Abstract
Enriching early life experiences (e.g., sport, art, music, volunteering, language learning) during a critical period of brain development may promote structural and functional brain changes that are still present decades later (>60 years). We assessed whether a greater variety of enriching early life activities (EELA) before age 13 years were associated with individual differences in cortical and subcortical (hippocampus and amygdala) structure and function later in life (older adults aged 60 to 80 years). Results indicated no association between EELA and amygdala and hippocampus volumes, but higher functional connectivity between the amygdala and the insula was associated with more variety of EELA. EELA was not associated with cortical thickness controlling for sex, but sex-specific associations with the right pars opercularis were found. EELA was further associated with variations in functional connectivity patterns of the orbitofrontal cortex with regions within the visual, somatosensory and limbic networks. Early life enriching activities appear to contribute to potential mechanisms of cognitive reserve (functional processes) more so than brain reserve (structure) later in life.
Keywords: Enrichment, Functional Connectivity, Multivoxel Pattern Analysis, Aging
1. Introduction
Engagement in cognitively and socially enriching lifestyle behaviors is associated with better cognitive and brain health (Johansen-Berg & Duzel, 2016; May, 2011). Numerous studies have demonstrated that certain activities such as socialization (Kok et al., 2018; Kotwal et al., 2016), bilingualism (Bak et al., 2014; Calabria et al., 2020), musical instrument learning (Mansens et al., 2018; Seinfeld et al., 2013) and physical activity (Erickson et al., 2011, 2019; Kramer & Erickson, 2007) have beneficial effects on brain structure and function. Evidence suggests this to be the case in children (Donnelly et al., 2009; Hillman et al., 2011; Kok et al., 2018; Schlaug et al., 2005), middle-aged adults (Chang et al., 2010; Gärtner et al., 2013; Morris et al., 2019) and older adults (Erickson et al., 2011; Kotwal et al., 2016; Mansky et al., 2020; Voss et al., 2010). Few studies however have taken an across the lifespan approach, to assess how early engagement (in childhood) in enriching activities can benefit later-life brain health. This may be highly important as engagement in enriching activities during brain development may lead to the augmentation of mechanisms of cognitive reserve and brain resilience (Richards & Deary, 2005), key to the maintenance and promotion of brain health with age (Barulli & Stern, 2013; Scarmeas & Stern, 2003). That is, during childhood, the brain may be particularly sensitive to environmental and psychosocial processes that can beneficially affect the development of neural mechanisms of plasticity (Richards & Deary, 2005).
Previous research on early life factors, such as enriching lifestyle activities (Chan et al., 2019; Moored et al., 2018; Schreiber et al., 2016), education and socioeconomic status (Staff et al., 2012) as well as childhood deprivation (Mackes et al., 2020) have demonstrated associations with later life cognitive health and brain structure, specifically, hippocampal and amygdala volumes. Suggesting that later life brain structure and function is susceptible to early life experiences. A study by Schreiber and colleagues (Schreiber et al., 2016) found that lifetime physical and cognitive activity was associated with lower cardiovascular risk and better episodic memory later in life, and education attainment was associated with greater hippocampal volume. Another study by Moored and colleagues (Moored et al., 2018), demonstrated that a greater variety of enriching early life activities (EELA) before age 13 was associated with greater hippocampal and amygdala volume in a sample of older (>60 years) African Americans, where sex-specific effects were demonstrated. Specifically, EELA was only associated with greater hippocampal volume in males. Indeed, sex differences in mechanisms of cognitive and brain reserve may be highly important for risk reduction of brain-related pathology (Subramaniapillai et al., 2021). Consequently, childhood engagement in certain enriching lifestyle behaviors may impact structural development during a critical period, and where such engagement is reflected in brain reserve mechanisms decades later, with sex-specific effects.
Sensory, visual and motor regions and networks may be particularly sensitive to early experience-dependent plasticity. Neuronal maturation and brain development occur rapidly in early childhood (Johnson, 1990), suggesting the brain is likely most sensitive to the effects of sensory experiences during this period. Whilst evidence shows that the brain retains the capacity for plasticity throughout life (Pascual-Leone et al., 2005), early enrichment may promote the maturation and development of neural networks (Hensch, 2005). For example, in musicians, evidence suggests that motor circuits are better structured than non-musicians and that there is perhaps a critical period for training influence of brain morphology, with different effects seen after age seven (Habib & Besson, 2009). In bilingual brains, early enriched bilingual environments result in larger language areas and stronger connectivity between distributed regions within the language network (Berken et al., 2017). Beyond single experiences, early environmental enrichment, where one is exposed to greater multisensory stimulation, physical activity and social interactions, is thought to exert profound effects on the maturation of the visual system (in rodents) (Baroncelli et al., 2010) and plasticity of hippocampal neurogenesis (Clemenson et al., 2015). This experience dependent plasticity of brain networks related to early enriched sensory inputs may be highly important in the development of complex behavioral and affective functioning across the lifespan (Richards & Deary, 2005).
Studying whether early enriched activities lead to long lasting differences in structure (brain reserve) and function (cognitive reserve) decades later will advance our understanding of how enriching lifestyle behaviors benefit brain health with advancing age. Distinguishing between brain reserve (passive structural characteristics) and cognitive reserve (active and dynamic functional processes) operationally in this research is important as they account for different variance in clinical and cognitive status (Stern et al., 2020). Further, calls to study sex-differences in cognitive and brain reserve research have been made (Subramaniapillai et al., 2021) given sex-difference are notable in the risk of age-related brain pathology (Irvine et al., 2012; Laws et al., 2018) and in their contributions to reserve mechanisms (Subramaniapillai et al., 2021).
We build upon the prior literature on childhood enrichment and later life brain health by asking whether the results from a previous study in a sample of African American older adults (Moored et al., 2018), generalize to other older populations. We hypothesized that more EELA would be associated with larger hippocampal and amygdala volume in our sample of older adults. We significantly extended these prior analyses through a comprehensive assessment of both sub-cortical (hippocampus and amygdala) and cortical (whole brain approach) structure and function, considering sex differences in each analysis.
2. Methods
2.1. Participants and study design
Participants in the study are a sub-sample of low-active (< 3 days of physical activity per week) but healthy older adults aged between 60–80 years who participated in a randomized controlled trial of exercise (NCT01472744). All outcome data are taken from baseline pre-intervention measurements. All participants provided informed consent and the University of Illinois Institutional Review Board approved all procedures used in the study. Inclusion criteria for the original study consisted of 1) >75% right- handed on the Edinburgh Handedness Questionnaire; (2) normal or corrected-to-normal vision of at least 20/40; (3) no color- blindness; (4) no history of stroke, transient ischemic attack, or head trauma; (5) >23 score on Mini-Mental State Examination (MMSE); (6) >21 score on Telephone Interview of Cognitive Status (TICS); (7) <10 score on Geriatric Depression Scale (GDS; (8) no contraindications to MRI. 202 of the original study participants (out of 247) who agreed to be recontacted were sent a letter gauging their interest in completing a short questionnaire about their early life experiences. Those interested were mailed a short, 10-item paper and pencil questionnaire (supplementary table 1), which were completed and returned via US postal service. Participants read and signed a new informed consent document pertaining to these new measures. Full demographic information about the included participants (N=88) are detailed in Table 1.
