Abstract
Colistin resistance is complex and multifactorial. DbcA is an inner membrane protein belonging to the DedA superfamily required for maintaining extreme colistin resistance of Burkholderia thailandensis. The molecular mechanisms behind this remain unclear. Here, we report that ∆dbcA displays alkaline pH/bicarbonate sensitivity and propose a role of DbcA in extreme colistin resistance of B. thailandensis by maintaining cytoplasmic pH homeostasis. We found that alkaline pH or presence of sodium bicarbonate displays a synergistic effect with colistin against not only extremely colistin resistant species like B. thailandensis and Serratia marcescens, but also a majority of Gram-negative and Gram-positive bacteria tested, suggesting a link between cytoplasmic pH homeostasis and colistin resistance across species. We found that lowering the level of oxygen in the growth media or supplementation of fermentable sugars such as glucose not only alleviated alkaline pH stress, but also increased colistin resistance in most bacteria tested, likely by avoiding cytoplasmic alkalinization. Our observations suggest a previously unreported link between pH, oxygen, and colistin resistance. We propose that maintaining optimal cytoplasmic pH is required for colistin resistance in a majority of bacterial species, consistent with the emerging link between cytoplasmic pH homeostasis and antibiotic resistance.
Subject terms: Chemical biology, Microbiology
Introduction
Colistin is a cationic antimicrobial peptide (CAMP) belonging to the polymyxin antibiotic family, which was first isolated from a Gram-positive soil bacteria Bacillus polymyxa1. A rise in multidrug resistance among bacterial pathogens and a lack of new antimicrobial drugs have led to renewed interest in reviving older antimicrobial agents such as polymyxin2. Despite its nephrotoxicity and neurotoxicity, polymyxin still remains one of the most effective CAMPs and is presently classified as “Reserve Group” of antibiotics, that can be used as a last resort antibiotic against pathogens resistant to most commonly used antibiotics3–5. However, the escalating use of colistin along with the rise in colistin resistance among several bacterial pathogens and the global spread of mobilized colistin resistance (mcr) genes are greatly worrisome6, 7.
Unlike most Gram negative bacteria, Burkholderia spp. display extremely high intrinsic polymyxin resistance8 and remain as important model system for understanding the molecular mechanism behind such a high level resistance. Several studies in Burkholderia have revealed that the extreme polymyxin resistance is likely multifactorial consisting of major and minor determinants of resistance9–14. One such unique genetic determinant of high level colistin resistance of Burkholderia thailandensis is DbcA (BTH_I1321), a highly conserved inner membrane protein belonging to DedA superfamily15. B. thailandensis DbcA is required for efficient lipid A modification with Ara4N and high level colistin resistance15, 16. DedA proteins have also been reported to be required for polymyxin and/or CAMP resistance of Salmonella enterica17, Neisseria meningitidis18, Klebsiella pneumoniae19, and Enterobacter cloacae20. The DedA family of membrane proteins is widely distributed in nature, found in all domains of life, whose physiological function remains unknown. Studies in Escherichia coli have shown that redundant DedA family proteins YqjA and YghB are required for a number of proton motive force dependent processes including twin arginine transport (TAT) dependent protein export and cell division21, antibiotic resistance dependent on efflux pump activity22, and survival at alkaline pH23.
While the exact mechanism of colistin killing is still unclear, there are many models proposing its mode of action. Colistin is polycationic and amphipathic in nature. These properties allow colistin to be electrostatically attracted to negatively charged phosphates of lipid A, thereby displacing divalent cations such as calcium and magnesium ions that normally bridge adjacent lipid A molecules and stabilize the outer membrane24. Disruption of outer membrane permeability promotes the uptake of the colistin itself, hence the term “self-promoted uptake”24. What happens next is not completely understood. It is believed that polymyxin can further disrupt the inner membrane phospholipid bilayer and induce the formation of pores, leading to leakage of cytoplasmic contents, and ultimately cell death by lysis25. A recent finding that polymyxin can target LPS in the inner membrane of polymyxin sensitive E. coli supports the notion that the bactericidal activity of polymyxin requires the disruption of inner membrane and cytoplasmic entry26. Polymyxin can increase cytoplasmic membrane permeability, as assessed by membrane depolarization in E. coli and Pseudomonas aeruginosa; however, bactericidal activity of polymyxin does not require membrane depolarization27, 28. While membrane depolarization induced by a protonophore CCCP has been shown to sensitize many Gram-negative species to polymyxin15, 29–32, membrane hyperpolarization has also been proposed to be responsible for increased polymyxin sensitivity in E. coli ∆phoP33, and Staphylococcus aureus ∆atpA34. Therefore, the role of membrane potential in polymyxin resistance is not clearly understood. PhoP is a broadly conserved response regulator of a two component system PhoPQ involved in many important processes such as proper maintenance of periplasmic redox state35–37, activation of type VI secretion system in intra and inter species bacterial competition through ROS production38 and polymyxin resistance through induction of genes involved in LPS modifications and maintenance of reversed membrane potential (more positive inside)33. AtpA encodes the alpha subunit of ATP synthase34. Our earlier report shows that maintaining membrane potential and extreme colistin resistance by B. thailandensis E264 and ∆dbcA is dependent upon the pH of the media16. Therefore, we measured colistin MIC of different bacteria grown at different pH to determine the effect of external pH in colistin toxicity. Sodium bicarbonate has been reported to sensitize S. aureus to polymyxin by alkalinizing the cytoplasm and altering the PMF39. Here, we explored the effect of sodium bicarbonate on colistin susceptibility of different bacterial species.
Another hypothesis of bactericidal activity of polymyxin is through oxidative stress. There are many reports suggesting that polymyxin can induce oxidative stress and cause cell death in many Gram-negative bacteria40–44. However, a few reports do not support this mechanism of killing by polymyxin, assessed by the equal killing by polymyxin at both aerobic and anaerobic conditions45 and the ability of colistin to effectively kill metabolically inactive and hypoxic subpopulation within the internal regions of biofilm in P. aeruginosa46. Whether bactericidal activity of colistin is dependent on oxygen is still unclear. We explored colistin resistance of different bacteria under normal, hypoxic, and anoxic conditions, and propose a link between pH and oxygen. We also show the dependence of colistin resistance on fermentable carbon source such as glucose and connect the effect of glucose back to pH homeostasis. Our study in Burkholderia ∆dbcA reveals a link between pH and colistin resistance not only in B. thailandensis, but also in other bacterial species. This study reveals an unexpected complexity of colistin resistance in both Gram-negative and Gram-positive bacteria.
Results
B. thailandensis ∆dbcA is sensitive to alkaline pH
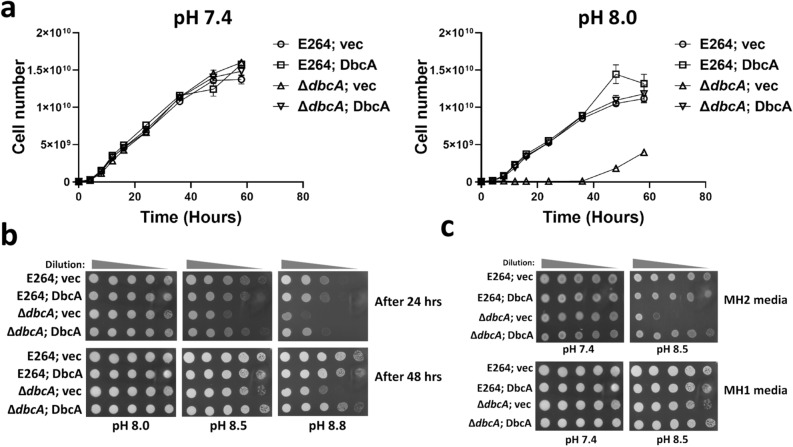
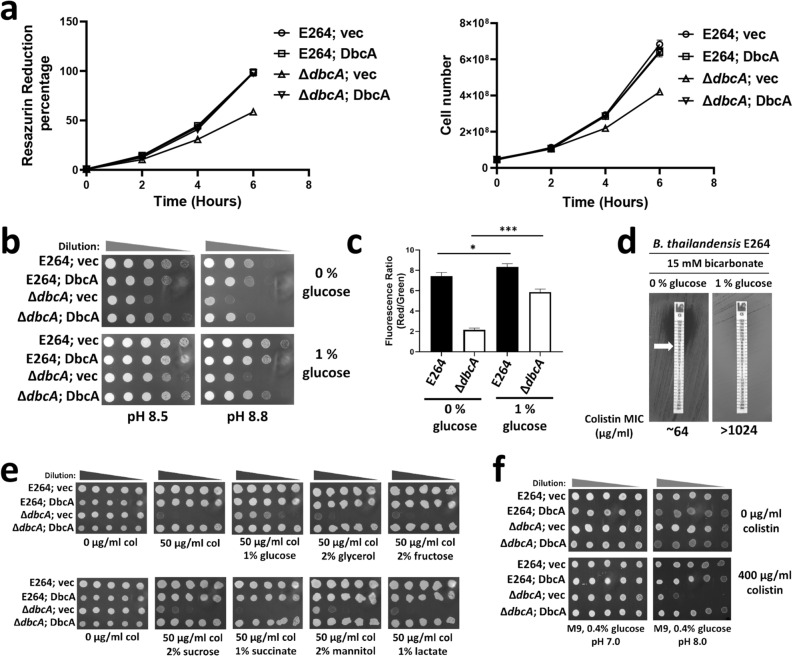
In light of the sensitivity of E. coli ∆yqjA to alkaline pH23, we tested whether B. thailandensis ∆dbcA is also alkaline pH sensitive. In our previous report, we found that ∆dbcA is more sensitive to divalent cations such as calcium and magnesium, and ∆dbcA also showed greater sensitivity to colistin in MH2 media compared to MH1 media16. Therefore, we looked at the alkaline pH sensitivity of ∆dbcA in MH1 broth to exclude the effects of divalent cations. Figure 1a shows that ∆dbcA has a growth defect at pH 8.0 compared to pH 7.4. However, on MH1 agar plates, the alkaline pH sensitivity of ∆dbcA requires pH 8.5 and above (Fig. 1b). ∆dbcA is also more sensitive to alkaline pH in presence of divalent cations as shown in the Fig. 1c.
