Abstract
RATIONALE:
While respiratory failure is a frequent and clinically significant outcome of COVID-19, cardiac complications are a common feature in hospitalized COVID-19 patients and are associated with worse patient outcomes. The cause of cardiac injury in COVID-19 patients is not yet known. Case reports of COVID-19 autopsy heart samples have demonstrated abnormal inflammatory infiltration of macrophages in heart tissues.
OBJECTIVE:
Generate an immuno-cardiac co-culture platform to model macrophage-mediated hyper-inflammation in COVID-19 hearts and screen for drugs that can block the macrophage-mediated inflammation.
METHODS AND RESULTS:
We systematically compared autopsy samples from non-COVID-19 donors and COVID-19 patients using RNA-seq and immunohistochemistry. We observed strikingly increased expression levels of CCL2 as well as macrophage infiltration in heart tissues of COVID-19 patients. We generated an immuno-cardiac co-culture platform containing human pluripotent stem cell (hPSC)-derived cardiomyocytes (CMs) and macrophages. We found that macrophages induce increased reactive oxygen species (ROS) and apoptosis in CMs by secreting IL-6 and TNF-α after SARS-CoV-2 exposure. Using this immuno-cardiac co-culture platform, we performed a high content screen and identified ranolazine and tofacitinib as compounds that protect CMs from macrophage-induced cardiotoxicity.
CONCLUSION:
We established an immuno-host co-culture system to study macrophage-induced host cell damage following SARS-CoV-2 infection and identified FDA-approved drug candidates that alleviate the macrophage-mediated hyper-inflammation and cellular injury.
GRAPHIC ABSTRACT:
A graphic abstract is available for this article.
Keywords: Human pluripotent stem cells, Cardiomyocyte, SARS-CoV-2, Inflammation, drug screening
Introduction
The current Coronavirus Disease 2019 (COVID-19) pandemic has already impacted millions of people worldwide, causing significant morbidity and mortality. The clinical symptoms of COVID-19 patients range from asymptomatic or a mild upper respiratory tract illness to severe viral pneumonia, cytokine release syndrome, acute respiratory distress syndrome, and death. Increasing amounts of evidence indicate that a hyper-inflammatory response to SARS-CoV-2 is associated with disease severity and death1–4. Patients with severe outcomes usually have elevated serum cytokine and chemokine levels and increased inflammatory markers1. Analyses of lung autopsy samples have reported mononuclear inflammation, infiltrated with lymphocytes and macrophages5–7. Recent studies also reported macrophage infiltration of several organs, including the liver8, heart9 and kidney10, 11. However, the detailed mechanism for how inflammatory cell infiltration causes host tissue damage is not clear.
While respiratory failure is a frequent and clinically significant outcome of COVID-19, cardiac complications are a common feature in hospitalized COVID-19 patients and are associated with worse patient outcomes2, 12. Acute cardiac injury was shown to be associated with a significantly greater mortality rate in COVID-19 patients than other risk factors such as age, chronic pulmonary disease or prior history of cardiovascular disease13, 14. The cause of cardiac injury in COVID-19 patients is not yet known. However, COVID-19 related Kawasaki disease-like symptoms have been reported, with many children suffering from cardiac dysfunction15. Recent studies analyzing COVID-19 post-mortem heart samples have demonstrated increased inflammatory infiltration of CD11b+16 and CD68+ macrophages9, 17, 18.
Macrophages include both tissue-resident and migrating macrophages19. Tissue-resident macrophages are widely distributed throughout the body and are mainly derived from embryonic progenitor macrophages. They are commonly immuno-suppressive and required for tissue homeostasis20. Migrating macrophages are mainly generated from monocytes circulating in the blood in response to secreted chemokines at the site of injury and are recruited into the injury site21. Recruited macrophages then undergo marked phenotypic and functional changes, and can be classified into pro-inflammatory or anti-inflammatory macrophages, with a continuum of macrophage polarization existing beyond these discrete categories22. Migratory macrophages and monocytes are recruited to the site of inflammation by chemokines including CCL2 that can bind to chemokine receptors such as CCR2 on the surface of these cells. Upon microbial stimulation, tissue-resident macrophages are significantly out-numbered by migrating macrophages. These recruited macrophages display a predominantly pro-inflammatory phenotype, secreting inflammatory cytokines, mediating leukocyte recruitment, and in the case of myocarditis causing oxidative damage to the heart23.
In our recent studies, we reported the detection of SARS-CoV-2 nucleocapsid (SARS-N) in the cardiomyocytes (CMs) of SARS-CoV-2 infected hamsters24. We further demonstrated that both hPSC-derived CMs and adult CMs are permissive to SARS-CoV-2 infection, which is consistent with recent reports25–27. In addition, we also showed that SARS-CoV-2 infected CMs recruit migrating monocytes by CCL2 secretion24. Here, we systematically examined autopsy samples from non-COVID donors and COVID-19 patients and found strikingly increased expression of CCL2 and inflammation-associated genes as well as increased macrophage infiltration in heart tissues from COVID-19 patients. We therefore designed an immuno-host platform containing hPSC-derived CMs co-cultured with hPSC-derived macrophages to explore the interaction between host cells and macrophages upon SARS-CoV-2 infection. Using this immuno-cardiac co-culture platform, we performed a high content screen and identified FDA-approved drugs that protect CMs from macrophage-induced apoptosis upon SARS-CoV-2 infection.
METHODS
Figures in the Data Supplement and a detailed Methods section are available online. Please see the Major Resources Table in the Supplemental Materials.
The Immuno-Cardiac Co-cultures
hPSC-derived CMs were dissociated with Accutase at 37°C followed by resuspending with fresh RPMI 1640-B27 plus Y-27632 and reseeding into 96-well plates. After 24 hr, the medium was switched to RMPI 1640-B27 without Y-27632. After 48 hr recovery, macrophages were added into hPSC-derived CMs in the same well and cultured for another 24 hr (short-term co-culture) or 7 days (long-term co-culture) before following analysis. Adult CMs were also seeded into plates for 48–96 hr and cultured with macrophages for another 24 hr before following analysis.
SARS-CoV-2
SARS-CoV-2, isolate USA-WA1/2020 (NR-52281) was deposited by the Center for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH. Infection of hPSC-derived or adult CMs and macrophages were performed in their respective cell culture growth media at the indicated multiplicities of infection (MOI) for 24 hr at 37°C, unless otherwise indicated.
All work involving SARS-CoV-2 was performed in the CDC/USDA-approved BSL-3 facility of the Global Health and Emerging Pathogens Institute at the Icahn School of Medicine at Mount Sinai in accordance with institutional biosafety requirements.
Data Availability
RNA-seq data is available from the GEO repository database with accession number GSE169241. Source code is available from https://github.com/shuibingchen/COVID-19_Hearts.
RESULTS
Human COVID-19 patient description
The patient characteristics are summarized in Table 1. Eleven non-COVID (COVID-19) and fifteen COVID (COVID-19) patient samples were used for either RNA-seq or staining. Five non-COVID and fifteen COVID patients had a prior history of cardiovascular-related disease(s) including hypertension (HTN, non-COVID: n = 5, COVID: n = 11), diabetes mellitus, (DM, healthy: n = 3, COVID: n = 9), hyperlipidemia (HL, non-COVID: n = 1, COVID: n = 9), non-ST elevation MI (NSTEMI, COVID: n = 1), and congenital heart (COVID: n = 1). One of the COVID patients had a previous history of atrial fibrillation. Two COVID patients developed new onset atrial fibrillation after diagnosis. New electrocardiographic findings were identified in COVID-19 patients, including new onset atrial fibrillation (n = 2), new onset ventricular fibrillation (n = 1) and ST-segment or T-wave changes (n = 5). Serum high-sensitivity troponin T levels were available for ten COVID-19 patients, with a median peak value of 4.2 ng/mL, range 0.00–93.85 ng/ml. Fourteen patients received one or more medications for COVID-19 including hydroxychloroquine (HCQ, 600 mg PO Q12 hr X 2 then 400 mg PO daily X 4 days)/chloroquine (CQ, 500 mg PO Q12 hr X 2 then 500 mg PO daily X 4 days, n = 8), steroids (n = 2), antibiotics (n = 12), and anticoagulation (n = 10).
