Abstract
The bacterial cell wall contains numerous surface-exposed proteins, which are covalently anchored and assembled by a sortase family of transpeptidase enzymes. The sortase are cysteine transpeptidases that catalyzes the covalent attachment of surface protein to the cell wall peptidoglycan. Among the reported six classes of sortases, each distinct class of sortase plays a unique biological role in anchoring a variety of surface proteins to the peptidoglycan of both pathogenic and non-pathogenic Gram-positive bacteria. Sortases not only exhibit virulence and pathogenesis properties to host cells, but also possess a significant role in gut retention and immunomodulation in probiotic microbes. The two main distinct functions are to attach proteins directly to the cell wall or assemble pili on the microbial surface. This review provides a compendium of the distribution of different classes of sortases present in both pathogenic and non-pathogenic Gram-positive bacteria and also the noteworthy role played by them in bacterial cell wall assembly which enables each microbe to effectively interact with its environment.
Keywords: Sortase, Cell wall, Gram-positive, Pathogenic, Non-pathogenic
1. Introduction
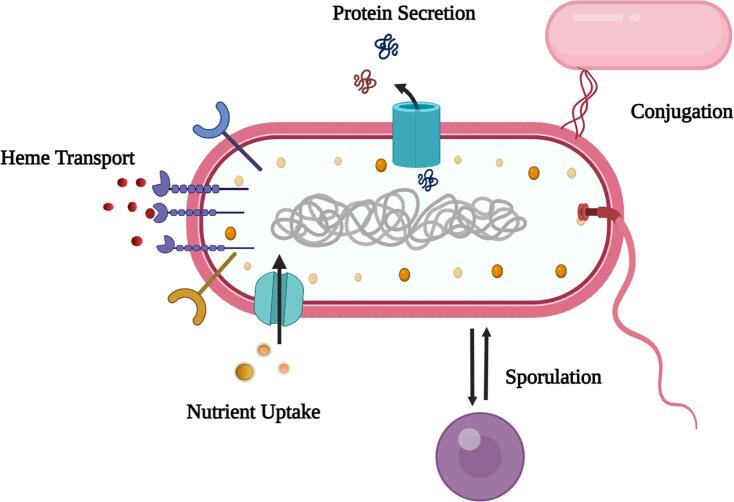
The cell wall of Gram-positive bacteria contains a multi-layered cellular component that acts as a cytoskeletal element for maintaining physical integrity and also acts as a scaffold for displaying a large number of surface proteins mediated by sortase enzymes. Sortases are membrane-bound transpeptidases that cleave the sorting signal of the secreted protein to form an isopeptide bond between the secreted protein and peptidoglycan. They are either responsible for covalently anchoring specific surface proteins or polymerizing pilin sub-units to form a proteinaceous structure termed pili (Hendrickx et al., 2011). Sortase-displayed surface structures play a pivotal role in displaying virulence and pathogenesis properties without affecting the growth and viability of cells. They are responsible for cell attachment, heme transport, nutrient uptake, sporulation and aerial hyphae formation (Cheng et al., 2009, Weiss et al., 2004) (Fig. 1). The surface proteins recognized by the sortase enzyme contain a C-terminal pentaglycine recognition motif followed by a stretch of hydrophobic amino acids and a positively charged tail (Schneewind et al., 1992).
Fig. 1.
General functions of sortases in a bacterial cell wall. Sortases are involved in pili formation, cell attachment to the host tissues, anchoring surface proteins to the cell wall, spore formation, uptake of nutrients and iron from the surrounding environment.
