Abstract
Background
Chlorhexidine gluconate (CHG) body washes and emollient application may modulate bacterial pathogen colonization and prevent neonatal hospital-acquired infections.
Methods
This pilot, non-randomized, open-label trial, enrolled preterm neonates (1000-1500g; day 1-3 of life) at a tertiary hospital in Cape Town, South Africa. Participants were sequentially allocated to 4 trial arms (n=20 each): 1% aqueous CHG (CHG), 1% CHG plus emollient (CHG+EM), emollient only (EM) and standard of care (SOC: no antiseptic/emollient). Trial treatment/s were applied daily for 10 days (d) post-enrolment, documenting neonatal skin condition score. Anterior nose, neck, umbilical and perianal swabs for bacterial culture were collected at d1, d3, d10 and d16 post-enrolment, (±1 day), reporting pathogen acquisition rates and semi-quantitative bacterial colony counts. (ClinicalTrials.gov identifier: NCT03896893; trial status: closed).
Findings
Eighty preterm neonates (mean gestational age 30 weeks [SD 2]) were enrolled between 4 March and 26 August 2019. The bacterial pathogen acquisition rate (comparing d1 and d16 swabs) varied from 33·9% [95%CI 22·9-47·0] at the umbilicus, 39·3% [95%CI 27·6-52·4] at the neck, to 71·4% [95%CI 58·5-81·7] at both the nose and perianal region. At d10, CHG babies had reduced bacterial density detected from neck, umbilicus, and perianal swabs compared to other groups (see Table 3). Following intervention cessation, colonization density was similar across all trial arms, but S. aureus colonization was more prevalent among EM and CHG+EM babies. Neonatal skin condition score improved in babies receiving emollient application (EM: -0·87 [95%CI 0·69-1·06] and CHG+EM: -0·73 [0·45-0·99]), compared to the SOC and CHG arms (Table 2); no CHG-related skin reactions occurred.
Interpretation
Bacterial colonization density was significantly reduced in babies receiving 1% CHG washes but colonization levels rebounded rapidly post-intervention. Emollient application improved skin condition but was associated with higher rates of S. aureus colonization.
Funding
South African Medical Research Council; National Institutes of Health (TW010682).
Keywords: chlorhexidine gluconate, infection prevention, neonatal unit, emollient, bacterial colonization, hospital-acquired infection
Abbreviations: AMR, antimicrobial resistance; ART, antiretroviral therapy; AE, adverse event; BSI, bloodstream infection; CFU, colony forming unit; CHG, chlorhexidine gluconate; CI, confidence interval; d, day; EM, emollient; ESBL, extended-spectrum B-lactamase; ACC, aerobic colony count; HAI, healthcare-associated infection; HIV, human immunodeficiency virus; IPC, infection prevention and control; KMC, kangaroo mother care; LMIC, low-to-middle income countries; nCPAP, nasal cannula positive airways pressure; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; SD, standard deviation; SOC, standard of care; spp, species; UIPC, Unit for Infection Prevention and Control; UTI, urinary tract infection; VLBW, very low birth weight
Research in context.
Evidence before this study
Hospitalised preterm neonates are at high risk of acquiring colonization with antibiotic-resistant bacteria and subsequently developing bloodstream infections. While 2% chlorhexidine gluconate (CHG) bathing is effective in preventing gram positive bacteraemia and central line associated bloodstream infections in adults and children, few studies have evaluated the safety and impact of CHG bathing in preterm neonates. Similarly, a Cochrane review of neonatal emollient therapy for the promotion of skin barrier integrity and prevention of bloodstream infections, was inconclusive. Evidence gaps remain regarding the optimal dose, duration, frequency of application, safety and impact of neonatal CHG bathing and emollient therapy.
Added value of this study
This pilot trial in hospitalized preterm neonates (1000-1500g birth weight) demonstrated that daily 1% CHG bathing significantly reduced overall bacterial colonization density. No CHG-related adverse events occurred. Emollient application significantly improved skin condition but was associated with higher rates of S. aureus colonization.
Implications of all the available evidence
Further studies are needed to define the optimal dose, duration, and frequency of CHG bathing with or without emollient therapy for reducing the density of bacterial colonization in hospitalized neonates. Infection prevention interventions including CHG bathing and emollient therapy are a research priority for preterm neonates in settings where gram negative bloodstream infection pathogens predominate.
Alt-text: Unlabelled box
1. Introduction
Hospitalised preterm neonates are at high risk of developing healthcare-associated (HA) and antimicrobial-resistant (AMR) infections [1]. Bacteria transferred at birth from the maternal vaginal tract and from the hospital environment, may lead to persistent neonatal colonization. Once colonization is established, these bacteria pose an ongoing risk of invasion and HAI [2,3]. For this reason, interventions targeting interruption or modulation of bacterial colonization may be effective in preventing HAI, including bloodstream infections (BSI) [4].
In low-middle income country (LMIC) neonatal units, bacterial colonization pressure is substantial. In Sub-Saharan African settings where healthcare facility-based births are common, early neonatal pathogen colonization occurs owing to sub-optimal water, sanitation, hygiene (WASH) and infection prevention (IPC) practices [5]. Neonatal multi-drug resistant gram negative pathogen acquisition rates ranging from 55 to 76% are reported from Kenya [6], Ghana [7], Morocco [8] and Cambodia [9], with a predominance of extended spectrum B-lactamase (ESBL)-producing K. pneumoniae.
Practices such as exclusive breastmilk feeding and kangaroo mother care (KMC) that promote neonatal colonization with normal flora are widely adopted in LMIC neonatal units [10]. Important IPC practices that interrupt pathogen transmission in neonatal units including hand hygiene, environmental and equipment cleaning, have not been consistently implemented in LMIC neonatal units [1]. Other interventions such as skin antisepsis and emollients (targeting reduced pathogen colonisation and promotion of neonatal skin integrity) have not been extensively studied, particularly in preterm neonates.
Chlorhexidine gluconate (CHG) is a topical antiseptic with broad bactericidal activity. CHG is widely used, typically at a concentration of 2% and often in combination with 70% isopropyl alcohol. Evidence from adult and paediatric intensive care units [11,12] supports the use of daily CHG bathing to prevent central line associated BSI and BSI with gram positive organisms. Despite the frequent use of CHG in neonatal units [13] for umbilical care and skin antisepsis prior to surgery and invasive procedures, whole body washing is seldom practiced owing to evidence gaps regarding the optimal dose, duration and treatment frequency and potential safety concerns in preterm infants (skin reactions) [14].
While evidence has shown benefits from umbilical cord application of CHG for neonates in community settings [15], [16], [17], more research is needed to determine whether CHG skin application could reduce HAI in hospitalized neonates. A recent Zambian study demonstrated significant reduction in hospital mortality and HAI rates among neonates >1500g birth weight who received a multi-modal IPC intervention that included 2% CHG bathing [18].
