Abstract
Background.
Quitting smoking is notoriously difficult. Models of nicotine dependence posit that strength of cognitive control contributes to maintaining smoking abstinence during smoking cessation attempts. We examine the role for large-scale functional brain systems associated with cognitive control in smoking lapse using a novel adaption of a well-validated behavioral paradigm.
Methods.
We use data from 17 daily smokers (5 female) after 12 hours of smoking abstinence. Participants completed up to 10 sequential, five-minute functional magnetic resonance imaging (fMRI) runs, within a single scanning session. After each run, participants decided whether to stay in the scanner in order to earn additional money or to terminate the session in order to smoke a cigarette (i.e., lapse) and forego additional monetary reward.
Results.
Cox regression results indicate that decreased segregation of the default mode system from the frontoparietal system undermines the ability to resist smoking.
Conclusions.
This study demonstrates the feasibility of modifying an established behavioral model of smoking lapse behavior for use in the imaging environment, and it provides initial evidence that this approach yields valuable information regarding fine-grained, time-varying changes in patterns of neural activity in the moments leading up to a decision to smoke. Specifically, results lend support to the hypothesis that large-scale functional brain systems associated with cognitive control resources in the moments leading up to potential as a mechanism underlying smoking relapse.
Keywords: connectivity, fMRI, lapse, nicotine, tobacco
1. INTRODUCTION
Quitting smoking is notoriously difficult, with the majority of cessation attempts ending in relapse (i.e., return to regular smoking) (1). Models of nicotine dependence posit that deficits in cognitive control contribute to continued smoking after a quit attempt in two key ways. A reduced ability to override impulses to smoke can bias decisions towards smoking over alternative, non-drug reinforcers (2) and can promote a return to smoking in order to ameliorate abstinence-related cognitive control deficits (3). In line with these proposals, smokers exhibit impaired inhibitory control and working memory relative to non-smokers (4,5); smokers show impaired cognitive control performance during smoking abstinence relative to smoking satiety (6); and poorer performance on working memory tasks and inhibitory control tasks is associated with more rapid smoking resumption following smoking abstinence (7). Here, we extend research on the role of cognitive control in smoking cessation by examining how functional brain systems associated with cognitive control are correlated with smoking lapse.
One of the best predictors of relapse following a smoking cessation attempt is experiencing a lapse (i.e., any smoking after initial cession) (8). Although lapses can represent just a single puff on a cigarette (9), the majority of participants who lapse go on to relapse and resume regular smoking (10). The first lapse during a cessation attempt, then, often represents a gateway towards relapse. Findings that reduced cognitive control is associated with relapse provide initial evidence that limitations in cognitive control are implicated in smoking lapses (11).
Yet, the nature of lapses, as defined by discrete moments embedded within cessation attempts, challenges the identification of their precipitants. A second challenge for studying smoking lapses is that the precipitants of lapses may themselves change from moment to moment. Indeed, in the case of cognitive control processes such as working memory, inhibitory control, and sustained attention, substantial day-to-day (12) and even moment-to-moment (13) fluctuations in performance have been observed. Within-person fluctuations have also been observed in the functional brain organization of systems involved in cognitive control (14). Observations of fluctuations in cognitive control and cognitive control-relevant functional brain systems encourage a consideration of functional brain systems in the moments immediately preceding lapses given that the status of processes (e.g., negative affect) in moments more proximal to lapses are more predictive of smoking lapses than data collected further back in time (e.g., hours before lapse versus day before lapse) (15).
Laboratory smoking lapse paradigms represent an efficient and cost-effective way to overcome the difficulty of isolating lapse behaviors as they occur (16). In an increasingly used lapse paradigm, smokers are exposed to known precipitants of smoking relapse behavior, including nicotine deprivation, alcohol, and stress (17-19). Smokers are then given the option of beginning tobacco self-administration or delaying self-administration by 5-minute increments for up to 50 minutes in exchange for monetary reinforcement. The delay period models smokers’ ability to resist smoking, with shorter relative to longer times to lapse reflective of lower abilities to resist smoking. This lapse paradigm demonstrates validity, showing sensitivity to the effects of medications with known clinical efficacy for smoking cessation (20) and, to date, has supported the role of alcohol, stress, nicotine and food deprivation, exposure to smoking environment cues, and the devaluation of monetary rewards in facilitating lapse behavior in the laboratory (17-24).
In the present study, we overcome the challenges associated with identifying the role of cognitive control-relevant functional brain systems in smoking lapses by observing participants as they engage in a laboratory smoking lapse paradigm, accompanied by functional Magnetic Resonance Imaging (fMRI). In addition to demonstrating the feasibility of this novel methodological approach, our goal is to provide insight into the role of cognitive control-relevant functional brain systems in efforts to resist the urge to smoke, specifically in the minutes preceding a lapse. We focus on interactions between two large-scale functional brain systems that are known to support cognitive control. The frontoparietal system is comprised of regions with roles in response suppression (25), working memory (26), and attentional control (27), processes relevant for changes in smoking behavior (7). The default mode system is characterized by a tendency to deactivate during many cognitive tasks and to activate at rest, as well as during self-referential and social tasks (28,29). Evidence suggests that the integrity of functional connectivity of the default mode and frontoparietal systems, as well as their interactions, is fundamental to cognitive control. In particular, greater strength of connectivity among the default mode and frontoparietal systems, indicating reduced segregation of activity between the two systems, is associated with poorer working memory and inhibitory control performance (30, 31). Given the association between cognitive control and the segregation of the default mode from the frontoparietal system, we hypothesized that greater default mode and frontoparietal system segregation would protect against lapses during a smoking lapse paradigm. Notably, by taking continuous fMRI measurements during the course of the lapse task, we also capture potentially time-varying changes in functional connectivity of the systems theorized to be associated with lapse behavior.