Table 1.
Participant Characteristics
| Overall | |
|---|---|
| N | 88 |
| Current age (mean (SD) | 65.81 (4.72) |
| Sex: Female (%) | 63 (71.6) |
| Race (%) | |
| Caucasian | 79 (89.7) |
| African American | 7 (8) |
| Asian/Pacific Island | 2 (2.3) |
| Years of education (mean (SD) | 15.89 (2.66) |
| Mother’s SES (mean (SD) | 5.93 (2.01) |
| Mini mental status exam (mean (SD) | 28.65 (1.36) |
| EELA composite score (mean (SD) | 4.16 (1.78) |
| EELA specific | |
| Musical instrument (%) | 58 (65.9) |
| Extracurricular lessons (%) | 62 (70.5) |
| Team sports (%) | 30 (34.1) |
| Foreign language (%) | 20 (22.7) |
| Volunteer at place of worship (%) | 72 (82.8) |
| Scouts (%) | 65 (73.9) |
| Family vacations (%) | 59 (67.0) |
| Current lifestyle activities (mean (SD) | 16.25 (3.58) |
2.2. Enriching early life activities
EELA were assessed through a retrospective questionnaire. The questionnaire asked for a “yes” or “no” answer to whether they participated in the following seven activities before the age of 13 years: Did you play a musical instrument? Did you take art, dance or musical lessons? Did you play team sports? Did you study a foreign language? Did you volunteer at a place of worship? Did you participate in scouting? Did you take family vacations? The main measure was comprised of the sum total of all “yes” answers with a resultant score ranging from 0-to-10, where a higher score meant more engagement in early life activities (Chan et al., 2019; Moored et al., 2018).
2.3. Covariates
Covariates included current age (in years), biological sex, the subjective socioeconomic status of the participants’ mother, education in number of years and engagement in current lifestyle activities. The mothers’ subjective socioeconomic status was measured using a ladder ranging from 1-to-10 asking “what did you consider the socioeconomic status of your mother?”, with 1 being ‘worst off’ and 10 being ‘best off’. This was measured in accordance with previous publications (Chan et al., 2019). Current engagement in lifestyle activities was measured as the number of later life activities via a 23-item questionnaire that probed participants engagement in a variety of activities ranging from volunteer work, gardening and cooking to crosswords, drawing and socialization. A detailed description of the questionnaire development can be found in a previous publication ((Carlson et al., 2012). We used the sum of “yes” answers as our covariate.
2.4. MRI acquisition
Participants undertook an MRI scanning session in a 3 Tesla Siemens Trio Tim system with a 12-channel head coil. High-resolution structural MRI scans were acquired using 3D MPRAGE T1-wighted sequences (TR = 1900 ms; TE = 2.32 ms; TI: 900 ms; flip angle = 9°; matrix = 256 × 256; FOV = 230 mm; 192 slices; resolution = 0.9 × 0.9 × 0.9 mm; GRAPPA acceleration factor 2). T2*-weighted resting state echoplanar imaging (EPI) data was obtained with the following parameters: (6min,TR=2s,TE=25ms,flipangle=80°, 3.4 × 3.4 mm2 in-plane resolution, 35 4 mm-thick slices acquired in ascending order, Grappa acceleration factor = 2, 64 × 64 matrix).
2.5. Preprocessing of structural MRI
Cortical reconstruction and Image segmentation and estimation of the subcortical volumes was performed using the freely available FreeSurfer software v.5.3 (http://surfer-nmr.mgh.harvard.edu/). A detailed description of the volume-based stream can be found in Fischl et al., 2002. Briefly, the automated pipeline removes nonbrain tissue (skullstripping), subcortical segmentation and estimation of total intracranial volume, whole brain volume and subcortical brain region volumes. Via this volume-based (subcortical) pipeline, FreeSurfer automatically labels subcortical structures via a final segmentation based on a subject-independent probabilistic atlas and subject-specific measured values (Fischl et al., 2002). From this we obtained the volumes of the left and right amygdala and hippocampus. Volumes are reported in mm3 and intracranial volume is included as a covariate in all sub-cortical structural analyses. For preprocessing of the cortex a 3-dimensional surface model was created using the “recon-all” surface-based stream. Automated Talairach transformation and intensity normalization were followed by nonbrain tissue removal, tessellation of the gray and white matter boundary and automated topology correction. Finally, surface deformation enabled the detection of tissue boundaries; grey–white and grey–CSF borders. The cortical surfaces were then inflated and registered to a spherical atlas that used individual cortical folding patterns to match cortical geometry across participants.
2.6. Volumetric and cortical thickness statistical analyses
To assess the independent associations between EELA and hippocampal and amygdala volumes multiple linear regression controlling for later life activities, age, biological sex, education and Mother’s SES, as well as estimated total intracranial volume (to account for individual differences in head size), were performed in R Version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). Model assumptions were checked using Q-Q and fitted vs residual plots and the normality of the residuals was formally checked using Shapiro-Wilk tests of normality. The significant influence of outliers was checked using Cooke’s distance with a cut off of 0.5 (no outliers removed). We present standardized beta coefficients (β) as the strength of the relationship between X and Y (that is, for every 1 unit (1 activity) increase in EELA there is a × standard deviation increase in our outcome). Model fitness is presented as adjusted R2 values and significance is considered at the p< .05 level. To assess differential effect in males and females associated with a unit increase in EELA (controlling for all covariates) we included an EELA by sex interaction term into the models.
To assess the effects of EELA on brain structure in cortical regions, cortical thickness was calculated at each vertex in the cortex as a measure of the distance between the white and pial surfaces. A Gaussian smoothing kernel of 10 mm full-width at half-maximum was applied. Vertex-wise General Linear Models (GLMs) were run for each hemisphere with cortical thickness as the dependant variable and EELA as the independent variable, controlling for all covariates. All results were corrected for multiple comparisons using a precached Monte-Carlo null-Z Simulation (10,000 repetitions) with a cluster-wise corrected p-value (CWP) of <0.05 for statistical significance. To assess differential effect in males and females associated with a unit increase in EELA (controlling for all covariates) we ran separate model contrasts of an interaction between EELA and sex.
