Abstract
Grape seed procyanidin extract (GSE) has been shown to exert antineoplastic properties in preclinical studies. Recently we reported findings from a modified phase I, open-label, dose-escalation clinical study conducted to evaluate the safety, tolerability, maximum tolerated dose (MTD), and potential chemopreventive effects of leucoselect phytosome (LP), a standardized GSE complexed with soy phospholipids to enhance bio-availability, in heavy active and former smokers. Three months of LP treatment significantly decreased bronchial Ki-67 labeling index (LI), a marker of cell proliferation on the bronchial epithelium. Because GSE is widely used as a supplement to support cardiovascular health, we evaluate the impact of oral LP on the fasting serum complex lipids metabolomics profiles in our participants. One month of LP treatment significantly increases eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the omega-3 polyunsaturated fatty acids (n-3 PUFA)s with well-established anti-cancer properties. LP also significantly increases unsaturated phosphatidylcholines (PC), likely from soy phospolipids in the phytosome and functioning as transporters for these PUFAs. Furthermore, 3 month of LP treatment significantly increases serum prostaglanding (PG) E3, a metabolite of EPA with anti-inflammatory and antineoplastic properties. Such increases in PGE3 correlate with reductions of bronchial Ki-67 LI (r = −0.9, p = 0.0374). Moreover, post-treatment plasma samples from trial participants significantly inhibit proliferations of human lung cancer cell lines A549 (adenocarcinoma), H520 (squamous cell carcinoma), DMS114 (small cell carcinoma), and 1198 (preneoplstic cell line). Our findings further support the potential utility of LP in reducing cardiovascular and neoplastic risks in heavy former and active smokers.
Keywords: Grape seed procyanidin extract, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), PGE3 and unsaturated phosphatidylcholines
INTRODUCTION
Preclinical studies demonstrate various antineoplastic effects of GSE against lung cancer (1–5). To faciliate clinical translation, we have selected an inexpensive, over the counter GSE preparation (leucoselect), standardized to smaller size oligomeric procyanidins (OPC) and complexed with soy phospholipids into phytosomes to improve bioavailability, for translation into a phase I lung cancer chemoprevention trial in heavy former or active smokers at high risk for lung cancer. This leucoselect phytosome (LP) has been shown to improve oxidative status, including the total antioxidant capacity of plasma, and reduce LDL susceptibility to oxidative stress in heavy smokers (6). We have recently reported the feasibility of LP as a potential lung cancer chemopreventive agent from the study, including a significant reduction of bronchial Ki-67, a marker of cell proliferation on the bronchial epithelium and a key surrogate endpoint biomarker for lung cancer chemoprevention trials (7).
Because GSE is widely used to promote cardiovascular health, we evaluate the effects of oral LP on the profiles of systemic complex lipid metabolomics by comparing matched pre- and post-treatment fasting serum samples from participants. LP treatment significantly increases serum EPA, DHA, and unsaturated PC. Furthermore, LP significantly increases fasting serum PGE3, a downstream eicosanoid derived from EPA, at the end of 3 months of treatment. Our findings support the continue investigation of LP for lung cancer prevention and treatment.
MATERIALS AND METHODS
Leucoselect phytosome clinical study design
A single arm, dose escalation, modified phase I lung cancer chemoprevention study of 3 months of oral LP, comprised of standardized OPC complexed with soy phospholipid or lecithin (1:2.6 w/w; Indena, Milan, Italy, supplied via Thorne Research), was conducted in high risk heavy active or ex-smokers 21 years of age or older with a smoking history of at least 30 pack-years (pky) as previously described (7). Written informed consent was obtained in accordance with the New Mexico VA Health Care System (NMVAHCS) Institutional Review Board, following the guidelines of Declaration of Helsinki, Belmont Report, and U. S. Common Rules. Qualified participants were treated with 1 capsule (cap), 450 mg/cap once a day, escalating weekly to 4 caps once a day for the rest of the treatment duration as tolerated. Fluorescence bronchoscopy with bronchial biopsies were performed at baseline and at the end of 3 months treatment. Serial fasting blood samples were collected at baseline, end of month 1 and end of month 3 treatment for comparative biomarker analysis. Blood samples were processed within 1 hour of collection, spun at 3000 rpm, 15 minutes for serum and 10 minutes for heparin plasma, aliquoted into cryovials and stored at −80°C until analysis.