We additionally collect self-ratings of affect, arousal, urge to smoke, and the extent to which participants resisted the urge to smoke during the course of the lapse task. Self-reports are much easier to obtain than fMRI measurements. Yet, an increasing body of literature indicates the promise of using indices of neural activity to predict smoking-related behaviors (32-34). Although fMRI measurements are costly to obtain, they overcome some limitations of self-reports, including social desirability effects (35) or a lack of conscious access to factors implicated in behavior (36), and allow the capture of activity in systems underlying multiple cognitive and affective functions simultaneously. Thus, as a second aim, we test whether associations between default mode and frontoparietal system segregation and lapse behavior is observed above and beyond self-ratings collected during the smoking lapse paradigm.
2. MATERIALS AND METHODS
2.1. Participants
Participants were 20 individuals (8 female) recruited using newspaper, radio, and internet advertisements, and flyers posted in the community. To be eligible for the study, individuals were required to be right handed, to be between the ages of 18 and 45 years, to report that they smoked at least 10 cigarettes per day for the past 24 months, to indicate that they were not currently planning to quit smoking or actively pursuing any form of smoking cessation treatment, and to have a baseline expired-air carbon monoxide (CO) level greater than 10 parts per million (ppm); the latter criterion was chosen in order to verify smoking status (BreathCo, Vitalograph, Lenexa, Kansas). Individuals were excluded if they reported any of the following during an initial telephone screening: current heavy use of illicit substances (defined as illicit drug use on 10 or more days in the past 30 days), current use of prescription medications that have been found to affect blood flow responses in the brain, current psychiatric diagnoses, chronic cardiovascular or respiratory problems, and/or any contraindications for magnetic resonance imaging (MRI). All procedures were approved by the Pennsylvania State University Institutional Review Board, and written informed consent was obtained from all participants.
2.2. Procedures
Demographic and smoking-related variables, including age, the average number of cigarettes smoked per day, and Fagerström Test of Cigarette Dependence (FTCD, 37), were collected at an initial baseline session. Participants then completed an fMRI session on a subsequent day. They were instructed to abstain from smoking and from using any nicotine-containing products for at least 12 hours prior to the scan session. Upon arriving for the fMRI experiment, participants reported the last time they smoked a cigarette and a CO sample was obtained to verify compliance with these instructions; compliance was defined as < 8 ppm or ≥ 50% reduction from their baseline CO level. Participants then completed the following surveys to assess affective state, nicotine withdrawal symptoms, and the urge to smoke: the Positive and Negative Affect Schedule (38), the Wisconsin Smoking Withdrawal Scale (39), and the Questionnaire of Smoking Urges-Brief (40).
After completion of the surveys, participants were given an overview of the remainder of the experimental visit. Specifically, they were told that they would be placed in the MRI scanner to complete tasks, where they would remain for up to 90 minutes, and that they would then complete additional questionnaires for approximately two hours after being removed from the scanner. Participants were explicitly informed that the entire visit would last four hours (including the two hours required to complete post-scan questionnaires), and that they would have to remain in the lab for this amount of time even if they finished the questionnaires early, to disincentivize ending the scan early to shorten the overall appointment duration..
Next, participants were given instructions for an fMRI reward task not reported on here before being placed in the scanner. Following the acquisition of anatomical data, participants then completed a 3.5-minute resting baseline scan and six runs of the reward task, each lasting approximately 5 minutes. At this point, participants could decide to leave the scanner and smoke a cigarette rather than taking part in the next in-scanner task. Participants deciding to stay in the scanner (n=17) performed an fMRI task modeling smoking lapse behavior adapted from prior behavioral research (20), which is the focus of the current study.
At the beginning of the fMRI smoking lapse task, participants were informed that they would be given the opportunity to smoke immediately after being removed from the scanner, but that they would be given the chance to earn extra money by delaying their removal to complete additional scans. Specifically, they were told that they could choose to remain in the scanner for up to 50 additional minutes, earning $1 for every five minutes that they remained in the scanner (i.e., up to $10 total), and that they would be asked to indicate via button press whether or not they would like to remain in the scanner before each five-minute scan began. After receiving instructions, participants provided visual analog scale ratings of their affect (from “unpleasant” to “pleasant”), their level of arousal (from “sleepy” to “aroused/activated”), and their urge to smoke (from “no urge at all” to “strongest urge ever”), with each scored on a 0-100 range.