Sortases are classified into six different classes (A-F), based on their primary sequences (Spirig et al., 2011) (Table 1). Class A sortase is well characterized and found mostly in low GC content Gram-positive bacteria. They play a housekeeping role in anchoring a variety of functionally distinct surface proteins with an LPXTG recognition sequence. Class B sortase actively participates in iron acquisition by recognizing iron transporter proteins with a NPQTN motif and covalently anchors them to the cell wall. Class C sortases are responsible for constructing complex pili polymers by recognizing the LPXTG motif. Class D sortases display proteins containing a LPXTA recognition motif on the cell wall that enables spore formation. GC-rich actinobacteria, in particular Corynebacterium and Streptomyces spp. contain Class E sortase which performs a similar function to Class A sortases. Class E sortases are involved in anchoring surface proteins and aerial hyphae proteins by recognizing a LAXTG sorting motif. Class F sortases were initially reported in Propionibacterium acnes which contains an LPXTG sorting signal similar to sortase A (Girolamo et al., 2019). All sortases contain a His-Cys-Arg as the catalytic triad, which catalyzes the transpeptidation reaction (Frankel et al., 2005, Frankel, 2007, Perry et al., 2002). The cysteine in the active site of the enzyme is involved in bond cleavage and formation of a stable thioacyl intermediate that is relieved by the nucleophilic attack of the amino group (pentaglycine crossbridge) in peptidoglycan synthesis precursors (Mazmanian et al., 1999). In site-directed mutagenesis, the replacement of cysteine (at position 184 in S. aureus) with an alanine abolishes sortase catalytic activity in vitro and in vivo. The dual acid/base role was carried out by His residue which donates a proton to the leaving amide nitrogen during the cleavage reaction and the second substrate accepts the proton from the amino group to allow the nucleophilic attack by the unprotonated amine. The Arg side chain is implicated in substrate binding and possibly in stabilisation of a presumed oxyanion intermediate (Frankel et al., 2007).
Table 1.
Sortase classification.
| Sortase Class | Cleavage site | Main Function | Bacterial genus |
|---|---|---|---|
| A | LPXTG | Surface protein anchoring | Staphylococcus, Listeria, Streptococcus, Bacillus, Clostridium, Entrobacter, Lactobacillus |
| B | (N/S/P)PXTG | Heme uptake | Bacillus, Listeria, Bacillus, |
| C | LPXTG | Pili assembly | Corynebacterium, Streptococcus, Clostridium, Actinomyces, Entrobacter, Lactobacillus |
| D | LPXTA | Spore formation | Bacillus |
| E | LAXTG | Aerial hyphae formation, Surface protein anchoring, Pilus attachment | Corynebacterium, Streptomyces, Actinomyces |
| F | LPXTG | Unknown | Propionibacterium |
Among different classes of sortases, the SrtA of Staphylococcus aureus is the pioneer for understanding the mechanism of these enzymes. After recognition of cell wall surface protein, SrtA catalyzes two sequential reactions: (i) thioesterification and (ii) transpeptidation. The enzyme first recognizes the pentaglycine sequence on the surface proteins, which are being secreted through the cytoplasmic membrane. The pentaglycine sequence contains an LPXTG motif at the C-terminus of the protein. In the second step, the SrtA cleaves the scissile bond between threonine and glycine residues to form an acyl-enzyme intermediate which subsequently transfers the carboxyl of threonine which is amide-linked to the pentaglycine cross-bridge of lipid II (Marraffini et al., 2006). Finally, the lipid II surface protein complex gets incorporated into the peptidoglycan by means of transglycosylation and transpeptidation reactions (Paterson and Mitchell, 2004, Spirig et al., 2011). The sortase enzyme accepts the nucleophiles which might vary in different Gram-positive bacteria, as the composition of peptidoglycan layers in the cell envelope vary from strain to strain..
Sortases are not only restricted to Gram-positive bacteria, however some sortase genes and its potential substrates are also found in Gram-negative bacteria which are not well characterized. Some Gram-negative bacteria such as Shewanella putrefascienes, Shewanella oneidensis, Microbulbifer degradans, Colwellia psychrerythraea and Bradyrhizobium japonicum consist of a gene encoding a single sortase-like protein and a potential sortase substrate (Comfort and Clubb, 2004).
