Abstract
This study was carried out to observed β-cyclodextrin (β-CD) inclusion complexes containing trans-cinnamaldehyde (CIN) by using DPPH, ABTS and FRAP assay. Antioxidative ability was compared between pure CIN and β-CD-CIN inclusion complexes and particle size, encapsulation efficiency, and temperature-dependent release of inclusion complexes were investigated. High concentration of β-CD (1.8%) as well as guest oil 1:3 molar ratio (β-CD:CIN) influenced on particle size bigger during self-assembly process. And particle sizes were increased as storage period. In the antioxidant capacity results, pure β-CD (1.8%) was antioxidative without CIN especially at FRAP assay. Antioxidant activity dramatically increased after 1:1 molar ratio (1.8% β-CD:CIN), especially at DPPH assay and ABTS•+ assay. In this study, β-CD complexation enhanced CIN solubility and affected increase the antioxidant activity of the CIN. Moreover, we need to consider that molar ratio of between β-CD concentration and CIN is effective manufacturing condition to improve antioxidant activity of β-CD-CIN inclusion complexes.
Keywords: β-Cyclodextrin, Inclusion complex, Trans-cinnamaldehyde, Antioxidant, Release
Introduction
Inclusion complexes formed by molecular self-assembly is one of encapsulation method which is useful to include hydrophobic essential oils (Chun et al., 2012; Szente and Szejtli, 2004). Self-assembly occurs by noncovalent interactions, including ionic bonds, hydrophobic interactions, hydrogen bonds, and van der Waals forces. The inclusion component is a unique form of chemical complex in which one molecule is included within another molecule or an aggregation of molecules (He et al., 2008; Stella and Rajewski, 1997; Szente and Szejtli, 2004). There are several advantages of inclusion complexes formed by cyclodextrin. First of all, cyclodextrins (CDs) are cyclic oligosaccharides consisting of six α-cyclodextrin, seven β-cyclodextrin, eight γ-cyclodextrin or more glucopyranose units linked by α-(1,4) bonds. CDs are hydrophilic outside and hydrophobic inside therefore they can encapsulate liphophilic component (Davaatseren et al., 2017; Melgarejo-Flores et al., 2013; Padukka et al., 2000; Ponce et al., 2010). β-CD is most effective due to its reasonable price among the CDs. CDs are carbohydrate wall materials and non-toxic ingredient which can be used as sweeteners (Adrián et al., 2020). CDs inclusion complexations can carry functional essential oils which are perfumed, antioxidant, antibacterial and so on. CDs complexation can improve stability of encapsulated component as well as food products against environments like light, heat, pH, and ionization state. CDs inclusion complexations protect against chemical reaction such as oxidation, volatility, browning reactions, and microbial contamination (Chun et al., 2012; Ponce et al., 2010; Szente and Szejtli, 2004).
Cinnamon oils (trans-cinnamaldehyde, CIN, 3-phenyl-2-propenal) are one of antioxidant and antimicrobial essential oil that gives cinnamon its flavor and odor (Jo et al., 2015a, b; Melgarejo-Flores et al., 2013). CIN is receiving increasing attention as an effective essential oil owing to its antibacterial and antioxidant properties in beverages such as fruit juice, milk, and edible film. Moreover, CIN is widely used as a flavoring agent (food additives) in foods such as gum, ice cream, candy, and beverages (Adrián et al., 2020; Jo et al., 2015a, b). However, there are several limitations that CIN is water insoluble, volatile and very sensitive to oxidation in the presence of light, high temperatures, oxygen, and humidity. Furthermore, its strong flavor or aroma is unfavorable sensorial properties.
To overcome limitations, CIN is commonly applied to encapsulation technology like emulsion system, cyclodextrin inclusion method, emulsion-diffusion method, etc. (Ayala-Zavala et al., 2013; Jo et al., 2015a; Lee et al., 2013). In various studies, β-CD inclusion complexes of essential oils like orange oils, oregano oils, eugenol, cinnamon oils, ginger oils, and fish oils were characterized physico-chemically and biologically by observing particle size, zeta potential, morphology structure, encapsulation efficiency, storage stability, and so on (Chun et al., 2012; Choi et al., 2010; Jo et al., 2015b; Kotronia et al., 2017; Torres-Alvarez et al., 2020). However, the activity of core material which is encapsulated component was not evaluated.