Emollient therapy may potentially reduce the risk for invasive bacterial infection in hospitalised neonates by enhancing skin integrity through incorporation of lipids. A single center study of sunflower seed oil application halved the incidence of sepsis in preterm neonates [19], however a Cochrane review of emollient efficacy for neonatal sepsis prevention was inconclusive [20]. In addition, the effect of combining emollient and antiseptic applications has not previously been evaluated.
CHG skin cleansing and emollient therapy are potentially useful, low-cost and feasible interventions for the prevention of hospital-acquired BSI in neonates. Given the paucity of data, we assessed the impact of 1% CHG bathing and emollient application on bacterial colonization dynamics in preterm neonates hospitalized in a middle-income country neonatal unit.
2. Methods
2.1. Trial design
We conducted a non-randomized, open-label, pilot clinical trial in a convenience sample of hospitalized neonates admitted to Tygerberg Hospital, Cape Town, South Africa between March and August 2019. The Stellenbosch University Health Research Ethics Committee and the Tygerberg Hospital management reviewed and approved the study protocol (N18/07/068; ClinicalTrials.gov identifier: NCT03896893). The manuscript was prepared in accordance with the CONSORT statement checklist for reporting of clinical trials.
2.2. Trial setting
Tygerberg Hospital is a 1384-bed public teaching hospital with a busy obstetric-neonatal service that manages approximately 8000 high-risk deliveries (37% low birth weight rate) and 3000 neonatal admissions annually. Despite being classified as an upper middle-income country by the World Bank, most patients using the public healthcare service are indigent and more typical of the population from low and lower middle-income countries. In 2017, the antenatal HIV prevalence in the Western Cape Province was 15·9% (95% CI: 14·2%–17·8%), with universal antiretroviral therapy in pregnancy and a national mother-to-child HIV infection transmission rate of 0·9% [23]. The neonatal unit (being the second largest neonatal inpatient unit in the country) comprises 132 beds including a 12-bed medical/surgical NICU, three high-dependency wards, and one kangaroo mother care ward. The neonatal unit provides medical and surgical care for sick, preterm (<37 weeks’ gestation) and/or low-birthweight (<2500 g) inborn and outborn neonates from surrounding district hospitals and midwife obstetric units. Prematurity, perinatal asphyxia and infection are the most common indications for admission. Critically ill neonates are nursed in the NICU, with availability of respiratory support (conventional ventilation, oscillation and nasal continuous positive airway pressure [nCPAP]), inotropic support and nitric oxide therapy). Given the extreme shortage of NICU beds, non-invasive ventilation (nCPAP and high-flow oxygen therapy) is used extensively in the high-dependency wards. Prior to this pilot trial, CHG bathing and emollient therapy was not used in the neonatal unit. 2% CHG in 70% alcohol was used for skin antisepsis prior to central line insertion for neonates >28 weeks gestational age. Aqueous 0·5% CHG body washes were occasionally used for methicillin-resistant Staphylococcus aureus (MRSA) decolonization during outbreaks in the neonatal wards. At our institution, the standard practise for per vaginal (PV) examinations in labour is to use sterile gloves without prior vaginal washes.
2.3. Participants
Neonates were eligible for enrolment if they fulfilled the following criteria: birth weight ≥1000g and ≤ 1500g (equivalent to gestational age 28–32 weeks); aged from day 1 - 4 of life; and anticipated length of hospital stay > 7 days. Neonates whose mothers were not present, unable or unwilling to provide consent for enrolment and neonates with any skin condition or congenital defect that could enhance CHG absorption were excluded. All potentially eligible neonates’ parents were approached for consent to trial participation and provided written informed consent.
2.4. Protocol for trial interventions and procedures
Preterm neonates (1000-1500g; day 1-4 of life) were sequentially allocated to 4 trial arms (n=20 each) with no randomization, no allocation concealment and no masking): 1% aqueous CHG (CHG); 1% CHG plus emollient (CHG+EM); emollient only (EM) and standard of care (SOC - no antiseptic/emollient). As this was a pilot trial with limited funding and minimal staffing, the trial design was intentionally simple. Sequential allocation to trial arms (starting with the CHG-containing arms) was chosen to facilitate closer observation for CHG skin reactions at the start of the trial, in case reduction in the CHG concentration to 0.5% was needed. Trial interventions (CHG, EM and CHG+EM) were applied daily from the neck down on weekdays by the trial investigators. Single named-patient containers of CHG +- emollient (where applicable) were used per patient. All interventions were discontinued 10 days post-enrolment. CHG was applied to 2 sterile cotton swabs with care taken to avoid pooling of CHG in skin folds. For neonates on the EM and CHG+EM arm, 2 grams of Aquaphor skin cream (Eucerin, Beiersdorf, Germany) was applied (as soon as the CHG had dried for babies on CHG + EM). Skin temperature (using an axillary thermometer) was recorded immediately before and 5 minutes after application of the trial intervention/s. Neonates on the CHG-containing arms were observed for skin reactions for 30 minutes following application of CHG.
2.5. Protocol for skin swab collection and laboratory processing
Swabs in liquid Amies transport medium (Sigma Transwab MWE) for bacterial culture were obtained from neonates’ anterior nares (nose), neck folds, umbilicus/umbilical stump and perianal area prior to application of trial interventions on day 1 (enrolment), day 3, day 10 and day 16 post-enrolment (all +- 1 day). The liquid transport medium in the swab tube was vortexed for 30 seconds and diluted 1:100 and 1:10 000 in normal saline. 25 mL of the fluid was inoculated onto blood and MacConkey agar plates, and 25 mL of each of the two dilutions was inoculated onto blood agar plates. All agar plates were incubated at 37oC for 24 hours, and for a further 24 hours if no growth was observed. Manual total, semiquantitative aerobic colony counts (ACC) were recorded for each specimen. Isolates were identified by Gram stain and then catalase, pyrrolidonyl aminopeptidase activity, and/or latex agglutination (Pastorex Staph-Plus; Bio-Rad, Redmond,WA) for gram positive organisms, or the automated Vitek-2 system (BioMerieux, Marcy-l’Étoile, France) for gram negative isolates.
2.6. Data definitions and sources
Patient records were reviewed daily to collect demographic, clinical, laboratory and antimicrobial prescription data; data were entered into an institutional-hosted REDCap database [27]. HA-BSI were defined as a positive blood culture yielding a known neonatal pathogen (based upon the categorization of the United States Centers for Disease Control and Prevention, US CDC) [25] obtained at ≥72 hours of life/hospitalization. Clinically-suspected infection included neonates with negative laboratory culture results, but symptoms, signs and/or raised markers of infection e.g. C-reactive protein >10mg/L, where at least 5 days of broad-spectrum antibiotic therapy was given.
