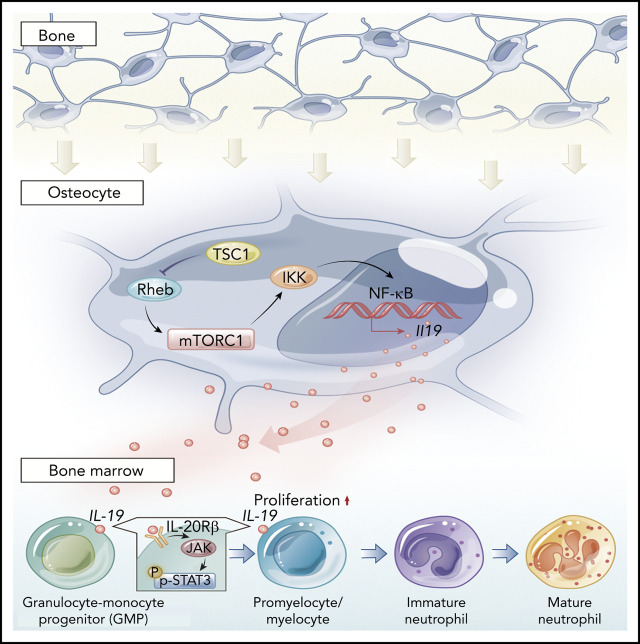
There is a Blood Commentary on this article in this issue.
Key Points
IL-19 produced by osteocytes stimulated granulopoiesis and neutrophil formation.
IL-19 is a promising therapeutic agent for the treatment of neutropenia.
Abstract
Osteocytes are the most abundant (90% to 95%) cells in bone and have emerged as an important regulator of hematopoiesis, but their role in neutrophil development and the underlying mechanisms remain unclear. Interleukin 19 (IL-19) produced predominantly by osteocytes stimulated granulopoiesis and neutrophil formation, which stimulated IL-19 receptor (IL-20Rβ)/Stat3 signaling in neutrophil progenitors to promote their expansion and neutrophil formation. Mice with constitutive activation of mechanistic target of rapamycin complex (mTORC1) signaling in osteocytes (Dmp1-Cre) exhibited a dramatic increase in IL-19 production and promyelocyte/myelocytic expansion, whereas mTORC1 inactivation in osteocytes reduced IL-19 production and neutrophil numbers in mice. We showed that IL-19 administration stimulated neutrophil development, whereas neutralizing endogenous IL-19 or depletion of its receptor inhibited the process. Importantly, low-dose IL-19 reversed chemotherapy, irradiation, or chloramphenicol-induced neutropenia in mice more efficiently than granulocyte colony-stimulating factor. This evidence indicated that IL-19 was an essential regulator of neutrophil development and a potent cytokine for neutropenia treatment.
Visual Abstract
Introduction
Neutrophils are the most abundant cell type among circulating white blood cells (60% to 70%) and immune cells in humans.1,2 They are produced in the bone marrow (BM) from hematopoietic stem cells (HSCs) that proliferate and differentiate into mature neutrophils, which are fully equipped with an armory of granules.3,4 These granules contain proteases that enable them to deliver lethal hits against invading microorganisms and form the first line of defense against invading pathogens, such as bacteria and fungi, playing a critical role in initiating inflammation and innate immunity.5,6 Patients with congenital neutrophil deficiencies or iatrogenic forms of neutropenia such as chemotherapy-induced neutropenia (CIN) suffer from severe infections that are often fatal, underscoring the importance of these cells in immune defense.7,8 Furthermore, they have a short half-life and an estimated 107 neutrophils in mice and 1011 in humans are produced each day.6,9 Therefore, the means to control the development, homeostasis, and functional activities of neutrophils is essential to mounting robust host defense responses.
Contextual signals from multiple cell types in the BM microenvironment or niche being critical for hematopoiesis and disease development is well established.10-13 The osteoblast was the first identified niche-supporting cell that produces many cytokines essential for normal hematopoiesis.13,14 Osteocytes are the most abundant (90% to 95%) and have the longest lifespan, yet are the least understood cells in bone.15 These matrix-embedded cells have emerged as master regulators of bone homeostasis via integrating mechanical signals and secreting sclerostin and receptor activator of NF-κB ligand to control osteoblast and osteoclast formation, respectively.16-19 Recent findings have suggested that osteocytes may control the hematopoietic microenvironment and contribute to hematopoiesis and inflammatory bone loss.20-22 However, the exact role of osteocytes in neutrophil development and the underlying mechanisms are unclear. Understanding the biology of granulopoiesis niches may facilitate the development of novel therapies to stimulate neutrophil production and prevent neutropenia.
Granulocyte colony-stimulating factor (G-CSF) is the primary cytokine driving granulopoiesis and is essential for tuning the production of neutrophils to meet the increased need during infection.23-25 Although G-CSF is widely used clinically to reduce CIN,26,27 it is not absolutely required for granulocytopoiesis because G-CSF–null mice have ∼25% residual granulocytopoiesis and generate fully mature neutrophils,28 indicating that alternative factors released by cells residing in the BM might stimulate granulocytopoiesis. In this study, we showed that interleukin-19 (IL-19) produced predominantly by osteocytes promote neutrophil progenitor expansion via stimulation of IL-19 receptor (IL-20 receptor β chain [IL-20Rβ])/Stat3 signaling. Strikingly, low doses of IL-19 recovered neutrophils after chemotherapy, irradiation, or chloramphenicol-induced neutropenia much more efficiently than G-CSF. Our findings revealed an unexpected but essential role for osteocytes in the regulation of neutrophil development and suggest that IL-19 is a potent therapeutic agent for the treatment of neutropenia.
Materials and methods
General methodology
No samples, mice, or data points were excluded from the reported analyses. Samples were randomized to experimental groups in C57BL/6 mice model (neutropenia of mice induced by arabinoside, radiation and chloramphenicol), but not in TSC1- or Rheb-knockout (KO) mice. Analyses were not performed in a blinded fashion.
Clinical specimens
Serum samples were obtained from acute myelocytic leukemia patients of prechemotherapy and postchemotherapy stages. Informed written consent was obtained from all subjects. The study protocols concerning human subjects were consistent with the principles of the Declaration of Helsinki, and were approved by the Clinical Research Ethics Committee of Southern Medical University and Zhujiang Hospital of Southern Medical University.
Mice
Conditional KO mice were produced by interbreeding TSC1fl/fl or Rhebfl/fl mice with Dmp1-cre mice. TSC1fl/fl and Dmp1-cre mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Rhebfl/fl mice were a generous gift from Bo Xiao (Sichuan University, Sichuan, China). TSC1fl/fl or Rhebfl/fl littermates were used as controls for all experiments. Routine genotyping of tail DNA was performed according to The Jackson Laboratory’s instructions. Sex-matched littermate mice 12 weeks of age (half male and half female) were used for experiments unless otherwise noted. Two-month-old C57BL/6 mice were purchased from the Laboratory Animal Centre of Southern Medical University (Guangzhou, China). All animals were provided with a standard diet and housed in pathogen-free cages at constant temperature and humidity. The circadian rhythm was maintained at 12 hours. The Southern Medical University Animal Care and Use Committee approved all procedures involving mice. All animal procedures involving animals and their care were conducted in accordance with the guidelines of Animal Use and Care of the National Institutes of Health.
Cells
Primary osteocytes were prepared from the long bones of mice as described previously.29 Primary osteoclasts were prepared from the BM cells of mice as described previously.30 Lymphocytes and monocytes were isolated from the BM of 8-week-old C57BL/6 mice using lymphocyte or monocyte Ficoll-Paque isolation (GE Healthcare, Little Chalfont, United Kingdom), respectively, and cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS). Endothelial cells were isolated from the BM of 8-week-old C57BL/6 mice using an Endothelium Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), and cultured in endothelial cell growth medium containing 10% FBS. NIH3T3 cells were cultured in Dulbecco modified Eagle medium containing 10% FBS.
Flow cytometry analysis
Cells were stained by standard protocols with the following antibodies (eBioscience, San Diego, CA, unless otherwise noted). BM cells were isolated as described previously,31 and cells were incubated with antibodies. For neutrophil development analysis, cells were stained with hematopoietic lineage, Sca-1, c-Kit, CD16/32, and CD34 (BD Biosciences, San Diego, CA). For cell-lineage analysis, cells were stained with Ter119, B220, and Sca-1. For neutrophil-proliferation analysis, cells were stained with 5-ethynyl-2′-deoxyuridine (Edu; Invitrogen, Carlsbad, CA) or CD11b and Gr-1. For IL-20Rβ level analysis, cells were stained for Ter119, Gr-1, F4/80, B220, and IL-20Rβ. For IL-20Rα (PA141167; Invitrogen) level analysis, cells were stained for Ter119, Gr-1, F4/80, B220, and IL-20Rα. For phosphorylated STAT3 (p-STAT3) level in neutrophil analysis, cells were stained with p-STAT3 and Gr-1. For lymphocyte analysis in spleen, cells were stained for CD19 and CD3e.
In vivo treatments
Recombinant murine IL-19 (R&D Systems, Minneapolis, MN) was reconstituted in 1× phosphate-buffered saline and injected intraperitoneally (IP) at a dose of 25 µg/kg per day into 12-week-old Dmp1-Rheb KO and control mice daily for 14 days. To block IL-19 (or IL-10) in vivo, 50 µg of purified IL-19 (or 50 µg of purified IL-10) antibody (GeneTex, Irvine, CA for IL-19; R&D Systems for IL-10) was injected into the marrow cavity of Dmp1-TSC1 KO mice and wild-type littermates by bilateral intratibial injection. Mice were euthanized after 14 days of treatment and the BM was collected for flow cytometry analysis.
Collection of conditioned medium from osteoclast, BMSC, lymphocyte, monocyte, and endothelial cell cultures
BM stem cells (BMSCs) were isolated as respectively previously described.32 Osteoclasts, lymphocytes, monocytes, and endothelial cells were cultured as above. Conditioned medium was collected when cells reached 80% to 90% confluence.
Coculture of mouse BM cells with conditioned medium from osteoclasts, BMSCs, lymphocytes, monocytes, and endothelial cells
C57BL/6 mouse total BM cells were plated in 6-well plates containing Dulbecco modified Eagle medium:F-12 with 10% FBS. Cocultures were cultured with fresh medium and osteoclast, lymphocyte, BMSC, monocyte, or endothelial cell culture conditioned medium (1:2); or with TSC1- or Rheb-null osteocyte culture conditioned medium (1:2) with or without recombinant murine IL-19 (50 ng/mL) or purified IL-19 antibody (50 μg/mL; GeneTex) at days 3 and 7. Cells were harvested on day 10. All samples were analyzed by flow cytometry.
