Abstract
Parkinson’s disease and other related diseases with alpha-synuclein pathology are associated with a long prodromal or preclinical stage of disease. Predictive models based on diagnosis of idiopathic rapid eye-movement (REM) sleep behavior disorder (iRBD) make it possible to identify people in the prodromal stage of synucleinopathy that have a high probability of future disease and provide an opportunity to implement neuroprotective therapies. However, rehabilitation providers may be unaware of iRBD and the motor abnormalities that indicate early motor system dysfunction related to alpha-synuclein pathology. Further, there is no existing rehabilitation framework to guide early interventions for people with iRBD. The purpose of this work is to 1) review extrapyramidal signs of motor system dysfunction in people with iRBD, and 2) propose a framework for early protective or preventive therapies in prodromal synucleinopathy using iRBD as a predictive marker. Longitudinal and cross-sectional studies indicate that the earliest emerging motor deficits in iRBD are bradykinesia, deficits performing activities of daily living, and abnormalities in speech, gait and posture. These deficits may emerge up to 12 years before a diagnosis of synucleinopathy. The proposed rehabilitation framework for iRBD includes early exercise-based interventions of aerobic exercise, progressive resistance training, and multimodal exercise with rehabilitation consultations to address exercise prescription, progression, and monitoring. This rehabilitation framework may be used to implement neuroprotective, multidisciplinary, and proactive clinical care in people with a high likelihood of conversion to Parkinson’s disease, dementia with Lewy bodies or multiple systems atrophy.
Keywords: rehabilitation, sleep disorders, prodromal, Parkinson’s disease, exercise, alpha-synuclein
INTRODUCTION
Traditional rehabilitation therapies for Parkinson’s disease (PD) are delivered when physical function is impaired and substantial neurodegeneration (~50–90% loss of nigral dopaminergic cells) has already occurred,1 likely limiting the effectiveness of physical interventions. However, it is now well recognized that PD is associated with a long prodromal2 or pre-diagnostic3 stage of disease, lasting years to decades, that may provide a window during which neuroprotective interventions can be proactively applied. A clinical population that is known to represent the early stages of alpha-synuclein pathology (i.e. prodromal synucleinopathy) and have a high likelihood of progression to overt neurodegenerative disease are people with idiopathic rapid eye movement (REM) sleep behavior disorder (iRBD).4 Thus, the presentation of iRBD alone in a patient necessitates the need for the initiation of therapies that slow or prevent progression of neurodegenerative alpha-synucleinopathy disease.5 Currently, there is no rehabilitation framework for how to treat people in the preclinical, prodromal or pre-diagnostic stages of synucleinopathy.
Early motor abnormalities observed in iRBD offer valuable insight regarding the onset and severity of motor system degeneration, and relevant early or protective therapies that may delay or mitigate future disability. The aims of this work are, first, to summarize the motor abnormalities present in people with iRBD and second, to propose a rehabilitative framework for physical and exercise-based therapies in people with prodromal synucleinopathy. This framework may be used to design effective neuroprotective trials and proactive clinical care in iRBD. While individuals with iRBD may progress to a variety of alpha-synucleinopathy phenotypes (e.g. Parkinson’s disease, dementia with Lewy bodies or multiple systems atrophy), the focus of the present work is largely centered on prior research in PD and clinical applications in prodromal or early PD.
Preclinical and Prodromal Synucleinopathy
Alpha-synucleinopathy is a subset of neurodegenerative disorders characterized by the presence of alpha-synuclein insoluble aggregates in neuronal or glial cells.6 While PD is the most common synucleinopathy, other phenotypes of synucleinopathy include dementia with Lewy bodies, and multiple systems atrophy.6,7 The time course of synucleinopathy may be separated into three stages of disease progression: preclinical, prodromal, and clinical (Table 1). The preclinical stage is considered the point at which the neurodegenerative process has begun, but there are no overt signs or symptoms of disease.4 The prodromal stage is considered the time period during which non-specific (non-motor) and/or specific (motor) signs of synucleinopathy or parkinsonism are present, but a clinical diagnosis cannot be made.4 Clinical synucleinopathy, for example PD, is diagnosed once the cardinal motor signs (i.e. bradykinesia, rigidity and/or resting tremor) have sufficiently manifested. Given that more than 30–70% of nigrostriatal dopaminergic neurons have already been lost at the time of PD diagnosis,8 it is vital that patients in the preclinical and prodromal stages of synucleinopathy be identified as early as possible in order to initiate interventions that can slow or prevent disease progression.
Table 1.
Operational Definitions
| Synucleinopathy | A subset of neurodegenerative disorders characterized by the presence of alpha-synuclein insoluble aggregates in neuronal or glial cells. Examples of clinical synucleinopathy include Parkinson’s disease, dementia with Lewy bodies or multiple systems atrophy. |
| Parkinsonism | A term used to describe a group of neurologic disorders that present with generalized bradykinesia, rigidity, and/or tremor. |
| Preclinical synucleinopathy | A stage of synucleinopathy neurodegeneration when the symptoms are largely absent. Biomarkers are necessary to identify people in the preclinical stage. |
| Prodromal synucleinopathy | A stage of synucleinopathy neurodegeneration when the clinical symptoms or signs of parkinsonism are evident (i.e bradykinesia), but the patient does not have an overt alpha-synucleinopathy as defined by current diagnostic criteria. |
| Clinical Parkinson’s Disease | A stage of neurodegeneration when the clinical signs of parkinsonism are observed: bradykinesia plus rest tremor and/or rigidity. |
| Phenoconversion | The timepoint of clinical conversion from prodromal synucleinopathy to an overt alpha-synucleinopathy neurodegenerative disease. |
| Rapid eye movement (REM) sleep without atonia (RSWA) | Loss of normal muscle atonia during rapid eye movement sleep, measured via electromyography |
| REM Sleep Behavior Disorder (RBD) | A sleep disorder parasomnia characterized by 1) elevated muscle activity during REM, or a loss of the usual REM sleep atonia (i. e. RSWA) and 2) dream enactment. |
| Idiopathic RBD | RBD occurring in the absence of a diagnosed neurologic disorder (such as Parkinson’s disease, dementia with Lewy bodies, or multiple systems atrophy) or possible cause. iRBD is considered a prodromal synucleinopathy. |
PD = Parkinson’s disease, REM = rapid eye movement, RSWA = REM sleep without atonia, RBD = REM sleep behavior disorder. Definitions are taken from McCann et al., 2014; Postuma & Berg, 2016; Schenck et al. 1986; Boeve et al. 2007.