Lipidomics by charged surface hybrid column (CSH)- electrospray (ESI) quadrupole time of flight mass spectrometer (QTOF) tandem mass spectrometry (MS/MS)
Extraction:
Serum was extracted using a previously described protocol (8). One organic phase aliquot was re-suspended in 100 μL of methanol:toluene (9:1, v/v) mixture containing 50 ng/mL CUDA (12-[(cyclohexylamino) carbonyl]amino]-dodecanoic acid) (Cayman Chemical). Samples were vortexed and sonicated for 5 min, centrifuged at 16,000 rcf and prepared for lipidomic analysis. Method blanks and pooled human plasma (BioreclamationIVT) were included as quality control samples.
Chromatographic and mass spectrometric conditions for lipidomic reverse phase liquid chromatography (RPLC)-high field quadrupole orbitrap mass spectrometer (QEHF) analysis
Using an Agilent 1290 Infinity Ultra-High-Performance Liquid Chromatography (UHPLC) system, re-suspended samples were injected at 3 and 5 μL for positive and negative ESI modes, respectively, onto a Waters Acquity UPLC CSH C18 (100 mm length × 2.1 mm id; 1.7 μm particle size) with CSH C18 pre-column (5 mm × 2.1 mm id; 1.7 μm particle size). The column was maintained at 65°C. To improve lipid coverage, different mobile phase modifiers were used for positive and negative ESI mode analysis (9). For positive ESI mode, 10 mM ammonium formate and 0.1% formic acid were used; for negative ESI mode, 10 mM ammonium acetate (Sigma–Aldrich) was employed. Both positive and negative ESI modes used the same mobile phase composition of (A) 60:40 v/v acetonitrile:water (LC-MS grade) and (B) 90:10 v/v isopropanol:acetonitrile. The gradient started at 0 min with 15% (B), 0–2 min 30% (B), 2–2.5 min 48% (B), 2.5–11 min 82% (B), 11–11.5 min 99% (B), 11.5–12 min 99% (B), 12–12.1 min 15% (B), and 12.1–15 min 15% (B). A flow rate of 0.6 mL/min was used. For data acquisition, positively charged lipids such as PC, lysoPC, were analyzed using an Agilent 6530 QTOF mass spectrometer at resolution R=10,000 while negatively charged lipids such as free fatty acids and phosphatidylinositols were analyzed using an Agilent 6550 QTOF mass spectrometer at resolution R=20,000.
Data processing using MS-DIAL
Untargeted lipidomic data processing was performed using MS-DIAL (10) for deconvolution, peak picking, alignment, and identification. The public LipidBLAST MS/MS spectra database was used, validated by retention time and m/z matching to authentic standards (11). Detected lipids were used for statistical analysis when they were positively detected in at least 50% of all samples in each group. Data were normalized by the sum-norm of all identified lipids (mTIC) (12), to scale each sample. Normalized peak heights were then submitted to R for statistical analysis.
Measurements of serum PGE3 and LTB5
PGE3 and LTB5 levels in matched fasting serum collected pre- and post-treatment were measured using specific enzyme-linked immunosorbent assay kit (MyBioSource, San Diego, CA, USA per manufacturer’s instructions.