2.7. Preprocessing of functional MRI
Preprocessing of the functional resting state data was performed using the CONN-toolbox v.19c (Whitfield-Gabrieli & Nieto-Castanon, 2012a), relying upon SPM v.12 (Wellcome Department of Imaging Neuroscience, UCL, London, UK) in MATLAB R2019a (The MathWorks Inc, Natick, MA, USA). The default preprocessing pipeline implemented in Conn was performed which consists of the following steps: functional realignment and unwarping, slice timing correction, outlier identification, segmentation (into grey matter, white matter and cerebrospinal fluid tissue) and normalization into standard Montreal Neurologic Institute (MNI) space with 2mm isotropic voxels for functional data and 1mm for anatomical data, using 4th order spline interpolation. Finally, functional scans were spatially smoothed using a 6mm Gaussian kernel. During the outlier detection step, acquisitions with framewise displacement above 0.9mm or global BOLD signal changes above 5 standard deviations were flagged as potential outliers using the Artefact Detection Tools (www.nitrc.org/projects/artifact_detect). We repeated our preprocessing with a more conservative 0.5mm scan-to-scan framewise displacement to ensure our results effectivity replicate (supplementary material). Two participants were removed from the final FC analyses for having >40 scans flagged. This cut off was determined based on preserving at least 5 minutes of scanning time (Van Dijk et al., 2009). Additionally, mean framewise displacement was calculated via the Jenkinson method (Jenkinson et al., 2002) and used as a covariate of no interest in all second level analyses. This was done to be over conservative given previous studies have shown high degree of motion-behavior correlations (Siegel et al., 2017), despite the fact that no motion variable was significantly correlated with EELA in our study (all p > 0.1). Denoising of the functional data was performed using a component-based correction method, CompCor (Behzadi et al., 2007) and temporal band-pass filtering (0.01–0.1Hz) to remove physiological, subject-motion and outlier artefacts. Linear regression was used to remove the effects of these artifacts on the BOLD time series for each voxel and each subject taking into account noise components from cerebral white matter and cerebrospinal areas, estimated subject-motion parameters (3 rotation and 3 translation parameters and 6 other parameters representing their first order time derivatives), scrubbing and constant and first-order linear session effects. Quality assurance plots of the preprocessing steps are illustrated in supplementary material (figures S1 to S4).
2.8. Seed-based correlations
The average time series from the left and the right amygdala and hippocampus (defined using the anatomical Harvard-Oxford atlas) were extracted. Then, Pearson’s correlation coefficients were computed between the time series in each ROI and the time series of all other voxels in the brain and converted to normally distributed z-scores using Fisher transformation prior to performing the second-level general linear model. Effect of EELA was entered as a covariate of interest in the second-level F-test (any effect of left or right structure) controlling for nuisance variables, age, sex, education, Mother’s SES, later life activities and mean framewise displacement, in separate general linear models for each structure. Result from these seed-based correlations are interpreted as an increase in Fischer transformed correlations associated with a 1 unit (activity) increase in EELA. A height-level statistical threshold of p<0.001, cluster threshold of p<0.05 family-wise error (FWE)-corrected, and k > 50 were used to determine significant clusters. An EELA by sex interaction contrast was also performed to assess the differential effect in males and females.
2.9. Multivoxel pattern analysis.
Multivoxel pattern analysis (MVPA) is data-driven connectivity approach to conduct whole-brain voxel-wise resting state functional connectivity analysis. MVPA was performed as implemented in the Conn toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012), per a number of previous publications (Anteraper et al., 2020; Arnold Anteraper et al., 2019; Guell et al., 2020; Muehlhan et al., 2020; Takamiya et al., 2020; Whitfield-Gabrieli et al., 2016). A detailed description of the methodology is provided in a previous publication (Arnold Anteraper et al., 2019). Our choice to implement MVPA was informed by the value of MVPA to detect discrete individual differences in distributed patterns of neural activation in older adults (Carp et al., 2011). MVPA creates pairwise connectivity patterns for each voxel and all other voxels in the brain resulting in multiple multivariate correlation spatial maps via principal component analysis (PCA). These spatial maps are used as a low-dimensional proxy for the entire pattern of connectivity between each voxel and the rest of the brain (Whitfield-Gabrieli et al., 2016). In this case, MVPA was done with 64 PCA components (representing the number of participant specific PCAs retained to characterize each participants’ voxel-to-voxel correlation structure) and 8 factors. This decision was based on a trade-off between the subject-to-component ratio (10:1) and the individual and cumulative explained variance for each N component (see supplementary material, figure 5). To ensure our results were not a function of the subject-to-component ratio we repeated our analysis over multiple MVPA factor numbers (supplementary figure 7). Second level MVPA analyses yield multivariate patterns of voxel clusters showing connectivity changes associated with EELA. A height-level statistical threshold of p<0.001, cluster threshold of p<0.05 family-wise error (FWE)-corrected, and k > 50 were used to determine significant clusters. Because MVPA is an omnibus test (Arnold Anteraper et al., 2019), post-hoc analyses were conducted to further investigate the connectivity patterns of the brain regions associated with EELA, identified by MVPA. Here, the MVPA clusters were taken as seeds in a seed-to-voxel analysis (voxel p<0.001 and FWE cluster-level p<0.05 correction, k > 50). Pearson’s correlation coefficients were computed for the MVPA time series and the time series of all other voxels in the brain and were converted to normally distributed z-scores using Fisher transformation prior to performing the second-level general linear model. For all functional connectivity analyses, current age, sex, education, Mother’s SES, mean framewise displacement and variety of later life lifestyle activities were included as covariates.
3. Results
3.1. Participant characteristics
Table 1 presents the participant characteristics. Our sample was majority female. At the group level participants engaged in multiple early life activities, where a majority of participants learned to play a musical instrument, took extracurricular lessons, volunteered at a place of worship, engaged in the scouts and went on family vacations. Regarding the subjective socioeconomic status of the mother, the group-level mean score lay around halfway between “worst off” to “best off”.
3.2. EELA and hippocampal and amygdala volume
Mean hippocampal volume for males was 8,434 mm3 ± 837sd and 8,153 mm3 ± 751sd in females. For amygdala volume, the mean in males was 3,133 mm3 ± 323sd and 2,856 mm3 ± 311 in females.
No significant relationship was found between EELA and hippocampal volume (β = −.02, SE = 4.77, p = .853, R2 = .154), either when controlling for or not later life experiences, total intracranial volume, age and sex. When we added a sex by EELA interaction term into the model, no significant interaction between males and females in the relationship between EELA and hippocampal volume was found (β = .045, SE = 2.101, p = .826, R2 = .108). Similarly, for amygdala volume, no significant effect of EELA (β = −.03, SE = 1.001, p = .843, R2 = .143) nor an EELA by sex interaction was found (β = .075, SE = 4.428, p = <.001, R2 = .097).
3.3. EELA and cortical thickness
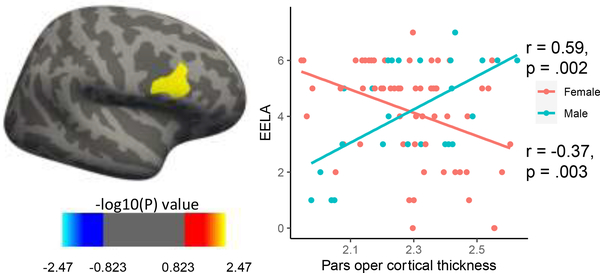
EELA was not associated with cortical thickness when controlling for or not controlling for all covariates. There was a significant cluster in the right pars opercular region of the frontal gyrus that showed differential effects in males and females associated with a unit increase in EELA, controlling for all covariates (cluster-wise corrected p<0.05 and vertex-wise threshold p<0.01) (Figure 1).
Figure 1.
Vertex-wise general linear model with an EELA by sex interaction, controlling for all covariates revealed a significant cluster in the right pars opercularis differentially associated with EELA in males comparted to females.