Participants then completed the following sequence for each of up to 10 five-minute runs. First, participants pushed one of two buttons to signify whether they would like to start the subsequent 5-minute run of the smoking lapse task or be removed from the scanner. Next, those who elected to remain in the scanner completed a 5-minute run of the task, during which they were asked to relax and remain as still as possible with their eyes open. (If the participant instead chose to be removed from the scanner, they were taken out of the MRI at that point and did not complete any additional runs of the task.) Finally, immediately after the run ended, participants used visual analog scales to rate their affect, level of arousal, and urge to smoke, as well as to rate how much they were trying to change or resist their urge to smoke during the previous run (from “not at all” to “very much”). Participants were given the opportunity to smoke after being removed from the scanner during a 10-minute break, after which they completed additional questionnaires (not reported here). The session concluded once participants had been in the lab for a total of four hours, at which point they were paid the money that they earned during the smoking lapse task (up to US$10) and the fMRI reward task (US$10) in cash. Participants also earned $40 ($10/hour) for completing the session, which was sent to them as a check via mail.
2.3. Data Preparation
A summary of our preparation and analysis of the functional imaging data from the smoking lapse task is as follows: we preprocessed and denoised the BOLD time series, after which we created an association matrix representing the functional connectivity among regions of the brain for each participant and each 5-minute block. We then quantified default mode and frontoparietal system segregation for each association matrix. We provide additional detail below.
2.3.1. Data acquisition.
Scanning was conducted at the Penn State Social, Life, and Engineering Sciences Imaging Center using a 3-Tesla Siemens Trio scanner (Siemens Corporation, NY). Prior to functional scanning, a high-resolution T1-weighted (T1w) anatomical image was acquired (256x256 matrix; FOV=256 mm2; 160 1-mm sagittal slices). During functional scanning, 34-slice oblique-axial functional images (3x3x3 mm voxels) were acquired using a standard echo-planar imaging pulse sequence [TR=2000 ms, TE=25 ms, FOV=192 mm, flip angle=80°].
2.3.2. Data preprocessing.
Initial preprocessing of the brain imaging data was performed using fMRIPrep 1.4.1rc1 (41). A detailed overview of the steps taken is provided in the supplement. We then denoised the fMRI data with a protocol based on studies that evaluated the performance of a wide variety of denoising pipelines in mitigating motion artifact in studies of BOLD functional connectivity (42) using the publicly available eXtensible Connectivity Pipeline (XCP) software (43). Six head motion regressors and three matter regressors (global signal, white matter, and cerebrospinal fluid), as well as their derivatives, quadratic terms, and the squares of their derivatives (36 regressors in total) were regressed from the time series. We also conducted despiking – identifying outliers in the intensity of each voxel’s detrended BOLD time series and interpolating over these outliers.
2.3.3. Creating an association matrix.
Using the preprocessed and denoised BOLD fMRI data, we created an association matrix representing the strength of functional connectivity between pairs of brain regions. We defined regions of the default mode and frontoparietal systems on a commonly applied parcellation scheme (44), the coordinates of which can be found in the Supporting Information. For each region, we extracted a time series of the BOLD signal separately for each individual. All regions were modeled as 10mm diameter spheres around the center coordinates. The extracted time series were the average time series for all voxels within the sphere. The spherical regions represented nodes in functional connectivity networks. Pairwise Pearson correlation coefficients between node time series were used as network edge weights. Similar to previous functional connectivity studies (e.g., 45), negative correlations were set to 0 to eliminate potential misinterpretation of negative edge weights.
2.3.4. Default mode and frontoparietal system segregation measure.
We calculated the strength of default mode and frontoparietal system segregation by taking the average of two system segregation measures (45,46). First, we computed a default mode system segregation measure:
| (1) |
where is the mean connectivity strength of edges between all pairs of nodes in the default mode system and is the mean connectivity strength of edges between all pairs of nodes that spanned the default mode and the frontoparietal systems. Higher values of the default mode system segregation indicate greater segregation of the default mode system from the frontoparietal system. We additionally computed the strength of frontoparietal system segregation from the default mode system switching the places of the default mode and frontoparietal systems in equation 1.
Both measures of system segregation contain a measure of between-system connectivity but are unique in the system for which they capture within-system connectivity. To provide an overall measure of default mode and frontoparietal system segregation, we take the average of the default mode system segregation and frontoparietal system segregation indices (see also 45 for use of a similar average system segregation index).
2.4. Data Analysis
2.4.1. Default mode and frontoparietal system segregation and lapse behavior.
We hypothesized that default mode and frontoparietal system segregation would be protective against deciding to leave the scanner in order to smoke a cigarette rather than remaining in the scanner in order to earn a monetary incentive. We adopted a survival analysis framework, a framework in which the outcome variable is the timing of an event, to test this hypothesis. In the current analyses, the relevant event is the decision to leave the scanner. In the present study, there were 9 event times, or moments at which a participant could decide to leave the scanner during the smoking lapse task (i.e., at the end of blocks 1 though 9). Of the 20 participants enrolled in the study, three participants decided to smoke rather than to begin the smoking lapse task. These left censored cases, in which the event occurred prior to or coincident with the start of the observation period, were not included in the analysis as they provided no fMRI data for the smoking lapse task. Of the remaining 17 participants, four did not choose to leave the scanner at any point during the 10 blocks. Survival analysis was developed in part to handle right-censored cases and, as such, these four right-censored cases are readily accommodated.