In this study, various molar ratios CIN was included into β-CD complexes and then physical properties of β-CD inclusion complexes were studied. Moreover, especially the antioxidant capacity of pure CIN and β-CD-CIN inclusion complexes was evaluated by using three different methods such as DPPH, ABTS, and FRAP.
Materials and methods
Preparation of β-CD-CIN inclusion complexes
β-cyclodextrin (β-CD, MW: 1135) purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan) was prepared at the concentrations of 0.3% and 1.8% (w/v) by dissolving in 55 °C distilled water for 1 h by using a shaking incubator with 250 rpm and then cooled down at room temperature. For β-CD inclusion complexes by molecular self-assembly, trans-cinnamaldehyde (CIN: 98%, MW: 132.16) obtained from Sigma-Aldrich® (USA) was added into β-CD solution as various mixing molar ratio (1:0.25, 1:0.5, 1:1, 1:2, and 1:3) with β-CD and mixed at 55 °C for 4 h in a shaking incubator with 250 rpm and then cooled down at room temperature. After completing inclusion processing, free CIN was removed by using n-hexane which was selected as an organic solvent to extract free CIN.
Particle size measurement
The average particle sizes were analyzed using the dynamic light scattering method (Zetasizer®, Nano ZS90; Malvern Instruments Ltd., Worcestershire, UK). Measurements were carried out at 25 °C in triplicate.
Encapsulation efficiency measurement
One mL of β-CD-CIN inclusion complexes (β-CD-CIN complexes) solution was mixed with 9 mL of n-hexane and vortexed vigorously to extract free CIN. The mixture was separated by centrifugation for 10 min at 3,000 rpm. Absorbance at 280 nm of the upper hexane layer containing the free CIN was measured using a UV/VIS spectrophotometer (Optizen, Seoul, Korea). The amount of free CIN was calculated using a previously measured standard curve and then percent encapsulation efficiency (EE%) was calculated by using the following equation (Eq. 1).
| 1 |
Temperature-dependent release study
There are many environmental stresses which affect food materials (food additives). Among various stresses, storage temperature was selected because pure cinnamaldehyde is a volatile organic compound and its evaporation starts at a low temperature (< 50 °C). Therefore, we preferentially examined the temperature-dependent release of CD-CIN-complexes at 25 °C and 37 °C. For temperature-dependent release study, 1 mL of each β-CD-CIN complexes solution was introduced into dialysis membrane (MWCO 1000 Da, Spectrum Laboratories, Inc., USA). Dialysate was distilled water and given temperatures were at 25 °C and 37 °C, respectively. At given time, dialyzed extracts were collected and then analyzed the optical density at 280 nm during each time interval. After removing dialysate and dialyzed extract, same amount of fresh dialysate was added.
Antioxidant capacity of pure CIN and CD-CIN complexes measured by DPPH, ABTS and FRAP
All chemicals were of analytical grade and were purchased from Sigma Aldrich (USA). All determinations were carried out at least in triplicate, on each occasion and at each separate concentration of the standard and samples.
DPPH assay
DPPH radical scavenging activity was determined by using a modification of the method by Mensor et al. (2001). Fifty mL of 0.3 mM DPPH was added with 125 mL of sample. After 30 min, the absorbance of the reaction mixture was measured at 518 nm by an absorbance microplate reader (VersaMax ELISA Microplate Reader, Molecular Devices, USA). Percentage antioxidant activity was compared against positive control which was the standard solution of ascorbic acid and then calculated using the following equation (Eq. 2)
| 2 |
ABTS•+ radical cation decolorization assay
ABTS•+ radical cation scavenging activity was determined by using the modified method of Re et al. (1999). ABTS (7.4 mM) was mixed with 2.45 mM potassium persulfate for 12–24 h in the dark and then absorbance of reaction mixture was adjusted with ethanol to 0.70 ± 0.02 at 732 nm ABTS•+ solution. An aliquot (20 µL) of sample was reacted with 180 µL ABTS (A732 nm = 0.70 ± 0.02) for one min then absorbance of reaction mixture was measured at 732 nm. Percentage antioxidant activity was compared against positive control which was standard solution of ascorbic acid and then calculated using the following equation (Eq. 3)
| 3 |
Ferric-ion reducing antioxidant power
The ferric reducing ability of plasma (FRAP) was determined as a measure of antioxidant capacity by using a modification of FRAP assay of Benzie and Strain (1996). To prepare FRAP reagent, 300 mM acetate, pH 3.6 glacial acetic acid buffer, 20 mM ferric chloride (FeCl3•6H2O), and 10 mM 4,6-tripryridyls-triazine (TPTZ) were prepared in 40 mM HCl. All three solutions were mixed together in the ratio 10:1:1 (v/v/v). The FRAP assay was performed that 10 μL of sample added 0.3 mL of reagent were incubated at 37 °C for 4 min. Absorbance at 593 nm was determined relative to a reagent blank. The total antioxidant capacity of samples was determined against a standard of known FRAP value, ferrous sulphate. Percentage antioxidant activity was compared against positive control which was a standard solution of ascorbic acid.
