Abstract
Background
The SARS-CoV-2 virus enters cells via Angiotensin-converting enzyme 2 (ACE2), disrupting the renin-angiotensin-aldosterone axis, potentially contributing to lung injury. Treatment with angiotensin receptor blockers (ARBs), such as losartan, may mitigate these effects, though induction of ACE2 could increase viral entry, replication, and worsen disease.
Methods
This study represents a placebo-controlled blinded randomized clinical trial (RCT) to test the efficacy of losartan on outpatients with COVID-19 across three hospital systems with numerous community sites in Minnesota, U.S. Participants included symptomatic outpatients with COVID-19 not already taking ACE-inhibitors or ARBs, enrolled within 7 days of symptom onset. Patients were randomized to 1:1 losartan (25 mg orally twice daily unless estimated glomerular filtration rate, eGFR, was reduced, when dosing was reduced to once daily) versus placebo for 10 days, and all patients and outcome assesors were blinded. The primary outcome was all-cause hospitalization within 15 days. Secondary outcomes included functional status, dyspnea, temperature, and viral load. (clinicatrials.gov, NCT04311177, closed to new participants)
Findings
From April to November 2020, 117 participants were randomized 58 to losartan and 59 to placebo, and all were analyzed under intent to treat principles. The primary outcome did not differ significantly between the two arms based on Barnard's test [losartan arm: 3 events (5.2% 95% CI 1.1, 14.4%) versus placebo arm: 1 event (1.7%; 95% CI 0.0, 9.1%)]; proportion difference -3.5% (95% CI -13.2, 4.8%); p = 0.32]. Viral loads were not statistically different between treatment groups at any time point. Adverse events per 10 patient days did not differ signifcantly [0.33 (95% CI 0.22–0.49) for losartan vs. 0.37 (95% CI 0.25–0.55) for placebo]. Due to a lower than expected hospitalization rate and low likelihood of a clinically important treatment effect, the trial was terminated early.
Interpretation
In this multicenter blinded RCT for outpatients with mild symptomatic COVID-19 disease, losartan did not reduce hospitalizations, though assessment was limited by low event rate. Importantly, viral load was not statistically affected by treatment. This study does not support initiation of losartan for low-risk outpatients.
Keywords: COVID-19, RAAS, Losartan, Angiotensin receptor blocker
Research in context.
Evidence before this study
Controversy exists surrounding the use of renin-angiotensin-aldosterone system (RAAS) modulation in COVID-19. Preclinical models in related viral pneumonias suggest a beneficial effect of RAAS inhibition, though some hypothesize these medications may increase the risk and severity of infections due to ACE2 induction. Observational studies and meta-analyses in COVID-19 to date have yielded mixed results.
Added value of this study
Losartan does not statistically significantly affect COVID-19 symptoms or viral load in mildly symptomatic outpatients. We observed no effect on hospitalization rate, with a 3.5% absolute increased rate (95% CI -4.8% - 13.2%) in participants treated with losartan.
Implications of all the available evidence
These data do not support initiation of losartan in newly diagnosed, mildly ill outpatients with COVID-19.
Alt-text: Unlabelled box
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused Coronavirus disease 19 (COVID-19) in over 85 million people globally including nearly 2 million deaths [1]. The overwhelming number of infections and hospitalizations strains the healthcare system, affecting even patients without COVID-19, creating a public health crisis.
The SARS-CoV-2 virus spike protein enters the cell via the angiotensin-converting enzyme 2 (ACE2) [2]. In states of health, ACE2 degrades angiotensin II (Ang II), a pulmonary vasoconstrictor and proinflammatory molecule, into angiotensin 1–7 and 1–9, which are lung-protective. Prior studies suggest patients with severe COVID-19 exhibit Ang II levels 5–10 times those of healthy individuals or with primary hypertension, and Ang II levels are associated with both viral load and lung injury [3]. Preclinical models of other viruses that utilize ACE2 (SARS and influenza) demonstrate reduction of lung injury following mitigation of this pathway [4], [5], [6]. We hypothesized treatment with an angiotensin receptor blocker, losartan, would decrease admission to the hospital secondary to and symptoms of COVID-19.
To this end, we conducted a pragmatic multicenter blinded randomized placebo-controlled clinical trial of symptomatic outpatients with COVID-19 not already treated with medications targeting the renin-angiotensin-aldoerone system (RAAS). The primary objective was to test if losartan treatment decreases hospitalization during the 15 days following enrollment. We also sought to determine if losartan improves self-reported dyspnea, functional status, or fever. As ARBs may induce ACE2 expression that could facilitate viral entry and replication, we tested the effect of losartan on SARS-CoV-2 viral load, though pragmatic considerations limited our ability to specifically test RAAS or ACE2 levels.
2. Methods
Trial design: This was a multicenter prospective double blind randomized placebo-controlled trial for the treatment of symptomatic outpatients with confirmed COVID-19 conducted across 3 hospital systems in Minnesota, United States (MHealth Fairview, Hennepin Healthcare, and Mayo Clinic) between April and November 2020 adhering to CONSORT guidelines [7]. Each of these healthcare systems serves between one and over a dozen hospitals, as well as dozens of clinic sites. All patients seeking testing at any of these locations had their results routed to central laboratories at each system. Specific eligibility for clinical testing varied over the course of the pandemic, with early restrictions based on limited availability following Centers for Disease Control (CDC) guidance, with later more widespread availability that varied by site.
Ethical approval and patient consent: The protocol was approved by a central institutional review board (Advarra Pro00042760) and underwent local context review. The study was conducted following good clinical practice guidelines under the oversight of an independent data safety monitoring board (DSMB). Participants provided written electronic informed consent prior to enrollment. The trial was conducted under the authority of the Food and Drug Administration (IND 148365), was registered on clinicaltrials.gov prior to study initialization (NCT04311177), and full protocol available from the corresponding author on request.
Participants: Consecutive patients presenting to a participating institution with a positive clinical SARS-CoV-2 result were screened for eligibility. Participants were eligible if they tested positive, were at least 18 years old, and were symptomatic within 24 h of informed consent. Eligibility initially required fever or upper respiratory symptoms, but this was expanded in July 2020 to include any CDC recognized symptoms [8]. Patients were excluded if they had >7 days of symptoms, were already taking an ACE-inhibitor or ARB, had prior adverse reactions to those medications, were pregnant or breastfeeding, were unwilling to use contraception or abstain from sex, had a history of dialysis, stage IV chronic kidney disease, or an estimated eGFR <30 ml/min/1.73 m2, a potassium >5.0 mmol/L, reported severe dehydration or reduced urine output over the prior 72 h, a history of cirrhosis, hepatitis B or C, or other severe liver disease expected to impair study drug metabolism, were prescribed aliskiren, had a measured systolic blood pressure of <110 mmHg at randomization, were enrolled in another blinded randomized clinical trial for COVID-19, or were unable to provide informed consent.