Table 1.
Patient characteristics.
| All cases | non-COVID | COVID-19 | |
|---|---|---|---|
| n | 24 | 9 | 15 |
| Age | 63.2 (27–95) | 55.6 (27–74) | 67.8 (30–95) |
| Male sex | 14 (58) | 6 (67) | 8 (53) |
| Duration of symptoms (days)a | 14.3 (5–49) | N/A | 14.3 (5–49) |
| Duration of hospitalization (days) | 9.1 (0–52) | N/A | 9.1 (0–52) |
| History of cardiovascular diseases | |||
| HTN | 16 (67) | 5 (56) | 11 (73) |
| DM | 12 (50) | 3 (33) | 9 (60) |
| HL | 10 (42) | 1 (11) | 9 (60) |
| NSTEMI | 1 (4) | 0 | 1 (7) |
| congenital heart | 1 (4) | 0 | 1 (7) |
| Prior atrial fibrillation | 1 (7) | N/A | 1 (7) |
| New-onset atrial fibrillation | 2 (13) | N/A | 2 (13) |
| New ECG changes | 8(53) | N/A | 8 (53) |
| Peak troponin (ng/mL)b | 4.2 (0.00–93.85) | N/A | 4.2 (0.00–93.85) |
| Drug treatment | |||
| HCQ/CQ | 8 (53) | N/A | 8 (53) |
| Steroids | 2 (13) | N/A | 2 (13) |
| Antibiotics | 12 (80) | N/A | 12 (80) |
| Anticoagulation | 10 (67) | N/A | 10 (67) |
Data are expressed as median (range) or n (%).
From onset of symptoms to death
Highest troponin value if multiple troponin values were available.
Macrophages are enriched in hearts of COVID-19 patients compared to non-COVID donors.
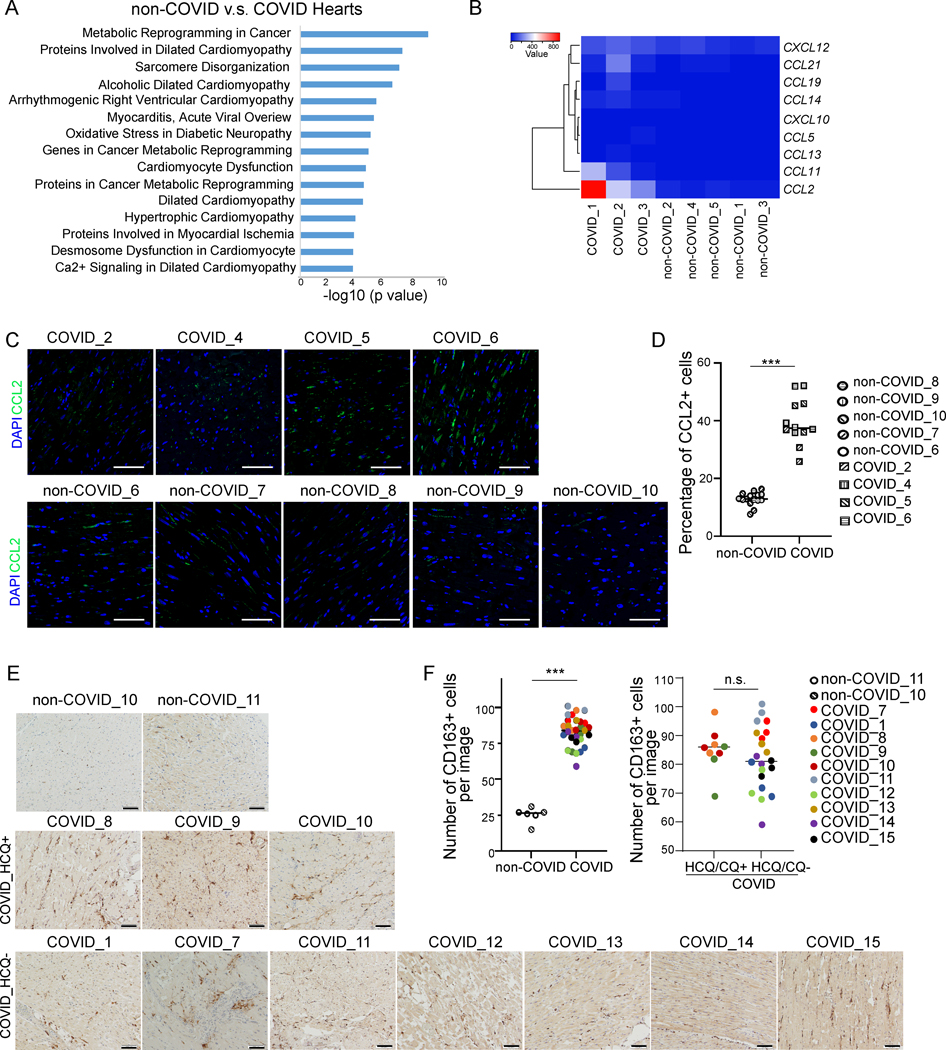
To examine the inflammatory response in cardiac tissues of COVID-19 patients, we first compared the transcriptional profiles of heart tissue samples obtained from autopsies of non-COVID donors or COVID-19 patients. In the multidimensional scaling (MDS) plot and heatmap, the profiles of non-COVID heart samples clustered separately from the COVID-19 heart samples (Figure IA and IB in the online-only Data Supplement). Elsevier Pathway analysis of the differentially expressed genes in COVID-19 versus non-COVID heart samples showed that genes involved in cardiac pathology were significantly enriched in COVID-19 patients, including dilated cardiomyopathy, sarcomere disorganization, alcoholic dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, myocarditis, cardiomyocyte dysfunction, dilated cardiomyopathy, and hypertrophic cardiomyopathy (Figure 1A). In addition, transcript levels for several chemokines were upregulated in COVID-19 heart samples (Figure 1B), and CCL2 exhibited significantly increased levels of expression (Figure IC in the online-only Data Supplement). This was further confirmed by significantly increased immunostaining levels for CCL2 in COVID-19 heart samples (Figure 1C and 1D).
Figure 1. Macrophages are enriched in the heart autopsy tissues of COVID-19 patients compared to non-COVID donors.
A, Elsevier Pathway analysis of top 1000 genes differentially expressed in non-COVID donors (N=5) versus COVID heart samples (N=3).
B, Heatmap of chemokines of heart tissues obtained from autopsied COVID patients (N=3) compared to non-COVID control donors (N=5). Data was presented as the absolute value.
C, D, Immunostaining (C) and quantification (D) of CCL2 in autopsy heart samples of non-COVID donors (N=5) and COVID patients (N=4). Three images of each samples were used for quantification. Scale bar= 50 μm.
E, F, Immunohistochemistry staining (E) and quantification (F) of CD163+ cells in autopsy heart samples of non-COVID donors (N=2), HCQ- COVID patients (N=7) and HCQ+ COVID patients (N=3). Three images per sample were used for quantification. Scale bar= 100 μm.
Data was presented as mean ± STDEV. P values were calculated by unpaired two-tailed Student’s t test. n.s. no significance and ***P < 0.001.
Since CCL2 is a well-known pro-inflammatory chemokine, we investigated the expression of genes involved in inflammation and found that expression levels for numerous inflammation-associated genes were upregulated in COVID-19 heart samples (Figure ID, IE and Table SI in the online-only Data Supplement). As CCL2 is a major chemoattractant for migrating macrophages, we examined the presence of macrophages and confirmed increased numbers of CD163+ cells in COVID-19 heart autopsy samples as compared to non-COVID donor samples (Figure 1E and 1F). No significant difference was detected between the number of CD163+ cells in heart autopsy samples of COVID-19 patients with and without HCQ/CQ treatment (Figure 1E and 1F), suggesting that the infiltration of CD163 cells is not due to or altered by HCQ/CQ treatment.