Among the various motor and non-motor biomarkers related to parkinsonism (see Heinzel et al. 2019 for review), a diagnosis of iRBD is recognized as the strongest predictor of future progression to PD4,10. iRBD is a parasomnia characterized by elevated muscle activity (REM sleep without atonia) and dream enactment behavior during REM sleep.11 Of the estimated 1% of the population affected by RBD,12 roughly 81%−90% of these individuals will eventually develop a neurodegenerative disorder and are therefore recognized as a prodromal or early stage of alpha-synucleinopathy.11 When RBD is idiopathic (iRBD), an estimated 74% of these individuals are predicted to develop a synucleinopathy disease within 12 years.5 Thus, iRBD provides a critical opportunity to study people with early stage synucleinopathy that have a high likelihood to progress to PD.
Furthermore, RBD or RSWA are present in roughly 40–55% of people with PD, and are associated with a more severe, rapid-declining sub-type of PD.13 In terms of the classical phenotypes, the co-expression of PD with RBD or RSWA is less likely to be a tremor predominant subtype and more likely to present as an akinetic-rigid subtype with greater rigidity,14 increased postural instability and gait disturbances and increased risk of falls.13 Clinically, it is important to identify patients with PD and RBD as they may benefit from early or intensive therapies due to their high-risk for accelerated functional decline.
ABNORMALITIES IN IRBD
Non-motor Abnormalities in iRBD
Non-motor features that predict future synucleinopathy in people with iRBD include loss of olfaction, impaired color acuity, constipation, sexual and urinary dysfunction, autonomic abnormalities, and cognitive decline.7,15 Based on the presence of non-motor impairments, longitudinal studies suggest that the prodromal phase may last 20–25 years.7,15 The earliest non-motor abnormalities are reported to be loss of olfaction and orthostatic hypotension.7 For a more in-depth discussion on non-motor abnormalities in people with iRBD, please see Högl et al. 2018.
Motor Abnormalities in iRBD
Roughly 34% of people with iRBD present with subtle extrapyramidal motor signs5,17 and such signs may occur as early as 6.3–12.9 years before phenoconversion to an neurodegenerative synucleinopathy disease.7,18 Importantly, motor signs are among the strongest predictor of future phenoconversion. People with iRBD and motor signs are more than three times as likely (Hazard Ratio = 3.16) to phenoconvert compared to people without motor signs.5 Motor signs identified in people with iRBD include bradykinesia, functional deficits, facial and speech abnormalities, gait and postural impairment, rigidity, and tremor. The evolution of motor signs has been shown to follow a chronological pattern starting after non-motor signs7,18 (Figure 1).
Figure 1.
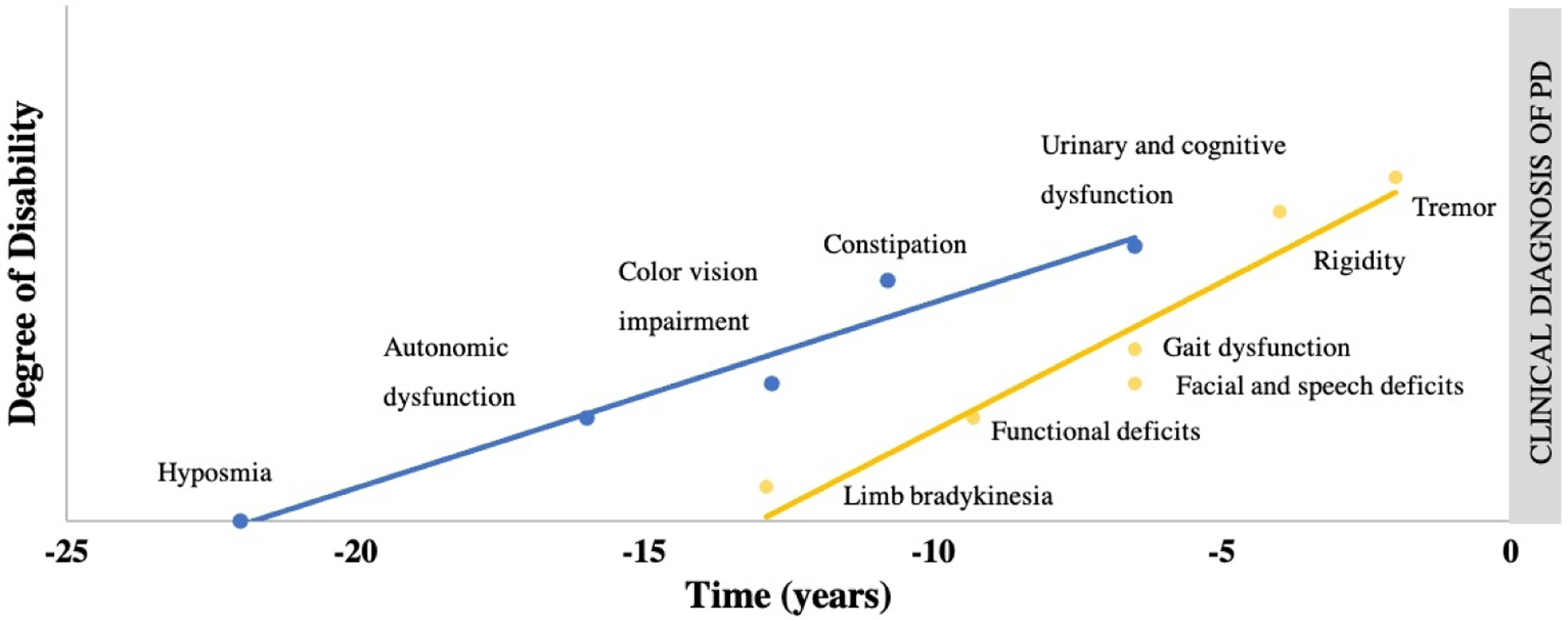
Estimated lead time of prodromal markers. Lead times for non-motor and motor signs are approximated from data found in Postuma 2012 and Fereshtehnejad et al. 2019. Limb bradykinesia lead time marked using quantitative data. Time in years refers to the prodromal stage with time point zero being the time of phenoconversion to an overt alpha-synucleinopathy such as Parkinson’s disease, dementia with Lewy bodies or multiple systems atrophy.
Limb Bradykinesia
Limb bradykinesia is the earliest motor feature to manifest in people with iRBD who are likely to convert to a neurodegenerative alpha-synucleinopathy.7,18 Clinical assessment of bradykinesia severity in people with iRBD is conducted using the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) using repetitive alternating movements of the fingers, hand, forearm and legs. Abnormal bradykinesia scores on the UPDRS-III manifest roughly 5 years prior to diagnosis.7,18 However, more sensitive quantitative measures of upper limb movement speed and accuracy, such as the alternate-tap test,19 may show deviation from age-normal scores 6–12 years before conversion.7
Parkinsonian bradykinetic behaviors have also been detected in iRBD using high-precision, quantitative methods. Instrumented analyses of index and thumb tapping have revealed decreased movement speed17 and a sequence effect20 in people with a short duration of iRBD symptoms (~5 years), suggesting early parkinsonian bradykinesia in people with iRBD. However, there are no longitudinal studies utilizing instrumented measures of bradykinesia in iRBD. It remains unclear how parkinsonian bradykinesia manifests initially, and if characteristics of bradykinesia, hypokinesia or akinesia can differentiate between healthy aging, demented or parkinsonian populations.