2.7. Protocol for neonatal skin scoring
A neonatal skin condition scale assessment (skin score) developed by Darmstadt et al was performed on weekdays before application of trial interventions until day 14 post-enrolment [19]. The skin score had a 3-criteria grading system (dryness, erythema, skin breakdown) with a minimum score of 3 and a maximum possible score of 9 (grading dryness: 1 = normal, no sign of dryness; 2 = dry skin, visible scaling; 3 = very dry skin, cracking/fissures; erythema: 1 = no evidence of erythema; 2 = visible erythema < 50% body surface; 3 = visible erythema > 50% body surface and breakdown: 1 = none; 2 = small localized areas; 3 = extensive breakdown).
2.8. Outcomes
Our primary outcome of interest was the change in pathogen colonization density at the four selected body sites from baseline (day 1) to days 3, 10 and 16 post-enrolment. Secondary outcomes were the rate of acquisition of pathogenic bacteria (gram negative and gram positive), the neonatal skin score and development of adverse events (AE's) or serious AE's e.g. hypothermia, CHG skin reactions, laboratory-confirmed and/or clinically-suspected infection. Neonates were observed until day 28 post-enrolment or until neonatal hospital transfer/discharge with telephonic follow-up on day 28 post-enrolment.
2.9. Statistical analysis
Descriptive analysis of neonatal and maternal demographic characteristics by trial arm was performed, reporting means with standard deviations or 95% confidence intervals for normally distributed data and medians with interquartile ranges for non-normally distributed data. Categorical variables were reported as counts and percentages. The proportion of neonates acquiring one or more new pathogen/s (comparing d1 and d16 swabs) was reported as the percentage with 95% confidence intervals (CI) for each body site swabbed. Density of skin colonization with pathogens was reported for all participants in log CFU/ml and compared between trial arms at each swabbing timepoint using the Kruskal-Wallis test. For all statistical tests performed, a p-value <0.05 was considered significant. All the statistical analyses were performed using STATA 16·0 (College Station, Texas 77845 USA).
2.10. Role of the funding source
The funders of the trial had no role in trial design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the trial and had final responsibility for the decision to submit.
3. Results
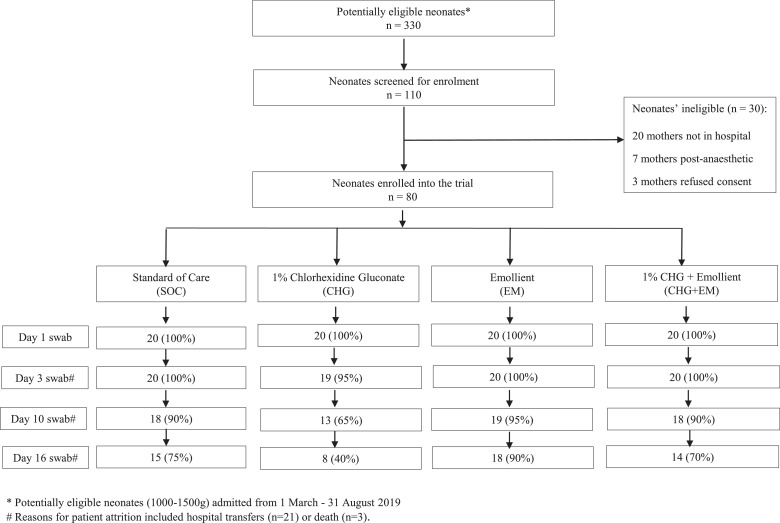
Of the 110/330 (33·3%) VLBW liveborn neonates admitted and screened for trial eligibility between 4 March and 26 August 2019, 80 (72·7%) were enrolled (Fig. 1). Neonates were enrolled to the trial at a median of 2 days of life (IQR 1-2), with no difference in the timing of enrolment by trial arm (p=0.721). Among the 80 enrolled participants, 40 (50%) were male, the mean gestational age was 30 (SD 2) weeks and the mean birth weight was 1253 (SD 150) grams (Table 1). Clinical factors and neonatal interventions around the time of trial enrolment that could contribute to bacterial colonization were documented e.g. receipt of antibiotics, use of non-invasive ventilation, surfactant therapy, kangaroo mother care (KMC) and expressed breastmilk (EBM) feeds (Table 1).
Figure 1.
Participant enrolment and trial design.
Legend: Figure 1 summarizes trial participant enrolment, trial arm assignment, skin swab collection (days 1, 3, 10, 16) and patient attrition. * Potentially eligible neonates (1000-1500g) admitted from 1 March - 31 August 2019. # Reasons for patient attrition included hospital transfers (n=21) or death (n=3).
Table 1.
Baseline characteristics of participants
| Intervention arms |
||||
|---|---|---|---|---|
| 1% chlorhexidine gluconate n=20 | Emollient n=20 | 1% CHG + emollientn=20 | Standard of Care n=20 | |
| Maternal | ||||
| Maternal age, mean (SD) | 28·7 (7·2) | 26·8 (6·0) | 27·6 (6·5) | 27·3 (7·6) |
| Maternal HIV infection, receiving ART, n (%) |
6 (30) | 4 (20) | 10 (50) | 10 (50) |
| Delivery by caesarean section, n (%) |
14 (70) | 14 (70) | 16 (80) | 15 (75) |
| Antenatal steroids received, n (%) | 18 (90) | 17 (85) | 17 (85) | 17 (85) |
| Membranes ruptured >18 hours pre-delivery, n (%) | 5 (25) | 0 (0) | 1 (5) | 3 (15) |
| Neonate (at birth) | ||||
| Male sex, n (%) | 11 (55) | 10 (50) | 7 (35) | 12 (60) |
| Gestational age at birth, mean (SD) |
30·4 (1·3) | 29·7 (1·6) | 30·5 (1·5) | 30·7 (2·3) |
| Birth weight, mean (SD) | 1251 (157) | 1197 (139) | 1277 (152) | 1287 (147) |
| Neonate (at enrolment) | ||||
| Day of life, median (IQR) | 2 (1-2) | 1 (1-3) | 2 (1-3·5) | 2 (2-2) |
| Presence of hyaline membrane disease, n (%) | 16 (80) | 17 (85) | 16 (80) | 13 (65) |
| Use of non-invasive ventilation, n (%) | 15 (75) | 18 (90) | 14 (70) | 16 (80) |
| Receiving antibiotics, n (%) | 12 (60) | 10 (50) | 9 (45) | 13 (65) |
| Receipt of surfactant therapy, n (%) | 3 (15) | 3 (15) | 1 (5) | 2 (10) |
| Neonate (during admission) | ||||
| *Required invasive ventilation, n (%) | 1 (5) | 1 (5) | 2 (10) | 1 (5) |
| *Central catheter placed, n (%) | 0 (0) | 5 (25) | 2 (10) | 3 (15) |
| *Parenteral nutrition given, n (%) | 0 (0) | 1 (5) | 2 (10) | 1 (5) |
| *Received blood transfusion, n (%) | 1 (5) | 1 (5) | 2 (10) | 1 (5) |
| Time to substantial# EBM feeds (days), mean (SD) | 2·9 (1·2) | 2·7 (1·8) | 3·0 (1·5) | 3·9 (3·0) |
| Daily weight gain in grams, median (IQR) | 10·2 (2·5-12·3) | 10·9 (6·6-16·4) | 9·7 (7·3-12·9) | 11·1 (5·3-16·9) |
| Receipt of intermittent KMC, n (%) | 19 (95) | 20 (100) | 16 (80) | 18 (90) |
| Time to initiation of KMC (days), median (IQR) | 4 (2-6) | 7·5 (4-17) | 4·5 (3·5-9) | 5 (3-6) |
SD – standard deviation, IQR – interquartile range, n (%) – number (percentage)*.