An immuno-cardiac co-culture platform reveals that, upon SARS-CoV-2 infection, macrophages induce increased ROS and apoptosis of CMs.
In recent studies, we reported the detection of viral nucleocapsid (SARS-N) in CMs of SARS-CoV-2 infected hamsters24. We further demonstrated that SARS-CoV-2 infected CMs recruit migrating monocytes by secretion of CCL224. To explore the interaction between CMs and macrophages upon SARS-CoV-2 infection, we created an immuno-cardiac co-culture platform using hPSC-derived CMs and macrophages.
CMs were derived from a MYH6:mCherry H9 hESC reporter line28 or a hiPSC line generated from healthy donor (Figure IF in the online-only Data Supplement) and over 90% of the cells expressed mCherry and stained positive with antibodies recognizing sarcomeric α-actinin and cardiac troponin T (cTNT) (Figure IG and IH in the online-only Data Supplement). Around 20% of hPSC-derived CMs expressed CCR2, a major receptor of CCL2 (Figure II in the online-only Data Supplement). In autopsy samples, we detected no significant difference of CCR2 expression between the atrium and ventricle (Figure IJ in the online-only Data Supplement). Furthermore, CCR2 expression in the heart autopsy samples of COVID-19 patients did not correlate with the severity of the patient outcome (Peak Troponin, Figure IK in the online-only Data Supplement).
Macrophages were derived from H9 or H1 hESC lines following a previously reported protocol29 (Figure IIA in the online-only Data Supplement) through a stepwise manner, including the generation of mesoderm, followed by hematopoietic progenitor cells, CD68+ monocytes (Figure IIB in the online-only Data Supplement) and finally CD11b+CD163+CD14+CD45+CD80- unstimulated macrophages (Figure IIC and IID in the online-only Data Supplement). The protocol was developed to differentiate hPSCs to macrophages comparable to peripheral blood-derived macrophages29. The hESC-derived monocytes and macrophages express CCR2 as indicated by qRT-PCR, immunostaining and flow cytometry (Figure IIE- III in the online-only Data Supplement). ACE2, the main cellular receptor for SARS-CoV-230, is highly expressed in H9 hESC-derived CMs and adult CMs, but not macrophages (Figure IIJ in the online-only Data Supplement).
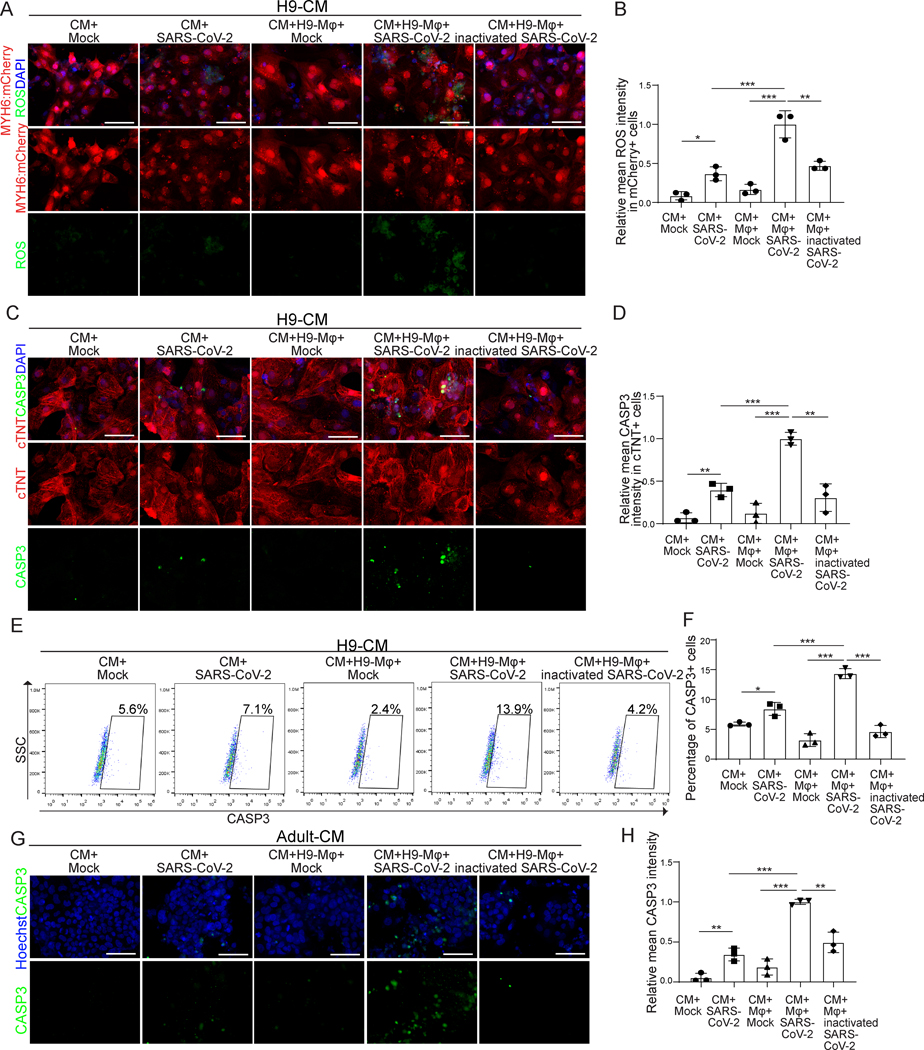
Previous studies reported that recruited monocyte-derived macrophages could induce oxidative damage in the heart following myocardial injury31. To examine if macrophages increase the damage to CMs in the presence of SARS-CoV-2, the immuno-cardiac cells were co-cultured for 24 hours and then exposed to different doses of SARS-CoV-2 (MOI=0.001, 0.01, 0.05, 0.1). At 24 hours post-infection (hpi), cells were either stained with a CellROX™ Green Reagent to detect ROS or antibody against cleaved caspase-3 (CASP3, Figure III in the online-only Data Supplement). At MOI=0.1, the presence of macrophages significantly increased both ROS (Figure 2A and 2B) and cell apoptosis (Figure 2C and 2D) of CMs in SARS-CoV-2 infected co-cultures. To quantify the cell apoptotic rate in CMs or macrophages, we co-cultured CMs derived from MYH6:mCherry H9 hESCs and macrophages derived from blue fluorescence protein (BFP)-labelled H1 hESCs. The percentage of CASP3+ cells in mCherry+ cells was significantly higher in the co-culture condition than the no-macrophage condition (Figure 2E and 2F, Figure IVA in the online-only Data Supplement). The apoptotic rate of macrophages was also increased in the co-culture condition (Figure IVB and IVC in the online-only Data Supplement). However, the overall apoptotic rate of macrophages was much lower than the one of CMs. We further monitored the cell necrotic rate of CMs and macrophages using the co-culture cells of CMs derived from MYH6:mCherry H9 hESCs and macrophages derived from BFP-labelled H1 hESCs by flow cytometry (% of RIP3+ cells). Although SARS-CoV-2 infection increased necrosis of CMs, it was not further increased in CMs or in macrophages in the co-culture condition (Figure V in the online-only Data Supplement), suggesting that the presence of macrophages does not impact CM necrosis. Moreover, we further validated that the presence of macrophages increased CM apoptosis upon SARS-CoV-2 infection using co-cultures containing CMs derived from an hiPSC line and macrophages derived from H1 hESCs (Figure VIA in the online-only Data Supplement). The same co-culture experiments were performed using adult human CMs and hPSC-derived macrophages. Similarly, the presence of macrophages also significantly increased cell apoptosis (Figure 2G and 2H) of adult CMs in SARS-CoV-2 infected cultures. Next, hPSC-derived CMs or adult human CMs were co-cultured with hPSC-derived macrophages for one week (long-term co-culture) and infected with SARS-CoV-2. Consistent with the short-term co-cultures, macrophages significantly increased cell apoptosis of both types of CMs in SARS-CoV-2 infected co-cultures (Figure VIB–VIE in the online-only Data Supplement). These data show that the presence of macrophages increased CM apoptosis upon SARS-CoV-2 infection.
