Abstract
Background
Investigation of the natural history of chronic obstructive pulmonary disease (COPD) has led to the recognition that individuals with higher than normal lung function may have lower risk of developing COPD. We tested the hypothesis that individuals with supernormal lung function have lower risk of COPD.
Methods
We followed 108,246 adults from the Copenhagen General Population Study recruited between 2003 and 2015 for clinical COPD outcomes until 2018. A subset of 16,892 attended another examination approximately 10 years later, allowing to investigate lung function decline and COPD development (forced expiratory volume in 1 se (FEV1)/forced vital capacity (FVC)<0·70 and FEV1<80% predicted with chronic respiratory symptom). Supernormal lung function was defined as FEV1>upper limit of normal (ULN).
Findings
At baseline, 3944(4%) had supernormal lung function, 91,938(85%) normal lung function, and 12,364(11%) had below normal lung function. Individuals with baseline supernormal versus normal lung function had higher FEV1 decline but did not differ in FEV1/FVC decline. None had COPD at 10 years in those with supernormal lung function, while 3% had in those with normal lung function. Early-life risk factors associated with COPD development and smoking exposure in different stages of life were less common in individuals with supernormal lung function. Compared to individuals with normal lung function, multivariable adjusted hazard ratios in those with supernormal lung function were 0·19(95% confidence interval:0·08–0·46) for acute obstructive lung disease hospitalisations, 0·56(0·45–0·69) for pneumonia hospitalisations, and 0·81(0·72–0·91) for all-cause mortality.
Interpretation
Supernormal lung function is associated with lower risk of developing COPD.
Funding
Herlev and Gentofte Hospital and Lundbeck Foundation.
Research in context.
Evidence before this study
Determining the association of early-life risk factors with higher than normal lung function (supernormal lung function) and its association with chronic obstructive pulmonary disease (COPD) could help us understand and identify factors related to optimum development and preservation of lung function from birth until old age. We searched PubMed for previous human studies published in English until March 10, 2021, using the MeSH search term: “Pulmonary Disease, Chronic Obstructive”, “Forced Expiratory Volume”, or “Spirometry” in combination with “Supernormal” or “Supranormal”. No previous study has investigated supernormal lung function against development of COPD.
Added value of this study
While 3% of individuals with normal lung function developed COPD during 10 years, none developed it in those with supernormal lung function. Early-life risk factors usually associated with development of COPD and smoking exposure in different stages of life were less common in those with supernormal versus normal lung function. Furthermore, individuals with supernormal lung function had lower risk of acute hospitalisations due to obstructive lung disease and pneumonia, and of all-cause mortality.
Implications of all the available evidence
Supernormal lung function is associated with lower risk of developing COPD. This information may be helpful during discussions between patients and doctors about future risk of developing chronic lung disease. Future guidelines on prevention, diagnosis, and treatment of COPD may wish to incorporate such knowledge.
Alt-text: Unlabelled box
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by the presence of chronic respiratory symptoms and airflow limitation, and is associated with significant morbidity and mortality in middle-aged and older adults [1]. It is now increasingly evident that lung function trajectories leading to development of airflow limitation in COPD may be determined early in life [2], [3], [4], [5], [6], [7], [8]. As we have advanced our understanding of the natural history of COPD, new and as of yet unanswered important questions have arisen. One such question is the health effects of higher than normal lung function (supernormal lung function) [2,3]. Recently, we showed that supernormal lung function, when defined as forced vital capacity (FVC) >upper limit of normal (ULN), is associated with lower morbidity and mortality in individuals in the general population [9]; however, while lower than normal lung function is an established risk factor for COPD [7], the association of supernormal lung function with obstructive lung diseases has not been explored before. This is an important question as determining the association of early-life risk factors with supernormal lung function and its association with COPD could help us understand factors related to optimum development and preservation of lung function from birth until old age.
We tested the hypothesis that individuals with supernormal lung function have lower risk of COPD. For this purpose, we used the Copenhagen General Population Study, a contemporary Danish population-based cohort.
2. Methods
2.1. Study design and population
YÇ and SA had full access to all data in the study and had final responsibility for the decision to submit for publication. We used the Copenhagen General Population Study, a contemporary Danish population-based cohort with ongoing enrolment [10], [11], [12]. In Denmark, all individuals are assigned a unique identification number (Central Person Registration number) at birth or immigration and recorded in the national Danish Civil Registration System. We therefore randomly invited individuals aged 20–100 living in the Capital Region of Denmark from the national Danish Civil Registration System to reflect the adult White Danish population of Danish descent (response-rate: 43%). All participants completed a questionnaire and underwent a physical examination. The questionnaire is a comprehensive and detailed assessment on health and lifestyle and includes also information on chronic disease and symptoms, as described previously [13]. Questionnaires were reviewed at the day of attendance by a healthcare professional together with the participant. The study was approved by Herlev and Gentofte Hospital and the regional ethics committee (approval number: H-KF-01–144/01) and was conducted according to the Declaration of Helsinki. All participants provided written informed consent. In the present study, we included 108 246 individuals with complete information on age, sex, height, and lung function, recruited between November 26, 2003 and April 28, 2015. In order to determine the predicted value for lung function, information on age, sex, and height is a necessity. An ongoing follow-up examination was initiated in March 31, 2014 where individuals are invited systematically based on region and previous participation date thereby allowing an approximately follow-up time of 10 years [14].
