Reflectance confocal microscopy (RCM) is a non-invasive imaging method that can visualize cellular details of the skin in vivo1. Through multiple clinical studies, RCM has been shown to provide high diagnostic accuracy for major skin cancers. In 2017, RCM was awarded common procedural terminology (CPT) reimbursement codes2. While clinical adoption of RCM has been steadily increasing, the high device cost (>$75,000) remains one of the key hurdles in widespread adoption of RCM. Previously, we have developed a portable confocal microscope (PCM) and demonstrated confocal imaging of skin lesions in Uganda3. Recently, we have developed an improved PCM device that uses near-infrared light4, similar to the standard RCM device (Vivascope 1500, Caliber ID).
We evaluated the near-infrared PCM device for imaging skin lesions commonly encountered in dermatology clinics. The PCM device (Fig. 1) had dimensions of 22 × 17.5 × 10 cm3 and weight of 1 kg, and built with a material cost of ~$5,000. Lateral and axial resolution was 1.6 and 6.0 μm, respectively, similar to the RCM resolution, 1.25 and 5 μm. Confocal images were acquired at the speed of 20 frames/sec, and transferred to a laptop. Ten skin lesions from five patients were imaged with the PCM device at the Banner-University Medicine Dermatology Clinic (Tucson, Arizona). Multiple areas were imaged for each skin lesion. Imaging depth was adjusted by gently changing the pressure on the skin. The same lesions were also imaged with the standard RCM device.
Figure 1.
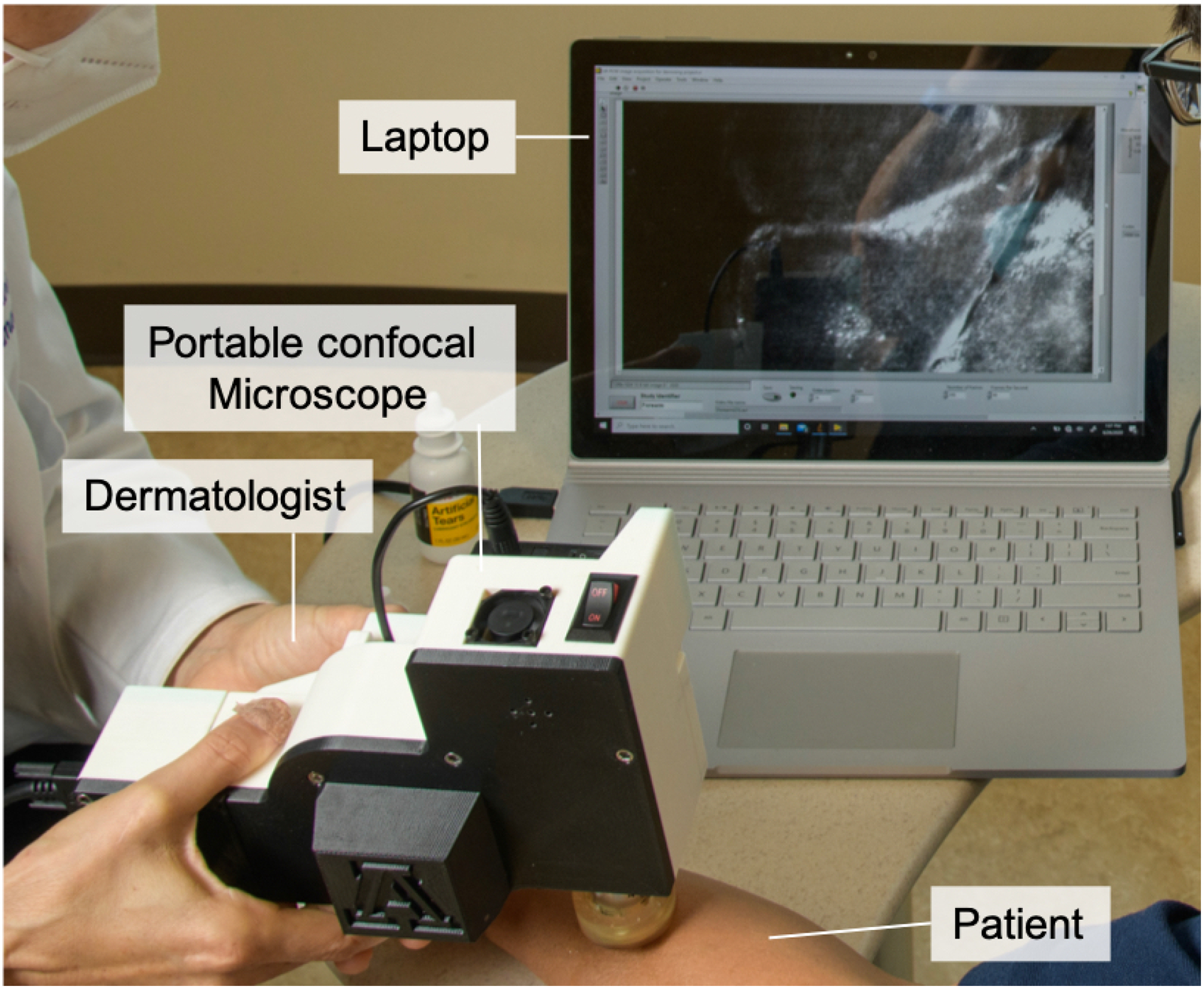
Photo of portable confocal microscope used in dermatology clinic.
