Abstract
The use of oral fluid (OF) to detect zoonotic pathogens in pigs has been only scarcely assessed. We evaluated OF as a potential specimen for detection by culture of methicillin-resistant Staphylococcus aureus (MRSA) and Yersinia enterocolitica, and the detection of antibodies against Salmonella spp. and hepatitis E virus (HEV) using commercial ELISAs. Samples from 33 pig farms were collected at the beginning and end of the fattening period. Results of the OF samples were compared with the results of serum samples and nasal swabs from individual pigs and pen floor fecal samples, using the Cohen kappa (κ) and the McNemar test. For Salmonella spp. antibodies, OF samples were negative, although the corresponding serum samples were positive. The detection of HEV antibodies in sera and OF had agreement at the first sampling, and poor and significant agreement at the second sampling (κ = 0.185, McNemar p = 0.238; κ = 0.088, McNemar p < 0.001). At both sampling times, the detection of MRSA in nasal swabs and OF showed agreement (κ = 0.466, McNemar p = 0.077; κ = 0.603, McNemar p = 1); agreement was seen for the detection of Y. enterocolitica in fecal and OF samples (κ = 0.012, McNemar p = 0.868; κ = 0.082, McNemar p = 0.061, respectively). According to the McNemar test, the use of pen-based OFs is more feasible for the detection of MRSA and Y. enterocolitica by culture than is detection of antibodies by commercial ELISA.
Keywords: hepatitis E virus, MRSA, pigs, saliva, Salmonella spp, Switzerland, Yersinia enterocolitica
Pigs can act as asymptomatic carriers of various zoonotic pathogens. In particular, the consumption of raw or undercooked pork and pork products can result in the transmission to humans of pathogens such as Salmonella spp. and Yersinia enterocolitica.4,39 Hepatitis E is not only a disease transmitted via contaminated drinking water in developing countries with poor sanitary conditions, but also a zoonotic disease; domestic pigs, wild boars, and perhaps other animal species are reservoirs for hepatitis E virus (HEV; Orthohepevirus A). The occurrence of HEV in domestic pigs and wild boars raises concern for food safety.12,26,34 Pigs also act as a reservoir for methicillin-resistant Staphylococcus aureus (MRSA), and people with pig exposure are at higher risk for MRSA colonization or infection.10,17,23
Surveillance for foodborne and zoonotic pathogens might be beneficial to prevent the contamination of carcasses by asymptomatic carrier animals harboring such agents. Therefore, monitoring programs have been established to detect biological hazards at the herd level before slaughter. Conventional sampling methods in pigs, such as blood sampling and swabbing, take much effort, consume time and money, and cause considerable stress to the animals. Oral fluid (OF)-based testing offers an opportunity to gain pig herd health data at the farm in a simple and animal-friendly way.30,33 Pigs are naturally attracted to new and flexible objects 42 ; in OF-based sampling, the pigs transfer OFs while chewing a rope.19,27,32,37
The immunoglobulin (Ig) fraction found in OF consists predominantly of IgA.5,11 Mucosal IgA antibodies are produced in plasma cells of local glandular tissue. 5 IgG and IgM are also present in OF, although in lower quantities than IgA, and are derived primarily from plasma through ultrafiltration.5,11 Pathogen-specific IgA, IgM, and IgG antibodies have been demonstrated in OF collected from diverse domestic animal species in response to infection, making OF a useful matrix for immunologic assays.30,31,33 Also, various infectious agents are known to be shed by pigs in OF (e.g., foot-and-mouth disease virus, classical swine fever virus, porcine reproductive and respiratory syndrome virus [PRRSV]). 31 OF testing is therefore considered an efficient and cost-effective approach for surveillance of viruses in swine herds.19,33,35 Nevertheless, little is known about the suitability of porcine OF for monitoring colonization by zoonotic bacteria, such as Salmonella spp., Y. enterocolitica, and MRSA, and infection by HEV.
We determined the feasibility of pen-based OF samples for generating data concerning carriage by pigs of potential zoonotic pathogens before slaughter. OF samples were taken twice; once when pigs were introduced on the fattening farm, and subsequently before the pigs were slaughtered. We investigated whether OF samples obtained under field conditions in pig herds on 33 fattening farms were suitable for the detection of antibodies against HEV and Salmonella spp. by commercial ELISAs. Given the possible colonization of the nasal cavity and oropharynx by MRSA and Y. enterocolitica, the detection of both was attempted by culture.14,36 We compared the results of OF herd sampling with the results obtained by nasal swabs for MRSA, or blood samples from individual animals for HEV and Salmonella spp., or pen floor fecal samples for Yersinia.