Figure 2. The immuno-cardiac co-culture platform-based assays reveal that, upon SARS-CoV-2 exposure, macrophages induce increased ROS and apoptosis of CMs.
A, B, Fluorescence images (A) and quantification (B) of ROS levels in H9-derived CMs in different conditions, including CM+mock, CM+SARS-CoV-2 (MOI=0.1), CM+macrophage+mock, CM+macrophage+ SARS-CoV-2 (MOI=0.1), CM+macrophage+ inactivated SARS-CoV-2 (MOI=0.1). Scale bar= 50μm.
C, D, Immunostaining assay (C) and quantification (D) of CASP3 of H9-derived CMs in different conditions as in (A). Scale bar= 50 μm.
E, F, Flow cytometry analysis (E) and quantification (F) of CASP3 in H9-derived CMs in different conditions as in (A). Scale bar= 50 μm.
G, H, Immunostaining assay (G) and quantification (H) of CASP3 in adult CMs in different conditions as in (A). Scale bar= 50 μm.
N=3 independent biological replicates. Data was presented as mean ± STDEV. P values between CM+mock, CM+SARS-CoV-2, CM+macrophage+mock and CM+macrophage+SARS-CoV-2 were calculated by two-way ANOVA analysis. P values between CM+macrophage+mock, CM+macrophage+SARS-CoV-2 and CM+macrophage+inactivated SARS-CoV-2 were calculated by one-way ANOVA analysis. *P < 0.05, **P < 0.01, ***P < 0.001.
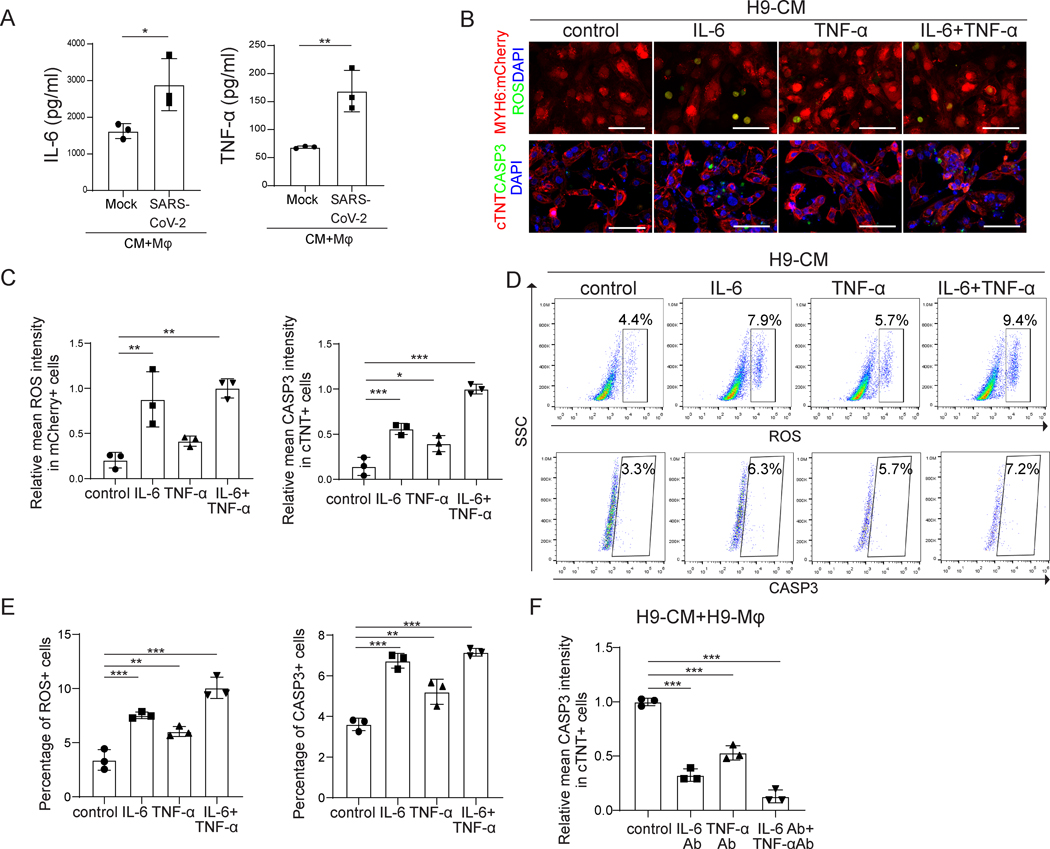
To determine how macrophages damage CMs in SARS-CoV-2 infected immuno-cardiac co-cultures, macrophages were exposed to SARS-CoV-2 and infectious viral particles in the cell culture media were measured over a time course of 48 hours. The amount of infectious virus detected in the supernatant decreased with time and was essentially undetectable by 48 hours post-infection (Figure VIIA in the online-only Data Supplement), suggesting that macrophages cannot be productively infected. Consistent with previous reports, macrophages might instead engulf SARS-CoV-2 to trigger the immune response21. Accordingly, macrophage transcript profiles were significantly altered following SARS-CoV-2 exposure (Figure VIIB and VIIC in the online-only Data Supplement). In particular, inflammatory genes (Figure VIID in the online-only Data Supplement) and cytokines (Figure VIIE in the online-only Data Supplement) were upregulated in macrophages following SARS-CoV-2 exposure. Secretion of cytokines into the cell culture supernatant, including IL-6 and TNF-α, but not IL-10, was confirmed by ELISA (Figure 3A and Figure VIIF–VIIG in the online-only Data Supplement). The amount of IL-6 and TNF-α secreted by macrophages after SARS-CoV-2 exposure is around 100-fold higher than levels secreted by infected CMs (Figure VIIH and VIII in the online-only Data Supplement), suggesting that macrophages are responsible for the majority of the increase of IL-6 and TNF-α secretion in infected co-cultures. To determine if IL-6 and TNF-α could induce ROS and apoptosis of CMs, hPSC-derived CMs were treated with 3.5 ng/ml IL-6, 0.25 ng/ml TNF-α or 3.5 ng/ml IL-6+0.25 ng/ml TNF-α for 24 hr. Staining confirmed that all three conditions increased ROS (Figure 3B and 3C) and apoptosis (Figure 3B and 3C, Figure VIIIA in the online-only Data Supplement) in hPSC-derived CMs, which was further validated by flow cytometry analysis (Figure 3D and 3E, Figure VIIIB–VIIIE in the online-only Data Supplement). Although the treatment with IL-6, TNF-α or IL-6+ TNF-α also increased the percentage of RIP3+ cells, the necrotic cell rate is lower than the apoptotic cell rate (Figure VIIIF and VIIIG in the online-only Data Supplement), suggesting that cell apoptosis is the major mechanism of IL-6 and/or TNF-α-induced CM death.
Figure 3. Macrophages induce increased ROS and apoptosis of CMs by secreting IL-6 and TNF-α.
A, ELISA assays were performed to examine the protein levels of IL-6 and TNF-α secreted by H9-derived CMs co-cultured with H9-derived macrophages exposed to mock or SARS-CoV-2 (MOI=0.1).
B, Confocal imaging of ROS in MYH6:mCherry+ H9-derived CMs and CASP3 in cTNT+ H9-derived CMs treated with 3.5 ng/ml IL-6, 0.25 ng/ml TNF-α, or 3.5 ng/ml IL-6+0.25 ng/ml TNF-α for 24 hr. Scale bar= 50 μm.
