Abstract
Background
Diabetic retinopathy (DR) is related to oxidative stress and insufficient intake of dietary antioxidants may be associated with the onset and progression of DR. This study aimed to detect the association between main dietary antioxidants intake and the risk for DR.
Methods
This is a cross-sectional study of a Chinese urban population. Four hundred and fifty-five subjects with type 2 diabetes were recruited and divided into diabetic patients without retinopathy (DWR) group and DR group based on their retinal status. CSMO (clinically significant macular oedema) was diagnosed by stereoscopic photography. Demographic and lifestyle characteristics were ascertained by questionnaire. General physical and ophthalmic examinations were completed for all subjects. Dietary antioxidants were assessed by 3-day food records. Subjects who have taken any type of vitamin supplements were excluded from the study. The association of dietary antioxidants with the risk for DR was analysed by logistic regression with adjustment of other factors. The dietary antioxidants levels of the CSMO subjects and non-CSMO subjects were compared using the Wilcoxon rank sum test.
Results
One hundred and nineteen subjects in DR group and 336 subjects in DWR group participated in the study. Only ten DR subjects had CSMO. The results showed that higher vitamin E (OR (95% CI):0.97 (0.95, 1.00), P = 0.036) and selenium (OR (95% CI):0.98 (0.96, 1.00), P = 0.017) intake appear to be the protective factors of DR. The dietary antioxidants levels of CSMO and non-CSMO subjects had no statistical differences (P > 0.05).
Conclusions
Dietary antioxidants intake, particularly vitamin E and selenium, were observed to have protective effects on DR.
Subject terms: Retinal diseases, Scientific community
Introduction
Diabetic retinopathy (DR), one of the microvascular complications in diabetes, remains the major cause of blindness in adults. With the growing prevalence of diabetes in recent years, the number of DR subjects in China had rapidly increased [1]. DR is a multi-factorial disease with a complex aetiology. Clinical studies have clearly documented that intensified glycaemic control reduces the occurrence and severity of DR [2]. But many subjects, even with a careful glycaemic control, are still affected by diabetic complications, including DR [3, 4]. The pathophysiology of this blinding disease is complicated, and the exact mechanism remains elusive. One of the possible mechanism is that sustained hyperglycaemia induces oxidative stress, which accelerates the progress of pathogenic lesions of DR [5]. Previous studies showed that administration of antioxidants can prevent diabetes-induced oxidative stress and the development of retinopathy in diabetic rats [6]. The effects of antioxidants on development of diabetic retinopathy in human remain to be controversial.
Diet control has always been one of the cornerstones in diabetes management. A recent research found that Mediterranean diet enriched with extra virgin olive oil, which has strong antioxidant effects, may protect against DR [7]. Fruits and vegetables rich in antioxidants were also found to reduce the risk for DR [8, 9]. However, previous study in non-Hispanic white and Hispanic adults in southern Colorado demonstrated that dietary intake of vitamin C, vitamin E, and β-carotene has no protective effect on DR [10]. In contrast, potential deleterious effects of those nutrient antioxidants were found in this study. Riboflavin was found to have the ameliorative effect on oxidative stress in diabetic mice. It is suggested that supplementation with dietary riboflavin might help to reduce diabetic complications [11]. Selenium is a component of antioxidant enzymes, namely glutathione peroxidase and thioredoxin reductase [12]. High-dose selenium was found to down-regulate vascular endothelial growth factor which can increase vascular permeability and stimulate retinal neovascularization [13, 14]. These evidences suggest that selenium may also play a role in preventing the progression of retinopathy.
The purpose of this study is to evaluate the effects of major dietary antioxidants that can be obtained from the Nutrition Calculator, including vitamin C, vitamin E, vitamin A, riboflavin and selenium, on risk for DR in a case–control cohort of Chinese subjects.
Subjects and methods
Study subjects and clinical evaluation
Data were collected from the Desheng Diabetic Eye Study, details of which have been described previously [15]. Subjects with type 2 diabetes mellitus (DM) were recruited between January 2010 and January 2011 from the Desheng community of urban Beijing. The study protocol was approved by the Ethics Committee of Beijing Tongren Hospital and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects before their enrolment. Diabetic subjects were recognised based on either a history of known type 2 DM or undergoing treatment for diabetes, excluding people who did not respond to the dietary survey and subjects with other major ocular diseases at baseline. Of the 516 subjects with type 2 DM were initially recruited, 495 had completed the 3-day food record questionnaire, whereas 21 (4.07%) subjects had no dietary data. Among the 495 subjects, 9 subjects with non-gradable fundus photographs were excluded. 31 subjects who had ever taken any type of vitamin supplements were also excluded from the statistics, due to lack records of intake frequency and duration of supplements.