PCM images visualized previously-validated RCM features for common skin lesions. For basal cell carcinoma (BCC), PCM clearly revealed the key diagnostic features such as tumor islands (Fig. 2a) and convoluted blood vessels (Fig. 2b) in a similar manner to the images obtained with the standard RCM device (Figs. 2d,e). In a PCM image obtained from another BCC lesion (Fig. 2c), hypopigmented tumor islands were visualized as dark silhouettes similarly to the image obtained with the standard RCM device (Fig. 2f).
Figure 2.
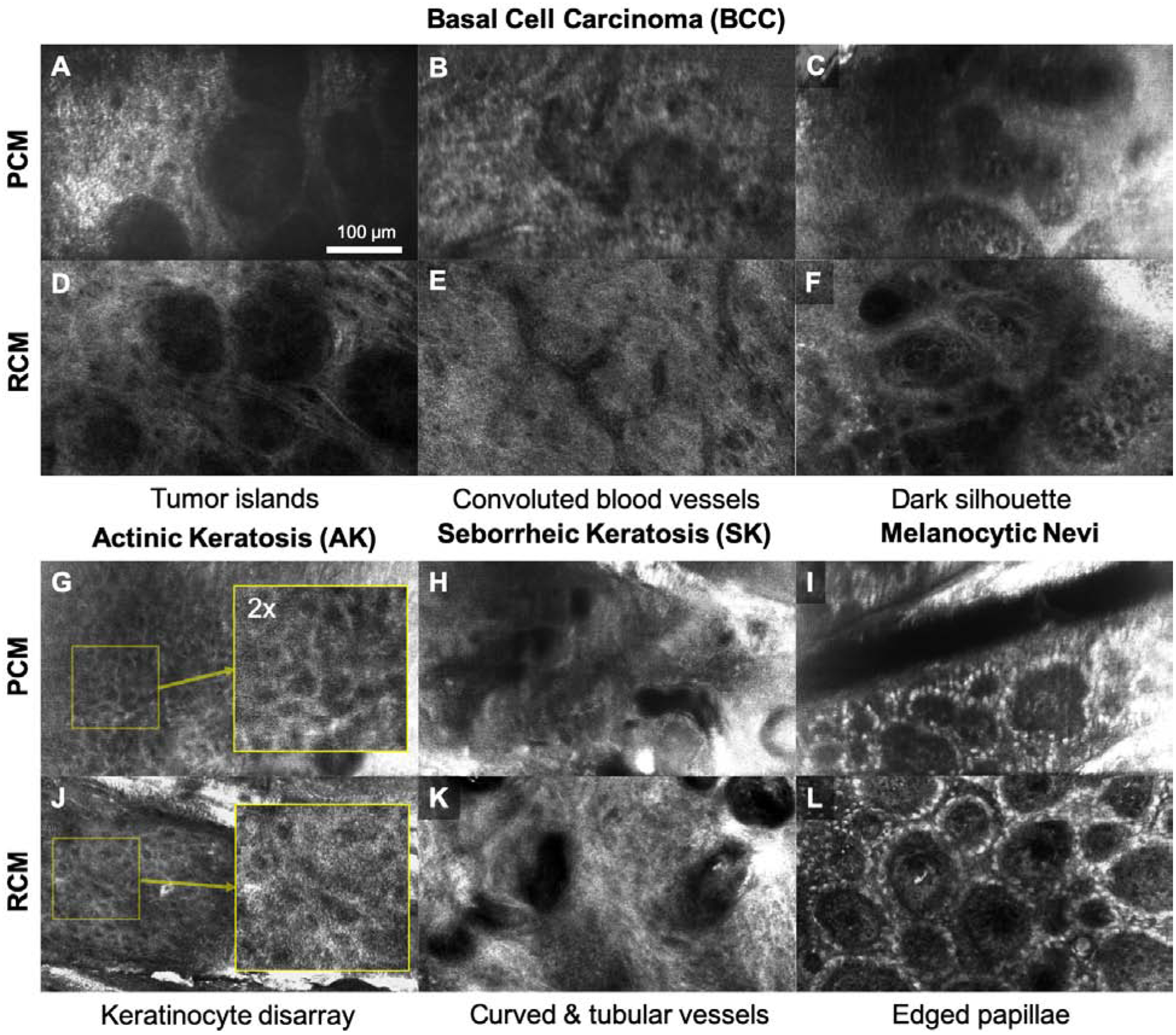
Portable confocal microscopy (PCM) and reflectance confocal microscopy (RCM) images of skin lesions commonly encountered in dermatology care. A-C, G-I: PCM images; D-F, J-L: RCM images.
Characteristic features of benign lesions were also revealed in PCM, irregularity in keratinocyte size and shape (Fig. 2g) in actinic keratosis (AK), curved and tubular vessels (Fig. 2h) in seborrheic keratosis (SK), and edged papillae (Fig. 2i) in melanocytic nevi, similarly to RCM images (Figs. 2j–l). The primary limitation we identified when using the PCM device is that the images sometimes appear blurry since stable connection between the device and skin was difficult to maintain. Efforts are undergoing to overcome this limitation, including increasing the imaging speed.
In conclusion, results from pilot imaging with PCM showed promise in visualizing key RCM features for common skin lesions. PCM working principle allows for adaptation of low-cost miniature optoelectrical components used in mobile devices, which can enable further miniaturization of PCM into a pen-sized, handheld device. Limitations of the pilot study include small number of patients and challenges in accurate spatial registration between PCM and RCM images. In the future, we will evaluate if PCM can visualize additional important RCM features and provide equivalent diagnostic accuracy to RCM.
