Abstract
Oesophageal cancer is the sixth leading cause of cancer death worldwide. This nationwide study analyses the survival results of oesophageal cancer under multidisciplinary team (MDT) care. We enrolled oesophageal cancer patients diagnosed between 2010 and 2015 with follow-up for at least 1 year. This study performed propensity score matching with a ratio of 1:1 between MDT participants and non-MDT participants. We performed conditional Cox proportional hazards model to research relative risk of survival and associated factors of survival. The adjusted survival curves were plotted. 8184 newly diagnosed oesophageal cancer patients were included. The favourable survival factors include participant status of MDT, gender, monthly salary, urbanization level, other catastrophic illness, stage of cancer, treatment methods, and service volume of physicians (P < 0.05). MDT participants showed lower risk of death (HR = 0.73; 95% CI 0.67–0.79). Further stratification analysis revealed that the incorporation of an MDT reduced the death risk of patients with stages 2, 3, and 4 cancer, with the greatest reduction observed in patients with stage 3 cancer (HR = 0.72; 95% CI 0.67–0.79). The risk of death was lower for oesophageal cancer patients who enrolled in MDT care.
Subject terms: Health care, Medical research, Oncology, Risk factors
Introduction
Globally, oesophageal cancer has the ninth highest incidence rate among all cancer types and is the sixth most common cause of cancer death1. Despite with ever-improving medical treatment, studies have revealed a low 5-year survival rate among oesophageal cancer patients2–4.
After the promulgation of the Cancer Control Act in Taiwan, the Ministry of Health and Welfare, initiated the Complete Cancer Care Quality Improvement Project in 2005 to promote cancer-prevention education, measures, and screening, provide education training on medical personnel, boost the quality of cancer treatment, and assist hospitals in the establishment of a multidisciplinary team (MDT) care model for cancer. An MDT care plan concerns the planning, assessment, review, and analysis of cancer treatment and care; consultation on nutrition, psychological issues, and medicine; health education; rehabilitation; preparation for discharge; continuous care after discharge; and integrative diagnosis and treatment of cancer5–8.
Due to its effect in improving clinical care outcomes according to the literature, the implementation of the MDT treatment strategy has been increasing in a number in places including European countries, the United States, and Australia9. However, only one study has focused its discussion of MDT care on the effect of improve on the survival rate of oesophageal cancer patients. That study was conducted in one British medical institution from 1991 and 2003; due to the small study population comprising 144 patients and before–after study design, it exhibited various research limitations and insufficient generalisability10.
Studies discussing cancers, such as oral, gastric, lung, colorectal, breast, and ovarian cancers, have revealed a conducive effect of MDTs on clinical care outcomes5,7,11–16. A study comparing patients with oral cancer who were managed and who were not managed by an MDT showed a lower death risk in those managed by an MDT11. A study of gastric cancer revealed the effect of MDTs in improving compliance with treatment guidelines and reducing inappropriate treatment recommendations, which thereby increased the survival of patients with gastric cancer17. Research in patients with stage 3 and stage 4 lung cancer showed a significantly lower death risk in those incorporating an MDT into the treatment plan than in those who did not5. A study of colorectal cancer also revealed that patients with colorectal cancer who incorporated an MDT into the treatment plan had a lower death risk than did those who did not, and that such a difference was particularly prominent among patients with stage 4 colorectal cancer18. According to Liao et al., colorectal patients incorporating an MDT into the treatment had fewer emergency department visits than did those without an MDT, indicating the higher quality of care received by those with an MDT19.
However, few studies have also revealed no significant effect of MDT intervention on the survival results. Research on lung and metastatic rectal cancer has shown no effect of MDT intervention on survival results improvement of cancer patients20,21. Other studies have noted the difficulties in assessing the effectiveness of the MDT treatment strategy; thus, this topic requires more research efforts8,9. Several American scholars suggested that future research should emphasise the various dimensions of the MDT treatment strategy, including its effectiveness and its relationship with patient survival22.
Overall, in addition to the appeals from scholars worldwide, the National Comprehensive Cancer Network had listed the MDT treatment strategy as one of the basic treatments for oesophageal cancer23. However, at the time of writing the present paper, only one study of MDT intervention focused on the survival of patients with oesophageal cancer10. The current medical evidence is insufficient for verifying the effect of MDT intervention on the survival results of oesophageal cancer patients, warranting further research on the topic. Therefore, based on data from the National Health Insurance Research Database (NHIRD) and Taiwan Cancer Registry Database (TCRD), a nationwide retrospective cohort research was conducted to research the effect of MDT intervention in improving the survival rate of oesophageal cancer patients.
Results
Features of oesophageal cancer patients adopting and not adopting the MDT treatment strategy
Before matching (Table 1), bivariate analysis revealed significance differences in the socioeconomic factor (monthly salary), environmental factor (urbanization level), health status (comorbidities), service volume of the physician, and hospital level between patients with oesophageal cancer with and without the cancer MDT treatment strategy (p < 0.05). According to Table 1, a higher proportion of patients with a high monthly salary decided to incorporate an MDT for oesophageal cancer treatment, with the highest proportion observed among those with a monthly salary between NT$36,301 and NT$45,800 and higher than NT$45,800. A high proportion of patients with oesophageal cancer incorporating an MDT was observed among those who lived in an area with urbanization levels 2, 3, 4, or 7. Regarding the health status (measured based on Deyo’s Charlson Comorbidity Index [CCI] and the presence of other catastrophic illnesses), patients with a favourable health status showed a higher tendency to adopt the MDT treatment strategy. Most of the patients received treatment in medical centres (60.39%). Compared with those visiting medical centres, a higher proportion of patients visiting regional and district hospitals adopted the MDT treatment strategy (medical centre: 32.09%; nonmedical centre: 43.71%). Regarding the service volume of the physician, patients whose physicians had a medium volume of service exhibited the highest tendency to adopt the MDT treatment strategy (39.73%).
Table 1.
Bivariate and logistic regression analysis: MDT participants and non-participants.
| Variables | Total | Non-MDT | MDT | χ2 | Adjusted | P valueb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n1 | % | n2 | % | P valuea | OR | 95% CI | |||