HIV – human immunodeficiency virus, ART – antiretroviral therapy.
EBM – expressed breastmilk, KMC – kangaroo mother care.
*at any point from enrolment to day 28 post-enrolment.
substantial expressed breastmilk feed >0·5 ml/hr.
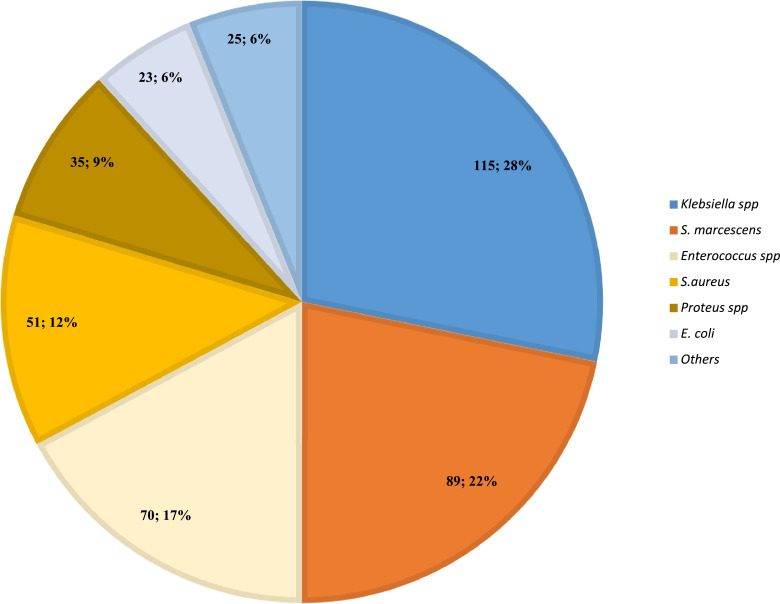
By day 10 post-enrolment, all neonates were colonized with gram negative bacilli at ≥1 body site, predominantly Enterobacterales. Klebsiella pneumoniae and Serratia marcescens accounted for 50% (204/408) of the pathogens cultured from skin swabs across all sites (Fig. 2). Among the 56 neonates still hospitalized at d16 post-enrolment, rates of new bacterial pathogen acquisition (comparing d1 and d16 swabs) varied from 33.9% [95%CI 22.9-47.0] at the umbilicus, 39.3% [95%CI 27.6-52.4] at the neck, to 71·4% [40/56; 95%CI 58.5-81.7] at both nose and perianal region. At d16 post-enrolment, the majority of the remaining 56 hospitalized neonates were colonized by one or more bacterial pathogens (87·5% [49/56; 95%CI 76·1-94·1] at the nose; 80·4% [45/56; 95%CI 68·0-88·8] at the neck; 75·0% [42/56; 95%CI 62·2-84·6] at the umbilical stump; and 100% [56/56; 95%CI 92·3-100] at the perianal region). The highest bacterial burden (combining all neonates with swabs available for timepoints d1, d3, d10 and d16) was measured from the perianal and neck swabs (3·7 [IQR 3·5-3·7] and 3·6 [IQR 3·5-3·7] log CFU/ml respectively), followed by the nose (3·0 [IQR 2·7-3·4] log CFU/ml) and umbilicus (2·9 [IQR 2·6-3·2] log CFU/ml).
Figure 2.
Spectrum of colonizing neonatal pathogens at day 10 post-enrolment (n=408).
Legend: Figure 2 depicts the number and percentage for each pathogen or species cultured from all superficial skin swabs (nose, neck, umbilicus, perianal region) collected at day 10 post-enrolment; Others = Acinetobacter, Enterobacter, Raoultella and Pseudomonas species (n=25).