3.4. EELA and amygdala and hippocampal functional connectivity
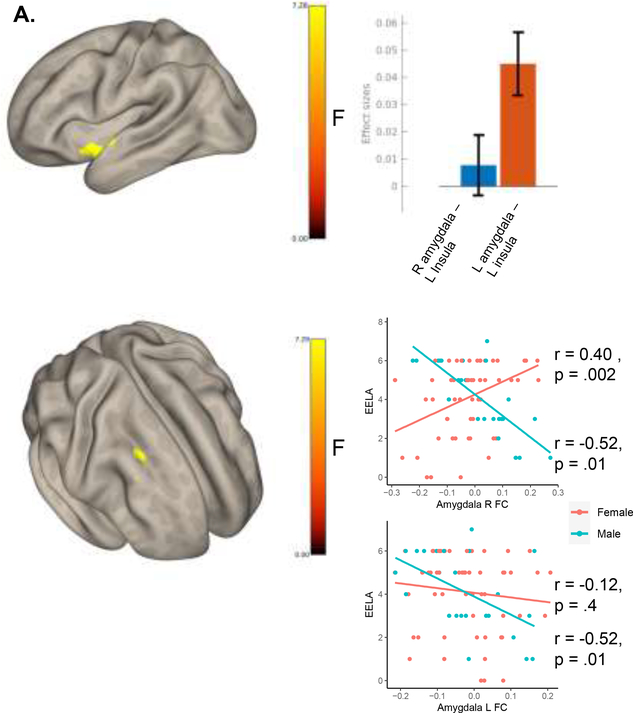
Seed-based correlations between the left or right hippocampus and the rest of the brain did not reveal any significant clusters significantly associated with EELA. Conversely, EELA was significantly associated with FC between bilateral amygdala and the left insular cortex (F(2,142) = 7.25, pfwe = <0.001, k = 225, peak MNI = −36 +00 −12; Figure 2A). A cluster in the homologous right insula cortex was also apparent at a less stringent cluster-threshold (supplementary material figure 6). Further, a significant cluster in the middle frontal gyrus showed differential connectivity with the amygdala in males compared to females associated with EELA (Figure 2B).
Figure 2.
A. Seed-based correlations revealed connectivity between the amygdala and the left insula associated with EELA (voxel p<0.001 and FEW cluster-level p<0.05 correction). This connectivity was strongest between the left amygdala and the left insula. At a less stringent threshold (voxel p <0.005) connectivity with bilateral insula was associated with EELA (supplementary figure 6). B. Results from a seed-based correlation analysis (seeding the left and right amygdala in an F-test) looking at the expected differential effect in males and females associated with a unit increase in EELA (controlling for all covariates). A Significant cluster (voxel p<0.001 and FDR cluster-level p<0.05 correction) in the right middle frontal gyrus (MNI: +30 +20 +38, k voxels = 92) showed differential connectivity with the left and right amygdala in males compared to females.
3.5. Multivoxel pattern analysis
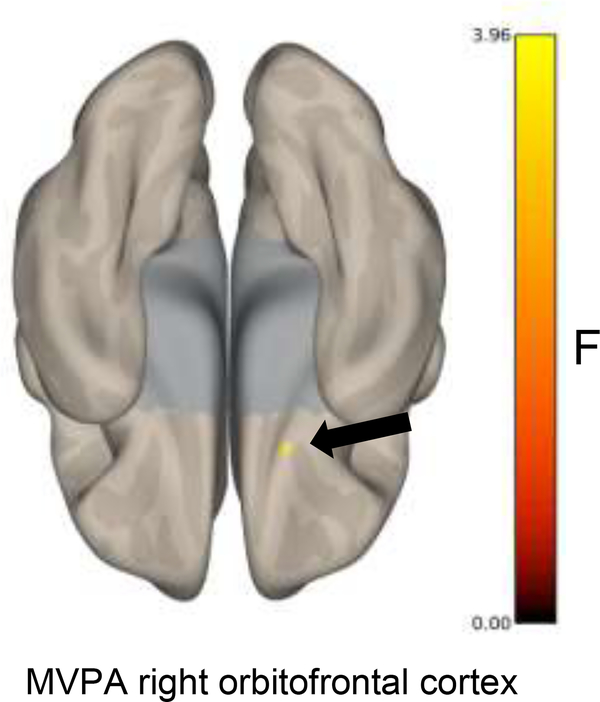
Our MPVA analysis (Figure 3) revealed significant differences in functional connectivity with one cluster in the right orbitofrontal cortex (peak MNI coordinates = +18 +12 −28, k = 64, F max = 4.64) associated with EELA (voxel p<0.001 and FWE cluster-level p<0.05 correction).
Figure 3.
Multivoxel pattern analysis revealed variations in functional connectivity with the orbitofrontal cortex associated with EELA (voxel p<0.001 and FWE cluster-level p<0.05 correction).
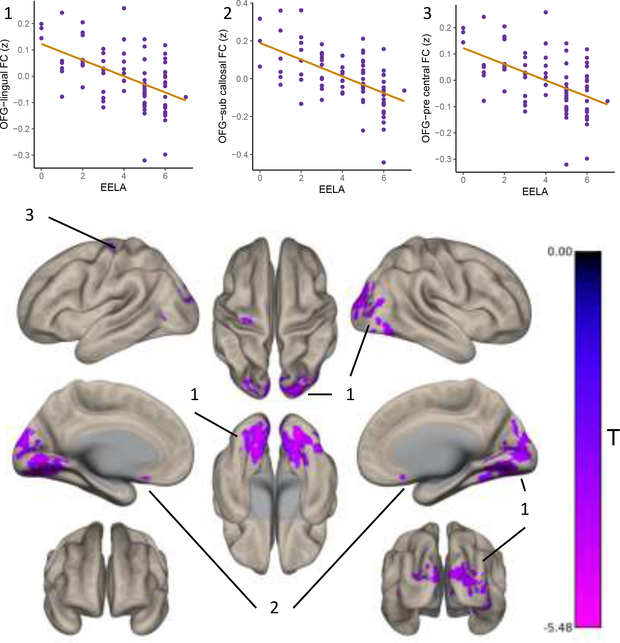
Post hoc characterization was performed by taking the time series from the MVPA cluster as an ROI in a seed-to-voxel analysis. The right orbitofrontal seed was anticorrelated with a cluster spanning occipital and lingual regions, a cluster in the subcallosal cortex and a cluster in the left precentral gyrus, as a function of EELA (voxel p<0.001 and FWE cluster-level p<0.05 correction; Figure 4). Table 2 outlines the overlap of the number of voxels per cluster that fall within canonical functional networks. A significant cluster in the brain stem showed differential connectivity with the orbitofrontal seed in males compared to females associated with a unit increase in EELA (peak MNI coordinates = +02 −36 −42, k = 116, F max = 3.44) where in males this relationship was negative and females positive.
Figure 4.
Post hoc seed-to-voxel analysis taking the orbitofrontal MVPA cluster as a seed, revealed that the variations in functional connectivity and EELA revealed by MVPA are driven by anticorrelations between the orbitofrontal cortex and distal visual and somatosensory regions as well as with a cluster in the subcallosal cortex (voxel p<0.001 and FEW cluster-level p<0.05 correction).
Table 2.