2.2. Supernormal lung function
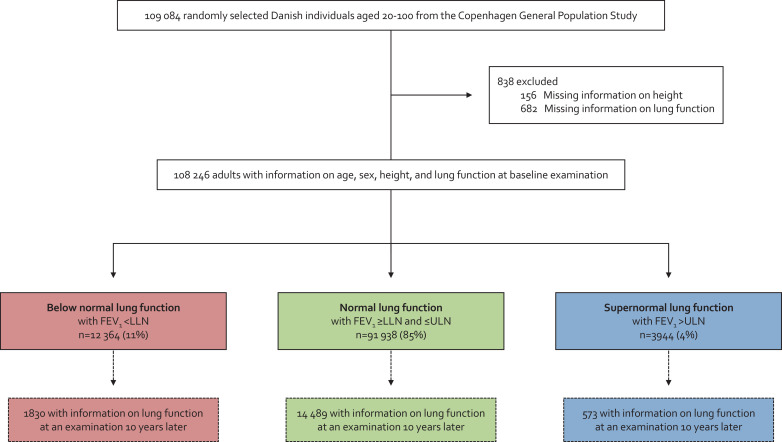
Lung function was determined using spirometry with pre-bronchodilator measurements of forced expiratory volume in 1 sec (FEV1) and FVC [15]. Spirometry use in the Copenhagen General Population Study has undergone a rigorous validation process before [16]. Predicted values of FEV1 and FVC were calculated according to internally derived reference equations based on a subsample of 11 288 healthy asymptomatic never-smoking individuals with age and height as covariates separately for men and women [15,16]. Since supernormal lung function has not been defined before [2,3], we chose ULN for FEV1, defined as the highest 5th percentile of the predicted value, calculated as the mean value plus 1·645 standard deviations. Supernormal lung function was defined as FEV1 >ULN, normal lung function as FEV1 ≥lower limit of normal (LLN) and ≤ULN, and below normal lung function was defined as FEV1 <LLN (Fig. 1). FEV1 was chosen to define supernormal lung function, as it is the single most important measurement used over the years to investigate the natural history of COPD [7]. A detailed description of lung function is provided in the supplement.
Fig. 1.
Flowchart. FEV1=forced expiratory volume in 1 sec. FVC=forced vital capacity. LLN=the lower limit of normal. ULN=upper limit of normal.
2.3. Outcomes
COPD was defined as presence of clinical airflow limitation with FEV1/FVC <0·70 and FEV1 <80% predicted plus at least one chronic respiratory symptom of chronic mucus hypersecretion, dyspnea, wheezing, and/or cough [12]. A subset of 16 892 individuals had information on lung function and chronic respiratory symptoms both at baseline and at an examination 10 years later (Fig. 1), allowing investigation of lung function decline and COPD development.
Clinical long-term outcomes included acute hospitalisations due to obstructive lung disease and pneumonia, while all-cause mortality was also ascertained. Acute hospitalisations due to obstructive lung disease (International Classification of Diseases [ICD]−10: J41-J46) and pneumonia (ICD-10: J12-J18) included all emergency department visits and hospital admissions with the mentioned primary discharge diagnosis. Information was available from the national Danish Patient Registry, which covers all public and private hospital contacts in Denmark, recorded in the period from November 26, 2003 until December 7, 2018. Information on vital status was obtained from the national Danish Civil Registration System, which contains date of death for all residents in Denmark, recorded in the period from November 26, 2003 until December 13, 2018. As follow-up was done using the above-mentioned register linkage based on the unique Central Person Registration number provided to everyone at birth or immigration, no person was lost to follow-up, and individuals who emigrated were censored at the date of emigration (n = 451). All diagnoses recorded in the registries are strictly made by a medical doctor according to national Danish laws using the World Health Organisations ICD-codes.
2.4. Covariates
Information on sex and age was obtained from the national Danish Civil Registration System. Body mass index (BMI) was calculated as measured weight divided by measured height squared (kg/m2). Smoking status was defined as never, former, or current smoking. Cumulative tobacco consumption was calculated in pack-years based on information on age at smoking initiation and cessation (for current smokers both at baseline and at the examination 10 years later), duration of tobacco consumption, and amount of consumed tobacco (number of daily consumed cigarettes, cheroots, and cigars and grams of weekly consumed pipe tobacco); a pack-year was 20 cigarettes or equivalent smoked daily for a year. Socioeconomic status was based on the longest acquired education after school and annual household income. Asthma was defined as an affirmative response to the question: “Do you have asthma?”. Treatment with airway medication was defined as an affirmative response to the question: “Do you take any kind of medication for asthma and/or bronchitis (including sprays and/or dry powders) daily or almost daily?”. Information on early-life risk factors were self-reported and collected at an examination 10 years after baseline.
2.5. Statistical analyses
Wilcoxon's rank-sum, Pearson's chi-squared, and Fisher's exact tests were used for group comparisons, i.e. individuals with supernormal, normal, and below normal lung function. Linear mixed-effects model with random-intercepts and -slopes and unstructured covariance matrices were used to determine the association between FEV1 and age in individuals with below normal, normal, and supernormal lung function, thereby being able to estimate annual lung function decline during 10 years of follow-up. Age was used as analysis time in this regard, as FEV1 decline may change with age as well as to make the results more interpretable [7]. Kaplan-Meier models were used to assess failure probability of acute hospitalisations and mortality during follow-up, and the difference was assessed using the log-rank test. Cox proportional hazard model was used to calculate hazard ratios for acute hospitalisations and mortality during follow-up. An approach with single failure-time analysis was used for all-cause mortality. For acute hospitalisations, we carried out multiple failure-time analysis using the Andersen-Gill approach, meaning that individuals were at risk of recurrent events [10]. To avoid counting a single event multiple times, we chose that hospitalised individuals during follow-up had to be clinically stable for at least four weeks after discharge before they could be considered at risk again for a subsequent event, in accordance with previous methodological recommendations [17]. We used analyses with left truncation at study entry and age as the underlying timescale. Analyses were adjusted for age (as timescale), sex, BMI, smoking status, cumulative tobacco consumption (pack-years), socioeconomic status, asthma, and treatment with airway medication. To account for missing data (< 0.5%), we performed multivariate imputation using chained equations to fill out missing values. As a form of sensitivity analysis, we investigated clinical long-term outcomes according to higher than normal lung function as a dose-response relationship by using restricted cubic splines with the median value as the reference, and three knots were chosen and placed according to Harrell's recommendation at the 10th, 50th, and 90th percentiles [18]. Since the clinical groups were derived using FEV1 and not FEV1/FVC, a small proportion of individuals with supernormal lung function had FEV1/FVC <LLN (n = 65). Thus, we characterised these individuals and excluded them in a sensitivity analysis. In another sensitivity analysis, we excluded also individuals with asthma. Analyses were performed using STATA/SE 13·1 for Windows (StataCorp, College Station, Texas, US), and a two-sided P-value <0·05 was considered significant.