Figure 1.
Alkaline pH sensitivity of B. thailandensis ∆dbcA. (a) Growth rate of B. thailandensis strains (E264; vec, E264; DbcA, ∆dbcA; vec, and ∆dbcA; DbcA) at different times in Mueller Hinton broth (MH1) with 100 µg/ml Tmp. The error bars indicate standard deviations of three biological replicates. (b) Spot assay of E264; vec, E264; DbcA, ∆dbcA; vec, and ∆dbcA; DbcA on MH1 agar media. 1:10 dilutions of indicated strains were spotted on NaOH adjusted MH1 media with 100 µg/ml Tmp and 0.001% rhamnose. Plates were analyzed after 24 or 48 h at 37 °C. (c) Comparison of alkaline pH sensitivity of B. thailandensis ∆dbcA in MH2 with MH1 agar media. MH2 contains ~ 20 to 25 mg/l of calcium and ~ 10 to 12.5 mg/l of magnesium, whereas MH1 contains no added calcium and magnesium salts. The pH was adjusted by either HCl or NaOH. Plates were analyzed after 48 h of growth at 37 °C.
External Alkaline pH can sensitize majority of bacteria tested to colistin
We previously showed that alkaline pH of the media can sensitize extremely colistin resistant bacteria like B. thailandensis to colistin16. We hypothesized that alkaline pH could also sensitize other bacterial species to colistin. We used different bacteria with different colistin resistance profiles and measured the colistin MIC at different pH. In a majority of bacteria including Gram-positive species such as S. aureus and B. bacillus, the colistin MIC decreased as the pH of the media increased (Fig. 2). Surprisingly, in colistin sensitive E. coli W3110 the colistin MIC slightly increased in alkaline pH media compared to the acidic media (Fig. 2). The colistin MIC of Serratia marcescens, on plate was ~ 3 µg/ml at pH 7.0 agar media. However, the MIC of S. marcescens in liquid media is more than 500 µg/ml (see Supplementary Fig. S1 online). The appearance of other colonies represented by a single black arrow (Fig. 2) are colistin heteroresistant colonies of S. marcescens32. We propose that these heteroresistant colonies of S. marcescens are responsible for the extreme colistin resistance of S. marcescens observed in liquid media. We observed that these heteroresistant colonies of S. marcescens do not appear as the pH of the media increased (Fig. 2). Moreover, alkaline pH appears to increase colistin sensitivity of isolated heteroresistant S. marcescens clones (see Supplementary Fig. S1 online). We also observed the appearance of heteroresistant colonies of K. pneumoniae47 at pH 5.5 agar plates that disappear at pH 7.0 and pH 8.0 (Fig. 2). These findings suggest that the heteroresistance to colistin is also dependent on the pH of the media.
Figure 2.
pH dependent colistin resistance of different bacterial species. Minimal inhibitory concentration (MIC) was determined for indicated strains on LB agar plates. The pH was adjusted with 100 mM MES for pH 5.5 and 100 mM Tris for pH 7.0, 8.0, and 8.5. Plates were analyzed after 24 h of growth at 37 °C. Approximate MICs are denoted by white arrows. Black arrows represent heteroresistant colonies of S. marcescens or K. pneumoniae.
Sodium bicarbonate can sensitize most bacteria to colistin
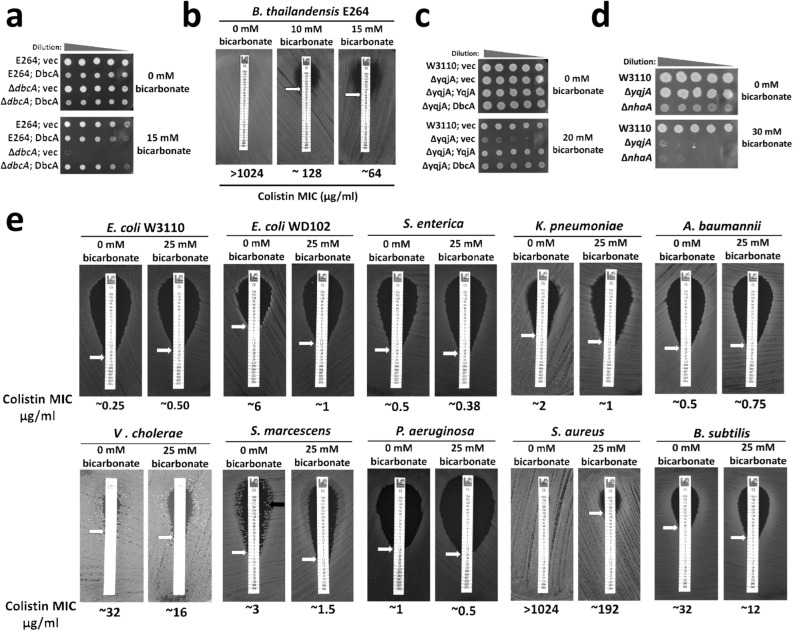
It has been reported that sodium bicarbonate can disrupt the PMF by alkalinizing the cytoplasm of S. aureus, increasing the susceptibility to polymyxin39. Since ∆dbcA is alkaline pH sensitive, we hypothesized that it may be sensitive to bicarbonate as well. While 15 mM sodium bicarbonate is not enough to drastically increase the external pH of the MH2 media, ∆dbcA was hypersensitive to sodium bicarbonate (Fig. 3a), suggesting that ∆dbcA has defective cytoplasmic pH homeostasis. Interestingly, sodium bicarbonate increased the sensitivity of wild type B. thailandensis to colistin (Fig. 3b), showing similarity between the effect of alkaline pH and sodium bicarbonate in colistin susceptibility. E. coli ∆yqjA is also sensitive to bicarbonate, and this phenotype can be rescued by the overexpression of DbcA (Fig. 3c), supporting the role of DbcA in maintaining cytoplasmic pH homeostasis. NhaA is a Na+/H+ antiporter and E. coli ∆nhaA is also alkaline pH sensitive48. E. coli ∆nhaA is also sodium bicarbonate sensitive (Fig. 3d), consistent with a previous report39, suggesting that bacterial strains unable to maintain cytoplasmic pH homeostasis are also sensitive to sodium bicarbonate, probably due to its cytoplasmic alkalinization effect.
Figure 3.
Effect of bicarbonate on colistin resistance. (a) Bicarbonate sensitivity of B. thailandensis ∆dbcA. Spot assay of B. thailandensis strains (E264; vec, E264; DbcA, ∆dbcA; vec, and ∆dbcA; DbcA) on MH2 agar media with 100 µg/ml Tmp, 0.002% rhamnose, and different concentrations of sodium bicarbonate as indicated. (b) Reduction in colistin MIC of B. thailandensis E264 by bicarbonate. The MIC was determined on MH2 agar plates with different concentrations of sodium bicarbonate as indicated. Approximate MICs are denoted by white arrows. Plates were analyzed after 48 h of growth at 37 °C. (c) Bicarbonate sensitivity of E. coli ∆yqjA and complementation of bicarbonate sensitivity of E. coli ∆yqjA by B. thailandensis dbcA. Spot assay of 1:10 dilutions of E. coli strains (W3110; vec, ∆yqjA; vec, ∆yqjA; YqjA, and ∆yqjA; DbcA) on LB agar media with 50 µg/ml Kan and 20 mM sodium bicarbonate as indicated. (d) Bicarbonate sensitivity of E. coli ∆nhaA. Spot assay of 1:10 dilutions of indicated strains on LB agar plate with and without 25 mM sodium bicarbonate. (e) Effect of bicarbonate on colistin MIC of different bacterial species. MIC was determined for indicated strains on LB agar plates with and without physiological concentration of bicarbonate (~ 25 mM). The pH of growth media was adjusted to pH 7.0 to avoid external pH fluctuations by sodium bicarbonate. Approximate MICs are denoted by white arrows.
We then measured the colistin MICs for other bacteria in the presence of bicarbonate and observed a similar pattern as alkaline pH. Bicarbonate decreased colistin MIC for a majority of bacteria tested (Fig. 3e). We also observed similar disappearance of heteroresistant colonies of S. marcescens with bicarbonate (Fig. 3e). Bicarbonate also increased the colistin sensitivity of heteroresistant S. marcescens (see Supplementary Fig. S1 online). Since the pH of the media was buffered to pH 7.0 using 100 mM Tris, we argue that the increased susceptibility to colistin is caused by the alkalinization of cytoplasmic pH, rather than the external pH.