Motor Experiences of Daily Living and Speech
Physical and functional deficits in people with iRBD have been identified using the UPDRS-II and III, with a deviation from normal scores beginning up to 9.8 years before phenoconversion.7 Basic activities of daily living demonstrate a deviation from normal, but near the time of phenoconversion (~1–3 years).7 Specific deficits related to activities of daily living include self-reported changes in handwriting, turning in bed, walking speed, and speech.
Items from the UPDRS-II and III that demonstrate predictive value in iRBD include corticobulbar mediated functions of speech and facial muscle tone. The lead time of facial and speech abnormalities has been estimated to range from 6.5–9.8 years.7,18 Although facial and speech abnormalities are noted perceptually in only 2% of people with RBD, quantitative speech abnormalities are estimated to be present in up to 88%.21 Specific vocal characteristics related to monopitch tone, inappropriate silences, and rate of follow-up speech segments have been shown to best discriminate between people with PD and controls, while people with iRBD demonstrate large individual variability.22 Speech abnormalities associated with iRBD are hypothesized to reflect bradykinesia and impaired muscle initiation (i.e. akinesia) in vocal musculature.22 When comparing instrumented voice assessments to other motor features such as tremor, hand tapping and posture, vocal quality was the best group discriminator between those with iRBD, PD and controls.23 These findings may indicate that instrumented evaluations of speech may yield a highly sensitive marker of future phenoconversion in iRBD. There are no published reports of quantitative speech analysis in longitudinal studies of iRBD.
Gait and Postural Abnormalities
In people with iRBD, reduced gait speed and postural abnormalities are present and resemble gait dysfunction in early PD. Reduced gait speed as measured by the Timed-up and Go outcome have been shown to first occur approximately 6 years before phenoconversion in people with iRBD.5,7,18,24 While instrumented studies of gait speed indicate mixed results,24–26 gait variability appears to be dysregulated in iRBD. Step time variability has been shown to be increased in iRBD cohorts, similar to age-matched individuals with PD.25 Yet, variability of gait characteristics have also been found to be reduced when at-home, long-duration gait bouts are assessed in iRBD compared to controls.24 These seemingly conflicting reports may reflect a lack of automaticity and flexibility of gait regulation in people with iRBD or result secondary to poor postural control.
Abnormal postural responses are also similar in people with iRBD and early PD. Gait tasks in combination with a cognitive task (i.e. dual-task gait) have been shown to unveil deficient postural compensation strategies in people with iRBD. During dual-task gait, people with iRBD increase step asymmetry and step width variability26 which is similar to changes seen in early PD. Additionally, during gait initiation, people with iRBD have been shown to demonstrate PD-like deficits with reduced anticipatory postural adjustments and fractionated muscle burst patterns.27 Considering that iRBD is associated with alpha-synuclein driven brainstem dysfunction,12 loss of postural control may be the earliest manifesting gait impairment as the pedunculopontine nucleus and other brainstem structures are integral to regulate gait and postural control.27 There are no longitudinal studies in iRBD using quantitative gait and postural response analyses.
Rigidity and Tremor
Clinically, rigidity and tremor are quantified using the UPDRS-III and are estimated to manifest 3.5–4.4 years and 1.3–1.5 years respectively before phenoconversion in people with iRBD.7,18 The relatively late manifestation of rigidity or tremor, when combined with bradykinesia, typically fulfills the diagnostic criteria for clinical PD and therefore offer little additional predictive value. There are no longitudinal studies utilizing instrumented measures of tremor or rigidity in people with iRBD.
FRAMEWORK FOR PRODROMAL EXERCISE AND REHABILITATION IN IRBD
In an effort to reduce the severity and slow the progression of neurodegenerative disease, it is imperative to identify persons with a high risk of future synucleinopathy or early signs of motor system dysfunction to implement neuroprotective interventions. Currently there are no known pharmacologic or nonpharmacologic interventions that are known to delay or prevent PD onset.28 However, there is compelling evidence from experiments in animal models of parkinsonism29 and trials in humans30 that exercise and physical medicine interventions may affect the expression and progression of disease with little risk of adverse effects. Thus, it is ideal to start exercise and physical medicine interventions during the prodromal stage or in people with iRBD.31,32 To date, a framework for exercise application or clinical management in iRBD has not been established. Here we propose a framework for implementing exercise and rehabilitative services in individuals with iRBD. This clinical framework includes the primary role of Sleep clinicians and Movement Disorders Neurologists to initiate consultative services from traditional rehabilitation providers. Rehabilitation providers can then initiate an intervention centered on exercise prescription, progression, and monitoring or address physical impairments as they manifest. The rationale for this framework comes from existing evidence in animal models, epidemiologic studies, and exercise interventions applied in early or mild PD cohorts.
Rationale for Neuroprotective Effects of Exercise in Prodromal Synucleinopathy
There is a surmounting body of evidence that indicates physical activity and exercise have neuroprotective effects that may reduce or mitigate the severity of neurodegenerative disease.33 The biologic mechanisms recognized to underlie exercise as a neuroprotective therapy include: enhanced neurogenesis, elevated neurotrophic factors, reduced neuroinflammation, and decreased oxidative stress.29
Exercise interventions in animal models of alpha-synuclein driven PD indicate there is a beneficial effect of exercise on motor and non-motor symptoms, while also mitigating degeneration of nigral systems. Two animal models of PD that are relevant to understanding how exercise therapy may be beneficial in iRBD are the rotenone and the adeno-associated virus alpha-synuclein model. Both the rotenone and the adeno-associated virus alpha-synuclein model lead to the development of alpha-synuclein in nigral neurons and the production of parkinsonian motor symptoms. Using the rotenone model, it has been shown that early aerobic exercise for two or six weeks was effective to prevent elevated alpha-synuclein levels while increasing tyrosine hydroxylase levels in the substantia nigra.34,35 In addition, aerobic exercise for two weeks following rotenone exposure was found to reduce the loss of serotonergic markers in the dorsal raphe and mitigate depressive behaviors.36 Rats that receive aerobic exercise following rotenone exposure also demonstrate improved motor performance in the rota-rod test compared to sedentary rats,35 suggesting preservation of motor function in exercising animals. A study of voluntary aerobic exercise using the adeno-associated virus alpha-synuclein model has also indicated that exercise reduces neuronal loss in the substantia nigra.37 While this study failed to demonstrate a beneficial effect of voluntary running on motor function, it did indicate beneficial effects of exercise on hippocampal-associated memory. Although the mechanism of neuroprotection from exercise is unclear, these findings support the idea that aerobic exercise can delay the neurodegenerative process related to alpha-synucleinopathy in the substantia nigra and dorsal raphe, while mitigating losses in motor and cognitive domains. For a full review of exercise-induced effects in animal models of PD, please see Crowley et al. 2019.