2.6. Role of the funding source
This study was funded by Herlev and Gentofte Hospital, Copenhagen University Hospital, and the Lundbeck Foundation. The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
3. Results
3.1. Clinical characteristics
Among 108 246 individuals from the general population, 3944 (4%) had supernormal lung function, 91 938 (85%) normal lung function, and 12 364 (11%) had below normal lung function (Fig. 1). At baseline examination, median FEV1 was 3·90 L (corresponding to 126% of predicted) for individuals with supernormal lung function, 3·00 L (98% of predicted) for individuals with normal lung function, and 2·02 L (69% of predicted) for individuals with below normal lung function (Table 1). Individuals with supernormal lung function were characterised by being younger (53·4 versus 57·9 and 61·0 years) and having a lower cumulative tobacco consumption (7·5 versus 14·8 and 30·0 pack-years) compared to individuals with normal and below normal lung function (all P-values < 0·05). Other noteworthy differences included lower prevalence of asthma (3% versus 5% and 16%) and treatment with airway medication (2% versus 4% and 19%) (all P-values < 0·05). These differences were less pronounced when compared to those with normal lung function and more pronounced when compared to those with below normal lung function.
Table 1.
Baseline characteristics according to lung function in 108 246 individuals in the general population.
| Below normal lung function (n = 12 364) | Normal lung function (n = 91 938) | Supernormal lung function (n = 3944) | |
|---|---|---|---|
| Age – years | 61·0 (51·8–69·3)* | 57·9 (47·9–67·2) | 53·4 (44·3–65·6)*,† |
| Male sex – no. (%) | 5738 (46)* | 41 504 (45) | 1429 (36)*,† |
| Weight – kg | 77·7 (66·7–90·0)* | 75·5 (65·7–86·1) | 73·5 (64·7–83·7)*,† |
| Height – cm | 171 (165–178) | 171 (165–178) | 172 (165–179)*,† |
| BMI – kg/m2 | 26·3 (23·4–29·7)* | 25·5 (23·2–28·3) | 24·9 (22·8–27·2)*,† |
| Lung function | |||
| FEV1 – L | 2·02 (1·58–2·47)* | 3·00 (2·49–3·60) | 3·90 (3·39–4·69)*,† |
| FEV1% predicted –% | 69 (60–75)* | 98 (90–106) | 126 (122–132)*,† |
| FVC – L | 2·94 (2·39–3·57)* | 3·87 (3·24–4·64) | 4·80 (4·19–5·81)*,† |
| FVC% predicted –% | 78 (70–85)* | 101 (92–109) | 125 (119–132)*,† |
| FEV1/FVC | 0·69 (0·61–0·76)* | 0·78 (0·74–0·82) | 0·81 (0·78–0·84)*,† |
| Smoking information | |||
| Never-smokers – no. (%) | 2960 (24)* | 40 292 (44) | 2026 (51)*,† |
| Former smokers – no. (%) | 5096 (41) | 37 429 (41) | 1575 (40) |
| Current smokers – no. (%) | 4276 (35)* | 13 851 (15) | 321 (8)*,† |
| Unknown– no. (%) | 32 (<1)* | 366 (<1) | 22 (<1)† |
| Tobacco consumption – pack-years‡ | 30·0 (15·0–44·0)* | 14·8 (5·3–27·7) | 7·5 (2·8–15·0)*,† |
| Poor socioeconomic status – no. (%) | 3634 (29)* | 15 161 (16) | 524 (13)*,† |
| Asthma – no. (%) | 1935 (16)* | 4519 (5) | 104 (3)*,† |
| Treatment with airway medication – no. (%) | 2359 (19)* | 4048 (4) | 94 (2)*,† |
Data presented as median (25th and 75th percentiles), or number (percent). Numbers may not sum to 100 due to rounding. Based on the Copenhagen General Population Study. BMI=body mass index. FEV1=forced expiratory volume in 1 sec. FVC=forced vital capacity.
P < 0·05 for comparison with normal lung function using Wilcoxon's rank sum or Pearson's chi-squared test.
P < 0·05 for comparison with below normal lung function using Wilcoxon's rank sum or Pearson's chi-squared test.
Includes only current and former smokers.
3.2. Lung function decline and development of airflow limitation
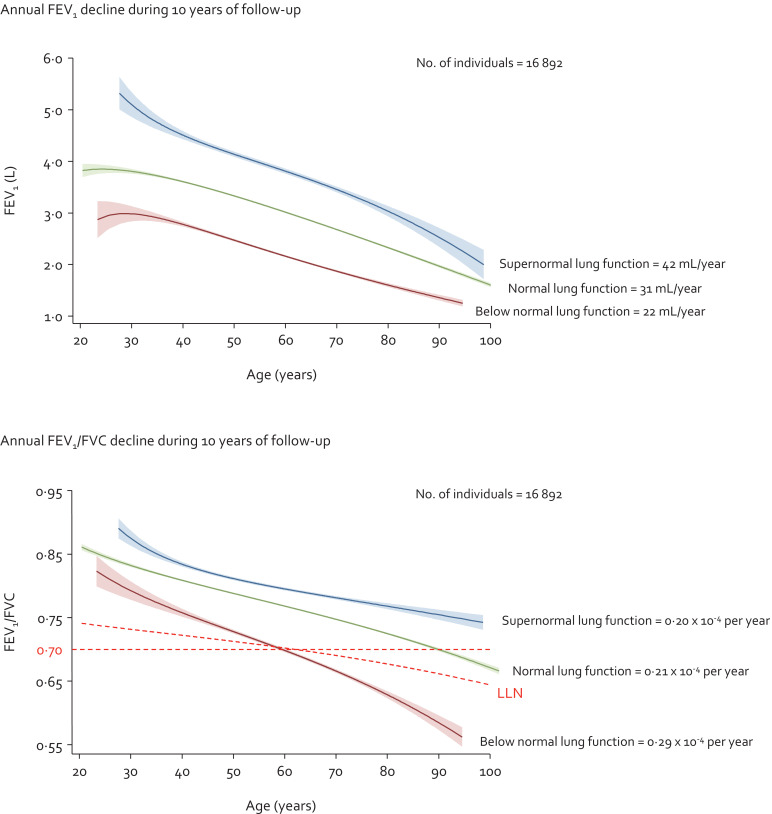
During 10 years follow-up (median: 9·9 years and interquartile range: 1·0 years), annual FEV1 decline in mL/year was highest for individuals with supernormal lung function and lowest for individuals with below normal lung function, while the opposite pattern was observed for annual FEV1/FVC decline (Fig. 2). Multivariable adjusted annual FEV1 decline was 42 mL/year for individuals with supernormal lung function, 31 mL/year for individuals with normal lung function, and 22 mL/year for individuals with below normal lung function. Corresponding respective annual FEV1/FVC decline was 0·21 × 10−4 per year, 0·20 × 10−4 per year, and 0·29 × 10−4 per year. All differences in FEV1 and FEV1/FVC declines between the groups had P-value <0.05 except for difference in FEV1/FVC decline between individuals with supernormal and normal lung function. In relative terms, the annual FEV1 and FEV1/FVC declines were overall similar with 1.1% and 0.2% per year for individuals with supernormal lung function, 1.0% and 0.3% per year for individuals with normal lung function, and 1.1% and 0.4% per year for individuals with below normal lung function. Results were similar without imputation. No individuals with supernormal lung function had COPD at baseline or at the examination 10 years later (Table 2). In those with normal lung function, <1% had COPD at baseline and 3% at the examination 10 years later, while in those with below normal lung function, the corresponding numbers were 25% and 34%, respectively.