Overlap of MVPA posthoc results with functional networks
| Voxels per cluster (k) per 7 networks | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak coordinates (MNI) | Peak brain region | Visual | Somatomotor | DAN | VAN | Limbic | FPCN | DMN | Total K |
| OFG mvpa cluster | |||||||||
| +28 −90 +14 | Bilateral lingual/occ | 3834 | 60 | 55 | 53 | 4817 | |||
| −02 +08 −10 | Subcallosal | 53 | 110 | ||||||
| −30 −20 +68 | L precentral | 60 | 70 | ||||||
L = left, occ = occipital. Note, a number of gray matter voxels per cluster lacked an a priori network label.
4. Discussion
In this study we did not find that more variety of EELA before age 13 was associated with larger hippocampal or amygdala volumes later in life (60–80 years). Conversely, more variety of EELA was associated with differences in the functional connectivity between the amygdala and the insula. We further demonstrated, using a whole brain data driven approach, that more variety of EELA was associated with variations in functional connectivity patterns of the orbitofrontal cortex. Post hoc analysis with this MVPA cluster showed that the variations in connectivity associated with EELA were being driven by functional connectivity with somatosensory regions. Importantly, sex-specific differences in the association between EELA and brain structure and function were seen.
A major finding in our study was the result that we did not replicate previous research (Moored et al., 2018), using an identical questionnaire, of an association between EELA and amygdala and hippocampal volumes. The discrepancies between our study and Moored et al., may lie with differences in the sample population, where our study consisted of mostly White healthy older adults, and the Moored et al., study consisted of predominantly African American or Black participants who were deemed to be at greater risk for cognitive decline (Mayeda et al., 2016). The discrepancies may be driven by differences in the mean number or distribution of EELA in each sample (Moored sample = 3.02, our sample = 4.12), as well as the frequency of EELA (something neither study assessed and a major limitation). Additionally, developmental differences between samples may have driven the discrepancies, where our sample had larger mean amygdala volumes (Moored male = 2,907 mm3, our sample male = 3,133 mm3; Moored female = 2,884 mm3, our sample female = 2,856 mm3) and hippocampal volumes (Moored male = 6,721mm3, our sample male = 8,434mm3; Moored female = 6,770mm3, our sample female = 8,153 mm3). These differences in EELA engagement and/or developmental differences may be reflective of disparities and differences in health and health behaviors, early socioeconomic and parental environments, and other contributors to reserve across the lifespan, such as opportunities for educational attainment and occupational complexity, which differ between racial and ethnic groups (Glymour & Manly, 2008; Pavalko & Caputo, 2013; Zhang et al., 2016).
We did not find any associations between EELA and cortical thickness at the whole group level controlling for sex. Yet we did find differential effects of EELA on cortical thickness in males compared to females in the pars opercularis, a node of the ventral attention network (Bernard et al., 2020), involved in response inhibition (Aron et al., 2014), and a region which has previously shown sex-specific differences in age-related pathology (Malpetti et al., 2017; Perneczky et al., 2007). While sex disparities in reserve contributing factors such as educational and occupational opportunities have improved over the last few decades, reports of differential effects of reserve contributors between men and women exist (Kok et al., 2018; Koran et al., 2017; Malpetti et al., 2017; Subramaniapillai et al., 2021). Further, gray and white matter volume in the inferior frontal gyrus has previously been associated with lifelong cognitive activity (education and occupational complexity) (Arenaza-Urquijo et al., 2013; Foubert-Samier et al., 2012). Consequently, our results extend previous research on enriching experiences and frontal lobe structural associations by showing a sex-specific relationship between a variety of EELA and later life brain structure in this region. Indeed, our results of functional connectivity also found sex-specific results, where differential functional connectivity between the amygdala and the right superior frontal gyrus and between the orbitofrontal gyrus and the brainstem was seen between males and females, further supporting sex-specific contributions of EELA to potential reserve mechanisms later in life.
While we did not see associations between EELA and hippocampal and amygdala volumes, we did find differences in the functional connectivity of the amygdala associated with EELA. The insula is implicated in wide-ranging functions from sensorimotor integration and olfacto-gustatory to socio-emotional and cognitive (Kurth et al., 2010). Connectivity between the amygdala and the insula has been consistently associated with emotion regulation (Jenkins et al., 2017; Ramasubbu et al., 2014; J. L. Stein et al., 2007; M. B. Stein et al., 2007; Tahmasian et al., 2013). Experience dependent plasticity of brain networks related to early enriched environments may be highly important in the development of complex behavioral and affective functioning across the lifespan (Richards & Deary, 2005). Consequently, our result is one potential mechanistic correlate of how EELA can contribute to mechanisms of cognitive reserve later in life.
At the whole brain functional connectivity level, our data-driven MVPA analysis showed one cluster in the orbitofrontal cortex whose connectivity patterns covaried with EELA. The orbitofrontal cortex is part of the ventral portion of the prefrontal cortex and its functions extend to motivational, emotional and social behaviors (Rolls, 2004). In adults, the orbitofrontal cortex is sensitive to experience dependent plasticity where cognitive training can increase orbitofrontal cortical thickness (Engvig et al., 2010). Our post hoc seed-to-voxel analysis revealed that the variations in orbitofrontal cortex connectivity patterns associated with EELA were being driven by functional connectivity with primary visual and somatosensory regions as well as a cluster in the limbic network (subcallosal). Previous studies have demonstrated consistent associations between long term (>6 weeks) activity-driven (motor training, musical training) changes in somatosensory and visual networks (Lee & Noppeney, 2011; Taubert et al., 2011). The connectivity between orbitofrontal cortex and visual/somatosensory areas associated with EELA were anticorrelated, where higher EELA was associated with higher anticorrelations between these regions. The EELA inventory assessed early activities that are highly somatosensory and visual in nature (musical instrument, sport, dance) and so the anticorrelations between the orbitofrontal cortex and visual regions are perhaps reflective of efficient multisensory processing in these networks (James et al., 2014; Rolls, 2004).
Beyond genetics, early social and maternal environments, education attainment, socioeconomic status and degree of lifestyle activities determine brain structure and function (Richards & Deary, 2005). It is possible therefore that greater engagement in EELA during a critical period of brain development (before 13 years) may lead to enhanced mechanisms of plasticity relevant for cognitive and brain reserve later in life. Controlling for sex in our study, EELA appears to be related to potential mechanisms of cognitive reserve (underlying functional brain processes that actively adapt to brain changes with age (Stern et al., 2020)) rather than brain reserve (passive concept related to momentary structural characteristics that buffer against brain aging (Stern et al., 2020)). Whether EELA acts upon mechanisms of plasticity during development that have durable lifespan effects or whether EELA promotes engagement in similar lifestyle activities across the lifespan, leading to a consistent upkeep of mechanisms of cognitive reserve (Richards & Deary, 2005; Schreiber et al., 2016), or a combination of both is unclear. Yet in our study we did not find a correlation between EELA and later life activity engagement and our results were not attenuated when controlling for later life activities, perhaps suggesting the former.