Materials and methods
Experimental design
Our study was conducted on 33 Swiss fattening farms, located in the area with the highest pig density in Switzerland, covering 9 Swiss cantons. Farms participated upon the request of the farm veterinarian (n = 12), pig trading companies (n = 10), the Swiss Pig Health Service (n = 2), or by the study director (n = 5). The farms were visited at the beginning and end of one fattening period (Table 1). Most of the farms (n = 20) were sampled either the day new fattening pigs arrived (day 0) or the next day (day 1). Eleven farms were sampled between days 2 and 4, and only 2 farms were sampled at another time because of farm management reasons. The newly arrived pigs weighed an average of 26 kg. Pigs were chosen haphazardly. In 23 farms, the same 1,409 ear-tagged animals were tested twice (beginning and end of fattening period) for the occurrence of Salmonella spp., Y. enterocolitica, and HEV using blood samples, nasal swabs, pen floor fecal samples, and OF samples (1 cotton rope per 20 pigs); 524 of these pigs were also tested for the occurrence of MRSA. In the 10 remaining farms, 294 pigs were tested twice (beginning and end of fattening period) only for the occurrence of MRSA using nasal swabs and OF specimens (Table 2). The second sampling took place 1 d to 3 wk before the pigs were slaughtered, at a weight of 80–100 kg and 5–6 mo old.
Table 1.
Overview of farms participating in sampling of pigs for various pathogens.
| Farm | No. of pigs sampled (1st/2nd sampling) | No. of pens sampled for OF (1st/2nd sampling) | Pig flow | Sampling times | Total no. of pigs on farm |
|---|---|---|---|---|---|
| 1 | 70/70 | 6/6 | All in, all out | 2× | 70 |
| 2 | 49/50 | 5/5 | All in, all out | 2× | 50 |
| 3 | 76/76 | 6/12 | Continuous | 2× | 88 |
| 4 | 55/55 | 2/3 | Continuous | 2× | 63 |
| 5 | 10/9 | 1/1 | Continuous | 2× | 10 |
| 6 | 40/38 | 1/1 | Continuous | 2× | 40 |
| 7 | 87/82 | 3/4 | All in, all out | 2× | 115 |
| 8 | 73/0 | 4/0 | Continuous | 1× | 90 |
| 9 | 36/36 | 1/2 | Continuous | 2× | 36 |
| 10 | 20/20 | 2/2 | Continuous | 2× | 20 |
| 11 | 87/80 | 5/6 | Continuous | 2× | 100–200 |
| 12 | 55/51 | 6/6 | All in, all out | 2× | 51–100 |
| 13 | 101/97 | 8/7 | All in, all out | 2× | 180 |
| 14 | 56/0 | 3/0 | All in, all out | 1× | 94 |
| 15 | 92/79 | 5/7 | Continuous | 2× | 125 |
| 16 | 68/67 | 2/2 | Continuous | 2× | 80 |
| 17 | 25/18 | 1/3 | Continuous | 2× | 30 |
| 18 | 119/119 | 27/27 | All in, all out | 2× | 442 |
| 19 | 47/0 | 2/0 | NA | 1× | 60 |
| 20 | 76/66 | 4/5 | Continuous | 2× | 100 |
| 21 | 40/40 | 4/4 | Continuous | 2× | 41 |
| 22 | 78/72 | 4/4 | Continuous | 2× | 125 |
| 23 | 49/48 | 2/2 | Continuous | 2× | 52 |
| 24 | 28/24 | 4/4 | Continuous | 2× | 56 |
| 25 | 29/29 | 3/3 | Continuous | 2× | 105 |
| 26 | 26/26 | 3/3 | All in, all out | 2× | 201–300 |
| 27 | 39/39 | 1/1 | Continuous | 2× | 1–50 |
| 28 | 25/25 | 1/6 | Continuous | 2× | 1–50 |
| 29 | 30/30 | 1/2 | Continuous | 2× | 51–100 |
| 30 | 27/21 | 2/2 | NA | 2× | NA |
| 31 | 30/22 | 2/2 | NA | 2× | NA |
| 32 | 30/27 | 1/2 | Continuous | 2× | 51–100 |
| 33 | 30/29 | 1/2 | Continuous | 2× | 51–100 |
NA = not assessed; OF = oral fluid.
Table 2.
Overview of the test results of either oral fluid samples or the conventional sample matrices on pen level at the first and second sampling.
| Pathogen | 1st sampling (3-mo-old pigs) | 2nd sampling (6-mo-old pigs) | ||||||
|---|---|---|---|---|---|---|---|---|
| Conventional sample matrices* | Oral fluid | Conventional sample matrices* | Oral fluid | |||||
| Total pos. pens | Total pens tested | Total pos. pens | Total pens tested | Total pos. pens | Total pens tested | Total pos. pens | Total pens tested | |
| Salmonella spp. (ELISA) | 24 (23.1) | 104 | 0 (0) | 104 | 49 (45.4) | 108 | 0 (0) | 108 |
| Hepatitis E virus (ELISA) | 20 (19.2) | 104 | 10 (9.6) | 104 | 82 (75.9) | 108 | 56 (51.9) | 108 |
| MRSA (culture) | 11 (9.1) | 121 | 5 (4.1) | 121 | 8 (5.9) | 136 | 9 (6.6) | 136 |
| Yersinia enterocolitica (culture) | 31 (29.8) | 104 | 27 (26.5) | 102 | 26 (26.3) | 99 | 12 (11.5) | 104 |
Numbers in parentheses are percentages. Pos. = positive.