Fig. 2.
Lung function decline in individuals with supernormal lung function in the general population. Based on the Copenhagen General Population Study. Linear mixed-effects model with random-intercepts and -slopes and unstructured covariance matrices. Analyses were multivariable adjusted for age, sex, body mass index, smoking status, cumulative tobacco consumption (pack-years), socioeconomic status, asthma, and treatment with airway medication. FEV1=forced expiratory volume in 1 sec. FVC=forced vital capacity.
Table 2.
COPD at baseline and at an examination 10 years later according to lung function in individuals in the general population.
| Below normal lung function (n = 1830) | Normal lung function (n = 14 489) | Supernormal lung function (n = 573) | |
|---|---|---|---|
| At baseline examination | |||
| FEV1/FVC <0·70 and FEV1 <80% predicted – no. (%) | 652 (36)* | 100 (<1) | 0 (0)*,† |
| Chronic respiratory symptoms – no. (%) | 1175 (64)* | 5338 (37) | 156 (27)*,† |
| COPD – no. (%) | 461 (25)* | 55 (<1) | 0 (0)† |
| At final examination 10 years later | |||
| FEV1/FVC <0·70 and FEV1 <80% predicted – no. (%) | 822 (45)* | 555 (4) | 0 (0)*,† |
| Chronic respiratory symptoms – no. (%) | 1203 (66)* | 5862 (40) | 159 (28)*,† |
| COPD – no. (%) | 628 (34)* | 389 (3) | 0 (0)*,† |
Data presented as number (%). Based on the Copenhagen General Population Study. COPD was defined as presence of FEV1/FVC < 0·70 and FEV1 < 80% predicted and at least one chronic respiratory symptom. COPD=chronic obstructive pulmonary disease. FEV1=forced expiratory volume in 1 sec. FVC=forced vital capacity.
P < 0·05 for comparison with normal lung function using Pearson's chi-squared or Fisher's exact test.
P < 0·05 for comparison with below normal lung function using Pearson's chi-squared or Fisher's exact test.
3.3. Early-life risk factors
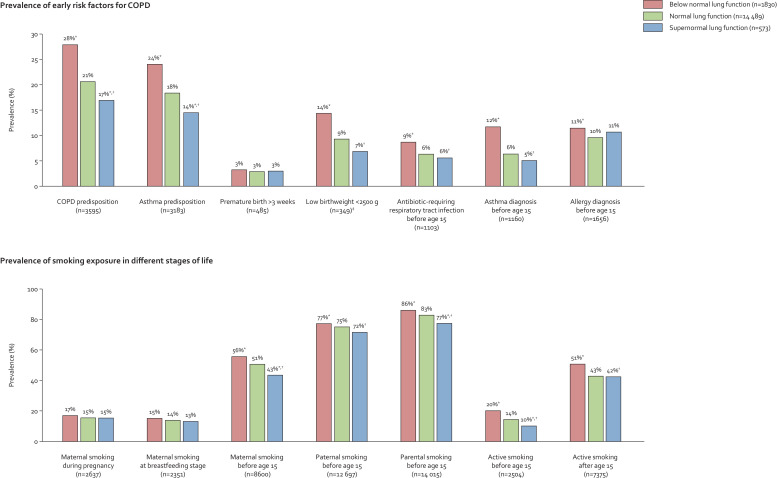
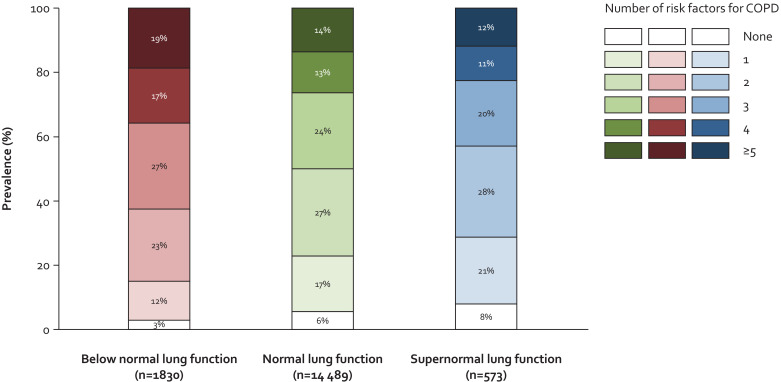
Individuals with supernormal lung function had a lower prevalence of early-life risk factors for COPD compared to individuals with normal and below normal lung function, which included COPD and asthma predisposition, low birthweight < 2500 g, antibiotic-requiring respiratory tract infections before age 15, and asthma diagnosis before age 15 (all P-values < 0·05) (Fig. 3). Furthermore, they also had a lower prevalence of smoking exposure in different stages of life except for maternal smoking during pregnancy and at breastfeeding stage (all P-values < 0·05). These differences were less pronounced when compared to those with normal lung function and more pronounced when compared to those with below normal lung function. Correspondingly, number of potential risk factors for COPD was lower for those with supernormal lung function compared to those with normal and below normal lung function (Fig. 4).
Fig. 3.