All subjects underwent a standardised evaluation consisting of a questionnaire, ocular and anthropometric examinations, as well as laboratory test. The questionnaire includes basic information (age, sex, ethnicity, income, education), lifestyle information (such as smoking, exercise and alcohol intake) and health status information (such as the use of insulin therapy and any history of systemic diseases). Ethnicity was divided into Han and other minorities. Exercise was divided into three groups according to the exercise intensity in the previous 7 days (group 0: have no exercise, group 1: have mild exercise without sensible perspiration, such as walking; group 2: have moderate or vigorous exercise with sensible perspiration). Smoking was also divided into three groups: group 0 (Never smoke), group 1(current smoker) and group 2 (former smoker). Drink only referred to current drinkers. Anthropometric parameters included bodyweight and height, waist and hip circumference and resting blood pressure which was measured three times, 5 min apart. Body mass index (BMI, kg/m2) [16] and waist-to-hip ratio (WHR) were calculated. A comprehensive ophthalmological examinations, including corrected visual acuity, slit-lamp biomicroscopy and fundus photography were performed [17, 18]. Seven fields 30° colour fundus photographs with stereoscopic images of the optic disc and macula were taken through the dilated pupils of each subject using a digital fundus camera (Zeiss Visucam Pro, Oberkochen, Germany). The Early Treatment Diabetic Retinopathy Study (ETDRS) grid was automatically centred on the fovea [19].
The overall retinopathy grade, including clinically significant macular oedema (CSMO), for each eye was determined according to the protocol described in the ETDRS [20]. All of the fundus photographs have been graded by a coauthor (XY) who has been trained at University of Wisconsin Fundus Photograph Reading Center. Subjects whose eyes had no signs of DR were assigned to the diabetic-without-retinopathy (DWR) group. Subjects whose eyes had DR worse than ‘questionable’ were assigned to the DR group. CSMO was assessed by stereoscopic photography. The ETDRS criteria for CSMO included the presence of any of the following three characteristics: (1) Thickening of the retina at or within 500 μm of the centre of the macula. (2) Hard exudates at or within 500 μm of the centre of the macula, if associated with thickening of adjacent retina. (3) A zone or zones of retinal thickening 1-disc area or larger, any part of which is within 1-disc diameter of the centre of the macula.
Dietary assessment
Dietary intakes were assessed using a 3-day records [21]. The dietary recording template was shown in Fig. 1. All subjects included had to fill a dietary record over three consecutive days except Saturday and Sunday. Common sets of household measures were used to help subjects to estimate food portion sizes. The nutrient quantities were calculated with Nutrition calculator V1.6 (ISBN: 7-900341-12-9). The database of this software is based on the China Food Composition 2002, including over 1400 kinds of food, which was compiled by Institute of Nutrition and Food Safety of China Centers for Disease Control [22].
Fig. 1. The dietary recording template.
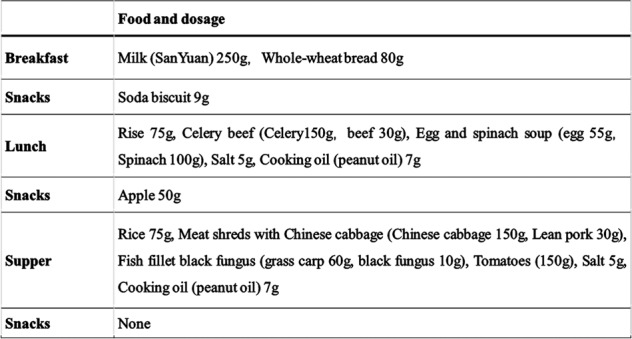
Three meals and snacks, with food portion sizes, should all be included in the record.
Serum and plasma biochemistry
Fasting blood samples were collected for measurement of C-reactive protein, fasting plasma glucose (FPG), glycosylated haemoglobin A1c (HbA1c), creatinine (CR), uric acid, lipid profile (levels of total cholesterol, triglycerides, and high-density and low-density lipoprotein cholesterol). All biochemical markers were measured using an automated system with reagents for routine biomarkers. HbA1c was assessed using a Hitachi analyser 7080 (Ibaraki, Japan). The first-void, midstream morning spot urine samples were collected and albuminuria was measured by immunonephelometry with a Roche/Cobas C501 analyser (Ibaraki, Japan). High albuminuria was defined as ≥20 mg/l [23].