Conventional sample matrices: detection of antibodies (IgG) against Salmonella spp. and hepatitis E virus in serum samples; culture of MRSA from nasal swabs; culture of Yersinia enterocolitica from pen floor fecal samples.
The sample size was based on the prevalence of each pathogen, the herd size, and the sensitivity and specificity of the tests used (Table 1). 7 The reported seroprevalences for Salmonella spp. and HEV infections in finishing pigs in Switzerland are 4% and 60%, respectively. 41 The prevalences of MRSA and Y. enterocolitica in Swiss fattening pigs were estimated to be ~20% and ~88%, respectively, therefore a smaller number of animals were sampled by nasal swabs.2,15,28 The farm visits were carried out according to the Swiss Animal Welfare guidelines (study LU 03/2014).
Oral fluid sample collection
The OF collection procedure has been described elsewhere.30,37 In brief, OF samples were collected by hanging in each pen a 3-strand twisted, 12-mm diameter, unbleached cotton rope (Seilerei Kislig). If the pens contained >20 pigs, a second rope was placed. The ropes were positioned in the pens at the shoulder height of the pigs for at least 45 min, after which the rope was inserted into a single-use plastic bag and OF was extracted manually. The samples were transported to the Division of Swine Medicine (University of Zurich, Zurich, Switzerland); the OF was decanted into a 10-mL tube, and later pipetted into 1.8-mL cryotubes (Sarstedt). OF was shipped to 2 different laboratories (Institute for Food Safety and Hygiene, University of Zurich, Switzerland and Institute of Veterinary Bacteriology, University of Bern, Switzerland) and tested for antibodies against Salmonella spp. and HEV by ELISA, and for Y. enterocolitica and MRSA by culture. Samples for culture were stored overnight at 4°C and shipped the next day to the laboratory. Samples for ELISA were stored at −20°C until analyzed.
Blood sample collection
Blood samples were centrifuged for 10 min at 1,000 × g, and sera were aliquoted into three 1.8-mL cryotubes (Sarstedt) and frozen at −20°C until analyzed. Sera were tested for antibodies against Salmonella spp. and HEV.
Nasal swab sample collection
For MRSA detection, nasal swabs were taken from both nares from 815 pigs on 33 different fattening farms using transport swabs with Amies medium (Thermo Fisher). The swabs were stored overnight at 4°C and shipped to the laboratory the following day.
Fecal sample collection
For Y. enterocolitica testing, fresh pooled fecal samples (5:1) were obtained from the floor of each pen in which sampled pigs were housed, placed in a clean 100-mL plastic tube, refrigerated at 4°C overnight, and shipped to the laboratory the following day.
Laboratory analysis
Detection of Y. enterocolitica by culture, as well as HEV and Salmonella spp. antibodies by ELISA, was performed at the Institute for Food Safety and Hygiene, Vetsuisse Faculty, University of Zurich, and for MRSA by culture at the Institute of Veterinary Bacteriology, Vetsuisse Faculty, University of Bern.
Commercial serum ELISA kits (pigtype Salmonella Ab, Qiagen; PrioCHECK HEV antibody ELISA kit, Prionics) were used to detect IgG antibodies against Salmonella spp. and HEV, respectively, in porcine OF and sera. The detection of antibodies against HEV in OF was validated by using oral fluids spiked with HEV antibody–positive serum samples (data not shown). OF samples were assayed according to the manufacturers’ instructions for serum samples, with the exception that OFs were centrifuged first at 16,110 × g for 60 s and then pipetted onto a microwell plate without dilution. The following steps were conducted as indicated by the manufacturer.