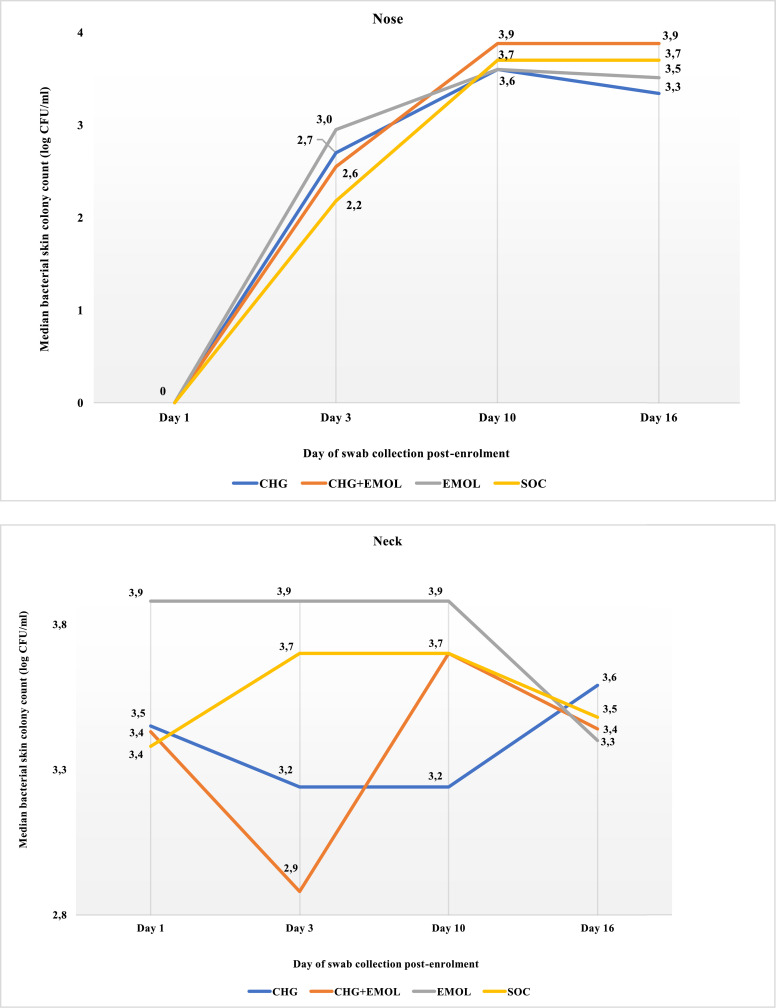
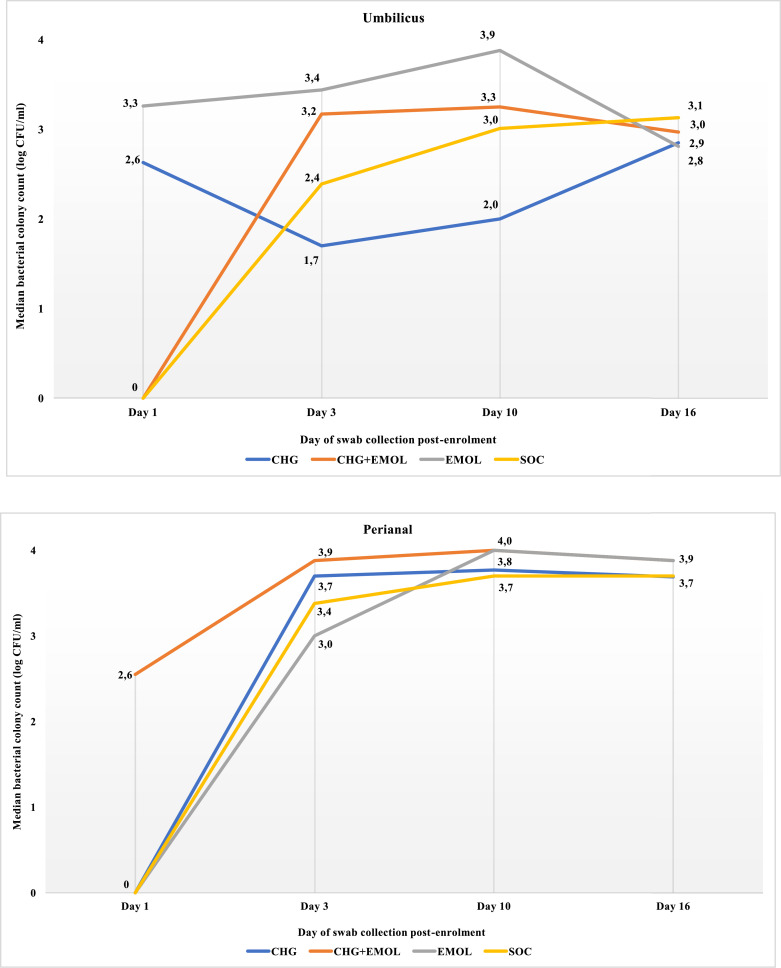
At day 3, CHG babies had reduced bacterial density detected from neck and umbilicus, with a difference of 0.5 and 0.7 log CFU respectively. At day 10, the difference in bacterial density between SOC and CHG was 0.5 and 1.0 log CFU for the neck and umbilicus respectively (Table 3, Figs. 3b and 3c). At day 16 (6 days after intervention cessation), there was no difference in bacterial colonization density by trial arm (Figs. 3a-d). At day 16, Staphylococcus aureus colonization was more prevalent among EM and CHG+EM babies than others (42/128 [32.8%] vs 13/92 [14.1%]; p=0.002).
Table 3.
Median bacterial colony count (log CFU/ml) by swab site and days post-enrolment
| Intervention arms |
Comparator | ||||
|---|---|---|---|---|---|
| 1% chlorhexidine gluconate (CHG) | Emollient (EM) | 1% CHG + emollient(CHG+EM) | Standard of Care (SOC) | p-value | |
| Swab site: nose (median, IQR) | |||||
| Day 1 | 0 (0-0) | 0 (0-2.8) | 0 (0-2·1) | 0 (0-2·2) | 0·513 |
| Day 3 | 2·70 (0-3·7) | 2·95 (0·9-3·5) | 2·55 (0-3·8) | 2·18 (0-3·6) | 0·861 |
| Day 10 | 3·60 (2·6-3·9) | 3·60 (3·4-4·0) | 3·88 (3·5-3·9) | 3·70 (3·0-3·9) | 0·390 |
| Day 16 | 3·34 (2·2-3·8) | 3·51 (3·1-3·9) | 3·88 (3·4-4·0) | 3·70 (2·2-3·7) | 0·292 |
| Swab site: neck (median, IQR) | |||||
| Day 1 | 3·45 (0-3·8) | 3·88 (2·2-3·9) | 3·43 (0-3·8) | 3·38 (2·8-3·7) | 0·082 |
| Day 3 | 3·24 (2·2-3·7) | 3·88 (3·6-4·0) | 2·88 (0-3·5) | 3·70 (3·7-3·9) | <0·001 |
| Day 10 | 3·24 (2·7-3·6) | 3·88 (3·4-4·0) | 3·70 (3·2-3·9) | 3·70 (3·7-3·9) | 0·003 |
| Day 16 | 3·59 (3·0-3·8) | 3·4 (2·9-3·7) | 3·44 (3·2-3·8) | 3·48 (3·0-3·7) | 0·795 |
| Swab site: umbilicus (median, IQR) | |||||
| Day 1 | 2·63 (0-3·4) | 3·26 (0-3·9) | 0 (0-3·3) | 0 (0-3·4) | 0·401 |
| Day 3 | 1·70 (0-3·3) | 3·44 (2·7-3·9) | 3·17 (2·0-3·6) | 2·39 (0-3·4) | 0·017 |
| Day 10 | 2·00 (0-2·5) | 3·88 (3·4-4·0) | 3·25 (2·3-3·9) | 3·01 (2·0-3·7) | <0·001 |
| Day 16 | 2·81 (0·9-3·6) | 2·85 (0-3·4) | 2·97 (0-3·2) | 3·13 (1·7-3·7) | 0·897 |
| Swab site: perianal (median, IQR) | |||||
| Day 1 | 0 (0-2·5) | 0 (0-2·4) | 2·55 (0-3.7) | 0 (0-1·2) | 0·062 |
| Day 3 | 3·70 (3·5-4·0) | 3·00 (1·8-3·9) | 3·88 (1·0-3·9) | 3·38 (0-3·7) | 0·136 |
| Day 10 | 3·77 (3·7-3·9) | 4·00 (3·9-4·0) | 4·00 (3·7-4·0) | 3·70 (3·7-3·9) | 0·004 |
| Day 16 | 3·69 (3·5-3·8) | 3·88 (3·7-4·0) | 3·88 (3·5-4·0) | 3·70 (3·7-4·0) | 0·304 |
The median bacterial colony count with interquartile range (IQR) is shown for each anatomical swab site and day of swab collection in log CFU/ml.