Statistical analysis
All data were collected by manual double entry using the EpiData Entry data entry programme to avoid potential sources of bias. Previous studies have reported OR value of dietary antioxidants ranging from 1.50 to 5.65 (P < 0.02) and R2 < 0.8 (P = 0.14–0.76) [10, 24]. Sample size was calculated using PASS (version 11.0.7) to achieve an OR of 3 and R2 = 0.5. Considering an acceptable 95% confidence interval (P = 0.05, power = 0.9), a target sample size of approximately 460 would be needed considering potential dropouts of the patients. Statistical analysis was performed using the R statistical analysis package. The χ2 test was used to compare categorical data of the two groups. The Shapiro–Wilk test was performed for normal distribution. Parametric variables were compared between groups by T-test. Nonparametric data were compared by using the Wilcoxon rank sum test. Variables with distributions that were not normally distributed were log-transformed before analysis. Binomial logistic regression was used for multivariate analysis. The odds ratio (OR) and 95% confidence intervals (CI) were calculated. The statistically significant level was set at P < 0.05.
Results
A total of 455 diabetic patients were assessed in the study, including 336 patients with retinopathy and 119 without retinopathy. Ten patients had CSMO. The mean age of DWR and DR subjects were 65.4 ± 8.8 and 63.2 ± 8.5 years (P = 0.36). The general characteristics of the study subjects are presented in Table 1. DWR group had more percentage of higher education subjects (P = 0.016) compared to DR group. DR patients had earlier diabetic onset age (P = 0.015) and longer DM duration (P < 0.001). DR was significantly associated with more insulin use, higher CR, FPG and HbA1c (P < 0.05).
Table 1.
Demographic characteristics and clinical findings according to divided groups.
| DWR n = 336 |
DR n = 119 |
P | |
|---|---|---|---|
| Age | 65.4 ± 8.8 | 63.2 ± 8.5 | 0.36c |
| Ethnics (han%) | 310 (92.3%) | 111 (93.3%) | 0.772d |
| Sex (man %) | 126 (37.5%) | 48 (40.3%) | 0.584d |
| Education (≥high school education) | 214 (63.7%) | 61 (51.3%) | 0.016d |
| Exercisea | 0.589d | ||
| 0 | 37 | 16 | |
| 1 | 206 | 75 | |
| 2 | 93 | 28 | |
| Smokeb | 0.999d | ||
| 0 | 235 | 83 | |
| 1 | 39 | 14 | |
| 2 | 62 | 22 | |
| Age onset | 55.6 ± 9.2 | 51.9 ± 9.7 | 0.015c |
| DM duration | 8.6 ± 5.7 | 13.1 ± 8.5 | <0.001e |
| ISU%f | 54 (16.1%) | 61 (51.3%) | <0.001d |
| BMI | 24.7 ± 3.6 | 25.3 ± 4.5 | 0.127e |
| WHR | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.115e |
| HDL | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.475e |
| LDL | 3.0 ± 0.8 | 3.2 ± 0.9 | 0.172e |
| Cholesterol | 5.2 ± 1.0 | 5.3 ± 0.3 | 0.346e |
| TG | 1.7 ± 1.7 | 1.6 ± 1.1 | 0.478e |
| CRP | 0.2 ± 0.3 | 0.3 ± 0.4 | 0.220e |
| CR | 65.6 ± 15.9 | 71.8 ± 30.8 | 0.006e |
| FPG | 7.4 ± 2.1 | 8.6 ± 2.6 | <0.001e |
| HbA1c | 6.6 ± 1.2 | 7.6 ± 1.5 | <0.001e |
Data are expressed as mean ± SD unless otherwise indicated.
CRP C-reactive protein, DM diabetes mellitus, DR diabetic retinopathy, DWR diabetes without retinopathy, FPG fasting plasma glucose, HbA1c glycosylated haemoglobin, HDL high density lipoprotein, LDL low density lipoprotein, TG triglyceride, WHR waist-hip ratio, ISU insulin.
agroup 0: have no exercise; group 1: have mild exercise without sensible perspiration, such as walking; group 2: have moderate or vigorous exercise with sensible perspiration.
bgroup 0: Never smoke, group 1: current smoker, group 2: former smoker.
cT-test.
dχ2 test.
eWilcoxon rank sum test.
fISU%: the percentage of the subjects using insulin.