Screening and consent: All positive clinical tests for SARS-CoV-2 in the electronic medical records (EMR) of participating institutions were screened in a multi-step process. First, automated screening excluded children, tests conducted >6 days prior, and at some sites, those already taking ACE-i or ARBs or with renal failure. Further screening was conducted via manual chart review by trained research personnel. Patients without apparent exclusions were eligible for phone contact at home. After phone contact review of inclusion and exclusion criteria, eligible patients underwent an informed consent discussion. Informed consent was performed remotely via telephone or video teleconference, and documentation of consent was performed using a 21 CFR part 11 compliant electronic consent (eConsent) platform (REDCap) [7], [8], [9], [10]. Following consent, patients underwent safety screening including blood pressure, serum potassium, creatinine, and either urine or serum pregnancy testing in women of childbearing age performed at a facility equipped to manage participants actively infected with SARS-CoV-2 or via mobile phlebotomy service.
Randomization and blinding: Site-specific randomization schema were created (TM, HV) using permuted blocks with randomly varying sizes stratified by site and age (>=60 or <60 years). This cutoff was chosen based on early data suggesting markedly different outcomes by age, in order to mitigate potential unequal allocation to each arm. Randomization was stratified by site and age, and randomization schedules generated using permuted blocks with randomly varying sizes of 2, 4 or 6 and stored in an Oracle database. To randomize, coordinators completed an eligibility form in RedCap. If the patient was eligible, a link to the randomization was displayed, which led coordinators to the secure randomization website, where they logged in using a unique user name and strong password. The coordinator input into the randomization web page the patient's ID, sex, age, eGFR; study site was linked to the coordinator user account so that a coordinator could only access that site's randomization schedule. Treatment group was assigned from the pre-generated schedule in sequential order by a process running in the web page's background in a 1:1 allocation ratio; the coordinator was blinded to treatment but was provided a printout of the dosage schedule for that patient. The coordinator notified the pharmacy that a patient had been randomized.
The pharmacist logged into the secure pharmacy website using a unique user name and strong password, from which they printed out a report of the patient's treatment assignment and dosage schedule. A pharmacist had access only to treatment assignments for patients randomized at their site. This pharmacist then prepared study drug and directly shipped to the patient within 24 h. Losartan tablets were over-encapsulated in microcrystalline methylcellulose in opaque capsules, while placebo tablets were matched capsules containing only methylcellulose. Apart from statisticians preparing the randomization schema (HV), all other study members including participants, research personnel, and clinical teams were blinded.
Intervention: The intervention was losartan 25 mg versus equally appearing placebo. Losartan was chosen given lower rates of associated cough compared to ACE inhibitors, and a well-established safety profile. Participants self-administered study drug orally twice daily for 10 days for participants with eGFR >60 mL/min/1.73 m2, and once daily for those with eGFR 30–60 mL/min/1.73 m2. The threshold for angiotensin receptor blockade is 20 mg daily, with twice daily dosing more effective than once daily due to a 6–9 h half-life of the active metabolite. This dose was chosen in lieu of a 50 mg twice daily (maximum) dose after the FDA raised safety concerns regarding the higher dose regimen. This dose is expected to provide 37% inhibition of the angiotensin receptor. A lower dose (once daily) was chosen in those with renal dysfunction due to an altered risk/benefit ratio due to delayed clearance [11]. Ten days was chosen based on the assumption that patients would take 2–7 days from symptom onset until diagnosis, and that early data suggested most hospitalizations occurred within 15 days of symptom onset. This duration maximized doses administered in this time frame, while mitigating risk of longer unnecessary treatment. Study drug was discontinued if the patient met the primary outcome of hospital admission or by a blinded investigator if serious drug-related adverse effects were suspected.
Safety monitoring and outcomes: Participants were provided a cuff and thermometer to measure daily blood pressures and temperatures. Participants were contacted every other day on study days 2–10 to review adherence and assess for adverse events following FDA guidance [using the GRADE system with blinded assessment for relatedness, and seriousness defined according to 21 CFR 312.32(a), with events reported within 2 days if serious, otherwise within 5 days] and outcomes. Oropharyngeal swabs for viral load were collected at randomization and self-collected by the participant on days 3, 6, 9, and 15, and shipped on ice the same day. Patients were educated by phone and patient-facing online video regarding collection technique. Blood samples for potassium and creatinine were obtained on study day 15. Patients were followed until day 28.
Data management: A REDCap database was utilized for data management. User access groups were created with study role appropriate limited access maintained by a study team member, limited to local sites, with a complete audit trial. Data available in the electronic medical record were abstracted by trained coordinators and entered into the database. Data obtained from phone interactions were entered by study coordinators. Following completed data entry, case report forms were locked. Independent study monitoring was performed to validate data elements.
Efficacy outcomes: The primary outcome was all-cause hospitalization within 15 days. The outcome was defined as a hospital admission order to the hospital from the primary clinical team. In an effort to design a pragmatic and generalizable trial in the context of a still poorly understood disease early, there were no clinical preclusions or indications that were required to meet the primary endpoint. Secondary outcomes included COVID-19 related emergency department or clinic presentations (adjudicated by a blinded investigator), ventilator free days, and death. We measured severity using an accepted 7-point ordinal scale [12]. Subjective assessments of functional status and dyspnea were obtained on days 0, 4, 10, and 28 using validated scales (SF-12 [13,14] and PROMIS [15,16]). As angiotensin II has pro-inflammatory effects and losartan is anti-inflammatory [17], we measured maximum daily temperatures as a surrogate of the inflammatory response. Finally, viral load was assessed on days 1, 3, 6, 9, and 15.
Viral load: Molecular detection of SARS-CoV-2 virus was performed with a CLIA-validated laboratory developed RT-qPCR test [18] based on the N1 and N2 viral gene primer-probe sets developed by the United States Centers for Disease Control (CDC). Nucleic acid extraction was performed with the Zymo Research (California, USA) Quick-RNA Viral Kit per manufacturer's protocol. The relative viral load (RVL) is calculated as follows: 2^[CtRP - (CtN1+CtN2)/2]. When CtN1 or CtN2 were undetected they were replaced with a value of 45, the maximum number of cycles. This calculation normalizes the raw Ct value for the SARS-CoV-2 viral targets (N1 and N2) to the Ct value of the human RP internal sample control. This provides a normalized relative value of the amount of viral RNA compared to total human nucleic acid within each sample to more accurately compare individual samples after compensation for sampling and extraction quality.
Statistical analysis: All analyses between groups were conducted under intention-to-treat principles. Demographics and baseline clinical characteristics are summarized using descriptive statistics. Differences in the primary outcome are summarized using the sample proportion with 95% exact confidence intervals and statistical significance evaluated using Barnard's unconditional exact test. Differences in continuous variables are presented as means and 95% Wald confidence intervals with significance evaluated using two-sample t-tests assuming unequal variances. Differences in longitudinal endpoints were evaluated using generalized estimating equations (GEE) fit by the geepack package with an identity link and independence working correlation structure. Each GEE included a randomization group by assessment time interaction and significance of overall randomization group effect was determined by a global Wald test. The GEEs for functional and dyspnea assessments were adjusted for the corresponding outcome on day 1, whereas the GEE for temperature includes day 1 in the longitudinal outcome itself. An ordinal logistic regression model with a randomization group effect was used for the 7-point ordinal endpoint. Longitudinal viral load was evaluated using a linear mixed model fit by the lme4 package with a randomization group by assessment time interaction and random participant and batch effects. Viral load outcomes were characterized in two ways, mean CtN1 and CtN2, and log base 10 relative viral load, log10{2(CtRP - (CtN1 + CtN2)/2)}, with undetected CtN1 and CtN2 assigned a value of 45, which reflects the maximum cycle threshold, and the overall treatment group effect evaluated by likelihood ratio test. Adverse events were analyzed by organ system and allocation, as events per person day for the first 28 days. All analyses were completed using R version 4.0.3 [19].