Early risk factors for COPD and smoking exposure in different stages of life in individuals with supernormal lung function in the general population. Based on the Copenhagen General Population Study. *P <0·05 for comparison with normal lung function using Pearson's chi-squared or Fisher's exact test. †P <0·05 for comparison with below normal lung function using Pearson's chi-squared or Fisher's exact test. ‡Information on birthweight was available on a subset of 3588 individuals. COPD=chronic obstructive pulmonary disease.
Fig. 4.
Number of risk factors for COPD in individuals with supernormal lung function in the general population. Based on the Copenhagen General Population Study. We included 11 risk factors for COPD: (1) genetic predisposition for COPD and/or asthma, (2) premature birth >3 weeks and/or low birthweight <2500 g, (3) antibiotic-requiring respiratory tract infection before age 15, (4) asthma diagnosis before age 15, (5) allergy before age 15, (6) maternal smoking during pregnancy, (7) maternal smoking at breastfeeding stage, (8) maternal smoking before age 15, (9) paternal smoking before age 15, (10) active smoking before age 15, and (11) active smoking after age 15. COPD=chronic obstructive pulmonary disease.
3.4. Clinical long-term outcomes
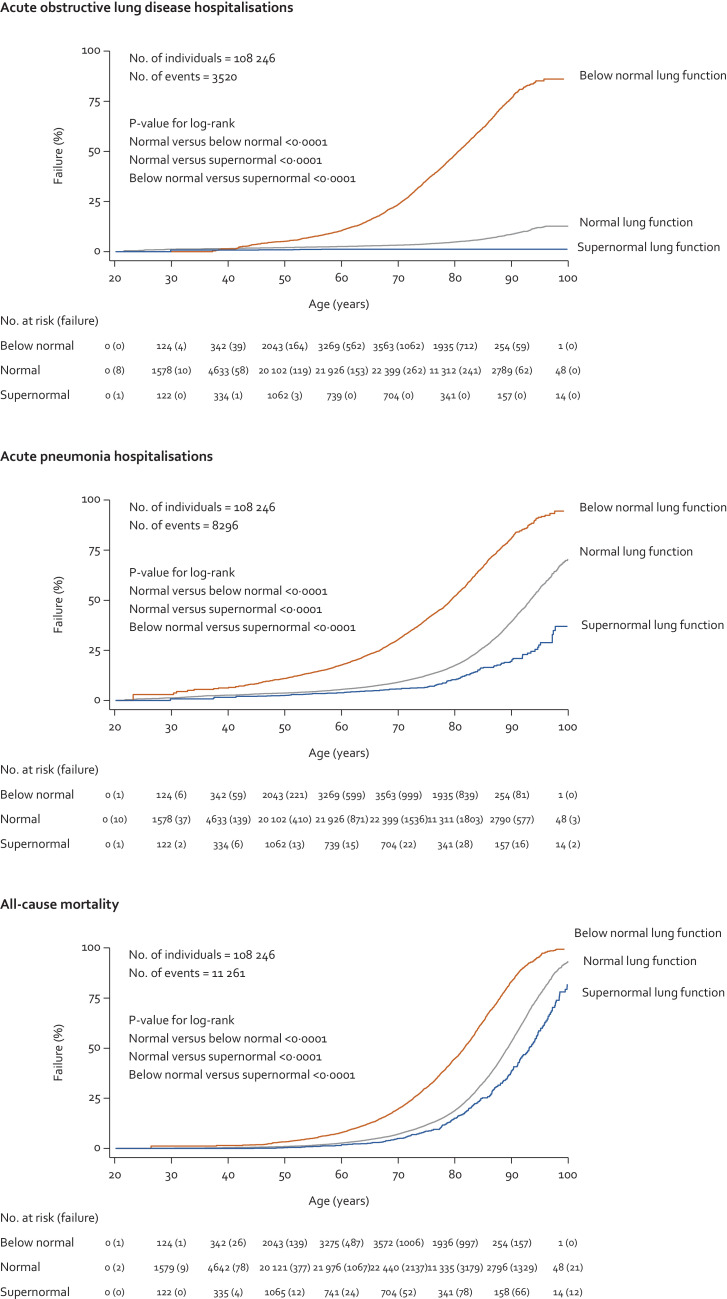
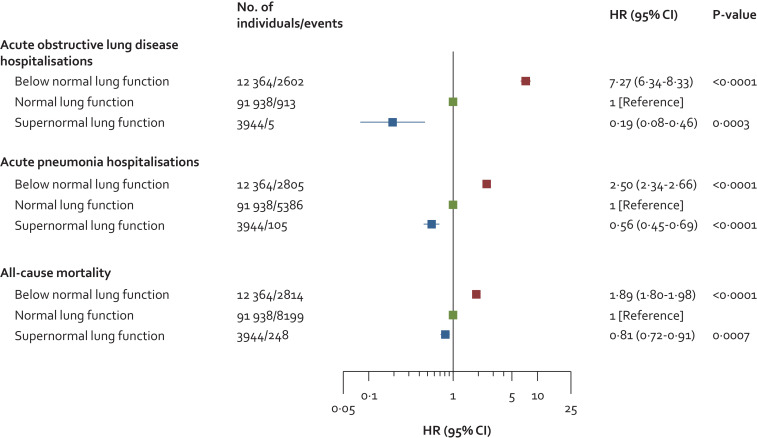
In the entire cohort of 108 246 individuals and during up to 15 years of follow-up (median: 9·3 years and interquartile range: 5·2 years), we observed 3520 acute obstructive lung disease hospitalisations, 8296 acute pneumonia hospitalisations, and 11 261 deaths. Individuals with supernormal lung function had lower risk of acute hospitalisations and mortality compared to individuals with normal and below normal lung function (Fig. 5). Compared to individuals with normal lung function, multivariable adjusted hazard ratios (HRs) in those with supernormal lung function were 0·19 (95% confidence interval: 0·08–0·46, P-value 0·0003) for obstructive lung disease hospitalisations, 0·56 (0·45–0·69, P-value < 0·0001) for pneumonia hospitalisations, and 0·81 (0·72–0·91, P-value 0·0007) for all-cause mortality (Fig. 6). Corresponding respective HRs in those with below normal lung function were 7·27 (6·34–8·33, P-value < 0·0001), 2·50 (2·34–2·66, P-value < 0·0001), and 1·89 (1·80–1·98, P-value < 0·0001). Results were similar without imputation.