A link between oxygen and pH homeostasis
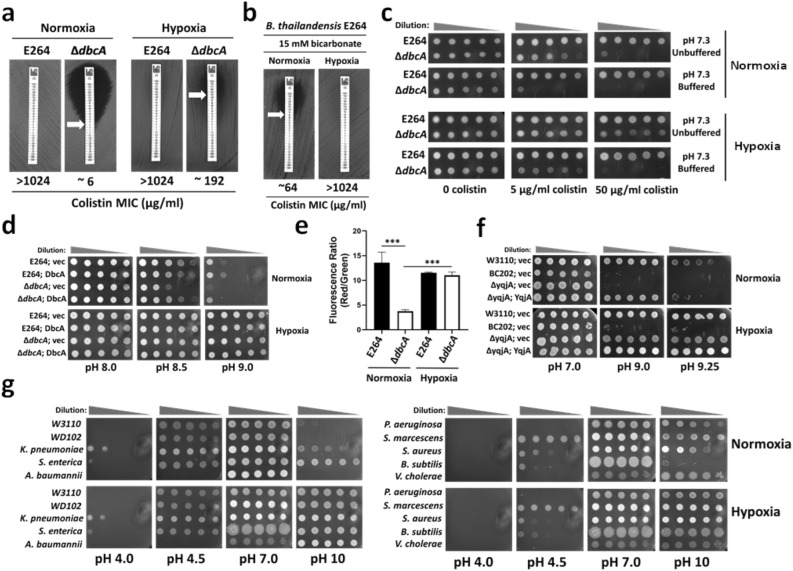
∆dbcA displayed a more severe growth defect in 25 ml broth when grown in a 250 ml flask compared to when grown in a test tube (Data not shown). We reasoned this could be the effect of aeration or oxygen levels and asked if altering the level of oxygen in growth media also affects the colistin susceptibility of ∆dbcA. To answer this, we measured the colistin MIC of B. thailandensis E264 and ∆dbcA at ambient oxygen levels (normoxia) and low oxygen levels (hypoxia). Surprisingly, we observed that the colistin MIC of ∆dbcA increased to ~ 192 µg/ml when grown in hypoxia (Fig. 4a). The reduction of colistin MIC of E264 by bicarbonate could also be compensated by hypoxia (Fig. 4b). The colistin sensitivity of ∆dbcA could be further lowered by media buffering and the compensation of colistin sensitivity of ∆dbcA by hypoxia could be suppressed by buffering the media (Fig. 4c). Burkholderia spp. are known to fluctuate the external pH as they grow, and they are known to acidify the media during stationary phase, regulated by quorum sensing49. Using a buffer and restraining the external pH fluctuation by ∆dbcA not only further increased its colistin sensitivity, but also suppressed the complementation of colistin sensitivity of ∆dbcA by hypoxia (Fig. 4c).
Figure 4.
Complementation of alkaline pH sensitivity or colistin sensitivity by hypoxia. (a) Partial compensation of colistin sensitivity of B. thailandensis ∆dbcA by hypoxia. The colistin MIC was determined for B. thailandensis strains (E264 and ∆dbcA) grown on MH2 agar plates with 100 µg/ml Tmp. (b) Reversal of bicarbonate induced sensitization of B. thailandensis E264 to colistin by hypoxia. (c) The effect of media buffering on colistin resistance. Spot assay of 1:10 dilutions of B. thailandensis strains (E264 and ∆dbcA) were grown in either normoxia or hypoxia on MH2 agar plates supplemented with indicated concentrations of colistin with unbuffered (pH 7.3 adjusted by HCl or NaOH) or buffered media (pH 7.3 adjusted with 70 mM BTP). (d) Compensation of alkaline pH stress by hypoxia in B. thailandensis strains. Spot assay of 1:10 dilutions of indicated strains on MH1 agar media with 100 µg/ml Tmp, 0.002% rhamnose with different pH media adjusted with HCl or NaOH and grown in either normoxia or hypoxia. (e) Compensation of membrane depolarization of B. thailandensis ∆dbcA by hypoxia. Membrane potential was measured for B. thailandensis strains (E264 and ∆dbcA) grown in either normoxia or hypoxia. ***; p < 0.001. (f) Compensation of alkaline pH stress by hypoxia in E. coli strains. Spot assay of 1:10 dilutions of indicated E. coli strains at different pH LB media and grown in either normoxia or hypoxia. 70 mM BTP was used to adjust the pH and 50 µg/ml Kan was used in the LB media for plasmid selection. (g) Compensation of alkaline pH stress by hypoxia in different bacterial species. Spot assay of 1:10 dilutions of indicated strains at different pH LB agar plates in either normoxia or hypoxia. 100 mM MES was used for pH 4.0 and 5, whereas 70 mM BTP was used for pH 8.0, 9.0. and 10.
We then investigated whether lowering the level of oxygen could rescue alkaline pH sensitivity of ∆dbcA. The alkaline pH sensitivity of ∆dbcA was corrected when grown under hypoxia (Fig. 4d). In fact, all strains tested grew better in alkaline pH media under hypoxia compared to normoxia (Fig. 4d). Hypoxia also corrected the lower membrane potential observed in ∆dbcA (Fig. 4e). E. coli ∆yqjA grew much better at pH 9.25 under hypoxia (Fig. 4f). However, BC20250 (E. coli ∆yqjA, ∆yghB) could not be rescued at pH 9.25 by hypoxia (Fig. 4f).
It was clear that lowering the amount of oxygen rescues the cells from alkaline stress. To explore this further, we measured the effect of hypoxia at extreme pHs, both alkaline and acidic. We grew several bacterial species at pH’s of 4.0 to 10 under normoxia and hypoxia. We observed that growth under hypoxia rescued all the bacterial strains against extreme alkaline pH whereas their sensitivity to extremely acidic pH stress remained the same under both growth conditions (Fig. 4g), suggesting that only alkaline stress can be alleviated by hypoxia.
In E. coli, alkaline stress increases the expression of non-proton pumping cytochrome bd, whereas the expression of proton pumping respiratory chain complexes decrease to minimize proton loss from the cytoplasm when generating PMF51. An E. coli cytochrome bd mutant was also reported to be alkaline pH sensitive52. In E. coli, the expression of cytochrome bd is regulated positively by a transcriptional regulator ArcA under hypoxia53. We propose that the induction of non-proton pumping pathways during hypoxia is responsible for rescuing the cells from alkaline pH stress by simply avoiding cytoplasmic proton loss. We reasoned that a E. coli cytochrome bd mutant should be sensitive to sodium bicarbonate and may be more sensitive to colistin as well. Indeed, E. coli ∆cydB was more sensitive to bicarbonate as well as colistin (see Supplementary Fig. S2 online) compared to its parent strain BW25113. This suggests that avoiding cytoplasmic proton loss could rescue cells from colistin toxicity.
Oxygen dependent colistin resistance in bacteria
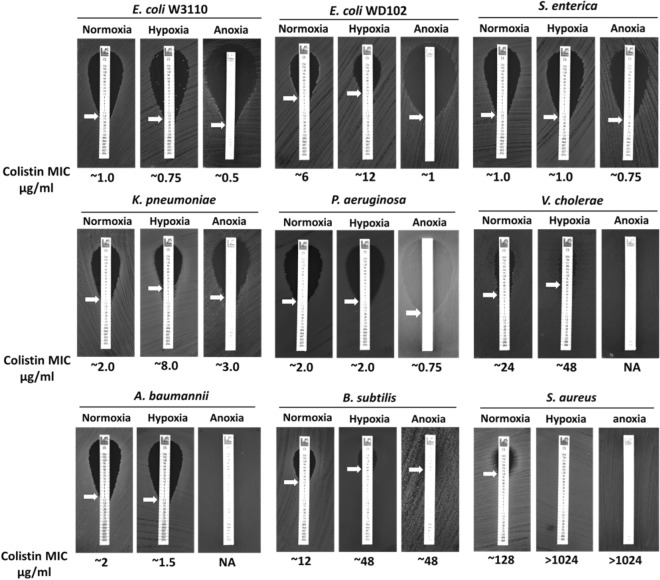
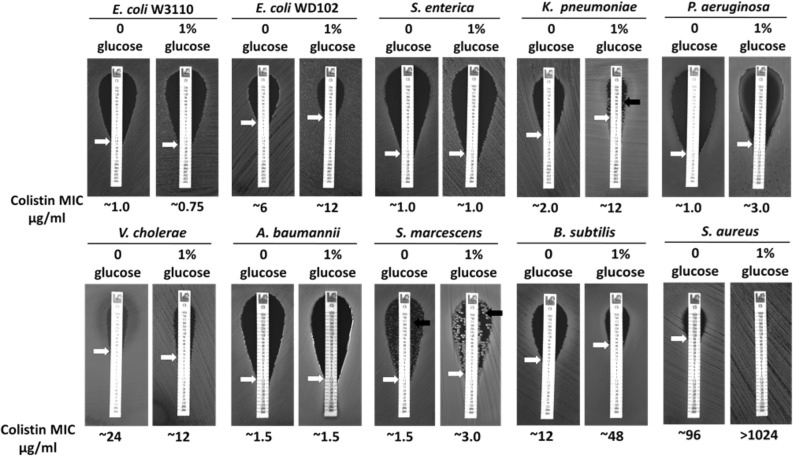
To examine whether the level of oxygen affects colistin susceptibility, we measured the colistin MIC of different bacteria grown under normoxic, hypoxic, and anoxic conditions. Growth under hypoxia increased the colistin MIC of E. coli WD102, K. pneumoniae, Vibrio cholerae, S. aureus, and Bacillus subtilis (Fig. 5). However, growth under anoxia decreased the colistin MIC of E. coli W3110, E. coli WD102, S. enterica, and P. aeruginosa. It should be noted that colistin MIC for several bacteria altered between buffered pH 7.0 LB agar plates (Fig. 2) and unbuffered pH 7.0 LB agar plates (Fig. 5—normoxia plates). E. coli, S. enterica, P. aeruginosa, V. cholerae, S. aureus, and B. subtilis displayed different colistin MIC in buffered pH 7.0 LB (Fig. 2) compared to unbuffered pH 7.0 LB (Fig. 5—normoxia plates), further supporting the notion that colistin toxicity is linked to the pH homeostasis.
Figure 5.
Oxygen dependent colistin resistance in different bacterial species. The colistin MIC was determined for indicated strains on LB agar plates. For anoxia, LB agar plates were supplemented with 20 mM sodium nitrate. Plates were analyzed after 24 h of growth at 37 °C. Approximate MICs are denoted by white arrows.