Power and sample size: At the time of study design, the observed hospitalization rate and expected event rate for the primary outcome in the control group was 15% [20]. We deemed an absolute reduction of 8% to be clinically important. A sample size of 528 is required to achieve 80% power to detect a difference between a hospitalization rate of 0.15 and 0.07, assuming a two-sided alpha of 0.05 with a continuity correction [21], which was inflated to a total enrollment of 580 to account for an approximately 10% loss to followup. We chose this target risk reduction as a clearly clinically meaningful target difference to justify a major change in practice pattern since we were not targeting relatively harder outcomes such as mortality or ventilator-free days. It is possible a smaller decrease might be clinically meaningful in the setting of a pandemic when hospital utilization is high.
Role of the funding source: This study was supported by Minnesota Partnership for Biotechnology and Medical Genomics (CON000000076883); the funder had no access to the dataset, and no role in the development, design, analysis, interpretation of results, or decision to publish. MAP, HV, TM, JK had access to the data. MAP made the decision to submit for publication.
3. Results
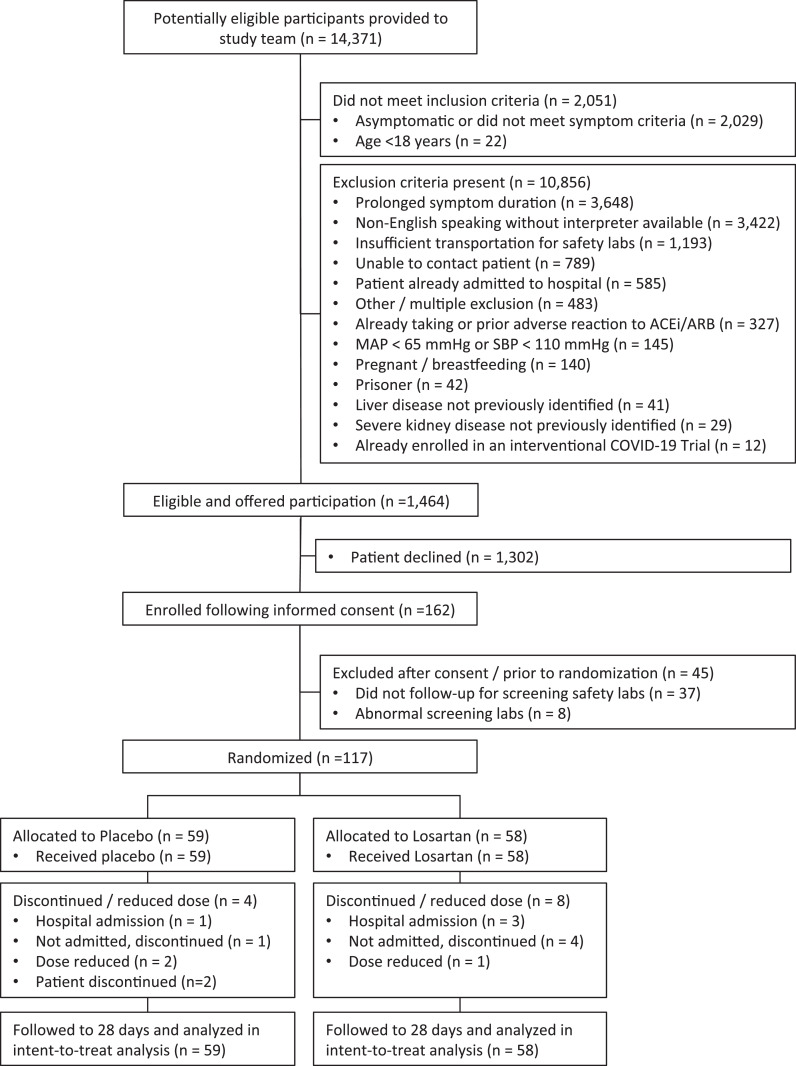
The study flow diagram is provided in Fig. 1. Ultimately 117 participants were randomized, 58 to the intervention and 59 to placebo. Demographics and clinical characteristics were well matched (Table 1) with the exception of a slight predominance of female patients allocated to losartan. Participants were mildly to moderately ill, young to middle age, and predominantly white with a low comorbidity burden. Symptoms at enrollment are summarized in Table 2.
Fig. 1.
Study flow diagram of patient enrollments and randomization
Table 1.
Trial demographics and comorbidities following intention to treat by randomization group. Binary and categorical variable summaries reflect No (%) {No Missing}, and continuous variable summaries reflect Median [25th, 75th percentiles] {No Missing}.
| Randomization Group |
||
|---|---|---|
| Placebo | Losartan | |
| Number Enrolled | 59 | 58 |
| Site of Enrollment | ||
| M Health | 24 (40.7%) | 23 (39.7%) |
| Hennepin | 25 (42.4%) | 26 (44.8%) |
| Mayo | 10 (16.9%) | 9 (15.5%) |
| Demographics | ||
| Female | 25 (42.4%) {0} | 33 (56.9%) {0} |
| Age | 37 [27, 46] {0} | 38 [29, 51] {1} |
| 18 – 34 | 27 (45.8%) | 20 (34.5%) |
| 35 – 54 | 23 (39%) | 31 (53.4%) |
| 55 – 64 | 9 (15.3%) | 3 (5.2%) |
| 65 – 89 | 0 (0%) | 3 (5.2%) |
| Missing | 0 (0%) | 1 (1.7%) |
| BMI | 27.5 [24.5, 32.0] {0} | 28.3 [24.8, 32.6] {0} |
| Normal weight (18.5 – 24.9) | 18 (30.5%) | 16 (27.6%) |
| Overweight (25 – 29.9) | 22 (37.3%) | 19 (32.8%) |
| Obese (30 – 55) | 19 (32.2%) | 23 (39.7%) |
| Race | ||
| Caucasian | 39 (66.1%) | 45 (77.6%) |
| Black or African American | 4 (6.8%) | 4 (6.9%) |
| Asian | 5 (8.5%) | 1 (1.7%) |
| Hispanic | 5 (8.5%) | 5 (8.6%) |
| Other/Unknown | 6 (10.2%) | 3 (5.2%) |
| Ethnicity | ||
| Non-Hispanic or Latino | 49 (83.1%) | 50 (86.2%) |
| Hispanic or Latino | 6 (10.2%) | 4 (6.9%) |
| Unknown | 4 (6.8%) | 4 (6.9%) |
| Insurance Type | ||
| Uninsured | 4 (6.8%) {0} | 5 (8.6%) {0} |
| Medicaid | 3 (5.1%) {0} | 3 (5.2%) {0} |
| Medicare | 0 (0%) {0} | 1 (1.7%) {0} |
| Private | 52 (88.1%) {0} | 49 (84.5%) {0} |
| Comorbid Conditions | ||
| Coronary Artery Disease | 0 (0%) {0} | 0 (0%) {1} |
| Hypertension | 3 (5.1%) {0} | 6 (10.3%) {1} |
| Congestive Heart Failure | 0 (0%) {0} | 0 (0%) {1} |
| Pulmonary Hypertension | 0 (0%) {0} | 0 (0%) {1} |
| Asthma | 8 (13.6%) {0} | 4 (6.9%) {1} |
| Chronic Obstructive Pulmonary Disorder | 0 (0%) {0} | 0 (0%) {1} |
| Chronic Bronchitis | 0 (0%) {0} | 0 (0%) {1} |
| Obstructive Sleep Apnea | 5 (8.5%) {0} | 3 (5.2%) {1} |
| Diabetes Mellitus | 3 (5.1%) {0} | 4 (6.9%) {1} |
| Tobacco or Nicotine User | 5 (8.5%) {0} | 7 (12.1%) {1} |
Table 2.