A number of limitations must be taken into account when interpreting our results. The questionnaire we deployed to measure EELA, while identical to that of previous studies (Chan et al., 2019; Moored et al., 2018) relied upon retrospective recall. Studies have shown though that free from emotion and retrospective impact bias, such questionnaires report low recall bias through asking simply whether one engaged or not in these actives, and has been shown to be very accurate, even after 50+ years (Berney & Blane, 1997). Additionally, a sample selection bias exists in our study as our included participants were initially recruited as part of a randomized control trial of exercise for cognitive and brain health, whose inclusion criteria required them to be sedentary (spent less than 3 days per week performing any time of physical activity). Whilst a limitation, this characterization may be reflective of the national US population at large (over a 3rd (34.8%) lead sedentary lifestyle (Du et al., 2019). Further, both MVPA clusters were relatively small in size, possibly reflective of our primary cluster threshold of p < 0.001. Nevertheless, this is in accordance with current recommendations on cluster-based thresholding in fMRI analyses (Woo et al., 2014). In addition, our resting state analyses were based on 6 minutes of scan time. While this has been suggested to be the minimum scan time necessary for resting state analyses (Van Dijk et al., 2009), it may be considered a short per more recent MRI protocols. Furthermore, we replicated our orbitofrontal finding when using different MVPA parameters (8 over 4 factors) which suggests our finding is not a function of this intermediate analysis choice. Further, our structural analysis looked at the gross hippocampal and amygdala regions as a whole. Future studies may find more sensitivity in analyzing the sub-regions of these two structures (Zheng et al., 2019). Lastly, our results are taken from a cross-sectional analysis and so we were unable to assess the longitudinal changes in functional connectivity and brain structure with advancing age. This also limits our interpretations of causal associations between EELA and later life functional connectivity. Whilst we did control for current engagement in a variety of lifestyle activities, future studies on longitudinal changes associated with EELA will gain stronger insights into how such modifiable lifestyle behaviors affect brain health with advancing age.
5. Conclusions
Together our results demonstrate that EELA before age 13 are associated more with potential mechanisms of cognitive reserve (function) than brain reserve (structure), decades later (60–80 years). However, potential sex-specific mechanisms of brain reserve were seen. Whilst longitudinal studies are required to fully assess the effect of enriching and modifiable lifestyle behaviors on brain health across the lifespan and with advancing age, our results suggest that strategies to increase the diversity of childhood activities will have long lasting effects on older adult’s brain health.
Supplementary Material
Highlights.
EELA is associated with amygdala function but not structure.
EELA affords primarily active (function) contributions to reserve later in life
Sex-specific differences in the EELA-structure/function relationships exist
Acknowledgments
We would like to thank Anya Knecht, Susan Houseworth, Nancy Dodge, Hilly Tracy, Robert Weisshappel and all of the Lifelong Brain and Cognition and Exercise Psychology Laboratory graduate students and staff for their help in participant recruitment and data collection.
Funding and conflict of interest statement
This work was supported by the National Institute on Aging at the National Institutes of Health (R37 AG025667). No author declares any potential or actual conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement
All data and code used in this study will be provided upon request to the corresponding author.
References
- Anteraper SA, Collin G, Guell X, Scheinert T, Molokotos E, Henriksen MT, Mesholam-Gately R, Thermenos HW, Seidman LJ, Keshavan MS, Gabrieli JDE, & Whitfield-Gabrieli S (2020). Altered resting-state functional connectivity in young children at familial high risk for psychotic illness: A preliminary study. Schizophrenia Research, 216, 496–503. 10.1016/j.schres.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mézenge F, Perrotin A, Desgranges B, Bartrés-Faz D, Eustache F, & Chételat G (2013). Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. NeuroImage, 83, 450–457. 10.1016/j.neuroimage.2013.06.053 [DOI] [PubMed] [Google Scholar]
- Arnold Anteraper S, Guell X, D’Mello A, Joshi N, Whitfield-Gabrieli S, & Joshi G (2019). Disrupted Cerebrocerebellar Intrinsic Functional Connectivity in Young Adults with High-Functioning Autism Spectrum Disorder: A Data-Driven, Whole-Brain, High-Temporal Resolution Functional Magnetic Resonance Imaging Study. Brain Connectivity, 9(1), 48–59. 10.1089/brain.2018.0581 [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, & Poldrack RA (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences, 18(4), 177–185. 10.1016/j.tics.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Bak TH, Nissan JJ, Allerhand MM, & Deary IJ (2014). Does bilingualism influence cognitive aging? Annals of Neurology, 75(6), 959–963. 10.1002/ana.24158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Sale A, & Maffei L (2010). Nurturing brain plasticity: Impact of environmental enrichment. Cell Death & Differentiation, 17(7), 1092–1103. 10.1038/cdd.2009.193 [DOI] [PubMed] [Google Scholar]
- Barulli D, & Stern Y (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17(10), 502–509. 10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berken JA, Gracco VL, & Klein D (2017). Early bilingualism, language attainment, and brain development. Neuropsychologia, 98, 220–227. 10.1016/j.neuropsychologia.2016.08.031 [DOI] [PubMed] [Google Scholar]
- Bernard F, Lemee J-M, Mazerand E, Leiber L-M, Menei P, & Minassian AT (2020). The ventral attention network: The mirror of the language network in the right brain hemisphere. Journal of Anatomy, 237(4), 632–642. 10.1111/joa.13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney LR, & Blane DB (1997). Collecting retrospective data: Accuracy of recall after 50 years judged against historical records. Social Science & Medicine, 45(10), 1519–1525. 10.1016/S0277-9536(97)00088-9 [DOI] [PubMed] [Google Scholar]
- Calabria M, Hernández M, Cattaneo G, Suades A, Serra M, Juncadella M, Reñé R, Sala I, Lleó A, Ortiz-Gil J, Ugas L, Ávila A, Ruiz IG, Ávila C, & Costa A (2020). Active bilingualism delays the onset of mild cognitive impairment. Neuropsychologia, 146, 107528. 10.1016/j.neuropsychologia.2020.107528 [DOI] [PubMed] [Google Scholar]
- Carlson MC, Parisi JM, Xia J, Xue Q-L, Rebok GW, Bandeen-Roche K, & Fried LP (2012). Lifestyle activities and memory: Variety may be the spice of life. The women’s health and aging study II. Journal of the International Neuropsychological Society: JINS, 18(2), 286–294. 10.1017/S135561771100169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Park J, Polk TA, & Park DC (2011). Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. NeuroImage, 56(2), 736–743. 10.1016/j.neuroimage.2010.04.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T, Parisi JM, Moored KD, & Carlson MC (2019). Variety of Enriching Early-Life Activities Linked to Late-Life Cognitive Functioning in Urban Community-Dwelling African Americans. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 74(8), 1345–1356. 10.1093/geronb/gby056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, Eiriksdottir G, Jonsdottir MK, Lopez OL, Harris TB, Gudnason V, & Launer LJ (2010). The Effect of Midlife Physical Activity on Cognitive Function Among Older Adults: AGES—Reykjavik Study. The Journals of Gerontology: Series A, 65A(12), 1369–1374. 10.1093/gerona/glq152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemenson GD, Deng W, & Gage FH (2015). Environmental enrichment and neurogenesis: From mice to humans. Current Opinion in Behavioral Sciences, 4, 56–62. 10.1016/j.cobeha.2015.02.005 [DOI] [Google Scholar]