In vivo siRNA knockdown of IL-20Rβ
The small interfering RNA (siRNA) against IL-20Rβ and a control scrambled siRNA were purchased from Santa Cruz Biotechnology. IL-20Rβ or negative control (NC) siRNAs were injected in the bilateral medullary cavity of 12-week-old Dmp1-TSC1 KO and wild-type mice (n = 10). Entranster in vivo RNA transfection reagent (Engreen Biosystem, Auckland, New Zealand) was used as a vehicle for siRNA delivery according to the manufacturer’s instructions. Six days after injection, mice were euthanized and BM was collected for flow cytometry analysis.
CIN mouse model and treatment
Two-month-old C57BL/6 female mice were used for experiments. Cytarabine (Sigma-Aldrich, St Louis, MO) was reconstituted with sterile phosphate-buffered saline and injected IP from day 1 to day 8 at a dose of 100 mg/kg to induce neutropenia. Recombinant murine IL-19 (25 µg/kg) and G-CSF (100 µg/kg; PeproTech, Rocky Hill, NJ) were twice-daily injected IP from day 2 to day 8.
Irradiation-induced neutropenia mouse model and treatment
Two-month-old C57BL/6 female mice received 15 exposures of 0.3 Gy per day whole-body irradiation from day 1 to day 15 to induce neutropenia. Recombinant murine IL-19 (25 µg/kg) and G-CSF (100 µg/kg) were injected IP from day 2 to day 15 once a day.
Chloramphenicol-induced neutropenia mouse model and treatment
Two-month-old C57BL/6 female mice were used for experiments. Chloramphenicol (Selleckchem, Houston, TX) was injected IP from day 1 to day 21 daily at a dose of 200 mg/kg to induce neutropenia. Recombinant murine IL-19 (25 µg/kg) and G-CSF (100 µg/kg) were injected IP from day 7 to day 21 once a day.
RNA sequencing and messenger RNA analysis
Complementary DNA (cDNA) libraries were constructed with osteocytes from Dmp1-TSC1 KO and control mice, and total RNA was used as the input material for a cDNA library. After total RNA was extracted, and the enriched RNAs were fragmented into short fragments and reverse transcribed into cDNAs, double-stranded cDNAs were synthesized by replacing deoxythymidine triphosphate with deoxyuridine triphosphate in the reaction buffer. Then uracil-N-glycosylase was used to digest the second-strand cDNAs. The products were selected, polymerase chain reaction (PCR) amplified, and sequenced by Gene Denovo Biotechnology Co (Guangzhou, China) with an Illumina HiSeq 4000 instrument.
The sequencing raw reads were obtained after removing of reads containing adapters, reads containing ploy-N, and low-quality reads from raw data. The clean reads of samples were then mapped by Tophat2 (version 2.1.1). Known and novel transcripts from the TopHat alignment results were identified with the Annotation Based Transcript (RABT) assembly in Cufflinks v2.1.1. The levels of transcript expression were normalized by using the fragments per kilobase of transcript per million mapped reads method. Messenger RNAs (mRNAs) with a P value <.05 and fold change ≥2 were then identified as significant differentially expressed genes (DEGs) by using the edgeR package.
Functional enrichment analysis
All DEGs and target genes of differentially expressed mRNAs were classified with Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis by using OmicShare tools (https://www.omicshare.com/tools/). P values <.05 in the results were considered significantly enriched.
Statistical analysis
All experiments were carried out in triplicate. Multiple comparisons were assessed by 1-way analysis of variance; otherwise, the unpaired Student t test was used for statistical analysis. Results were considered significant at *P < .05, **P < .01, and ***P < .001.
Results
Osteocyte mTORC1 regulates neutrophil formation in mice
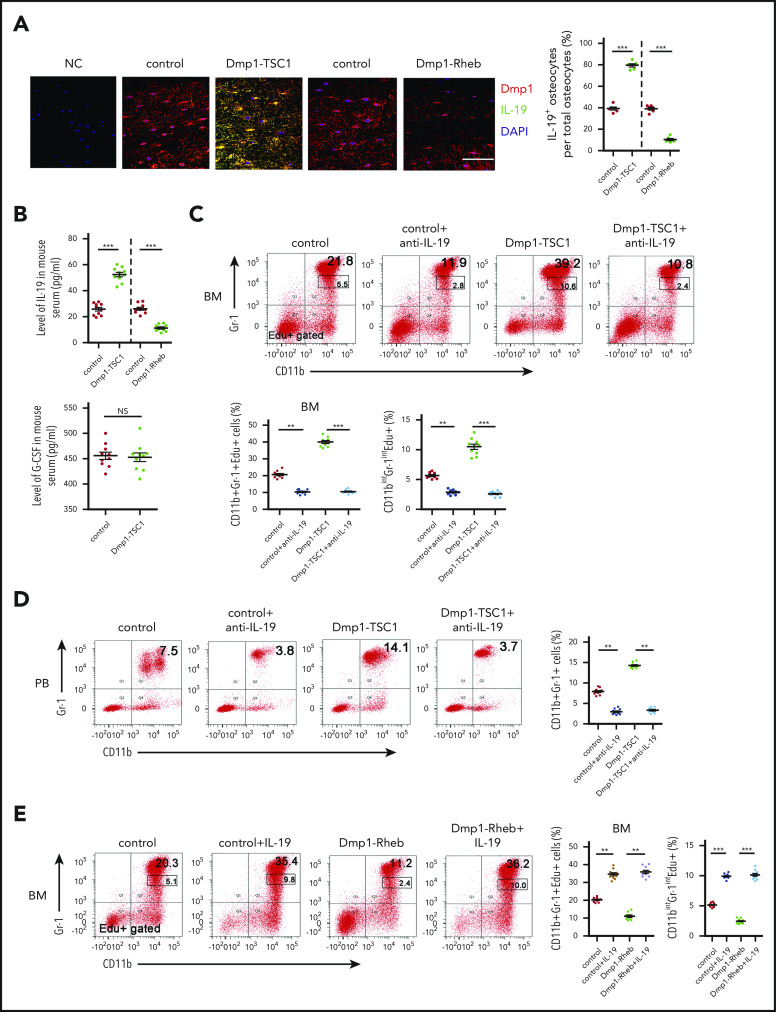
Mechanistic target of rapamycin complex 1 (mTORC1) is a master regulator of cellular growth, proliferation, and metabolism in response to nutrients, growth factors, and stresses.33,34 We generated mice with mTORC1 activation in osteocytes by crossing Tsc1 (mTORC1 inhibitor)-loxP mice with Dmp1-Cre mice (Dmp1-TSC1). These mice exhibited a hyperactivation of mTORC1 in osteocytes, as evidenced by increased phosphorylation of S6 (S235/236) (Figure 1A-C), and increases in bone mass, cancellous bone, and cortical bone thickness (supplemental Figure 1A-B; supplemental Table 1; available on the Blood Web site). Interestingly, a routine blood examination revealed a dramatic increase in neutrophils in Dmp1-TSC1 mice compared with their littermate controls (Figure 1D), but the spleens and the numbers of red blood cells and platelets were unaffected (supplemental Figure 2; supplemental Table 2). Fluorescent-activated cell sorting (FACS) analysis indicated that the number of CD11b+Gr-1+ granulocytic cells (mainly neutrophils) was increased markedly in the peripheral blood (PB) and BM of Dmp1-TSC1 mice (Figure 1E). In contrast, no significant differences in monocytes, lymphocytes, and erythroid lineages were observed between Dmp1-TSC1 and control mice (supplemental Table 2). These findings suggested that mTORC1 activation in osteocytes specifically stimulated granulocytopoiesis and increases neutrophil numbers in mice.
Figure 1.
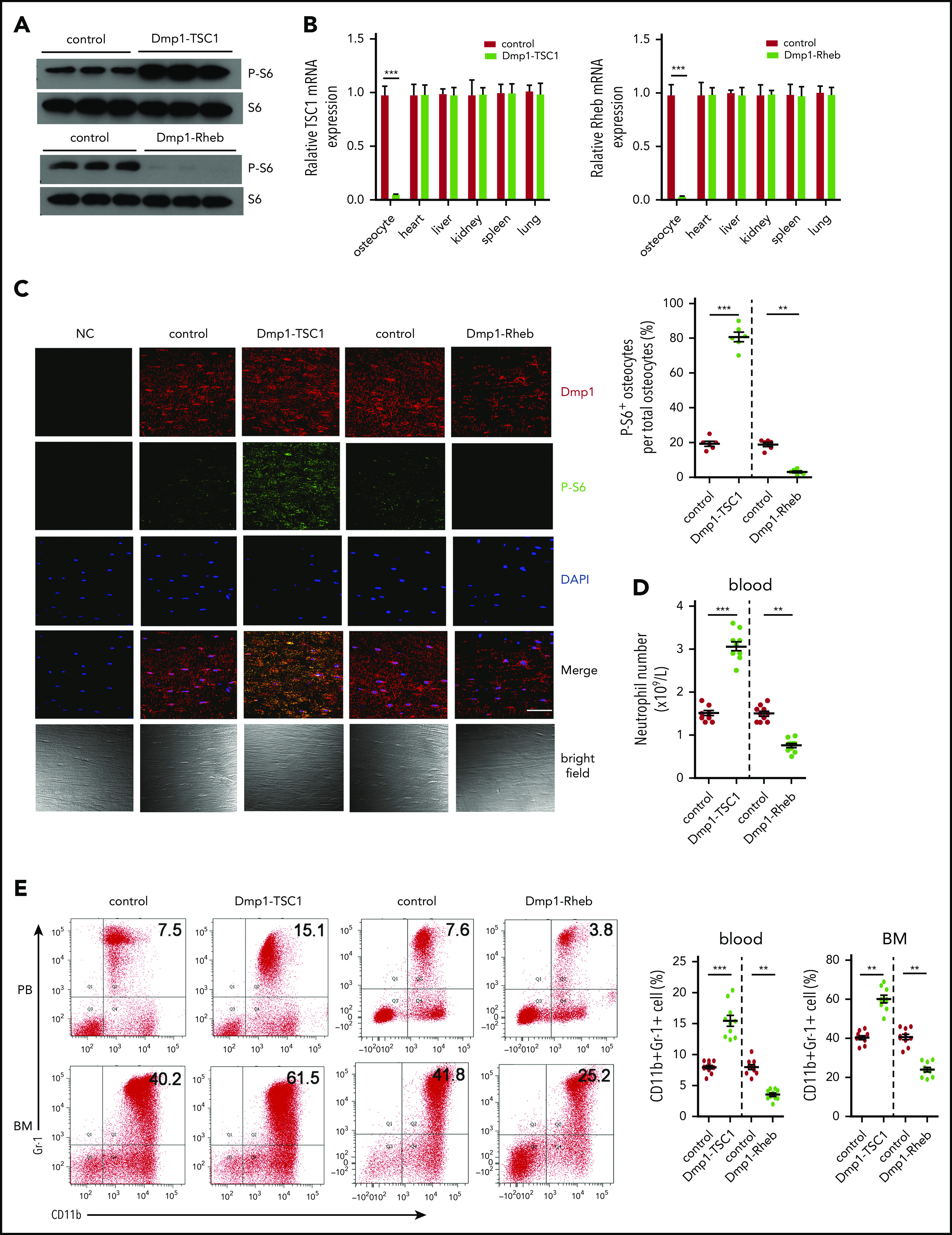
Osteocyte mTORC1 in mice regulates granulopoiesis. (A) The level of P-S6 (S235/236) in primary osteocytes isolated from 12-week-old Dmp1-TSC1, Dmp1-Rheb, and control mice was detected by western blotting. (B) The TSC1 and Rheb mRNA expression in osteocytes, heart, liver, kidney, spleen, and lung tissues from 12-week-old Dmp1-TSC1, Dmp1-Rheb, and control mice was detected by qPCR. (C) Immunofluorescence microscopy of femur sections of 12-week-old Dmp1-TSC1, Dmp1-Rheb, and control mice. Cells were stained with anti-Dmp1 (red) and anti–P-S6 (green). Blue, nuclei visualized by 4′,6-diamidino-2-phenylindole (DAPI) stain. Scale bar, 50 µm. (D) The total number of PB neutrophils in 12-week-old Dmp1-TSC1, Dmp1-Rheb, and control mice (mean ± standard deviation [SD]; n = 10). (E) FACS analysis of BM and PB CD11b+Gr-1+ neutrophils in 12-week-old Dmp1-TSC1, Dmp1-Rheb, and control mice (mean ± SD; n = 10). Data are mean ± SD of 3 independent experiments. **P < .01; ***P < .001.
