced the architectural destruction cells |
[19] |
3.9. Antiviral Activity
The O. elongatum EOs, cultivated in northern Morocco, were studied for the inactivation of Murine norovirus (MNV-1) (Table 4), which is a human norovirus surrogate. Interestingly, the EOs from leaves and flowering tops showed antiviral activities of 0.87-0.50 log10TCID50/mL reduction and 0.75 log10TCID50/mL reduction, respectively [6].
3.10. Other Biological Activities
Besides the antiparasitic and antiviral activities, the antioxidant effect of endemic O. elongatum was examined in the Rif in northern of Morocco (Table 4) [1, 17]. The authors evaluated the vasodilator activity of O. elongatum leaves, extracted by methanol and ethyl acetate on a Wistar rat mesenteric vascular bed precontracted with norepinephrine. Measurement of the perfusion pressure of the rat mesenteric bed revealed that the methanolic extracts (PP = 50 mmHg) gave more active substances than the leaves of O. elongatum extracted in ethyl acetate (PP = 20 mmHg) [17]. Furthermore, the food conservation aspect of flowering tops of the studied plant on fresh pomegranate juice was demonstrated [10]. The findings demonstrated that the EOs combined with heat reduce the growth of natural flora presented in the pomegranate juice and thus improve the juice conservation process, while nutritional and organoleptic qualities were also preserved. Moreover, the impact against corrosion inhibition of O. elongatum leaves and flowers, using a mixture of methanol/chloroform, was deeply elaborated by applying the electrochemical impedance spectroscopy (EIS), mass loss method, and adsorption isotherms method [18]. According to their electrochemical parameters measurement, it was shown that the use of extracts decreased the corrosion potential (Ecorr), which ranged from −399.446 mV/ESC to −365.607 mV/ESC. A similar decrease was observed with corrosion current (jcorr). Nonetheless, a significant increase of charge transfer resistance (Rct) was noted, with the increase of the OEE concentration (the ability of layer protection from corrosion on the mild steel) [18]. Another important impact of O. elongatum was surveyed by Douhri et al. [19].
Scientists have shown the hepatoprotective effect of methanolic leaf extracts of this species at different doses against the toxicity induced by carbon tetrachloride (CCl4) in rats. The biochemical examination of serum hepatic biomarkers showed a significant decrease in serum aminotransferase levels, the canalicular enzyme, and alkaline phosphatase and reduction in the destruction of hepatic cell architecture at the dose of 2000 mg/kg/d [19].
3.11. Toxicology
O. elongatum is an aromatic plant well known for its flavor and widely consumed in Morocco as a condiment and food preservative [24]. From a toxicological point of view, only one study investigated the toxicological properties of O. elongatum extract by evaluating acute oral toxicity [19]. The results showed a slight change in behavior with loss of appetite and temporary sedation without any change in pathophysiological and neurological activity with an LD50 greater than 3000 mg/kg. Jenner et al. [27] also reported the same signs in rats when testing carvacrol, the major component of O. elongatum, in an acute oral toxicity test. The LD50 in this test was 810 mg/kg, suggesting that carvacrol is the active ingredient responsible for the behavioral change caused by this plant [27].