Statistical analysis
The data were analyzed by using one-way ANOVA analysis of variance. An analysis of variance was performed on all the variables using PASW statistics 18 (SPSS Inc., Chicago, IL, USA). Differences among the means were compared using Tukey-HSD (Tukey’s Honestly Significant Difference) Test (p < 0.05).
Results and discussion
Particle size of β-CD-CIN complexes
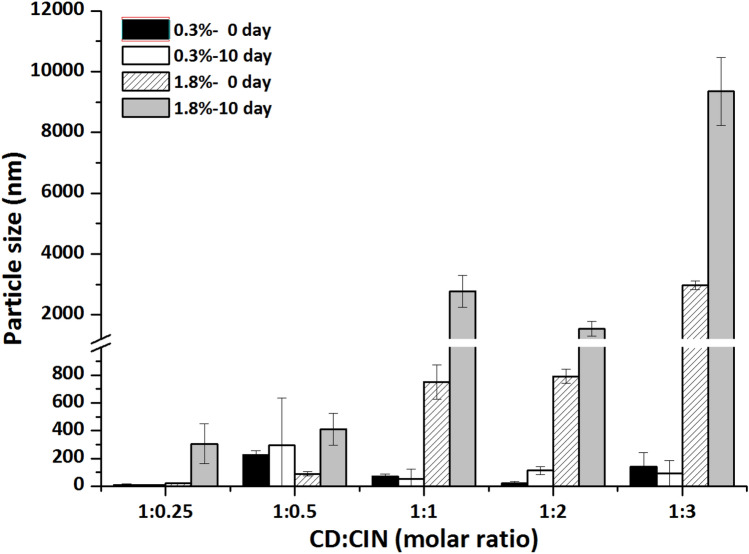
As Fig. 1 shows, particle size of β-CD-CIN complexes were varied by β-CD concentration and storage period. Higher concentration (1.8%) and longer storage time made inclusion complexes bigger. Moreover, increase of CIN molar ratio significantly affected particle sizes of β-CD-CIN complexes and this tendency was shown apparently at 1.8% β-CD-CIN complexes. The particle size of 0.3% CD-CIN complexes were not increased over 1 µm but 1.8% CD-CIN complexes were increased near 10 µm at 1:3 molar ratio (CD:CIN) and 10 days of storage period. CD complexes are getting bigger during the self-assembly because a high CD concentration is not solved entirely, and there is still insoluble CD. Moreover, if the molar ratio is not suitable between CD and core materials, in other words, if the cavity of CD is not sufficient for core material, many CD complexes located around core material for including core-material. These affect the mean particle size value, and CD-core-complexes are larger aggregates depend on assembly time. Furthermore, over self-assembly time induces self-aggregation. This phenomenon was also observed at the previous study. In the studies of Chun et al. (2012) and Jo et al. (2015a), they manufactured cyclodextrin inclusion complexes with essential oil (eugenol and trans-cinnamaldehyde) by various formulations and then characterized its physical properties during storage. High concentration of CD as well as guest oil influenced particle size bigger during the self-assembly process (Chun et al., 2012; Jo et al., 2015a).
Fig. 1.