Cell Cultures
As models to evaluate the anti-neoplastic bioactivity of oral LP against lung cancer, the human NSCLC cell lines A549, H520, small cell lung cancer cell line DMS114 (purchased from ATCC; Manassas, VA., in 2019, 2015, and 2016, respectively), and the bronchial preneoplastic cell line 1198 (generously provided by Dr. Andres Klein-Santos, Fox Chase Cancer Center, Philadelphia, PA, received in 2013) were co-cultured in vitro with matched, pre- and post-3 months treatment fasting heparinized plasma from 6 study participants. Experiments involving A549 was initiated within 6 months of purchase and was not further authenticated. ATCC uses Short Tandem Repeat profiling for cell line authentication. H520, DSM114 and 1198 cells were not further authenticated. Cells were last tested for mycoplasma in 2017. Cells were maintained as monolayers in an atmosphere of 5% CO2 in air at 370C in 25-cm2 tissue culture flasks containing cell line specific culture medium as previously described (4, 13). Only Cells within passage 3–6 at 70–80% confluence were used. Aliquots (100 μl) of 6 × 104 cells/ml of A549 cells, 10 × 104 cells/ml of H520 cells, 12 × 104 cells/ml of DMS114 cells, or 10 × 104 cells/ml of 1198 cells were plated in 96 well plates and incubated at 370C for 2 h, followed by addition of 10, 10, 5, or 5 μl heparinized plasma, respectively, and incubated for 44 hours. Cells were then subjected to PrestoBlue cell viability/proliferation assay.
PrestoBlue Cell viability/proliferation Assay
To quantify cellular proliferation in conditioned cells, PrestoBlue HS cell viability reagent (Invitrogen, Eugene, Oregon) at 1/10th of cell culture volume was added to conditioned cells per well then incubated for 20 minutes at 37°C, 5% CO2, to measure the reduction of the reagent by metabolically active cells according to the manufacturer’s instructions.
Statistical Analysis
The effects of LP treatment on complex lipid metabolomics were determined by comparing baseline values with those obtained at 1 month of treatment using Wilcoxon signed-rank test to assess the difference between treatment effect on each compound. Benjamini-Hochberg procedure (14) was used to control the false discovery rate. Fold changes, defined as median average of post-treatment divided by the median average of pre-treatment, were calculated for each compound. Chemical similarity enrichment analysis (15) was performed using the raw p-value and fold change of each compound. Kolmogorov-Smirnov test was used to test the difference at compound set enrichment level. All statistical analyses were conducted using R.
The effects of LP treatment on PGE3 and LTB5 and plasma co-cultures with human lung cancer and preneoplastic cells were determined by comparing matched pre- and post-3 months treatment values from each of the 6 participants who completed 3 months treatment. Fold or percent change of each biomarker from each participant was calculated first by normalizing matched post-treatment to baseline pre-treatment values, followed by paired t tests. Data were expressed as the mean ± SEM in all circumstances where mean values were compared. Differences were considered significant when p <0.05. Pearson correlation coefficients were computed for modulations of serum EPA, DHA and PGE3 with modulations of bronchial Ki-67 by LP from these participants.