Figure 3.
Median bacterial colony count (log CFU/ml) at the nose by days post-enrolment.
Legend: The median bacterial colonization density at the nose was compared at four time points for each trial arm using the Kruskal-Wallis test (Day 1 p=0.513; Day 3 p=0.861; Day 10 p=0.390; Day 16 p =0.292).
Figure 3b Median bacterial colony count (log CFU/ml) at the neck by days post-enrolment
Legend: The median bacterial colonization density at the neck was compared at four time points for each trial arm using the Kruskal-Wallis test (Day 1 p=0.082; Day 3 p<0.001; Day 10 p=0.003; Day 16 p =0.795).
Figure 3c Median bacterial colony count (log CFU/ml) at the umbilicus by days post-enrolment
Legend: The median bacterial colonization density at the umbilicus was compared at four time points for each trial arm using the Kruskal-Wallis test (Day 1 p=0.401; Day 3 p=0.017; Day 10 p<0.001; Day 16 p =0.897).
Figure 3d Median bacterial colony count (log CFU/ml) at the peri-rectum by days post-enrolment
Legend: The median bacterial colonization density at the perianal area was compared at four time points for each trial arm using the Kruskal-Wallis test (Day 1 p=0.062; Day 3 p=0.136; Day 10 p=0.004; Day 16 p =0.304).
Neonatal skin condition score improved in babies receiving emollient application (EM: -0.87 [95%CI -0.69--1.06]; and CHG+EM: -0.73 [-0.45--0.99]), compared to the SOC and CHG arms (Table 2). No CHG-related skin reactions occurred. Two babies in the EM arm developed a transient macular rash deemed unrelated to the emollient. Hypothermia (grade 2; temperature 35·0○C – 35·5○C) occurred in 2 neonates on the CHG arm but was rapidly reversed by active re-warming under a radiant heater. Two non-infection related SAEs occurred: spontaneous intestinal perforation (SOC arm) and pulmonary haemorrhage (EM arm).
Table 2.
Neonatal skin score, adverse events and neonatal outcome by trial arm.
| Intervention arms |
Comparator | ||||
|---|---|---|---|---|---|
| Total cohort n=80 | 1% chlorhexidine gluconate n=20 | Emollient n=20 | 1% CHG + emollientn=20 | Standard of Care n=20 | |
| Neonatal skin condition score# | |||||
| Baseline skin score, mean (SD) | 4·05 (0·71) | 4·19 (0·87) | 3·89 (0·66) | 3·95 (0·60) | 4·15 (0·67) |
| Change in skin score from baseline to final score (95% CI), p-value | + 0·28 (0·02-0·53) p=0·032 |
+ 0·38 (-0·81-0·50) p=0·081 |
- 0·87 (-0·69--1·06) p<0·001 |
- 0·73 (-0·45–-0·99) p<0·001 |
+ 0·19 (-0·60-0·21) p=0·335 |
| Adverse events, SAEs, final outcome | |||||
| Post-intervention temperatures* in degrees Celsius, mean (SD) | 36·6 (0·4) | 36·6 (0·3) | 36·7 (0·3) | 36·6 (0·3) | 36·7 (0·4) |
| Adverse events hypothermia (grade 2) transient macular rash CHG skin reaction |
2 (2·5) 2 (2·5) 0 |
2 (10) 0 0 |
0 2 (10) - |
0 0 0 |
0 0 - |
| Serious adverse events (non-infectious) spontaneous intestinal perforation pulmonary hemorrhage |
1 (1·3) 1 (1·3) |
0 0 |
0 1 (5) |
0 0 |
1 (5) 0 |
| Serious adverse events (infectious) presumed bacterial infection bloodstream infection urinary tract infection hospital-acquired pneumonia necrotizing enterocolitis |
12 (15) 7 (8·8) 1 (1·3) 1 (1·3) 3 (3·7) |
0 2 (10) 1 (5) 1 (5) 1 (5) |
7 (35) 2 (10) 0 0 0 |
2 (10) 0 0 0 2 (10) |
3 (15) 3 (15) 0 0 0 |
| Day 28 post-enrolment outcome still hospitalized transferred out discharged home died |
38 (47·5) 34 (42·5) 5 (6·3) 3 (3·7) |
9 (45) 5 (25) 3 (15) 3 (15) |
11 (55) 8 (40) 1 (5) 0 (0) |
7 (35) 13 (65) 0 (0) 0 (0) |
11 (55) 8 (40) 1 (5) 0 (0) |
A neonatal skin condition score (Darmstadt et al)19 was performed using a 3-criteria grading system (dryness, erythema, skin breakdown) with a minimum (best) score of 3 and a maximum (worst) possible score of 9.
pooled post-intervention temperature (in degrees Celsius) was calculated as the sum of each day's temperature measurement divided by the total days of observation per patient.
Infection-related events occurred in 30% of the cohort (24/80), including 3 BSI, 3 presumed sepsis (SOC), 2 BSI, 1 urinary tract infection, 1 necrotizing enterocolitis (NEC) and 1 HA-pneumonia (CHG), 2 BSI with meningitis, 7 presumed sepsis (EM) and 2 NEC, 2 presumed sepsis (CHG+EM). All 3 neonates who died were in the CHG arm and had received a mean of 4 CHG baths at the time of demise. One neonate (1400g) developed fulminant Klebsiella sepsis on day 6 of life and demised within 48 hours of blood culture collection despite ventilation and inotropic support in the NICU. Another neonate (1030g) developed necrotizing enterocolitis (NEC) with intestinal perforation on day 8 of life and demised despite maximal support in the NICU. The third neonate (1230g) developed perforated NEC on day 10 of life and demised from Klebsiella sepsis in the NICU 4 days after laparotomy for excision of necrotic bowel.
Total antibiotic exposure per neonate was 3 days (IQR 2-6.5 days) with no difference by trial arm (p=0.103). Ampicillin plus gentamicin (n=44), piperacillin-tazobactam plus amikacin (n= 35) and meropenem with/without vancomycin (n=15), were the most frequently used antibiotic regimens.
No protocol violations occurred during the trial. Two participants in the CHG arm had a single CHG application omitted when undergoing laparotomies for NEC with intestinal perforation. In 2 participants, one day of CHG application was omitted owing to a low temperature measurement (<36 degrees Celsius) prior to CHG application, although they were able to resume CHG baths the following day.