Baseline vitals and symptomatology by randomization group. Categorical variable summaries reflect n (%), and continuous variable summaries reflect mean (std dev) {n missing}.
| Randomization Group |
||
|---|---|---|
| Placebo | Losartan | |
| Number Enrolled | 59 | 58 |
| Baseline Vitals | ||
| Systolic Blood Pressure | 133 (16) {0} | 132 (14) {0} |
| Diastolic Blood Pressure | 82 (10) {0} | 82 (11) {0} |
| Mean Arterial Pressure | 99 (11) {0} | 98 (11) {0} |
| Temperature (℉) | 98.3 (0.9) {16} | 98.2 (1.1) {17} |
| Heart Rate | 80 (14) {11} | 80 (13) {12} |
| Respiratory Rate (Breaths/min) | 17 (1) {30} | 17 (1) {32} |
| Oxygen Saturation | 98 (1) {10} | 97 (5) {10} |
| Creatinine (mg/dL) | 0.9 (0.2) {0} | 1 (0.6) {0} |
| Potassium (mmol/dL) | 5.5 (12) {0} | 3.9 (0.6) {0} |
| Symptomatology | ||
| Days with Symptoms Before Seeking Care | 1.7 (1.4) {0} | 1.7 (1.5) {0} |
| 0 Days | 10 (16.9%) | 10 (17.2%) |
| 1 Day | 24 (40.7%) | 24 (41.4%) |
| 2 Days | 10 (16.9%) | 11 (19%) |
| 3 Days | 10 (16.9%) | 7 (12.1%) |
| 4 – 7 Days | 5 (8.5%) | 6 (10.3%) |
| Cough (Dry) | 46 (78%) {0} | 40 (69%) {0} |
| Muscle Aches (myalgias) | 44 (74.6%) {1} | 45 (77.6%) {0} |
| Headache | 43 (72.9%) {0} | 42 (72.4%) {2} |
| Fatigue / Malaise | 38 (64.4%) {3} | 42 (72.4%) {2} |
| Fever | 35 (59.3%) {2} | 34 (58.6%) {2} |
| Sore Throat | 28 (47.5%) {1} | 24 (41.4%) {3} |
| Runny Nose (Rhinorrhea) | 21 (35.6%) {0} | 31 (53.4%) {0} |
| Sinus congestion | 23 (39%) {8} | 27 (46.6%) {10} |
| Loss of taste | 22 (37.3%) {3} | 22 (37.9%) {3} |
| Loss of smell | 19 (32.2%) {2} | 19 (32.8%) {4} |
| Cough (with sputum production) | 12 (20.3%) {2} | 21 (36.2%) {1} |
| Shortness of Breath | 13 (22%) {0} | 17 (29.3%) {1} |
| Diarrhea | 17 (28.8%) {3} | 12 (20.7%) {1} |
| Joint Pain | 9 (15.3%) {3} | 16 (27.6%) {1} |
| Vomiting/Nausea | 12 (20.3%) {2} | 11 (19%) {1} |
| Other symptom | 11 (18.6%) {9} | 11 (19%) {11} |
| Chest Pain | 7 (11.9%) {1} | 12 (20.7%) {2} |
| Abdominal Pain | 4 (6.8%) {3} | 13 (22.4%) {4} |
| Wheezing | 6 (10.2%) {1} | 6 (10.3%) {0} |
| Lymphadenopathy | 6 (10.2%) {4} | 5 (8.6%) {5} |
| Altered Mental Status | 2 (3.4%) {1} | 4 (6.9%) {0} |
| Bleeding (Hemorrhage) | 0 (0%) {2} | 0 (0%) {2} |
| Skin rash | 1 (1.7%) {3} | 2 (3.4%) {2} |
| Inability to walk | 1 (1.7%) {1} | 0 (0%) {0} |
| Skin ulcers | 0 (0%) {5} | 0 (0%) {3} |
| Conjunctivitis | 0 (0%) {3} | 1 (1.7%) {4} |
| Seizures | 0 (0%) {0} | 0 (0%) {0} |
| Cough (with hemoptysis) | 0 (0%) {1} | 0 (0%) {2} |
Only 4 patients (3.4%) met the primary outcome of all-cause hospitalization at or before day 15, lower than the expected rate of 11% (15% in the control vs. 7% hypothesized in the treatment group), all occurring before August 15, 2020. We did not observe a statistically significant difference in the primary outcome between the arms, with 3 events in the losartan arm (5.2%; 95% CI 1.1, 14.4%) versus 1 in the placebo arm (1.7%; 95% CI 0.0, 9.1%), corresponding to an absolute difference of -3.5% (95% CI -13.2, 4.8%; p = 0.320) favoring placebo (Table 3). The clinical presentation and indications for admission are provided in Table 4. Due to the low primary efficacy event rate as well as decreasing rates of enrollment due to decreasing local COVID-19 positivity rates coupled with significantly lower rates of willingness to participate in clinical trials due to development of new treatments and pending availability of vaccines (moving the expected completion date well into 2022 or beyond), the trial was terminated early by the investigators prior to treatment allocation unblinding in consultation with the DSMB. This consultation occurred prior to the preplanned efficacy assessment at 50% enrollment, and only rolling safety data were available to the DSMB at the time of this decision.
Table 3.
Primary and secondary outcomes, by treatment allocation, with 95% exact confidence intervals.
| Randomization Group |
|||
|---|---|---|---|
| Placebo (n = 59) | Losartan (n = 58) | Difference | |
| Primary outcome | |||
| All cause hospitalization (15 days), % | 1.7 (0.0, 9.1) | 5.2 (1.1, 14.4) | -3.5 (-13.2, 4.8) |
| Secondary outcomes | |||
| Additional hospitalizations, (15-28 days), % | 0.0 (0.0, 6.1) | 0.0 (0.0, 6.2) | 0.0 (-6.8, 6.7) |
| Additional ED/clinic visits (28 days), % | 6.8 (1.9, 16.5) | 8.6 (2.9, 19.0) | -1.8 (-13.3, 9.4) |
| Intensive care unit admission, % | 1.7 (0.0, 9.1) | 1.7 (0.0, 9.2) | 0.0 (-8.2, 8.1) |
| Death, % | 0.0 (0.0, 6.1) | 0.0 (0.0, 6.2) | 0.0 (-6.8, 6.7) |
ED – Emergency department
Table 4.