For the detection of MRSA, 1 mL of OF was centrifuged for 10 min at 1,970 × g, and the supernatant was discarded. OF sediment and nasal swabs were transferred into tubes containing 10 mL of Mueller–Hinton broth supplemented with 6.5% NaCl. The following steps were performed as described previously. 28 S. aureus was identified using matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS; Biotyper 3.0, MBT Compass Library DB-5989 MSP; Bruker Daltonics) following the direct transfer protocol recommended by the manufacturer. MRSA isolates were confirmed by PCR for the mecA gene as described previously. 38
Y. enterocolitica was detected by mixing 1 mL of OF or 1 g of feces in 10 mL of phosphate-buffered saline (PBS) with 1% mannitol and 0.15% bile salts (PMB).16,25 After 2 wk of cold enrichment at 4°C, 10 μL of the enrichment was plated on cefsulodin–irgasan–novobiocin agar (Thermo Fisher), and the plates were incubated at 30°C for 24–48 h. Colonies with typical morphology were subcultured on blood agar and then tested for urease; this method is ISO-certified for identifying Yersinia spp.21,22
Statistical analysis
Data recording and editing were performed (Excel 2007; Microsoft); statistical analyses were performed (Statistical Software: release 15.1; StataCorp). The results of conventional sampling methods were compared with the results from OF. A pen was assessed positive for a pathogen and antibodies if an individual serum sample or the pen-based OF sample was positive. Results from sera and OF were compared at the pen level. The sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were assessed, setting serum samples, nasal swabs, or fecal samples as the gold standard. Given an ordinal distribution, the variables were not normally distributed. No statistical analysis or transformation for normal distribution was performed. The level of significance was set at 5%. Agreement of results obtained from OF and serum samples for ELISA, and OF from nasal swabs or fecal samples for culture, were determined using the McNemar test. The Cohen kappa (κ) was used to assess associations between positive ELISA and culture results and OF.13,40
Results
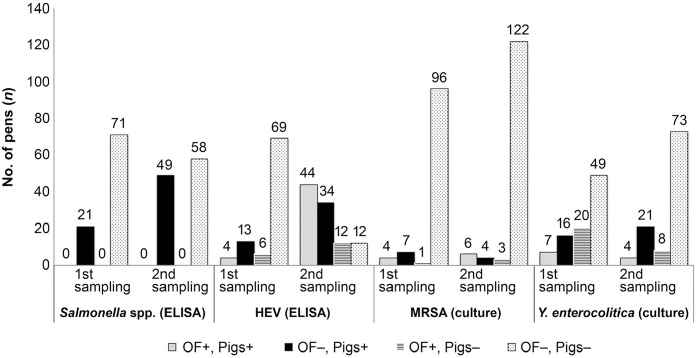
From the initial 1,409 pigs, 1,173 pigs were retested at the second sampling for antibodies to Salmonella spp., HEV, and Y. enterocolitica (Fig. 1). An additional 294 pigs were tested for MRSA at the first sampling time, and 272 of these pigs were retested a second time (Fig. 1). In farms 8, 14, and 19, second sampling could not be performed.
Figure 1.
Number of pens that tested positive for antibodies to Salmonella spp. and hepatitis E virus (HEV) by ELISA, and positive for methicillin-resistant Staphylococcus aureus (MRSA) and Yersinia enterocolitica by culture: comparison of ELISA and culture results obtained on oral fluid (OF) with those obtained on other matrices (nasal swabs, sera, feces) from animals in the same herd. At the first sampling, 92 pens were tested for Salmonella spp. and HEV antibodies, and for Y. enterocolitica; 108 pens were tested for MRSA. At the second sampling, 107 pens were tested for Salmonella spp. and HEV antibodies, and for Y. enterocolitica; 135 pens were tested for MRSA. + = positive; − = negative.
In most pens, pigs interacted quickly with the rope provided, yet in several pens no OF was harvested. In farms 7 and 13, the same pigs would not chew the rope at either the first or the second sampling time. In farms 2, 5, 6, and 30, pigs would not chew on the rope the first day they arrived on the farm but chewed eagerly on the second day.
The apparent seroprevalence of Salmonella spp. in samples of individual animals was 0%–48.9% at the first sampling, and 0%–31.7% at the second sampling (Table 2, Suppl. Table 1). At both times, no Salmonella spp. IgG was detected in OF (Fig. 1, Table 2, Suppl. Table 1). The seroprevalence for HEV was up to 16.6% in serum samples and 83.3% in OF at the first sampling, and up to 97.9% in serum samples and 100% in OF at the second sampling (Table 2, Suppl. Table 1). The MRSA and Y. enterocolitica prevalence in nasal swabs or fecal samples and OF was up to 100% at the farm level at both times (Table 2, Suppl.Table 2).
Test performance measures of the different pathogens in OF samples compared to specimens sampled conventionally (serum, nasal swabs, and fecal samples) revealed variable results (Fig. 1, Table 3). For Salmonella spp. IgG, no positive predictive value and positive likelihood ratio could be calculated because no antibodies were detected for this pathogen in OF. The detection of specific IgG against HEV showed poor agreement between sera and OF at the first sampling (κ = 0.185, McNemar p = 0.238) and poor agreement at the second sampling (κ = 0.088, McNemar p < 0.001). The detection of MRSA showed moderate agreement between nasal swabs and OF at the first sampling (κ = 0.466, McNemar p = 0.077) and substantial agreement at the second sampling (κ = 0.604, McNemar p = 1). The detection of Y. enterocolitica showed poor agreement between fecal samples and OF at the first sampling (κ = 0.012, McNemar p = 0.868) and poor agreement at the second sampling (κ = 0.082, McNemar p = 0.061).