A participant’s likelihood of staying in the scanner may be associated with different types of predictors, both time-invariant (e.g., age) and time-varying (e.g., default mode and frontoparietal system segregation within each scanning block). We estimate the survival function, reflecting the cumulative loss of all participants in the sample. Formally, the survival function is expressed as
| (2) |
and gives the probability that a participant will stay in the scanner past time t. We also estimate the hazard rate, which is the risk of leaving the scanner given that the participant has stayed in the scanner up to a specific time, and we determine whether the function differs systematically in relation to predictor variables. We use a Cox regression model to examine how the hazard rate is related to default mode and frontoparietal system segregation during each scanning block of the smoking lapse task. We fit a cox regression model specified as
| (3) |
where the hazard of deciding to leave the scanner at time t depends on the product of the baseline hazard h0(t) and an exponentiated linear function of q predictors that may be time-invariant or time-varying. We include the time-invariant predictor of age, β1, and the time-varying predictor of default mode and frontoparietal system segregation during each scanning block directly preceding the decision, β2, the value of which varies from scanning block to block.
Of greatest interest was the test of whether the parameter β2 was different than 0 (i.e., that there is an association between default mode and frontoparietal system segregation and the hazard of choosing to leave the scanner to smoke a cigarette). Parameters were transformed into a more easily interpreted hazard ratio metric (HR = exp[β]), which can be interpreted as the change in the risk of leaving the scanner if the parameter in question rises by one unit: HR = 1.00 indicates no association between the predictor and outcome variable, HR > 1 indicates higher hazard of event occurrence for higher values of the predictor, and HR < 1 indicates lower hazard of even occurrence for higher values of the predictor. HRs can also be interpreted as percent change in hazard as 100 x [HR-1]. We included age as a time-invariant covariate because previous work indicates that default mode and frontoparietal system segregation decreases with age (e.g.,45). All predictor variables were standardized to increase the interpretability of the resulting coefficients.
We fit the model using PROC PHREG by implementing the counting process style of input. The discrete nature of event quantification (block by block) resulted in tied times during which participants decided to leave the scanner. We used the “tie = exact” option in SAS Proc PHREG to accommodate these tied events.
2.4.2. In-scanner motion and smoking behavior.
In an additional model, we tested the extent to which associations between default mode and frontoparietal system segregation and lapse behavior remained significant when controlling for participant motion during each scanning block and the number of cigarettes smoked per day reported at baseline.
2.4.3. Self-reports and smoking lapse behavior.
We added time-varying self-ratings of affect, arousal, and urge to smoke prior to each scan block, and post-scan block self-ratings of how much participants were trying to change or resist their urge to smoke during the previous run, to the model specified in equation 3. This allowed us to examine the extent to which the default mode and frontoparietal system segregation measure was associated with lapse behavior controlling for these self-reports.
2.4.4. Time-invariant default mode and frontoparietal system segregation and lapse behavior.
Our use of a measure of default mode and frontoparietal system segregation from each scanning block reflects an assumption that the time-varying nature of segregation is important for predicting lapse behavior. An alternative possibility is that a time-invariant segregation measure would be sufficient to predict lapse behavior. To examine this possibility, we took the average measure of each participants’ default mode and frontoparietal system segregation values across their repeated measures and used this average measure as a time-invariant version of default mode and frontoparietal system segregation. We used this time-invariant default mode and frontoparietal system segregation measure as a predictor of time to smoking lapse instead of the time-varying default mode and frontoparietal system segregation measure in a model similar to that shown in equation 3.
2.4.5. Additional analyses.
Additional analyses that are tangential to the main manuscript but that may be of interest to some readers are included in the supplement. These include analyses using alternative constructions of the segregation measure and a consideration of the salience system, given previous work implicating a role for the salience network in cognitive control. We observe no evidence for a role for the salience system in leaving the scanner in the present study.
3. RESULTS
3.1. Participant characteristics and descriptive statistics
As detailed above, 17 participants (5 female) provided data for the survival analysis. The mean age of these participants was 24.41 years (SD=6.90). The self-identified racial composition of the usable sample was as follows: 82% White, 6% Asian, and 12% unreported. Participants reported smoking an average of 13.41 (SD=3.62) cigarettes per day and had a baseline CO level of 19.53 ppm (SD = 7.19). Additional characterization of the sample may be found in Table S1.
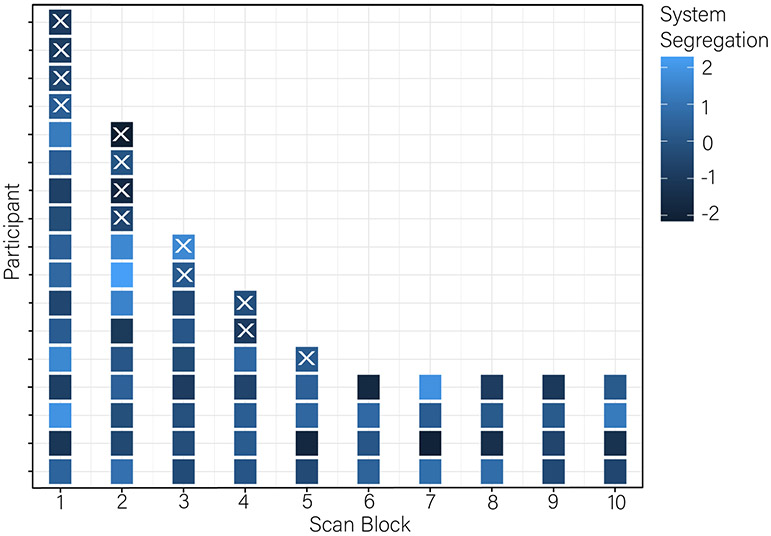
We provide descriptive statistics of key model variables in Table 1. Figure 1 shows the survival times for each participant (presence of square on horizontal lines) grouped by scanning block, in addition to the value of default mode and frontoparietal system segregation within each block for each participant. Right censored cases (n=4) do not have Xs at the end of their rows to indicate that the event of interest (smoking lapse) was not observed in these participants.