RESULTS
Effects of one month of oral LP treatment on complex lipid metabolomics profiles in fasting serum
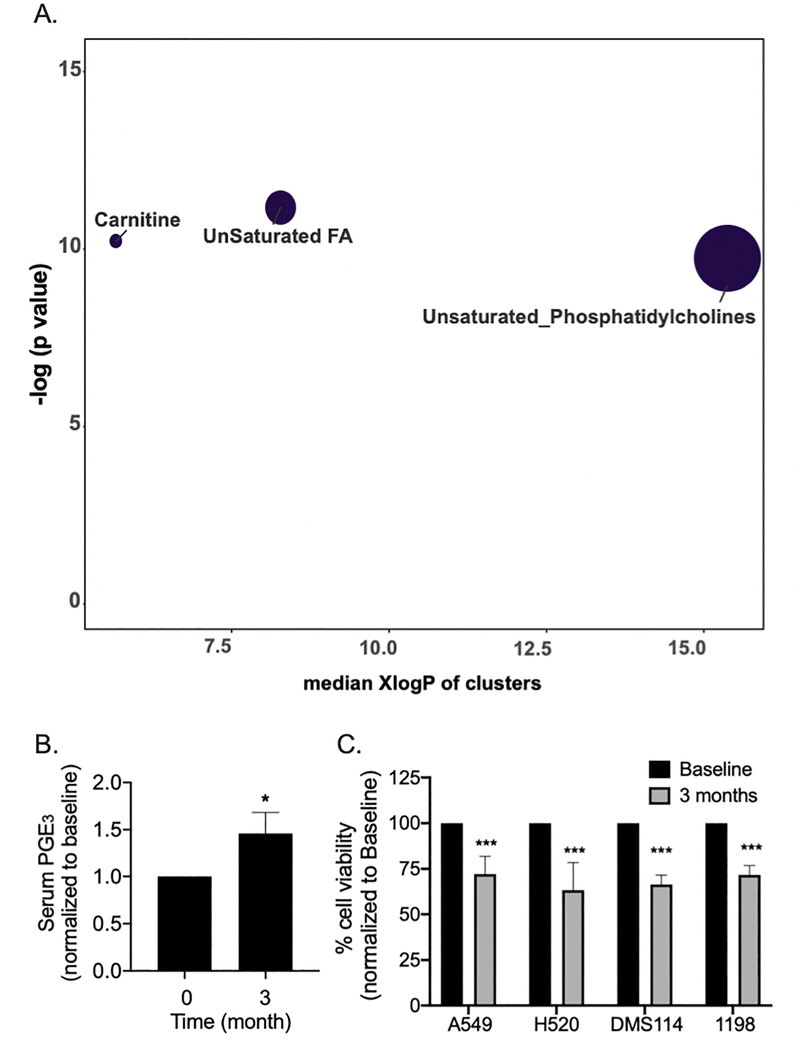
To determine the effects of oral LP treatment on the systemic metabolomics profiles of complex lipids, matched pre- and post-1 month-treatment fasting serum samples were compared. A total of 8 sets of paired samples were available for evaluation. Among the 23 clusters of compounds, 3 clusters were found to be significantly altered: 1) Unsaturated fatty acid (FA), 2) Unsaturated PC, and 3) Carnitine (Table 1A and Figure 1A).
Table 1A.
Systemic effects of 1 month of LP treatment on complex lipid metabolomics (n = 8)
| Cluster name | Cluster size | p-values | FDR |
|---|---|---|---|
| Unsaturated FA | 14 | 0.000014 | 0.00032 |
| Unsaturated Phosphatidylcholines | 75 | 0.000049 | 0.00038 |
| Carnitine | 6 | 0.000037 | 0.00038 |
| Cholesterol Esters | 9 | 1 | 1 |
| Diglycerides | 8 | 1 | 1 |
| Galactosylceramides | 6 | 1 | 1 |
| Lactosylceramides | 4 | 1 | 1 |
| Lysophospholipids | 3 | 1 | 1 |
| NewCluster_14 | 6 | 1 | 1 |
| NewCluster_18 | 5 | 1 | 1 |
| NewCluster_19 | 4 | 1 | 1 |
| Phosphatidylethanolamines | 16 | 1 | 1 |
| Phospholipid Ethers | 5 | 1 | 1 |
| Plasmalogens | 11 | 1 | 1 |
| Saturated Ceramides | 3 | 1 | 1 |
| Saturated FA | 7 | 1 | 1 |
| Saturated_Lysophosphatidylcholines | 8 | 1 | 1 |
| Saturated_Phosphatidylcholines | 7 | 1 | 1 |
| Saturated Triglycerides | 5 | 1 | 1 |
| Sphingomyelins | 23 | 1 | 1 |
| Unsaturated Ceramides | 12 | 1 | 1 |
| Unsaturated Lysophosphatidylcholines | 14 | 1 | 1 |
| Unsaturated_Triglycerides | 51 | 1 | 1 |
Fig. 1.
A). Impact Plot of modulations of Complex Lipidomics in fasting serum by LP treatement. Oral LP treamtent significantly altered unsaturated FA, unsaturated PC, and carnitine clusters. B). Three months of oral LP treatment significantly increased PGE3 levels in fasting serum of study participants by an average of 26 % (66.2 + 10.3 pg/ml at baseline vs. 96.3 + 17.0 pg/ml at 3 months). Fold change of each biomarker from each participant was calculated first by normalizing matched post-treatment to baseline pre-treatment values. Columns, mean; bars, SEM (n = 6). *, P < 0.05. C). To determine the systemic, antineoplastic bioactivity of oral administration of LP, A549, H520, DMS114 and 1198 cells were treated with matched fasting heparinized plasma obtained from subjects pre- and post-3 months of oral LP treatment (Plasma: culture medium = 1:10 for A549 and H520, 1:20 for DMS114 and 1198). Post-treatment plasma significantly reduced proliferation of A549, H520, DMS114 and 1198 cells in comparison to pre-treatment plasma. Percent change of cell viability from each participant was calculated first by normalizing matched post-treatment to baseline pre-treatment values. Mean; bars, SEM (n = 6). ***, P < 0.001.