Epidemiologic studies also indicate that physical activity and exercise may play a protective role in reducing the risk of PD (for review, see Fang et al. 2018). A relationship between increased physical activity and decreased prevalence of prodromal non-motor symptoms in aging adults has been recently reported.39 A large longitudinal trial has provided further evidence that even a medium level of physical activity lowers the risk of developing PD.40 Following PD diagnosis, regular physical activity has been associated with slower declines in functional mobility and health-related quality of life,41 indicating the value for early exercise and physical activity intervention.
Exercise as an Intervention for Prodromal Synucleinopathy
Several promising exercise interventions have been studied in PD that may translate to iRBD. The following interventional recommendations have been drawn from randomized controlled trials in samples of people with early PD (disease duration of less than 5 years) and intervention of at least 12 weeks. Due to a paucity of exercise research in early PD, studies of cohorts with an average disease duration of 5–7 years are also considered. There is an immediate need for research to be conducted in individuals with prodromal synucleinopathy or early PD to better understand the impacts of different exercise types on improving or slowing the progression of parkinsonism and synucleinopathy.
Aerobic training
Aerobic exercise is recommended as a prodromal intervention for iRBD to promote neuroprotection and possibly mitigate or delay the onset of synucleinopathy disease. Aerobic exercise in early PD has been shown to increase aerobic capacity, endurance, and slow the progression of motor symptoms.42–44 A study comparing treadmill training in de novo PD patients (i.e., before medications are initiated) at high or moderate intensities (80% or 60% maximum heart rate, respectively) for 6 months showed that only high-intensity aerobic exercise was superior to usual care with respect to the UPDRS-III measures of progression.42 Home-based stationary cycling in early PD for 6 months has also been shown to significantly reduce the rate of progression of motor symptoms and improve cardiovascular fitness compared to stretching and relaxation.43 Recently, cycling for 3 months has been shown to generate increased cortico-striatal neuroplasticity and dopamine release compared to stretching exercises.44 Further, evidence for aerobic exercise to mitigate the loss of motor function was found in mild PD cohorts who complete 3–6 months of Nordic walking exercise.45,46 Improvement in alternating movements following 6 months of moderate intensity aerobic exercise has been noted in mild PD,46 suggesting a possible therapy for early onset bradykinesia in iRBD. In summary, evidence from aerobic exercise trials in early PD, as well as in parkinsonian animal models, suggests that aerobic exercise may impart beneficial effects on motor function by preserving dopaminergic and cortico-striatal systems in the presence of disease.34,35,44 For a review of aerobic exercise in PD, please see Schootemeijer et al. 2020.
Prescription of aerobic exercise in iRBD should include factors of intensity, duration and frequency. Moderate to high intensity exercise with a duration of 30–45 minutes a minimum of 3 times per week is recommended.42,43 If safely tolerated, high-intensity (>70% maximal heart rate) aerobic exercise should be recommended over moderate-intensity aerobic exercise.42 Common modes of aerobic exercise include cycling, treadmill excise and walking programs; however, patient preference should be prioritized if intensity and duration goals can be met.
Progressive resistance training
Progressive resistance training (i.e. resistance exercise that increases the resistance load or intensity over time) is recommended in iRBD as it may reduce the progression of both motor and cognitive symptoms. Recent reviews in PD indicate that progressive resistance exercise has a potent effect on muscle strength, physical function and parkinsonian symptoms in mild to moderate PD.47,48 To our knowledge, there are no published resistance exercise trials in early PD cohorts with an average disease duration of less than 5 years. However, long-term resistance exercise studies in mild to moderate PD, with an average disease duration of 5–7 years, have shown that there are beneficial effects on strength,49 physical function50,51 and Parkinsonian motor symptoms.50,52–54 The longest resistive exercise trial to date in PD was two years in duration and revealed progressive resistance exercise, but not non-progressive general exercise that included some strengthening, significantly reduced clinical measures of motor severity, improved physical function, reduced bradykinesia, improved cognitive function, and increased strength.52,53 Resistance exercise has also been found to improve standardized ratings of limb bradykinesia,54 peak velocity of ballistic arm movements53 and functional gait speed.50,55 Relevant to the motor abnormalities observed in iRBD, resistance exercise may be effective to address early-onset limb bradykinesia and gait hypokinesia. Yet, it is unclear if progressive resistance exercise has disease modifying effects despite the consistent reports of mitigating parkinsonian symptoms.48
Progressive resistance training protocols for iRBD should be prescribed 2–3x/week, for 20–60 minutes or more.52,55 Specifically, progressive resistance exercise that systematically increases the intensity of muscular demand over time is recommended to maximize neuromuscular gains.52 Considerations should be given to extensor muscle focused exercises as extensor muscle weakness tends to be greater than flexor weakness in people with PD56 and may contribute to postural abnormalities, fall risk and loss of physical function. Additionally, resistance exercises may be most beneficial when progressive resistance and instability are combined to maximize effects on physical function and neuromuscular adaptation.57
Multimodal exercise
Multimodal exercise programs performed individually or in a group recreational exercise setting are recommended for people with iRBD as they may improve physical function, quality of life and reduce sedentary habits. In early PD, multimodal exercise programs have been found to be effective to mitigate progressive mobility loss. Particularly dance and yoga appear to be effective at improving parkinsonian motor symptoms, physical function and gait speed when delivered with a dose of 60 minutes, 2 times per week.58–60 Sardinian dance training compared to usual care in early PD has been shown to improve motor symptoms, functional mobility, balance, and fatigue.59 When expanding the list of studies to those including people with PD for 5–7 years, we found further support for Tango dance61 and yoga,62 as well as for Tai Chi/Qigong.63,64 Tango dance lessons given twice weekly in mild-moderate PD has been shown to have a variety of beneficial effects such as improved balance, functional mobility, walking speed and slowing the progression of parkinsonian motor symptoms.61 Tai Chi/Qigong are associated with mixed effects on motor symptoms but may be effective to improve balance and physical function.63,64 Other multimodal exercise programs that may be applicable in prodromal synucleinopathy, but are not discussed due to variations in study design or disease duration, include group activities such as boxing,65 sensorimotor agility programs,66 individualized home exercise programs,67 and physical therapy.68
For patients with iRBD who show no overt motor abnormalities, multimodal exercise may be especially effective to promote sustained independent community exercise. To promote adherence to long-term exercise participation, recommendations should be made for a mode of exercise that is appealing and likely to be completed by the individual. Therefore, factors that ensure consistent exercise participation (3 days per week at minimum) should be prioritized and recommendations for mode of exercise centered on patient preference. Clinically, it may be especially important for a multimodal excise prescription in iRBD to include remote or in-person consultative services with a rehabilitation expert.