Colistin resistance is dependent upon the carbon source
We reported that B. thailandensis ∆dbcA had lower membrane potential than the parent strain E26415. It is possible that ∆dbcA also has lower metabolic activity. We used a redox indicator dye, resazurin to measure the metabolic activity of ∆dbcA. Active bacterial populations can reduce the resazurin dye from the oxidized form (blue; absorbance at 570 nm) to the reduced form (red; absorbance at 600 nm), giving a quantifiable measure of bacterial metabolic activity54. We found that ∆dbcA had lower resazurin reduction activity coupled with slower growth at pH 7.5 MH1 media compared to other strains (Fig. 6a). We then supplemented the growth media with glucose as a source of metabolic energy to see if that could rescue the alkaline pH sensitivity of ∆dbcA. Addition of glucose could not only partially rescue the alkaline pH sensitivity of ∆dbcA (Fig. 6b), but also could partially correct the lower membrane potential of ∆dbcA (Fig. 6c). It should be noted that glucose supplementation could also significantly increase the membrane potential of E264 (Fig. 6c) and improve its survivability at extreme alkaline pH (Fig. 6b). Similar to hypoxia, supplementation of glucose also compensated alkaline pH stress of a majority of bacterial species tested (see Supplementary Fig. S3 online). The reduction of colistin MIC of E264 by bicarbonate could also be compensated by glucose supplementation (Fig. 6d), all suggesting that the compensation of glucose supplementation is also linked to the pH.
Figure 6.
Glucose dependent colistin resistance. (a) Lower metabolic activity of B. thailandensis ∆dbcA. Relative metabolic activity was measured by the percentage of the reduced form of Resazurin within 6 h of growth. 5 × 107 cells of overnight cultures of indicated strains were inoculated in 25 ml fresh MH1 broth, with 100 µg/ml Tmp, 0.015% Resazurin dye and grown for 6 h. Resazurin reduction percentage for each strain was calculated (left) and the cell number was also determined (right) for each time point. (b) Compensation of alkaline pH stress by glucose supplementation in the growth media. Spot assay of 1:10 dilutions of all four B. thailandensis strains on MH1 media with 100 µg/ml Tmp, 0.002% rha, and 0 and 1% glucose. The pH was adjusted with either HCl or NaOH. (c) Partial compensation of membrane depolarization of B. thailandensis ∆dbcA by glucose. Membrane potential was measured for B. thailandensis strains (E264 and ∆dbcA) grown in MH2 media with different amounts of glucose as indicated. *; p < 0.05, and ***; p < 0.001. (d) Reversal of bicarbonate induced sensitization of B. thailandensis E264 to colistin by glucose supplementation. MIC was determined on MH2 agar plates with 15 mM sodium bicarbonate and different concentrations of glucose as indicated. (e) Effect of different carbon sources in colistin resistance. Spot assay of 1:10 dilutions of indicated B. thailandensis strains in MH2 agar plates with 100 µg/ml Tmp, 0.002% rha, and different carbon sources. Plates were analyzed after 48 h of growth at 37 °C. (f) Colistin sensitivity of B. thailandensis ∆dbcA in M9 media. 1:10 dilutions of indicated strains were spotted on M9 agar plates with 0.4% glucose with or without 400 µg/ml colistin adjusted to different pH using either HCl or NaOH.
We then tested whether supplementation of different carbon sources (glucose, glycerol, fructose, sucrose, succinate, mannitol, lactate, acetate, and citrate) could compensate the colistin sensitivity of ∆dbcA. We found that while supplementation of glucose slightly compensates the colistin sensitivity of ∆dbcA in MH2 agar media (Fig. 6e), acetate and citrate reduced the colistin MIC of ∆dbcA (see Supplementary Fig. S4 online). In fact, ∆dbcA was slightly more sensitive to acetate and citrate compared to the WT E264 (see Supplementary Fig. S4 online). Surprisingly, the colistin MIC of ∆dbcA in M9 agar media with 0.4% glucose was more than 1024 µg/ml, similar to WT E264 (see Supplementary Fig. S4 online). This is consistent with a previous finding, where susceptibility of several bacterial species to polymyxin was increased in rich medium compared to minimal medium55. During the growth of B. thailandensis in rich media, such as LB, the pH of the supernatant increases initially and later decreases during the late stationary phase49. It has been proposed that the deamination of amino acids in LB media leads to the production of ammonia, that is responsible for medium alkalinization49. We propose that the medium alkalinization in rich media is responsible for the colistin hypersensitivity of ∆dbcA.
When the initial pH of the M9 media with 0.4% glucose is adjusted to pH 8.0, ∆dbcA was much more sensitive to colistin (Fig. 6f), supporting our hypothesis that the medium alkalinization is sensitizing ∆dbcA to colistin. We also measured the external pH fluctuation by E264 and ∆dbcA with different sugars. We found that with glucose, the pH of the supernatant dropped compared to other sugars (see Supplementary Fig. S5 online). We then measured the colistin MIC of different bacterial species with glucose supplementation. We found that addition of glucose increased the colistin MIC for E. coli WD102, K. pneumoniae, P. aeruginosa, S. marcescens, B. subtilis, and S. aureus (Fig. 7) all supporting a general link between cytoplasmic pH and colistin sensitivity. Our observation is consistent with previous finding regarding the effect of glucose in polymyxin resistance of P. aeruginosa56.
Figure 7.
Effect of glucose supplementation in colistin MIC of different bacterial species. The colistin MIC was determined for indicated strains on LB agar plates with either 0% or 1% glucose. Plates were analyzed after 24 h of growth at 37 °C. Approximate MICs are denoted by white arrows. A black arrow represents heteroresistant colonies of S. marcescens or K. pneumoniae.
Discussion
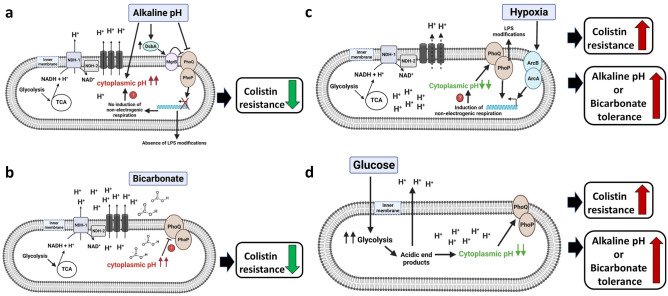
There is emerging evidence regarding the link between cytoplasmic pH and antibiotic resistance. In E. coli, cytoplasmic alkalinization by chloroquine was shown to decrease norfloxacin resistance57. Chloroquine can also decrease colistin MIC of both E. coli W3110 and E. coli WD102 (see Supplementary Fig. S6 online). It has been reported that intracellular alkalinization caused by kanamycin, chlorpromazine, and norfloxacin can cause cell death in Mycobacterium smegmatis, while increasing proton influx by either media acidification or by using protonophore can block the antibiotic lethality58. Our study supports this emerging hypothesis and suggests that colistin resistance of a number of bacterial species can be attenuated by intracellular alkalinization by either alkali challenge or adding bicarbonate. Similarly, colistin sensitivity can be rescued by growing cells under conditions, such as acidic pH, hypoxia, or glucose supplementation, all of which could reduce cytoplasmic alkalinization. This is the first report to our knowledge that shows a link between pH, oxygen and colistin resistance in bacteria. The findings of this study are depicted Fig. 8.
Figure 8.
Model depicting the link between cytoplasmic pH and colistin resistance. Only the bacterial cytoplasmic membrane is depicted for simplicity. (a) While E. coli maintains its cytoplasmic pH at ~ 7.2 when grown at pH 7.0, growth at pH 8.0 can increase the cytoplasmic pH to ~ 7.688. Increasing cytoplasmic pH can be toxic to cells unless compensated by cytoplasmic pH homeostasis mechanisms, one of which includes induction of non-proton pumping respiratory pathways51. Polymyxin has been shown to inhibit the non-proton pumping respiratory complex NDH-259, 60. Growth at alkaline pH in minimal media inhibits the expression of PhoPQ dependent genes69, 70. DsbA is a periplasmic disulfide-bond formation protein, which is induced by alkaline pH80, 89. MgrB is a small inner membrane peptide that directly binds PhoQ and mediates negative feedback on the PhoPQ circuit36. DsbA has been reported to negatively regulate PhoPQ probably through disulfide bond formation in MgrB35. Inhibition of PhoPQ can reduce colistin resistance either through inhibition of LPS modifications or through inhibition of non-proton pumping respiratory pathways33, which could further increase the cytoplasmic pH and increase colistin toxicity. (b) Sodium bicarbonate has been reported to increase the cytoplasmic pH of S. aureus39. This increase in cytoplasmic pH may be responsible for reduced colistin resistance. The cytoplasmic domain of PhoQ is responsible for sensing acidic cytoplasmic pH and activating PhoP in S. Typhimurium90. Therefore, it is possible that bicarbonate may repress PhoPQ by causing an alkaline cytoplasmic pH leading to sensitivity to colistin. (c) Growth in hypoxia stimulates ArcAB, which can induce non-proton pumping respiratory complex such as cytochrome bd53. This could reduce cytoplasmic proton loss and decrease the cytoplasmic pH, increasing alkaline pH tolerance. Cytoplasmic acidification could further activate PhoPQ and increase colistin resistance. (d) Glucose supplementation of B. thailandensis can acidify the growth media, which may explain the similar increase in alkaline pH tolerance as hypoxia. This hypothesis is supported by the observed cytoplasmic acidification by glucose supplementation in Bacillus pseudofirmus86. The cytoplasmic acidification by glucose could also stimulate PhoPQ.
Why alkaline pH stress/bicarbonate decreases colistin resistance remains an important question. It has been reported that polymyxin is a non-competitive inhibitor of the type II NADH-quinone oxidoreductase (NDH-2) respiratory enzyme in E. coli, Acinetobacter baumannii, K. pneumoniae59, and M. smegmatis60. NDH-2 is an alternative NADH quinone reductase, which oxidizes NADH coupled to the reduction of quinone without pumping protons across the inner membrane61. It is possible that upon inhibition of non-proton pumping pathways by polymyxin, cells rely solely on proton pumping pathways that would be devastating under alkaline pH condition, unless compensated by increasing proton influx or switching to hypoxic metabolism or fermentation, which avoid intracellular alkalinization. The polymyxin B-induced inhibition of NDH-2 and the metabolic shift from aerobic to fermentation in K. pneumoniae supports this hypothesis62. However not all bacteria have NDH-2, including B. thailandensis63, 64. The reduced colistin MIC of E. coli ∆cydB (see Supplementary Fig. S2 online) also suggests that induction of non-proton pumping cytochrome bd can increase colistin resistance. Whether polymyxin also inhibits the non-proton pumping pathway at cytochrome bd level, or ubiquinone level, is unknown.