Clinical scenario, indications for hospital admission, and supplemental oxygen-free days for participants meeting the primary outcome.
| Randomization Group | Clinical presentation and indication for admission | O2 free days |
|---|---|---|
| Placebo | Dyspnea, fever, hypoxia to 90% | 7.3 |
| Losartan | Dyspnea, weakness, fall, hypoxia to 85% | 0 |
| Losartan | Dyspnea, cough, fever, hypoxia to 92% | 12.0 |
| Losartan | Dyspnea with normal vital signs, blood tinged sputum, viral pneumonitis without pulmonary embolism | 23.2 |
Secondary outcomes by treatment allocation are also shown in Table 3. There were no additional hospitalizations between days 15 and 28. There were 4 additional participants in the placebo arm and 5 additional participants in the losartan arm who presented to an ED/clinic within 28 days. Only 1 participant in each arm was admitted to the intensive care unit, and none died. The composite outcome of any visit within 28 days did not differ between groups [8 in the losartan arm (13.8%; 95% CI 6.1, 25.4%) versus 5 in the placebo arm (8.5% 95% CI 2.8, 18.7%); difference of -5.1% (95% CI -17.4, 7.1%; p = 0.529)]. There was no statistically significant variation in mechanical ventilatory support and very few patients required supplemental oxygen due to very low admission rates. As such, these data were not analyzed further for differences in ventilator-free or therapeutic oxygen-free days, as these data were captured in the severity of illness scale, though oxygen-free days can be found in Table 3.
When ascertaining the ordinal disease severity scale, functional limitations for those out of the hospital were only assessed on Day 15, and there were few with limitations or worse (<=5), 2 (3.6%) in the placebo group and 7 (12.7%) in the losartan arm. The unadjusted odds ratio estimate was -1.3 (95% CI -3.3, 0.2; p = 0.0803) favoring placebo, and after adjusting for age and assigned gender the odds ratio estimate was -1.4 (95% CI -3.4, 0.2; p = 0.0962). Due to partial withdrawals and loss to follow-up, there were 4 placebo participants and 3 losartan participants missing ordinal outcomes on Days 7, 15 and 28. All were alive and out of the hospital, but whether or not they had functional limitations was unknown. Assuming participants with missing values on Day 15 had functional limitations did not substantively alter this finding. Analyses of disease severity on Days 7 and 28 were not conducted as functional limitations for participants out of the hospital were not assessed and there were only four participants admitted to the hospital prior to Day 28. One placebo participant was hospitalized on mechanical ventilation on days 7 and 15, and was hospitalized but required no supplemental oxygen on Day 28. One losartan participant was admitted and discharged prior to Day 7. Another was hospitalized on Day 7 with supplemental oxygen, but was discharged prior to Day 15. The last was hospitalized on Day 7, requiring a non-invasive mechanical ventilation or high flow device, and on Day 15, requiring supplemental oxygen, but discharged prior to Day 28.
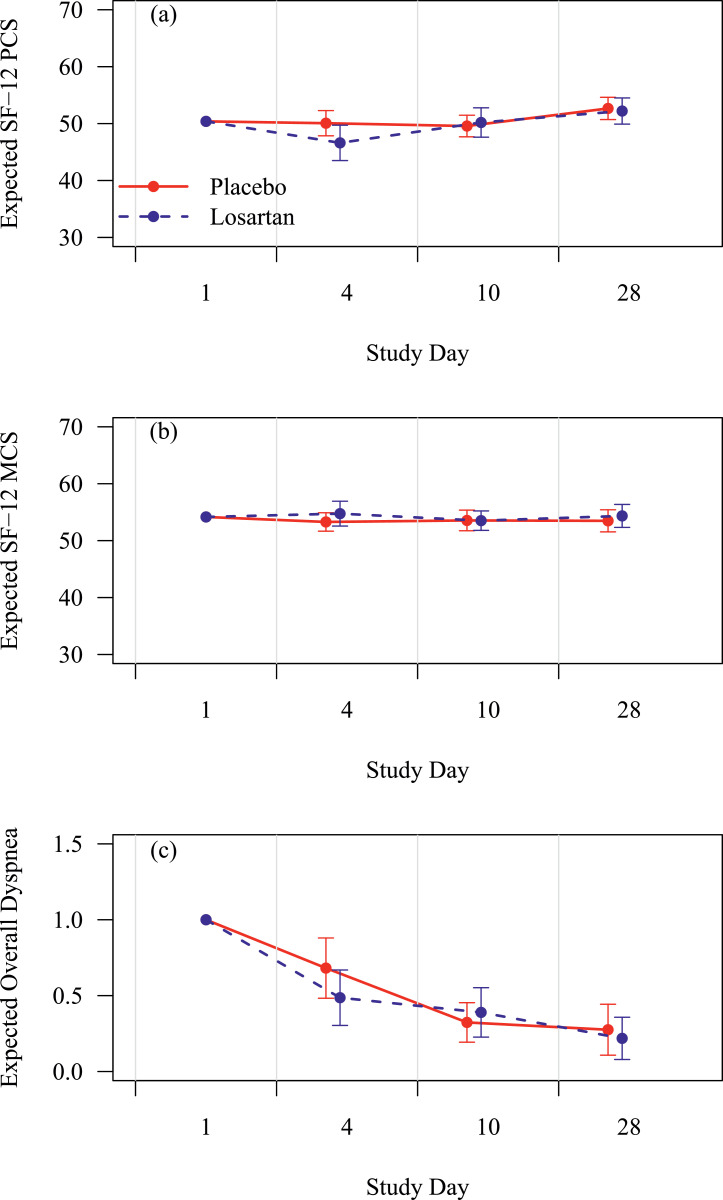
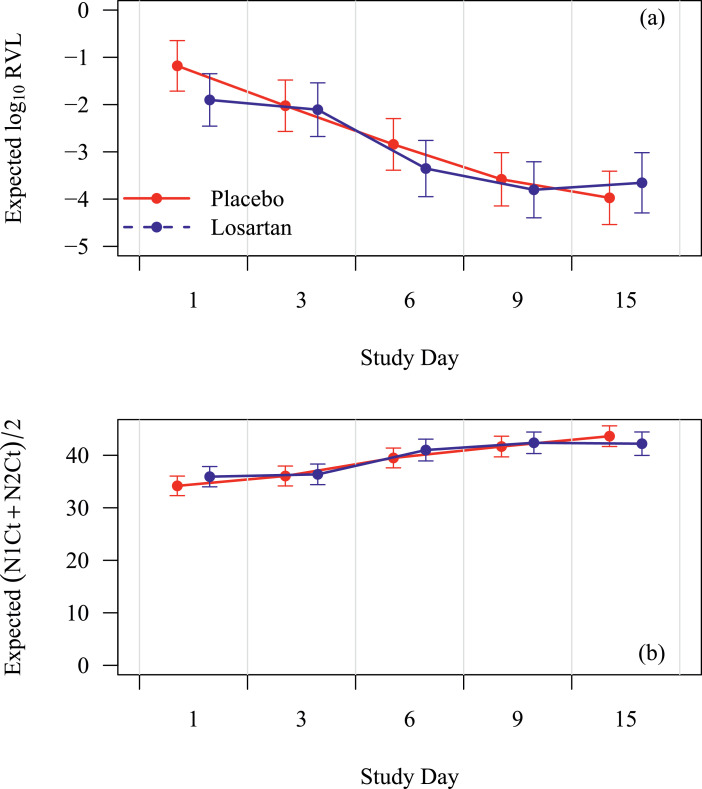
Losartan did not have a statistically significantly impact on functional status or dyspnea (Fig. 2). Missingness rates were 2–26% for overall dyspnea (increasing over time) versus 22–45% for SF-12 scores due to partial compliance, early modification to the survey instruments, and losses to follow-up. Supplemental analyses using multiple imputation of 20 complete datasets based on predictive mean matching did not substantively alter these findings. Maximum daily temperature did not statistically differ by treatment allocation (p = 0.12, not shown). Viral loads are illustrated in Fig. 3, and no statistically significantly difference by treatment allocation was observed at any point.