Dietary calories and antioxidants intake of the two groups are shown in Table 2. The data showed that DR patients had lower calories intake than DWR patients, but this difference was not statistically significant (P > 0.05). Dietary antioxidants, vitamin C, vitamin E, riboflavin and selenium in DR group were lower than those in DWR patients, but the difference was not statistical significance (P > 0.05).
Table 2.
Daily dietary calories and antioxidants intake of the two groups.
| DWR n = 336 |
DR n = 119 |
P | |
|---|---|---|---|
| Calories (kcal) | 1315 (1064.8, 1585.5) | 1270 (1063, 1478.5) | 0.113 |
| Carbohydrate (g) | 159.8 (134.2, 200.8) | 159 (127.8, 184.9) | 0.223 |
| Protein (g) | 53.2 (41.6, 66) | 51.2 (42.6, 60.5) | 0.182 |
| Fat (g) | 46.6 (36.3, 60.7) | 44.2 (33.5, 54.9) | 0.148 |
| Antioxidants | |||
| Vitamin C (mg) | 83 (55.3, 120.9) | 78.1 (51.5, 115.3) | 0.402 |
| Vitamin E (mg) | 18.5 (14.2, 24.5) | 17.5 (13.6, 21.8) | 0.111 |
| Vitamin A (μgRE) | 469 (283.2, 737.2) | 478 (278.5, 751.5) | 0.905 |
| Riboflavin (mg) | 0.755 (0.57, 1.01) | 0.72 (0.575, 0.9) | 0.237 |
| Selenium (μg) | 36.2 (29.6, 48.3) | 35.8 (27, 43.5) | 0.082 |
Data were compared by using the Wilcoxon rank sum test.
RE retinol equivalents.
The percentage of calories sources (carbohydrate, fat and protein) were analysed in the multiple logistic regression. After adjusted of multiple factors, including gender, ethnics, use of insulin, glycosylated haemoglobin, hypertension and exercise, dietary vitamin E (OR (95%CI): 0.97 (0.95, 1.00), P = 0.036) and selenium (OR (95%CI): 0.98 (0.96, 1.00), P = 0.017) intake were inversely associated with the risk for DR (Table 3).
Table 3.
Adjusted regression models of diet calories and antioxidants as predictors of DR.
| OR (95%CI) | P | |
|---|---|---|
| Calories* | ||
| Carbohydrate (%) | 1.01 (0.98, 1.04) | 0.468 |
| Protein (%) | 1.01 (0.93, 1.09) | 0.823 |
| Fat (%) | 0.99 (0.96, 1.02) | 0.404 |
| Antioxidants | ||
| Vitamin C | 1.00 (0.99, 1.01) | 0.413 |
| Vitamin E | 0.97 (0.95, 1.00) | 0.036 |
| Vitamin A | 1.00 (1.00, 1.01) | 0.423 |
| Riboflavin | 0.57 (0.27, 1.18) | 0.129 |
| Selenium | 0.98 (0.96, 1.00) | 0.017 |
Gender, ethics, use of insulin, glycosylated haemoglobin, hypertension and exercise were adjusted for all those nutrients.
*The calorie percentages of carbohydrate, fat and protein were analysed in this multiple logistic regression.
Average dietary antioxidants levels in DR subjects without CSMO were higher than those in DR patients with CSMO, but the difference was not statistically significant (P > 0.05) between the two groups when compared using the Wilcoxon rank sum test.
Discussion
This was the first cross-sectional study to determine the association between daily dietary antioxidants and the risk for DR among Chinese urban population. The data showed that vitamin E and selenium were the protective factors for DR, whereas vitamin C, vitamin A and riboflavin were not significantly associated with the risk for DR. Diabetic patients with CSMO had more intake of dietary antioxidants, but difference was not statistically significant.
Dietary antioxidants may have the beneficial effect on DR based on the molecular mechanisms in animal and clinical studies. Previous studies on diabetic rats demonstrate that feeding antioxidants can prevent the development of retinopathy [25–27]. Nutritional supplementation of antioxidants also helps to maintain normal retinal function, mitochondrial homoeostasis and inflammatory mediators [27]. However, results from human studies have not reached a consensus. Previously studies of effects of dietary antioxidants on DR included flavonoid, lutein, zeaxanthin, vitamin C, carotene, vitamin E and selenium. Vitamins C and E, carotene, selenium, flavonoid and lutein were indicated to be the protective factors for DR in some of the previous studies [8, 9, 28, 29]. But in large population studies, dietary vitamins C and E, and lutein are not associated with the risk for DR, while decreased risk for retinopathy was found among users of vitamin C or E supplements or complex supplements compared with reported users of no supplements [30].