Table 1.
Correlations and Descriptive Statistics of Key Study Variables
| Variables | System Seg |
DMN Seg |
FPN Seg |
DMN- FPN |
DMN WN |
FPN WN |
Age | Cigs/ Day |
FTCD | Motion | Affect | Arousal | Urge | Resist |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. System Sega | - | |||||||||||||
| 2. DMN Sega | 0.78 | - | ||||||||||||
| 3. FPN Sega | 0.79 | 0.23 | - | |||||||||||
| 4. DMN-FPNa | −0.56 | −0.76 | −0.12 | − | ||||||||||
| 5. DMN WNa | 0.55 | 0.61 | 0.25 | 0.04 | - | |||||||||
| 6. FPN WNa | 0.33 | −0.24 | 0.75 | 0.54 | 0.32 | - | ||||||||
| 7. Age | −0.49 | −0.59 | −0.19 | 0.46 | −0.30 | 0.10 | - | |||||||
| 8. Cigs/Day | −0.15 | −0.15 | −0.09 | −0.05 | −0.30 | −0.11 | −0.15 | - | ||||||
| 9. FTCD | −0.11 | −0.07 | −0.10 | 0.04 | 0.02 | −0.05 | −0.16 | 0.40 | - | |||||
| 10. Motiona | 0.00 | −0.06 | 0.05 | −0.03 | −0.15 | 0.05 | 0.01 | 0.26 | −0.20 | - | ||||
| 11. Affecta | −0.17 | −0.24 | −0.02 | −0.06 | −0.47 | −0.14 | 0.42 | −0.15 | −0.52 | −0.27 | - | |||
| 12. Arousala | −0.06 | −0.35 | 0.24 | 0.11 | −0.34 | 0.25 | −0.01 | 0.14 | 0.10 | 0.03 | 0.10 | - | ||
| 13. Urgea | 0.28 | 0.09 | 0.34 | −0.18 | −0.05 | 0.15 | −0.52 | 0.01 | 0.37 | −0.47 | −0.01 | 0.30 | - | |
| 14. Resista | 0.18 | −0.03 | 0.32 | −0.18 | −0.23 | 0.11 | −0.33 | 0.08 | 0.34 | −0.32 | −0.01 | 0.29 | 0.91 | − |
| Mean | 0.35 | 0.35 | 0.35 | 0.09 | 0.14 | 0.15 | 26.31 | 13.76 | 2.94 | 1.09 | 40.59 | 27.94 | 43.10 | 36.43 |
| SD | 0.07 | 0.08 | 0.11 | 0.01 | 0.01 | 0.02 | 7.90 | 3.71 | 1.71 | 0.06 | 24.26 | 18.46 | 33.82 | 32.35 |
Notes:
Intraindividual mean of the time series (up to 10 scan blocks per person); Seg=segregation; DMN = default mode system; FPN = frontoparietal system; N=17.
Figure 1.
Survival times (x-axis) for each participant (separate lines on y-axis). Event times (leaving the scanner to smoke a cigarette) are indicated by an X. Four participants remained in the scanner for the entire 50 minutes. Each scan run for each participant is colored to indicate the magnitude of default mode and frontoparietal system segregation (z-scored and residualized to account for correlation with age). Lighter colors indicate greater segregation.
3.2. Default mode and frontoparietal system segregation and lapse behavior
We estimated the baseline survival function with an unconditional (baseline hazard) model. We then added age and the default mode and frontoparietal system segregation variable to the model. Goodness of model fit was tested using a likelihood ratio test that compared the fit of the model with age and default mode and frontoparietal system segregation as predictors relative to the unconditional model. The likelihood ratio test was significant, χ2(2)=6.67, p=0.04, indicating that the model with age and default mode and frontoparietal system segregation fit the data better than the unconditional model.
Results of the model (Table 2) indicate that the extent of default mode and frontoparietal system segregation in the scanning block immediately preceding the decision to stay or leave the scanner was associated with the choice to leave the scanner in order to smoke a cigarette, β2=−0.76, p=0.04. As hypothesized, with one standard deviation increase in the segregation variable (predictor variables were standardized prior to model estimation), participants were 0.47 times (HR=0.47) as likely, or 53% less likely (percent change = 100 x [0.47- 1.00]=−53%), to choose to leave the scanner in order to smoke a cigarette.
Table 2.