C, Quantification of ROS in MYH6:mCherry+ H9-derived CMs and CASP3 in cTNT+ H9-derived CMs treated with 3.5 ng/ml IL-6, 0.25 ng/ml TNF-α, or 3.5 ng/ml IL-6+0.25 ng/ml TNF-α for 24 hr analyzed by immunostaining.
D, Flow cytometry analysis of ROS in MYH6:mCherry+ H9-derived CMs and CASP3 in MYH6:mCherry+ H9-derived CMs treated with 3.5 ng/ml IL-6, 0.25 ng/ml TNF-α, or 3.5 ng/ml IL-6+0.25 ng/ml TNF-α for 24 hr. Scale bar= 50 μm.
E, Quantification of ROS in MYH6:mCherry+ H9-derived CMs and CASP3 in MYH6:mCherry+ H9-derived CMs treated with 3.5 ng/ml IL-6, 0.25 ng/ml TNF-α, or 3.5 ng/ml IL-6+0.25 ng/ml TNF-α for 24 hr analyzed by flow cytometry.
F, Quantification of CASP3 in cTNT+ H9-derived CMs in CM+macrophage+SARS-CoV-2 condition treated with control or IL-6 neutralizing antibody, TNF-α neutralizing antibody, or IL-6 neutralizing antibody+TNF-α neutralizing antibody.
N=3 independent biological replicates. Data was presented as mean ± STDEV. P values were calculated by one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001.
We also evaluated the response of adult human CMs to cytokine treatments. Consistent with the results obtained with hPSC-derived CMs, IL-6 and/or TNF-α treatment increased ROS (Figure IXA and IXB in the online-only Data Supplement) and apoptosis (Figure IXC and IXD in the online-only Data Supplement) in adult human CMs. Finally, using IL-6 and/or TNF-α neutralizing antibodies, SARS-CoV-2 induced CM apoptosis was partially blocked in both hPSC-derived and adult CMs (Figure 3F, Figure IXE and IXF in the online-only Data Supplement). Although some CMs expressed CCR2, CCL2 alone did not affect the ROS or apoptotic rate of hPSC-derived CMs in the absence of macrophages, as shown by both immunostaining and flow cytometry (Figure IXG–IXJ in the online-only Data Supplement). Taken together, we show that macrophages secrete IL-6 and TNF-α upon SARS-CoV-2 infection of the immuno-cardiac co-cultures, which increases ROS levels and induces apoptosis of CMs.
An immuno-cardiac co-culture platform based high content screen identifies FDA-approved drugs ranolazine and tofacitinib for preventing macrophage-mediated hyper-inflammation in CMs.
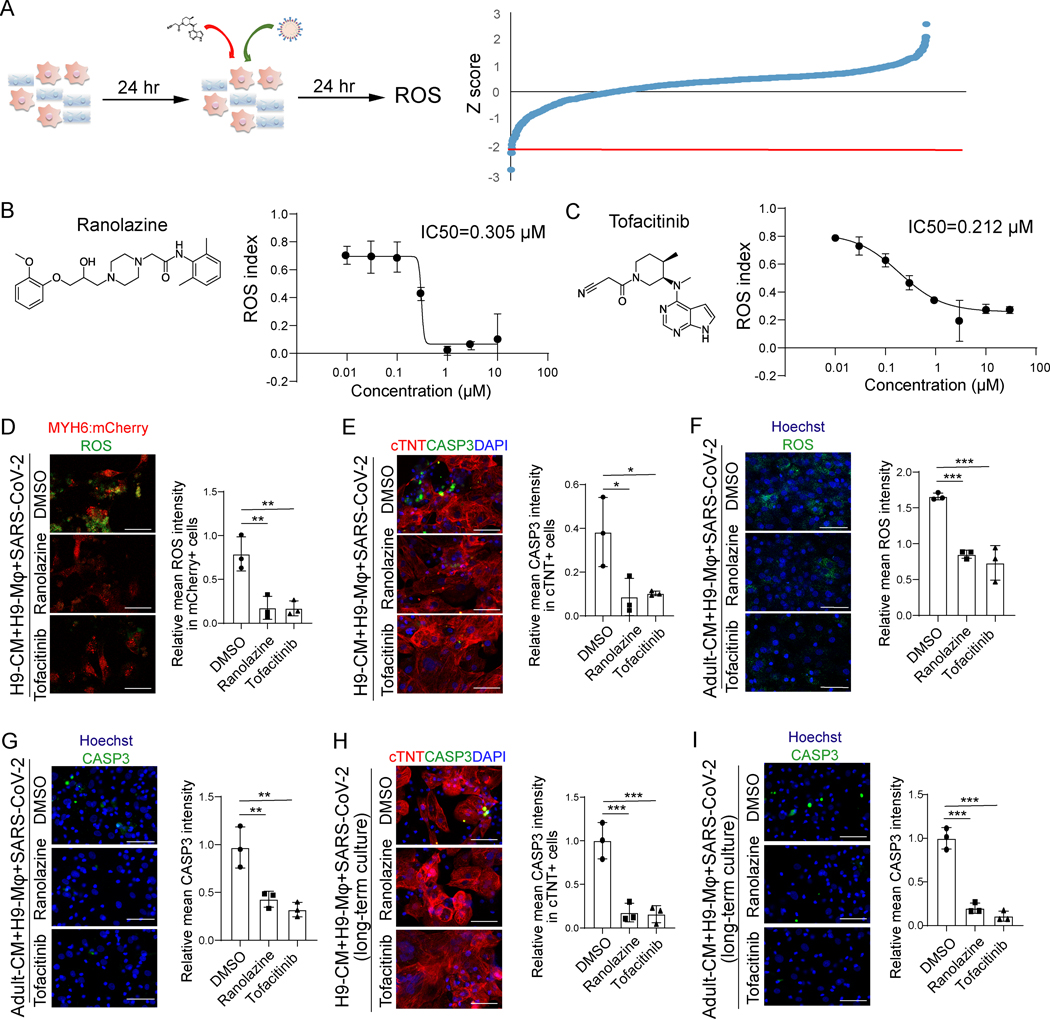
A high content screen was performed to identify drug candidates that could abrogate macrophage-mediated CM apoptosis. In brief, the immuno-cardiac co-culture platform containing hPSC-derived CMs and macrophages was treated with compounds from the Prestwick library that contains 1280 FDA-approved drugs, followed by SARS-CoV-2 infection (MOI=0.1, Figure 4A). At 24 hpi, cells were stained with ROS indicator dye, fixed and analyzed using the ImageXpress high content analysis system. The ROS index was calculated by the equation:
Figure 4. An immuno-cardiac co-culture platform based high content screen identifies FDA-approved drug candidates that protect CMs from macrophage-mediated cell apoptosis.
A, Scheme of the high content screen and primary screening results.
B, Chemical structure and inhibitory curve of ranolazine.
C, Chemical structure and inhibitory curve of tofacitinib.
D, Immunostaining and quantification of relative mean ROS intensity in MYH6:mCherry+ CMs under 10 μM ranolazine or 0.5 μM tofacitinib treated virus-immuno-cardiac co-culture platform containing H9-derived CMs and H9-derived macrophages infected with SARS-CoV-2 virus (MOI=0.1). Scale bar= 50 μm.
E, Confocal imaging and quantification of relative mean CASP3 intensity in cTNT+ CMs under 10 μM ranolazine or 0.5 μM tofacitinib treated virus-immuno-cardiac co-culture platform containing H9-derived CMs and H9-derived macrophages infected with SARS-CoV-2 virus (MOI=0.1). Scale bar= 50 μm.
F, Immunostaining and quantification of relative mean ROS intensity in adult CMs under 10 μM ranolazine or 0.5 μM tofacitinib treated virus-immuno-cardiac co-culture platform containing adult human CMs and H9-derived macrophages infected with SARS-CoV-2 virus (MOI=0.1). Scale bar= 50 μm.