FRAMEWORK FOR INTEGRATING REHABILITION INTO IRBD CARE
Clinical practice guidelines in PD suggest that physical therapy (PT), occupational therapy (OT), and speech language pathology (SLP) should be considered early after diagnosis.69–71 This antiquated approach fails to recognize the clear evidence that PD is a characterized by years to decades of progressive neurodegeneration and loss of motor function. Further, considering the growing evidence that aerobic exercise may be neuroprotective, in addition to the known cardiovascular and mental health benefits of physical activity, rehabilitation services should be integrated into care for people with prodromal synucleinopathy. The evidence is clear that functional deficits, bradykinesia, gait impairment and voice deficits are commonly present in iRBD indicating impending phenoconversion to a neurodegenerative synucleinopathy such as PD, dementia with Lewy bodies or multiple systems atrophy. Therefore, rehabilitation services should be started before diagnosis of a neurodegenerative alpha-synucleinopathy disease in people with iRBD. Staging of services should be based on symptom progression due to the predictive value these signs have in identifying those at risk of future neurodegenerative synucleinopathy (Table 2).
Table 2.
Timeline of services in rehabilitation framework
| Clinical Early PD | |||
|---|---|---|---|
| Presentation | iRBD and non-motor signs | iRBD with subtle motor and/or functional deficits | iRBD with bradykinesia + rigidity or tremor |
| Sleep Clinician Role | Sleep Clinician provide education re: risk of neurodegenerative disease, exercise/lifestyle modification | Referral to Movement Disorders Neurologist |
Movement Disorders Neurologist initiate clinical care for early PD |
| Exercise Prescription | Aerobic, resistance and multimodal exercise | ||
| Rehabilitation Role | Consultative services with PT for exercise prescription if not physically active Consultative services with OT for safe sleep environment recommendations |
Refer to PT, OT, and SLP for baseline evaluations of physical function, physical activity monitoring, and individualized exercise recommendations to target observed deficits. | Initiate clinical care for early PD including PT, OT, and SLP referrals for baseline evaluations of physical function, physical activity monitoring, and individualized exercise recommendations to target observed deficits. |
iRBD = idiopathic REM sleep behavior disorder; PD = Parkinson’s disease; PT = physical therapy; OT = occupational therapy; SLP = speech language pathology.
Early Prodromal Synucleinopathy
People with iRBD in the earliest stages of prodromal synucleinopathy disease may present only with RSWA and/or dream enactment and no other predictive signs. These people are primarily managed by their Sleep clinician. In addition to the standard care established for iRBD, we recommend that Sleep clinicians educate the patient on the risk of future neurodegenerative disease and the evidence regarding the benefits of exercise.72 In individuals who are sedentary or would like assistance designing an exercise plan, consultative visits with a physical therapist may increase their exercise participation.67,68 Therefore, we recommend consultative services with PT for a subset of iRBD patients who need an exercise prescription or have comorbidities that require activity adaptation. Strategies to promote increased exercise may include motivational interviewing, general exercise education, behavioral change elements and mobile technology/app-based telemedicine in sedentary or inactive individuals.67
Late Stage Prodromal Synucleinopathy
Patients with iRBD who exhibit motor and non-motor signs that strongly predict future phenoconversion to an a neurodegenerative synucleinopathy should be evaluated for subtle parkinsonism. When mild bradykinesia is observed, we recommend that the Sleep clinician consider referral to a Movement Disorders Specialist and a knowledgeable physical therapist. Early referral to PT can promote increased participation in physical activity and exercise, and possibly mitigate the progression of motor signs such as bradykinesia.46,53,54
In later stages of prodromal synucleinopathy, when functional deficits are reported by the patient, the Sleep clinician or Movement Disorders Specialist should consider involving the full in interdisciplinary physical medicine and rehabilitation team, including PT, OT, and SLP.31 The interdisciplinary team may then address and monitor the progression of functional deficits (e.g. handwriting changes, walking changes, speech changes). Because consultation with all three disciplines may overwhelm an individual with iRBD, we recommend taking an interdisciplinary or transdisciplinary approach.73 For example, if an individual with iRBD and motor symptoms is referred to PT for exercise promotion, the physical therapist should screen for deficits in the domains of motor experiences of daily living and speech. If there are deficits, the physical therapist can provide education and referrals to OT and SLP as appropriate.
We propose that physical therapists, occupational therapists, and speech-language pathologists utilize a similar approach in people with iRBD manifesting mild motor deficits as they do in early clinical PD.69–71 Rehabilitation should include a comprehensive discipline-specific evaluation of physical function with attention to establishing a baseline performance assessment using standardized outcome measures and identifying the presence of any deficits. Treatments should include addressing any observed functional deficits, education on the role of exercise and multidisciplinary therapy, and prescribing/monitoring exercise programs.
Potential rehabilitative treatment strategies for iRBD have been described31 and include services from PT, OT and SLP. PT treatments should address exercise prescription to improve or maintain aerobic function, strength, balance, agility, mobility, and posture. In the early stages of the disease, treatments should focus more on restoration of function, but may also include compensatory strategies. OT treatments should focus on maintaining or improving all domains of occupational performance and quality of life including employment, instrumental and basic activities of daily living, particularly related to fine motor control. OT treatments may include restorative exercises, environmental adaptations, and behavioral compensatory strategies. In addition, fall prevention and safety should be addressed by PT and OT. SLP may address domains of speech and voice, while also monitoring cognition and swallowing, as early impairments in these areas should be considered indicative of possible dementia with Lewy bodies or multiple system atrophy and immediately communicated to the treating neurologist.
Transitioning to Care for Clinical PD
Once early clinical PD has been diagnosed, we recommend interdisciplinary rehabilitation treatment following treatment guidelines for PT, OT, and SLP as stated in previously published clinical practice guidelines.69–71 We refer readers to these guidelines and the several published real-world examples of how interdisciplinary rehabilitation programs can be implemented.68,74
FUTURE DIRECTIONS AND CONSIDERATIONS
The existing literature in iRBD indicates that motor abnormalities and subtle parkinsonism can be observed for greater than 10 years prior to phenoconversion from iRBD to a neurodegenerative alpha-synucleinopathy; therefore, protective or early therapies in iRBD should be tailored to promote neuroprotection and address the manifesting motor features. Several considerations and limitations are relevant to the implementation of early or protective therapies in iRBD.
One important consideration of the proposed rehabilitation framework is that it applies to patients with iRBD who represent prodromal synucleinopathy and may convert to not only PD, but also to dementia with Lewy bodies and multiple system atrophy. Interestingly, the available predictive models do not indicate a substantial difference in prodromal motor signs between those that convert to PD, dementia with Lewy bodies or multiple systems atrophy.5,7 This observation may suggest that the proposed framework for rehabilitative services in iRBD may be more widely applicable in a variety of alpha-synucleinopathies.
