The main risk factors for age-related macular degeneration (AMD) are age, genetics, metabolic syndrome, inflammation and smoking. Age and genetics cannot be changed, but the other three factors appear to be modifiable to prevent AMD in the early stages. Nevertheless, AMD treatment has focused on the advanced stages of the disease, particularly the use of anti-VEGF antibody and its derivatives for wet AMD patients. For dry AMD, some candidate drugs are currently in phase II or phrase III clinical trials with some potential to ameliorate macular geographic atrophy, yet their ultimate efficacy remains uncertain.
Intervention in AMD pathogenesis in the very early stages may provide a better prevention and treatment regimen. Thus, a reasonable approach would be to investigate the commonalities of the modifiable factors, namely metabolic syndrome, inflammation and smoking, to pinpoint the potential initiation factors. A survey of the literature revealed little information and very few experimental data addressing the initiation stage of AMD. One study that included 232 patients negated the relevance of serum uric acid in the overall incidence of AMD and linked high serum uric acid (HUA) with choroid neovascularization only at a relatively late stage of AMD [1]. In contrast, a more recent cohort study positively associated gout and AMD among 1,684,314 patients [2]. Therefore, the previous literature has not substantiated the role of hyperuricemia in AMD pathogenesis, particularly in the early stage. Neither of these studies addressed the role of HUA in early AMD or the potential underlying mechanism. In this comment, we identify hyperuricemia as a potential initiation factor for AMD and propose two pathways with a detailed discussion of the mechanism.
Metabolic syndrome links hyperlipidaemia, hyperuricemia and AMD
Hyperlipidaemia is a known risk factor for both HUA and AMD. Conversely, uric acid (UA) can upregulate several lipid profiles, including very low density lipoprotein. With an increase in very low density lipoprotein, organic anion transporter 1 (OAT1) expression in RPE is suppressed, leading to reduced UA excretion [3]. This scenario is supported by data from hyperuricemic rats, which showed decreased OAT1 expression in RPE [4]. It is likely that hyperlipidaemia impedes UA excretion, resulting in UA accumulation and RPE degeneration [3]. Therefore, metabolic syndrome could be the platform by which hyperlipidaemia links HUA and AMD.
Similar inflammatory activation in AMD and gout
Inflammatory status is inevitable in both HUA and AMD. During inflammation, the renin angiotensin system (RAS) is activated and subsequently induces inflammatory biomarkers, such as TNF-α, IL-6 and IL-1β in RPE cells [5]. These markers are also regarded as inducers for choroidal neovascularization. Besides, NOD-, LRR- and pyrin domain–containing protein 3 (NLRP3) inflammasome is present in human RPE cells and has been shown to be activated by dry AMD hallmarks such as lipofuscin and drusen [6]. NLRP3 inflammasome is also likely initiated by IL-1β and monosodium urate (MSU) crystals in RPE cells, leading to the secretion of pro-inflammatory cytokines, which is similar to the inflammatory response in gout [7]. This evidence reveals a strong correlation between HUA and AMD and suggests that the accumulation of UA crystals may be an initiation factor in AMD pathogenesis.
Non-conclusive evidence for smoking as an early AMD initiating factor
Smoking is regarded as the most modifiable risk factor for AMD. It causes general hypoxia in the ocular tissues, followed by cellular stress and inflammation, leading to AMD progression [8]. Could smoking be another potential link between HUA and AMD? Current data from population studies is ambiguous. While some studies have indicated a positive linkage between smoking and HUA [8, 9], others have shown an inverse association [10]. Thus, whether smoking is another factor linking HUA and AMD remains obscure and is worth further investigation.