| Total | 8184 | 100.00 | 5181 | 63.31 | 3003 | 36.69 | |||||
| Gender | 0.300 | ||||||||||
| Female | 517 | 6.32 | 316 | 61.12 | 201 | 38.88 | – | – | – | – | |
| Male | 7667 | 93.68 | 4865 | 63.45 | 2802 | 36.55 | 0.88 | 0.73 | 1.07 | 0.193 | |
| Age at diagnosed (year) | 0.015 | ||||||||||
| < 45 | 690 | 8.43 | 443 | 64.20 | 247 | 35.80 | - | - | - | - | |
| 45–54 | 2601 | 31.78 | 1601 | 61.55 | 1000 | 38.45 | 1.12 | 0.94 | 1.34 | 0.219 | |
| 55–64 | 2789 | 34.08 | 1755 | 62.93 | 1034 | 37.07 | 1.04 | 0.87 | 1.24 | 0.690 | |
| 65–74 | 1253 | 15.31 | 803 | 64.09 | 450 | 35.91 | 1.00 | 0.82 | 1.23 | 0.971 | |
| ≧ 75 | 851 | 10.40 | 579 | 68.04 | 272 | 31.96 | 0.86 | 0.69 | 1.07 | 0.185 | |
| Monthly salary (NTD) | < 0.001 | ||||||||||
| ≦ 17,280 | 2269 | 27.72 | 1495 | 65.89 | 774 | 34.11 | – | – | – | – | |
| 17,281–22,800 | 3395 | 41.48 | 2158 | 63.56 | 1237 | 36.44 | 1.10 | 0.98 | 1.24 | 0.096 | |
| 22,801–28,800 | 630 | 7.70 | 387 | 61.43 | 243 | 38.57 | 1.15 | 0.96 | 1.39 | 0.139 | |
| 28,801–36,300 | 711 | 8.69 | 462 | 64.98 | 249 | 35.02 | 1.03 | 0.86 | 1.23 | 0.767 | |
| 36,301–45,800 | 761 | 9.30 | 427 | 56.11 | 334 | 43.89 | 1.50 | 1.26 | 1.78 | < 0.001 | |
| ≧ 45,801 | 418 | 5.11 | 252 | 60.29 | 166 | 39.71 | 1.33 | 1.07 | 1.66 | 0.011 | |
| Urbanization level | < 0.001 | ||||||||||
| Level 1 | 1855 | 22.67 | 1109 | 59.78 | 746 | 40.22 | 1.00 | – | – | – | |
| Level 2 | 2356 | 28.79 | 1474 | 62.56 | 882 | 37.44 | 0.82 | 0.72 | 0.93 | 0.002 | |
| Level 3 | 1524 | 18.62 | 1012 | 66.40 | 512 | 33.60 | 0.72 | 0.62 | 0.83 | < 0.001 | |
| Level 4 | 1346 | 16.45 | 906 | 67.31 | 440 | 32.69 | 0.68 | 0.58 | 0.79 | < 0.001 | |
| Level 5 | 238 | 2.91 | 125 | 52.52 | 113 | 47.48 | 1.21 | 0.91 | 1.60 | 0.191 | |
| Level 6 | 447 | 5.46 | 282 | 63.09 | 165 | 36.91 | 0.81 | 0.65 | 1.01 | 0.066 | |
| Level 7 | 418 | 5.11 | 273 | 65.31 | 145 | 34.69 | 0.76 | 0.61 | 0.96 | 0.019 | |
| Other catastrophic illness | 0.023 | ||||||||||
| No | 7782 | 95.09 | 4905 | 63.03 | 2877 | 36.97 | 1.00 | – | – | – | |
| Yes | 402 | 4.91 | 276 | 68.66 | 126 | 31.34 | 0.86 | 0.69 | 1.08 | 0.199 | |
| Charlson Comorbidity index | 0.001 | ||||||||||
| 0 | 4734 | 57.84 | 2926 | 61.81 | 1808 | 38.19 | 1.00 | – | – | – | |
| 1 | 1924 | 23.51 | 1229 | 63.88 | 695 | 36.12 | 0.92 | 0.82 | 1.03 | 0.151 | |
| 2 | 844 | 10.31 | 558 | 66.11 | 286 | 33.89 | 0.86 | 0.73 | 1.00 | 0.056 | |
| ≧ 3 | 682 | 8.33 | 468 | 68.62 | 214 | 31.38 | 0.76 | 0.63 | 0.91 | 0.003 | |
| Cancer stage | < 0.001 | ||||||||||
| I | 343 | 4.19 | 222 | 64.72 | 121 | 35.28 | 1.00 | – | – | – | |
| II | 1521 | 18.59 | 905 | 59.50 | 616 | 40.50 | 1.18 | 0.92 | 1.52 | 0.188 | |
| III | 4397 | 53.73 | 2775 | 63.11 | 1622 | 36.89 | 1.01 | 0.80 | 1.28 | 0.926 | |
| IV | 1923 | 23.50 | 1279 | 66.51 | 644 | 33.49 | 0.86 | 0.67 | 1.10 | 0.235 | |
| Service volume of physicians | < 0.001 | ||||||||||
| Low (< 25%) | 2030 | 24.80 | 1320 | 65.02 | 710 | 34.98 | 1.00 | – | – | – | |
| Middle (25–75%) | 4158 | 50.81 | 2506 | 60.27 | 1652 | 39.73 | 1.28 | 1.15 | 1.44 | < 0.001 | |
| High (> 75%) | 1996 | 24.39 | 1355 | 67.89 | 641 | 32.11 | 1.04 | 0.90 | 1.19 | 0.636 | |
| Hospital level | < 0.001 | ||||||||||
| Medical centers | 4942 | 60.39 | 3356 | 67.91 | 1586 | 32.09 | 1.00 | – | – | – | |
| Non-medical centers | 3242 | 39.61 | 1825 | 56.29 | 1417 | 43.71 | 1.68 | 1.52 | 1.86 | < 0.001 | |
| Hospital ownership | 0.063 | ||||||||||
| Non-public | 5650 | 69.04 | 3539 | 62.64 | 2111 | 37.36 | 1.00 | – | – | – | |
| Public | 2534 | 30.96 | 1642 | 64.80 | 892 | 35.20 | 1.02 | 0.92 | 1.14 | 0.655 | |
MDT multidisciplinary team, OR odds ratio, NTD new Taiwan dollar.
Effect of MDT on the survival of oesophageal cancer patients
Propensity score matching was conducted at a ratio of 1:1. Logistic regression was employed to establish a model; the dependent variable was whether the patient adopted the MDT treatment strategy, and the independent variables comprised demographic factors (sex and age), socioeconomic factor (monthly salary), environmental factor (urbanization level), health status (CCI, other catastrophic illnesses, and oesophageal cancer stage), and characteristics of the main hospital that the patient visited (hospital level and hospital ownership). Logistic regression analysis was conducted to obtain the propensity score of each patient, followed by propensity score matching at a 1:1 ratio. The final research sample included 2953 patients with oesophageal cancer in the experimental group (patients adopting the MDT treatment strategy) and 2,953 patients in the control group (patients not adopting the MDT treatment strategy) (in Table 2).
Table 2.
Bivariate analysis of factors affecting MDT participant status after propensity score matching.
| Variables | 1: 1 matching | SMD | |||||
|---|---|---|---|---|---|---|---|
| Total | Non-MDT | MDT | |||||
| N | % | n1 | % | n2 | % | ||
| Total | 5906 | 100.00 | 2953 | 50.00 | 2953 | 50.00 | |
| Gender | |||||||
| Female | 373 | 6.32 | 178 | 6.03 | 195 | 6.60 | 0.071 |
| Male | 5533 | 93.68 | 2775 | 93.97 | 2758 | 93.40 | 0.005 |
| Age at diagnosed (year) | |||||||
| < 45 | 486 | 8.23 | 248 | 8.40 | 238 | 8.06 | 0.042 |
| 45–54 | 1966 | 33.29 | 990 | 33.53 | 976 | 33.05 | 0.061 |
| 55–64 | 2046 | 34.64 | 1026 | 34.74 | 1020 | 34.54 | 0.035 |
| 65–74 | 865 | 14.65 | 418 | 14.16 | 447 | 15.14 | 0.029 |
| ≧ 75 | 543 | 9.19 | 271 | 9.18 | 272 | 9.21 | 0.061 |
| Monthly salary (NTD) | |||||||
| ≦ 17,280 | 1606 | 27.19 | 834 | 28.24 | 772 | 26.14 | 0.034 |
| 17,281–2,2800 | 2421 | 40.99 | 1202 | 40.70 | 1219 | 41.28 | 0.016 |
| 22,801–28,800 | 472 | 7.99 | 231 | 7.82 | 241 | 8.16 | 0.024 |
| 28,801–36,300 | 510 | 8.64 | 261 | 8.84 | 249 | 8.43 | 0.040 |
| 36,301–45,800 | 598 | 10.13 | 283 | 9.58 | 315 | 10.67 | 0.065 |
| ≧ 45,801 | 299 | 5.06 | 142 | 4.81 | 157 | 5.32 | 0.019 |
| Urbanization level | |||||||
| Level 1 | 1460 | 24.72 | 741 | 25.09 | 719 | 24.35 | 0.047 |
| Level 2 | 1715 | 29.04 | 839 | 28.41 | 876 | 29.66 | 0.041 |
| Level 3 | 1020 | 17.27 | 511 | 17.30 | 509 | 17.24 | 0.008 |
| Level 4 | 872 | 14.76 | 434 | 14.70 | 438 | 14.83 | 0.040 |
| Level 5 | 204 | 3.45 | 102 | 3.45 | 102 | 3.45 | 0.004 |
| Level 6 | 342 | 5.79 | 177 | 5.99 | 165 | 5.59 | 0.021 |
| Level 7 | 293 | 4.96 | 149 | 5.05 | 144 | 4.88 | 0.064 |
| Charlson Comorbidity index | |||||||
| 0 | 3610 | 61.12 | 1835 | 62.14 | 1775 | 60.11 | 0.019 |
| 1 | 1368 | 23.16 | 683 | 23.13 | 685 | 23.20 | 0.018 |
| 2 | 525 | 8.89 | 245 | 8.30 | 280 | 9.48 | 0.027 |
| ≧ 3 | 403 | 6.82 | 190 | 6.43 | 213 | 7.21 | 0.010 |
| Other catastrophic illness | |||||||
| No | 5673 | 96.05 | 2845 | 96.34 | 2828 | 95.77 | 0.003 |
| Yes | 233 | 3.95 | 108 | 3.66 | 125 | 4.23 | 0.075 |
| Cancer stage | |||||||
| I | 236 | 4.00 | 116 | 3.93 | 120 | 4.06 | 0.080 |
| II | 1154 | 19.54 | 558 | 18.90 | 596 | 20.18 | 0.049 |
| III | 3215 | 54.44 | 1618 | 54.79 | 1597 | 54.08 | 0.029 |
| IV | 1301 | 22.03 | 661 | 22.38 | 640 | 21.67 | 0.004 |
| Hospital level | |||||||
| Medical centers | 3155 | 53.42 | 1571 | 53.20 | 1584 | 53.64 | 0.050 |
| Non-medical centers | 2751 | 46.58 | 1382 | 46.80 | 1369 | 46.36 | 0.026 |
| Hospital ownership | |||||||
| Non-public | 4092 | 69.29 | 2025 | 68.57 | 2067 | 70.00 | 0.042 |
| Public | 1814 | 30.71 | 928 | 31.43 | 886 | 30.00 | 0.012 |
MDT multidisciplinary team, NTD New Taiwan Dollar.