4. Discussion
In this pilot clinical trial enrolling preterm neonates, 1% CHG bathing significantly reduced bacterial pathogen colonization density at the neck, umbilicus and perianal areas but not the nose. Colonization density rebounded toward baseline following cessation of CHG bathing at all sites. Neonatal skin condition score improved in babies receiving emollient application but was associated with a higher prevalence of S. aureus colonization. Acquisition of pathogens occurred rapidly with all neonates becoming colonized with gram negative bacilli at ≥1 body site. Klebsiella pneumoniae and Serratia marcescens accounted for half of all bacteria cultured from skin swabs.
Invasive bacterial infections including BSI, are a major contributor to in-hospital morbidity in hospitalized preterm neonates. Furthermore, hospital-acquired and multidrug-resistant bacterial infections are increasingly reported as a leading cause of death in LMIC neonatal units [21]. Interventions that reduce pathogen colonisation and promote skin integrity may potentially reduce bacterial infection risk in hospitalized neonates. However, the threshold of colonization density reduction required to prevent progression from colonization to invasive bacterial infection is unknown. Similarly, the most effective and safe neonatal dose, dosing interval and duration of application for antiseptics and emollients is yet to be determined.
Studies in an adult cohort [22] and a small neonatal sample (40 infants, mean gestational age 34 weeks) [23] measured the residual skin concentration following use of 2% CHG-impregnated body wipes, demonstrating significantly reduced skin bacterial burden over time. Residual antiseptic activity persisted for 24 hours following CHG bathing and then declined to undetectable levels with gradual rebound of skin bacterial density over 1-3 days. In both studies, the overall bacterial skin colonization density was low (1-2 log CFU/ml) and dominated by gram positive organisms. These factors limit the utility and generalisability of these findings in LMIC neonatal units where gram negative pathogens predominate and bacterial colonization pressure is high. Our trial illustrated that 71% of potential pathogens identified from skin swabs collected at d10 post-enrolment were gram negative bacteria. Overall bacterial colonization density was high exceeding 3 log CFU/ml at most skin sites. In our trial, the nose was the only site not achieving a significant reduction in bacterial colonization density by day 10. Our postulated reason is that CHG baths are applied from the neck down, thus explaining unchanged bacterial colonization density at the nose.
In two pragmatic real-world trials, daily CHG bathing reduced BSI rates in hospitalised neonates. In an Indian trial that enrolled 140 neonates, daily 0·25% CHG baths given until day 7 of life resulted in a non-significant reduction in proven BSI prevalence (6·9% vs 3·6%; p=0.195) [24]. A large Zambian prospective cohort trial using an infection control bundle including weekly 2% CHG bathing, demonstrated significant reduction in suspected sepsis and proven BSI rates in neonates with birth weight >1000 g [18,25].
Although concerns persist regarding the potential for CHG to cause dermatological reactions and systemic absorption in neonates [14], we observed no skin reactions or neurological manifestations. Application of CHG did however cause moderate hypothermia in 2 participants that was easily and rapidly corrected. CHG bathing may be most effective if commenced soon after birth, allowing the antiseptic to exert its effect before high bacterial colonization loads are established. However, this would require that LMIC neonatal units have the means to ensure neonatal thermal care before and during the intervention. The emollient therapy was equally well-tolerated with only 2 possibly trial-related instances of transient macular skin rash. In both emollient-containing trial arms, neonates’ skin condition score (a proxy for skin barrier health) improved significantly. However, the finding of increased S. aureus colonisation rates at final swab is concerning and biologically plausible. Both CHG bathing and emollient therapy are potentially maternally-administered interventions. Future trials should consider involving neonates’ mothers in treatment application, once the optimal strength and frequency of CHG application and its safety is established.
Serious adverse events (none trial related) occurred frequently in this cohort and affected 24/80 (30%) participants, highlighting the volatile clinical course and vulnerability of preterm neonates. Infection-related SAE's occurred in all trial arms; proven BSI was the most frequent infection-related SAE type. The deaths of the three neonates on the 1% CHG arm (BSI, NEC, NEC + BSI) were deemed unrelated to the trial intervention.
Strengths of this trial include the application of CHG/emollient therapy in an understudied population (preterm infants 1000-1500g) from a LMIC neonatal unit where gram negative pathogens predominate as causes of BSI. The impact and safety of the intervention/s was prospectively studied to report detailed information on clinical parameters (temperature and skin score), microbiological outcomes (bacterial colonisation density) and neonatal outcomes (AE's, SAE's and outcome 28 days post-enrolment). Study limitations include the pilot trial design (non-randomised), small sample size, lack of correlation of skin bacterial density with CHG skin residual concentration, inability to differentiate overall bacterial colony count from pathogen colonization density, and a loss of 30% of the original cohort by day 16 post-enrolment owing to hospital transfers and deaths. Given the high rate of participant loss from hospital transfers in this pilot trial, future neonatal CHG bathing studies should ensure a large enough sample size to maintain statistical power, account for any missing data, and explore the influence of missing data using sensitivity analyses. Furthermore, this trial was not designed or powered to assess the impact of the intervention (CHG/emollient) on rates of suspected and culture-proven bacterial bloodstream infection, and therefore relative risks by trial arm were not calculated for these outcomes. A further limitation was the lack of adjustment for potential confounders, which was not performed owing to the absence of established predictors of neonatal pathogen colonization density.
CHG and emollient therapy are promising interventions to decrease the risk of bacterial infection in hospitalised preterm neonates. More studies are needed from LMIC neonatal units where skin bacterial colonisation density is high and gram negative, antimicrobial-resistant pathogens predominate. In particular, the most effective but safe dose and dosing interval for CHG skin antisepsis in preterm neonates needs to be determined. The question of whether addition of emollient therapy improves skin condition and safety of CHG application in preterm neonates also warrants further study.
Contributors
AD, AB, MFC, ACW and SC conceptualised the trial. AD and IA recruited patients, collected the clinical data and obtained the skin swabs. ACW and SP advised on the laboratory specimen processing methods. SP conducted the laboratory processing and reporting of the skin swabs. AD analysed the trial data and prepared the first draft of the manuscript. All authors read, edited and approved the final manuscript.
Data Sharing statement
The datasets generated during and/or analysed during the trial are available from the corresponding author on reasonable request.
Funding
South African Medical Research Council; National Institutes of Health (TW010682).
Declaration of Competing Interest
SC declares participation in a multi-site trial of CHG sponsored by Sage Pharmaceuticals in 2016. The other authors declare no competing interests.