4. Biological Mechanism Insights into O. elongatum Main Compounds
The potent anti-inflammatory activity of the extracts encouraged the authors to isolate the main compounds (thymol, carvacrol, limonene, α-pinene, and linalool), which might be responsible for the anti-inflammatory effect. The reported studies showed that thymol inhibits inducible lymphocyte proliferation [28], reduces edema and leukocyte influx to injured areas [29], and induces membrane stabilization (84.11%) in human red blood cell membrane stabilization assay [30]. Moreover, this molecule had inhibitory effects on various inflammatory mediators such as IL-1β, IL-6, TNF-α, and TNF-β [31]. It also decreased c-Fos, NFAT-1, and NFAT-2 expression, with inhibition of inducible phospho-SAPK/JNK and phospho-STAT3 levels [32]. Molecular investigations showed that thymol also inhibits TLR4 upregulation and suppresses IKK, iκbα, and p65 phosphorylation [33]. Additionally, carvacrol has also shown an anti-inflammatory effect [34]. This compound was reported to be able to inhibit the production of PGE2 (inflammatory mediator catalyzed by COX1) and suppress COX-2 promoter activity by activating PPARα and PPARγ. It also reduced the expression of LPS-induced COX-2 mRNA and protein, suggesting that the action of carvacrol on COX-2 is mediated through its agonistic effect on PPARγ [35]. Moreover, Yoon et al. [36] showed that limonene decreased the production of proinflammatory cytokines and inflammatory mediators in macrophages by the inhibition of LPS-induced NO and PGE2, which decreased iNOS and COX-2 expression [36]. This monoterpene also exhibited an anti-inflammatory effect via the inhibition of some signaling pathways leading to the inflammatory process in leukemia (HL-60) cell lines, such as ROS, monocyte chemoattractant protein-1 (MCP-1), NF-κB, and p38 mitogen-activated protein kinase (MAPK) [37]. On the other hand, α-pinene exhibited potent activity in the inflammatory process and neuropathic pain [38]. It inhibited ear edema at 0.15 h (120–135% vs. 175%), paw edema at 12 h (146 ± 6%), and a decrease in COX-2 (115 ± 74% vs. 202 ± 20%) [39] and reduced the level of IL-6 in the hippocampus, cortex, and striatum [40]. In addition, linalool is another monoterpene, which also exhibited an anti-inflammatory effect. This compound significantly reduced hypersensitivity and paw edema at doses of 50 and 200 mg/kg of carrageenan-induced edema model in rats [41, 42].
Several studies showed the antidiabetic effect of different compounds identified in O. elongatum. The authors showed that carvacrol exhibits antidiabetic effects via several mechanisms such as reduction in blood glucose and insulin levels, decrease in (HOMA-IR) index, and decrease in the expressions of the mRNA of gluconeogenic genes, PEPCK, and G6Pase [43]. Additionally, carvacrol may also decrease glucose levels by lowering HbA1c, G6Pase, and FBPase activities. It also promoted the activities of glucokinase and glucose-6-phosphate dehydrogenase in the liver and protected pancreatic islets [44]. This monoterpene inhibited the activity of α-amylase (IC50 = 152.3 ± 1.21 μg mL−1), α-glucosidase (IC50 = 94.02 ± 0.78 μg mL−1) [45], and β-galactosidase [46]. On the other hand, limonene ameliorates glucose homeostasis by increasing hepatic glycogen with a decrease in plasma glucose and HbA1c levels and suppresses the activities of gluconeogenic enzymes (G6Pase and FBPase) [47]. Moreover, it ameliorates the reduction of FBG level and glucose tolerance along with the activation of PPARα signaling [48]. In addition, using two different cell lines, C2C12 skeletal muscle cells [49] and 3T3-L1 preadipocytes [50], limonene has been shown to improve glucose absorption by increasing phosphorylation of activated protein kinase B (Akt) and promoting p38 mitogen-activated protein kinase (p38MAPK) [50].
Furthermore, linalool also showed an antidiabetic effect [51, 52]. This compound was reported to be able to decrease blood glucose, HbA1c, fructosamine, IL-6 and TNF-α, and area under the curve of (AUCglucose) glucose value and increase insulin level [52]. On the other hand, thymol was also able to treat hyperglycemia by normalizing blood sugar, plasma insulin, HbA1c, and insulin resistance index [53]. Rhayour et al. [54] investigated the expression levels of genes involved in insulin transcription in STZ-induced diabetic rats and reported an increase in expression of the Mafa and Pdx1 genes.
The major compounds of this plant are limonene, linalool, carvacrol, and thymol. In fact, these molecules have shown in some studies a significant antibacterial power [55–57]. Rhayour et al. [58] examined the mechanism of action of thymol on bacteria E. coli and Bacillus subtilis as the model of Gram-positive and Gram-negative bacteria. This action was demonstrated by the release of absorbent substances at 260 nm. This release of substances associated with rapid bacterial mortality could be the consequence of lesions on the envelopes induced by antibacterial agents (Figure 3). Another study [59] showed that carvacrol affects cell membranes of bacteria by changing the composition of fatty acids, which subsequently affects the fluidity and permeability of the membrane. On the other hand, several studies have indicated that linalool alters normal cell morphology, destroys the cell wall and cell membrane, inhibits the growth of P. aeruginosa, and even leads to its death [60]. However, the mechanism of action of limonene against cytoplasmic membranes of microorganisms results in a loss of membrane integrity (Figure 3), inhibition of respiratory enzymes, and dissipation of the proton motive force [61].