- Donnelly JE, Greene JL, Gibson CA, Smith BK, Washburn RA, Sullivan DK, DuBose K, Mayo MS, Schmelzle KH, Ryan JJ, Jacobsen DJ, & Williams SL (2009). Physical Activity Across the Curriculum (PAAC): A randomized controlled trial to promote physical activity and diminish overweight and obesity in elementary school children. Preventive Medicine, 49(4), 336–341. 10.1016/j.ypmed.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Liu B, Sun Y, Snetselaar LG, Wallace RB, & Bao W (2019). Trends in Adherence to the Physical Activity Guidelines for Americans for Aerobic Activity and Time Spent on Sedentary Behavior Among US Adults, 2007 to 2016. JAMA Network Open, 2(7), e197597–e197597. 10.1001/jamanetworkopen.2019.7597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, & Walhovd KB (2010). Effects of memory training on cortical thickness in the elderly. NeuroImage, 52(4), 1667–1676. 10.1016/j.neuroimage.2010.05.041 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE, & Committee*, F. 2018 P. A. G. A. (2019). Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Medicine & Science in Sports & Exercise, 51(6), 1242–1251. 10.1249/MSS.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, & Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, & Dale AM (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- Foubert-Samier A, Catheline G, Amieva H, Dilharreguy B, Helmer C, Allard M, & Dartigues J-F (2012). Education, occupation, leisure activities, and brain reserve: A population-based study. Neurobiology of Aging, 33(2), 423.e15–423.e25. 10.1016/j.neurobiolaging.2010.09.023 [DOI] [PubMed] [Google Scholar]
- Gartner H, Minnerop M, Pieperhoff P, Schleicher A, Zilles K, Altenmüller E, & Amunts K (2013). Brain morphometry shows effects of long-term musical practice in middle-aged keyboard players. Frontiers in Psychology, 4. 10.3389/fpsyg.2013.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, & Manly JJ (2008). Lifecourse Social Conditions and Racial and Ethnic Patterns of Cognitive Aging. Neuropsychology Review, 18(3), 223–254. 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Guell X, Arnold Anteraper S, Gardner AJ, Whitfield-Gabrieli S, Kay-Lambkin F, Iverson GL, Gabrieli J, & Stanwell P (2020). Functional Connectivity Changes in Retired Rugby League Players: A Data-Driven Functional Magnetic Resonance Imaging Study. Journal of Neurotrauma, 37(16), 1788–1796. 10.1089/neu.2019.6782 [DOI] [PubMed] [Google Scholar]
- Habib M, & Besson M (2009). What do Music Training and Musical Experience Teach Us About Brain Plasticity? Music Perception, 26(3), 279–285. 10.1525/mp.2009.26.3.279 [DOI] [Google Scholar]
- Hensch TK (2005). Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience, 6(11), 877–888. 10.1038/nrn1787 [DOI] [PubMed] [Google Scholar]
- Hillman CH, Kamijo K, & Scudder M (2011). A review of chronic and acute physical activity participation on neuroelectric measures of brain health and cognition during childhood. Preventive Medicine, 52, S21–S28. 10.1016/j.ypmed.2011.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K, Laws KR, Gale TM, & Kondel TK (2012). Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. Journal of Clinical and Experimental Neuropsychology, 34(9), 989–998. 10.1080/13803395.2012.712676 [DOI] [PubMed] [Google Scholar]
- James CE, Oechslin MS, Ville DVD, Hauert C-A, Descloux C, & Lazeyras F (2014). Musical training intensity yields opposite effects on grey matter density in cognitive versus sensorimotor networks. Brain Structure and Function, 219(1), 353–366. 10.1007/s00429-013-0504-z [DOI] [PubMed] [Google Scholar]
- Jenkins LM, Stange JP, Barba A, DelDonno SR, Kling LR, Briceño EM, Weisenbach SL, Phan KL, Shankman SA, Welsh RC, & Langenecker SA (2017). Integrated cross-network connectivity of amygdala, insula, and subgenual cingulate associated with facial emotion perception in healthy controls and remitted major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience, 17(6), 1242–1254. 10.3758/s13415-017-0547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, & Duzel E (2016). Neuroplasticity: Effects of Physical and Cognitive activity on brain structure and function. NeuroImage, 131, 1–3. 10.1016/j.neuroimage.2016.03.081 [DOI] [PubMed] [Google Scholar]
- Johnson MH (1990). Cortical Maturation and the Development of Visual Attention in Early Infancy. Journal of Cognitive Neuroscience, 2(2), 81–95. 10.1162/jocn.1990.2.2.81 [DOI] [PubMed] [Google Scholar]
- Kok R, Prinzie P, Bakermans-Kranenburg MJ, Verhulst FC, White T, Tiemeier H, & IJzendoorn M. H. van. (2018). Socialization of prosocial behavior: Gender differences in the mediating role of child brain volume. Child Neuropsychology, 24(6), 723–733. 10.1080/09297049.2017.1338340 [DOI] [PubMed] [Google Scholar]
- Koran MEI, Wagener M, Hohman TJ, & for the Alzheimer’s Neuroimaging Initiative. (2017). Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging and Behavior, 11(1), 205–213. 10.1007/s11682-016-9523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal AA, Kim J, Waite L, & Dale W (2016). Social Function and Cognitive Status: Results from a US Nationally Representative Survey of Older Adults. Journal of General Internal Medicine, 31(8), 854–862. 10.1007/s11606-016-3696-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, & Erickson KI (2007). Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends in Cognitive Sciences, 11(8), 342–348. 10.1016/j.tics.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, & Eickhoff SB (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function, 214(5), 519–534. 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws KR, Irvine K, & Gale TM (2018). Sex differences in Alzheimer’s disease. Current Opinion in Psychiatry, 31(2), 133–139. 10.1097/YCO.0000000000000401 [DOI] [PubMed] [Google Scholar]
- Lee H, & Noppeney U (2011). Long-term music training tunes how the brain temporally binds signals from multiple senses. Proceedings of the National Academy of Sciences, 108(51), E1441–E1450. 10.1073/pnas.1115267108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackes NK, Golm D, Sarkar S, Kumsta R, Rutter M, Fairchild G, Mehta MA, Sonuga-Barke EJS, & Team, on behalf of the E. Y. A. F. (2020). Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proceedings of the National Academy of Sciences, 117(1), 641–649. 10.1073/pnas.1911264116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpetti M, Ballarini T, Presotto L, Garibotto V, Tettamanti M, & Perani D (2017). Gender differences in healthy aging and Alzheimer’s Dementia: A 18F-FDG-PET study of brain and cognitive reserve. Human Brain Mapping, 38(8), 4212–4227. 10.1002/hbm.23659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansens D, Deeg DJH, & Comijs HC (2018). The association between singing and/or playing a musical instrument and cognitive functions in older adults. Aging & Mental Health, 22(8), 970–977. 10.1080/13607863.2017.1328481 [DOI] [PubMed] [Google Scholar]