Our data suggests that the pH, bicarbonate, oxygen, and glucose dependent colistin toxicity is observed mainly for colistin resistant Gram-negative bacterial species such as E. coli WD102, K. pneumoniae, V. cholerae, B. thailandensis and S. marcescens as well as Gram-positive bacterial species including S. aureus and B. subtilis. All these colistin resistant Gram-negative species have a conserved PhoPQ or PmrAB systems65–68. It is possible that alteration of lipid A modifications at alkaline pH is responsible for reduction of colistin MIC. It has been reported that PhoPQ-dependent gene expression increases upon decreasing the pH of the media in S. typhimurium69 and S. marcescens68. In S. typhimurium, lipid A modifications with Ara4N and phosphoethanolamine (PEtN) were suppressed at pH 8.0 N-minimal media with 10 mM Mg2+ not only in the WT strain, but also in the PhoPc and pmrAc mutants70. However, it should be noted that these experiments in S. typhimurium and S. marcescens were performed using minimal media. While Salmonella PhoP-dependent gene expressions in murine RAW264.7 macrophages has been correlated with acidification of the phagosome, acidification of LB broth in vitro did not show a similar level of PhoP-dependent gene expression71. Our experiment also shows that the growth of S. typhimurium at different pH LB media only showed a slight decrease in colistin MIC from 0.5 µg/ml at pH 5.5 to 0.3 µg/ml at pH 8.0 (Fig. 2). Therefore, measuring the expression of PhoPQ or PmrAB-dependent genes at different pH in LB media will help explain whether the decrease in colistin MIC with alkali challenge is due to the reduced expression of PhoPQ or PmrAB systems. The decrease in colistin MIC of B. cenocepacia MH55 (ArnT suppressor mutant strain)10 and K. pneumoniae arnT::Tn72 (see Supplementary Fig. S7 online) by alkaline pH supports lipid A modification independent colistin resistance. Our previous report shows that B. thailandensis E264 has similar level of Ara4N modified lipid A species grown at different pH media (pH 5.5, 7.0, and 7.5)16. However, E264 is more sensitive at pH 7.5 media compared to pH 7.0 (see Supplementary Fig. S7 online). Whether suppression of lipid A modifications at alkaline pH is responsible for reduced colistin MIC in other Gram-negative bacterial species remains to be investigated. Quantitative measurement of modified lipid A species of different bacterial species at different pH LB media will help address this hypothesis.
Our findings suggest that the external pH effect on colistin MIC can be independent of lipid A modification with Ara4N. However, it is also possible that alkaline pH could suppress other LPS modifications beside Ara4N addition by ArnT that could be responsible for pH dependent colistin resistance. In addition, with intact PhoPQ or PmrAB systems, it is possible that the pH dependent colistin resistance is mediated directly or indirectly through PhoPQ or PmrAB, independent of lipid A modifications. The PhoPQ system has been shown to regulate many processes including cytoplasmic pH regulation such as induction of non-proton pumping pathways33 that could prevent cytoplasmic proton loss and increase colistin resistance (Fig. 8a). Whether bicarbonate also inhibits PhoPQ by cytoplasmic alkalinization remains to be investigated (Fig. 8b). The reduction of colistin MIC in Gram-positive bacteria, such as B. subtilis and S. aureus at alkaline pH (Fig. 2) is also not clear. Similar to LPS modifications by Gram-negative bacteria, Gram-positive bacteria can also alter their cell surface charge either by modifying their negatively charged cell wall teichoic acids with positively charged D-alanine mediated by dltABCD operon or by masking their anionic membrane phosphatidylglycerols with cationic L-lysine mediated by the enzyme MprF73. Interestingly, it has been reported that growth at alkaline pH significantly reduced the alanine ester content of teichoic acids only in chemostat culture of S. aureus, probably due to their removal by the hydrolysis of the base-labile ester linkages at high pH74, 75. This may explain the reduced colistin MIC of S. aureus and B. subtilis at alkaline pH. The enzyme MprF and dltABCD operons are regulated by GraXSR three component system76 and whether GraXSR can sense the change in pH remains to be investigated.
Reduction in membrane potential at alkaline pH may explain the reduced colistin MIC. The lower membrane potential observed in ∆dbcA at pH 7.5 was corrected by either acidic pH16, or hypoxia (Fig. 4e), or supplementation of glucose (Fig. 6c), paralleled with increase in colistin resistance (Figs. 4a, 6e). The increased sensitivity towards colistin due to lower membrane potential is also supported by other reports that show reducing membrane potential by CCCP can sensitize many bacterial species to colistin15, 32. It is also possible that PMF dependent secondary transporters required for colistin efflux are inefficient at alkaline pH due to a reduced proton gradient and lower PMF51, 52. However, addition of sodium bicarbonate has been shown to increase the cytoplasmic pH of S. aureus with a subsequent increase in ∆ѱ, and it is this increase in ∆ѱ (membrane hyperpolarization) that has been proposed to be responsible for increased colistin sensitivity39. This is further supported by S. aureus ∆atpA and E. coli ∆phoP, both of which show membrane hyperpolarization and increased sensitivity towards polymyxin33, 34.
We propose that a pH effect, rather than membrane potential, could explain colistin resistance more accurately. It has been proposed that the bactericidal activity of polymyxin requires the respiratory generation of PMF in E. coli and membrane depolarization by CCCP exposure was shown to improve survival against colistin33. It should be noted that the aerobic generation of PMF could increase the cytoplasmic alkalinization over time and could be worse at alkaline pH unless compensated by proton influx. Brief exposure to CCCP could in fact acidify the cytoplasm58 and avoid cytoplasmic alkalinization to a toxic level, thus improving colistin resistance. However, the continuous presence of CCCP in the growth media sensitizes many bacterial species to colistin probably due to lower metabolic activity and inhibition of PMF dependent transporters required for colistin resistance15, 32. The compensation of reduction of colistin MIC of E264 by CCCP by either acidic pH, glucose supplementation, or hypoxia supports the notion that the CCCP effect is also linked to pH homeostasis (see Supplementary Fig. S8 online). The decrease in colistin resistance of S. aureus by bicarbonate may also be due to increase in cytoplasmic pH to a toxic level39. However, the inhibition of aerobic respiration by bicarbonate could also be responsible39. It should be noted that polymyxin itself could inhibit oxygen consumption in E. coli77 and S. typhimurium78. There is evidence that alteration of oxygen consumption is responsible for either bactericidal or bacteriostatic effect of antibiotics in bacteria79. It is possible that alkaline pH or bicarbonate affects oxygen consumption and could be responsible for increased sensitivity towards colistin, which may itself inhibit oxygen consumption. Measuring oxygen consumption rates at different pH media or with bicarbonate supplementation should provide more insights regarding the above hypothesis.
It may be argued that increased oxidative stress at alkaline pH may be responsible for increased colistin susceptibility. However, oxidative stress-induced genes are activated at acidic pH in E. coli K-12 rather than alkaline80. Polymyxin has also been reported to induce oxidative stress40–44. Although the increase in colistin MIC of B. thailandensis ∆dbcA, E. coli WD102, K. pneumoniae, V. cholerae, B. subtilis and S. aureus under hypoxia supports this notion, reduced colistin MIC of E. coli WD102, K. pneumoniae, and P. aeruginosa in anoxia do not support the oxidative stress hypothesis (Fig. 5). Increase in colistin susceptibility in anaerobic conditions in P. aeruginosa biofilms has also been reported81, consistent with our own data that shows reduced colistin MIC of P. aeruginosa in anoxia, hence questioning the validity of oxidative stress hypothesis. Surprisingly, the exposure to oxidative stress (3.5 mM H2O2) increased the colistin MIC of P. aeruginosa PAO1 by 2–fourfold82. Therefore, we argue that a small decrease in colistin MIC of P. aeruginosa PAO1 in alkaline pH (Fig. 2) is probably not due to oxidative stress. Here, we propose an alternative explanation. Induction of non-proton pumping pathway under hypoxia may help bacteria avoid increase in cytoplasmic pH to a toxic level, thus compensating extreme alkaline pH as well as colistin sensitivity (Figs. 4, 5). However, in anoxic condition, generation of PMF by nitrate reduction coupled with continuous proton pumping may increase the possibility of losing cytoplasmic protons and increase sensitivity towards colistin.
Carbon starvation during growth under alkaline pH media may be responsible for alkaline pH or colistin sensitivity. E. coli XylE is a PMF dependent sugar transporter belonging to MFS superfamily and sharing 63% sequence similarity with human GLUT183. In B. subtilis, polymyxin B decreases intracellular levels of carbon metabolites and ATP, while addition of glucose increases survival against polymyxin B by increasing ATP levels84. A metabolomics study of colistin treated Mycobacterium tuberculosis suggests that colistin induces a shift towards glucose utilization for energy and upregulation of glyoxylate cycle85. It is possible that the lower PMF at alkaline pH negatively affects PMF dependent sugar transporters such as E. coli XylE and cause sugar starvation, which could be exacerbated by colistin. Our data shows that supplementation of glucose improves bacterial survival against alkaline pH stress or colistin (Fig. 7 and see Supplementary Fig. S3 online), supporting the above hypothesis. We also measured the colistin sensitivity of ∆dbcA with other carbon sources and found that the colistin sensitivity compensation was only seen with fermentable carbon source-glucose (see Supplementary Fig. S6 online). The acidification of the media with glucose supplementation (see Supplementary Fig. S5 online) suggests that the glucose effect is also linked to the pH. We propose that glucose could decrease the internal pH by making acidic end products. During a pH shift from 8.5 to 10.5, alkaliphilic bacteria Bacillus pseudofirmus OF4 displayed cytoplasmic pH of 9.2 in the presence of glucose instead of pH 10.5 in the presence of malate86 supporting our hypothesis that supplementation of glucose could acidify the cytoplasm or avoid cytoplasmic alkalinization and hence improve survival against colistin. The reduction of colistin MIC of ∆dbcA by acetate or citrate (see Supplementary Fig. S4 online) might also be linked to the cytoplasmic pH homeostasis. Acetate and citrate could directly feed into Krebs’s cycle and increase electron transport chain (ETC) activity, hence pumping out cytoplasmic protons by ETC complexes and increasing the possibility of cytoplasmic alkalinization to a toxic level.