Cox regression results testing association between default mode and frontoparietal system segregation and age on hazard of choosing to leave the scanner to smoke
| Predictor | Estimate | Standard Error |
p | Hazard Ratio | 95% Confidence Interval of Hazard Ratio |
|---|---|---|---|---|---|
| System segregation | −0.76 | 0.38 | 0.04 | 0.47 | 0.22 – 0.98 |
| Age | −1.05 | 0.66 | 0.11 | 0.35 | 0.10 – 1.27 |
| −2 Log Likelihood | 38.50 | ||||
| AIC | 42.50 |
Note: AIC= Akaike Information Criteria. N = 17 persons. Likelihood ratio test: χ2(2)=6.67, p=0.04.
3.3. In-scanner motion and smoking behavior
Follow-up analyses indicate that the association between default mode and frontoparietal system segregation and the choice to leave the scanner was robust to including participant motion and cigarettes per day (Table S2) and FTCD score at baseline (Table S3).
3.4. Self-reports and smoking lapse behavior
The association between default mode and frontoparietal system segregation remained significant (β=−1.11, p=0.03) when time-varying self-reports of affect, arousal, and urge to smoke prior to each scan block, and post scan block self-ratings of how much participants were trying to change or resist their urge to smoke during the previous run were included as covariates (Table S4). Included in the same model, no significant independent associations emerged between self-reports and lapse behavior. The association between default mode and frontoparietal system segregation also remained significant when each self-rating was included as the only self-rating in the model (Tables S5-S8). In these models, with each self-rating in separate models, both higher urges to smoke reported prior to the scan block (β=1.68, p=0.03) and greater reported efforts to change or resist the urge to smoke during the previous run (β=1.17, p=0.04) were associated with a higher likelihood of deciding to leave the scanner.
Repeated measure correlations between the default mode and frontoparietal system segregation measure and the self-report scales indicated no significant associations between segregation and urge to smoke, r(53)=0.01, p=0.92, arousal, r(53)=0.05, p=0.71, or efforts to resist smoking urges, r(53)=−0.001, p=0.99. Affect had the strongest correlation with segregation, r(53)=0.26, p=0.06, such that greater default mode and frontoparietal system segregation was associated with more positive affect prior to the scan block. This correlation was not significant, though affect was significant correlated with the default mode system segregation measure, r(53)=0.33, p=0.02.
3.3. Time-invariant default mode and frontoparietal system segregation and smoking lapse
There was no evidence (Table S9) that the association between default mode and frontoparietal system segregation and time to smoking lapse was significant when using a time-invariant version of default mode and frontoparietal system segregation, β=−0.61, p=0.15. This suggests that added value of capturing temporal dynamics in system segregation across time.
3. DISCUSSION
One of the best predictors of smoking relapse is the experience of a lapse during a cessation attempt (10). To provide insight into the antecedents of smoking lapses, we examined the association between cognitive control-relevant functional brain systems and smoking lapse behavior. In line with our hypothesis, decreased default mode and frontoparietal system segregation undermined the ability to resist smoking in a sample of daily smokers who were deprived of nicotine for over 12 hours.
The current study extends a laboratory paradigm modeling smoking lapse behavior (20) into the neuroimaging setting. Capturing BOLD fMRI during an attempt to resist smoking to earn a monetary incentive allowed us to test the role for large-scale functional brain networks associated with cognitive control in prompting lapse behavior. Our focus on connectivity among the default mode and frontoparietal systems reflects the importance of these systems in cognitive control abilities, with findings that segregation of these systems from one another supports accurate cognitive control performance (30, 31). In the context of cigarette-smoking specifically, improvements in cognitive withdrawal symptoms after nicotine replacement are associated with increased inverse coupling between default mode and frontoparietal systems (47). Our findings are consistent with behavioral studies indicating that poorer cognitive control task performance is associated with more rapid smoking resumption following smoking abstinence (7) and further build upon them by considering brain dynamics in real time during decisions to resist smoking.
Interestingly, self-ratings of affect, arousal, and urge to smoke prior to scan blocks and post-scan block ratings of how much participants resisted the urge to smoke were not independently significantly associated with decisions to leave the scanner in order to smoke a cigarette. When self-ratings were considered separately from all other self-ratings, both higher urges to smoke reported prior to a scan block and greater reported efforts to change or resist the urge to smoke during the previous run were associated with a higher likelihood of deciding to leave the scanner. In the context of these significant associations between self-ratings and decisions to leave the scanner, system segregation remained a significant predictor of lapse behavior. These findings add to a body of literature indicating the promise of neural activity in predicting smoking-related behaviors (32-34). For example, existing work has observed associations between BOLD activity in the left dorsolateral prefrontal cortex and posterior cingulate during an N-back working memory task and the ability to remain abstinent during a 7-day quit attempt (34). With these findings, neuroimaging is emerging as a tool to predict behavior, providing an alternative to efforts to predict future behavior through self-reports that may contain biases stemming from social desirability effects (35) or that may fail to predict behavior due to a lack of conscious access to factors implicated in behavior (36).