G, Confocal imaging and quantification of relative mean CASP3 intensity in adult CMs under 10 μM ranolazine or 0.5 μM tofacitinib treated virus-immuno-cardiac co-culture platform containing adult human CMs and H9-derived macrophages infected with SARS-CoV-2 virus (MOI=0.1). Scale bar= 50 μm.
H, Confocal imaging and quantification of relative mean CASP3 intensity in cTNT+ CMs under 10 μM ranolazine or 0.5 μM tofacitinib treated virus-immuno-cardiac co-culture platform containing H9-derived CMs and H9-derived macrophages infected with SARS-CoV-2 virus for long term co-culture (7 days co-culture, MOI=0.1). Scale bar= 50 μm.
I, Confocal imaging and quantification of relative mean CASP3 intensity in adult CMs under 10 μM ranolazine or 0.5 μM tofacitinib treated virus-immuno-cardiac co-culture platform containing adult human CMs and H9-derived macrophages infected with SARS-CoV-2 virus for long term co-culture (7 days co-culture, MOI=0.1). Scale bar= 50 μm.
N=3 independent biological replicates. Data was presented as mean ± STDEV. P values were calculated by one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001.
and plotted for each drug (Figure 4A). Several drug candidates, including ranolazine (IC50= 0.305 μM, Figure 4B) and tofacitinib (IC50= 0.212 μM, Figure 4C) were found to decrease the ROS index in a dose-dependent manner. Other known anti-oxidative reagents, such as trolox and resveratrol, also decreased the ROS index (Figure XA in the online-only Data Supplement). To validate the screen, the immuno-cardiac co-culture platform containing hPSC-derived CMs and macrophages was infected with SARS-CoV-2 (MOI=0.1) and both 10 μM ranolazine and 0.5 μM tofacitinib were found to decrease ROS (Figure 4D and Figure XB in the online-only Data Supplement) as well as the rates of CM apoptosis (Figure 4E and Figure XC in the online-only Data Supplement). Consistent with these results, treatment of infected co-cultures using adult human CMs also inhibited the ROS rate (Figure 4F) and apoptosis rate (Figure 4G, Figure XD in the online-only Data Supplement). In addition, both ranolazine and tofacitinib decreased the apoptosis rate of long-term co-cultures of hPSC-derived CMs or adult CMs with hPSC-derived macrophages after SARS-CoV-2 infection (Figure 4H and 4I).
RNA-seq was used to examine the transcriptional changes of CMs upon drug treatment and virus infection. To perform the assay, macrophages were cultured on the upper insert chamber of the trans-well plate and hPSC-derived CMs were cultured on the bottom of the trans-well plate. Co-cultures were exposed to SARS-CoV-2 at MOI=0.1. 24 hours later, the hPSC-derived CMs were collected and analyzed by RNA-seq. Consistent with the staining results, ranolazine or tofacitinib decreased the expression of genes associated with ROS and cell apoptosis (Figure 5A–5D). Ranolazine or tofacitinib treatment also increased the expression of sarcomeric genes and ion channels (Figure 5E and 5F)32.
Figure 5. Gene expression analysis of drug treated CMs.
A, B, Heatmap of ROS-related genes in 10 μM ranolazine (A) or 0.5 μM tofacitinib (B) treated H9-derived CMs co-cultured with H9-derived macrophages infected with SARS-CoV-2 virus using trans-well plates (MOI=0.1).
C, D, Heatmap of apoptosis-related genes in 10 μM ranolazine (C) or 0.5 μM tofacitinib (D) treated H9-derived CMs co-cultured with H9-derived macrophages infected with SARS-CoV-2 virus using trans-well plates (MOI=0.1).
E, F, Heatmap of cardiomyocyte related genes in 10 μM ranolazine (E) or 0.5 μM tofacitinib (F) treated H9-derived CMs co-cultured with H9-derived macrophages infected with SARS-CoV-2 virus using trans-well plates (MOI=0.1).
Ranolazine and tofacitinib protect CMs from macrophage-induced damage through different mechanisms.
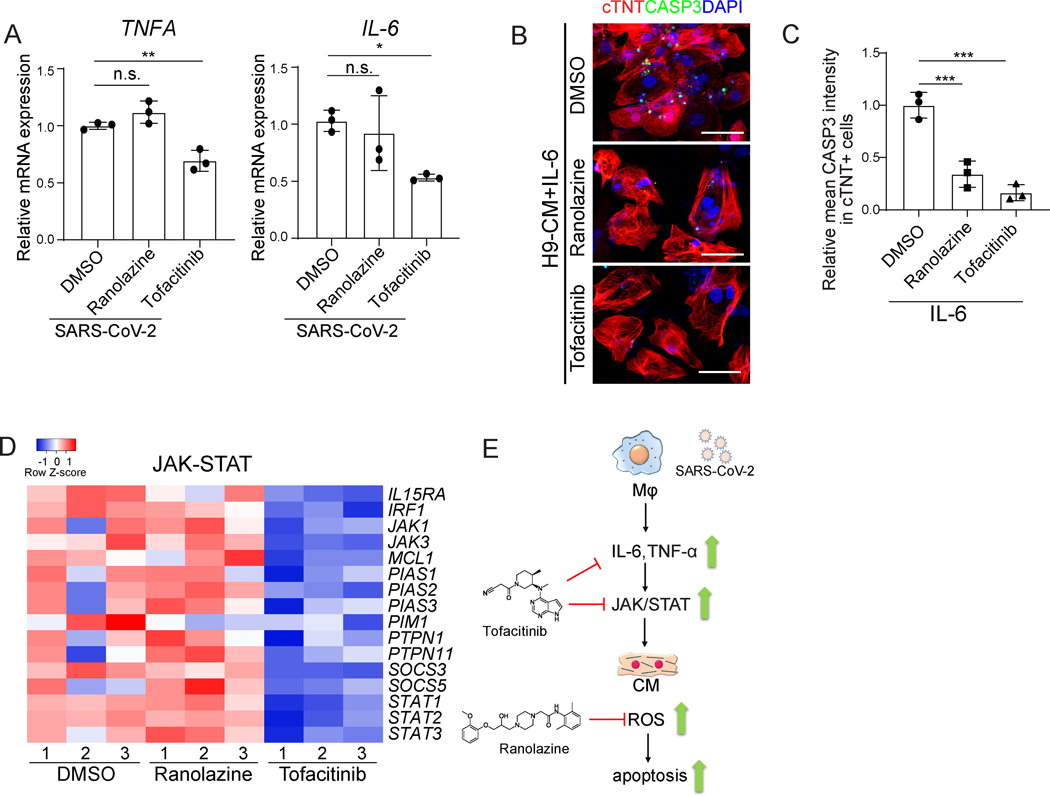
Ranolazine or tofacitinib could possibly block the secretion of IL-6 and/or TNFα, affecting downstream signaling pathways, or rescue cell survival. To distinguish these possibilities, we first examined the IL6 and TNFA mRNA expression of macrophages upon SARS-CoV-2 exposure in the presence or absence of ranolazine or tofacitinib. Tofacitinib, but not ranolazine, significantly blocked the upregulation of IL6 and TNFα in macrophages upon SARS-CoV-2 exposure (Figure 6A). Both ranolazine and tofacitinib rescued IL-6 mediated apoptosis of hPSC-derived CMs (Figure 6B and 6C), which suggests that ranolazine and tofacitinib might also affect the downstream pathways or rescue cell survival. Tofacitinib is a JAK inhibitor33, which has been approved by the FDA for rheumatoid arthritis in patients with an inadequate response or intolerance to methotrexate34. Transcript profiling validates that JAK-STAT associated genes were downregulated in tofacitinib, but not in ranolazine treated conditions (Figure 6D). Ranolazine has been shown to protect CMs from doxorubicin-induced oxidative stress35. Consistent with this result, transcript profiling suggested that ranolazine treatment causes a decrease in expression levels of ROS-associated genes (Figure 5A). Together, the results suggest that tofacitinib functions by blocking the secretion of IL-6 and TNFα, inhibiting JAK-STAT pathway, while ranolazine protects CMs by lowering ROS levels (Figure 6E).