How DbcA maintain cytoplasmic pH homeostasis and extreme colistin resistance of B. thailandensis is still unclear. Structural and biochemical studies of this interesting family of membrane transporters will help answer these questions. Future investigations including direct measurement of cytoplasmic pH under different growth conditions will further validate our model (Fig. 8) and enhance our understanding of alkaline pH homeostasis and polymyxin resistance. Our work shows how different factors such as pH, level of oxygen, and source of carbon in the growth media can influence colistin resistance of several bacterial species and hence emphasizes a need to take caution while measuring colistin MIC in a laboratory setting.
Materials and methods
Culture conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown in either LB medium (1% tryptone, 0.5% yeast extract, and 1% NaCl), Mueller Hinton Broth (MH1), Cation-adjusted Mueller Hinton Broth 2 (MH2) (Sigma). MH1 or MH2 as indicated was used exclusively with B. thailandensis and LB was used for all other strains. Colistin (Col), trimethoprim (Tmp), rhamnose (rha), 2,6 Diaminopimelic acid (DAP), kanamycin (kan), Resazurin, sodium bicarbonate, glucose, glycerol, fructose, sucrose, mannitol, sodium lactate, sodium acetate, chloroquine diphosphate salt, and sodium citrate were purchased from VWR or MilliporeSigma. Cultures were grown at 37 °C shaking at 225 rpm. BD GasPak EZ Gas Generating Container Systems with either microaerophilic or anaerobe sachets were purchased from VWR. Colistin MIC strips were purchased from Liofilchem, Inc.
Table 1.
Bacterial strains and plasmids used in this study.
| Strains | Description | Source or reference |
|---|---|---|
| Escherichia coli W3110 | Wild Type, F−, λ−, IN (rrnD-rrnE)1, rph-1 | E. coli genetic stock center, Yale University |
| Escherichia coli W3110; vec | W3110 transformed with pBBR1MCS-2 | 15 |
| Escherichia coli ∆nhaA | W3110, ∆nhaA-737::kan | 22 |
| Escherichia coli BW25113 | Wild Type, lacIq rrnBT14 ∆lacZWJ16 hsdR514 ∆araBADAH33 ∆rhaBADLD78 | 91 |
| Escherichia coli BW25113 ∆cydB | Kanamycin-resistant Keio collection mutant | 92 |
| Escherichia coli BC202KS | W3110 ∆yqjA::TetR ∆yghB781::KanS | 15 |
| Escherichia coli BC202; vec | BC202KS transformed with pBBR1MCS-2 | This study |
| Escherichia coli ∆yqjA | W3110; ∆yqjA::TetR | 50 |
| Escherichia coli ∆yqjA; vec | ∆yqjA transformed with pBBR1MCS-2 | This study |
| Escherichia coli ∆yqjA; YqjA | ∆yqjA transformed with pRP102 | This study |
| Escherichia coli ∆yqjA; DbcA | ∆yqjA transformed with pRP101 | This study |
| Escherichia coli RHO3 | SM10(lambda pir), kanS; ∆asd::FRT ∆aphA::FRT | 93 |
| Burkholderia thailandensis E264; vec | E264 transformed with pSCrhaB2 TmpR | 15 |
| Burkholderia thailandensis E264 ΔdbcA; vec | ΔdbcA::FRT transformed with pSCrhaB2 TmpR | 15 |
| Burkholderia thailandensis E264; DbcA | E264 transformed with pSCdbcA | 15 |
| Burkholderia thailandensis E264 ΔdbcA; DbcA | ΔdbcA::FRT transformed with pSCdbcA | 15 |
| Escherichia coli WD102 | W3110, pmrAC, zjd-2211: Tn10 | 94 |
| Klebsiella pneumoniae ST258 | A clinical isolate from Public Health England resistant to polymyxin | 19 |
| Klebsiella pneumoniae arnT::Tn | A transposon insertion mutant carrying a chloramphenicol resistance determinant (T30) | 72 |
| Salmonella enterica subsp. Enterica 14028 | Virulent wild type strain | ATCC |
| Pseudomonas aeruginosa PAO1 | Wild type strain | 95, 96 |
| Vibrio cholerae C6706 | O1 El Tor strain | 97 |
| Serratia marcescens 13880 | Wild type strain | ATCC |
| Staphylococcus aureus subsp. aureus Rosenbach, 6538 | Wild type strain | ATCC |
| Bacillus subtilis 6051 | Wild type strain | ATCC |
| Burkholderia cenocepacia K56-2 | ET12 clone related to J2315, CF clinical isolate | BCRRC, B. cepacia Research and Referral Repository for Canadian CF Clinics |
| Burkholderia cenocepacia MH55 | K56-2, ΔarnT-arnBC+ lptGD31H (lptGS) | 98 |
| Plasmids | ||
| pBBR1MCS-2 | Expression vector; RK2 ori, lacZa, T3 and T7 promoters, KanR | 99 |
| pRP101 | pBBR1MCS-2 expressing dbcA | This study |
| pRP102 | pBBR1MCS-2 expressing EcyqjA | This study |
| pSCrhaB2 | Expression vector; oripBBR1rhaR, rhaS, PrhaBTmpRmob + | 100 |
| pSCdbcA | pSCrhaB2 expressing dbcA with His6 tag at C terminus | 15 |
Transformation and complementation analysis
Transformation of E. coli was carried out using a heat shock87, while transformation of B. thailandensis was carried out using biparental conjugation16. Briefly, both recipient B. thailandensis and donor E. coli RHO3 strains carrying TmpR plasmid(s) to be transferred were inoculated by thoroughly spreading them on LB plates supplemented with 200 µg/ml DAP and incubated at 37 °C. After ~ 18 h of incubation, a loopful of bacterial cells from conjugation plates were streaked on LB with 100 µg/ml Tmp plates for selection and DAP was excluded on these plates to select against the donor strain, RHO3. After 48 h of incubation at 37 °C, isolated TmpR colonies were used for colony PCR using plasmid specific primers16 to confirm the introduction of TmpR plasmids into Burkholderia recipient strains.
Susceptibility assays
For testing the susceptibility on solid medium, overnight cultures of bacterial strains were adjusted to 3 × 108 cells/ml. Five microliters of serially log10-diluted cells was then spotted on LB/MH1/MH2 agar plates containing various concentrations of antibiotics, sodium bicarbonate, or other chemical agents as indicated in figure captions. Growth was analyzed after incubation for 24–72 h at 37 °C as indicated. The colistin MIC was measured using Liofilchem® MIC Test Strips. Overnight cultures were adjusted to 1 × 108 cells/ml for B. thailandensis strains and 5 × 107 cells/ml for all other strains in fresh LB/MH2 and a sterile swab was used to create a lawn of cells. Then the MIC strip was applied to the plates and the growth was evaluated after 24–48 h at 37 °C. All experiments were repeated at least three times and highly reproducible.
Measurement of membrane potential
Measurement of membrane potential was performed using JC-1 dye15. Briefly, 5 × 107 cells from the overnight cultures were inoculated in 25 ml of fresh MH2 broth in 250 ml flask and grown for about 5 h at 37 °C shaking incubator with glucose if required. For hypoxic growth, a bacterial culture was inoculated in 10 ml MH2 broth in 50 ml flask that was placed in BD GasPak EZ Gas Generating Container Systems with microaerophilic sachets (VWR) and grown for 5 h at 37 °C with shaking. Similarly, a 50 ml flask with a 10 ml bacterial inoculation was grown at normal oxygen condition for normoxia control. Then, ~ 6 × 108 cells were taken from each culture, washed with permeabilization buffer, PB (10 mM Tris, pH 7.5, 1 mM EDTA, 10 mM glucose) and, finally, resuspended in PB buffer. 3 µM of JC-1 dye was added, incubated in the dark at 37 °C for 30 min. Cells were washed and resuspended in PB buffer and fluorescence measurements were carried out using a JASCO FP-6300 spectrofluorometer. Membrane potential is determined by the ratio of red (595 nm) to green (530 nm) fluorescence with excitation of 488 nm.
Measurement of metabolic activity
The metabolic activity was measured using a commercially available resazurin dye (VWR). Measuring cell-viability by resazurin protocol by Tip Biosystems was used to quantitatively measure the resazurin reduction percentage. 5 × 107 cells of overnight bacterial cultures were inoculated in 25 ml fresh MH1 broth, with 100 µg/ml Tmp, and 0.015% resazurin dye and grown at 37 °C shaking incubator for 6 h. Initial absorbance of resazurin at 570 nm (reduced form) and 600 nm (oxidized form) was reported before starting the incubation. After every hour, one ml of culture was removed, centrifuged, and the supernatant was used to measure absorbance at 570 and 600 nm. Pelleted cells were resuspended in PBS and cell number was measured for the same time points.
Statistical analysis
Experiments were repeated three times with three biological replicates. The data presented in the graphs indicate the mean ± standard deviation (SD) value for three independent replicates of each treatment. GraphPad Prism 9.0 was used to produce graphs and calculate the statistical significances by unpaired Student’s t-test.
Supplementary Information
Acknowledgements
This work was supported entirely with private funding by the LSU Foundation Biotransport Research Support Fund (WTD). We thank Dr. Marcia Newcomer and Dr. Scott Herke (LSU Genomics Facility) for technical support. We also thank Ginger Brininstool for providing us with Serratia marcescens and Bacillus subtilis.
Author contributions
W.T.D. and P.R.P. Conception and design of the study, acquisition, analysis, and interpretation of the data and writing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92718-7.