Particle size of CD-CIN complexes at 0 day and 10 day of storage period
Encapsulation efficiency and retention amount of CIN
The encapsulation efficiency (%) and the amount of encapsulated CIN (µg/mL) were calculated before measuring antioxidant capacity (Table 1). The amount of encapsulated CIN (µg/mL) was increased depend on CIN molar ratio. In the study of Jo et al. (2015b), the encapsulation efficiency of guest compound was > 60% which was quite higher than other studies. However, the CIN encapsulation efficiency was lower as < 50% in this study. Especially, 0.3% β-CD-CIN complexes just showed 1.50–5.88% encapsulation efficiency. The encapsulation efficiency of molecular inclusion complexation is influenced by the various conditions such as CD type, CD concentration, the molar ratio of CD and core material, self-assembly time, etc. The high concentration of CD is a larger cavity than the low concentration CD. In this study, 1.8% β-CD complexes could include more CIN and have higher encapsulation efficiency. In the characterization of β-CD-CIN complexes, the antioxidant activity of both 0.3% and 1.8% β-CD-CIN complexes were evaluated for comparison, but a temperature-dependent (25 °C and 37 °C) release study was carried out with only 1.8% β-CD-CIN complexes. The released amounts of CIN were at 25 °C and 37 °C around 5.5% and 7.5%, respectively (Fig. 2). As mentioned, CIN is very volatile and easily oxidized so this could be generated during processing. Moreover, 0.3% β-CD-CIN complexes were very low loading amount because molar ratio of β-CD and CIN was used to make inclusion complexes through self-assembly. Namely, big molecular weight difference between β-CD (Mw 1135) and CIN (Mw 132.16) made that small amount of CIN was included into β-CD.
Table 1.
Encapsulation efficiency of CIN depeding on various concentration of CD or CIN
| CD (%) | CD:CIN (molar ratio) | Encapsulation efficiency (%) | Encapsulated CIN (µg/mL) |
|---|---|---|---|
| 0.3 | 1:0.25 | 2.35 | 2 |
| 1:0.5 | 5.88 | 10 | |
| 1:1 | 3.03 | 10 | |
| 1:2 | 4.18 | 28 | |
| 1:3 | 1.5 | 15 | |
| 1.8 | 1:0.25 | 20.51 | 100 |
| 1:0.5 | 47.59 | 470 | |
| 1:1 | 34.41 | 750 | |
| 1:2 | 41.25 | 1650 | |
| 1:3 | 45.39 | 2720 |
Fig. 2.
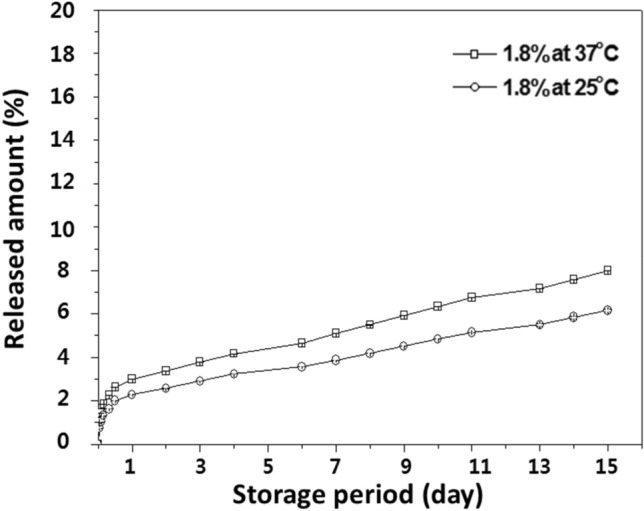
Temperature-dependent release study of 1.8% CD-CIN complexes
DPPH radical scavenging activity
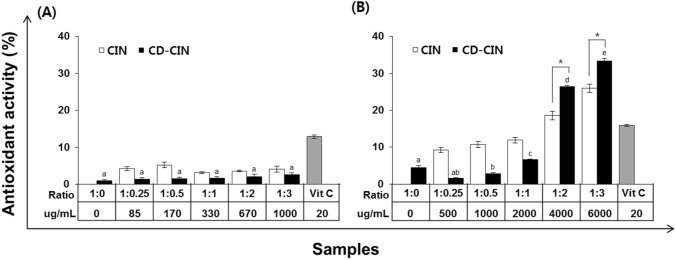
DPPH radical scavenging activity has been generally used for the measurement of antioxidant activity. In this study, pure CIN and β-CD-CIN complexes were compared their antioxidant activity and positive control was ascorbic acid. In Fig. 3(A), pure CIN and 0.3% β-CD-CIN complexes were under 10% antioxidant activity and it is three times lower than positive control. And antioxidant activity of β-CD-CIN complexes were lower than pure CIN except 1:3 molar ratio (β-CD:CIN) sample. Figure 3(B) shows that all 1.8% β-CD-CIN complexes were higher antioxidant activity than pure CIN. Moreover, 1:3 molar ratio β-CD-CIN complexes was similar with positive control as 33% antioxidant activity.