HUA as the convergent risk factor for AMD
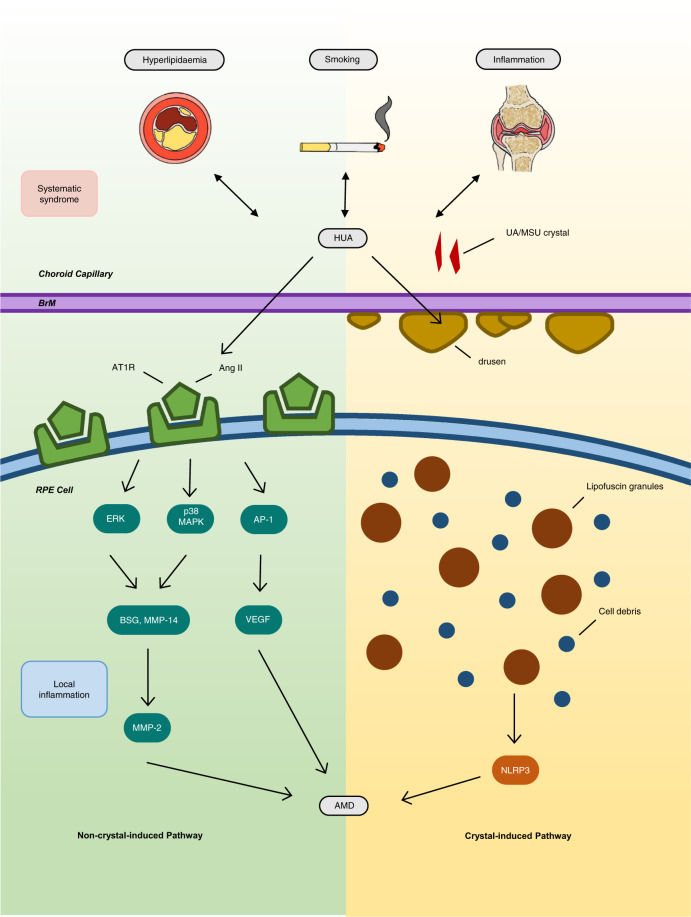
Based on the above modifiable risk factors, HUA may initiate early AMD pathogenesis that results from metabolic syndrome, with the additional possible involvement of smoking. Therefore, two alternative pathways can be proposed, namely, ‘crystal-induced AMD’ and ‘non-crystal-induced AMD’, depending on whether UA or MSU crystals are deposited in the retina.
Crystal-induced vs. non-crystal–induced pathway
A possibility exists that MSU crystals are deposited in the retina; this is supported by a case report of a chronic gout patient who showed crystal-like lesions at the termination of retinal arterioles and the RPE cells [11]. Furthermore, a recent observational cohort study associated gout and AMD with a hazard ratio of 1.39 (95% CI, 1.35, 1.43) among 1,684,314 patients [2]. This crystal-induced pathway is likely manifested with inflammation induced by UA or MSU crystals formed within the choroid capillaries–Bruch’s membrane–RPE complex (Fig. 1), similar to the pathogenesis of gouty arthritis.
Fig. 1. Two possible pathways for pathogenesis of ‘hyperuricemic AMD’.
AMD age-related macular degeneration, Ang II angiotensin II, AP-1 activator protein 1, AT1R angiotensin II type 1 receptor, BrM Bruch’s membrane, BSG basigin, ERK extracellular signal-regulated kinase, GA geographic atrophy, HUA hyperuricemia, MAPK mitogen-activated protein kinase, MMP matrix metalloproteinase, MSU monosodium urate, NLRP3 NOD-, LRR- and pyrin domain- containing protein 3, RPE retinal pigmented epithelium, UA uric acid, VEGF vascular endothelial growth factor.
AMD pathogenesis may also occur without crystal formation (Fig. 1). Recent publications have linked RAS activation, through a cascade of events, to elevated MMP-2 expression with subsequent extracellular matrix protein degradation in both Bruch’s membrane and the RPE basement membrane [12].
More direct evidence needed for ‘hyperuricemic AMD’
Based on these two possible pathways, ‘hyperuricemic AMD’ may exist and needs to be confirmed. For crystal-induced pathogenesis, UA crystal deposits in the choroid capillaries–Bruch’s membrane–RPE complex should be identified. For the non-crystal pathway, the link between HUA, RAS components and AMD pathogenesis demands direct cause–effect evidence for definite confirmation.