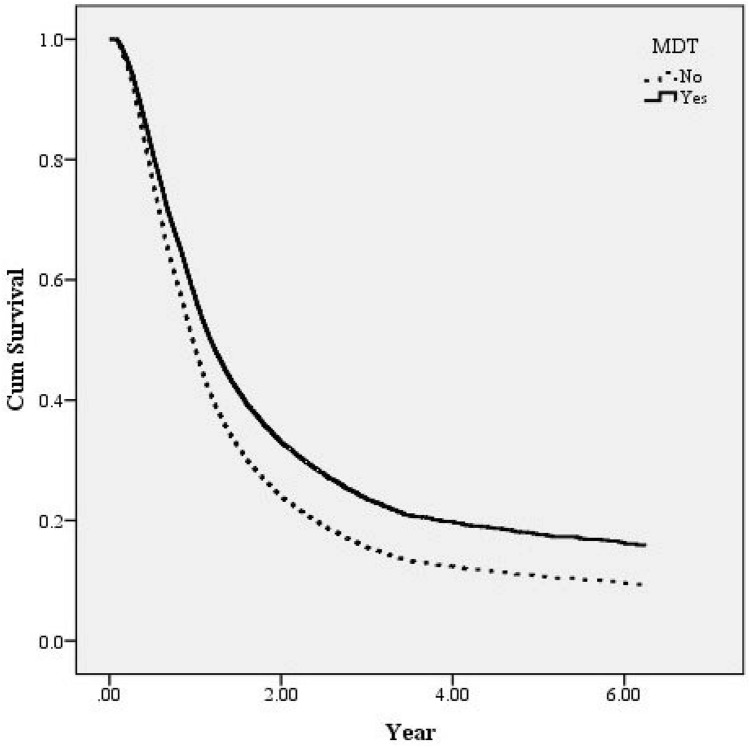
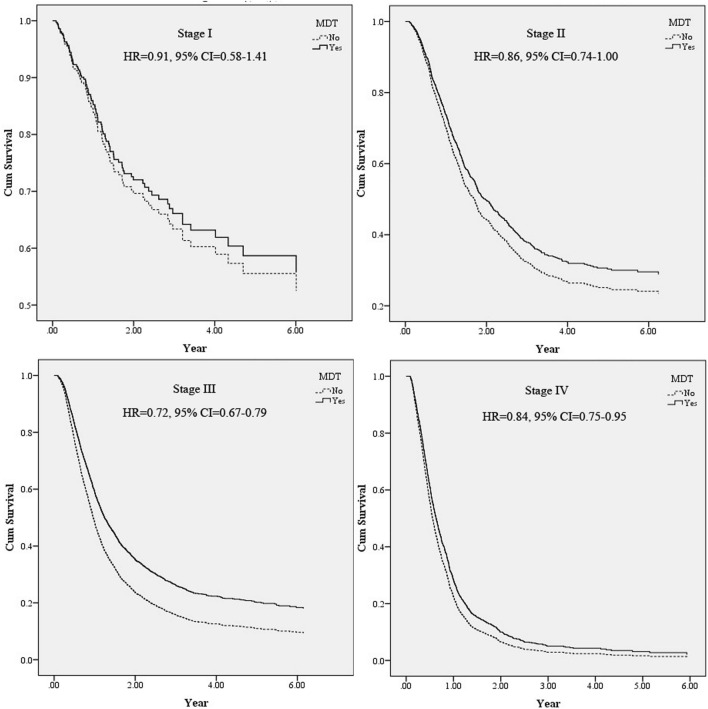
According to Table 3, the death rate was lower in patients adopting the MDT treatment strategy (72.10%) than in those not adopting the MDT treatment strategy (76.36%). A conditional Cox proportional hazard model was employed for statistical analyses to explore the survival rates of those with and without an MDT. As shown in Table 3, the death risk of patients adopting the MDT treatment strategy was 0.73 times that of patients not adopting the MDT treatment strategy (95% confidence interval [CI] 0.67–0.79). With relevant variables controlled for (Fig. 1), adjusted survival curves were generated for patients with and without an MDT. These patients were further divided according to the oesophageal cancer stage for stratification analysis, which showed that the incorporation of an MDT significantly reduced death risk for patients with stages 2, 3, and 4 cancer; the reduction was particularly marked for patients with stage 3 cancer [hazard ratio (HR) = 0.72; 95% CI 0.67–0.79; Fig. 2].
Table 3.
Analysis of MDT participant status and other factors affecting survival of esophageal patients.
| Variables | Total | Survival | Death | P valuea | Adjusted | P valueb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n1 | % | n2 | % | HR | 95% CI | ||||
| Total | 5906 | 100.00 | 1522 | 25.77 | 4384 | 74.23 | |||||
| MDT participant status | < 0.001 | ||||||||||
| No | 2953 | 50.00 | 698 | 23.64 | 2255 | 76.36 | |||||
| Yes | 2953 | 50.00 | 824 | 27.90 | 2129 | 72.10 | 0.73 | 0.67 | 0.79 | < 0.001 | |
| Gender | < 0.001 | ||||||||||
| Female | 373 | 6.32 | 145 | 38.87 | 228 | 61.13 | – | – | – | – | |
| Male | 5533 | 93.68 | 1377 | 24.89 | 4156 | 75.11 | 1.45 | 1.01 | 2.09 | 0.043 | |
| Age at diagnosed (year) | 0.001 | ||||||||||
| < 45 | 486 | 8.23 | 108 | 22.22 | 378 | 77.78 | – | – | – | – | |
| 45–54 | 1966 | 33.29 | 464 | 23.60 | 1502 | 76.40 | 1.29 | 0.88 | 1.89 | 0.189 | |
| 55–64 | 2046 | 34.64 | 584 | 28.54 | 1462 | 71.46 | 1.19 | 0.84 | 1.68 | 0.333 | |
| 65–74 | 865 | 14.65 | 233 | 26.94 | 632 | 73.06 | 1.30 | 0.90 | 1.87 | 0.163 | |
| ≧ 75 | 543 | 9.19 | 133 | 24.49 | 410 | 75.51 | 1.33 | 0.83 | 2.15 | 0.239 | |
| Monthly salary (NTD) | < 0.001 | ||||||||||
| ≦ 17,280 | 1606 | 27.19 | 316 | 19.68 | 1290 | 80.32 | – | – | – | – | |
| 17,281–22,800 | 2421 | 40.99 | 625 | 25.82 | 1796 | 74.18 | 0.74 | 0.55 | 1.01 | 0.047 | |
| 22,801–28,800 | 472 | 7.99 | 147 | 31.14 | 325 | 68.86 | 0.76 | 0.49 | 1.18 | 0.214 | |
| 28,801–36,300 | 510 | 8.64 | 140 | 27.45 | 370 | 72.55 | 0.65 | 0.45 | 0.94 | 0.023 | |
| 36,301–45,800 | 598 | 10.13 | 196 | 32.78 | 402 | 67.22 | 0.60 | 0.29 | 1.22 | 0.159 | |
| ≧ 45,801 | 299 | 5.06 | 98 | 32.78 | 201 | 67.22 | 0.42 | 0.23 | 0.75 | 0.003 | |
| Urbanization level | 0.310 | ||||||||||
| Level 1 | 1460 | 24.72 | 404 | 27.67 | 1056 | 72.33 | – | – | – | – | |
| Level 2 | 1715 | 29.04 | 437 | 25.48 | 1278 | 74.52 | 0.93 | 0.62 | 1.41 | 0.737 | |
| Level 3 | 1020 | 17.27 | 283 | 27.75 | 737 | 72.25 | 1.02 | 0.56 | 1.84 | 0.958 | |
| Level 4 | 872 | 14.76 | 195 | 22.36 | 677 | 77.64 | 0.98 | 0.51 | 1.90 | 0.954 | |
| Level 5 | 204 | 3.45 | 47 | 23.04 | 157 | 76.96 | 0.51 | 0.29 | 0.91 | 0.022 | |
| Level 6 | 342 | 5.79 | 81 | 23.68 | 261 | 76.32 | 0.68 | 0.40 | 1.16 | 0.159 | |
| Level 7 | 293 | 4.96 | 75 | 25.60 | 218 | 74.40 | 1.19 | 0.64 | 2.19 | 0.584 | |
| Other catastrophic illness | 0.591 | ||||||||||
| No | 5673 | 96.05 | 1457 | 25.68 | 4216 | 74.32 | – | – | – | – | |
| Yes | 233 | 4.96 | 65 | 27.90 | 168 | 72.10 | 1.78 | 1.16 | 2.74 | 0.009 | |
| Charlson Comorbidity index | 0.280 | ||||||||||
| 0 | 3610 | 61.12 | 917 | 25.40 | 2693 | 74.60 | – | – | – | – | |
| 1 | 1368 | 23.16 | 363 | 26.54 | 1005 | 73.46 | 1.02 | 0.78 | 1.33 | 0.891 | |
| 2 | 525 | 8.89 | 134 | 25.52 | 391 | 74.48 | 0.98 | 0.67 | 1.43 | 0.912 | |