In our study, vitamin E and selenium were found to be the protective factors of DR. In the past 20 years, the consumption of several kinds of cooking oil are the main source of vitamin E, and animal offal and meats are the main source of selenium in Chinese population [22]. Vitamin E is the major antioxidant in lipid phase. The potential benefit of vitamin E may be due to its free radical scavenger activity outside the cell through non-enzymatic mechanisms [31]. It can also improve the action of insulin in patients with insulin resistance [32]. A previous study suggested vitamin E supplementation could significantly decrease the incidence of diabetic retinopathy in both type 1 and 2 diabetes [33]. Another study indicated that pharmacological doses of vitamin E was associated with decreased HbA1c levels in type 1 diabetic patients [34], which could lead to a reduction of DR. In the other study, supplementation of taurine, vitamin E and selenium for 4 months was found to reduce the biochemical retinal alterations in diabetic rat in poor metabolic control [35]. In type 1 diabetic patients, vitamin E could normalise diabetic retinal hemodynamics, compared with control levels [31, 36]. A previous human study with large sample, including 1353 subjects with type 2 diabetes, demonstrated no association of retinopathy with intake of vitamin E from food alone or from food and supplements combined, while decreased risk for retinopathy was found with the users of vitamin E supplements or complex supplements [24]. Another study, including 387 diabetic participants from southern Colorado, even reported a deleterious effect of nutrient antioxidants [10]. Probably due to different dietary patterns, the average intake of vitamin E and C in our study is far more than the intake in those previous two human studies. It is plausible that protective effects of dietary vitamin E depends on its dosage, the relatively small quantity of vitamin E intake in previous study might not be sufficient to protect the retina in diabetic patients. It has been showed that plasma protein mask the dietary polyphenols, and reduce their radical scavenging potential in type 2 diabetics [37]. Thus, for diabetic patients, the effective quantity of vitamin E should be higher than that for normal persons without diabetes. Besides, in our study, all of the participants come from urban community, where they can easily get instructions for glucose control. The HbA1c levels for DWR and DR group were 6.6 ± 1.2 and 7.6 ± 1.5, respectively. Well-controlled blood glucose may decrease the masking effect of antioxidants [37]. After it disarms a free radical, vitamin E becomes a weak free radical itself, or prooxidant. However, vitamin C can help to turn vitamin E free radical back into antioxidant [38, 39]. Thus, combining with lower levels of vitamin C supplementation in those two studies, vitamin E could be a risk factor for DR as a pro-oxidant. Moreover, the classic Chinese diet is considered the healthy diet with lower fat and higher fibre intake [40]. That may contribute to the observation of the protective effect of vitamin E on DR in our study.
In consistent with our study, a review in 1984 has mentioned that high-dose selenium, supplied with vitamin C and E, was found to slow the progression of visual loss in DR and macular degeneration [28]. Several researches of diabetic rat reported that selenium could reduce biochemical retinal alterations, blunt the increment in serum glucose, ameliorate the oxidative stress in liver, and have protective effects against diabetes-induced brain and erythrocyte oxidative injuries through regulation of the antioxidant level and cytokine production [35, 41, 42]. On the other hand, high-dose selenium in proliferative retinopathies may reflect down-regulation of vascular endothelial growth factor [29]. Those results demonstrate the possible protective effect of selenium on DR. In human research, selenium was found to have beneficial effects on antioxidant, insulin resistance and B cell function in diabetic nephropathy patients [43]. The mean plasma selenium level in type 1 diabetic patients was significantly lower than normal controls, and a significantly lower plasma selenium contents was found in diabetics with poor metabolic control compared with those with good or average control [44]. However, in a hospital-based case–control study including 847 participants, serum selenium levels are positively associated with the prevalence of diabetes [45]. One possible reason may be the decreased utility of selenium in diabetic patients. In another study, including 5423 subjects in Hunan, a positive correlation between dietary selenium intake and the prevalence of diabetes was demonstrated [46]. However the results of the Selenium and Vitamin E Cancer Trial ruled out any significant relationship between supplementary selenium and the risk of type 2 diabetes [47]. Selenium may only be the protective factor for DR, but not DM. In the above two studies, the average selenium intake level is higher than that in our study. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice [48], which may be the reason for the contradictory results.