Figure 6. Ranolazine and Tofacitinib decrease ROS and CASP3 in the immuno-cardiac co-culture platform through different mechanisms.
A, qRT-PCR analysis of expression levels for TNFA and IL6 in ranolazine or tofacitinib treated H9-derived macrophages exposed to SARS-CoV-2 (MOI=0.1).
B, C, Confocal imaging (B) and quantification (C) of relative mean CASP3 intensity in cTNT+ H9-derived CMs. CMs were treated with 10 μM ranolazine or 0.5 μM tofacitinib and then treated with IL-6. Scale bar= 50 μm.
D, Heatmap of JAK/STAT signaling pathway related genes in 10 μM ranolazine or 0.5 μM tofacitinib treated H9-derived CMs co-cultured with H9-derived macrophages infected with SARS-CoV-2 virus using trans-well plates (MOI=0.1).
E, Proposed mechanism of action.
N=3 independent biological replicates. Data was presented as mean ± STDEV. P values were calculated by one-way ANOVA. n.s. no significance, *P < 0.05, **P < 0.01, and ***P < 0.001.
DISCUSSION
Immune cell mediated hyper-inflammation is believed to contribute to the severity and outcome of COVID-19. Single cell RNA-seq of bronchoalveolar lavage fluid immune cells from patients with varying severity of COVID-19 identified an abundance of proinflammatory macrophages in severe COVID-19 patients36. Recent studies analyzing lung autopsy samples have reported mononuclear inflammation, infiltrated with lymphocytes and macrophages5–7. Here, we systematically examined heart autopsy samples from COVID-19 patients and non-COVID controls and found macrophage infiltration in heart autopsy samples of COVID-19 patients. Consistently, CCL2 was found to be upregulated in heart and lung autopsy samples from COVID-19 patients37. CCL2, also known as monocyte chemoattractant protein 1 (MCP-1), is a chemokine that facilitates the migration and infiltration of monocytes to sites of inflammation produced by either tissue injury or infection. CCR2 is the major receptor for CCL2. Recent studies identified both CCR2+ and CCR2- macrophages in human heart, of which migrating CCR2+ macrophages play a pro-inflammatory role38, 39. CD163 is expressed in both CCR2+ and CCR2- macrophages and CCR2+ monocytes in human heart38. Consistently, we detected an increase of CD163+ cells in heart autopsy samples of COVID-19 patients. Together, the data suggest that CCL2 might play an important role in macrophage infiltration of COVID-19 patient hearts.
To study the macrophage-host cell interaction upon SARS-CoV-2 infection, we created an immuno-host co-culture platform using hPSC-derived or adult CMs co-cultured with hPSC-derived macrophages. In the presence of SARS-CoV-2, macrophages secrete IL-6 and TNF-α, which causes increased apoptosis of CMs. Clinically, increased levels of IL-6 and TNF-α were detected in severe but not moderate cases of COVID-1940, 41. Thus, a combination treatment blocking both IL-6 and TNF-α might be useful to attenuate toxicity. It is worth noting that IL-6 plays a double edge role in cardiac injury, since it can provide cardiac protective activity at certain phases of cardiac injury 42. Thus, timing would be critical for using IL-6 and TNF-α blocking therapy.
Finally, an immuno-cardiac based high content screen using FDA-approved drugs identified ranolazine and tofacitinib as protecting CMs from macrophage-induced ROS and apoptosis upon SARS-CoV-2 infection. The mechanistic studies suggested that tofacitinib functions by blocking TNF-α and IL-6 generation and inhibiting the JAK/STAT pathway, a pathway downstream of IL-6, while ranolazine blocks the increase of IL-6/TNF-α-dependent ROS in a CM-dependent manner (Figure 6E). Consistently, previous studies showed that tofacitinib blocks TNF-α generation/secretion43–45. Ranolazine, a piperazine derivative, is a well-tolerated medication with beneficial metabolic properties without affecting heart rate or blood pressure. Ranolazine is recommended for patients with stable ischemic heart disease if unable to use acceptable doses of β-blockers46. In addition, ranolazine was shown to improve glycemic control in type 2 diabetic patients47. Tofacitinib citrate has been approved by the FDA to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and ulcerative colitis. JAK inhibitors have recently been considered to treat COVID-19 patients48. We provide the first experimental evidence of the anti-inflammatory activity of a JAK inhibitor on SARS-CoV-2 infected cells.
Although this study provides a proof-of-principle co-culture system to study the macrophage-mediated damage of CMs, some other cell types, such as cardiac fibroblasts and endothelial cells, might contribute to the heart damage of COVID-19 patients. In addition to macrophages, other cell types in the heart, such as cardiac fibroblasts secrete IL-6 (Burger A et al. American Journal of Physiology-Heart and Circulatory Physiology. 2001). Moreover, IL-6 receptor and TNF-α receptor are expressed in various cell types in the heart, such as endothelium. Thus, additional studies should further dissect the contribution of other types of cells, such as cardiac fibroblasts and endothelial cells, to the heart damage of COVID-19 patients.
Supplementary Material
Novelty and Significance.
What is Known?
Increased numbers of macrophages were found in lung autopsy samples of COVID-19 patients compared with non-COVID-19 patients.
While respiratory failure is a frequent and clinically significant outcome of COVID-19 patients, cardiac complications are a common feature in hospitalized COVID-19 patients and are associated with worse patient outcomes.
What New Information Does This Article Contribute?
We systematically compared autopsy samples from non-COVID-19 donors and COVID-19 patients using RNA-seq and immunohistochemistry and found that macrophages are enriched in heart tissues of COVID-19 patients.
Our study reports the first immuno-cardiac co-culture platform to study macrophage-mediated hyper-inflammation related to SARS-CoV-2 infection.
The co-culture platform was adapted to a high content screen and identified two FDA-approved drugs that protect CMs from macrophage-mediated cardiotoxicity upon SARS-CoV-2 infection.
By comparing heart samples from non-COVID and COVID patient donors, we found increased numbers of macrophages in COVID patient hearts. Therefore, we developed an immuno-host co-culture platform containing hPSC-derived or adult CMs, to evaluate the impact of macrophages on host cells upon SARS-CoV-2 infection. We showed that macrophages secrete increased levels of IL-6 and TNF-α which increased apoptosis of CMs. Finally, we performed a drug screen and identified candidate therapeutics for consideration of repurposing for clinical use to block macrophage-mediated hyper-inflammation of COVID-19 patients.
Acknowledgments
Source of Funding.
This work was supported by the American Heart Association (18CSA34080171, S.C., T.E.), Department of Surgery, Weill Cornell Medicine (T.E., S.C.), and NIH (NIDDK, DP3DK111907, R01DK116075, R01DK119667, R01 DK119667–02S1, R01 DK124463, and U01 DK127777, S.C., NCI R01CA234614, NIAID 2R01AI107301 and NIDDK R01DK121072 and 1RO3DK117252), Bill and Melinda Gates Foundation (S.C., T.E., R.E.S, B.tO,), Department of Medicine, Weill Cornell Medicine (R.E.S.), by the Defense Advanced Research Projects Agency (DARPA-16–35-INTERCEPT-FP-006, B.T.) and by the Jack Ma Foundation (D.D.H), National Institutes of Health (R21AI149033, J.K.L.), National Heart, Lung, And Blood Institute of the National Institutes (F31HL149295, J.A.A). S.C and R.E.S. are supported as Irma Hirschl Trust Research Award Scholars. T.E. is supported by an Outstanding Investigator Award from the NHLBI (R35 HL135778).