| ≧ 3 | 403 | 6.82 | 108 | 26.80 | 295 | 73.20 | 0.99 | 0.58 | 1.69 | 0.963 | |
| Cancer stage | < 0.001 | ||||||||||
| I | 236 | 4.00 | 144 | 61.02 | 92 | 38.98 | – | – | – | – | |
| II | 1154 | 19.54 | 445 | 38.56 | 709 | 61.44 | 1.18 | 0.70 | 2.01 | 0.533 | |
| III | 3215 | 54.44 | 824 | 25.63 | 2391 | 74.37 | 2.75 | 1.78 | 4.26 | < 0.001 | |
| IV | 1301 | 22.03 | 109 | 8.38 | 1192 | 91.62 | 6.49 | 3.88 | 10.85 | < 0.001 | |
| Treatment | < 0.001 | ||||||||||
| Surgery | 476 | 8.06 | 218 | 45.80 | 258 | 54.20 | – | – | – | – | |
| Radiotherapy | 303 | 5.13 | 33 | 10.89 | 270 | 89.11 | 2.41 | 1.66 | 3.49 | < 0.001 | |
| Chemotherapy | 247 | 4.18 | 44 | 17.81 | 203 | 82.19 | 2.62 | 1.76 | 3.89 | < 0.001 | |
| Surgery + radiotherapy | 287 | 4.86 | 59 | 20.56 | 228 | 79.44 | 1.09 | 0.76 | 1.56 | 0.647 | |
| Surgery + chemotherapy | 283 | 4.79 | 58 | 20.49 | 225 | 79.51 | 1.96 | 1.36 | 2.82 | < 0.001 | |
| Radiotherapy + chemotherapy | 1649 | 27.92 | 375 | 22.74 | 1274 | 77.26 | 0.93 | 0.72 | 1.20 | 0.552 | |
| Surgery + radiotherapy + chemotherapy | 2661 | 45.06 | 735 | 27.62 | 1926 | 72.38 | 0.78 | 0.60 | 1.00 | 0.051 | |
| Service volume of physicians | |||||||||||
| Low (< 25%) | 1492 | 25.26 | 284 | 19.03 | 1208 | 80.97 | – | – | – | – | |
| Middle (25–75%) | 3109 | 52.64 | 807 | 25.96 | 2302 | 74.04 | 0.96 | 0.83 | 1.11 | 0.561 | |
| High (> 75%) | 1305 | 22.10 | 431 | 33.03 | 874 | 66.97 | 0.81 | 0.67 | 0.97 | 0.023 | |
| Hospital level | < 0.001 | ||||||||||
| Medical centers | 3155 | 53.42 | 865 | 27.42 | 2290 | 72.58 | – | – | – | – | |
| Non-medical centers | 2751 | 46.58 | 657 | 23.88 | 2094 | 76.12 | 0.74 | 0.31 | 1.77 | 0.501 | |
| Hospital ownership | < 0.001 | ||||||||||
| Non-public | 4092 | 69.29 | 1029 | 25.15 | 3063 | 74.85 | – | – | – | – | |
| Public | 1814 | 30.71 | 493 | 27.18 | 1321 | 72.82 | 0.83 | 0.67 | 1.03 | 0.089 | |
MDT multidisciplinary team, HR hazard ratio, NTD new Taiwan dollar.
Figure 1.
Survival curves of esophageal patients according to MDT participant status. The cumulative survival of esophageal patients among 2953 MDT patients and 2953 non-participants. The survival curves were controlled by gender, age, monthly salary, urbanization level, comorbidities, other catastrophic illness, level of hospital, hospital ownership, service volume of attending physicians. The survival rates of MDT participants were significantly higher than those of MDT non-participants (adjusted HR = 0.73, 95% CI 0.67–0.79).
Figure 2.
Stratification analysis of the patients according to the oesophageal cancer stage showed the incorporation of an MDT significantly reduced death risk for patients with stages 2, 3, and 4 cancer; the reduction was particularly marked for patients with stage 3 cancer (HR = 0.72; 95% CI 0.67–0.79).
Table 4 presents a relatively low death risk among patients with stages 2, 3, and 4 cancer who adopted the MDT treatment strategy compared with their same-stage counterparts without an MDT. The effect of an MDT was the most substantial among patients with stage 3 cancer, with the death rates for those adopting and not adopting the MDT treatment strategy being 70.95% and 77.75%, respectively. The patients who adopted the MDT treatment strategy exhibited a lower death rate than did those not adopting the MDT treatment strategy, regardless of the service volume of their physician. Regarding the hospital level, among patients visiting medical centres, those with an MDT (68.81%) showed a substantially lower death rate than did those without an MDT (76.38%). Regarding the ownership of the main hospital that each patient visited, an MDT was observed to considerably reduce patients’ death rate in public hospitals (with MDT: 67.49%; without MDT: 77.97%).
Table 4.
Comparison of the survival of esophageal patients between MDT participants and non-participants.