Fig. 5.
Kaplan-Meier curves for acute hospitalisations and mortality in individuals with supernormal lung function in the general population. Based on the Copenhagen General Population Study. Analyses used left truncation and age as underlying timescale.
Fig. 6.
Risk of acute hospitalisations and mortality in individuals with supernormal lung function in the general population. Based on the Copenhagen General Population Study. Cox proportional hazard models used left truncation and age as underlying timescale. Analyses were multivariable adjusted for age (as timescale), sex, body mass index, smoking status, cumulative tobacco consumption (pack-years), socioeconomic status, asthma, and treatment with airway medication.
3.5. Sensitivity analyses
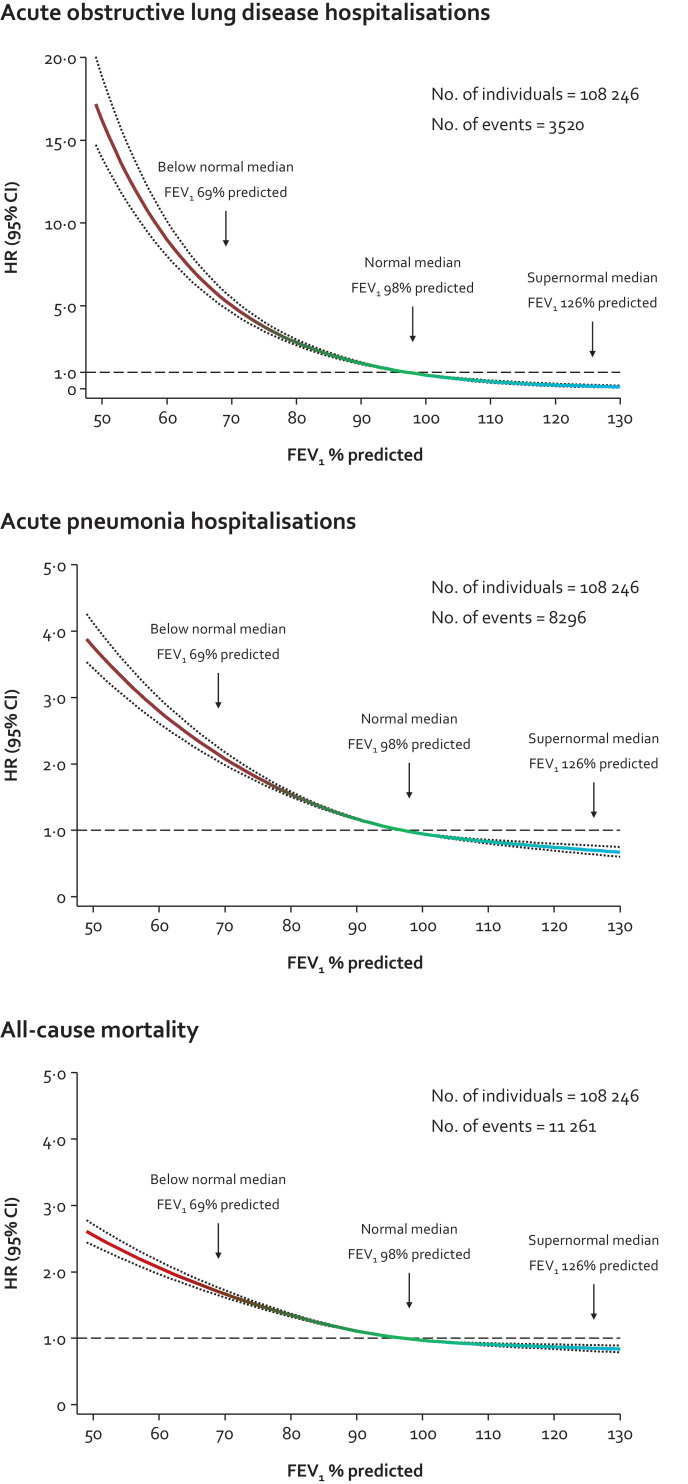
By investigating the dose-response relationship between FEV1% predicted and clinical outcomes using spline curves rather than grouping lung function, we found that the lower risk of clinical outcomes was apparent already from the median in those with normal lung function (Fig. 7).
Fig. 7.
Risk of acute hospitalisations and mortality in individuals according to lung function in the general population. Based on the Copenhagen General Population Study. Cox proportional hazard models used left truncation and age as underlying timescale in restricted cubic splines with median as the reference and three knots chosen according to Harrell's recommendation at the 10th, 50th, and 90th percentiles. Individuals at the bottom and top percentile were included in the actual analyses but excluded from the graphs for visual purposes. Analyses were multivariable adjusted for age (as timescale), sex, body mass index, smoking status, cumulative tobacco consumption (pack-years), socioeconomic status, asthma, and treatment with airway medication.
Among individuals with supernormal lung function, 65 (2%) had FEV1/FVC <LLN despite FEV1 ≥ULN and compared to those with FEV1/FVC ≥LLN, these individuals were characterised by being older and having a lower weight and height but higher FVC (Table S1 in the supplement). Otherwise, no differences could be detected including smoking status and cumulative tobacco consumption. Interestingly, similar but less pronounced differences in age, weight, height, and FVC could also be detected in those with normal lung function (Table S1). When individuals with FEV1/FVC <LLN were excluded from those with supernormal lung function, risk of clinical long-term outcomes was similar (compare Fig. S1 with Fig. 6). Likewise, results were similar when individuals with asthma were excluded (compare Table S2 with Table 2 and Fig. S2 with Fig. 6). All sensitivity analyses were similar without imputation.
4. Discussion
While 3% of individuals with normal lung function developed COPD during 10 years follow-up, none developed COPD in those with supernormal lung function. Potential early-life risk factors usually associated with development of COPD and smoking exposure in different stages of life were less common in those with supernormal versus normal lung function. Furthermore, individuals with supernormal lung function had lower risk of acute hospitalisations due to obstructive lung disease and pneumonia, and lower risk of all-cause mortality.