Effects of one month of oral LP treatment on EPA, DHA, and unsaturated PC in fasting serum
Key coumpounds significantly altered/increased by LP treatment in the fasting serum of study participants include EPA and DHA in the unsaturated FA cluster. In addition, LP treatment significantly increased a total of 3 unsaturated PC, with PC(36:5) A as the key compound within that cluster. Moreover, plasmalogen PC(p-18:1/18:3) was also significantly increased (Table 1B). Pearson correlation of modulations of EPA and DHA with modulations of bronchial Ki-67 LI showed a trend toward statistical significance (Table 1C).
Table 1B.
Key compounds in the unsaturated FA, the unsaturated PC clusters, and a plasmalogen that were significantly increased by LP treatment.
| Compound Name | Cluster name | Pubchem. ID | Fold change | p-value |
|---|---|---|---|---|
| FA (20:5) (EPA) | Unsaturated FA | 446284 | 1.2 | 0.031 |
| FA (22:6) (DHA) | Unsaturated FA | 445580 | 1.3 | 0.047 |
| PC(39:6) | Unsaturated_Phosphatidylcholines | 52922637 | 1.3 | 0.031 |
| PC(p-18:1/18:3) | Plasmalogen | 53480747 | 1.4 | 0.047 |
Table 1C.
Pearson corrrelation coefficients for modulations of PGE3, EPA, or DHA with modulations of bronchial Ki-67 LI by LP (n = 6).
| Ki-67 LI | r value | p value |
|---|---|---|
| PGE3 | −0.900 | 0.037 |
| EPA | −0.794 | 0.109 |
| DHA | −0.709 | 0.178 |
Effects of 3 months of oral LP treatment on fasting serum PGE3 and LTB5
EPA can function as a substrate for cyclooxygenase (COX)s to synthesize unique 3-series PG compounds, especially PGE3, which tends to have antiproliferative and anti-inflammatory activities. To determine if the increase in systemic EPA by LP treatment might lead to an increase in PGE3, the levels of PGE3 in matched pre- and post-treatment fasting serum samples were measured. LP treatment significantly increased PGE3 levels by an average of 45% (Fig. 1B). As EPA could be a precursor for LTB5, the levels of LTB5 were also measured. LP treatment did not significantly change LTB5 levels. Increases of PGE3 significantly correlated with decreases of bronchial Ki-67 LI (Table 1C).
Post-treatment fasting plasma samples inhibit proliferations of human lung cancer and preneoplastic cells
To evaluate the systemic, antineoplastic effects from oral LP treatment, A549, H520, DMS114 and 1198 human cell lines were co-cultured with matched pre- and post-3 months treatment fasting plasma. Post-treatment plasma samples significantly inhibited cell proliferations in comparison with pre-treatment plasma (Fig. 1C).
DISCUSSION
In this correlative study of our recently published, modified phase 1 lung cancer chemoprevention study with LP in heavy former and active smokers, we demonstrate that once a day oral LP results in significant increases of n-3 PUFAs EPA and DHA, unsaturated PC, and PGE3 in fasting serum. Post-treatment fasting plasma samples also significantly inhibit proliferations of human lung cancer and preneoplastic cell lines in vitro.