Future rehabilitative and exercise-based studies in iRBD are needed. Clinical trials to examine the efficacy of neuroprotective interventions in iRBD have been designed75 and sample sizes estimated.5 While the time to phenoconversion is the ideal primary outcome in therapeutic trials,75 exercise-based trials should also include outcomes sensitive to the progression of parkinsonian motor signs evident in iRBD (i.e. MDS-UPDRS) and instrumented measures of bradykinesia, rigidity, tremor and voice. These outcomes should be assessed in the absence of cholinergic, dopaminergic or noradrenergic medication that may mask exercise-induced benefits on the motor system52 and compared to cohort data in people with PD and coexisting RBD to control for differences in PD phenotype.
Finally, it remains unknown if exercise-based interventions can halt or mitigate the neurodegenerative process associated with alpha-synucleinopathy. While it is clear from previous trials that exercise can slow motor symptom progression in people with PD,42,52,61 exercise may mask motor symptoms without interfering with age and time-dependent neurodegenerative processes. Therefore, it is imperative that future studies include biomarkers of neurodegeneration16 to assess the effectiveness of interventions to prevent or slow progression of disease.
SUMMARY AND CONCLUSION
Extrapyramidal or subtle parkinsonian signs manifest in people with iRBD years to decades before phenoconversion to an degenerative alpha-synucleinopathy disease.7,18 A survey of the literature indicates that specific motor signs evident in iRBD include bradykinesia, impaired motor experiences of daily living, speech abnormalities, and gait/posture deficits. The proposed rehabilitation framework for iRBD includes early exercise-based interventions of aerobic exercise, progressive resistance training, and multimodal exercise with consultative services provided by rehabilitation experts to address exercise prescription, progression, and monitoring.
Lastly, there is an immediate need for exercise and rehabilitation research to be conducted in individuals with prodromal synucleinopathy to better understand the role of exercise, physical activity and early rehabilitation services on alpha-synuclein driven neurodegeneration. Using the clinical population of iRBD, it is now feasible to evaluate neuroprotective interventions; however, development of effective therapies will require increased awareness of iRBD by clinicians and require collaboration between specialists in the fields of neurology, sleep medicine, and physical medicine. The proposed framework is recommended for clinicians who wish to integrate rehabilitative services into the care plan for patients who show signs of prodromal or subclinical synucleinopathy.
Acknowledgments:
This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494 and NIH NINDS grant RO1 NS088679. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
REFERENCES
- 1.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136(8):2419–2431. doi: 10.1093/brain/awt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzel S, Roeben B, Ben-Shlomo Y, et al. Prodromal Markers in Parkinson’s Disease: Limitations in Longitudinal Studies and Lessons Learned. Front Aging Neurosci. 2016;8(JUN):1–10. doi: 10.3389/fnagi.2016.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darweesh SKL, Verlinden VJA, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson’s disease. Brain. 2017;140(2):429–441. doi: 10.1093/brain/aww291 [DOI] [PubMed] [Google Scholar]
- 4.Postuma RB, Berg D. Advances in markers of prodromal Parkinson disease. Nat Rev Neurol. 2016;12(11):622–634. doi: 10.1038/nrneurol.2016.152 [DOI] [PubMed] [Google Scholar]
- 5.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain. 2019;142(3):744–759. doi: 10.1093/brain/awz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCann H, Stevens CH, Cartwright H, Halliday GM. α-Synucleinopathy phenotypes. Parkinsonism Relat Disord. 2014;20(SUPPL.1):S62–S67. doi: 10.1016/S1353-8020(13)70017-8 [DOI] [PubMed] [Google Scholar]
- 7.Fereshtehnejad S-M, Yao C, Pelletier A, Montplaisir JY, Gagnon J-F, Postuma RB. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain. 2019;142(7):2051–2067. doi: 10.1093/brain/awz111 [DOI] [PubMed] [Google Scholar]
- 8.Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67(6):715–725. doi: 10.1002/ana.21995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464–1470. doi: 10.1002/mds.27802 [DOI] [PubMed] [Google Scholar]
- 10.Cova I, Priori A. Diagnostic biomarkers for Parkinson’s disease at a glance: where are we? J Neural Transm. 2018;125(10):1417–1432. doi: 10.1007/s00702-018-1910-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howell MJ, Schenck CH. Rapid Eye Movement Sleep Behavior Disorder and Neurodegenerative Disease. JAMA Neurol. 2015;72(6):707. doi: 10.1001/jamaneurol.2014.4563 [DOI] [PubMed] [Google Scholar]
- 12.Dauvilliers Y, Schenck CH, Postuma RB, et al. REM sleep behaviour disorder. Nat Rev Dis Prim. 2018;4(1):19. doi: 10.1038/s41572-018-0016-5 [DOI] [PubMed] [Google Scholar]
- 13.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79(10):1117–1121. doi: 10.1136/jnnp.2008.149195 [DOI] [PubMed] [Google Scholar]
- 14.Linn-Evans ME, Petrucci MN, Amundsen Huffmaster SL, et al. REM sleep without atonia is associated with increased rigidity in patients with mild to moderate Parkinson’s disease. Clin Neurophysiol. 2020;131(8):2008–2016. doi: 10.1016/j.clinph.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postuma RB, Gagnon JF, Pelletier A, Montplaisir J. Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Mov Disord. 2013;28(5):597–604. doi: 10.1002/mds.25445 [DOI] [PubMed] [Google Scholar]
- 16.Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration — an update. Nat Rev Neurol. 2018;14(1):40–55. doi: 10.1038/nrneurol.2017.157 [DOI] [PubMed] [Google Scholar]
- 17.Yamada G, Ueki Y, Oishi N, et al. Nigrostriatal dopaminergic dysfunction and altered functional connectivity in rem sleep behavior disorder with mild motor impairment. Front Neurol. 2019;10(JUL):1–12. doi: 10.3389/fneur.2019.00802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain. 2012;135(6):1860–1870. doi: 10.1093/brain/aws093 [DOI] [PubMed] [Google Scholar]
- 19.Nutt JG, Lea ES, Van Houten L, Schuff RA, Sexton GJ. Determinants of tapping speed in normal control subjects and subjects with Parkinson’s disease: Differing effects of brief and continued practice. Mov Disord. 2000;15(5):843–849. doi: [DOI] [PubMed] [Google Scholar]