References
- 1.Koyama Y. A new antibiotic'colistin'produced by spore-forming soil bacteria. J. Antibiot. 1950;3:457–458. [Google Scholar]
- 2.World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. (World Health Organization, 2014).
- 3.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents. 2005;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Hsia Y, et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health. 2019;7:e861–e871. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- 6.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti Infect. Ther. 2012;10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 7.Elbediwi M, et al. Global burden of colistin-resistant bacteria: Mobilized Colistin Resistance Genes Study (1980–2018) Microorganisms. 2019 doi: 10.3390/microorganisms7100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loutet SA, Valvano MA. Extreme antimicrobial Peptide and polymyxin B resistance in the genus burkholderia. Front. Microbiol. 2011;2:159. doi: 10.3389/fmicb.2011.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loutet SA, Mussen LE, Flannagan RS, Valvano MA. A two-tier model of polymyxin B resistance in Burkholderia cenocepacia. Environ. Microbiol. Rep. 2011;3:278–285. doi: 10.1111/j.1758-2229.2010.00222.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamad MA, Di Lorenzo F, Molinaro A, Valvano MA. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia. Mol. Microbiol. 2012;85:962–974. doi: 10.1111/j.1365-2958.2012.08154.x. [DOI] [PubMed] [Google Scholar]
- 11.El-Halfawy OM, Valvano MA. Chemical communication of antibiotic resistance by a highly resistant subpopulation of bacterial cells. PLoS ONE. 2013;8:e68874. doi: 10.1371/journal.pone.0068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar N, et al. Crystal structures of the Burkholderia multivorans hopanoid transporter HpnN. Proc. Natl. Acad. Sci. USA. 2017;114:6557–6562. doi: 10.1073/pnas.1619660114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmerk CL, Bernards MA, Valvano MA. Hopanoid production is required for low-pH tolerance, antimicrobial resistance, and motility in Burkholderia cenocepacia. J. Bacteriol. 2011;193:6712–6723. doi: 10.1128/JB.05979-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernier SP, Son S, Surette MG. The Mla pathway plays an essential role in the intrinsic resistance of Burkholderia cepacia complex species to antimicrobials and host innate components. J. Bacteriol. 2018 doi: 10.1128/JB.00156-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panta PR, et al. A DedA family membrane protein is required for Burkholderia thailandensis colistin resistance. Front. Microbiol. 2019 doi: 10.3389/fmicb.2019.02532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panta PR, Doerrler WT. A Burkholderia thailandensis DedA family membrane protein is required for proton motive force dependent lipid a modification. Front. Microbiol. 2021 doi: 10.3389/fmicb.2020.618389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Cromie MJ, Hsu FF, Turk J, Groisman EA. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 2004;53:229–241. doi: 10.1111/j.1365-2958.2004.04107.x. [DOI] [PubMed] [Google Scholar]
- 18.Tzeng YL, et al. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J Bacteriol. 2005;187:5387–5396. doi: 10.1128/JB.187.15.5387-5396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jana B, et al. The secondary resistome of multidrug-resistant Klebsiella pneumoniae. Sci. Rep. 2017;7:42483. doi: 10.1038/srep42483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Feng Y, Zong Z. Heterogeneous resistance to colistin in Enterobacter cloacae complex due to a new small transmembrane protein. J. Antimicrob. Chemother. 2019;74:2551–2558. doi: 10.1093/jac/dkz236. [DOI] [PubMed] [Google Scholar]
- 21.Sikdar R, Doerrler WT. Inefficient Tat-dependent export of periplasmic amidases in an Escherichia coli strain with mutations in two DedA family genes. J. Bacteriol. 2010;192:807–818. doi: 10.1128/JB.00716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Doerrler WT. Members of the conserved DedA family are likely membrane transporters and are required for drug resistance in Escherichia coli. Antimicrob. Agents Chemother. 2014;58:923–930. doi: 10.1128/AAC.02238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Doerrler WT. Escherichia coli YqjA, a member of the conserved DedA/Tvp38 membrane protein family, is a putative osmosensing transporter required for growth at alkaline pH. J. Bacteriol. 2015;197:2292–2300. doi: 10.1128/JB.00175-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hancock RE. Peptide antibiotics. The Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 25.Hancock, R. E., Falla, T. & Brown, M. in Advances in Microbial Physiology Vol. 37 135–175 (Elsevier, 1995). [DOI] [PubMed]
- 26.Sabnis A, et al. Colistin kills bacteria by targeting lipopolysaccharide in the cytoplasmic membrane. Elife. 2021 doi: 10.7554/eLife.65836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Dhillon P, Yan H, Farmer S, Hancock RE. Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000;44:3317–3321. doi: 10.1128/aac.44.12.3317-3321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daugelavicius R, Bakiene E, Bamford DH. Stages of polymyxin B interaction with the Escherichia coli cell envelope. Antimicrob. Agents Chemother. 2000;44:2969–2978. doi: 10.1128/aac.44.11.2969-2978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park YK, Ko KS. Effect of carbonyl cyanide 3-chlorophenylhydrazone (CCCP) on killing Acinetobacter baumannii by colistin. J. Microbiol. 2015;53:53–59. doi: 10.1007/s12275-015-4498-5. [DOI] [PubMed] [Google Scholar]
- 30.Ni W, et al. Effects of efflux pump inhibitors on colistin resistance in multidrug-resistant gram-negative bacteria. Antimicrob. Agents Chemother. 2016;60:3215–3218. doi: 10.1128/AAC.00248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osei Sekyere J, Amoako DG. Carbonyl cyanide m-chlorophenylhydrazine (CCCP) reverses resistance to colistin, but not to carbapenems and tigecycline in multidrug-resistant enterobacteriaceae. Front. Microbiol. 2017;8:228. doi: 10.3389/fmicb.2017.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron SA, Rolain JM. Efflux pump inhibitor CCCP to rescue colistin susceptibility in mcr-1 plasmid-mediated colistin-resistant strains and Gram-negative bacteria. J. Antimicrob. Chemother. 2018;73:1862–1871. doi: 10.1093/jac/dky134. [DOI] [PubMed] [Google Scholar]
- 33.Alteri CJ, Lindner JR, Reiss DJ, Smith SN, Mobley HL. The broadly conserved regulator PhoP links pathogen virulence and membrane potential in Escherichia coli. Mol. Microbiol. 2011;82:145–163. doi: 10.1111/j.1365-2958.2011.07804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vestergaard M, et al. Inhibition of the ATP synthase eliminates the intrinsic resistance of Staphylococcus aureus towards polymyxins. mBio. 2017 doi: 10.1128/mBio.01114-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippa AM, Goulian M. Perturbation of the oxidizing environment of the periplasm stimulates the PhoQ/PhoP system in Escherichia coli. J. Bacteriol. 2012;194:1457–1463. doi: 10.1128/JB.06055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippa AM, Goulian M. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 2009;5:e1000788. doi: 10.1371/journal.pgen.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato A, Tanabe H, Utsumi R. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: Identification of extracellular Mg2+-responsive promoters. J. Bacteriol. 1999;181:5516–5520. doi: 10.1128/JB.181.17.5516-5520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storey D, et al. Klebsiella pneumoniae type VI secretion system-mediated microbial competition is PhoPQ controlled and reactive oxygen species dependent. PLoS Pathog. 2020;16:e1007969. doi: 10.1371/journal.ppat.1007969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farha MA, French S, Stokes JM, Brown ED. Bicarbonate alters bacterial susceptibility to antibiotics by targeting the proton motive force. ACS Infect. Dis. 2018;4:382–390. doi: 10.1021/acsinfecdis.7b00194. [DOI] [PubMed] [Google Scholar]
- 40.Lima MR, et al. Evaluation of the interaction between polymyxin B and Pseudomonas aeruginosa biofilm and planktonic cells: Reactive oxygen species induction and zeta potential. BMC Microbiol. 2019;19:115. doi: 10.1186/s12866-019-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Halfawy OM, Valvano MA. Putrescine reduces antibiotic-induced oxidative stress as a mechanism of modulation of antibiotic resistance in Burkholderia cenocepacia. Antimicrob. Agents Chemother. 2014;58:4162–4171. doi: 10.1128/AAC.02649-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Z, Zhu Y, Qin W, Yin J, Qiu J. Oxidative stress induced by polymyxin E is involved in rapid killing of Paenibacillus polymyxa. Biomed. Res. Int. 2017;2017:5437139. doi: 10.1155/2017/5437139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han ML, et al. Comparative metabolomics and transcriptomics reveal multiple pathways associated with polymyxin killing in Pseudomonas aeruginosa. mSystems. 2019 doi: 10.1128/mSystems.00149-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sampson TR, et al. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob. Agents Chemother. 2012;56:5642–5649. doi: 10.1128/AAC.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brochmann RP, et al. Bactericidal effect of colistin on planktonic Pseudomonas aeruginosa is independent of hydroxyl radical formation. Int. J. Antimicrob. Agents. 2014;43:140–147. doi: 10.1016/j.ijantimicag.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol. Microbiol. 2008;68:223–240. doi: 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- 47.Jayol A, Nordmann P, Brink A, Poirel L. Heteroresistance to colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrob. Agents Chemother. 2015;59:2780–2784. doi: 10.1128/AAC.05055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padan E, Landau M. Sodium–proton (Na(+)/H(+)) antiporters: Properties and roles in health and disease. Met. Ions Life Sci. 2016;16:391–458. doi: 10.1007/978-3-319-21756-7_12. [DOI] [PubMed] [Google Scholar]
- 49.Goo E, et al. Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc. Natl. Acad. Sci. USA. 2012;109:19775–19780. doi: 10.1073/pnas.1218092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompkins K, Chattopadhyay B, Xiao Y, Henk MC, Doerrler WT. Temperature sensitivity and cell division defects in an Escherichia coli strain with mutations in yghB and yqjA, encoding related and conserved inner membrane proteins. J. Bacteriol. 2008;190:4489–4500. doi: 10.1128/JB.00414-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krulwich TA, Sachs G, Padan E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011;9:330–343. doi: 10.1038/nrmicro2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avetisyan AV, Bogachev AV, Murtasina RA, Skulachev VP. Involvement of a d-type oxidase in the Na(+)-motive respiratory chain of Escherichia coli growing under low delta mu H+ conditions. FEBS Lett. 1992;306:199–202. doi: 10.1016/0014-5793(92)80999-w. [DOI] [PubMed] [Google Scholar]
- 53.Cotter PA, Melville SB, Albrecht JA, Gunsalus RP. Aerobic regulation of cytochrome d oxidase (cydAB) operon expression in Escherichia coli: roles of Fnr and ArcA in repression and activation. Mol. Microbiol. 1997;25:605–615. doi: 10.1046/j.1365-2958.1997.5031860.x. [DOI] [PubMed] [Google Scholar]
- 54.O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 55.Haas G, Sevag M. Critical role of amino acids on the sensitivity and development of resistance to polymyxin B. Arch. Biochem. Biophys. 1953;43:11–24. doi: 10.1016/0003-9861(53)90079-4. [DOI] [PubMed] [Google Scholar]
- 56.Conrad RS, Wulf RG, Clay DL. Effects of carbon sources on antibiotic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1979;15:59–66. doi: 10.1128/AAC.15.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyes-Fernandez EZ, Schuldiner S. Acidification of cytoplasm in Escherichia coli provides a strategy to cope with stress and facilitates development of antibiotic resistance. Sci. Rep. 2020;10:9954. doi: 10.1038/s41598-020-66890-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartek IL, et al. Antibiotic bactericidal activity is countered by maintaining pH homeostasis in Mycobacterium smegmatis. mSphere. 2016 doi: 10.1128/mSphere.00176-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deris ZZ, et al. A secondary mode of action of polymyxins against Gram-negative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. J. Antibiot (Tokyo) 2014;67:147–151. doi: 10.1038/ja.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mogi T, et al. Polymyxin B identified as an inhibitor of alternative NADH dehydrogenase and malate: Quinone oxidoreductase from the Gram-positive bacterium Mycobacterium smegmatis. J .Biochem. 2009;146:491–499. doi: 10.1093/jb/mvp096. [DOI] [PubMed] [Google Scholar]
- 61.Matsushita K, Ohnishi T, Kaback HR. NADH-ubiquinone oxidoreductases of the sEscherichia coli aerobic respiratory chain. Biochemistry. 1987;26:7732–7737. doi: 10.1021/bi00398a029. [DOI] [PubMed] [Google Scholar]
- 62.Ramos PI, et al. The polymyxin B-induced transcriptomic response of a clinical, multidrug-resistant Klebsiella pneumoniae involves multiple regulatory elements and intracellular targets. BMC Genomics. 2016;17:737. doi: 10.1186/s12864-016-3070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andreae, C. A. Understanding the Role of Anaerobic Respiration in Burkholderia thailandensis and B. pseudomallei Survival and Virulence. (2014).