| Variables | Non-MDT | P value | MDT | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Survival | Death | Total | Survival | Death | |||||||||
| N | % | n1 | % | n2 | % | N | % | n1 | % | n2 | % | |||
| Total | 2953 | 100.00 | 698 | 23.64 | 2255 | 76.36 | 2953 | 100.00 | 824 | 27.90 | 2129 | 72.10 | < 0.001 | |
| Gender | < 0.001 | < 0.001 | ||||||||||||
| Female | 178 | 6.03 | 65 | 36.52 | 113 | 63.48 | 195 | 6.60 | 80 | 41.03 | 115 | 58.97 | ||
| Male | 2775 | 93.97 | 633 | 22.81 | 2142 | 77.19 | 2758 | 93.40 | 744 | 26.98 | 2014 | 73.02 | ||
| Age at diagnosed (year) | 0.003 | 0.156 | ||||||||||||
| < 45 | 248 | 8.40 | 54 | 21.77 | 194 | 78.23 | 238 | 8.06 | 54 | 22.69 | 184 | 77.31 | ||
| 45–54 | 990 | 33.53 | 201 | 20.30 | 789 | 79.70 | 976 | 33.05 | 263 | 26.95 | 713 | 73.05 | ||
| 55–64 | 1026 | 34.74 | 280 | 27.29 | 746 | 72.71 | 1020 | 34.54 | 304 | 29.80 | 716 | 70.20 | ||
| 65–74 | 418 | 14.16 | 99 | 23.68 | 319 | 76.32 | 447 | 15.14 | 134 | 29.98 | 313 | 70.02 | ||
| ≧ 75 | 271 | 9.18 | 64 | 23.62 | 207 | 76.38 | 272 | 9.21 | 69 | 25.37 | 203 | 74.63 | ||
| Monthly salary (NTD) | < 0.001 | < 0.001 | ||||||||||||
| ≦ 17,280 | 834 | 28.24 | 148 | 17.75 | 686 | 82.25 | 772 | 26.14 | 168 | 21.76 | 604 | 78.24 | ||
| 17,281–2,2800 | 1202 | 40.70 | 299 | 24.88 | 903 | 75.12 | 1219 | 41.28 | 326 | 26.74 | 893 | 73.26 | ||
| 22,801–28,800 | 231 | 7.82 | 63 | 27.27 | 168 | 72.73 | 241 | 8.16 | 84 | 34.85 | 157 | 65.15 | ||
| 28,801–36,300 | 261 | 8.84 | 65 | 24.90 | 196 | 75.10 | 249 | 8.43 | 75 | 30.12 | 174 | 69.88 | ||
| 36,301–45,800 | 283 | 9.58 | 89 | 31.45 | 194 | 68.55 | 315 | 10.67 | 107 | 33.97 | 208 | 66.03 | ||
| ≧ 45,801 | 142 | 4.81 | 34 | 23.94 | 108 | 76.06 | 157 | 5.32 | 64 | 40.76 | 93 | 59.24 | ||
| Charlson comorbidity index | 0.046 | 0.559 | ||||||||||||
| 0 | 1835 | 62.14 | 421 | 22.94 | 1414 | 77.06 | 1775 | 60.11 | 496 | 27.94 | 1279 | 72.06 | ||
| 1 | 683 | 23.13 | 175 | 25.62 | 508 | 74.38 | 685 | 23.20 | 188 | 27.45 | 497 | 72.55 | ||
| 2 | 245 | 8.30 | 57 | 23.27 | 188 | 76.73 | 280 | 9.48 | 77 | 27.50 | 203 | 72.50 | ||
| ≧ 3 | 190 | 6.43 | 45 | 23.68 | 145 | 76.32 | 213 | 7.21 | 63 | 29.58 | 150 | 70.42 | ||
| Cancer stage | < 0.001 | < 0.001 | ||||||||||||
| I | 116 | 3.93 | 71 | 61.21 | 45 | 38.79 | 120 | 4.06 | 73 | 60.83 | 47 | 39.17 | ||
| II | 558 | 18.90 | 212 | 37.99 | 346 | 62.01 | 596 | 20.18 | 233 | 39.09 | 363 | 60.91 | ||
| III | 1618 | 54.79 | 360 | 22.25 | 1258 | 77.75 | 1597 | 54.08 | 464 | 29.05 | 1133 | 70.95 | ||
| IV | 661 | 22.38 | 55 | 8.32 | 606 | 91.68 | 640 | 21.67 | 54 | 8.44 | 586 | 91.56 | ||
| Treatment | < 0.001 | < 0.001 | ||||||||||||
| Surgery | 312 | 10.57 | 138 | 44.23 | 174 | 55.77 | 164 | 5.55 | 80 | 48.78 | 84 | 51.22 | ||
| Radiotherapy | 172 | 5.82 | 19 | 11.05 | 153 | 88.95 | 131 | 4.44 | 14 | 10.69 | 117 | 89.31 | ||
| Chemotherapy | 149 | 5.05 | 28 | 18.79 | 121 | 81.21 | 98 | 3.32 | 16 | 16.33 | 82 | 83.67 | ||
| Surgery + radiotherapy | 140 | 4.74 | 24 | 17.14 | 116 | 82.86 | 147 | 4.98 | 35 | 23.81 | 112 | 76.19 | ||
| Surgery + chemotherapy | 166 | 5.62 | 31 | 18.67 | 135 | 81.33 | 117 | 3.96 | 27 | 23.08 | 90 | 76.92 | ||
| Radiotherapy + chemotherapy | 877 | 29.70 | 171 | 19.50 | 706 | 80.50 | 772 | 26.14 | 204 | 26.42 | 568 | 73.58 | ||
| Surgery + radiotherapy + Chemotherapy | 1137 | 38.50 | 287 | 25.24 | 850 | 74.76 | 1524 | 51.61 | 448 | 29.40 | 1076 | 70.60 | ||
| Service volume of physicians | < 0.001 | < 0.001 | ||||||||||||
| Low (< 25%) | 798 | 27.02 | 132 | 16.54 | 666 | 83.46 | 694 | 23.50 | 152 | 21.90 | 542 | 78.10 | ||
| Middle (25–75%) | 1484 | 50.25 | 365 | 24.60 | 1119 | 75.40 | 1625 | 55.03 | 442 | 27.20 | 1183 | 72.80 | ||
| High (> 75%) | 671 | 22.72 | 201 | 29.96 | 470 | 70.04 | 634 | 21.47 | 230 | 36.28 | 404 | 63.72 | ||
| Hospital level | 0.010 | < 0.001 | ||||||||||||
| Medical centers | 1571 | 53.20 | 371 | 23.62 | 1200 | 76.38 | 1584 | 53.64 | 494 | 31.19 | 1090 | 68.81 | ||
| Non-medical centers | 1382 | 46.80 | 327 | 23.66 | 1055 | 76.34 | 1369 | 46.36 | 330 | 24.11 | 1039 | 75.89 | ||
| Hospital ownership | 0.148 | < 0.001 | ||||||||||||
| Non-public | 2025 | 68.57 | 493 | 24.35 | 1532 | 75.65 | 70.00 | 536 | 25.93 | 1531 | 74.07 | |||
| Public | 928 | 31.43 | 205 | 22.09 | 723 | 77.91 | 30.00 | 288 | 32.51 | 598 | 67.49 | |||
MDT participant observation period:1.59 ± 1.39 years (median: 1.14 years); Non-participant observation period:1.34 ± 1.38 years (median: 0.87 years).
MDT multidisciplinary team, NTD New Taiwan Dollar.
Other relevant factors affecting the survival of oesophageal cancer patients
A conditional Cox proportional hazard model (Table 3) was performed to observe the relative risk of survival in patients who adopted and did not adopt the MDT treatment strategy as well as to explore relevant factors affecting patient survival. The following factors had a significant effect on patients’ survival (p < 0.05): gender, monthly salary, urbanization level, other catastrophic illnesses, oesophageal cancer stage, treatment methods, and service volume of the physician. By contrast, patients’ age, CCI, hospital level, and hospital ownership showed no significant effect on patient survival (p > 0.05). In the Table 3, the death risks of all age groups were greater than 70% in the studied period. Those who developed oesophageal cancer at an age less than 45 years had the highest death risk (77.78%), followed by those who developed the cancer at 75 years or older. However, with relevant variables controlled for, the death risk of those who developed cancer at each age group was not significantly different from that of those who developed cancer at less than 45 years (P > 0.05). The analysis of patients’ socioeconomic status revealed the highest death rate (80.32%) among those whose monthly salary was NT$17,280 or lower. With relevant variables controlled for, the death risk of patients with a monthly salary of NT$28,801–36,300 was 0.65 times that of patients with a monthly salary of NT$17,280 or lower (95% CI 0.45–0.94). Patients with a monthly salary of NT$45,801 or higher had a death risk 0.42 times that of patients with a monthly salary of NT$17,280 or lower (95% CI 0.23–0.75). Regarding the health status, the death risk of patients with other catastrophic illnesses was 1.78 times that of patients without such an illness (95% CI 1.16–2.74). In the study period between 2010 and 2015, the survival rate of patients with stage 1 oesophageal cancer was 61.02%; the survival rate was significantly lower in patients with stage 2 cancer (38.56%); that of patients with stage 3 cancer was 25.63%; and that of patients with stage 4 cancer was extremely low at 8.38%. With relevant variables controlled for, the death risk increased as cancer progressed: the death risks of patients with stages 3 and 4 cancer were respectively 2.75 (95% CI 1.78–4.26) and 6.49 (95% CI 3.88–10.85) times that of patients with stage 1. According to the analysis of the service volume of the physician, patients whose physician had a higher service volume showed a lower death risk. The death risk of patients whose physician had a high service volume was 0.81 times that of patients whose physician had a low service volume (95% CI 0.67–0.97).
Discussion
Characteristics of patients with oesophageal cancer adopting and not adopting the MDT treatment strategy
This study is the first nationwide cohort research discussing the effect of MDTs of the survival results of newly diagnosed oesophageal cancer patients and the characteristics of such patients who adopted and did not adopt the MDT treatment strategy. This study recruited 14,563 patients newly diagnosed with oesophageal cancer from 2010 to 2015, among which 12,908 had received a surgery, chemotherapy, or radiotherapy within 1 year of their diagnosis. Subsequently, patients who adopted the MDT treatment strategy were matched at a ratio of 1:1 with those who did not adopt the MDT treatment strategy, finalising the sample to 2953 patients with MDT care and 2953 without MDT care. According to the results, whether patients adopted the MDT treatment strategy was associated with the following factors (p < 0.05): monthly salary, urbanization level of residence, cancer stage, level of the main hospital visited, and service volume of the physician. Studies have verified the association between whether a patient adopted the MDT treatment strategy and the patient’s disease severity, level of the main hospital visited, and the service volume of the physician5,11. The proportion of patients with a high monthly salary who adopted the MDT treatment strategy was higher compared with their low-monthly-salary counterparts, which was probably because patients with higher income and their families had a stronger will and better ability to seek medical help. The proportion of patients living in high-urbanization-level areas who adopted the MDT treatment strategy was higher than patients living in low-urbanization-level areas; this may be because hospitals with a sufficient scale and capacity to practice MDTs were mostly located in high urbanization level regions.