Normal lung development is characterised by a rapid increase in lung function during childhood and a peak in early adulthood around the ages of 20–25 years followed by a plateau phase for approximately 5–10 years and a subsequent steady normal age-related decline [3]. Theoretically, exposure to different risk factors for COPD during different stages of life may affect development and preservation of normal lung function from birth until old age [19]. Current understanding indicates that development of airflow limitation in COPD seems to follow two major lung function trajectories characterised by low maximally attained lung function in early adulthood and accelerated lung function decline [7]. Recently, it has been hypothesised that a third trajectory of individuals with supernormal lung function, characterised by a lung function with high peak in early adulthood and slower age-related decline in late adulthood, may exist [2]. Since lung function is continuously, and approximately normally, distributed in the general population, the proportion of individuals with supernormal lung function is a product of an arbitarly chosen threshold, thus it cannot be doubted that individuals with supernormal lung function exist. In the present study, we demonstrate that individuals with supernormal lung function have a lower prevalence of potential risk factors for impaired lung development, e.g. disposition to obstructive lung disease, low birth weight [20], [21], [22], [23], [24], passive exposure to parental smoking [25,26], respiratory tract infections [21,[27], [28], [29]], and asthma. [30], [31], [32] This is also in keeping with the notion that supernormal lung function may originate in early life. Supporting results were also observed in a previous study of three population-based birth cohorts, where children with persistently high lung function seem to follow the same lung function trajectory from birth until old age [5,6]. Furthermore, early-life risk factors for COPD were less common in those with persistently high lung function, of whom less than 1% had COPD at 53 years of age [6]. For comparison, in the present study none of the individuals with baseline supernormal lung function developed COPD at the examination 10 years later. Lastly, we were able to show that rather than being a phenomenon of distinct lung function classes, the association of lung function with outcomes is continuous, where lower or higher risk of outcomes is apparent already for small differences from below and above the median values of FEV1% predicted. The obvious question is whether individuals with higher than normal lung function have supernormal lung function or may instead represent ideal lung function and everyone else is inferior to this.
It may somewhat be surprising for some that there was not a single risk factor among clinical characteristics and early-life risk factors that could really predict having supernormal lung function. However, we believe that having supernormal lung function (or higher than normal lung function) does not depend on just one favourable risk factor but instead a combination of several. Although individuals with higher than normal lung function only display small differences for several risk factors when compared to those with normal lung function, the substantial difference in lung function may arise by combining all these small differences. Interestingly, these differences in risk factors were less pronounced when compared to those with normal lung function, but more pronounced when compared to those with below normal lung function, thereby achieving an even higher difference in lung function. Not only genetics, but also type and degree of environmental exposure may influence development of higher than normal lung function.
Interestingly, during 10 years of follow-up, FEV1 decline in mL/year was highest for individuals with supernormal lung function and lowest for those with below normal lung function. Since accelerated FEV1 decline is usually a sign of ongoing disease process in COPD, this finding, at first glance, seems to contradict the favourable risk profile of individuals with supernormal lung function. However, the higher FEV1 decline may not be a sign of accelerated decline but instead a larger attained lung capacity, as it needs to be relative to FEV1/FVC. Indeed, individuals with below normal lung function had the lowest FEV1 decline among all three clinical groups due to their small baseline lung capacity, but still had a higher prevalence of COPD at the examination 10 years later due to the higher FEV1/FVC decline. This was also more apparent using relative decline in each group, as these values were similar. Surprisingly, our results indirectly indicate that factors associated with accelerated decline such as active smoking may also be related to lung growth when exposed to them during childhood. It is not always obvious that these two processes share risk factors; however, this is certainly a topic that could be researched more.
Another important aspect to consider is that individuals with supernormal lung function may be protected against development of COPD compared to those with normal lung function by experiencing fewer respiratory events or attacks, which is supported by the lower risk acute obstructive lung disease and pneumonia hospitalisations. This idea is also supported by the fact that patients with COPD experiencing frequent and severe exacerbations compared to those experiencing few and mild exacerbations have a course with much more advanced disease progression [33].
Strengths of the present study include a large contemporary population-based cohort with randomly selected individuals with a long follow-up. Strengths also include no losses to follow-up due to complete Danish health registries, and information on clinically relevant outcomes for COPD. Importantly, since individuals were recruited randomly from the national Danish Civil Registration System, where all individuals in Denmark are recorded, study findings can be extrapolated to the Danish general population, especially due to the large sample-size with less influence of random variation. In addition, since lung function is biologically and statistically continuously distributed in any general population regardless of nationality and ethnicity, study findings can also be extrapolated to other general populations with a similar context of health care, ethnicity, and socioeconomic level.
A potential limitation of the present study is that post-bronchodilator spirometry was not used to define COPD. Thus, some individuals may have a reversible form of airflow limitation indicating asthma. However, asthma may precede and contribute to COPD development [19]. In addition, COPD was defined as presence of clinical airflow limitation with FEV1/FVC <0·70 and FEV1 <80% of predicted plus at least one chronic respiratory symptom, which is usually used as an inclusion criterion for clinical trials with COPD and as an exclusion criterion for clinical trials with asthma.
Implications of the present study relate to the importance of lung health for development of COPD. Although the benefits of high lung function may be less prioritised than the detrimental effects of low lung function in a clinical setting, the present study should be viewed as a first step in exploring the association between best possible lung function and risk of COPD. Identifying favourable risk factors for high lung function will help societies to promote and secure a future generation with improved lung health from birth to old age to avoid COPD and many other chronic diseases where lung function plays a key role such as asthma and cardiovascular disease.
In conclusion, supernormal lung function among individuals in the general population is associated with lower risk of developing COPD. The results were consistent when using early-life risk factor burden, hospitalisations, and mortality as outcome measures.
Declaration of Competing Interest
YÇ reports personal fees from Boehringer Ingelheim, AstraZeneca, and Sanofi Genzyme outside of the submitted work. JV reports personal fees from GlaxoSmithKline, Chiesi Pharmaceuticals, Boehringer-Ingelheim, Novartis, Almirall, AstraZeneca, Bioxydyn, and Ferring outside of the submitted work. PL reports grants and personal fees from Almirall, grants and personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from Novartis, grants and personal fees from GlaxoSmithKline, personal fees from Nycomed, personal fees from Pfizer, personal fees from Mundipharma outside of the submitted work. BGN and SA have nothing to disclose. The views expressed are those of the authors.