There are two major classes of PUFAs: the omega-3 (n-3) and the omega 6 (n-6). Three n-3s have been most studied: alpha-linolenic acid (ALA, containing 18 carbon), EPA (20 carbons) and DHA (22 carbons). ALA is an essential FA that needs to be obtained from the diet. ALA can be converted into EPA and then to DHA, but the conversion is limited (reported rate of 15% primarily in the liver). Therefore, consumption of EPA and DHA from foods or dietary supplements is required to practically increase their levels in the body. ALA is present in plant oils such as soybean, flaxseed and canola oils. Whereas DHA and EPA are present in fish, fish oils and Krill oils, but are originally synthesized by microalgae, then consumed by fish (16).
EPA and DHA are long chain n-3 PUFAs with anti-inflammatory and immunomodulatory properties. They are believed to benefit cardiac, musculoskeletal, gastrointestinal and immune systems in humans (17). Epidemiologic and preclinical findings also support their anti-cancer properties. For example, n-3 FAs reduce onset of different cancers and protect against late stage cancers in carcinogen induced mouse tumors, human tumor xenografts on mouse, and spontaneous mouse tumors induced by transgenes. A higher intake of n-3 PUFAs is linked to a reduced risk of skin, colorectal, lung, prostate and breast cancers in humans (18).
The anticancer activities of EPA and DHA are partially associated with their effects on modulating eicosanoid metabolism (19), including inhibition of PGE2 production. PGE2 is derived from the n-6 Arachidonic acid (AA) precursors liberated from phospholipids in the cell memberane and converted into PG by COX. Ample studies have implicated proinflammatory and procarcerous effects of the inducible COX-2/PGE2 pathways in lung cancer (20). We previously demonstrated that GSE might simultaneously function as a natural COX-2 inhibitor and prostacyclin (PGI2) inducer. PGI2 is known for its antineoplastic and anti-platelet properties, capable of improving endobronchial dysplasia in former smokers (21). In this study, we demonstrate that oral LP significantly increases serum PGE3, likely through the increase of precursor EPA. EPA can function as a selective COX-2 inhibitor through competitive inhibition of the n-6 AA binding to COXs, resulting in a decrease production of PGE2, while concomitantly generates PGE3. PGE3 has anitproliferative and anti-inflammatory activities, can potentially antagonize tumor promoting effects of PGE2 in tumorigenic cells. Whereas the modulations of serum PGE3 significantly correlate with the modulations of Ki-67 LI, the modulations of EPA and DHA only show a trend toward significant correlations with modulations of bronchial Ki-67 LI, likely due to insufficient sample sizes.
Since our participants were specifically instructed to not alter their dietary intake nor start new dietary supplements during the course of the study, we speculate that the increases in DHA and EPA post-LP treatment likely are associated with the phytosome or lecithin component of LP. Lecithin is comprised of mixtures of glycerophospholipids including PC (22). it is conceivable that the phytosome increases systemic PC, which function as transporters that enhance absorption and bioavailability of EPA and DHA from usual diet of the participants. Such a notion is supported by a study showing that combined intake of dietary crude lecithin with DHA increases systemic availability of DHA in rats (23). Long chain n-3 PUFAs may also be synthesized from ALA by a progressive series of enzymatic desaturation and chain elongation steps (24). To this end, significant increases in the carnitine clusters of metabolites in the lipidomics likely reflect increases in carbon input from phytosome and transport of long-chain fatty acids into mitochondria for subsequent β-oxidation. The mechanisms involved in modulations of the carnitine cluster of metabolites and their potential anti-cancer roles in LP treatment remain to be elucidated. Significant change in the plasmalogen PC(p-18:1/18:3) also indicates differences in peroxisome metabolism, an organelle that is specifically active in PUFA metabolism that generates plasmalogen precursors (25).
Our findings illustrate the novel, additive effects of phytosome in LP, beyond its original intended purposes of enhancing absorption, intercellular transport and systemic bioavailability of GSE. To our knowledge, this is the first report demonstrating the additional, potential benefits of the carrier phytosome in humans. These findings, along with the significant reduction of Ki-67 LI in the proximal bronchi, and favorable modulations of a variety of other eicosanoids from our previous reports (7), further support the notion that oral administration of LP is capable of dampening the driving forces of cancerizations systemically and in the lungs (Fig. 2). Our findings support the continued clinical investigations of LP as an anti-neoplastic and chemopreventive agent against lung cancer.