Effect of MDTs on the survival of oesophageal cancer patients
The proportion of people with cancer has been growing over the last few years. Globally, much research attention has been paid to oesophageal cancer in particular—the world’s sixth most common cause of cancer death. Prior researches had predominantly examined risk factors of oesophageal cancer or the effect of different treatments on patient survival24–26. By the time of writing the present study, only one small-scale before–after study focused on the effect of MDT intervention on the survival of oesophageal cancer patients10. Since there is a lack of strong evidence to support the effectiveness of MDTs for oesophageal cancer patients, investigating data from the TCRD and NHIRD, this nationwide, retrospective cohort research was performed to analyse the effect of MDT intervention in improving survival rate of patients with oesophageal cancer.
An MDT is aimed at benefiting both medical service providers and patients, improving the satisfaction and psychological state of patients and bringing together relevant medical providers for the joint formulation of care plans. These features have contributed to the growing popularity of MDTs in countries such as the United Kingdom, the United States, Australia, and European countries9. By incorporation of MDTs into the care plan for cancer patients improves the results of medical care. This is mostly because MDT intervention is a shift from the conventional care model that involves only a single medical department towards an integrative care model that engages specialists from relevant departments, including physicians, surgeons, oncologists, pathologists, radiologists, dietitians, physiatrists, nurses, and social workers, and MDT intervention involves regular meetings among these specialists to discuss and follow-up the status of patients with cancer8. In particular, due to the high complexity involved and the high probability of comorbidities, the diagnosis and treatment processes for cancer patients require a combination of various diagnostic and treatment methods. Accordingly, MDTs facilitate collaboration among medical teams, enhance compliance with treatment guidelines, reduce inappropriately treatment recommendations, shorten diagnostic time, boost the accuracy of diagnoses, and thus increase the survival of patients with cancer5–7,22.
In this study, we used the 1:1 propensity score matching method to minimize the selection bias between the two groups of patients (with and without an MDT) for eliminating the effects of confounding factors on the patients’ adoption of the cancer MDT strategy. As shown in Table 3, the death risk of patients adopting the MDT treatment strategy was 0.73 times that of patients not adopting the MDT treatment strategy. Accordingly, with relevant factors controlled for, patients with oesophageal cancer adopting the MDT treatment strategy had a lower death risk than did those not adopting the MDT treatment strategy. The study results are consistent with the finding of various studies that MDTs improved the survival rate in patients with different cancers5,11,17,27. However, only one study discussed the effect of MDT intervention on the survival results of oesophageal cancer patients; that research was performed in a medical institution in the United Kingdom from 1991 to 2003 and had a study population of only 144 patients. The same study revealed that MDT intervention improved the survival results of oesophageal cancer patients, with an increase of the 5-year survival rate from 10 to 52% (p < 0.05). Nevertheless, the before-after design of the study created various research limitations and hindered the generalisability of the results10. Studies in oral, gastric, lung, colorectal, breast and ovarian cancers have all indicated a beneficial effect of MDTs on clinical care outcomes5,14,27–29.
Associated factors of the survival of oesophageal cancer patients
According to Table 3, the survival of oesophageal cancer patients was significantly affected (p < 0.05) by the demographic factor (gender), health status (other catastrophic illnesses), socioeconomic factor (monthly salary), environmental factor (urbanization level), cancer stage, treatment, and service volume of the physician.
The study results showed that male patients had a death risk 1.45 times higher than female patients (95% CI 1.01–2.09), which is consistent with the findings of a previous study30. Patients with a higher monthly salary exhibited a lower death risk; specifically, the death risk of those with a monthly salary of NT$45,801 or more was 0.42 times that of those with a monthly salary of NT$17,280 or less (95% CI 0.23–0.75). Hence, socioeconomic factors affect the survival of oesophageal cancer patients. The National Programme of Cancer Registries Patterns of Care Study lead by American scholar Byers argued that a low socioeconomic status results in a less favourable prognosis in patients with cancer; this is because a low socioeconomic status may lead to delayed diagnoses and passive cancer treatment31. In Taiwan, cancer is categorised as a catastrophic illness as per the National Health Insurance Act; thus, patients with cancer are partially exempt from covering the medical costs involved in treating cancers. Despite the exemption, the association between a low socioeconomic status and unfavourable prognosis in patients with cancer remained in the present study. Another Taiwanese study also revealed better prognosis in high-socioeconomic-status patients with oesophageal cancer than in their low-socioeconomic-status counterparts32. Additionally, high-socioeconomic-status patients with oesophageal cancer, even if living in a low-socioeconomic-status area, had a higher chance of receiving esophagectomy32.
According to the analysis of patients’ health status, those with other catastrophic illnesses had a death risk that was 1.78 times higher than that of those without such illnesses (95% CI 1.16–2.74). This finding is consistent with previous findings33. Regarding the cancer stage, patients at a later cancer stage had a higher death risk; the death risks of patients with stage 3 and stage 4 cancer were respectively 2.75 (95% CI 1.78–4.26) and 6.49 (95% CI 3.88–10.85) times that of patients with stage 1 cancer. The prognosis of oesophageal cancer had a close association with the cancer stage of a patient at diagnosis; those in the early stage at diagnosis exhibited more favourable prognoses34. In Taiwan, oesophageal cancer is usually diagnosed at stage 2, 3, or even 4; thus, the prognosis is mostly unfavourable. Despite advancements in surgical techniques and postoperative care over recent years, the postoperative 5-year survival rate for oesophageal cancer has improved only slightly by 10–20%35.
According to analysis results for the service volume of the physician, a low death risk was observed among patients whose physicians had a high service volume; specifically, these patients had a death risk 0.81 times that of patients whose physicians had a low service volume (95% CI 0.67–0.97). Studies have demonstrated a positive effect of physicians’ service volume on patient care outcomes, which is probably attributable to the abundant care experience accumulated and excellent techniques honed by providing services and performing a large number of surgeries. Research has also shown that for physicians or hospitals with a high service volume, medical teams with abundant experience possess a higher skill level and better ability to execute treatment plans, in turn contributing to more favourable treatment outcomes and a lower patient death risk36,37. Previous research and the present study propose the consistent finding that high service and surgery volumes of physicians are associated with a low death rate of patients.
There were limitations in this study. First, our research collected data from the TCRD and NHIRD, and the discussion was limited to variables contained in the two databases. Therefore, other factors (e.g., smoking, drinking, or exercise habit) could not be included in this study, which are potentially related to the survival results of oesophageal cancer patients. Second, our research did not classify patients into groups with different pathological cell types, which could have contributed to the different survival rates. Third, the databases did not reveal whether patients had completed the entire treatment.
In conclusion, MDT intervention significantly reduced the death risk of patients with oesophageal cancer (HR = 0.73). Patients with the following characteristics had a less favourable prognosis: males, low socioeconomic status, presence of other catastrophic illnesses, low service volume of the physician and late stage of cancer.
Materials and methods
Data source
In this retrospective nationwide cohort study, we investigate data of oesophageal cancer patients from the TCRD, and mortality outcome from Cause of Death Data which were released by the Ministry of the Interior. Then, the NHIRD data from 2008 to 2016 were used for subsequent analysis of relevant variables. NHIRD contains comprehensive healthcare data of more than 23 million civilians who were representative of 99.7% of the residents of Taiwan11.
Study design
We explored the TCRD during January 2010 to December 2015 for oesophageal cancer patients and the Cause of Death Data during January 2010 to December 2016 for mortality outcome. The study cohort retrieved patients with incidental oesophageal cancer (ICD-9-CM: C150) who received treatment in a hospital for their oesophageal cancer within a year after oesophageal cancer was diagnosed. The treatments included surgical treatment, chemotherapy or radiation. The exclusion criteria include.
Patients who had other coexisting cancers or who had developed other cancers were excluded: Because this study examined survival rate differences between patients with oesophageal cancer who adopted and did not adopt the MDT treatment strategy, the presence of other cancers could exert an influence on the survival of these patients.
Patients receiving palliative were excluded: This study explored survival rate differences between patients with oesophageal cancer who adopted and did not adopt the MDT treatment strategy. Therefore, including patients who received palliative treatment after the diagnosis, which indicates the absence of curative intent treatment, in the comparison between the two aforementioned groups is inappropriate.