Fig. 3.
Antioxidant activity comparison by DPPH assay between CIN and CD-CIN complexes formulated with various molar ratio of CD and CIN; (A) 0.3% CD and (B) 1.8% CD. Values represented mean ± SD. Means with different superscripts (a–b) differ significantly (p < 0.05)
Host or guest material concentration and form of guest material influenced antioxidant activity. In other words, DPPH radical scavenging activity was increased depend on CIN concentration and it was strongly observed at 1.8% β-CD-CIN complexes. And 1.8% β-CD-CIN complexes were more effective than pure CIN. The 0.3% β-CD-CIN complexes were produced with a small amount CIN through the molecular inclusion method. Moreover, most of CIN were not included into β-CD as showing low encapsulation efficiency (< 6%). The reasons that 1.8% β-CD-CIN complexes were more effective are pure 1.8% β-CD solution was slightly higher antioxidant activity than 0.3% β-CD solution and higher encapsulation efficiency was observed. 1.8% β-CD-CIN complexes were more effective than pure CIN unlike 0.3% β-CD-CIN complexes. Small amount of pure CIN was slightly miscible in water without water soluble carrier but at over of certain concentration, pure CIN could not dissolved entirely. It was observed that the solubility of CIN could be improved by forming β-CD-CIN complexes. Ferreira et al. (2013) observed that the antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. In the result of radical scavenging activity toward DPPH, mangiferin and β-CD inclusion complex showed higher antioxidant activity when compared with free mangiferin.
ABTS•+ radical cation decolorization assay (ABTS•+ assay)
ABTS•+ assay has been also used for measurement of antioxidant activity like DPPH assay. Pure CIN and β-CD-CIN complexes were compared their antioxidant activity and positive control was ascorbic acid. The results of ABTS•+ assay showed similar trend with DPPH assay at 0.3% β-CD-CIN complexes that pure CIN was higher antioxidant activity than β-CD-CIN complexes. And there were no significant different as CIN ratio (p > 0.05). For the 1.8% β-CD-CIN complexes, β-CD-CIN complexes showed higher antioxidant activity than pure CIN at all samples in DPPH assay, but in ABTS•+ assay, only 1:2 and 1:3 molar ratio of β-CD-CIN complex showed higher as over 20% which was higher than positive control. And it was also observed when β-CD-CIN molar ratio was increased from 1:1 to 1:2, antioxidant activity dramatically increased both pure CIN and β-CD-CIN complexes (p < 0.05).
Ferric-ion reducing antioxidant power (FRAP assay)
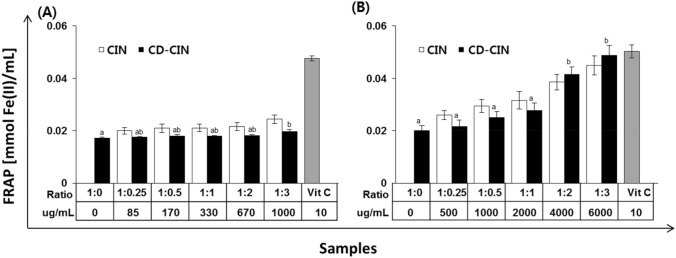
The results of FRAP assay showed similar trend with ABTS•+ assay at 0.3% β-CD-CIN complexes that CIN was higher antioxidant activity than β-CD-CIN complexes and there were no significant different depend on molar ratio between CIN and β-CD (p > 0.05). Moreover, at 1.8% CD-CIN complexes, 1:2 and 1:3 (β-CD:CIN) molar ratio of β-CD-CIN complex showed significant higher antioxidant activity. Antioxidant activity of β-CD-CIN complex were increased as CIN molar ratio but there were no significant difference among1:0.25, 1:0.5, 1:1 and between 1:2 and 1:3 β-CD-CIN complex.