The use of RAS inhibitors has been suggested as a new treatment for AMD [12]. However, a recent case-control database analysis revealed no regression of AMD pathogenesis after administration [13]. Therefore, dual crystal-induced and non-crystal–induced pathways may contribute concomitantly to AMD pathogenesis, which explains why merely inhibiting the non-crystal–induced pathway does not block AMD pathogenesis. Whatever the afflicting route is, the ultimate strategy for ‘hyperuricemic AMD’ would be to alleviate HUA at the initiation stage. However, current consensus doesn’t deem HUA as an important issue in AMD pathogenesis. Once the role of HUA being concerned, the clinical criteria of HUA need to be reconsidered not only for gout and chronic kidney disease, but also for early AMD assessment.
Author contributions
All authors contribute to literature survey, interpretation, construct the concept and writing up of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Subramani S, Khor SE, Livingstone BI, Kulkarni UV. Serum uric acid levels and its association with age-related macular degeneration (ARMD) Med J Malays. 2010;65:36–40. [PubMed] [Google Scholar]
- 2.Singh JA, Cleveland JD. Gout and the risk of age-related macular degeneration in the elderly. PLoS ONE. 2018;13:e0199562. doi: 10.1371/journal.pone.0199562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu L, Shi Y, Zhuang S, Liu N. Recent advances on uric acid transporters. Oncotarget. 2017;8:100852–62. doi: 10.18632/oncotarget.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu N, Wang L, Yang T, Xiong C, Xu L, Shi Y, et al. EGF receptor inhibition alleviates hyperuricemic nephropathy. J Am Soc Nephrol. 2015;26:2716–29. doi: 10.1681/ASN.2014080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurihara T, Ozawa Y, Ishida S, Okano H, Tsubota K. Renin-angiotensin system hyperactivation can induce inflammation and retinal neural dysfunction. Int J Inflam. 2012;2012:581695. doi: 10.1155/2012/581695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73:1765–86. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, et al. Gout. Nat Rev Dis Prim. 2019;5:69. doi: 10.1038/s41572-019-0115-y. [DOI] [PubMed] [Google Scholar]
- 8.Burke BT, Köttgen A, Law A, Grams M, Baer AN, Coresh J, et al. Gout in older adults: the atherosclerosis risk in communities study. J Gerontol A Biol Sci Med Sci. 2016;71:536–42. doi: 10.1093/gerona/glv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera-Paredez B, Macías-Kauffer L, Fernandez-Lopez JC, Villalobos-Comparán M, Martinez-Aguilar MM, de la Cruz-Montoya A, et al. Influence of genetic and non-genetic risk factors for serum uric acid levels and hyperuricemia in mexicans. Nutrients. 2019;11:1136–55. doi: 10.3390/nu11061336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Yan S, Li C, Zhao S, Lv J, Wang F, et al. Risk factors for gout developed from hyperuricemia in China: a five-year prospective cohort study. Rheumatol Int. 2013;33:705–10. doi: 10.1007/s00296-012-2439-8. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Brenner JE, Foster WJ. Retinal complications of gout: a case report and review of the literature. BMC Ophthalmol. 2018;18:11. doi: 10.1186/s12886-018-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pons M, Cousins SW, Alcazar O, Striker GE, Marin-Castano ME. Angiotensin II-induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via the angiotensin II receptor type 1-mitogen-activated protein kinase 1 pathway in retinal pigment epithelium: implications for age-related macular degeneration. Am J Pathol. 2011;178:2665–81. doi: 10.1016/j.ajpath.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas AS, Redd T, Hwang T. Effect of systemic beta-blockers, ACE inhibitors, and angiotensin receptor blockers on development of choroidal neovascularization in patients with age-related macular degeneration. Retina. 2015;35:1964–8. doi: 10.1097/IAE.0000000000000603. [DOI] [PubMed] [Google Scholar]