Fig. 2.
Effect of losartan on (2a) SF-12 Physical Component Score (PCS); (2b) SF-12 Mental Component Score (MCS); and (2c) PROMIS overall dyspnea, respectively. X-axis is study day and y-axis is score on the instrument. Placebo is in red lines and losartan in blue lines with 95% CIs at each assessment. Losartan did not statistically significantly affect these outcomes overall or at any time point. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Fig. 3.
Effect of losartan on (3a) relative viral load (log10 scale); (3b) mean cycle threshold (Ct), respectively. X-axis is study day and y-axis is relative viral load (RVL) or threshold cycle (Ct). Relative viral load (RVL) is corrected to human marker DNA to control for specimen quality. Mean cycle threshold (Ct) is inversely related to viral load (high viral loads have a low Ct). Placebo is in red lines and losartan in blue lines with 95% CIs at each assessment. Losartan did not statistically significantly affect the cycle threshold or relative viral load overall or at any time point. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
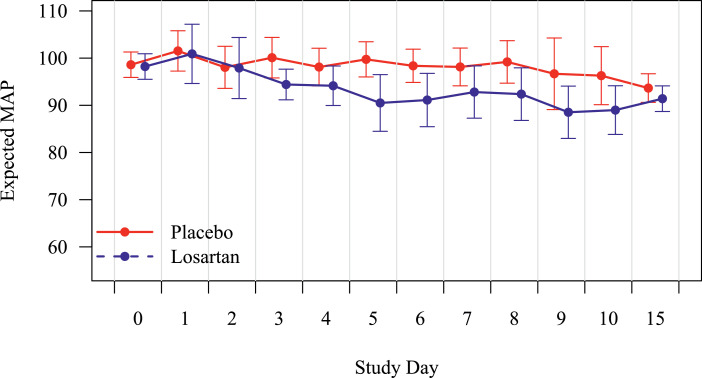
The treatment was well tolerated, and the majority of participants completed the entire course, 84% in the losartan arm vs. 93% of those assigned to placebo. No statistically significant differences were observed in the number of SAEs or AEs per participant. Organ specific AE and SAE rates per day for the first 28 days are provided in Table 5, as well as by anticipated / unanticipated for losartan and/or COVID, and finally by relatededness, all judged by blinded investigators Table 6. Mean arterial pressure was reduced from baseline at day 15 in both arms, but the difference in reductions was not statistically significant (p = 0.725, not shown). By generalized modeling, losartan appeared to cause a transient, non-statistically significant reduction in blood pressure (p = 0.16, Fig. 4). No participants in either group required evaluation for a hypotensive event, developed kidney injury or hyperkalemia.
Table 5.
Per person day rate of Adverse Events and Serious Adverse Events during first 28 days with estimates (95% CI) and p-value based on negative binomial regression with a likelihood ratio test.
| Type of AE/SAE | Losartan (n = 58) | Placebo (n = 59) | P-value |
|---|---|---|---|
| Respiratory | 0.008 (0.004,0.014) | 0.016 (0.01,0.025) | 0.0518 |
| ENT | 0.005 (0.002,0.011) | 0.002 (0.001,0.006) | 0.1912 |
| Skin | 0.002 (0.001,0.007) | 0 (NA*) | NA* |
| Renal | 0.001 (0.000,0.006) | 0.003 (0.001,0.007) | 0.443 |
| Gastrointestinal | 0.003 (0.001,0.011) | 0.002 (0.000,0.008) | 0.7465 |
| Cardiovascular | 0.006 (0.003,0.012) | 0.004 (0.002,0.009) | 0.3852 |
| Constitutional | 0.004 (0.002,0.010) | 0.008 (0.004,0.015) | 0.2117 |
| Neurologic | 0.002 (0.001,0.007) | 0.001 (0.000,0.005) | 0.6161 |
| Any | 0.033 (0.022,0.049) | 0.037 (0.025,0.055) | 0.6806 |
Events are reported as rates per person-day because the patients could have had more than one event.
*Because there were no Skin AEs/SAEs in the Placebo arm, the MLE does not exist (it's on the boundary of the parameter space) and thus asymptotic confidence intervals and p-values fail.
Table 6.
Per person day rate of Adverse Events (AEs) during first 10 and 28 days with estimates (95% CI) and p-value based on negative binomial regression with a likelihood ratio test. AEs were assessed by a blinded investigator, and expected AEs for COVID-19 and losartan are listed and analyzed below. For potentially related AEs to the intervention, the likelihood (unrelated, unlikely, possibly, probably, or definitely) was assessed based on the blinded investigator's judgment.
| Losartan (n = 58) | Placebo (n = 59) | P-value | |
|---|---|---|---|
| First 10 Days Per-Person Per-Day AE Rates | |||
| Expected Covid-19 | 0.138 (0.1,0.189) | 0.181 (0.134,0.244) | 0.2137 |
| Expected Losartan (Any Relatedness) | 0.064 (0.046,0.089) | 0.062 (0.045,0.086) | 0.8776 |
| Expected Losartan (Unlikely or More Related) | 0.05 (0.034,0.074) | 0.041 (0.027,0.063) | 0.4913 |
| Expected Losartan (Possibly or More Related) | 0.012 (0.006,0.026) | 0.012 (0.006,0.026) | 0.9871 |
| Expected Losartan (Probably Related) | 0 (0,Inf) | 0.002 (0,0.012) | 0.2405 |
| First 28 Days Per-Person Per-Day AE Rates | |||
| Expected Covid-19 | 0.058 (0.044,0.078) | 0.077 (0.059,0.1) | 0.1711 |
| Expected Losartan (Any Relatedness) | 0.027 (0.02,0.037) | 0.028 (0.021,0.038) | 0.8688 |
| Expected Losartan (Unlikely or More Related) | 0.022 (0.015,0.033) | 0.021 (0.014,0.031) | 0.8048 |
| Expected Losartan (Possibly or More Related) | 0.005 (0.002,0.01) | 0.005 (0.002,0.01) | 0.9814 |
| Expected Losartan (Probably Related) | 0 (0,Inf) | 0.001 (0,0.005) | 0.2369 |
Fig. 4.
Effect of losartan on mean blood pressure over study days 1–15. X-axis is day of treatment, and y-axis is mean arterial pressure (MAP) in mmHg. Placebo is in red and losartan in blue with 95% CIs at each assessment. While losartan did not statistically significantly affect these outcomes overall or at any time point, a non-significant reduction of up to 10 mmHg was observed at approximately day 5. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
To our knowledge this is the first randomized blinded clinical trial testing the effect of initiation of a RAAS inhibitor to naive outpatients with COVID-19. This study also represents one of the first placebo-controlled interventional trials of an oral medication in outpatients with COVID-19. We tested the hypothesis that blockade of angiotensin II activity with losartan would decrease both hospitalization and respiratory symptoms of COVID-19. Based on the 95% confidence interval, we can rule out a treatment effect larger than a 4.8% absolute decrease in the hospitalization rate in favor of losartan, less than our a priori threshold for clinical significance. Given that the 95% confidence interval falls outside our a priori threshold for targeted effect size, one could interpret this trial as informatively negative. This, however, is contingent on whether or not a clinician considers a 4.8% absolute reduction in hospitalization rate as clinically meaningful or not. Our interpretation is that while this reduction may be clinically meaningful during times of resource strain or in resource poor environments, the relatively soft nature of the outcome might not justify practice change in the current COVID-19 clinical practice environment, even if true.