Nonstandard Abbreviation and Acronyms
- CM
Cardiomyocyte
- COVID-19
Coronavirus disease 2019
- CCL2
C-C motif chemokine ligand 2
- CQ
Chloroquine
- HCQ
Hydroxychloroquine
- hPSC
Human pluripotent stem cells
- NSTEMI
Non-ST elevation MI
- HTN
Hypertension
- DM
Diabetes mellitus
- HL
Hyperlipidemia
- ROS
Reactive oxygen species
- CASP3
Caspase3
- FDA
Food and drug administration
- RIP3
Receptor-interacting protein kinase 3
- CCR2
C-C motif chemokine receptor 2
- cTNT
Troponin T2, cardiac type
- Ab
Antibody
Footnotes
DISCLOSURES
R.E.S. is on the scientific advisory board of Miromatrix Inc and is a consultant and speaker for Alnylam Inc. The other authors have no conflicts of interest.
References.
- 1.Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, china. Lancet. 2020;395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with covid-19 in wuhan, china: A retrospective cohort study. Lancet. 2020;395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, china. JAMA Intern Med. 2020;180:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Valle DM, Kim-schulze S, Hsin-hui H et al. An inflammatory cytokine signature helps predict covid-19 severity and death. medRxiv. 2020:2020.2005.2028.20115758 [Google Scholar]
- 5.Carsana L, Sonzogni A, Nasr A et al. Pulmonary post-mortem findings in a series of covid-19 cases from northern italy: A two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nienhold R, Ciani Y, Koelzer VH et al. Two distinct immunopathological profiles in autopsy lungs of covid-19. medRxiv. 2020:2020.2006.2017.20133637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes BJ, Adrover JM, Baxter-Stoltzfus A et al. Targeting potential drivers of covid-19: Neutrophil extracellular traps. J Exp Med. 2020;217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Shi L, Wang Y et al. Pathological findings of covid-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8:420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of covid-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diao B, Wang C, Wang R et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (sars-cov-2) infection. medRxiv. 2020:2020.2003.2004.20031120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merad M, Martin JC. Pathological inflammation in patients with covid-19: A key role for monocytes and macrophages. Nature Reviews Immunology. 2020;20:355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, china. JAMA. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi S, Qin M, Shen B et al. Association of cardiac injury with mortality in hospitalized patients with covid-19 in wuhan, china. JAMA Cardiol. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (covid-19). JAMA Cardiol. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during covid-19 pandemic. Lancet. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escher F, Pietsch H, Aleshcheva G et al. Detection of viral sars-cov-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavazzi G, Pellegrini C, Maurelli M et al. Myocardial localization of coronavirus in covid-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindner D, Fitzek A, Brauninger H et al. Association of cardiac infection with sars-cov-2 in confirmed covid-19 autopsy cases. JAMA Cardiol. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginhoux F, Jung S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nature reviews. Immunology. 2014;14:392–404 [DOI] [PubMed] [Google Scholar]
- 20.Honold L, Nahrendorf M. Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res. 2018;122:113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merad M, Martin JC. Pathological inflammation in patients with covid-19: A key role for monocytes and macrophages. Nature reviews. Immunology. 2020;20:355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews. Immunology. 2011;11:723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, Rosenthal N, Kovacic JC. The macrophage in cardiac homeostasis and disease: Jacc macrophage in cvd series (part 4). Journal of the American College of Cardiology. 2018;72:2213–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Nilsson-Payant BE, Han Y, et al. SARS-CoV-2 Infected Cardiomyocytes Recruit Monocytes by Secreting CCL2. Res Sq. 2020 [Google Scholar]
- 25.Bojkova D, Wagner JUG, Shumliakivska M et al. Sars-cov-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Han Y, Nilsson-Payant BE et al. A human pluripotent stem cell-based platform to study sars-cov-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A, Garcia G jr., Wang Y, Plummer JT, Morizono K, Arumugaswami V, Svendsen CN. Human ipsc-derived cardiomyocytes are susceptible to sars-cov-2 infection. Cell Rep Med. 2020;1:100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai SY, Ghazizadeh Z, Wang HJ et al. A human embryonic stem cell reporter line for monitoring chemical-induced cardiotoxicity. Cardiovasc Res. 2020;116:658–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao X, Yakala GK, van den Hil FE, Cochrane A, Mummery CL, Orlova VV. Differentiation and functional comparison of monocytes and macrophages from hipscs with peripheral blood derivatives. Stem cell reports. 2019;12:1282–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann M, Kleine-Weber H, Schroeder S et al. Sars-cov-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao DJ. Macrophages in cardiovascular homeostasis and disease. Circulation. 2018;138:2452–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu XQ, Soo SY, Sun W, Zweigerdt R. Global expression profile of highly enriched cardiomyocytes derived from human embryonic stem cells. Stem Cells. 2009;27:2163–2174 [DOI] [PubMed] [Google Scholar]
- 33.Changelian PS, Flanagan ME, Ball DJ et al. Prevention of organ allograft rejection by a specific janus kinase 3 inhibitor. Science. 2003;302:875–878 [DOI] [PubMed] [Google Scholar]
- 34.Traynor K. Fda approves tofacitinib for rheumatoid arthritis. Am J Health Syst Pharm. 2012;69:2120. [DOI] [PubMed] [Google Scholar]
- 35.Tocchetti CG, Carpi A, Coppola C et al. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur J Heart Fail. 2014;16:358–366 [DOI] [PubMed] [Google Scholar]
- 36.Liao M, Liu Y, Yuan J et al. Single-cell landscape of bronchoalveolar immune cells in patients with covid-19. Nat Med. 2020;26:842–844 [DOI] [PubMed] [Google Scholar]
- 37.Han Y, Duan X, Yang L, et al. Identification of sars-cov-2 inhibitors using lung and colonic organoids. Nature. 2021;589:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajpai G, Schneider C, Wong N et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018;24:1234–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajpai G, Bredemeyer A, Li W et al. Tissue resident ccr2- and ccr2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019;124:263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G, Wu D, Guo W et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to covid-19 based on an analysis of data of 150 patients from wuhan, china. Intensive Care Med. 2020;46:846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fontes JA, Rose NR, Čiháková D. The varying faces of il-6: From cardiac protection to cardiac failure. Cytokine. 2015;74:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurung P, Dahal S, Chaudhary P, Guragain D, Karmacharya U, Kim JA, Jeong BS. Potent inhibitory effect of bj-3105, a 6-alkoxypyridin-3-ol derivative, on murine colitis is mediated by activating ampk and inhibiting nox. Int J Mol Sci. 2020;21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Gong FY, Li ZJ, Gong Z, Zhou Z, Ma SY, Gao XM. A study on the risk of fungal infection with tofacitinib (cp-690550), a novel oral agent for rheumatoid arthritis. Sci Rep. 2017;7:6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Baos S, Gratal P, Barrasa JI, Lamuedra A, Sanchez-Pernaute O, Herrero-Beaumont G, Largo R. Inhibition of pstat1 by tofacitinib accounts for the early improvement of experimental chronic synovitis. J Inflamm (Lond). 2019;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rayner-Hartley E, Sedlak T. Ranolazine: A contemporary review. J Am Heart Assoc. 2016;5:e003196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckel RH, Henry RR, Yue P, Dhalla A, Wong P, Jochelson P, Belardinelli L, Skyler JS. Effect of ranolazine monotherapy on glycemic control in subjects with type 2 diabetes. Diabetes Care. 2015;38:1189–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spinelli FR, Conti F, Gadina M. Hijaking sars-cov-2? The potential role of jak inhibitors in the management of covid-19. Sci Immunol. 2020;5 [DOI] [PubMed] [Google Scholar]
- 49.Blanco-Melo D, Nilsson-Payant BE, Liu WC et al. Imbalanced host response to sars-cov-2 drives development of covid-19. Cell. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Winter JCF. Using the student’s t-test with extremely small sample sizes. Practical Assessment, Research, and Evaluation. 2013;10:10 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data is available from the GEO repository database with accession number GSE169241. Source code is available from https://github.com/shuibingchen/COVID-19_Hearts.