We further conditionally inactivated mTORC1 in osteocytes by crossing Rheb (mTORC1 upstream activator)-loxP mice with Dmp1-Cre mice (Dmp1-Rheb). These mice exhibited mTORC1 inactivation in osteocytes and osteoporosis (Figure 1A-C; supplemental Figure 1C-D; supplemental Table 3). Significantly, Dmp1-Rheb mice showed a decrease in neutrophils (Figure 1D-E), whereas no significant differences were observed in the development of monocytes, lymphocytes, and erythroid lineages between Dmp1-Rheb and control mice (supplemental Table 2). These results demonstrated that mTORC1 inhibition in osteocytes reduced neutrophils in mice BM.
Osteocytes stimulate neutrophil progenitor proliferation in vitro and in vivo
During the process of granulocytopoiesis, a common myeloid progenitor (CMP) arises from HSC development into megakaryocyte and erythrocyte progenitors (MEPs) and granulocyte–monocyte progenitor cells (GMPs), which give rise to either a monocyte (monoblast) or a granulocyte precursor cell myeloblast. Myeloblasts differentiate to promyelocytes (PMs) and subsequently myelocytes (MCs) at which point proliferation ceases. After maturation, they are released to the PB as mature polymorphonuclear granulocytes.3,4 We found that the percentage of BM GMPs was increased in Dmp1-TSC1 mice (Figure 2A), whereas it was decreased in Dmp1-Rheb mice compared with their littermate controls (Figure 2B). In contrast, CMPs, MEPs, and HSCs (Lin−Sca-1+c-Kit+ [LSK]) remained unchanged in both Dmp1-TSC1 and Dmp1-Rheb mice (Figure 2A-D). These findings suggested that osteocyte mTORC1 may affect granulocytopoiesis by regulating the expansion of GMPs and neutrophil progenitors. The Edu staining of BM cells confirmed that the proliferation of PMs/MCs (CD11bintGr-1int) was increased markedly in Dmp1-TSC1 but was impaired in Dmp1-Rheb mice (Figure 2E-F). This evidence demonstrated that osteocyte mTORC1 stimulated the expansion of neutrophil lineages (CD11b+Gr-1+ cells) in mice.
Figure 2.
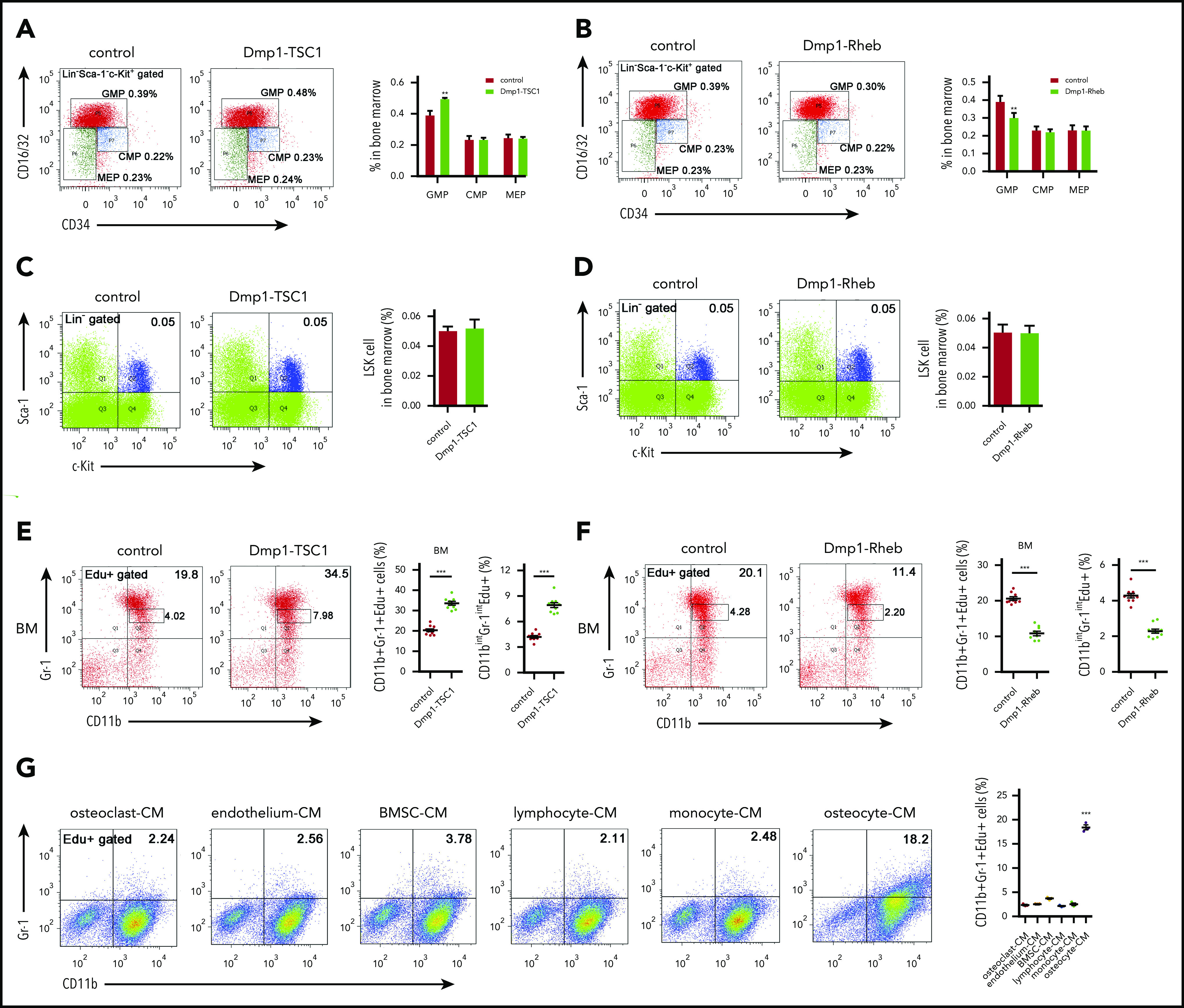
Osteocytes stimulate neutrophil development in vitro and in vivo. (A) FACS analysis of myeloid progenitor cell populations of BM in 12-week-old Dmp1-TSC1 and control mice (mean ± SD; n = 10). (B) FACS analysis of myeloid progenitor cell populations of BM in 12-week-old Dmp1-Rheb and control mice (mean ± SD; n = 10). (C) FACS analysis of LSK cells of BM in 12-week-old Dmp1-TSC1 and control mice (mean ± SD; n = 10). (D) FACS analysis of LSK cells of BM in 12-week-old Dmp1-Rheb and control mice (mean ± SD; n = 10). (E) FACS analysis of CD11b+Gr-1+ and CD11bintGr-1int (PM/MC neutrophil) cell proliferation in the BM of 12-week-old Dmp1-TSC1 and control mice (mean ± SD; n = 10). (F) FACS analysis of CD11b+Gr-1+ and CD11bintGr-1int cell proliferation in the BM of 12-week-old Dmp1-Rheb and control mice (mean ± SD; n = 10). (G) BM cells from 12-week-old C57BL/6 mice were cultured in conditioned medium (CM) from osteoclasts, osteocytes, monocytes, BMSCs, lymphocytes, or endothelial cells for 10 days. FACS analysis of CD11b+Gr-1+ cell proliferation (mean ± SD; n = 3). Data are mean ± SD of 3 independent experiments. **P < .01; ***P < .001.
To determine whether osteocytes regulate CD11b+Gr-1+ cell proliferation in vitro, BM cells were cultured in conditional medium from primary osteocyte, osteoclast, endothelial cell, BMSC, lymphocyte, and monocyte culture supernatants. Only osteocytes exhibited a great capacity to stimulate CD11b+Gr1+ cell proliferation in BM culture (Figure 2G; supplemental Figure 3). According to a previous report,35 and because osteocytes develop from osteoblasts and osteoblast precursor cells, we also tested the neutrophils of OCN-TSC1, OSX-TSC1 KO, and control mice. We observed no changes in neutrophils of OCN-TSC1 and OSX-TSC1 KO compared with those of control mice (supplemental Figure 4). Furthermore, conditional medium from osteocytes of Dmp1-TSC1 mice markedly elevated CD11b+Gr1+ cell number in BM culture (supplemental Figure 5). These results suggested that osteocytes may modulate neutrophil progenitor proliferation both in vivo and in vitro.