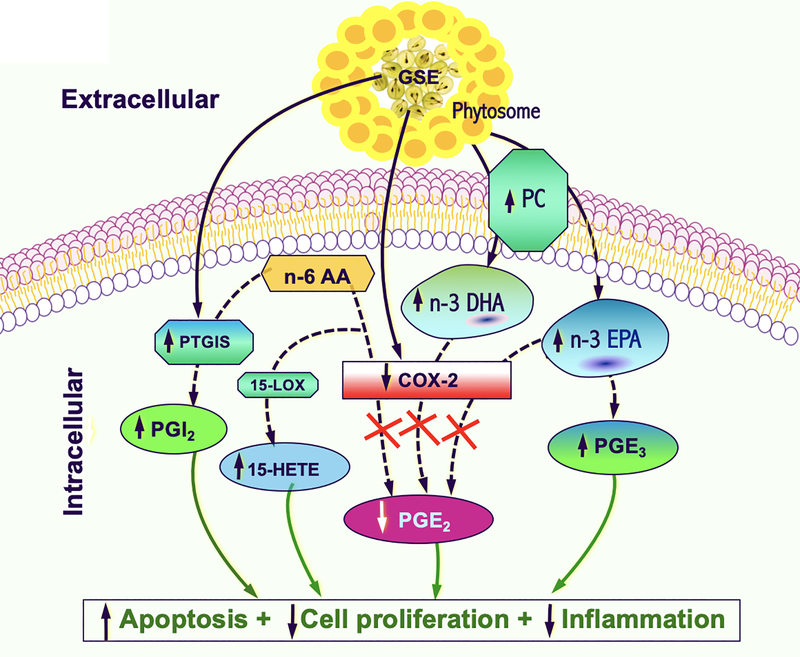
Fig. 2.
Proposed mechanistic diagram of the effects of LP treatment on EPA, DHA, PC and major eicosanoids against lung cancer. The phytosome portion of LP likely contains high levels of PC and serves as transporters for n-3 PUFAs EPA and DHA. Increases in EPA and DHA function as competitive inhibitors of COX-2. In addition, an increase in EPA results in an increase in downstream PGE3. Other GSE-mediated modulations of major eicosanoids signaling pathways previously described by our group are also depicted (a decrease in PGE2 due to COX-2 inhibition by GSE, while an increase in prostacyclin synthase (PTGIS) by GSE results in an overall increase in PGI2. Furthermore, an increase in 15-HETE is likely due to shunting of the n-6 AA precursor toward the 15-LOX pathway in the setting of COX-2 inhibition. Collectively, through modulations of these eicosanoids and n-3 PUFAs, LP increases apoptosis, decreases cell proliferation and inflammation, thereby reduces the driving forces of cancerization.
Prevention Relevance Statement.
In this correlative study of leucoselect phytosome (LP) for lung cancer chemoprevention in heavy active and former smokers, we demonstrate for the first time, favarble modulations of omega-3 polyunsaturated fatty acid and downstream prostaglandin E3 in fasting serum, further supporting the chemopreventive potential of LP against lung cancer.
Acknowledgement
We wish to thank A. Vargas and K. Park for their excellent technical assistance; Indena, Inc. and Thorne research, Inc., for generously supplying the LP. This work has been supported by grants from the National Institute of Health: (National Cancer Institute R21CA173211 to JTM), (NIH U2C ES030158 to OF); and VA Merit Review: (BX002258, BX004092 and CX002028 to JTM). This research was partially supported by the University of New Mexico Comprehensive Cancer Center Support Grant NCI P30CA118100. JTM is an Associate of the Kidney Institute of New Mexico.