Figure 3.
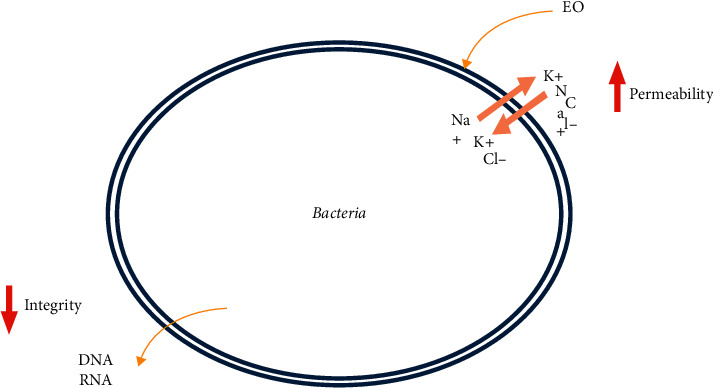
Antibacterial action of O. elongatum bioactive compounds.
Numerous studies have been published on the anticancer activity of the main compounds of oregano EOs such as limonene, carvacrol, and thymol [62–64]. Islam et al. [63] determined that the mechanism of action of thymol in a cancer cell caused severe DNA damage through several mechanisms (e.g., ROS induction and subsequent increase in oxidative stress and/or mitochondrial dysfunction or nuclear factor of activated T-cells (NFAT-2) pathway), which eventually upregulates Bax/Bcl-2 protein expression and results in the cytochrome- (cyto-) c release from the mitochondria (intrinsic pathway). In another work, carvacrol treatment induced cell apoptosis, possibly through the activation of the mitochondrial apoptotic, MAPK, and PI3K/Akt signaling pathways. Taken together, our results indicate that carvacrol might be a promising natural product in the management of colon cancer [65].
5. Conclusion
Morocco is a country rich in plant resources with a specific diversity of medicinal plants used in the treatment and prevention of several illnesses. This study provides evidence that the Moroccan O. elongatum L. species possesses active principles that exhibit marked therapeutic effects confirming and justifying the popular uses of these plants to treat certain diseases as antibacterial, antifungal, antiviral, antioxidant, vasodilator, corrosion inhibitor, and hepatoprotective agents. The current study represents useful documentation that can provide sufficient support for clinical trials of O. elongatum L. Although preliminary studies have confirmed their therapeutic effect, further investigations should be carried out, in particular, to ensure the safety of the treatment.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Abdelaali Balahbib and Abdelhakim Bouyahya contributed equally to this work.
References
- 1.Oualili H., Nmila R., Chibi F. Chemical composition and antioxidant activity of Origanum elongatum essential oil. Pharmacognosy Research. 2019;11(3):p. 283. [Google Scholar]
- 2.Bakha M., Al Faiz C., Daoud M., et al. Genome size and chromosome number for six taxa of Origanum genus from Morocco. Botany Letters. 2017;164(4):361–370. doi: 10.1080/23818107.2017.1395766. [DOI] [Google Scholar]
- 3.Boukhira S., Bousta F., Moularat S., Abdellaoui A., Ouaritini Z. B., Bousta D. Evaluation of the preservative properties of origanum elongatum essential oil in a topically applied formulation under a challenge test. Phytothérapie. 2018;18(2):92–98. doi: 10.3166/phyto-2018-0067. [DOI] [Google Scholar]
- 4.El Harsal A., Ibn Mansour A., Skali Senhaji N., et al. Influence of extraction time on the yield, chemical composition, and antibacterial activity of the essential oil from origanum elongatum (E. & M.) harvested at Northern Morocco. Journal of Essential Oil Bearing Plants. 2018;21(6):1460–1474. doi: 10.1080/0972060x.2019.1572545. [DOI] [Google Scholar]
- 5.Ramzi H., Ismaili M. R., Aberchane M., Zaanoun S. Chemical characterization and acaricidal activity of Thymus satureioides C. & B. and Origanum elongatum E. & M. (Lamiaceae) essential oils against Varroa destructor Anderson & Trueman (Acari: Varroidae) Industrial Crops and Products. 2017;108:201–207. doi: 10.1016/j.indcrop.2017.06.031. [DOI] [Google Scholar]
- 6.Moussaoui N., Sanchez G., Khay E. O. Antibacterial and antiviral activities of essential oils of northern moroccan plants. British Biotechnology Journal. 2013;3(3):318–331. doi: 10.9734/bbj/2013/3596. [DOI] [Google Scholar]
- 7.Figuérédo G., Cabassu P., Chalchat J.-C., Pasquier B. Studies of mediterranean oregano populations. vi: chemical composition of essential oils of origanum elongatumemberger et maire from Morocco. Journal of Essential Oil Research. 2006;18(3):278–280. doi: 10.1080/10412905.2006.9699087. [DOI] [Google Scholar]