Osteocytes regulate the expansion of neutrophil cell lineages via secretion of IL-19
The findings herein suggested that osteocyte mTORC1 might stimulate neutrophil formation in a paracrine manner. To screen the contribution of osteocyte-derived signaling molecules to neutrophil development, a global mRNA expression profile in TSC1-deficient or control osteocytes was developed by RNA sequencing (National Center for Biotechnology Information Sequence Read Archive [NCBI SRA: PRJNA590045]). The analysis of DEG transcript levels revealed a significant difference in Dmp1-TSC1 KO and control mice osteocytes. There were 2017 significant DEGs, including 471 upregulated and 1546 downregulated mRNAs in the Dmp1-TSC1 KO compared with the control mice. To elucidate the functional roles of DEGs, we performed KEGG pathway enrichment analysis for the DEGs. The pathway enrichment revealed DEGs involved in hematopoietic cell lineage, extracellular matrix–receptor interaction, and cytokine-cytokine receptor interaction (supplemental Figure 6). Unexpectedly, levels of G-CSF and granulocyte macrophage–colony-stimulating factor were unaltered in TSC1-deficient osteocytes (supplemental Table 4). However, IL-19, a cytokine with diverse but poorly defined biological functions,36,37 was elevated 150-fold in TSC1-null osteocytes. Many different cell types, such as keratinocytes, epithelial cells, fibroblasts, monocytes/macrophages, and B cells, have been reported to secrete IL-19.36,38 Surprisingly, quantitative PCR (qPCR) analysis of IL-19 mRNA from cells residing in the BM revealed that osteocytes produced more IL-19 mRNA than osteoclasts, endothelial cells, BMSCs, and lymphocytes (supplemental Figure 7A). mTORC1 activation strongly stimulated, whereas mTORC1 inhibition impaired, IL-19 expression in TSC1- or Rheb-null osteocytes, respectively (supplemental Figure 7B,D). Although monocytes produced some IL-19, there was a difference in IL-19 mRNA in Dmp1-TSC1 and Dmp1-Rheb KO mice compared with control mice (supplemental Figure 7C). The level of IL-19 in conditional medium from osteocytes, but not osteoblasts, was greater in OSX-TSC1 and OCN-TSC1 mice than in control mice (supplemental Figure 7E-F). IL-19 and Dmp1 double staining confirmed that IL-19 was predominantly expressed by osteocytes and regulated by mTORC1 in bone (Figure 3A; supplemental Figure 8). Consistently, serum IL-19, but not G-CSF, was elevated in Dmp1-TSC1 mice (Figure 3B). Although IL-10 was elevated 811-fold in TSC1-null osteocytes (supplemental Figure 6; supplemental Table 4), injection of IL-10 neutralization antibody did not rescue the neutrophil phenotype of Dmp1-TSC1 KO mice, thus indicating that IL-10 was not responsible for the increased neutrophils in Dmp1-TSC1 mice (supplemental Figure 9). Considering that bone is the largest organ in the human body and osteocytes are the most abundant (90% to 95%) and have the longest lifespan in bone,15 our results suggested that osteocytes were the predominant source of IL-19 in the BM and produced IL-19 in an mTORC1-dependent manner.
Figure 3.
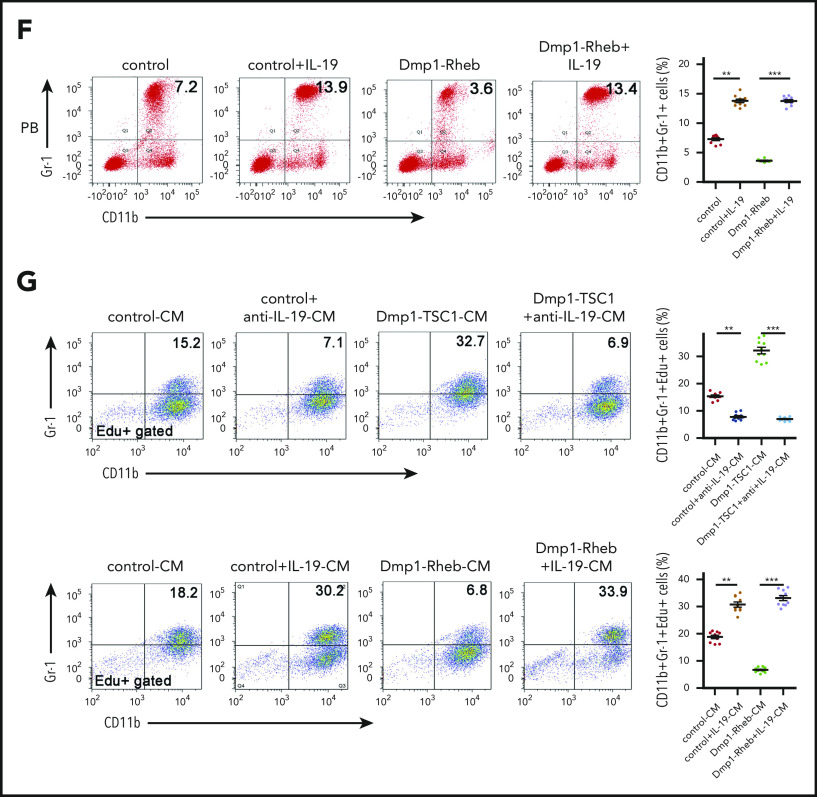
IL-19 secreted predominantly by osteocytes regulates granulopoiesis. (A) Immunofluorescence microscopy of femur sections of 12-week-old Dmp1-TSC1, Dmp1-Rheb, and control mice. Cells were stained with anti-Dmp1 (red) and anti–IL-19 (green). Blue, nuclei visualized by DAPI stain. Scale bar, 50 µm. (B) Level of IL-19 or G-CSF in serum of 12-week-old Dmp1-TSC1, Dmp1-Rheb, and control mice was detected by enzyme-linked immunosorbent assay (ELISA) (mean ± SD; n = 10). (C) FACS analysis of CD11b+Gr-1+ and CD11bintGr-1int cell proliferation in the BM of 12-week-old Dmp1-TSC1 and control mice with bilateral intratibial injection into the marrow cavity with purified IL-19 antibody (25 μg/kg per day) for 14 days (mean ± SD; n = 10). (D) FACS analysis of PB CD11b+Gr-1+ neutrophils in purified IL-19 antibody–treated 12-week-old mice (mean ± SD; n = 10). (E) FACS analysis of CD11b+Gr-1+ and CD11bintGr-1int cell proliferation in the BM of 12-week-old Dmp1-Rheb and control mice IP injected with recombinant murine IL-19 (25 µg/kg per day) for 14 days (mean ± SD; n = 10). (F) FACS analysis of PB CD11b+Gr-1+ neutrophils in recombinant IL-19–treated 12-week-old mice (mean ± SD; n = 10). (G) FACS analysis of the proliferation of CD11b+Gr-1+ cells cultured in CM from 12-week-old Rheb- or TSC1-loss osteocyte cultures treated with IL-19 or IL-19 antibody (mean ± SD; n = 10). Data are mean ± SD of 3 independent experiments. **P < .01; ***P < .001. NS, not significant.
To define the role of IL-19 in neutrophil formation in vivo, we injected recombinant mouse IL-19 or antibodies against IL-19 into mouse BM for 2 weeks. IL-19 neutralization antibody reduced the CD11b+Gr-1+ cell number and PM/MC (CD11bintGr-1int) cell proliferation in Dmp1-TSC1 mice (Figure 3C-D). In contrast, IL-19 increased the number of CD11b+Gr-1+ cells and restored PM/MC (CD11bintGr-1int) cell proliferation in Dmp1-Rheb mice (Figure 3E-F). Importantly, addition of IL-19 antibody to BM cultures abolished the stimulation of CD11b+Gr-1+ cell proliferation (CD11b+Gr-1+Edu+) by TSC1-null osteocyte-conditioned medium (Figure 3G). Incubation of BM cultures with IL-19 restored the ability of Rheb-null osteocyte-conditioned medium to stimulate CD11b+Gr-1+ cell proliferation (Figure 3G). These findings indicated that osteocytes stimulated CD11b+Gr-1+ cell expansion and neutrophil formation via production of IL-19 both in vitro and in vivo.
IL-19 promotes neutrophil progenitor expansion via IL-20Rβ/STAT3 signaling
IL-19 can signal through receptor complexes containing IL-20Rβ and the IL-20 receptor α chain (IL-20Rα) and activate the JAK and STAT pathway, particularly STAT3.36,37 Consistent with the effects of IL-19 on hematopoietic cells, neutrophil lineages including PMs/MCs (CD11bintGr-1int), immature neutrophils (CD11blowGr-1high), and mature neutrophils (CD11bhighGr-1high)39 expressed high levels of IL-20Rβ (Figure 4A-B). Furthermore, GMP expressed 6.4- and 3.0-fold more IL-20Rβ than CMP and MEP, respectively. This IL-19 receptor expression pattern is consistent with the effect of IL-19 on these progenitor types (supplemental Figure 10). As previously reported,40,41 IL-20Rα was nearly undetectable in all examined hematopoietic cells (Figure 4C-D). Furthermore, p-STAT3 and Gr-1 double staining and FACS analysis showed that STAT3 phosphorylation (Figure 4E-F; supplemental Figure 11) was increased in Gr-1+ cells from Dmp1-TSC1 mouse BM, whereas the level was lower in Dmp1-Rheb mouse BM Gr-1+ cells (Figure 4E,G; supplemental Figure 11). IL-19 treatment increased STAT3 phosphorylation in BM Gr-1+ cells (Figure 4H-I; supplemental Figure 12). Importantly, injection of IL-20Rβ siRNAs into mouse BM suppressed IL-20Rβ expression and STAT3 phosphorylation in Gr-1+ cells (Figure 4J,L; supplemental Figure 13), and inhibited CD11b+Gr-1+ cell expansion in mice (Figure 4K). IL-20Rβ siRNA also abolished the increase in p-STAT3 and neutrophil formation in Dmp1-TSC1 mice (Figure 4M). These findings indicated that IL-19 activated IL-20Rβ/Stat3 signaling in neutrophil lineages to promote their expansion and neutrophil formation.
Figure 4.