While underpowered and not statistically significant, it should be noted that in terms of both hospital admission and any healthcare encounter, placebo outperformed losartan, and a risk of harm cannot be ruled out. Surrogate indicators of lung injury (dyspnea and functional status) and inflammation (temperature) do not support significant impact on these mechanisms of action. It is unlikely losartan affects a clinically significant reduction in hospitalization of low-risk outpatients with COVID-19.
While antivirals [12], anti-inflammatories [12,22], and improved processes of care have likely improved outcomes [23] for hospitalized patients with COVID-19, effective outpatient treatments remain limited with few oral treatments available. Monoclonal antibodies have received emergency use authorization from the United States Food and Drug Administration (FDA) [23], [24], [25] though it remains unclear who should receive this treatment. Administration of intravenous infusions to outpatients creates logistical challenges, contributing to slow adoption of this treatment [26]. While vaccine progress is astounding, vaccination programs take time, especially in less developed nations, and widespread distrust among portions of the public remains [27], meaning immunization efforts will continue for several years. As such, there remains a pressing need for low cost, oral, safe, adjuvant therapeutic options.
This trial was designed and powered based on early observations regarding the hospitalization rate of patients with COVID-19 from China and later New York in February-March of 2020. The initial goal was to complete a highly pragmatic trial, as early data suggested a higher outcome rate than subsequently observed. The expected 15% hospitalization rate was markedly lower in our study, which contributed substantially to the decision to terminate the trial early. The source of this discrepancy is multifactorial. First, early testing availability was limited, introducing selection bias and higher initial hospitalization rates. We now understand there exists widespread infection amongst asymptomatic and minimally symptomatic individuals [28]. Unfortunately, by the time these data were recognized and well understood, the trial was already well underway. While we limited enrollment to those with symptoms, the severity of illness of trial participants was lower than expected. Consideration was given to limiting enrollment to a higher risk cohort partially through the trial, but overall reluctance to change enrollment criteria partially through the study as it was not part of the a priori design, and our experience and challenges with trial enrollment precluded this as a feasible solution. Moreover, the remotely conducted trial design likely introduced further selection bias. The enrolled participants skewed younger, less ethnically diverse, and with fewer comorbidities than the target population despite significant efforts to reach out to older and more diverse populations. Anecdotally, we enrolled a moderate proportion of healthcare workers with better access to diagnostic laboratory testing earlier in the pandemic, who exhibited a greater likelihood to consent to trial participation. Combined, these factors contributed to the lower than expected event rate. Nevertheless, conclusions regarding the effect size of losartan in this less severely-ill cohort remain valid, and demonstrate supportive care alone may be sufficient when considering the effect on short-term COVID-19 outcomes in mildly ill patients.
It is worth considering the potential utility of the intervention given the low enrollment rate. About 15% of patients were excluded for being asymptomatic, and would not likely benefit from the treatment. Other exclusions on Fig. 1 that would suggest against clinical utility such as prior use of ACEi/ARB, low blood pressure, prior medication reaction, or liver disease represent a small proportion, another 10–15% of patients. All of the remaining patients were not enrolled specifically for study related barriers – prolonged symptom duration (reflecting early, slow testing), non-English speakers (limited due to interpreter service availability), insufficient transportation for safety monitoring (rarely practiced during clinical use of losartan for even longer time periods outside of clinical trials), or patient's declining to participate. This suggests the intervention could be relevant to practice, if efficacious.
We hypothesized that if losartan were to reduce hospitalization, this would be achieved through the reduction of angiotensin II induced lung injury. We captured dyspnea using self-reported a 5-question dyspnea symptom inventory (PROMIS) [29,30] and functional status using a validated instrument often utilized to assess quality of life in dyspneic patients (SF-12) [31]. We measured maximum daily temperature as a proxy of systemic inflammation. None of these measures differed significantly by treatment allocation, arguing against the clinical significance of losartan to affect this mechanism of disease in this population, either due to irrelevance of this pathway in patients unlikely to suffer serious short-term sequelae of COVID-19 or ineffectiveness of losartan to blunt this response.
Despite the well understood safety profile of losartan, this trial provides important data regarding the effect of ARBs in the setting of COVID-19. A particularly important observation is that losartan did not affect viral load. Prior observations demonstrate ARBs induce expression of ACE2 [32]. An increased density of receptors promote viral entry, replication, and paradoxically worsen symptoms. This initially led to concerns regarding the safety of this class of medications in COVID-19 [33,34], though subsequent preclinical studies suggest against these concern [35]. While large observational studies of other RAAS targeted clinical trials have not demonstrated a negative clinical impact of these treatments, the effect of RAAS inhibition on viral load within a clinical trial has not been previously reported [36], [37], [38], [39]. his trial data augment our understanding and support the preclinical and observational data to date.
This study provides insight into opportunities and challenges of conducting remote clinical trials during a pandemic. The protocol was designed to minimize face-to-face contact and maintain quarantine, particularly when personal protective equipment was scarce. While remote consent facilitated these desires, it may have introduced a selection bias against non-English speakers, older patients less comfortable with technology, and economically disadvantaged patients [40]. We unexpectedly encountered patients either not sick enough consent to a clinical trial or too sick to present for safety laboratory testing. Ongoing and future remote trials should consider mobile phlebotomy teams or dried blood spot testing.
There are several important limitations to consider. This trial was underpowered to detect small differences in hospitalization given the mild illness of participants. Given the issues surrounding remote consent, fear of an unknown disease, and logistical challenges, there exists concern for selection bias and drawing conclusions applicable to a higher risk group of outpatients or inpatients would be inappropriate. While we might expect similar results across different ARBs or even ACE inhibitors, there may be important in-class differences. Regarding viral load, it is possible the short course of treatment is insufficient to affect ACE2 expression, and our results may not be applicable to those taking these medications chronically. Critically, due to limitations in funding and a focus on a pragmatic design, we were not able to assess the effect of the intervention on the RAAS pathways in question, either systemically or in the lungs, though this remains an area of further investigation. Finally, we acknowledge that studies stopped early for futility have been an area of debate for 40 years [41]. We made the difficult decision of stopping early because of the observed hospitalization rates, logistical challenges, and concern of more equitable allocation of potential study participants into larger national outpatient trial efforts more likely to identify an effective therapy.
In summary, treatment of mildly symptomatic outpatients with 10 days of losartan did not reduce subsequent hospitalization rates. Losartan treatment did not improve functional status, reduce self-reported dyspnea, and or affect viral load. These data do not support the initiation of losartan in ACE-i or ARB naive outpatients with mild COVID-19 disease.
Funding
This study was supported by Minnesota Partnership for Biotechnology and Medical Genomics (CON000000076883).