There are many methods to represent antioxidant activity, and they have a different mechanism such as scavenging activity, reducing antioxidant power, and lipid peroxidation. Moreover, a synthetic antioxidant, a natural antioxidant, or free radicals are used depending on the antioxidant activity assay. It is necessary to use various techniques for better precise results because each method has advantages and disadvantages. In this study, antioxidant capacity of β-CD-CIN complexes measured by DPPH, ABTS and FRAP. First, presence of β-CD (1.8%) was effective to show higher antioxidant activity. As Figs. 3, 4 and 5 show, β-CD solution at 1:0 molar ratio (β-CD:CIN) showed antioxidant activity and higher β-CD (1.8%) was more effective. At 1.8% β-CD-CIN complexes, there was a significant change in ABTS•+ and FRAP assay (p < 0.05). Second, as the β-CD-CIN molar ratio increased from 1:1 to 1:2 (β-CD:CIN), the antioxidant activity dramatically increased both CIN and β-CD-CIN complexes, especially at DPPH assay and ABTS•+ assay. In this study, it was certainly observed that β-CD complexation enhanced CIN solubility and it affected the increase of CIN antioxidant activity. However, antioxidant capacity depended on β-CD concentration and CIN molar ratio. It was researched that β-CD complexation method improved the antioxidant capacity of hydrophobic functional guest materials (cinnamon oil, eugenol, limonene, polyphenol, etc.) when evaluated the effect of ethanol extracts of 5 spices. CDs shapes toroidal and are hydrophobic interior and hydrophilic exterior because the secondary and primary hydroxyl groups (−OH) of CD are exposed to the solvent. Therefore, the interior of the toroids is less hydrophilic, and the exterior of toroids is more hydrophilic than interior. In β-CD and CIN molecular complexation, exposed -OH group of CD and -OH group of CIN can form the hydrogen bond which may contribute to antioxidant activity (Stražišar et al., 2008). However, it could be considered that the molar ratio of β-CD and CIN can affect chemical interaction between β-CD and CIN and the power of interaction influence the level of antioxidant capacity during β-CD and CIN molecular complexation. In the study of Kim et al. (2004), DPPH radical scavenging activity, the extract of clove and cinnamon showed the most effective among 5 spices. β-CD molecular inclusion with hydrophobic compounds has been studied and there are lots of scientific results but the research of β-CD molecular inclusion should be continuously carried out because β-CD and various essential oils need their own optimal formulation to be effective inclusion complexes and their function is also investigated.
Fig. 4.
Antioxidant activity comparison by ABTS assay between CIN and CD-CIN complexes formulated with various molar ratio of CD and CIN; (A) 0.3% CD and (B) 1.8% CD. Values represented mean ± SD. Means with different superscripts (a–b) differ significantly (p < 0.05)
Fig. 5.
Antioxidant activity comparison by FRAP assay between CIN and CD-CIN complexes formulated with various molar ratio of CD and CIN; (A) 0.3% CD and (B) 1.8% CD. Values represented mean ± SD. Means with different superscripts (a–b) differ significantly (p < 0.05)
Acknowledgements
No funding to declare.
Declarations
Conflict of interest
All authors have no conflict of interest to report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yeon-Ji Jo, Email: jo.yeonji.1986@gmail.com.
Han-Seul Cho, Email: johansel@paran.com.
Ji-Yeon Chun, Email: chunjiyeon@jejunu.ac.kr.
References
- Adrián M, Silvia NO, Francisco GC, José Manuel LN. Applications of cyclodextrins in food science-A review. Trends in Food Science and Technology. 2020;104:132–143. doi: 10.1016/j.tifs.2020.08.009. [DOI] [Google Scholar]
- Ayala-Zavala JF, Silva-Espinoza BA, Cruz-Valenzuela MR, Leyva JM, Ortega-Ramírez LA, Carrazco-Lugo DK, Pérez-Carlón JJ, Melgarejo-Flores BG, González-Aguilar GA, Miranda MRA. Pectin-cinnamon leaf oil coatings add antioxidant and antibacterial properties to fresh-cut peach. Flavour and Fragrance Journal. 2013;28:39–45. doi: 10.1002/ffj.3125. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducint ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Choi MJ, Ruktanonchai U, Min SG, Chun JY, Soottitantawat A. Physical characteristics of fish oil encapsulated by β-cyclodextrin using an aggregation method or polycaprolactone using an emulsion-diffusion method. Food Chemistry. 2010;119:1694–1703. doi: 10.1016/j.foodchem.2009.09.052. [DOI] [Google Scholar]