- 20.Krupička R, Krýže P, Neťuková S, et al. Instrumental analysis of finger tapping reveals a novel early biomarker of parkinsonism in idiopathic rapid eye movement sleep behaviour disorder. Sleep Med. 2020;75:45–49. doi: 10.1016/j.sleep.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 21.Rusz J, Hlavnička J, Tykalová T, et al. Quantitative assessment of motor speech abnormalities in idiopathic rapid eye movement sleep behaviour disorder. Sleep Med. 2016;19(2016):141–147. doi: 10.1016/j.sleep.2015.07.030 [DOI] [PubMed] [Google Scholar]
- 22.Rusz J, Hlavnicka J, Tykalova T, et al. Smartphone Allows Capture of Speech Abnormalities Associated with High Risk of Developing Parkinson’s Disease. IEEE Trans Neural Syst Rehabil Eng. 2018;26(8):1495–1507. doi: 10.1109/TNSRE.2018.2851787 [DOI] [PubMed] [Google Scholar]
- 23.Arora S, Baig F, Lo C, et al. Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology. 2018;91(16):E1528–E1538. doi: 10.1212/WNL.0000000000006366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Din S, Yarnall AJ, Barber TR, et al. Continuous Real-World Gait Monitoring in Idiopathic REM Sleep Behavior Disorder. J Parkinsons Dis. 2019. doi: 10.3233/JPD-191773 [DOI] [PubMed] [Google Scholar]
- 25.Mcdade EM, Boot BP, Christianson TJH, et al. Subtle gait changes in patients with REM sleep behavior disorder. Mov Disord. 2013;28(13):1847–1853. doi: 10.1002/mds.25653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehgoetz Martens KA, Matar E, Hall JM, et al. Subtle gait and balance impairments occur in idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2019;(May 2019):1–7. doi: 10.1002/mds.27780 [DOI] [PubMed] [Google Scholar]
- 27.Alibiglou L, Videnovic A, Planetta PJ, Vaillancourt DE, MacKinnon CD. Subliminal gait initiation deficits in rapid eye movement sleep behavior disorder: A harbinger of freezing of gait? Mov Disord. 2016;31(11):1711–1719. doi: 10.1002/mds.26665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–1266. doi: 10.1002/mds.27372 [DOI] [PubMed] [Google Scholar]
- 29.Crowley EK, Nolan YM, Sullivan AM. Exercise as a therapeutic intervention for motor and non-motor symptoms in Parkinson’s disease: Evidence from rodent models. Prog Neurobiol. 2019;172(March 2018):2–22. doi: 10.1016/j.pneurobio.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 30.Schootemeijer S, van der Kolk NM, Bloem BR, de Vries NM. Current Perspectives on Aerobic Exercise in People with Parkinson’s Disease. Neurotherapeutics. 2020. doi: 10.1007/s13311-020-00904-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson BP, Westlake KP. Link Between Parkinson Disease and Rapid Eye Movement Sleep Behavior Disorder With Dream Enactment: Possible Implications for Early Rehabilitation. Arch Phys Med Rehabil. 2018;99(2):411–415. doi: 10.1016/j.apmr.2017.08.468 [DOI] [PubMed] [Google Scholar]
- 32.McCarter SJ, Boeve BF, Graff-Radford NR, Silber MH, St Louis EK. Neuroprotection in idiopathic REM sleep behavior disorder: a role for exercise? Sleep. 2019;42(6):1–3. doi: 10.1093/sleep/zsz064 [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Yan T, Chu JMT, et al. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab Investig. 2019;99(7):943–957. doi: 10.1038/s41374-019-0232-y [DOI] [PubMed] [Google Scholar]
- 34.Almeida MF, Silva CM, Chaves RS, et al. Effects of mild running on substantia nigra during early neurodegeneration. J Sports Sci. 2018;36(12):1363–1370. doi: 10.1080/02640414.2017.1378494 [DOI] [PubMed] [Google Scholar]
- 35.Shin MS, Kim TW, Lee JM, Ji ES, Lim BV. Treadmill exercise alleviates nigrostriatal dopaminergic loss of neurons and fibers in rotenone-induced Parkinson rats. J Exerc Rehabil. 2017;13(1):30–35. doi: 10.12965/jer.1734906.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin MS, Kim TW, Lee JM, Sung YH, Lim BV. Treadmill exercise alleviates depressive symptoms in rotenone-induced Parkinson disease rats. J Exerc Rehabil. 2017;13(2):124–129. doi: 10.12965/jer.1734966.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley EK, Nolan YM, Sullivan AM. Neuroprotective effects of voluntary running on cognitive dysfunction in an α-synuclein rat model of Parkinson’s disease. Neurobiol Aging. 2018;65:60–68. doi: 10.1016/j.neurobiolaging.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 38.Fang X, Han D, Cheng Q, et al. Association of Levels of Physical Activity With Risk of Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Netw open. 2018;1(5):e182421. doi: 10.1001/jamanetworkopen.2018.2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes KC, Gao X, Molsberry S, Valeri L, Schwarzschild MA, Ascherio A. Physical activity and prodromal features of Parkinson disease. Neurology. 2019;93(23):e2157–e2169. doi: 10.1212/WNL.0000000000008567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang F, Trolle Lagerros Y, Bellocco R, et al. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain. 2015;138(2):269–275. doi: 10.1093/brain/awu323 [DOI] [PubMed] [Google Scholar]
- 41.Rafferty MR, Schmidt PN, Luo ST, et al. Regular Exercise, Quality of Life, and Mobility in Parkinson’s Disease: A Longitudinal Analysis of National Parkinson Foundation Quality Improvement Initiative Data. J Parkinsons Dis. 2017;7(1):193–202. doi: 10.3233/JPD-160912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schenkman M, Moore CG, Kohrt WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with De Novo Parkinson disease a phase 2 randomized clinical trial. JAMA Neurol. 2018;75(2):219–226. doi: 10.1001/jamaneurol.2017.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Kolk NM, de Vries NM, Kessels RPC, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. 2019;18(11):998–1008. doi: 10.1016/S1474-4422(19)30285-6 [DOI] [PubMed] [Google Scholar]
- 44.Sacheli MA, Neva JL, Lakhani B, et al. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov Disord. 2019;34(12):1891–1900. doi: 10.1002/mds.27865 [DOI] [PubMed] [Google Scholar]
- 45.Cugusi L, Solla P, Serpe R, et al. Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation. 2015;37(2):245–254. doi: 10.3233/NRE-151257 [DOI] [PubMed] [Google Scholar]
- 46.Reuter I, Mehnert S, Leone P, Kaps M, Oechsner M, Engelhardt M. Effects of a flexibility and relaxation programme, walking, and nordic walking on parkinson’s disease. J Aging Res. 2011;2011. doi: 10.4061/2011/232473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung CLH, Thilarajah S, Tan D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin Rehabil. 2016;30(1):11–23. doi: 10.1177/0269215515570381 [DOI] [PubMed] [Google Scholar]