Although not associated with lapse behavior, there was some evidence for a correlation between self-ratings of affect and default mode system segregation. More positive affect was reported prior to scans during which segregation between the default mode and frontoparietal systems was greater than usual. Positive affect has been theorized to inhibit craving by facilitating self-regulation and, consistent with this perspective, high positive affect is associated with reduced cravings during tobacco, alcohol, and opioid withdrawal (48). Results of the present study, coupled with findings that positive mood is associated with increased flexibility in large-scale brain networks (49), are consistent with the perspective that positive mood facilitates self-regulation via modulating functional connectivity associated with cognitive control. Notably, however, functional connectivity predicted lapse behavior above and beyond self-rating of affect (Table S6) suggesting that while positive affect may modulate the extent of default mode and frontoparietal system segregation, it is not sufficient to predict lapse behavior on its own.
Taken together, the findings have potentially significant implications for both methodology and for understanding, and eventually treating addiction. First, we show the feasibility of taking a behavioral smoking lapse task, which has provided key insights into the antecedents of smoking lapse behavior (17-24), into the neuroimaging environment. This opens the door for future research aiming to characterize neural correlates associated with smoking lapses which, to date, has been difficult due to the nature of lapses, defined by discrete moments embedded within cessation attempts. Understanding the brain processes that precede lapses may suggest novel intervention possibilities. Second, findings that fluctuations in patterns of functional connectivity associated with cognitive control, but not person-averaged, time-invariant patterns of functional connectivity, were associated with smoking lapses highlight the importance of considering the time-varying nature of antecedents to smoking lapses for the treatment of addiction. Ecological momentary assessment designs have long-considered the time-varying nature of certain antecedents of smoking lapses, intensively measuring hypothesized antecedents to lapses many times a day for many days. Despite the rich temporal detail available through ecological momentary assessment, we note that the cadence of measurement in the present study (5-minute increments) was much finer than what is typical for an EMA study. These findings encourage us to consider the timescales over which cognitive control and other lapse-related processes may be fluctuating in situ and the extent to which typical sampling rates are sufficient to provide insight into proposed antecedents to lapses.
The study findings should be interpreted in the context of study strengths and limitations. First, there is a risk that findings could fail to replicate in future work due to the small number of participants and the relatively limited nature of the screening for potentially confounding factors (e.g., psychiatric diagnosis, substance use). Regarding sample size, however, we note that a strength of the intensive repeated measures design is that findings are based on 71 imaging runs despite a sample size of 17 individuals. Data were collected under minimal task demands (i.e., during resting state), building on work indicating the ability to extract functional connectivity indices relevant for understanding cognitive control from rest (30, 31). Yet, lapse episodes in ecologically valid contexts are often provoked by both internal states (e.g., negative affect) and external stimuli (e.g., smoking cues) that were not incorporated into the study design. For example, in behavioral analogues of the current paradigm, participants sit in front of cigarettes while they decide whether to forgo smoking in order to earn monetary reward or to forgo the additional monetary reward in order to smoke. Although having cigarettes present would be technically challenging (but not impossible; 50) to achieve in the neuroimaging environment, there is a substantial body of fMRI drug cue-reactivity work that could provide satisfactory analogues. We additionally note that the minimal demands associated with this version of the lapse task provides a baseline against which additional manipulations of interest may be added (e.g., stress, alcohol) in the future, as has been done in behavioral work (17,19).
4.1. Conclusions
In summary, default mode and frontoparietal system segregation was associated with a reduced risk of lapsing during a laboratory smoking lapse task. Results lend support to the hypothesis that large-scale functional brain systems associated with cognitive control are implicated in smoking lapse behavior and point to the importance of cognitive control as a mechanism underlying smoking relapse.
Supplementary Material
Citation diversity statement.
Recent work in neuroscience and other fields has identified a bias in citation practices such that papers from women and other minorities are under-cited relative to the number of such papers in the field. Here we sought to proactively consider choosing references that reflect the diversity of the field in thought, form of contribution, gender, and other factors. We used automatic classification of gender based on the first names of the first and last authors, with possible combinations including male/male, male/female, female/male, and female/female (https://github.com/dalejn/cleanBib). Excluding self-citations to the first and last authors of our current paper, the references contain 38.30 % male/male, 21.30% male/female, 21.83% female/male, and 19.10% female/female. We look forward to future work that could help us to better understand how to support equitable practices in science.