End-stage patients with mortality outcome within a month after the diagnosis were excluded: These patients may not be able to receive MDT treatment in time to reflect the benefits of MDTs.
Patients with stage 0 oesophageal cancer were excluded: According to an American study, the 5-year survival rate of stage 0 oesophageal cancer patients is higher than 90%38. Additionally, stage 0 oesophageal cancer is usually diagnosed and treated by specialists of a single medical department. Therefore, the intervention of MDTs may not provide much benefit to the survival results of stage 0 oesophageal cancer patients, who were thus excluded from the study.
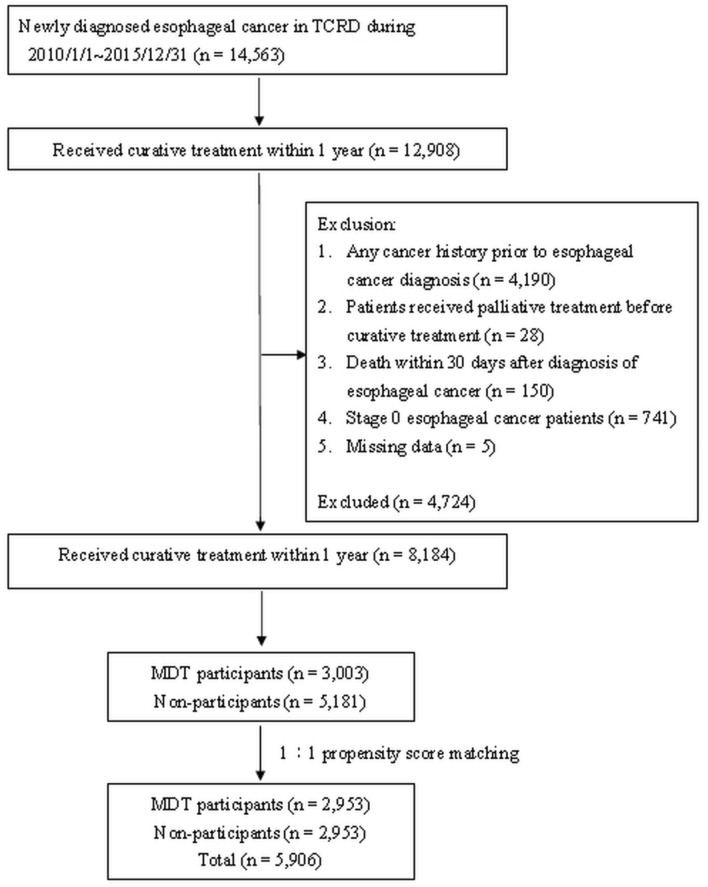
To mitigate the selection bias between the groups adopting and not adopting the MDT treatment strategy, this study employed 1:1 propensity score matching and a logistic regression model to predict whether a patient would adopt the MDT treatment strategy or not. The matching approach was used to control for the effects of confounding factors on the adoption of MDTs in the treatment plan, thereby increasing the consistency between patients adopting and not adopting the MDT treatment strategy. The selection process of study participants is showed as in Fig. 3.
Figure 3.
Flowchart of MDT participants and non-MDT participants enrolled from Taiwan Cancer Registry Database in Taiwan during 2010–2015.
Since the patient identifications in the National Health Insurance Research Database have been scrambled and de-identified by the Taiwan government for academic research use, the informed consent was waived by the Research Ethics Committee of the Changhua Christian Hospital. The research was conducted in accordance with the 1964 Declaration of Helsinki and amendments and was approved by the institutional review board of the Changhua Christian Hospital (IRB No. 181259), Taiwan.
Variables of interest
Since medical providers in Taiwan can have revenues by MDT care with appropriate medical records, this study claimed the aforementioned information to defined the MDT group. The control variables were as follows: demographic characteristics (age and sex), socioeconomic factor (monthly salary), environmental factor (urbanization level), health status (comorbidities, cancer stage and other catastrophic illnesses), and the features of the main hospital and physician visited (hospital ownership, hospital level, and service volume of the physician).
The urbanization level of patients’ workplace and residence was determined according to the locations of units from where health insurance was purchased for them and with reference to ‘Incorporating Development Stratification of Taiwan Townships into Sampling Design of Large Scale Health Interview Survey’ by Liu et al. in 2006. Liu et al. classified urbanization into Levels 1 to 7 by conducting a cluster analysis with the following variables: proportion of the population with a junior college degree or higher, population density, proportion of the population 65 years or older, proportion of the population working in agriculture, and number of Western medicine doctors per 100,000 residents. Level 7 represents the least urbanized areas; otherwise, level 1 represents the most urbanized areas39.
Regarding the health status, the comorbidities of patients were classified into 17 categories in accordance with Deyo’s CCI. ICD-9-CM codes commissioned to the principal and additional diagnoses of patients were converted into weighted scores, which were then summed to obtain the CCI score40. CCI scores were classified into the following four levels in this study: 0, 1, 2, and 3 or higher.
The cancer stage referred to the stage of cancer at the time of diagnosis defined by the American Joint Committee on Cancer, which comprised four stages, namely stages 1, 2, 3, and 4.
The main hospitals visited referred to the medical institutions where each of the patients was diagnosed with oesophageal cancer. The hospitals were classified into three hospital levels (i.e., medical centres, regional hospitals, and district hospitals) and two ownership types (i.e., public and private hospitals).
Definition of the service volume of the physician refers to the numbers of oesophageal cancer patients treated by the physician of each studied patient in the year when the patient received treatment for oesophageal cancer. For subsequent analyses, the service volume was divided by quartiles into the following three levels: low (lower than 25%), medium (25–75%), and high (higher than 75%).
Main outcome measurements
The dependent variable, namely whether patients with oesophageal cancer survived or not, was determined by obtaining the dates of death of patients from the Cause of Death Data from January 2010 to December 2016. With relevant variables controlled for, adjusted survival curves for patients with and without an MDT were generated.
Statistical analyses
This retrospective and longitudinal cohort study used SAS 9.4 for data organisation and statistical analyses. A Chi-square test was performed to determine whether oesophageal patients adopting or not adopting MDT care were statistically different in terms of the following variables: demographic characteristics (age and sex), socioeconomic factor (monthly salary), environmental factor (urbanization level), health status (comorbidities and other catastrophic illnesses), cancer stage, treatment methods, and main hospital visited (hospital level and hospital ownership).
To minimize the selection bias between study subjects adopting and not adopting the MDT treatment strategy, propensity score matching was conducted at a 1:1 ratio. Logistic regression was executed to build a model. The dependent variable was whether patients adopted the MDT treatment strategy, and the independent variables were the demographic characteristics (age and sex), socioeconomic factor (monthly salary), environmental factor (urbanization level), health status (comorbidities and other catastrophic illnesses), cancer stage, treatment methods, and main hospital visited (hospital level and hospital ownership). Accordingly, propensity score matching method was performed to control for the effects of confounding factors on patients’ adoption of the MDT treatment strategy and thereby enhanced the consistency between patients adopting and not adopting the MDT treatment strategy. A Chi-square test was then performed to examine differences of different variables between those adopting and not adopting the MDT treatment strategy.
A conditional Cox proportional hazard model, with relevant variables controlled for, was conducted to determine the relative risk of survival of patients adopting and not adopting the MDT treatment strategy on a weekly basis. The analysis result was presented in HR and 95% CI. The dependant variable was whether a patient survived or not; the independent variable was whether a patient adopted the MDT treatment strategy; and the control variables were a patient’s demographic characteristics, socioeconomic factor, environmental factor, health status, and oesophageal cancer stage, treatment methods, as well as the features of the main hospital and physician visited. With relevant variables controlled for, adjusted survival curves were generated to present differences between the survival curves of patients who adopted and did not adopt the MDT treatment strategy.
Acknowledgements
We are grateful to Health Data Science Center, China Medical University Hospital for providing administrative, technical and funding support.
Author contributions
W.C.T., Y.C.H., S.Y.H., Y.S.T., and P.T.K. designed the study. Y.C.H., P.T.K., L.T.C., Y.S.T., and W.C.T. collected data. Y.C.H., P.T.K., S.Y.H., and W.C.T. were responsible for study conceptualization and developing the analytical plan. L.T.C. and P.T.K. analyzed data. Y.C.H. and Y.S.T. drafted the manuscript. Y.C.H., P.T.K., and W.C.T. revised manuscript. P.T.K. and W.C.T. obtained funding sources. All authors read and approved the final manuscript.