Acknowledgments
Author Contribution
This study was funded by Herlev and Gentofte Hospital, Copenhagen University Hospital, and the Lundbeck Foundation.
Funding
This study was funded by Herlev and Gentofte Hospital, Copenhagen University Hospital, and the Lundbeck Foundation.
Data sharing statement
Additional data regarding technical details, statistical code, and derivative data are available from the principal investigator at shoaib.afzal@regionh.dk on reasonable request. According to national Danish laws, only summary-level data and scripts can be shared. Data access for further analyses may be possible through direct collaborative agreement or through locally managed access arranged through the study's principal investigator.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100974.
Appendix. Supplementary materials
References
- 1.Çolak Y., Afzal S., Nordestgaard B.G., Vestbo J., Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5:426–434. doi: 10.1016/S2213-2600(17)30119-4. [DOI] [PubMed] [Google Scholar]
- 2.Agusti A., Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7:358–364. doi: 10.1016/S2213-2600(18)30529-0. [DOI] [PubMed] [Google Scholar]
- 3.Agusti A., Hogg J.C. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1248–1256. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- 4.Agusti A., Noell G., Brugada J., Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5:935–945. doi: 10.1016/S2213-2600(17)30434-4. [DOI] [PubMed] [Google Scholar]
- 5.Belgrave D.C.M., Granell R., Turner S.W. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6:526–534. doi: 10.1016/S2213-2600(18)30099-7. [DOI] [PubMed] [Google Scholar]
- 6.Bui D.S., Lodge C.J., Burgess J.A. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 7.Lange P., Celli B., Agusti A. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 8.Martinez F.D. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 9.Çolak Y., Nordestgaard B.G., Vestbo J., Lange P., Afzal S. Relationship between supernormal lung function and long-term risk of hospitalisations and mortality: a population-based cohort study. Eur Respir J. 2020 doi: 10.1183/13993003.04055-2020. [DOI] [PubMed] [Google Scholar]
- 10.Çolak Y., Afzal S., Nordestgaard B.G., Vestbo J., Prevalence LP. Characteristics, and prognosis of early chronic obstructive pulmonary disease: the copenhagen general population study. Am J Respir Crit Care Med. 2019;201:671–680. doi: 10.1164/rccm.201908-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Çolak Y., Afzal S., Nordestgaard B.G., Lange P. Majority of never-smokers with airflow limitation do not have asthma: the copenhagen general population study. Thorax. 2016;71:614–623. doi: 10.1136/thoraxjnl-2015-208178. [DOI] [PubMed] [Google Scholar]
- 12.Çolak Y., Nordestgaard B.G., Vestbo J., Lange P., Afzal S. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J. 2019;54 doi: 10.1183/13993003.00734-2019. [DOI] [PubMed] [Google Scholar]
- 13.Çolak Y., Nordestgaard B.G., Laursen L.C., Afzal S., Lange P., Dahl M. Risk factors for chronic cough among 14,669 individuals from the general population. Chest. 2017;152:563–573. doi: 10.1016/j.chest.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Çolak Y., Afzal S., Nordestgaard B.G., Marott J.L., Lange P. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: the copenhagen general population study. Eur Respir J. 2018;52 doi: 10.1183/13993003.00616-2018. [DOI] [PubMed] [Google Scholar]
- 15.Çolak Y., Afzal S., Nordestgaard B.G., Vestbo J., Lange P. Young and middle-aged adults with airflow limitation according to lower limit of normal but not fixed ratio have high morbidity and poor survival: a population-based prospective cohort study. Eur Respir J. 2018;51 doi: 10.1183/13993003.02681-2017. [DOI] [PubMed] [Google Scholar]
- 16.Løkke A., Marott J.L., Mortensen J., Nordestgaard B.G., Dahl M., Lange P. New Danish reference values for spirometry. Clin Respir J. 2013;7:153–167. doi: 10.1111/j.1752-699X.2012.00297.x. [DOI] [PubMed] [Google Scholar]
- 17.Aaron S.D., Donaldson G.C., Whitmore G.A., Hurst J.R., Ramsay T., Wedzicha J.A. Time course and pattern of COPD exacerbation onset. Thorax. 2012;67:238–243. doi: 10.1136/thoraxjnl-2011-200768. [DOI] [PubMed] [Google Scholar]
- 18.Harrell F.E. 1st Ed. Springer; New York: 2001. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. [Google Scholar]
- 19.Postma D.S., Bush A., van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385:899–909. doi: 10.1016/S0140-6736(14)60446-3. [DOI] [PubMed] [Google Scholar]
- 20.Barker D.J., Godfrey K.M., Fall C., Osmond C., Winter P.D., Shaheen S.O. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaheen S.O., Sterne J.A., Tucker J.S., Florey C.D. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998;53:549–553. doi: 10.1136/thx.53.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle L.W., Andersson S., Bush A. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med. 2019;7:677–686. doi: 10.1016/S2213-2600(18)30530-7. [DOI] [PubMed] [Google Scholar]
- 23.Lawlor D.A., Ebrahim S., Davey Smith G. Association of birth weight with adult lung function: findings from the British Women's Heart and Health Study and a meta-analysis. Thorax. 2005;60:851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rona R.J., Gulliford M.C., Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306:817–820. doi: 10.1136/bmj.306.6881.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerra S., Stern D.A., Zhou M. Combined effects of parental and active smoking on early lung function deficits: a prospective study from birth to age 26 years. Thorax. 2013;68:1021–1028. doi: 10.1136/thoraxjnl-2013-203538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y.F., Gilliland F.D., Berhane K. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162:2097–2104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- 27.Burrows B., Knudson R.J., Lebowitz M.D. The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis. 1977;115:751–760. doi: 10.1164/arrd.1977.115.5.751. [DOI] [PubMed] [Google Scholar]
- 28.Chan J.Y., Stern D.A., Guerra S., Wright A.L., Morgan W.J., Martinez F.D. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135:607–616. doi: 10.1542/peds.2014-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston I.D., Strachan D.P., HR A. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med. 1998;338:581–587. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 30.Bisgaard H., Jensen S.M., Bonnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 31.McGeachie M.J., Yates K.P., Zhou X. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sears M.R., Greene J.M., Willan A.R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 33.Dransfield M.T., Kunisaki K.M., Strand M.J. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.