Table 3.
Overview of the analyzed test performance measures of various pathogens in oral fluid samples compared to conventional samples (serum, nasal swabs, and fecal samples).
| Pathogen | First sampling | Second sampling | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SE | SP | PPV | NPV | LRp | LRn | κ | p | SE | SP | PPV | NPV | LRp | LRn | κ | p | |
| IgG anti-Salmonella ELISA | 0 (0) | 1(0) | NA | 0.772 (0.043) | NA | 1 (0) | NA | NA | 0 (0) | 1 (0) | NA | 0.558 (0.051) | NA | 1 (0) | NA | NA |
| IgG anti-hepatitis E virus ELISA | 0.250 (0.108) | 0.921 (0.031) | 0.400 (0.145) | 0.854 (0.039) | 3.167 (0.584) | 0.814 (0.148) | 0.185 | 0.238 | 0.543 (0.055) | 0.522 (0.104) | 0.800 (0.054) | 0.245 (0.061) | 1.136 (0.240) | 0.876 (0.233) | 0.088 | <0.001 |
| MRSA culture | 0.364 (0.145) | 0.990 (0.010) | 0.800 (0.179) | 0.932 (0.025) | 35.273 (1.072) | 0.643 (0.228) | 0.466 | 0.077 | 0.600 (0.155) | 0.974 (0.015) | 0.667 (0.157) | 0.966 (0.017) | 23.400 (0.063) | 0.411 (0.388) | 0.604 | 1 |
| Y.e. culture | 0.292 (0.093) | 0.710 (0.055) | 0.259 (0.084) | 0.742 (0.054) | 1.006 (0.370) | 0.997 (0.152) | 0.012 | 0.868 | 0.208 (0.082) | 0.885 (0.036) | 0.357 (0.128) | 0.784 (0.044) | 1.806 (0.507) | 0.895 (0.112) | 0.082 | 0.061 |
Numbers in parentheses are standard errors. IgG = immunoglobulin G; κ = Cohen kappa; LRn = negative likelihood ratio; LRp = positive likelihood ratio; MRSA = methicillin-resistant Staphylococcus aureus; NA = not assessed; NPV = negative predictive value; p-value, McNemar test; PPV = positive predictive value; SE = sensitivity; SP = specificity; Y.e. = Yersinia enterocolitica.
Discussion
OF is used as an alternative to serum for testing purposes, especially for monitoring, surveillance, and detection of pathogens and immunoglobulins at the herd level.24,30,33 OF offers a cost-effective approach because >80% of the pigs in the same pen are represented when presenting the rope for 30 to 45 min.19,37 Guidelines for OF sampling for the presence of PRRSV on farm demonstrate that using a fixed spatial sampling method, the probability of detecting PRRSV in one barn using 2 OF samples is 43% when the prevalence is 25%. 35 In several studies, OF samples were considered to be useful for detecting zoonotic pathogens in pigs. OF is useful in detecting Erysipelothrix spp., Streptococcus suis, and influenza A virus in pigs.3,8,9,18
In some pens, no interaction with the rope was recorded, and no OF was harvested. The reason behind this unequal interest in the rope can only be guessed. In pens with fully slatted floors, increasing the number of ropes will usually lead to an increase in the total chewing-time in pigs, but no such effect is known in straw-bedded pens, which are customary in Switzerland. 37 Therefore, we could not assess if straw bedding has an effect on the pig–rope interaction in our study. In some farms, pigs did not chew the rope at arrival. This behavior was probably caused by stress and distraction caused by many new impressions. If OF is used for monitoring and surveillance purposes, it is essential that pigs from all kinds of farms chew on the ropes provided. Alternatively, pigs could be sampled before they leave the farrowing site or some days after arrival at the fattening farm. Also, the time of day might be an influencing factor, given the fact that pigs are more active during the day. If a monitoring program for herd health prior to slaughter is established using OF, the collection should be done during the fattening period, but only after an adjustment period or at the end of the fattening period.