Funding
This study was supported by the grants (DMR-110-151) from China Medical University, Taiwan. None of the funding sources had any role or input into the design and conduct of the study or approval of the manuscript.
Data availability statement
Regarding the data availability, data were obtained from the National Health Insurance Research Database published by the Ministry of Health and Welfare, Taiwan. Due to legal restrictions imposed by the Taiwan government related to the Personal Information Protection Act, the database cannot be made publicly available. All researchers can apply for using the databases to conduct their studies. Requests for data can be sent as a formal proposal to the Health and Welfare Data Science Center of the Ministry of Health and Welfare (http://www.mohw.gov.tw/EN/Ministry/Index.aspx). Any raw data are not allowed to be brought out from the Health and Welfare Data Science Center. The restrictions prohibited the authors from making the minimal data set publicly available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pei-Tseng Kung and Wen-Chen Tsai.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Y, et al. Esophageal cancer in patients under 50: A SEER analysis. J. Thorac. Dis. 2018;10:2542–2550. doi: 10.21037/jtd.2018.05.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan C-C, et al. Effects of multidisciplinary team care on the survival of patients with different stages of non-small cell lung cancer: A national cohort study. PLoS ONE. 2015;10:e0126547. doi: 10.1371/journal.pone.0126547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt HM, et al. Thoracic multidisciplinary tumor board routinely impacts therapeutic plans in patients with lung and esophageal cancer: A prospective cohort study. Ann. Thorac. Surg. 2015;99:1719–1724. doi: 10.1016/j.athoracsur.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Richardson B, et al. The effect of multidisciplinary teams for rectal cancer on delivery of care and patient outcome: Has the use of multidisciplinary teams for rectal cancer affected the utilization of available resources, proportion of patients meeting the standard of care, and does this translate into changes in patient outcome? Am. J. Surg. 2016;211:46–52. doi: 10.1016/j.amjsurg.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: Are they effective in the UK? Lancet Oncol. 2006;7:935–943. doi: 10.1016/S1470-2045(06)70940-8. [DOI] [PubMed] [Google Scholar]
- 9.Hong NJ, Wright FC, Gagliardi AR, Paszat LF. Examining the potential relationship between multidisciplinary cancer care and patient survival: an international literature review. J. Surg. Oncol. 2010;102:125–134. doi: 10.1002/jso.21589. [DOI] [PubMed] [Google Scholar]
- 10.Stephens MR, et al. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis. Esophagus. 2006;19:164–171. doi: 10.1111/j.1442-2050.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsai WC, Kung PT, Wang ST, Huang KH, Liu SA. Beneficial impact of multidisciplinary team management on the survival in different stages of oral cavity cancer patients: Results of a nationwide cohort study in Taiwan. Oral Oncol. 2015;51:105–111. doi: 10.1016/j.oraloncology.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Y, et al. The efficiency of electronic list-based multidisciplinary team meetings in management of gastrointestinal malignancy: A single-center experience in Southern China. World J. Surg. Oncol. 2018;16:146. doi: 10.1186/s12957-018-1443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDermid E, et al. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis. Off. J. Assoc. Coloproctol. Great Britain Ireland. 2009;11:291–295. doi: 10.1111/j.1463-1318.2008.01580.x. [DOI] [PubMed] [Google Scholar]
- 14.Munro A, Brown M, Niblock P, Steele R, Carey F. Do Multidisciplinary Team (MDT) processes influence survival in patients with colorectal cancer? A population-based experience. BMC Cancer. 2015;15:686. doi: 10.1186/s12885-015-1683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton E, et al. Surgical management of recurrent ovarian cancer: The advantage of collaborative surgical management and a multidisciplinary approach. Gynecol. Oncol. 2011;120:29–32. doi: 10.1016/j.ygyno.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: Retrospective, comparative, interventional cohort study of 13 722 women. BMJ (Clin. Res. Ed.) 2012;344:e2718. doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boniface MM, et al. Multidisciplinary management for esophageal and gastric cancer. Cancer Manag. Res. 2016;8:39–44. doi: 10.2147/CMAR.S101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu YH, Kung PT, Wang ST, Fang CY, Tsai WC. Improved patient survivals with colorectal cancer under multidisciplinary team care: A nationwide cohort study of 25,766 patients in Taiwan. Health Policy (Amsterdam, Netherlands) 2016;120:674–681. doi: 10.1016/j.healthpol.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Liao CM, Kung PT, Wang YH, Tsai WC. Effects of multidisciplinary team on emergency care for colorectal cancer patients: A nationwide-matched cohort study. Medicine. 2017;96:e7092. doi: 10.1097/md.0000000000007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Ucar AE, et al. The beneficial effects of specialist thoracic surgery on the resection rate for non-small-cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 2004;46:227–232. doi: 10.1016/j.lungcan.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Lordan JT, Karanjia ND, Quiney N, Fawcett WJ, Worthington TR. A 10-year study of outcome following hepatic resection for colorectal liver metastases—The effect of evaluation in a multidisciplinary team setting. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2009;35:302–306. doi: 10.1016/j.ejso.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Taplin SH, et al. Reviewing Cancer Care Team Effectiveness. J. Oncol. Pract. 2015;11:239–246. doi: 10.1200/JOP.2014.003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajani JA, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019;17:855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 24.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 25.Lordick F, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016;27:v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 26.Monig S, et al. Early esophageal cancer: The significance of surgery, endoscopy, and chemoradiation. Ann. N. Y. Acad. Sci. 2018 doi: 10.1111/nyas.13955. [DOI] [PubMed] [Google Scholar]
- 27.Wang YH, et al. Effects of multidisciplinary care on the survival of patients with oral cavity cancer in Taiwan. Oral Oncol. 2012;48:803–810. doi: 10.1016/j.oraloncology.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Shylasree TS, et al. Survival in ovarian cancer in Wales: Prior to introduction of all Wales guidelines. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2006;16:1770–1776. doi: 10.1111/j.1525-1438.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 29.Boxer MM, Vinod SK, Shafiq J, Duggan KJ. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer. 2011;117:5112–5120. doi: 10.1002/cncr.26149. [DOI] [PubMed] [Google Scholar]
- 30.Chen MF, Yang YH, Lai CH, Chen PC, Chen WC. Outcome of patients with esophageal cancer: A nationwide analysis. Ann. Surg. Oncol. 2013;20:3023–3030. doi: 10.1245/s10434-013-2935-4. [DOI] [PubMed] [Google Scholar]
- 31.Byers TE, et al. The impact of socioeconomic status on survival after cancer in the United States. Cancer. 2008;113:582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 32.Wu CC, et al. The effect of individual and neighborhood socioeconomic status on esophageal cancer survival in working-age patients in Taiwan. Medicine. 2016;95:e4140. doi: 10.1097/md.0000000000004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Edge SB, et al. AJCC cancer staging manual. 7. Berlin: Springer; 2010. [Google Scholar]
- 35.Cheng YF, et al. Esophageal squamous cell carcinoma and prognosis in Taiwan. Cancer Med. 2018 doi: 10.1002/cam4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birkmeyer JD, et al. Surgeon volume and operative mortality in the United States. N. Engl. J. Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 37.Lee CC, Ho HC, Chou P. Multivariate analyses to assess the effect of surgeon volume on survival rate in oral cancer: A nationwide population-based study in Taiwan. Oral Oncol. 2010;46:271–275. doi: 10.1016/j.oraloncology.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Headrick JR, et al. High-grade esophageal dysplasia: Long-term survival and quality of life after esophagectomy. Ann. Thorac. Surg. 2002;73:1697–1702. doi: 10.1016/S0003-4975(02)03496-3. [DOI] [PubMed] [Google Scholar]
- 39.Liu CY, et al. Incorporating development stratification of TAIWAN townships into sampling design of large scale health interview survey. J. Health Manag. 2006;4:1–22. doi: 10.29805/JHM.200606.0001. [DOI] [Google Scholar]
- 40.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Regarding the data availability, data were obtained from the National Health Insurance Research Database published by the Ministry of Health and Welfare, Taiwan. Due to legal restrictions imposed by the Taiwan government related to the Personal Information Protection Act, the database cannot be made publicly available. All researchers can apply for using the databases to conduct their studies. Requests for data can be sent as a formal proposal to the Health and Welfare Data Science Center of the Ministry of Health and Welfare (http://www.mohw.gov.tw/EN/Ministry/Index.aspx). Any raw data are not allowed to be brought out from the Health and Welfare Data Science Center. The restrictions prohibited the authors from making the minimal data set publicly available.