- 64.Melo AM, Bandeiras TM, Teixeira M. New insights into type II NAD(P)H:quinone oxidoreductases. Microbiol. Mol. Biol. Rev. 2004;68:603–616. doi: 10.1128/MMBR.68.4.603-616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubin EJ, Herrera CM, Crofts AA, Trent MS. PmrD is required for modifications to Escherichia coli endotoxin that promote antimicrobial resistance. Antimicrob. Agents Chemother. 2015;59:2051–2061. doi: 10.1128/AAC.05052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng HY, Chen YF, Peng HL. Molecular characterization of the PhoPQ–PmrD–PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 2010;17:60. doi: 10.1186/1423-0127-17-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Destoumieux-Garzon D, Duperthuy M, Vanhove AS, Schmitt P, Wai SN. Resistance to antimicrobial peptides in vibrios. Antibiotics (Basel) 2014;3:540–563. doi: 10.3390/antibiotics3040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barchiesi, J., Castelli, M. E., Di Venanzio, G., Colombo, M. I. & Garcia Vescovi, E. The PhoP/PhoQ system and its role in Serratia marcescens pathogenesis. J Bacteriol194, 2949–2961. 10.1128/JB.06820-11 (2012). [DOI] [PMC free article] [PubMed]
- 69.Prost LR, et al. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol. Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Gibbons HS, Kalb SR, Cotter RJ, Raetz CR. Role of Mg2+ and pH in the modification of Salmonella lipid A after endocytosis by macrophage tumour cells. Mol. Microbiol. 2005;55:425–440. doi: 10.1111/j.1365-2958.2004.04409.x. [DOI] [PubMed] [Google Scholar]
- 71.Alpuche Aranda CM, Swanson JA, Loomis WP, Miller SI. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramage B, et al. Comprehensive arrayed transposon mutant library of Klebsiella pneumoniae outbreak strain KPNIH1. J. Bacteriol. 2017;199:66. doi: 10.1128/JB.00352-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin J, et al. Mechanisms of bactericidal action and resistance of polymyxins for Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2020;104:3771–3780. doi: 10.1007/s00253-020-10525-y. [DOI] [PubMed] [Google Scholar]
- 74.Archibald AR, Baddiley J, Heptinstall S. The alanine ester content and magnesium binding capacity of walls of Staphylococcus aureus H grown at different pH values. Biochimica et Biophysica Acta BBA-Biomembranes. 1973;291:629–634. doi: 10.1016/0005-2736(73)90468-9. [DOI] [PubMed] [Google Scholar]
- 75.MacArthur AE, Archibald A. Effect of culture pH on the D-alanine ester content of lipoteichoic acid in Staphylococcus aureus. J. Bacteriol. 1984;160:792. doi: 10.1128/jb.160.2.792-793.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang SJ, et al. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect. Immun. 2012;80:74–81. doi: 10.1128/IAI.05669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wahn K, Lutsch G, Rockstroh T, Zapf K. Morphological and physiological investigations on the action of polymyxin B on Escherichia coli. Arch. Mikrobiol. 1968;63:103–116. doi: 10.1007/BF00412165. [DOI] [PubMed] [Google Scholar]
- 78.Teuber M. Action of polymyxin B on bacterial membranes. Arch. Microbiol. 1974;100:131–144. doi: 10.1007/BF00446313. [DOI] [PubMed] [Google Scholar]
- 79.Lobritz MA, et al. Antibiotic efficacy is linked to bacterial cellular respiration. Proc. Natl. Acad. Sci. USA. 2015;112:8173–8180. doi: 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maurer LM, Yohannes E, Bondurant SS, Radmacher M, Slonczewski JL. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 2005;187:304–319. doi: 10.1128/JB.187.1.304-319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kolpen M, et al. Increased bactericidal activity of colistin on Pseudomonas aeruginosa biofilms in anaerobic conditions. Pathog. Dis. 2016;74:ftv086. doi: 10.1093/femspd/ftv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohamed FA, Shaker GH, Askoura MM. Oxidative stress influences Pseudomonas aeruginosa susceptibility to antibiotics and reduces its pathogenesis in host. Curr. Microbiol. 2020;77:479–490. doi: 10.1007/s00284-019-01858-7. [DOI] [PubMed] [Google Scholar]
- 83.Jeckelmann JM, Erni B. Transporters of glucose and other carbohydrates in bacteria. Pflugers Arch. 2020;472:1129–1153. doi: 10.1007/s00424-020-02379-0. [DOI] [PubMed] [Google Scholar]
- 84.Yu WB, Pan Q, Ye BC. Glucose-induced cyclic lipopeptides resistance in bacteria via ATP maintenance through enhanced glycolysis. iScience. 2019;21:135–144. doi: 10.1016/j.isci.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koen N, van Breda SV, Loots DT. Metabolomics of colistin methanesulfonate treated Mycobacterium tuberculosis. Tuberculosis (Edinb) 2018;111:154–160. doi: 10.1016/j.tube.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 86.Padan E, Bibi E, Ito M, Krulwich TA. Alkaline pH homeostasis in bacteria: New insights. Biochimica et biophysica acta BBA-biomembranes. 2005;1717:67–88. doi: 10.1016/j.bbamem.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Froger A, Hall JE. Transformation of plasmid DNA into E. coli using the heat shock method. J. Vis. Exp. 2007 doi: 10.3791/253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zarkan A, et al. Indole pulse signalling regulates the cytoplasmic pH of E. coli in a memory-like manner. Sci. Rep. 2019;9:3868. doi: 10.1038/s41598-019-40560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gonzales-Siles L, Karlsson R, Kenny D, Karlsson A, Sjöling Å. Proteomic analysis of enterotoxigenic Escherichia coli (ETEC) in neutral and alkaline conditions. BMC Microbiol. 2017;17:1–17. doi: 10.1186/s12866-016-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi J, Groisman EA. Acidic pH sensing in the bacterial cytoplasm is required for Salmonella virulence. Mol. Microbiol. 2016;101:1024–1038. doi: 10.1111/mmi.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006;2:20060008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopez CM, Rholl DA, Trunck LA, Schweizer HP. Versatile dual-technology system for markerless allele replacement in Burkholderia pseudomallei. Appl. Environ. Microbiol. 2009;75:6496–6503. doi: 10.1128/AEM.01669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trent MS, et al. Accumulation of a polyisoprene-linked amino sugar in polymyxin-resistant Salmonella typhimurium and Escherichia coli: Structural characterization and transfer to lipid A in the periplasm. J. Biol. Chem. 2001;276:43132–43144. doi: 10.1074/jbc.M106962200. [DOI] [PubMed] [Google Scholar]
- 95.Holloway BW. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 1955;13:572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- 96.Jacobs MA, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamad MA, Di Lorenzo F, Molinaro A, Valvano MA. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia (dagger) Mol. Microbiol. 2012;85:962–974. doi: 10.1111/j.1365-2958.2012.08154.x. [DOI] [PubMed] [Google Scholar]
- 99.Kovach ME, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 100.Cardona ST, Valvano MA. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid. 2005;54:219–228. doi: 10.1016/j.plasmid.2005.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.