- 8.Figueredo G. Clermont-Ferrand, France: Université Blaise Pascal-Clermont-Ferrand II; 2007. Etude chimique et statistique de la composition d’huiles essentielles d’origans (Lamiaceae) cultivés issus de graines d’origine méditerranéenne. Theses. [Google Scholar]
- 9.Bouharb H., El Badaoui K., Zair T., El amri J., Chakir S., Alaoui T. Sélection de quelques plantes médicinales du Zerhoun (Maroc centrale) pour l’activité antibactérienne contre Pseudomonas aeruginosa. Journal of Applied Biosciences. 2014;78:6685–6693. doi: 10.4314/jab.v78i0.3. [DOI] [Google Scholar]
- 10.Moussaoui N. E., Khay E. O., Amajoud N., et al. Effect of Origanum elongatum essential oil and heating on pomegranate juice quality. International Journal of Current Microbiology and Applied Sciences. 2016;5(4):1–8. doi: 10.20546/ijcmas.2016.504.001. [DOI] [Google Scholar]
- 11.Douhri H., Raissouni I., Amajoud N., et al. Antibacterial effect of ethanolic extracts of Moroccan plant against Escherichia coli. Journal of Materials and Environmental Sciences. 2017;8(12):4408–4414. doi: 10.26872/jmes.2017.8.12.465. [DOI] [Google Scholar]
- 12.Amakran A., Hamoudane M., Ramdan B. Antifungal activity of the essential oil of origanum elongatum on candida, aspergillus and rhizopus species. Journal de Mycologie Medicale. 2014;2(24):p. e78. doi: 10.1016/j.mycmed.2014.01.078. [DOI] [Google Scholar]
- 13.Coskun S., Girisgin O., Kürkcüoglu M., et al. Acaricidal efficacy of Origanum onites L. essential oil against Rhipicephalus turanicus (Ixodidae) Parasitology Research. 2008;103(2):259–261. doi: 10.1007/s00436-008-0956-x. [DOI] [PubMed] [Google Scholar]
- 14.Degerli S., Tepe B., Celiksoz A., Berk S., Malatyali E. In vitro amoebicidal activity of Origanum syriacum and Origanum laevigatum on Acanthamoeba castellanii cysts and trophozoites. Experimental Parasitology. 2012;131(1):20–24. doi: 10.1016/j.exppara.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Machado M., Dinis A. M., Salgueiro L., Cavaleiro C., Custódio J. B. A., Sousa M. d. C. Anti-Giardia activity of phenolic-rich essential oils: effects of Thymbra capitata, Origanum virens, Thymus zygis subsp. sylvestris, and Lippia graveolens on trophozoites growth, viability, adherence, and ultrastructure. Parasitology Research. 2010;106(5):1205–1215. doi: 10.1007/s00436-010-1800-7. [DOI] [PubMed] [Google Scholar]
- 16.Abattouy N., Valero López A., Romero M. C. Actividad IN VIVO del aceite esencial de Origanum elongatum frente a larvas L3 de Anisakis pegreffii. IN VIVO Activity of Essential Oil Origanum Elongatum Against Larva L3 of Anisakis Pegreffii. 2010;51:107–111. [Google Scholar]
- 17.Yousfi K. E., Greche H., Misbahi H., Cheikh R. B. Phytochemical evaluation and vasodilatory activity of O. elongatum, C. salviifolius and C. laurifolius. Moroccan Journal of Chemistry. 2020;8(1):226–234. [Google Scholar]
- 18.El Attari H., Chefira K., Rchid H. Corrosion inhibition study of the origanum elongatum extract: electrochemical, gravimetric and adsorption isotherms studies in 0.5 m sulfuric acid. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2019;10(1) [Google Scholar]
- 19.Douhri B., Idaomar M., Senhaji N. S., Ennabili A., Abrini J. Hepatoprotective effect of origanum elongatum against Carbon Tetrachloride (CCl4) induced toxicity in rats. European Journal of Medicinal Plants. 2014;4(1):14–28. doi: 10.9734/ejmp/2014/5132. [DOI] [Google Scholar]
- 20.Ietswaart J. H., Else V. A Taxonomic Revision of the Genus Origanum (Labiatae) The Hague, The Netherlands: The Hague: Leiden University Press; 1980. [Google Scholar]
- 21.Kintzios S. E. Oregano. In: Peter K. V., editor. In Handbook of Herbs and Spices. 2nd. Sawston, UK: Woodhead Publishing; 2012. pp. 417–436. [DOI] [Google Scholar]
- 22.Simonnet X., Quennoz M., Bellenot D., Pasquier B. Evaluation agronomique et chimique de différentes espèces d’origan. Revue suisse de viticulture, arboriculture et horticulture. 2011;43(6):344–349. [Google Scholar]
- 23.Euro+MedPlantBase. The information resource for euro-mediterranean plant diversity. 2006. http://ww2.bgbm.org/EuroPlusMed/query.asp.