- Chun JY, You SK, Lee MY, Choi MJ, Min SG. Characterization of β-cyclodextrin self-aggregates for eugenol encapsulation. International Journal of Food Engineering. 2012;8:17. [Google Scholar]
- Davaatseren M, Jo YJ, Hong GP, Hur HJ, Park S, Choi MJ. Studies on the anti-oxidative function of trans-cinnamaldehyde-included β-cyclodextrin complex. Molecules. 2017;22:1868. doi: 10.3390/molecules22121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira FdR, Valentim IB, Ramones ELC, Trevisan MTS, Olea-Azar C, Perez-Cruz F, de Abreu FC, Goulart MOF. Antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. LWT-Food Science and Technology. 2013;51:129–134. doi: 10.1016/j.lwt.2012.09.032. [DOI] [Google Scholar]
- He Y, Fu P, Shen X, Gao H. Cyclodextrin-based aggregates and characterization by microscopy. Micron. 2008;39:495–516. doi: 10.1016/j.micron.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Jo YJ, Chun JY, Kwon YJ, Min SG, Hong GP, Choi MJ. Physical and antimicrobial properties of trans-cinnamaldehyde nanoemulsions in water melon juice. LWT - Food Science and Technology. 2015;60:444–451. doi: 10.1016/j.lwt.2014.09.041. [DOI] [Google Scholar]
- Jo YJ, Cho HS, Choi MJ, Min SG, Chun JY. Effect of Various Concentration of beta-cyclodextrin inclusion complexes containing trans-cinnamaldehyde by molecular self-assembly. International Journal of Food Engineering. 2015;11:619–627. doi: 10.1515/ijfe-2015-0030. [DOI] [Google Scholar]
- Kim J, Kim SA, Yun WK, Kim EJ, Woo MK, Lee MS. Antioxidative effect of ethanol extract for 5 kinds of spice. Journal of the Korean Society of Food Science and Nutrition. 2004;33:1426–1431. doi: 10.3746/jkfn.2004.33.9.1426. [DOI] [Google Scholar]
- Kotronia M, Kavetsou E, Loupassaki S, Kikionis S, Vouyiouka S, Detsi A. Encapsulation of oregano (Origanum onites L.) essential oil in β-cyclodextrin (β-CD): Synthesis and characterization of the inclusion complexes. Bioengineering (Basel) 2017;4:74. doi: 10.3390/bioengineering4030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Min SG, You SK, Choi MJ, Hong GP, Chun JY. Effect of β-cyclodextrin on physical properties of nanocapsules manufactured by emulsion–diffusion method. International Journal of Food Engineering. 2013;119:588–594. doi: 10.1016/j.jfoodeng.2013.06.018. [DOI] [Google Scholar]
- Melgarejo-Flores BG, Ortega-Ramírez LA, Silva-Espinoza BA, González-Aguilar GA, Miranda MRA, Ayala-Zavala JF. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biology and Technology. 2013;86:321–328. doi: 10.1016/j.postharvbio.2013.07.027. [DOI] [Google Scholar]
- Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TCD, Coube CS, Leitão SG. Screening of brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Padukka I, Bhandari B, D'Arcy B. Evaluation of various extraction methods of encapsulated oil from beta-cyclodextrin-lemon oil complex powder. Journal of Food Composition and Analysis. 2000;13:59–70. doi: 10.1006/jfca.1999.0820. [DOI] [Google Scholar]
- Ponce Cevallos PA, Buera MP, Elizalde BE. Encapsulation of cinnamon and thyme essential oils components (cinnamaldehyde and thymol) in beta-cyclodextrin: Effect of interactions with water on complex stability. Journal of Food Engineering. 2010;99:70–75. doi: 10.1016/j.jfoodeng.2010.01.039. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Stella VJ, Rajewski RA. Cyclodextrins. Their future in drug formulation and delivery. Pharmaceutical Research. 1997;14:556–567. doi: 10.1023/A:1012136608249. [DOI] [PubMed] [Google Scholar]
- Stražišar M, Andrenšek S, Šmidovni A. Effect of β-cyclodextrin on antioxidant activity of coumaric acids. Food Chemistry. 2008;110:636–642. doi: 10.1016/j.foodchem.2008.02.051. [DOI] [Google Scholar]
- Szente L, Szejtli J. Cyclodextrins as food ingredients. Trends in Food Science & Technology. 2004;15:137–142. doi: 10.1016/j.tifs.2003.09.019. [DOI] [Google Scholar]
- Torres-Alvarez C, Castillo S, Sánchez-García E, González CA, Galindo-Rodríguez SA. Inclusion complexes of concentrated orange oils and β-cyclodextrin: Physicochemical and biological characterizations. Molecules. 2020;25:5109. doi: 10.3390/molecules25215109. [DOI] [PMC free article] [PubMed] [Google Scholar]