We evaluated the detection of anti-HEV and anti-Salmonella antibodies in OF specimens using commercial ELISAs that were not validated for OF. We modified the ELISA procedures only by the dilution of the OF samples for the detection of Salmonella spp. and HEV. Using a lower dilution (1:2) and overnight incubation of OF samples for detecting antibodies against Salmonella spp. might improve the performance of the ELISA. 1 We did not incubate the sample overnight, and we sampled from naturally infected pigs with a mean seroprevalence of 4.4%. The low percentage of positive pigs in our study and the use of a non-validated ELISA might have influenced our results. However, further investigations are needed to provide reliable results in detecting antibodies against Salmonella spp. in OF by using an ELISA validated for serum. OF is feasible for the detection of antibodies against HEV in human saliva samples. 29 Therefore, we tested negative OF spiked with a positive serum sample; HEV-specific antibodies were detectable (unpublished data). The validity of this approach was supported by prior evidence that pigs have detectable levels of antibodies in OF.5,11
Several hypotheses can be made for the negative outcomes in OF of the ELISAs for the detection of antibodies against Salmonella spp. One reason could be a lower diagnostic sensitivity of the method, given lower concentrations of IgG antibodies in OF compared to matched serum samples.11,24 Therefore, the ELISA for the detection of Salmonella spp. and HEV antibodies was performed without dilution. Additionally, the predominant antibody in OF is IgA, but the test kits used detect IgG antibodies. Given that OF is thought to be a filtrate of serum, IgG should be present in OF, but in lower concentrations.5,11 It is known that the sample material may affect the results of OF testing. For example, OF collected with cotton-based materials contains lower amounts of IgA compared to whole saliva samples in human OF, as well as in pig OF, but has no diminished effect on the amount of IgG. 27 Furthermore, the ELISAs that we used are validated for serum and meat juice, not OF. OF is a different matrix, and, in addition to the lower antibody concentration, there might be inhibitory factors that reduce the amount of antibody in OF.11,20 If OF is stored, the amounts of detectable antibodies decrease over time. 32
We used the Cohen kappa and the McNemar test to assess the agreement of conventional sample matrices and OF for the detection of HEV antibodies and the detection by culture of MRSA and Y. enterocolitica. Although the McNemar test failed to reject the null hypothesis of equal detection rates for the tested specimens, with the exception of the second sampling for HEV antibodies, the Cohen kappa showed only poor agreement of HEV antibodies or Y. enterocolitica culture between the corresponding samples. The reliability of the Cohen kappa for our study remains questionable in light of the high variability of farm-wise prevalence of positive pens as measured in the conventional sample matrices. Therefore, we considered the McNemar test more suitable for assessment of agreement between the conventional sample matrices and OF.
More pens tested positive for antibodies against HEV in serum samples than in OF. However, there was no significant disagreement between the 2 sample matrices. In farms 1 and 12, all pigs tested negative in serum for HEV, but OF tested positive. In several farms, positive sera were common, and OFs were either in agreement with sera or not. Further investigations are needed to provide feasible detection of antibodies against Salmonella spp. and HEV. The discrepancy of the ELISA results might be the result of cross-reaction with antibodies against different pathogens or in processing of OF. For example, ELISAs for detection of antibodies against PRRSV or Erysipelothrix spp. in OF seem to give good results after modification of the assay.3,18,24 It might be that the discrepancy of the results from the ELISAs used is the result of differences in the immune response generated by the pathogens (HEV, Salmonella spp.) tested.
A parallel study including this set of samples showed that antibodies against Toxoplasma gondii could be detected in OF from infected pigs by immunoblot (IB) techniques, and that IgA seemed to be a more adequate target than IgG. 6 In that study, positive IB results were obtained in pooled OF samples from groups with high rates (>90%) of pigs seropositive to T. gondii, but not in groups with ≤13% seropositive pigs, suggesting that this approach might be used as a screening tool to determine high exposure to T. gondii in a farm. 6 We showed that the use of OF as a screening tool for pig herd health for the tested pathogens using commercial ELISA kits, which are not adapted to the matrix of OF, is not promising at this point. Therefore, further research is needed into the use of ELISAs and in the detection of IgG against various pathogens in OF. It will be important to sample individual animals or all animals in a pen to ascertain the percentage of positive animals needed for pen-based OF samples to test positive.
We cultured OF in an effort to detect MRSA and Y. enterocolitica.22,28 This approach was valid given that both pathogens might be colonizing the oral cavity. We suspected that these 2 pathogens would also be present in OF given the proximity of the colonized organs. Only for the detection of MRSA in OF and nasal swabs from individual pigs by culture was substantial agreement found between the test results of the different specimens. MRSA are easily transmitted between animals and intermittently colonize the nasal cavity. Therefore, the MRSA status might change during the lifespan of a pig. 2 MRSA are natural occupants of mucous membranes and the skin of the pig and might be more easily transmitted via the oral cavity. Y. enterocolitica persists deep in the tonsils and is intermittently transmitted via feces and might be transmitted in oral secretions. 36 Interestingly, the percentage of detected Y. enterocolitica was higher in OF than in fecal samples at the second sampling. However, the McNemar test demonstrated agreement between OF and the reference matrices. The Cohen kappa and McNemar test showed different levels of agreement, given the high variance of positive pens in our study. The results for Y. enterocolitica cultural testing via OF might be beneficial. However, further investigation is needed to determine if OF is a beneficial matrix for the detection of Y. enterocolitica.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211021599 for Evaluation of oral fluids for surveillance of foodborne and zoonotic pathogens in pig farms by Franziska Schott, Karolin Hoffmann, Eleonora Sarno, Patrick D. Bangerter, Roger Stephan, Gudrun Overesch, Michael Haessig, Xaver Sidler and Robert Graage in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank the participating farmers for participating in this study, and Prionics for their technical support.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Our study was funded by the Federal Food Safety and Veterinary Office (FSVO) of Switzerland.