- 48.Mak MKY, Wong-Yu ISK. Exercise for Parkinson’s disease. Int Rev Neurobiol. 2019;147:1–44. doi: 10.1016/bs.irn.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 49.Shulman LM, Katzel LI, Ivey FM, et al. Randomized clinical trial of 3 types of physical exercise for patients with parkinson disease. JAMA Neurol. 2013;70(2):183–190. doi: 10.1001/jamaneurol.2013.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ni M, Signorile JF, Balachandran A, Potiaumpai M. Power training induced change in bradykinesia and muscle power in Parkinson’s disease. Park Relat Disord. 2016;23(2016):37–44. doi: 10.1016/j.parkreldis.2015.11.028 [DOI] [PubMed] [Google Scholar]
- 51.Prodoehl J, Rafferty MR, David FJ, et al. Two-year exercise program improves physical function in Parkinson’s disease: The PRET-PD randomized clinical trial. Neurorehabil Neural Repair. 2015;29(2):112–122. doi: 10.1177/1545968314539732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013;28(9):1230–1240. doi: 10.1002/mds.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.David FJ, Robichaud JA, Vaillancourt DE, et al. Progressive resistance exercise restores some properties of the triphasic EMG pattern and improves bradykinesia: The PRET-PD randomized clinical trial. J Neurophysiol. 2016;116(5):2298–2311. doi: 10.1152/jn.01067.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vieira de Moraes Filho A, Chaves SN, Martins WR, et al. Progressive resistance training improves bradykinesia, motor symptoms and functional performance in patients with parkinson’s disease. Clin Interv Aging. 2020;15:87–95. doi: 10.2147/CIA.S231359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson’s disease: A preliminary study. Park Relat Disord. 2009;15(10):752–757. doi: 10.1016/j.parkreldis.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 56.Robichaud JA, Pfann KD, Comella CL, Brandabur M, Corcos DM. Greater impairment of extension movements as compared to flexion movements in Parkinson’s disease. Exp Brain Res. 2004;156(2):240–254. doi: 10.1007/s00221-003-1782-0 [DOI] [PubMed] [Google Scholar]
- 57.Silva-Batista C, Corcos DM, Barroso R, et al. Instability Resistance Training Improves Neuromuscular Outcome in Parkinson’s Disease. Med Sci Sports Exerc. 2017;49(4):652–660. doi: 10.1249/MSS.0000000000001159 [DOI] [PubMed] [Google Scholar]
- 58.Cheung C, Bhimani R, Wyman JF, et al. Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson’s disease: A pilot randomized controlled trial. Pilot Feasibility Stud. 2018;4(1):1–11. doi: 10.1186/s40814-018-0355-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solla P, Cugusi L, Bertoli M, et al. Sardinian Folk Dance for Individuals with Parkinson’s Disease: A Randomized Controlled Pilot Trial. J Altern Complement Med. 2019;25(3):305–316. doi: 10.1089/acm.2018.0413 [DOI] [PubMed] [Google Scholar]
- 60.Elangovan N, Cheung C, Mahnan A, Wyman JF, Tuite P, Konczak J. Hatha yoga training improves standing balance but not gait in Parkinson’s disease. Sport Med Heal Sci. 2020;2(2):80–88. doi: 10.1016/j.smhs.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duncan RP, Earhart GM. Randomized Controlled Trial of Community-Based Dancing to Modify Disease Progression in Parkinson Disease. Neurorehabil Neural Repair. 2012;26(2):132–143. doi: 10.1177/1545968311421614 [DOI] [PubMed] [Google Scholar]
- 62.Ni M, Signorile JF, Mooney K, et al. Comparative effect of power training and high-speed yoga on motor function in older patients with parkinson disease. Arch Phys Med Rehabil. 2016;97(3):345–354.e15. doi: 10.1016/j.apmr.2015.10.095 [DOI] [PubMed] [Google Scholar]
- 63.Xiao CM, Zhuang YC. Effect of health Baduanjin Qigong for mild to moderate Parkinson’s disease. Geriatr Gerontol Int. 2016;16(8):911–919. doi: 10.1111/ggi.12571 [DOI] [PubMed] [Google Scholar]
- 64.Choi H-J, Garber CE, Jun T-W, Jin Y-S, Chung S-J, Kang H-J. Therapeutic Effects of Tai Chi in Patients with Parkinson’s Disease. ISRN Neurol. 2013;2013:1–7. doi: 10.1155/2013/548240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Combs SA, Diehl MD, Chrzastowski C, et al. Community-based group exercise for persons with Parkinson disease: A randomized controlled trial. NeuroRehabilitation. 2013;32(1):117–124. doi: 10.3233/NRE-130828 [DOI] [PubMed] [Google Scholar]
- 66.King LA, Horak FB. Delaying mobility disability in people with parkinson disease using a sensorimotor agility exercise program. Phys Ther. 2009;89(4):384–393. doi: 10.2522/ptj.20080214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellis TD, Cavanaugh JT, DeAngelis T, et al. Comparative effectiveness of mhealth-supported exercise compared with exercise alone for people with Parkinson disease: Randomized controlled pilot study. Phys Ther. 2019;99(2):203–216. doi: 10.1093/ptj/pzy131 [DOI] [PubMed] [Google Scholar]
- 68.Rafferty MR, MacDonald J, Byskosh A, et al. Using Implementation Frameworks to Provide Proactive Physical Therapy for People With Parkinson Disease: Case Report. Phys Ther. 2019;99(12):1644–1655. doi: 10.1093/ptj/pzz129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keus S, Munneke M, Graziano M, et al. European Physiotherapy Guideline for Parkinson’s Disease.; 2014.
- 70.Sturkenboom I, Thijssen M, Gons-Van Elsacker J, et al. Guidelines for Occupational Therapy in Parkinson’s Disease Rehabilitation.; 2008. www.ParkinsonNet.nl. Accessed September 15, 2020.
- 71.Kalf H, De Swart B, Bonnier-Baars M, et al. Guidelines for Speech-Language Therapy in Parkinson’s Disease.; 2008. www.ParkinsonNet.nl. Accessed September 15, 2020.
- 72.St. Louis EK. Prognostic Counseling for Patients With Idiopathic/Isolated REM Sleep Behavior Disorder: Should We Tell Them What’s Coming? Yes. Mov Disord Clin Pract. 2019;6(8):667–668. doi: 10.1002/mdc3.12814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norrefalk JR. How do we define multidisciplinary rehabilitation? J Rehabil Med. 2003;35(2):100–101. doi: 10.1080/16501970306118 [DOI] [PubMed] [Google Scholar]
- 74.Shrubsole K Implementation of an integrated multidisciplinary Movement Disorders Clinic: applying a knowledge translation framework to improve multidisciplinary care. Disabil Rehabil. 2019. doi: 10.1080/09638288.2019.1691666 [DOI] [PubMed] [Google Scholar]
- 75.Videnovic A, Ju YES, Arnulf I, et al. Clinical trials in REM sleep behavioural disorder: Challenges and opportunities. J Neurol Neurosurg Psychiatry. 2020;91(7):740–749. doi: 10.1136/jnnp-2020-322875 [DOI] [PMC free article] [PubMed] [Google Scholar]