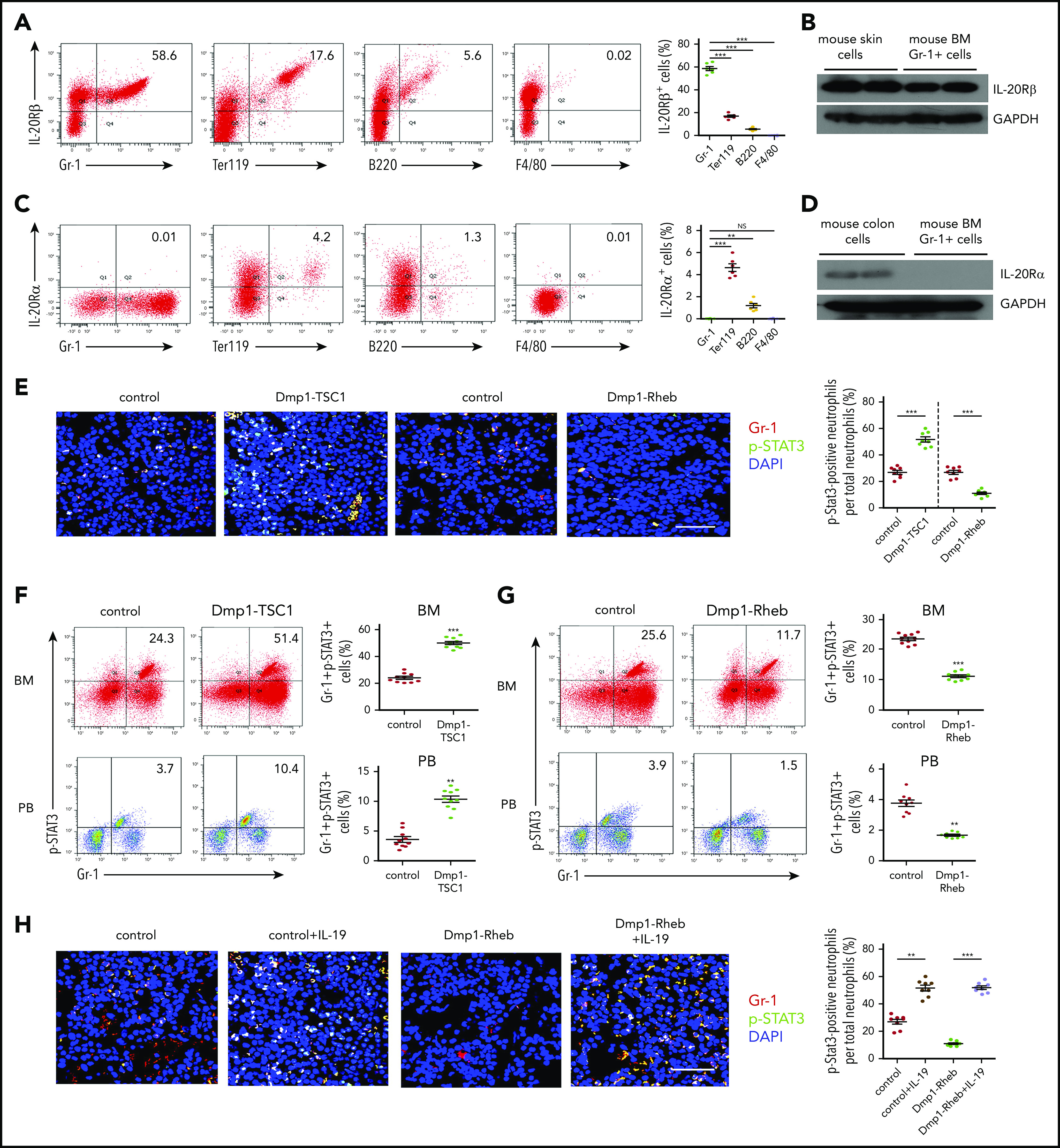
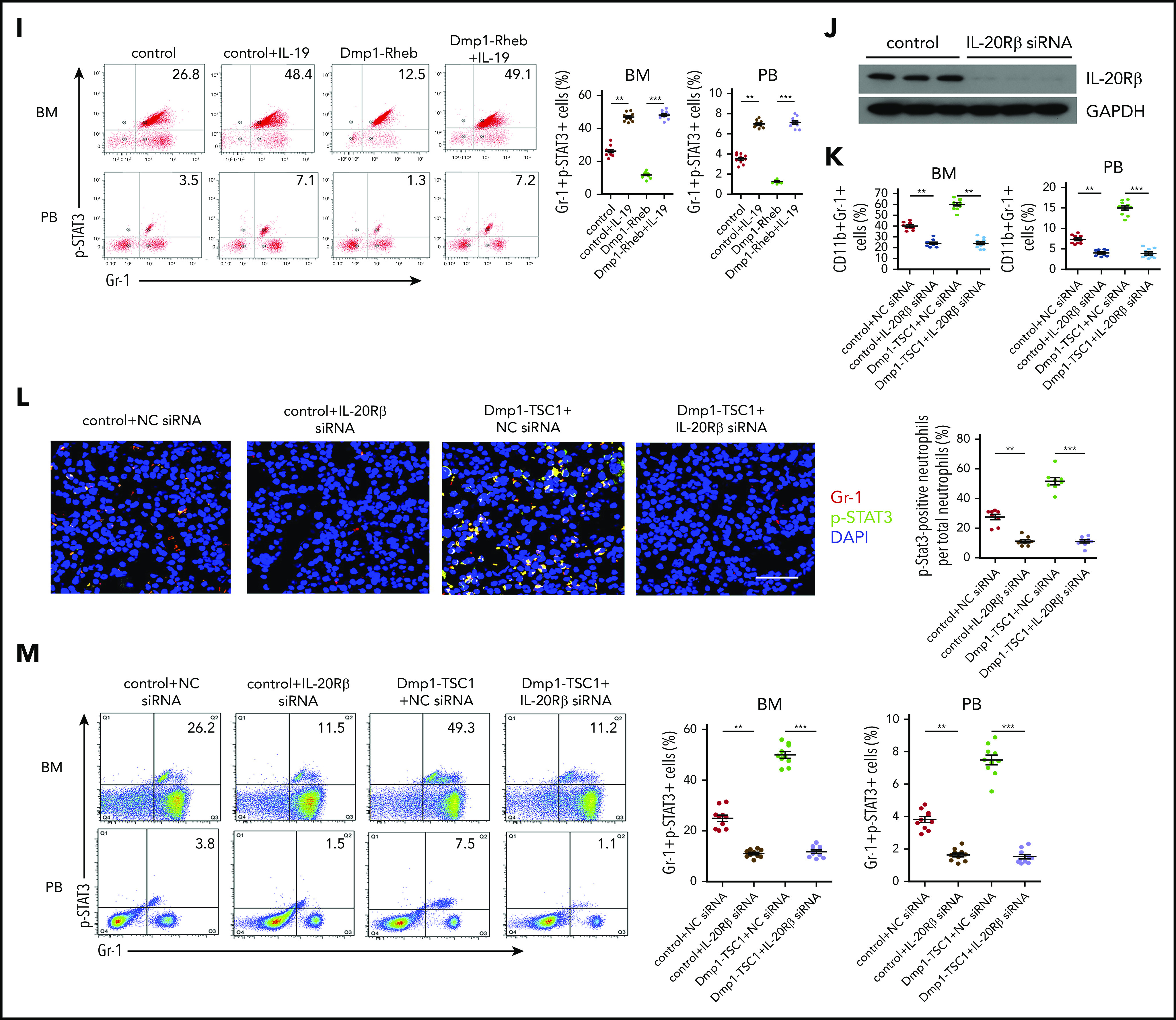
IL-19 promotes granulopoiesis through IL-20Rβ/STAT3 signaling. (A) FACS analysis of IL-20Rβ levels in Gr-1+, Ter119+, B220+, and F4/80+ cells in the BM of 12-week-old mice (mean ± SD; n = 6). (B) Expression levels of IL-20Rβ in skin cells and BM Gr-1+ cells in 12-week-old mice were detected by western blotting. (C) FACS analysis of IL-20Rα in Gr-1+, Ter119+, B220+, and F4/80+ cells in the BM of 12-week-old mice (mean ± SD; n = 6). (D) Expression levels of IL-20Rα in colon cells and BM Gr-1+ cells in 12-week-old mice were detected by western blotting. (E) Immunofluorescence microscopy of femur sections of 12-week-old Dmp1-TSC1, Dmp1-Rheb, and control mice. Cells were stained with anti–Gr-1 (red) and anti–p-STAT3 (green). Blue, nuclei visualized by DAPI stain. Scale bar, 30 µm. (F) FACS analysis of BM and PB Gr-1+p-STAT3+ cells in 12-week-old Dmp1-TSC1 and control mice (mean ± SD; n = 10). (G) FACS analysis of BM and PB Gr-1+p-STAT3+ cells in 12-week-old Dmp1-Rheb and control mice (mean ± SD; n = 10). (H) Immunofluorescence microscopy of femur sections of 12-week-old Dmp1-Rheb and control mice IP injected with recombinant murine IL-19 (25 µg/kg per day) for 14 days. Cells were stained with anti–Gr-1 (red) and anti–p-STAT3 (green). Blue, nuclei visualized by DAPI stain. Scale bar, 30 µm. (I) FACS analysis of BM and PB Gr-1+p-STAT3+ cells in recombinant IL-19–treated 12-week-old mice (mean ± SD; n = 10). (J) Dmp1-TSC1 and control mice with bilateral intratibial injection into the marrow cavity with IL-20Rβ or negative control (NC) siRNAs for 10 days. The efficiency of IL-20Rβ siRNAs for IL-20Rβ in BM cells was measured by western blotting. (K) FACS analysis of BM and PB CD11b+Gr-1+ cells in IL-20Rβ siRNA-treated 12-week-old mice (mean ± SD; n = 10). (L) Immunofluorescence microscopy of femur sections of 12-week-old Dmp1-TSC1 and control mice treated with IL-20Rβ or NC siRNAs. Cells were stained with anti–Gr-1 (red) and anti–p-STAT3 (green). Blue, nuclei visualized by DAPI stain. Scale bar, 30 µm. (M) FACS analysis of BM and PB Gr-1+p-STAT3+ cells in 12-week-old Dmp1-TSC1 and control mice treated with IL-20Rβ or NC siRNAs (mean ± SD; n = 10). Data are mean ± SD of 3 independent experiments. **P < .01; ***P < .001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
mTORC1 activates NF-κB signaling to induce IL-19 transcription in osteocytes
Transcription factor NF-κB was reported to regulate Il-19 gene transcription and be activated or inhibited by mTORC1 in different cell types.42,43 We next examined whether mTORC1 stimulates IL-19 expression through NF-κB. As expected, NF-κB activity was strongly activated in TSC1-null osteocytes with constitutive mTORC1 activation, as manifested by the markedly increased phosphorylation (S536) and nuclear localization of p65 NF-κB, accompanied by enhanced phosphorylation of IKK-β (S180/181) and S6 (S235/236) (Figure 5A-B). In contrast, NF-κB was inhibited in Rheb-null osteocytes in which mTORC1 was inhibited (Figure 5C-D). Importantly, chemical inhibition of NF-κB abolished the increased IL-19 mRNA in TSC1-null osteocytes (Figure 5E). Phosphatidic acid (PA), an mTORC1 activator, strongly stimulated IL-19 expression, which was blocked by the NF-κB inhibitor (Figure 5F). These results indicated that mTORC1 stimulated IL-19 expression through activation of NF-κB signaling.