ORCID iDs: Franziska Schott  https://orcid.org/0000-0003-2296-2000
https://orcid.org/0000-0003-2296-2000
Robert Graage  https://orcid.org/0000-0003-4563-8297
https://orcid.org/0000-0003-4563-8297
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Franziska Schott, Department of Farm Animals, Division of Swine Medicine, University of Zurich, Zurich, Switzerland.
Karolin Hoffmann, Institute of Veterinary Pathology, University of Zurich, Zurich, Switzerland.
Eleonora Sarno, Institute for Food Safety and Hygiene, University of Zurich, Zurich, Switzerland.
Patrick D. Bangerter, Office for Consumer Protection Canton Aargau, Veterinary Service, Aarau, Switzerland (Bangerter)
Roger Stephan, Institute for Food Safety and Hygiene, University of Zurich, Zurich, Switzerland.
Gudrun Overesch, Institute of Veterinary Bacteriology, University of Bern, Bern, Switzerland.
Michael Haessig, Administrative Department for Farm Animal Diagnostics, University of Zurich, Zurich, Switzerland.
Xaver Sidler, Department of Farm Animals, Division of Swine Medicine, University of Zurich, Zurich, Switzerland.
Robert Graage, Department of Farm Animals, Division of Swine Medicine, University of Zurich, Zurich, Switzerland.
References
- 1. Atkinson BM, et al. Detection of Salmonella-specific antibody in swine oral fluids. Porcine Health Manag 2019;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bangerter PD, et al. Longitudinal study on the colonisation and transmission of methicillin-resistant Staphylococcus aureus in pig farms. Vet Microbiol 2016;183:12–134. [DOI] [PubMed] [Google Scholar]
- 3. Bender JS, et al. Characterization of Erysipelothrix species isolated from clinically affected pigs, environmental samples, and vaccine strains from six recent swine erysipelas outbreaks in the United States. Clin Vaccine Immunol 2010;17:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berends BR, et al. Impact on human health of Salmonella spp. on pork in The Netherlands and the anticipated effects of some currently proposed control strategies. Int J Food Microbiol 1998;44:219–229. [DOI] [PubMed] [Google Scholar]
- 5. Brandtzaeg P. Secretory immunity with special reference to the oral cavity. J Oral Microbiol 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campero LM, et al. Detection of antibodies to Toxoplasma gondii in oral fluid from pigs. Int J Parasitol 2020;50:349–355. [DOI] [PubMed] [Google Scholar]
- 7. Cannon RM, Roe RT. Detecting the presence of a disease. In: Cannon and Roe Livestock Disease Surveys: A Field Manual for Veterinarians. Australian Government Publishing Service, 1982:14–18. https://www.aphis.usda.gov/animal_health/surveillance_toolbox/docs/epi_surv/cannon_roe_1982_livestock_disease_surveys.pdf [Google Scholar]
- 8. Cheong Y, et al. Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method. J Vet Sci 2017;18:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demeter S, et al. Detection of influenza A virus on porcine oral fluid samples. J Vet Diagn Invest 2011;23:241–247. [DOI] [PubMed] [Google Scholar]
- 10. de Neeling AJ, et al. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet Microbiol 2007;122(3-4):366–372. [DOI] [PubMed] [Google Scholar]
- 11. Escribano D, et al. Validation of three commercially available immunoassays for quantification of IgA, IgG, and IgM in porcine saliva samples. Res Vet Sci 2012;93:682–687. [DOI] [PubMed] [Google Scholar]
- 12. European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J 2016;15:5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feuer E, Kessler LG. Test statistics and sample size for a two-sample McNemar. Biometrics 1989;45:629–636. [PubMed] [Google Scholar]
- 14. Fetsch A, et al. Co-colonization and clonal diversity of methicillin-sensitive and methicillin-resistant Staphylococcus aureus in sows. Vet Microbiol 2016;185:7–14. [DOI] [PubMed] [Google Scholar]
- 15. Fredriksson-Ahomaa M, et al. Prevalence of pathogenic Yersinia enterocolitica in pigs slaughtered at a Swiss abattoir. Int J Food Microbiol 2007;119:207–212. [DOI] [PubMed] [Google Scholar]
- 16. Fukushima H, et al. Mice and moles inhabiting mountainous areas of Shimane Peninsula as sources of infection with Yersinia pseudotuberculosis. J Clin Microbiol 1990;28:2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goerge T, et al. MRSA colonization and infection among persons with occupational livestock exposure in Europe: prevalence, preventive options and evidence. Vet Microbiol 2017;200:6–12. [DOI] [PubMed] [Google Scholar]
- 18. Giménez-Lirola L, et al. Improving ante mortem diagnosis of Erysipelothrix rhusiopathiae infections by use of oral fluids for bacterial, nucleic acid, and antibody detection. J Microbiol Methods 2013;92:113–121. [DOI] [PubMed] [Google Scholar]