Funding:
National Institute of Health/Fogarty International Center (R21TW010221)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: CG and DK are inventors on US patent applications (University of Arizona, assignee) on the portable confocal microscopy technology presented. The University of Arizona has a technology-licensing agreement with ArgosMD on the presented technology. CG and DK have rights to receive royalties as a result of this licensing agreement. DK serves as a scientific advisor to ArgosMD.
IRB approval status: The University of Arizona IRB has reviewed and approved this study (approval # 1408448583).
References
- 1.Milind Rajadhyaksha SG, Zavislan James M., Anderson R. Rox, Webb Robert H.. In vivo confocal scanning laser microscopy of human skin II : Advances in Instrumentation and comparison with histology. J Invest Dermatol 1999;Vol.113:293–303. [DOI] [PubMed] [Google Scholar]
- 2.Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC , Nehal KS. Reflectance confocal microscopy of skin in vivo: From bench to bedside. Lasers in surgery and medicine 2017;49:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman EE, Semeere A, Laker-Oketta M, Namaganda P, Osman H, Lukande R et al. Feasibility and implementation of portable confocal microscopy for point-of-care diagnosis of cutaneous lesions in a low-resource setting. J Am Acad Dermatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong C, Stratton DB, Curiel-Lewandrowski CN , Kang D. Speckle-free, near-infrared portable confocal microscope. Applied optics 2020;59:G41–G6. [DOI] [PMC free article] [PubMed] [Google Scholar]