Figure 5.
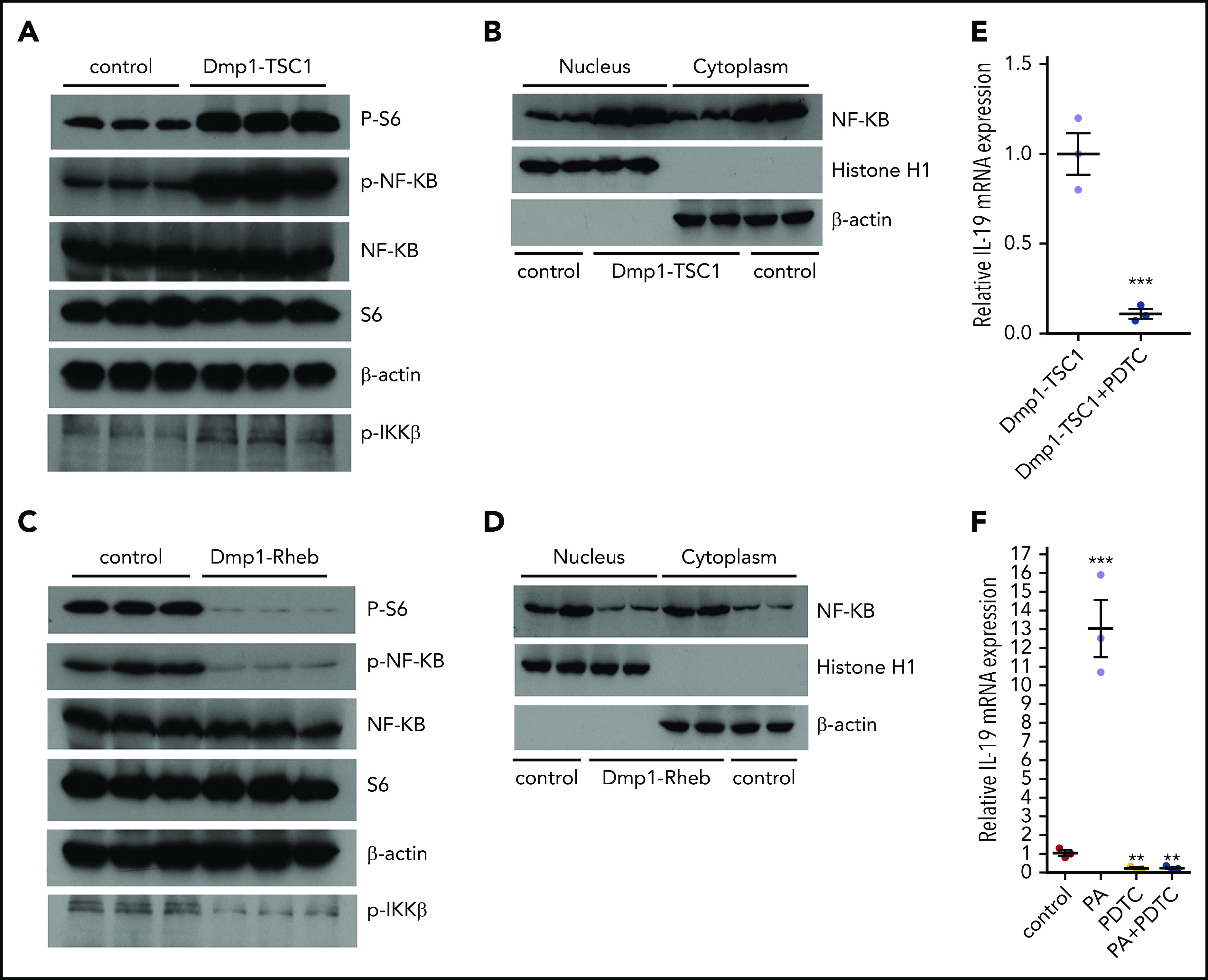
mTORC1 activates IL-19 transcription by promotion of NF-κB signaling. (A) Expression of p-NF-κB (Ser536) and p-IKK-β (Ser180/181) was detected by western blotting in TSC1-loss and control osteocytes. (B) The expression of NF-κB in the nucleus and cytoplasm of TSC1-loss and control osteocytes. β-Actin and histone H1 were used as cytoplasm and nuclear protein markers, respectively. (C) The expression of p-NF-κB (Ser536) and p-IKK-β (Ser180/181) were detected by western blotting in Rheb-loss and control osteocytes. (D) The expression of NF-κB in the nucleus and cytoplasm in Rheb -loss and control osteocytes. (E) The level of IL-19 mRNA in TSC1-loss osteocytes exposed to NF-κB inhibitor (pyrrolidine dithiocarbamate [PDTC]; 50 µM, 24 hours) measured by qPCR (mean ± SD; n = 3). (F) IL-19 mRNA levels were detected by qPCR in control osteocytes exposed to PDTC (50 µM, 24 hours) or (and) mTORC1 activator PA (50 µM, 24 hours) (mean ± SD; n = 3). Data are mean ± SD of 3 independent experiments. **P < .01; ***P < .001.
IL-19 is a potent cytokine for the treatment of neutropenia
Based on the strong granulocytic effects of IL-19 described previously, we assessed its potential application in the treatment of neutropenia in mice. Severe neutropenia was induced in C57BL/6 mice by administering a high dose of the antineoplastic chemotherapy reagent cytosine arabinoside (100 mg/kg) for 8 days (Figure 6A-B; supplemental Figure 14). IL-19 efficiently recovered neutrophil formation in mice with severe CIN. Strikingly, IL-19 was much more effective than G-CSF because a low dose (25 μg/kg per day) of IL-19 yielded more neutrophils in the PB and CD11b+Gr-1+ cells in BM than G-CSF (100 μg/kg per day) (Figure 6A-B; supplemental Tables 5 and 6), without affecting G-CSF secretion in arabinoside-induced neutropenia (Figure 6C). Irradiation and radiotherapy could also induce myelosuppression and neutropenia. Similarly, IL-19 reversed radiation-induced neutropenia with more efficiency than G-CSF (Figure 6D-E; supplemental Figure 15). The emergence of resistant organisms has increased the use of chloramphenicol. Chloramphenicol-induced neutropenia is another type of iatrogenic neutropenia. We treated C57BL/6 mice with a high dose of chloramphenicol (200 mg/kg per day) for 21 days to induce severe neutropenia (Figure 7A). Consistently, IL-19 increased the number of neutrophils in the PB and CD11b+Gr-1+ cells in the BM and successfully reversed chloramphenicol-induced neutropenia (Figure 7B; supplemental Figure 16). Interestingly, serum from cancer patients who have undergone chemotherapy had reduced activity to promote neutrophil formation in cultured BM (Figure 7C), accompanied with a downregulation of serum IL-19 (Figure 7D). The IL-19 level was also reduced in both osteocytes and serum of all 3 neutropenia model mice (supplemental Figure 17). These findings demonstrated that IL-19 efficiently reversed chemotherapy, irradiation, or chloramphenicol-induced neutropenia, suggesting that IL-19 is a promising candidate for neutropenia treatment.
Figure 6.
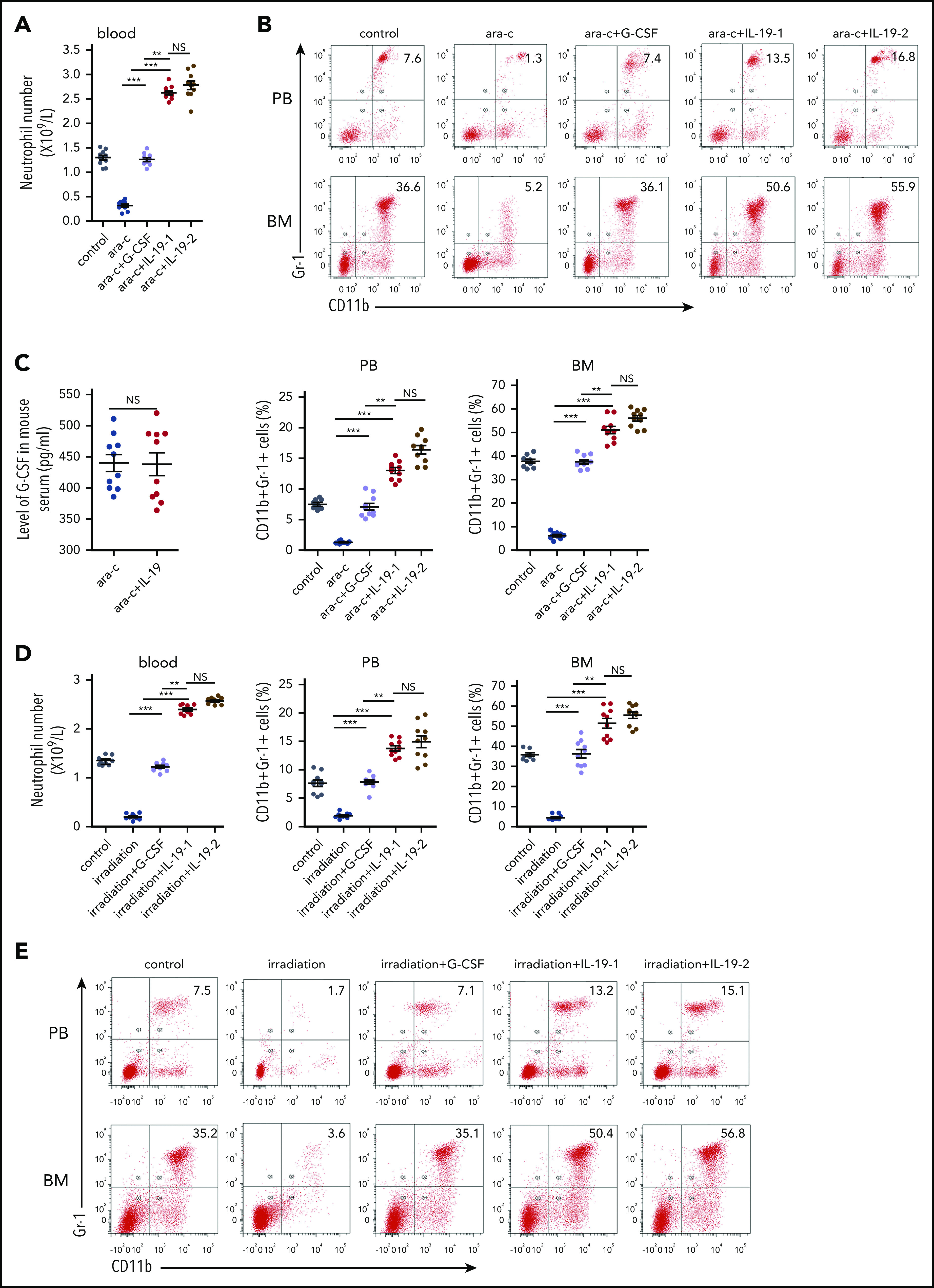
IL-19 prevented chemotherapy- and irradiation-induced neutropenia. (A) Two-month-old C57BL/6 male mice were injected with cytarabine (100 mg/kg per day) followed by twice-daily injection of IL-19-1 (25 µg/kg), IL-19-2 (50 µg/kg), or G-CSF (100 µg/kg) from approximately 24 hours after cytarabine injection; mice were euthanized on day 9. The total number of neutrophils in PB was detected (mean ± SD; n = 10). (B) FACS analysis of BM and PB CD11b+Gr-1+ neutrophils in treated mice (mean ± SD; n = 10). (C) Level of G-CSF in serum of C57BL/6 mice (half male and half female) was detected by ELISA (mean ± SD; n = 10). (D) Two-month-old C57BL/6 male mice received 15 exposures of 0.3 Gy per day whole-body irradiation from day 1 to day 15 to induce neutropenia. Mice received injections of IL-19-1 (25 µg/kg), IL-19-2 (50 µg/kg), and G-CSF (100 µg/kg per day) from ∼24 hours after irradiation and were euthanized on day 16. The total number of neutrophils in the PB was detected (mean ± SD; n = 10). (E) FACS analysis of BM and PB CD11b+Gr-1+ neutrophils in treated mice (mean ± SD; n = 10). Data are mean ± SD of 3 independent experiments. **P < .01; ***P < .001.