In our study, no association was found between DR with vitamin C, A and riboflavin. In a previous study, vitamin C and carotene have been found to be associated with the decreased risk of DR [8]. But in a population study, vitamin C was not associated with DR [24]. In another study, even a deleterious effect was observed [10]. In type 2 diabetic mice, it is suggested that supplementation with dietary riboflavin might help in the reduction of diabetic complications [11]. But no study has reported on association between riboflavin and risk of DR in human.
A recent study in 62 patients with diabetic macular oedema showed that intravitreal ranibizumab combined with antioxidant supplementation reduced central subfield macular thickness after 2 years of follow-up, compared to ranibizumab alone in patients with diabetic macular oedema [49]. In a large clinical study with 235 diabetic patients, a daily dosage of 600 mg alpha-lipoic acid did not prevent the occurrence of CSMO in diabetic patients [50]. In our study, only ten patients had CSMO. The small sample size limited the analysis of association between dietary antioxidants and risk for CSMO. But the result presented that CSMO patients had lower levels of dietary antioxidants, which was consistent with the previous study. Larger sample size was needed for further analysis.
Our study is a cross-sectional study. Lack of follow-up results prevents us to predict the causality between diet antioxidants and risk for DR. Consequently, prospective studies are needed to determine if food antioxidants have the protective effect on DR. Since most of the food was cooked, some of the antioxidants might be lost during cooking. Accurate nutrition data about cooked foods are needed. Low CSMO numbers could be related with detection method in this study. The sample size of this study was relatively small and it leads to low statistical power. Further studies with a large simple size in Chinese population will help to give a definitive answer to the issue we are concerned.
Taken together, the results of this study support the hypothesis that a diet rich in antioxidants, vitamin E and selenium, might help to prevent the development of DR in Chinese populations. However, the adequate dose of supplementation should be carefully assessed and future studies should focus on potential toxicity of the antioxidants.
Summary
What was known before
Dietary antioxidants may play a role in preventing the progression of retinopathy. The results of previous studies are inconsistent. To date, the relationship between dietary antioxidants and DR has not been evaluated in Chinese population.
What this study adds
A diet rich in antioxidants might help to prevent the development of DR in Chinese.
Acknowledgements
Foundations are supported by the Beijing Natural Science Foundation (No. 7131007), the National Natural Science Foundation of China (No. 81070734), and the Beijing Education Commission (No. KZ201110025028).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Group UPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 4.Gæde P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617–22.. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 5.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retinal Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustata GT, Rosca M, Biemel KM, Reihl O, Smith MA, Viswanathan A, et al. Paradoxical effects of green tea (Camellia sinensis) and antioxidant vitamins in diabetic rats improved retinopathy and renal mitochondrial defects but deterioration of collagen matrix glycoxidation and cross-linking. Diabetes. 2005;54:517–26.. doi: 10.2337/diabetes.54.2.517. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Lopez A, Babio N, Martinez-Gonzalez MA, Corella D, Amor AJ, Fito M, et al. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes complications: a post hoc analysis of a randomized trial. Diabetes Care. 2015;38:2134–41.. doi: 10.2337/dc15-1117. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Tanaka S, Horikawa C, et al. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology. 2013;24:204–11.. doi: 10.1097/EDE.0b013e318281725e. [DOI] [PubMed] [Google Scholar]