Acknowledgements
The authors thank the neonates, their parents and the staff of the Tygerberg Hospital Neonatal Unit. The authors acknowledge and thank clinical and laboratory colleagues who rendered assistance with identification of potential participants for recruitment and laboratory specimen processing. Trial data were collected and managed using REDCap electronic data capture tools hosted at Stellenbosch University.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100946.
Appendix. Supplementary materials
References
- 1.Ramasethu J. Prevention and treatment of neonatal nosocomial infections. Matern Health Neonatol Perinatol. 2017;3:5. doi: 10.1186/s40748-017-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folgori L, Tersigni C, Hsia Y. The relationship between Gram-negative colonization and bloodstream infections in neonates: a systematic review and meta-analysis. Clin Microbiol Infect. 2018;24(3):251–257. doi: 10.1016/j.cmi.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Bulabula ANH, Dramowski A, Mehtar S. Transmission of multidrug-resistant Gram-negative bacteria from colonized mothers to their infants: a systematic review and meta-analysis. J Hosp Infect. 2020;104(1):57–67. doi: 10.1016/j.jhin.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Zingg W, Hopkins S, Gayet-Ageron A. Health-care-associated infections in neonates, children, and adolescents: an analysis of paediatric data from the European Centre for Disease Prevention and Control point-prevalence survey. Lancet Infect Dis. 2017;17(4):381–389. doi: 10.1016/S1473-3099(16)30517-5. [DOI] [PubMed] [Google Scholar]
- 5.Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365(9465):1175–1188. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 6.Kagia N, Kosgei P, Ooko M. Carriage and Acquisition of Extended-spectrum beta-Lactamase-producing Enterobacterales Among Neonates Admitted to Hospital in Kilifi, Kenya. Clin Infect Dis. 2019;69(5):751–759. doi: 10.1093/cid/ciy976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labi AK, Bjerrum S, Enweronu-Laryea CC. High carriage rates of multidrug-resistant gram-negative bacteria in neonatal intensive care units from Ghana. Open Forum Infect Dis. 2020;7(4):ofaa109. doi: 10.1093/ofid/ofaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arhoune B, Oumokhtar B, Hmami F. Rectal carriage of extended-spectrum beta-lactamase- and carbapenemase-producing Enterobacteriaceae among hospitalised neonates in a neonatal intensive care unit in Fez, Morocco. J Glob Antimicrob Resist. 2017;8:90–96. doi: 10.1016/j.jgar.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Crellen T, Turner P, Pol S. Transmission dynamics and control of multidrug-resistant Klebsiella pneumoniae in neonates in a developing country. Elife. 2019;8 doi: 10.7554/eLife.50468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bee M, Shiroor A, Hill Z. Neonatal care practices in sub-Saharan Africa: a systematic review of quantitative and qualitative data. J Health Popul Nutr. 2018;37(1):9. doi: 10.1186/s41043-018-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HY, Lee WK, Na S, Roh YH, Shin CS, Kim J. The effects of chlorhexidine gluconate bathing on health care-associated infection in intensive care units: a meta-analysis. J Crit Care. 2016;32:126–137. doi: 10.1016/j.jcrc.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Milstone AM, Elward A, Song X. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381(9872):1099–1106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamma PD, Aucott SW, Milstone AM. Chlorhexidine use in the neonatal intensive care unit: results from a national survey. Infect Control Hosp Epidemiol. 2010;31(8):846–849. doi: 10.1086/655017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman AK, Aucott SW, Milstone AM. Safety of chlorhexidine gluconate used for skin antisepsis in the preterm infant. J Perinatol. 2012;32(1):4–9. doi: 10.1038/jp.2011.148. [DOI] [PubMed] [Google Scholar]
- 15.Sinha A, Sazawal S, Pradhan A, Ramji S, Opiyo N. Chlorhexidine skin or cord care for prevention of mortality and infections in neonates. Cochrane Database Syst Rev. 2015;(3) doi: 10.1002/14651858.CD007835.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semrau KEA, Herlihy J, Grogan C. Effectiveness of 4% chlorhexidine umbilical cord care on neonatal mortality in Southern Province, Zambia (ZamCAT): a cluster-randomised controlled trial. Lancet Glob Health. 2016;4(11):e827–ee36. doi: 10.1016/S2214-109X(16)30215-7. [DOI] [PubMed] [Google Scholar]
- 17.Sankar MJ, Chandrasekaran A, Ravindranath A, Agarwal R, Paul VK. Umbilical cord cleansing with chlorhexidine in neonates: a systematic review. J Perinatol. 2016;36(Suppl 1):S12–S20. doi: 10.1038/jp.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mwananyanda L, Pierre C, Mwansa J. Preventing bloodstream infections and death in Zambian neonates: impact of a low-cost infection control bundle. Clin Infect Dis. 2019;69(8):1360–1367. doi: 10.1093/cid/ciy1114. [DOI] [PubMed] [Google Scholar]
- 19.Darmstadt GL, Badrawi N, Law PA. Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial. Pediatr Infect Dis J. 2004;23(8):719–725. doi: 10.1097/01.inf.0000133047.50836.6f. [DOI] [PubMed] [Google Scholar]
- 20.Cleminson J, McGuire W. Topical emollient for preventing infection in preterm infants. Cochrane Database Syst Rev. 2016;(1) doi: 10.1002/14651858.CD001150.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madhi S.A.PJ, Baillie V. Unraveling specific causes of neonatal mortality using minimally invasive tissue sampling: an observational study. Clin Infect Dis. 2019;69(Suppl 4):S351–SS60. doi: 10.1093/cid/ciz574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popovich KJ, Lyles R, Hayes R. Relationship between chlorhexidine gluconate skin concentration and microbial density on the skin of critically ill patients bathed daily with chlorhexidine gluconate. Infect Control Hosp Epidemiol. 2012;33(9):889–896. doi: 10.1086/667371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson J, Suwantarat N, Colantuoni E. The impact of chlorhexidine gluconate bathing on skin bacterial burden of neonates admitted to the Neonatal Intensive Care Unit. J Perinatol. 2019;39(1):63–71. doi: 10.1038/s41372-018-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta B, Vaswani ND, Sharma D, Chaudhary U, Lekhwani S. Evaluation of efficacy of skin cleansing with chlorhexidine in prevention of neonatal nosocomial sepsis - a randomized controlled trial. J Matern Fetal Neonatal Med. 2016;29(2):242–247. doi: 10.3109/14767058.2014.996126. [DOI] [PubMed] [Google Scholar]
- 25.Westling T, Cowden C, Mwananyanda L. Impact of chlorhexidine baths on suspected sepsis and bloodstream infections in hospitalized neonates in Zambia. Int J Infect Dis. 2020;96:54–60. doi: 10.1016/j.ijid.2020.03.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.