Data sharing statement
Deidentified data are in the process of being deposited on the Data Repository for the University of Minnesota (DRUM), and the corresponding author can be contacted for data access.
CRediT authorship contribution statement
Michael A. Puskarich: Writing – original draft, Conceptualization, Funding acquisition, Formal analysis, Data curtion. Nathan W. Cummins: Conceptualization, Funding acquisition, Formal analysis, Data curtion. Nicholas E. Ingraham: Conceptualization. David A. Wacker: Funding acquisition. Ronald A. Reilkoff: Funding acquisition. Brian E Driver: Funding acquisition, Data curtion. Michelle H. Biros: . Fernanda Bellolio: Funding acquisition, Data curtion. Jeffrey G. Chipman: Conceptualization. Andrew C. Nelson: Data curtion. Kenneth Beckman: . Ryan Langlois: . Tyler Bold: . Matthew T. Aliota: Funding acquisition. Timothy W. Schacker: Conceptualization. Helen T. Voelker: Formal analysis. Thomas A Murray: Formal analysis. Joseph S. Koopmeiners: Data curtion. Christopher J. Tignanelli: Data curtion.
Declaration of Competing Interest
The authors have no financial conflicts of interest to disclose. All the authors report grants from Minnesota Partnership for Biotechnology and Medical Genomics during the conduct of the study. MAP reports also grants from Bill and Melinda Gates Foundation and grants from NHLBI, outside the submitted work.
Acknowledgments
We would like to acknowledge the data safety monitoring board members, John Younger, MD, Scott Halpern MD, PhD, Raymond Townsend, MD, Scott Emerson, MD, PhD, and Jeremy Kahn, MD. Additionally, we would like to thank Dave Anklarlo, our project manager, and lead coordinators Julie Scherber and Alex Dunn, whose tireless efforts helped make this trial possible.
References
- 1.Coronavirus update (Live): 86,321,500 cases and 1,865,798 deaths from COVID-19 virus pandemic - Worldometer. Accessed January 5, 2021. https://www.worldometers.info/coronavirus/.
- 2.Ingraham N.E., Barakat A.G., Reilkoff R. Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.00912-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai Y., Kuba K., Penninger JM. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. 2008;93(5):543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou Z., Yan Y., Shu Y. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz K.F., Altman D.G., Moher D., Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. Symptoms of coronavirus. Published January 4, 2021. Accessed January 5, 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- 9.Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sica D.A., Gehr T.W., Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797–814. doi: 10.2165/00003088-200544080-00003. [DOI] [PubMed] [Google Scholar]
- 12.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biehl M., Kashyap R., Ahmed A.H. Six-month quality-of-life and functional status of acute respiratory distress syndrome survivors compared to patients at risk: a population-based study. Crit Care. 2015;19(1):1–8. doi: 10.1186/s13054-015-1062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Website. Accessed January 5, 2021. https://www.jstor.org/stable/3766749?seq=1
- 15.Hinchcliff M., Beaumont J.L., Thavarajah K. Validity of two new patient reported outcome measures in systemic sclerosis: the PROMIS-29 profile and the FACIT-dyspnea. Arthritis Care Res. 2011;63(11):1620. doi: 10.1002/acr.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yount S.E., Atwood C., Donohue J. Responsiveness of PROMIS® to change in chronic obstructive pulmonary disease. J Patient Rep Outcomes. 2019;3(1):1–13. doi: 10.1186/s41687-019-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Ortega M., Ruperez M., Esteban V., Rodriguez-Vita J., Sanchez-Lopez E., Egido J. Modulation of angiotensin II effects, a potential novel approach to inflammatory and immune diseases. Curr Med Chem Anti-Inflamm Anti-Allergy Agents. 2003;2(4):379–394. doi: 10.2174/1568014033483626. [DOI] [Google Scholar]
- 18.Nelson A.C., Auch B., Schomaker M. Analytical validation of a COVID-19 qRT-PCR detection assay using a 384-well format and three extraction methods. Cold Spring Harbor Lab. 2020 doi: 10.1101/2020.04.02.022186. Published online April 52020.04.02.022186. [DOI] [Google Scholar]
- 19.Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ URL. [Google Scholar]
- 20.Wu Z., McGoogan JM. Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 21.Fleiss J.L., Tytun A., Ury H.K. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics. 1980;36:343–346. [PubMed] [Google Scholar]
- 22.RECOVERY Collaborative Group. Horby P., Lim W.S. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. Published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asch D.A., Sheils N.E., Islam M.N. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2020;22 doi: 10.1001/jamainternmed.2020.8193. Published online December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An EUA for bamlanivimab—a monoclonal antibody for COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.24415. Published online. [DOI] [Google Scholar]
- 25.Chen P., Nirula A., Heller B. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2029849. Published online October 28, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinreich D.M., Sivapalasingam S., Norton T. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2020;17 doi: 10.1056/NEJMoa2035002. Published online December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris R. Low demand for antibody drugs against COVID-19. NPR. Published December 22, 2020. Accessed January 5, 2021. https://www.npr.org/sections/health-shots/2020/12/22/948874701/low-demand-for-antibody-drugs-against-covid-19.
- 28.Lindholt M.F., Jørgensen F.J., Bor A., Petersen MB. Willingness to use an approved COVID-19 vaccine: cross-national evidence on levels and individual-level predictors. doi:10.31234/osf.io/8kn5f [DOI] [PMC free article] [PubMed]
- 29.Wallace M., James A.E., Silver R. Rapid transmission of severe acute respiratory syndrome coronavirus 2 in detention facility, Louisiana, USA, May-June, 2020. Emerg Infect Dis. 2021;27(2) doi: 10.3201/eid2702.204158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkinson C., Layte R., Jenkinson D. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19(2) doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 31.[No title]. Accessed January 6, 2021. http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Dyspnea_Scoring_Manual.pdf.
- 32.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 33.Tignanelli C.J., Ingraham N.E., Sparks M.A. Antihypertensive drugs and risk of COVID-19? Lancet Respir Med. 2020;8(5):e30–e31. doi: 10.1016/S2213-2600(20)30153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/s2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C., Ye D., Mullick A.E. Effects of renin-angiotensin inhibition on ACE2 (Angiotensin-Converting Enzyme 2) and TMPRSS2 (Transmembrane protease serine 2) expression: insights Into COVID-19. Hypertension. 2020;76(4):e29–e30. doi: 10.1161/HYPERTENSIONAHA.120.15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duarte M., Pelorosso F.G., Nicolosi L., et al. Telmisartan for treatment of Covid-19 patients: an open randomized clinical trial. Preliminary report. medRxiv. Published online August 13, 2020:2020.08.04.20167205.
- 37.First randomized trial backs safety of common heart drugs in COVID-19 patients. Accessed January 5, 2021. https://www.escardio.org/The-ESC/Press-Office/Press-releases/LOPES.
- 38.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds H.R., Adhikari S., Pulgarin C. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaton E., Stang J., Biros M. The use of electronic consent for COVID-19 clinical trials: lessons for emergency care research during a pandemic and beyond. Acad Emerg Med. 2020;27(11):1183–1186. doi: 10.1111/acem.14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter S.D., Han H., Guyatt G.H. A systematic survey of randomised trials that stopped early for reasons of futility. BMC Med Res Methodol. 2020;20(1):1–11. doi: 10.1186/s12874-020-0899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]