- 19. Graage R, et al. Influence of age, group size and the presence of porcine reproductive and respiratory syndrome virus on the collection of oral fluids. Vet J 2019;244:13–15. [DOI] [PubMed] [Google Scholar]
- 20. Gutiérrez A, et al. Proteomic analysis of porcine saliva. Vet J 2011;187:356–362. [DOI] [PubMed] [Google Scholar]
- 21. Hilbert F, et al. Rapid urease screening of Yersinia on CIN agar plates. Int J Food Microbiol 2003;84:111–115. [DOI] [PubMed] [Google Scholar]
- 22. Joutsen S, et al. Pathogenic Yersinia enterocolitica O:3 isolated from a hunted wild alpine ibex. Epidemiol Infect 2013;141:612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khanna T, et al. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet Microbiol 2008;128:298–303. [DOI] [PubMed] [Google Scholar]
- 24. Kittawornrat A, et al. Detection of porcine reproductive and respiratory syndrome virus (PRRSV) antibodies in oral fluid specimens using a commercial PRRSV serum antibody enzyme-linked immunosorbent assay. J Vet Diagn Invest 2012;24:262–269. [DOI] [PubMed] [Google Scholar]
- 25. Martinez PO, et al. Variation in the prevalence of enteropathogenic Yersinia in slaughter pigs from Belgium, Italy, and Spain. Foodborne Pathog Dis 2011;8:445–450. [DOI] [PubMed] [Google Scholar]
- 26. Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol 2010;140:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen C, et al. Effect of collection material and sample processing on pig oral fluid testing results. Vet J 2013;198:158–163. [DOI] [PubMed] [Google Scholar]
- 28. Overesch G, et al. The increase of methicillin-resistant Staphylococcus aureus (MRSA) and the presence of an unusual sequence type ST49 in slaughter pigs in Switzerland. BMC Vet Res 2011;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pisanic N, et al. Development of an oral fluid immunoassay to assess past and recent hepatitis E virus (HEV) infection. J Immunol Methods 2017;448:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prickett J, et al. Detection of porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest 2008;20:156–163. [DOI] [PubMed] [Google Scholar]
- 31. Prickett JR, Zimmerman JJ. The development of oral fluid-based diagnostics and applications in veterinary medicine. Anim Health Res Rev 2010;11:207–216. [DOI] [PubMed] [Google Scholar]
- 32. Prickett JR, et al. Stability of porcine reproductive and respiratory syndrome virus and antibody in swine oral fluid. J Swine Health Prod 2010;18:187–195. [Google Scholar]
- 33. Ramirez A, et al. Efficient surveillance of pig populations using oral fluids. Prev Vet Med 2012;104:292–300. [DOI] [PubMed] [Google Scholar]
- 34. Rein DB, et al. The global burden of hepatitis E genotypes 1 and 2 in 2005. Hepatology 2012;55:988–997. [DOI] [PubMed] [Google Scholar]
- 35. Rotolo ML, et al. Sampling guidelines for oral fluid-based surveys of group-housed animals. Vet Microbiol 2017;209:20–29. [DOI] [PubMed] [Google Scholar]
- 36. Schaake J, et al. Essential role of invasin for colonization and persistence of Yersinia enterocolitica in its natural reservoir host, the pig. Infect Immun 2014;82:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seddon YM, et al. Optimising oral fluid collection from groups of pigs: effect of housing system and provision of ropes. Vet J 2012;193:180–184. [DOI] [PubMed] [Google Scholar]
- 38. Stegger M, et al. Rapid PCR detection of Staphylococcus aureus clonal complex 398 by targeting the restriction-modification system carrying sau1-hsdS1. J Clin Microbiol 2011;49:732–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tauxe RV, et al. Yersinia enterocolitica infections and pork: the missing link. Lancet 1987;1:1129–1132. [DOI] [PubMed] [Google Scholar]
- 40. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360–363. [PubMed] [Google Scholar]
- 41. Wacheck S, et al. Seroprevalence of anti-hepatitis E virus and anti-Salmonella antibodies in pigs at slaughter in Switzerland. J Food Prot 2012;75:1483–1485. [DOI] [PubMed] [Google Scholar]
- 42. Weerd HA, et al. Effects of species-relevant environmental enrichment on the behaviour and productivity of finishing pigs. Appl Anim Behav Sci 2006;99:230–247. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211021599 for Evaluation of oral fluids for surveillance of foodborne and zoonotic pathogens in pig farms by Franziska Schott, Karolin Hoffmann, Eleonora Sarno, Patrick D. Bangerter, Roger Stephan, Gudrun Overesch, Michael Haessig, Xaver Sidler and Robert Graage in Journal of Veterinary Diagnostic Investigation