Footnotes
Conflict of Interests: The authors declare that there is no conflict of interests.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
REFERENCES
- 1.Sharma S, Meeran S, Katiyar S. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E(2) and prostaglandin E(2) receptors. Molecular Cancer Therapeutics 2010; 9:569–80. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar S, Meeran S, Katiyar N, Katiyar S. Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clinical Cancer Research 2009; 15:821–31. [DOI] [PubMed] [Google Scholar]
- 3.Mao JT, Xue B, Smoake J, Lu QY, Park H, Henning SM, et al. MicroRNA-19a/b mediates grape seed procyanidin extract-induced anti-neoplastic effects against lung cancer. J Nutr Biochem 2016; 34:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao JT, Smoake J, Park HK, Lu QY, Xue B. Grape Seed Procyanidin Extract Mediates Antineoplastic Effects against Lung Cancer via Modulations of Prostacyclin and 15-HETE Eicosanoid Pathways. Cancer Prev Res (Phila). 2016;9:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue B, Lu QY, Massie L, Qualls C, Mao JT. Grape seed procyanidin extract against lung cancer: the role of microrna-106b, bioavailability, and bioactivity. Oncotarget 2018; 9:15579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigna GB, Costantini F, Aldini G, Carini M, Catapano A, Schena F, et al. Effect of a standardized grape seed extract on low-density lipoprotein susceptibility to oxidation in heavy smokers. Metab Clin Exp 2003; 52:1250–7. [DOI] [PubMed] [Google Scholar]
- 7.Mao JT, Lu QY, Xue B, Neis P, Zamora FD, Lundmark L, et al. A Pilot Study of a Grape Seed Procyanidin Extract for Lung Cancer Chemoprevention. Cancer Prev Res (Phila) 2019;12:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008;49:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cajka T & Fiehn O Increasing lipidomic coverage by selecting optimal mobile-phase modifiers in LC–MS of blood plasma. Metabolomics 2016; 12:34. [Google Scholar]
- 10.Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods 2015; 12:523–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kind T, Liu K, Lee D, DeFelice B, Meissen JK, Fiehn O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat Methods 2013; 10:755–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan S, Kind T, Cajka T,Hazen SL, Tang WHW, Kaddurah-Daouk R, et al. Systematic Error Removal Using Random Forest for Normalizing Large-Scale Untargeted Lipidomics Data. Anal Chem 2019; 91:3590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao JT, Nie WX, Tsu IH, Jin YS, Rao JY, Lu QY, et al. White tea extract induces apoptosis in non-small cell lung cancer cells: the role of peroxisome proliferator-activated receptor-{gamma} and 15-lipoxygenases. Cancer Prev Res (Phila) 2010; 3:1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamini Yoav, and Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 1995; 57: 289–300. [Google Scholar]
- 15.Barupal DK, and Fiehn O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Scientific Reports 2017; 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris WS. Omega-3 fatty acids. In: Coates PM, Betz JM, Blackman MR, et al. , eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:577–86. [Google Scholar]
- 17.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc 2009;109:668–79. [DOI] [PubMed] [Google Scholar]
- 18.Jing K, Wu T, Lim K. Omega-3 polyunsaturated fatty acids and cancer. Anticancer Agents Med Chem 2013;13:1162–77. [DOI] [PubMed] [Google Scholar]
- 19.Yang P, Jiang Y, Fischer SM. Prostaglandin E3 metabolism and cancer. Cancer Lett. 2014; 348:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao JT, Fishbein MC, Adams B, Roth MD, Goodglick L, Hong L, et al. Celecoxib decreases Ki-67 proliferative index in active smokers. Clin Cancer Res 2006; 12:314–20. [DOI] [PubMed] [Google Scholar]
- 21.Keith RL, Blatchford PJ, Kittelson J, Minna JD, Kelly K, Massion PP, et al. Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prev Res (Phila) 2011; 4:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith I, Hong-shum L. Complex mixture of phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidic acid, glycolipids, etcet al. eds. 2011. Food Additives Data Book (2nd ed.). Chichester, West Sussex. Wiley-Blackwell. P.334. [Google Scholar]
- 23.van Wijk N, Balvers M, Cansev M, Maher TJ, Sijben JW, Broersen LM. Dietary Crude Lecithin Increases Systemic Availability of Dietary Docosahexaenoic Acid with Combined Intake in Rats. Lipids. 2016;51:833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci 2015; 7:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2012;1822:1442–52. [DOI] [PubMed] [Google Scholar]