- 24.Christaki E., Bonos E., Giannenas I., Florou-Paneri P. Aromatic plants as a source of bioactive compounds. Agriculture. 2012;2(3):228–243. doi: 10.3390/agriculture2030228. [DOI] [Google Scholar]
- 25.Benabid A. Flore et ecosystemes du Maroc: evaluation et preservation de la biodiversite. 2000.
- 26.Belmehdi O., El Harsal A., Benmoussi M., Laghmouchi Y., Skali Senhaji N., Abrini J. Effect of light, temperature, salt stress and pH on seed germination of medicinal plant Origanum elongatum (Bonnet) Emb. & Maire. Biocatalysis and Agricultural Biotechnology. 2018;16:126–131. doi: 10.1016/j.bcab.2018.07.032. [DOI] [Google Scholar]
- 27.Jenner P. M., Hagan E. C., Taylor J. M., Cook E. L., Fitzhugh O. G. Food flavourings and compounds of related structure I. acute oral toxicity. Food and Cosmetics Toxicology. 1964;2:327–343. doi: 10.1016/s0015-6264(64)80192-9. [DOI] [PubMed] [Google Scholar]
- 28.Amirghofran Z., Hashemzadeh R., Javidnia K., Golmoghaddam H., Esmaeilbeig A. In vitroimmunomodulatory effects of extracts from three plants of theLabiataefamily and isolation of the active compound(s) Journal of Immunotoxicology. 2011;8(4):265–273. doi: 10.3109/1547691x.2011.590828. [DOI] [PubMed] [Google Scholar]
- 29.Pascual M. E., Slowing K., Carretero E., Sánchez Mata D., Villar A. Lippia: traditional uses, chemistry and pharmacology: a review. Journal of Ethnopharmacology. 2001;76(3):201–214. doi: 10.1016/s0378-8741(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 30.Sheorain J., Mehra M., Thakur R., Grewal S., Kumari S. In vitro anti-inflammatory and antioxidant potential of thymol loaded bipolymeric (tragacanth gum/chitosan) nanocarrier. International Journal of Biological Macromolecules. 2019;125:1069–1074. doi: 10.1016/j.ijbiomac.2018.12.095. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y.-M., Chao T.-Y., Chang W.-C., Chang M. J., Lee M.-F. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. Journal of Food and Drug Analysis. 2016;24(3):556–563. doi: 10.1016/j.jfda.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gholijani N., Gharagozloo M., Farjadian S., Amirghofran Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. Journal of Immunotoxicology. 2016;13(2):157–164. doi: 10.3109/1547691x.2015.1029145. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q., Cheng F., Xu Y., et al. Thymol alleviates lipopolysaccharide-stimulated inflammatory response via downregulation of RhoA-mediated NF-κB signalling pathway in human peritoneal mesothelial cells. European Journal of Pharmacology. 2018;833:210–220. doi: 10.1016/j.ejphar.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Landa P., Kokoska L., Pribylova M., Vanek T., Marsik P. In vitro anti-inflammatory activity of carvacrol: inhibitory effect on COX-2 catalyzed prostaglandin E2 biosynthesisb. Archives of Pharmacal Research. 2009;32(1):75–78. doi: 10.1007/s12272-009-1120-6. [DOI] [PubMed] [Google Scholar]
- 35.Hotta M., Nakata R., Katsukawa M., Hori K., Takahashi S., Inoue H. Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. Journal of Lipid Research. 2010;51(1):132–139. doi: 10.1194/jlr.m900255-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon W.-J., Lee N. H., Hyun C.-G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. Journal of Oleo Science. 2010;59(8):415–421. doi: 10.5650/jos.59.415. [DOI] [PubMed] [Google Scholar]
- 37.Wiseman D. A., Werner S. R., Crowell P. L. Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21cip1and p27kip1in human pancreatic adenocarcinoma cells. Journal of Pharmacology and Experimental Therapeutics. 2007;320(3):1163–1170. doi: 10.1124/jpet.106.111666. [DOI] [PubMed] [Google Scholar]