Figure 7.
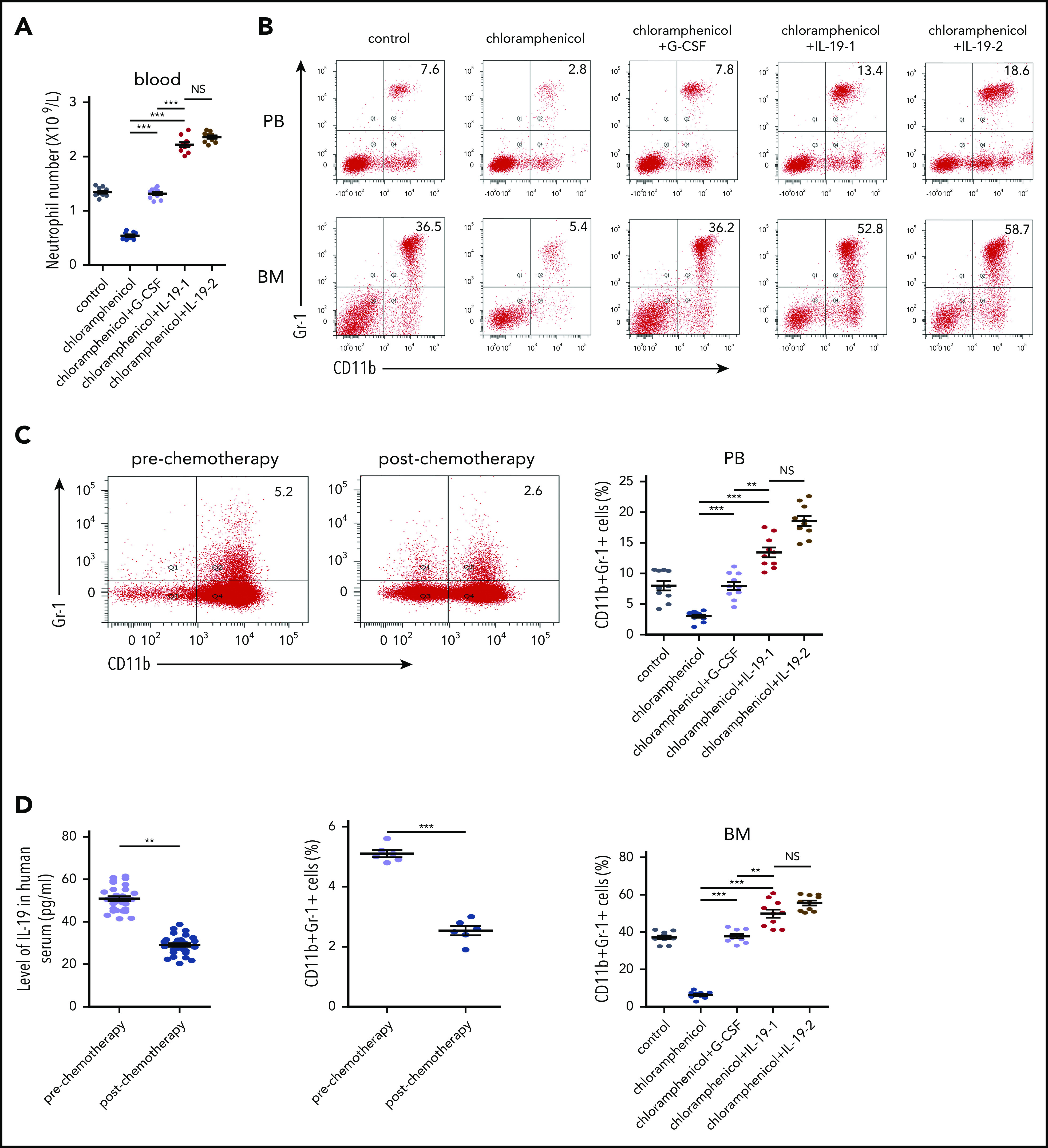
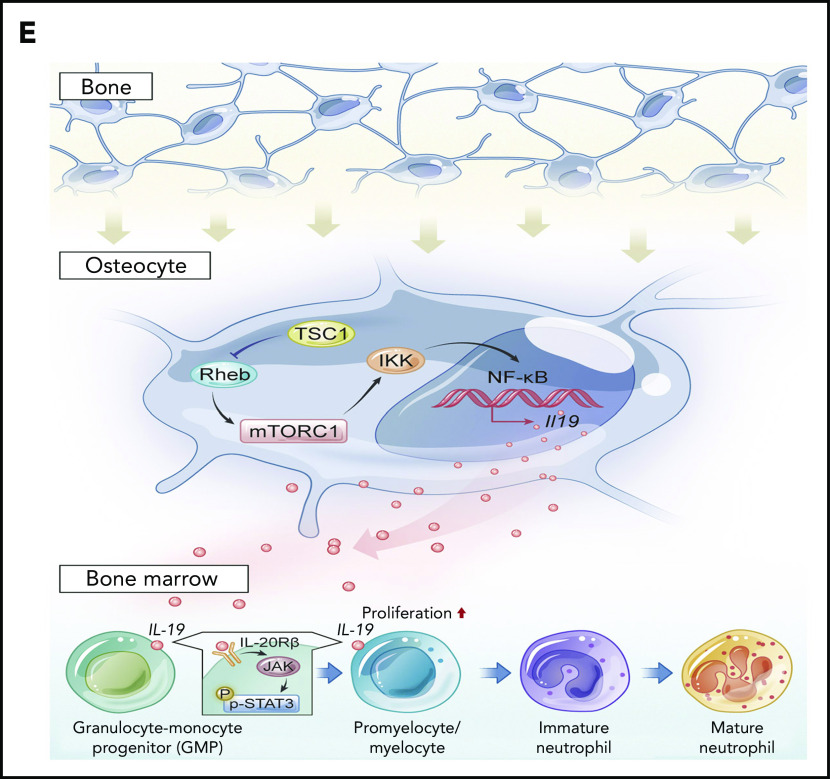
IL-19 prevented chloramphenicol-induced neutropenia. (A) Two-month-old C57BL/6 male mice were injected with chloramphenicol (200 mg/kg per day) followed by injection of IL-19-1 (25 µg/kg), IL-19-2 (50 µg/kg), or G-CSF (100 µg/kg per day) starting from 6 days after chloramphenicol injection; mice were euthanized on day 22. The total number of neutrophils in PB was detected (mean ± SD; n = 10). (B) FACS analysis of BM and PB CD11b+Gr-1+ neutrophils in treated mice (mean ± SD; n = 10). (C) C57BL/6 mouse total BM cells were cultured with serum of patients before and after chemotherapy with cytarabine at days 3 and 7; cells were harvested on day 10. FACS analysis of CD11b+Gr-1+ neutrophils in treated BM cells (mean ± SD; n = 6). (D) The level of IL-19 in the serum of patients pre- and postchemotherapy with cytarabine (mean ± SD; n = 10). Data are mean ± SD of 3 independent experiments. (E) Schematic model of the regulation of neutrophil development by osteocytes via secretion of IL-19. **P < .01; ***P < .001.
Discussion
Osteocytes differentiate from osteoblasts, and extend long cell processes that connect with neighboring osteocytes, or communicate directly with osteoblasts and osteoclasts, as well as BM cells, by extending dendrites to the marrow space and by secreting molecules such as sclerostin, fibroblast growth factor-23, and receptor activator of NF-κB ligand, which act locally or on distant organs.15-17 In addition to control adaptive bone remodeling, recent studies have identified osteocytes as a major component of the BM microenvironment and important regulators of hematopoiesis.20,21,44,45 Osteocytes are essential for G-CSF–stimulated mobilization of hematopoietic stem/progenitor cells,46 and play critical roles in lymphopoiesis, as ablation of osteocytes in mice leads to severe lymphopenia.45 Mice lacking Gsα in osteocytes (Dmp1-Cre) develop striking myeloproliferation with increased myeloid cells and platelets.20 Although G-CSF is increased twofold in these mice, how osteocytes control hematopoiesis or the granulopoiesis niche is still unclear.20 The exact role of osteocytes in neutrophil formation needs to be defined. We found that mice with ablation of TSC1 in osteocytes (Dmp1-cre), but not in mature osteoblasts (Ocn-cre) or osteoprogenitors (Osx-cre), produced a high level of IL-19 and neutrophils,33,34 implying a specific function for IL-19 secreted predominantly by osteocytes in the granulopoiesis niche and neutrophil development. Our in vivo and in vitro evidence demonstrated the essential role of osteocytes in supporting CD11b+Gr-1+ cell expansion, and neutrophil formation via IL-19 production and IL-20Rβ/Stat3-signaling activation, underscoring the important contribution of osteocyte-produced IL-19 to neutrophil formation (Figure 7E).