- 9.Mahoney SE, Loprinzi PD. Influence of flavonoid-rich fruit and vegetable intake on diabetic retinopathy and diabetes-related biomarkers. J Diabetes Complic. 2014;28:767–71.. doi: 10.1016/j.jdiacomp.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Mayer-Davis EJ, Bell RA, Reboussin BA, Rushing J, Marshall JA, Hamman RF. Antioxidant nutrient intake and diabetic retinopathy: the San Luis Valley Diabetes Study. Ophthalmology. 1998;105:2264–70.. doi: 10.1016/S0161-6420(98)91227-1. [DOI] [PubMed] [Google Scholar]
- 11.Alam MM, Iqbal S, Naseem I. Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch Biochem Biophys. 2015;584:10–9. doi: 10.1016/j.abb.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 12.SG P, PH G. Recent developments in selenium metabolism and chemical speciation: a review. J Trace Elem Med Biol. 1999;13:193–214. doi: 10.1016/s0946-672x(99)80037-6. [DOI] [PubMed] [Google Scholar]
- 13.Jiang C, Ganther H, Lu J. Monomethyl selenium-specific inhibition of MMP-2 and VEGF expression: implications for angiogenic switch regulation. Mol Carcinog. 2000;29:236–50.. doi: 10.1002/1098-2744(200012)29:4<236::aid-mc1006>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008;27:608–21.. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Li Y-Y, Yang X-F, Gu H, Liu X-P, Snellingen T, Liu N-P. The Beijing Desheng Diabetic Eye Study: rationale, design, methodology and baseline data. Int J Ophthalmol. 2018;11:108–16. doi: 10.18240/ijo.2018.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang FH, Liang YB, Peng XY, Wang JJ, Zhang F, Wei WB, et al. Risk factors for diabetic retinopathy in a rural Chinese population with type 2 diabetes: the Handan Eye Study. Acta Ophthalmol. 2011;89:e336–e43.. doi: 10.1111/j.1755-3768.2010.02062.x. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107:237–43. doi: 10.1001/archopht.1989.01070010243030. [DOI] [PubMed] [Google Scholar]
- 18.Rudnisky CJ, Tennant MT, Weis E, Ting A, Hinz BJ, Greve MD. Web-based grading of compressed stereoscopic digital photography versus standard slide film photography for the diagnosis of diabetic retinopathy. Ophthalmology. 2007;114:1748–54.. doi: 10.1016/j.ophtha.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Group ETDRSR. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 20.ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. Ophthalmology. 1991;98:823–33. [PubMed] [Google Scholar]
- 21.Crawford PB, Obarzanek E, Morrison J, Sabry Z. Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9-and 10-year-old girls. J Am Dietetic Assoc. 1994;94:626–30.. doi: 10.1016/0002-8223(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Nutrition and Food Safety CC. China Food Composition 2002. Beijing, China: Peking University Medical Press; 2002. [Google Scholar]
- 23.Lepore G, Maglio ML, Nosari I, Dodesini AR, Trevisan R. Cost-effectiveness of two screening programs for microalbuminuria in type 2 diabetes. Diabetes Care. 2002;25:2103–4. doi: 10.2337/diacare.25.11.2103. [DOI] [PubMed] [Google Scholar]
- 24.Millen AE, Klein R, Folsom AR, Stevens J, Palta M, Mares JA. Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2004;79:865–73.. doi: 10.1093/ajcn/79.5.865. [DOI] [PubMed] [Google Scholar]
- 25.Fennell JP, Brosnan MJ, Frater AJ, Hamilton CA, Alexander MY, Nicklin SA, et al. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther. 2002;9:110–7. doi: 10.1038/sj.gt.3301633. [DOI] [PubMed] [Google Scholar]
- 26.Zhu HL, Stewart AS, Taylor MD, Vijayasarathy C, Gardner TJ, Sweeney HL. Blocking free radical production via adenoviral gene transfer decreases cardiac ischemia-reperfusion injury. Mol Ther: J Am Soc Gene Ther. 2000;2:470–5. doi: 10.1006/mthe.2000.0193. [DOI] [PubMed] [Google Scholar]
- 27.Kowluru RA, Zhong Q, Santos JM, Thandampallayam M, Putt D, Gierhart DL. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr Metab. 2014;11:8. doi: 10.1186/1743-7075-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu BJ, Hu YN, Lin S, Ma WJ, Li XR. Application of Lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol. 2011;4:303–6. doi: 10.3980/j.issn.2222-3959.2011.03.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarty M. The putative therapeutic value of high-dose selenium in proliferative retinopathies may reflect down-regulation of VEGF production by the hypoxic retina. Med Hypotheses. 2005;64:159–61.. doi: 10.1016/j.mehy.2002.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Sahli MW, Mares JA, Meyers KJ, Klein R, Brady WE, Klein BE, et al. Dietary intake of lutein and diabetic retinopathy in the Atherosclerosis Risk in Communities Study (ARIC) Ophthalmic Epidemiol. 2016;23:99–108. doi: 10.3109/09286586.2015.1129426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bursell SE, Clermont AC, Aiello LP, Aiello LM, Schlossman D, Feener EP, et al. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22:1245–51.. doi: 10.2337/diacare.22.8.1245. [DOI] [PubMed] [Google Scholar]