- 38.Quintão N., da Silva G., Antonialli C., Rocha L., Filho V., Cicció J. Chemical composition and evaluation of the anti-hypernociceptive effect of the essential oil extracted from the leaves of ugni myricoideson inflammatory and neuropathic models of pain in mice. Planta Medica. 2010;76(13):1411–1418. doi: 10.1055/s-0029-1240891. [DOI] [PubMed] [Google Scholar]
- 39.Li X.-J., Yang Y.-J., Li Y.-S., Zhang W. K., Tang H.-B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. Journal of Ethnopharmacology. 2016;179:22–26. doi: 10.1016/j.jep.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Khoshnazar M., Bigdeli M. R., Parvardeh S., Pouriran R. Attenuating effect of α-pinene on neurobehavioural deficit, oxidative damage and inflammatory response following focal ischaemic stroke in rat. Journal of Pharmacy and Pharmacology. 2019;71(11):1725–1733. doi: 10.1111/jphp.13164. [DOI] [PubMed] [Google Scholar]
- 41.Batista P. A., de Paula Werner M. F., Oliveira E. C., et al. The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. The Journal of Pain. 2010;11(11):1222–1229. doi: 10.1016/j.jpain.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Peana A. T., D’Aquila P. S., Panin F., Serra G., Pippia P., Moretti M. D. L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9(8):721–726. doi: 10.1078/094471102321621322. [DOI] [PubMed] [Google Scholar]
- 43.Woon K. G., Kyung J. H., Do Yeon K., Hyun C. S. Beneficial effects of carvacrol on insulin resistance in high fat diet-induced diabetic mice. Proceedings of the Fall General Meeting and Academic Conference 2013; October 2013; San Francisco, CA, USA. p. p. 281. [Google Scholar]
- 44.Ezhumalai M., Radhiga T., Pugalendi K. V. Antihyperglycemic effect of carvacrol in combination with rosiglitazone in high-fat diet-induced type 2 diabetic C57BL/6J mice. Molecular and Cellular Biochemistry. 2014;385(1–2):23–31. doi: 10.1007/s11010-013-1810-8. [DOI] [PubMed] [Google Scholar]
- 45.Govindaraju S., Arulselvi P. I. Characterization of coleus aromaticus essential oil and its major constituent carvacrol forin vitroantidiabetic and antiproliferative activities. Journal of Herbs, Spices & Medicinal Plants. 2018;24(1):37–51. doi: 10.1080/10496475.2017.1369483. [DOI] [Google Scholar]
- 46.Wang J., Hu X., Ai W., et al. Phytol increases adipocyte number and glucose tolerance through activation of PI3K/Akt signaling pathway in mice fed high-fat and high-fructose diet. Biochemical and Biophysical Research Communications. 2017;489(4):432–438. doi: 10.1016/j.bbrc.2017.05.160. [DOI] [PubMed] [Google Scholar]
- 47.Murali R., Saravanan R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomedicine & Preventive Nutrition. 2012;2(4):269–275. doi: 10.1016/j.bionut.2012.08.008. [DOI] [Google Scholar]
- 48.Jing L., Zhang Y., Fan S., et al. Preventive and ameliorating effects of citrus d-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. European Journal of Pharmacology. 2013;715(1–3):46–55. doi: 10.1016/j.ejphar.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 49.Soundharrajan I., Kim D. H., Srisesharam S., Kuppusamy P., Sivanesan R., Choi K. C. Limonene promotes osteoblast differentiation and 2-deoxy- d -glucose uptake through p38MAPK and Akt signaling pathways in C2C12 skeletal muscle cells. Phytomedicine. 2018;45:41–48. doi: 10.1016/j.phymed.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Soundharrajan I., Kim D. H., Srisesharam S., Kuppusamy P., Choi K. C. R-limonene enhances differentiation and 2-Deoxy-D-Glucose uptake in 3t3-l1 preadipocytes by activating the akt signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2018;2018:1–10. doi: 10.1155/2018/4573254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.More T. A., Kulkarni B. R., Nalawade M. L., Arvindekar A. U. Antidiabetic activity of linalool and limonene in streptozotocin- induced diabetic rat: a combinatorial therapy approach. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6(8):p. 6. [Google Scholar]