- Mansky R, Marzel A, Orav EJ, Chocano-Bedoya PO, Grunheid P, Mattle M, Freystatter G, Stahelin HB, Egli A, & Bischoff-Ferrari HA (2020). Playing a musical instrument is associated with slower cognitive decline in community-dwelling older adults. Aging Clinical and Experimental Research. 10.1007/s40520-020-01472-9 [DOI] [PubMed] [Google Scholar]
- May A (2011). Experience-dependent structural plasticity in the adult human brain. Trends in Cognitive Sciences, 15(10), 475–482. 10.1016/j.tics.2011.08.002 [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, & Whitmer RA (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12(3), 216–224. 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moored KD, Chan T, Varma VR, Chuang Y-F, Parisi JM, & Carlson MC (2018). Engagement in Enriching Early Life Activities is Associated with Larger Hippocampal and Amygdala Volumes in Community-Dwelling Older Adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 10.1093/geronb/gby150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris TP, Tormos Muñoz J-M, Cattaneo G, Solana-Sánchez J, Bartrés-Faz D, & Pascual-Leone A (2019). Traumatic Brain Injury Modifies the Relationship Between Physical Activity and Global and Cognitive Health: Results From the Barcelona Brain Health Initiative. Frontiers in Behavioral Neuroscience, 13. 10.3389/fnbeh.2019.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlhan M, Alexander N, Trautmann S, Weckesser LJ, Vogel S, Kirschbaum C, & Miller R (2020). Cortisol secretion predicts functional macro-scale connectivity of the visual cortex: A data-driven Multivoxel Pattern Analysis (MVPA). Psychoneuroendocrinology, 117, 104695. 10.1016/j.psyneuen.2020.104695 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, & Merabet L (2005). The Plastic Human Brain Cortex. Annual Review of Neuroscience, 28(1), 377–401. 10.1146/annurev.neuro.27.070203.144216 [DOI] [PubMed] [Google Scholar]
- Pavalko EK, & Caputo J (2013). Social Inequality and Health Across the Life Course. American Behavioral Scientist, 57(8), 1040–1056. 10.1177/0002764213487344 [DOI] [Google Scholar]
- Perneczky R, Drzezga A, Diehl-Schmid J, Li Y, & Kurz A (2007). Gender differences in brain reserve. Journal of Neurology, 254(10), 1395. 10.1007/s00415-007-0558-z [DOI] [PubMed] [Google Scholar]
- Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola I, & Goodyear B (2014). Reduced Intrinsic Connectivity of Amygdala in Adults with Major Depressive Disorder. Frontiers in Psychiatry, 5. 10.3389/fpsyt.2014.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, & Deary IJ (2005). A life course approach to cognitive reserve: A model for cognitive aging and development? Annals of Neurology, 58(4), 617–622. 10.1002/ana.20637 [DOI] [PubMed] [Google Scholar]
- Rolls ET (2004). The functions of the orbitofrontal cortex. Brain and Cognition, 55(1), 11–29. 10.1016/S0278-2626(03)00277-X [DOI] [PubMed] [Google Scholar]
- Scarmeas N, & Stern Y (2003). Cognitive Reserve and Lifestyle. Journal of Clinical and Experimental Neuropsychology, 25(5), 625–633. 10.1076/jcen.25.5.625.14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Norton A, Overy K, & Winner E (2005). Effects of music training on the child’s brain and cognitive development. Annals of the New York Academy of Sciences, 1060, 219–230. 10.1196/annals.1360.015 [DOI] [PubMed] [Google Scholar]
- Schreiber S, Vogel J, Schwimmer HD, Marks SM, Schreiber F, & Jagust W (2016). Impact of lifestyle dimensions on brain pathology and cognition. Neurobiology of Aging, 40, 164–172. 10.1016/j.neurobiolaging.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinfeld S, Figueroa H, Ortiz-Gil J, & Sanchez-Vives MV (2013). Effects of music learning and piano practice on cognitive function, mood and quality of life in older adults. Frontiers in Psychology, 4. 10.3389/fpsyg.2013.00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Mitra A, Laumann TO, Seitzman BA, Raichle M, Corbetta M, & Snyder AZ (2017). Data Quality Influences Observed Links Between Functional Connectivity and Behavior. Cerebral Cortex (New York, N.Y.: 1991), 27(9), 4492–4502. 10.1093/cercor/bhw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, & Whalley LJ (2012). Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Annals of Neurology, 71(5), 653–660. 10.1002/ana.22631 [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, & Meyer-Lindenberg A (2007). A validated network of effective amygdala connectivity. NeuroImage, 36(3), 736–745. 10.1016/j.neuroimage.2007.03.022 [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, & Paulus MP (2007). Increased Amygdala and Insula Activation During Emotion Processing in Anxiety-Prone Subjects. American Journal of Psychiatry, 164(2), 318–327. 10.1176/ajp.2007.164.2.318 [DOI] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, & Vuoksimaa E (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia, 16(9), 1305–1311. 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniapillai S, Almey A, Natasha Rajah M, & Einstein G (2021). Sex and gender differences in cognitive and brain reserve: Implications for Alzheimer’s disease in women. Frontiers in Neuroendocrinology, 60, 100879. 10.1016/j.yfrne.2020.100879 [DOI] [PubMed] [Google Scholar]
- Tahmasian M, Knight DC, Manoliu A, Schwerthöffer D, Scherr M, Meng C, Shao J, Peters H, Doll A, Khazaie H, Drzezga A, Bäuml J, Zimmer C, Förstl H, Wohlschläger AM, Riedl V, & Sorg C (2013). Aberrant Intrinsic Connectivity of Hippocampus and Amygdala Overlap in the Fronto-Insular and Dorsomedial-Prefrontal Cortex in Major Depressive Disorder. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamiya A, Kishimoto T, Hirano J, Nishikata S, Sawada K, Kurokawa S, Yamagata B, Kikuchi T, & Mimura M (2020). Neuronal network mechanisms associated with depressive symptom improvement following electroconvulsive therapy. Psychological Medicine, 1–8. 10.1017/S0033291720001518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Lohmann G, Margulies DS, Villringer A, & Ragert P (2011). Long-term effects of motor training on resting-state networks and underlying brain structure. NeuroImage, 57(4), 1492–1498. 10.1016/j.neuroimage.2011.05.078 [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, & Buckner RL (2009). Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory, Properties, and Optimization. Journal of Neurophysiology, 103(1), 297–321. 10.1152/jn.00783.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo A, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, & Kramer AF (2010). Plasticity of Brain Networks in a Randomized Intervention Trial of Exercise Training in Older Adults. Frontiers in Aging Neuroscience, 2. 10.3389/fnagi.2010.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli Ghosh S. S., Nieto-Castanon A, Saygin Z, Doehrmann O, Chai XJ, Reynolds GO, Hofmann SG, Pollack MH, & Gabrieli JDE (2016). Brain connectomics predict response to treatment in social anxiety disorder. Molecular Psychiatry, 21(5), 680–685. 10.1038/mp.2015.109 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli, & Nieto-Castanon A (2012a). Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012b). Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hayward MD, & Yu Y-L (2016). Life Course Pathways to Racial Disparities in Cognitive Impairment among Older Americans. Journal of Health and Social Behavior, 57(2), 184–199. 10.1177/0022146516645925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Li C, Zhang D, Cui D, Wang Z, & Qiu J (2019). Study on the sub-regions volume of hippocampus and amygdala in schizophrenia. Quantitative Imaging in Medicine and Surgery, 9(6), 1025–1036. 10.21037/qims.2019.05.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code used in this study will be provided upon request to the corresponding author.