- 32.Upritchard JE, Sutherland WH, Mann JI. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23:733–8. doi: 10.2337/diacare.23.6.733. [DOI] [PubMed] [Google Scholar]
- 33.A BJ, Jain A, Vitamin E. Its beneficial role in diabetes mellitus (DM) and its complications. J Clin diagnostic Res: JCDR. 2012;6:1624–8. doi: 10.7860/JCDR/2012/4791.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain SK, McVie R, Jaramillo JJ, Palmer M, Smith T. Effect of modest vitamin E supplementation on blood glycated hemoglobin and triglyceride levels and red cell indices in type I diabetic patients. J Am Coll Nutr. 1996;15:458–61.. doi: 10.1080/07315724.1996.10718624. [DOI] [PubMed] [Google Scholar]
- 35.Di Leo MA, Ghirlanda G, Silveri NG, Giardina B, Franconi F, Santini SA. Potential therapeutic effect of antioxidants in experimental diabetic retina: a comparison between chronic taurine and vitamin E plus selenium supplementations. Free Radic Res. 2003;37:323–30.. doi: 10.1080/1071576021000055271. [DOI] [PubMed] [Google Scholar]
- 36.Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev. 2010;131:276–86.. doi: 10.1016/j.mad.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Cao H, Xie Y, Chen X. Type 2 diabetes diminishes the benefits of dietary antioxidants: evidence from the different free radical scavenging potential. Food Chem. 2015;186:106–12.. doi: 10.1016/j.foodchem.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 38.Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol. 2000;36:2185–91.. doi: 10.1016/s0735-1097(00)00980-3. [DOI] [PubMed] [Google Scholar]
- 39.Rafighi Z, Arab S, Yusof RM, Shiva A. The effect of vitamin C and E on lipid profile in people with type 2 diabetes mellitus. Glob J Health Sci. 2011;3:69–74. [Google Scholar]
- 40.Campbell TC, Parpia B, Chen J. Diet, lifestyle, and the etiology of coronary artery disease: the Cornell China study. Am J Cardiol. 1998;82:18–21. doi: 10.1016/s0002-9149(98)00718-8. [DOI] [PubMed] [Google Scholar]
- 41.Aly H, Mantawy M. Comparative effects of zinc, selenium and vitamin E or their combination on carbohydrate metabolizing enzymes and oxidative stress in streptozotocine-induced diabetic rats. Eur Rev Med Pharmacol Sci. 2012;16:66–78. [PubMed] [Google Scholar]
- 42.Kahya MC, Naziroğlu M, Çiğ B. Melatonin and selenium reduce plasma cytokine and brain oxidative stress levels in diabetic rats. Brain Inj. 2015;29:1490–6. doi: 10.3109/02699052.2015.1053526. [DOI] [PubMed] [Google Scholar]
- 43.Bahmani F, Kia M, Soleimani A, Asemi Z, Esmaillzadeh A. Effect of selenium supplementation on glycemic control and lipid profiles in patients with diabetic nephropathy. Biol trace Elem Res. 2016;172:282–9. doi: 10.1007/s12011-015-0600-4. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz C, Alegria A, Barbera R, Farre R, Lagarda J. Selenium, zinc and copper in plasma of patients with type 1 diabetes mellitus in different metabolic control states. J Trace Elem Med Biol. 1998;12:91–5. doi: 10.1016/s0946-672x(98)80031-x. [DOI] [PubMed] [Google Scholar]
- 45.Lu C-W, Chang H-H, Yang K-C, Kuo C-S, Lee L-T, Huang K-C. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Res Care. 2016;4:e000253. doi: 10.1136/bmjdrc-2016-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei J, Zeng C, Gong Q-Y, Yang H-B, Li X-X, Lei G-H, et al. The association between dietary selenium intake and diabetes: a cross-sectional study among middle-aged and older adults. Nutr J. 2015;14:18–23.. doi: 10.1186/s12937-015-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011;14:2327–36.. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lafuente M, Ortin L, Argente M, Guindo JL, Lopez-Bernal MD, Lopez-Roman FJ, et al. Combined intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema: two-year randomized single-blind controlled trial results. Retina. 2017;37:1277–86. doi: 10.1097/IAE.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 50.Haritoglou C, Gerss J, Hammes HP, Kampik A, Ulbig MW, Group RS. Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica. 2011;226:127–37.. doi: 10.1159/000329470. [DOI] [PubMed] [Google Scholar]