- 52.Deepa B., Anuradha C. V. Linalool, a plant derived monoterpene alcohol, rescues kidney from diabetes-induced nephropathic changes via blood glucose reduction. Diabetologia Croatica. 2011;17 [Google Scholar]
- 53.Saravanan S., Pari L. Role of thymol on hyperglycemia and hyperlipidemia in high fat diet-induced type 2 diabetic C57BL/6J mice. European Journal of Pharmacology. 2015;761:279–287. doi: 10.1016/j.ejphar.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 54.Saadat Brujeni A. Thymol effect on the expression of Mafa and Pdx1 genes in streptozotocin-induced diabetic rats. Razi Journal of Medical Sciences. 2019;26(10) [Google Scholar]
- 55.Hąc-Wydro K., Flasiński M., Romańczuk K. Essential oils as food eco-preservatives: model system studies on the effect of temperature on limonene antibacterial activity. Food Chemistry. 2017;235:127–135. doi: 10.1016/j.foodchem.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 56.Churklam W., Chaturongakul S., Ngamwongsatit B., Aunpad R. The mechanisms of action of carvacrol and its synergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control. 2020;108 doi: 10.1016/j.foodcont.2019.106864.106864 [DOI] [Google Scholar]
- 57.Duarte A., Luís Â., Oleastro M., Domingues F. C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control. 2016;61:115–122. doi: 10.1016/j.foodcont.2015.09.033. [DOI] [Google Scholar]
- 58.Rhayour K., Bouchikhi T., Tantaoui-Elaraki A., Sendide K., Remmal A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components onescherichia coliand bacillus subtilis. Journal of Essential Oil Research. 2003;15(4):286–292. doi: 10.1080/10412905.2003.9712144. [DOI] [Google Scholar]
- 59.Swamy M. K., Akhtar M. S., Sinniah U. R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evidence-based Complementary and Alternative Medicine. 2016;2016 doi: 10.1155/2016/3012462.3012462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Cai J., Chen H., et al. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microbial Pathogenesis. 2020;141 doi: 10.1016/j.micpath.2020.103980.103980 [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z., Vriesekoop F., Yuan Q., Liang H. Effects of nisin on the antimicrobial activity of d-limonene and its nanoemulsion. Food Chemistry. 2014;150:307–312. doi: 10.1016/j.foodchem.2013.10.160. [DOI] [PubMed] [Google Scholar]
- 62.Baranauskaite J., Kubiliene A., Marksa M., et al. The influence of different oregano species on the antioxidant activity determined using HPLC postcolumn DPPH method and anticancer activity of carvacrol and rosmarinic acid. BioMed Research International. 2017;2017 doi: 10.1155/2017/1681392.1681392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Islam M. T., Khalipha A. B. R., Bagchi R., et al. Anticancer activity of thymol: a literature-based review and docking study with Emphasis on its anticancer mechanisms. IUBMB Life. 2019;71(1):9–19. doi: 10.1002/iub.1935. [DOI] [PubMed] [Google Scholar]
- 64.Sekhar K. C., Rajanikanth A., Bobby M. N., Kanala J. R. A review on anticancer potential of natural drugs: hispolon and limonene. International Journal of Current Microbiology and Applied Sciences. 2018;7(11):3253–3263. doi: 10.20546/ijcmas.2018.711.375. [DOI] [Google Scholar]
- 65.Fan K., Li X., Cao Y., et al. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anti-Cancer Drugs. 2015;26(8):813–823. doi: 10.1097/cad.0000000000000263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