IL-19 belongs to the IL-20 subfamily of cytokines, which includes IL-19, IL-20, IL-22, IL-24, and IL-26, is primarily produced by leukocytes, and acts on epithelial tissues to enhance epithelial innate immunity.36-38 It is now clear that IL-19 is produced not only by activated immune cell subsets (monocytes, macrophages, B cells) but also to a similar extent by activated tissue cells, such as keratinocytes, epithelial cells, fibroblasts, smooth muscle cells, and synovial tissues.38 Although first reported in 1999, no clear role for IL-19 has yet been defined. Several human diseases and their animal models have implicated a role for IL-19 in psoriasis, inflammatory bowel disease, atherogenesis, endotoxic shock, rheumatoid arthritis, and cancer.36,46-48 However, key questions remain, especially with respect to the nature of its receptor and contradictory functions on leukocytes and inflammation.36-38 Although recombinant IL-19 has been shown to induce monocytes to produce proinflammatory cytokines, such as IL-6 and tumor necrosis factor α (TNF-α),49 and anti-inflammatory cytokine IL-10,50 its roles in immune cells have not been extensively investigated. The role of IL-19 in neutrophil formation is unknown. Although the effect of genetic IL-19 ablation on neutrophil numbers is currently lacking,47 our results clearly showed that IL-19 secreted predominantly by osteocytes stimulated CD11b+Gr1+ cell expansion and neutrophil formation both in vivo and in vitro, highlighting its role in innate immunity. IL-19 may function through the IL-20Rα/Rβ complex. However, no apparent IL-20Rα expression was detected in hematopoietic and immune cells in previous studies40,41 nor in our results. Given the fact that IL-20Rβ is always expressed by these cells37 and that it is IL-20Rβ, but not IL-20Rα, that binds IL-19 directly in solution,51 it is plausible that IL-19 predominantly derived from osteocytes may target neutrophil progenitors via IL-20Rβ, although the involvement of additional undefined receptors cannot be ruled out. Supporting this notion, there were reduced numbers of dermal neutrophils in IL-23–treated IL-20Rβ–null mice.51 Additionally, our finding that knocking down IL-20Rβ eliminated the effects of IL-19 on neutrophils also indicated an important role for IL-20Rβ in this process. Furthermore, together with the finding that endogenous IL-19 predominantly from osteocytes of Dmp1-Rheb mice, as well as injected recombinant IL-19, had no significant effects on serum G-CSF levels, our results collectively suggested that the IL-19–IL-20Rβ axis may function to promote neutrophil formation in a G-CSF–independent way.
Neutropenia can be secondary to irradiation and cytotoxic drugs such as antibiotics and chemotherapy.52 CIN has a high incidence rate and is considered an unpreventable side effect of many types of cancer treatment; CIN can lead to infection, increased length of hospital stay, discontinuation of cancer treatment, and increased morbidity and mortality.26 The administration of myeloid growth factors (eg, G-CSF) is the only approved treatment for the prevention of CIN; however, their high cost, specific indications and contraindications, and potential side effects limit their application to only a relatively small subset of patients at the highest risk of complications, such as infection.53 Novel agents that increase neutrophil counts or protect marrow progenitors could be used as a stand-alone preventive strategy or as an adjunct to G-CSF. Evidence from the present in vivo study suggested that IL-19 may be an attractive option for the treatment of neutropenia as it is more effective than G-CSF in the treatment of chemotherapy-, irradiation-, or chloramphenicol-induced neutropenia. The minimum effective dose of IL-19 used in this study was very low, which can prevent potential adverse effects and reduce the cost. Further studies are warranted to evaluate the full therapeutic potential of IL-19 (or combinatorial approaches) in the treatment of neutropenia.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (82070110, 81991511, 81625015, 81530070, and 81871745), and the Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110104002).
Footnotes
Data can be found at https://data.mendeley.com/datasets/x78chtp975/1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.B. and M.X. designed the research and wrote the paper; M.X., W.Z., W.L., L.M., J.Y., and L.H. performed the experiments and analyzed the data; S.Z., Y.Z., A.L., and Q.S. performed mouse experiments and collected and analyzed the data; Y.L. helped to collect the data; G.X. helped to formulate the hypothesis; and Z.Z. and X.B. conceived the project and wrote the paper.
Conflict-of-disclosure: The authors declare no competing financial interests.
Correspondence: Xiaochun Bai, Department of Cell Biology, School of Basic Medical Sciences, The Third Affiliated Hospital, Southern Medical University, Shatai Nan Rd 1023-1063, Baiyun District, Guangzhou, China 510515; e-mail: baixc15@smu.edu.cn; and Zhipeng Zou, Academy of Orthopedics, Guangdong Province, Guangdong Provincial Key Laboratory of Bone and Joint Degeneration Diseases, The Third Affiliated Hospital of Southern Medical University, Shatai Nan d 1023-1063, Baiyun District, Guangzhou, China 510515; e-mail: zzp@smu.edu.cn.
REFERENCES
- 1.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602-611. [DOI] [PubMed] [Google Scholar]
- 2.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159-175. [DOI] [PubMed] [Google Scholar]
- 3.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657-670. [DOI] [PubMed] [Google Scholar]
- 4.Kondo M, Wagers AJ, Manz MG, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759-806. [DOI] [PubMed] [Google Scholar]
- 5.Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN. Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell. 2019;177(3):541-555.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14(5):302-314. [DOI] [PubMed] [Google Scholar]
- 7.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459-489. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla MA, Gillio AP, Ruggeiro M, et al. Effects of recombinant human granulocyte colony-stimulating factor on neutropenia in patients with congenital agranulocytosis. N Engl J Med. 1989;320(24):1574-1580. [DOI] [PubMed] [Google Scholar]
- 9.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58(3):705-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Y, Nilsson SK. Bone, microenvironment and hematopoiesis. Curr Opin Hematol. 2012;19(4):250-255. [DOI] [PubMed] [Google Scholar]
- 11.Rankin EB, Wu C, Khatri R, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149(1):63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201(11):1771-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836-841. [DOI] [PubMed] [Google Scholar]
- 14.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841-846. [DOI] [PubMed] [Google Scholar]
- 15.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315-323. [DOI] [PubMed] [Google Scholar]
- 19.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165-176. [DOI] [PubMed] [Google Scholar]
- 20.Fulzele K, Krause DS, Panaroni C, et al. Myelopoiesis is regulated by osteocytes through Gsα-dependent signaling. Blood. 2013;121(6):930-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Divieti Pajevic P, Krause DS. Osteocyte regulation of bone and blood. Bone. 2019;119:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metzger CE, Narayanan SA. The role of osteocytes in inflammatory bone loss. Front Endocrinol (Lausanne). 2019;10:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisbart RH, Golde DW, Clark SC, Wong GG, Gasson JC. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985;314(6009):361-363. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34(2):81-89. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8(7):533-544. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy [published correction appears in N Engl J Med. 2013;369(3):293]. N Engl J Med. 2013;368(12):1131-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Carbonero R, Mayordomo JI, Tornamira MV, et al. Granulocyte colony-stimulating factor in the treatment of high-risk febrile neutropenia: a multicenter randomized trial. J Natl Cancer Inst. 2001;93(1):31-38. [DOI] [PubMed] [Google Scholar]
- 28.Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737-1746. [PubMed] [Google Scholar]
- 29.Stern AR, Stern MM, Van Dyke ME, Jähn K, Prideaux M, Bonewald LF. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques. 2012;52(6):361-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia WF, Tang FL, Xiong L, et al. Vps35 loss promotes hyperresorptive osteoclastogenesis and osteoporosis via sustained RANKL signaling. J Cell Biol. 2013;200(6):821-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933-1936. [DOI] [PubMed] [Google Scholar]
- 32.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946-1949. [DOI] [PubMed] [Google Scholar]
- 33.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155-162. [DOI] [PubMed] [Google Scholar]
- 34.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease [published correction appears in Cell. 2017;169(2):361-371]. Cell. 2017;168(6):960-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim J, Burclaff J, He G, Mills JC, Long F. Unintended targeting of Dmp1-Cre reveals a critical role for Bmpr1a signaling in the gastrointestinal mesenchyme of adult mice. Bone Res. 2017;5(1):16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallagher G. Interleukin-19: multiple roles in immune regulation and disease. Cytokine Growth Factor Rev. 2010;21(5):345-352. [DOI] [PubMed] [Google Scholar]
- 37.Autieri MV. IL-19 and other IL-20 family member cytokines in vascular inflammatory diseases. Front Immunol. 2018;9:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines–from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14(12):783-795. [DOI] [PubMed] [Google Scholar]
- 39.Liu G, Hu X, Sun B, et al. Phosphatase Wip1 negatively regulates neutrophil development through p38 MAPK-STAT1. Blood. 2013;121(3):519-529. [DOI] [PubMed] [Google Scholar]
- 40.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168(11):5397-5402. [DOI] [PubMed] [Google Scholar]
- 41.Wolk K, Witte K, Witte E, et al. Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J Leukoc Biol. 2008;83(5):1181-1193. [DOI] [PubMed] [Google Scholar]
- 42.Xiao M, Wang Y, Tao C, et al. Osteoblasts support megakaryopoiesis through production of interleukin-9. Blood. 2017;129(24):3196-3209. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Jia L, Lin W, et al. Epstein-Barr virus-encoded latent membrane protein 1 upregulates glucose transporter 1 transcription via the mTORC1/NF-κB signaling pathways. J Virol. 2017;91(6):e02168-e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asada N, Katayama Y, Sato M, et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12(6):737-747. [DOI] [PubMed] [Google Scholar]
- 45.Sato M, Asada N, Kawano Y, et al. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab. 2013;18(5):749-758. [DOI] [PubMed] [Google Scholar]
- 46.Myles IA, Fontecilla NM, Valdez PA, et al. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013;14(8):804-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azuma YT, Matsuo Y, Kuwamura M, et al. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis. 2010;16(6):1017-1028. [DOI] [PubMed] [Google Scholar]
- 48.Kragstrup TW, Andersen T, Heftdal LD, et al. The IL-20 cytokine family in rheumatoid arthritis and spondyloarthritis. Front Immunol. 2018;9:2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao YC, Liang WG, Chen FW, Hsu JH, Yang JJ, Chang MS. IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. J Immunol. 2002;169(8):4288-4297. [DOI] [PubMed] [Google Scholar]
- 50.Jordan WJ, Eskdale J, Boniotto M, et al. Human IL-19 regulates immunity through auto-induction of IL-19 and production of IL-10. Eur J Immunol. 2005;35(5):1576-1582. [DOI] [PubMed] [Google Scholar]
- 51.Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmblad J, Papadaki HA. Chronic idiopathic neutropenias and severe congenital neutropenia. Curr Opin Hematol. 2008;15(1):8-14. [DOI] [PubMed] [Google Scholar]
- 53.Matikas A, Georgoulias V, Kotsakis A. Emerging agents for the prevention of treatment induced neutropenia in adult cancer patients. Expert Opin Emerg Drugs. 2016;21(2):157-166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.