Acknowledgements
S.J.W. acknowledges support from the National Institute on Drug Abuse (R01DA041438 and R21DA045853). D.S.B. and D.M.L. acknowledge support from the John D. and Catherine T. MacArthur Foundation, the Alfred P. Sloan Foundation, the ISI Foundation, the Paul Allen Foundation, the Army Research Laboratory (W911NF-10-2-0022), the Army Research Office (Bassett-W911NF-14-1-0679, Grafton-W911NF-16-1-0474, DCIST- W911NF-17-2-0181), the Office of Naval Research, the National Institute of Mental Health (2-R01-DC-009209-11, R01 – MH112847, R01-MH107235, R21-M MH-106799), the National Institute of Child Health and Human Development (1R01HD086888-01), National Institute of Neurological Disorders and Stroke (R01 NS099348), and the National Science Foundation (BCS-1441502, BCS-1430087, NSF PHY-1554488 and BCS-1631550). D.M.L. acknowledges support from the National Institute on Drug Abuse (K01DA047417). D.M.L, D.S.B, and E.B.F acknowledge support from the Army Research Office (W911NF-18-1-0244). The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, Schwartz R. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6:e011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans DE, To CN, Ashare RL. The role of cognitive control in the self-regulation and reinforcement of smoking behavior. Nicotine Tob Res. 2018;21:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenstein JE, Kassel JD. The effects of smoking and smoking abstinence on verbal and visuospatial working memory capacity. Exp Clin Psychopharm. 2009;17:78–90. [DOI] [PubMed] [Google Scholar]
- 5.Spinella M Correlations between orbitofrontal dysfunction and tobacco smoking. Addict Biol. 2002;7:381–384. [DOI] [PubMed] [Google Scholar]
- 6.Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo-controlled experimental study of nicotine: II—Effects on response inhibition and executive functioning. Psychopharmacology. 2007;190:457–467. [DOI] [PubMed] [Google Scholar]
- 7.Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchner TR, Shiffman S, Wileyto EP. Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. J Abnorm Psychol. 2012;121:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addict Behav. 1990;15:105–114. [DOI] [PubMed] [Google Scholar]
- 10.Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: a report from the Normative Aging Study. Addict Behav. 1992;17:367–377. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brose A, Schmiedek F, Lövdén M, Lindenberger U. Daily variability in working memory is coupled with negative affect: the role of attention and motivation. Emotion; 2012;12:605–617. [DOI] [PubMed] [Google Scholar]
- 13.Esterman M, Rosenberg MD, Noonan SK. Intrinsic fluctuations in sustained attention and distractor processing. J Neurosci. 2014;34:1724–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun U, Schäfer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci. 2015;112:11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. [DOI] [PubMed] [Google Scholar]
- 16.Lerman C, LeSage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. [DOI] [PubMed] [Google Scholar]
- 17.McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacology. 2011;25:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahler CW, Metrik J, Spillane NS, Day A, Leventhal AM, McKee SA, … Rohsenow DJ. Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory analogue task. 2014. Psychopharmacology;231:4649–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson JG, Oliver JA, Hallyburton MB, Sweitzer MM, Conklin CA, McClernon FJ. Smoking environment cues reduce ability to resist smoking as measured by a delay to smoking task. Addictive Behaviors. 2017:67,49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leeman RF, O’Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology. 2010;212:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson SJ, Delgado MR, McKee SA, Grigson PS, MacLean RR, Nichols TT, Henry SL. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cogn Affect Behav Neurosci. 2014; 14:1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridderinkhof KR, Van Den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognition. 2004;56:129–140. [DOI] [PubMed] [Google Scholar]
- 26.Mars RB, Grol MJ. Dorsolateral prefrontal cortex, working memory, and prospective coding for action. J Neurosci. 2007;27:1801–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shomstein S, Kravitz DJ, Behrmann M. Attentional control: Temporal relationships within the fronto-parietal network. Neuropsychologia. 2012;50:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. [DOI] [PubMed] [Google Scholar]
- 29.Shulman GL, Fiez JA, Corbetta M, Buckner R, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. [DOI] [PubMed] [Google Scholar]
- 30.Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28:1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy AC, Bertolero MA, Papadopoulos L, Lydon-Staley DM, and Bassett DS (2019): Multimodal network dynamics underpinning working memory. Nat Comm. 2020;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper N, Tompson S, O’Donnell MB, Emily BF. Brain activity in self-and value-related regions in response to online antismoking messages predicts behavior change. J Media Psychol. 2015;27:93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falk EB, Berkman ET, Whalen D, Lieberman MD. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychol. 2011;30:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth-Kewley S, Larson GE, Miyoshi DK. Social desirability effects on computerized and paper-and-pencil questionnaires. Comput Hum Behav. 2007;21:463–477. [Google Scholar]
- 36.Nisbett R, Wilson T. Telling more than we can know: Verbal reports on mental processes. Psychol Rev. 1977;84:231–259. [Google Scholar]
- 37.Fagerström K Determinants of tobacco use and renaming the FTND to the Fagertröm Test for Cigarette Dependence. Nicotine Tob Res. 2012;14: 75–78. [DOI] [PubMed] [Google Scholar]
- 38.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 39.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7:354–361. [DOI] [PubMed] [Google Scholar]
- 40.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. [DOI] [PubMed] [Google Scholar]
- 41.Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lydon-Staley DM, Ciric R, Satterthwaite TD, Bassett DS (2019): Evaluation of confound regression strategies for the mitigation of micromovement artifact in studies of dynamic resting-state functional connectivity and multilayer network modularity. Network Neurosci. 2019;3:427–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciric R, Rosen AF, Erus G, Cieslak M, Adebimpe A, Cook PA, et al. Mitigating head motion artifact in functional connectivity MRI. Nat Protoc. 2018;13:2801–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci. 2014;111:E4997–E5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen JR, D'Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 2016;36:12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage. 2010;52:590–599. [DOI] [PubMed] [Google Scholar]
- 48.Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. J Abnorm Psychol. 1992;101:617–629. [DOI] [PubMed] [Google Scholar]
- 49.Betzel RF, Satterthwaite TD, Gold JI, Bassett DS. Positive affect, surprise, and fatigue are correlates of network flexibility. Sci Rep. 2017;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson SJ, Sayette MA, Fiez JA. Quitting-unmotivated and quitting-motivated cigarette smokers exhibit different patterns of cue-elicited brain activation when anticipating an opportunity to smoke. J Abnorm Psychol. 2012;121: 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.