Although there are many studies on sortases, referencing virulence and colonization factors, there are only a few reports on sortases that display proteins in non-pathogenic bacteria which includes food grade microbes of the lactic acid bacteria (LAB) and in Corynebacterium glutamicum, an industrial important microbe for the production of amino acids. In non-pathogenic bacteria, especially in probiotics, the sortase plays a pivotal role in eliciting health benefits to the host. Distinctive sortases in pathogenic and non-pathogenic bacteria are shown in Table 2 and this review provides a description about the virulence and functional aspects of sortases reported in each Gram-positive species that has been published.
Table 2.
Distinctive sortases in pathogenic and non-pathogenic bacteria.
| Bacterial Species | Sortase protein | Putative CWSS proteins | References |
|---|---|---|---|
| Actinomyces oris | SrtA, SrtC (SrtC1 and SrtC2) | 14 | (Wu et al., 2011, Wu et al., 2012) |
| Bacillus anthracis | SrtA, SrtB, SrtC | 10 | (Gaspar et al., 2005, Maresso et al., 2006, Marraffini et al., 2006) |
| Bacillus cereus | SrtA, SrtB, SrtC (SrtC1 and SrtC2), SrtD | 2 | (Budzik et al., 2007) |
| Bacillus subtilis | YhcS | 2 | (Duc Nguyen et al., 2011) |
| Bifidobacterium bifidum | SrtA | 14 | (Westermann et al., 2012, Wei et al., 2016) |
| Clostridium perfringens | SrtB | 13 | (Boekhorst et al., 2005, Van Leeuwen et al., 2014) |
| Corynebacterium diphtheriae | SrtA, SrtB, SrtC, SrtD, SrtE, SrtF | 17 | (Mandlik et al., 2007) |
| Corynebacterium glutamicum | SrtE | 1 | (Susmitha et al., 2019) |
| Enterobacter faecalis | SrtA, SrtC | 41 | (Kemp et al., 2007) |
| Lactobacillus plantarum | SrtA | 32 | (Pretzer et al., 2005) |
| Lactobacillus rhamnosus | SrtA, SrtC (SrtC1 and SrtC2) | 6 | (Douillard et al., 2014) |
| Lactococcus lactis | SrtA, SrtC | 14 | (Dieye et al., 2010) |
| Lactobacillus salivarius | SrtA | 10 | (Van Pijkeren et al., 2006) |
| Lactobacillus casei | SrtA (SrtA1 and SrtA2), SrtC (SrtC1 and SrtC2) | 23 | (Muñoz-Provencio et al., 2012) |
| Lactobacillus acidophilus | SrtA | 12 | (Call et al., 2015) |
| Lactobacillus gasseri | SrtA | 12 | (Call et al., 2015) |
| Lactobacillus delbrueckii | SrtA | 2 | (Van Pijkeren et al., 2006) |
| Listeria monocytogenes | SrtA, SrtB | 43 | (Bierne et al., 2002, Garandeau et al., 2004) |
| Propionibacterium acnes | SrtF | 4 | (Girolamo et al., 2019, Lodes et al., 2006) |
| Staphylococcus aureus | SrtA, SrtB | 22 | (Mazmanian et al., 2000, Mazmanian et al., 2002) |
| Streptococcus suis | SrtA, SrtB, SrtC, SrtD, SrtE | 3 | (Osaki et al., 2002, Lu et al., 2011) |
| Streptococcus pyogenes | SrtA, SrtB | 15 | (Barnett and Scott, 2002) |
| Streptococcus agalactiae | SrtA, SrtC (SrtC1, SrtC2, SrtC3 and SrtC4) | 35 | (Lalioui et al., 2005, Dramsi et al., 2006) |
| Streptococcus pneumoniae | SrtA, SrtC (SrtC1, SrtC2 and SrtC3) | 16 | (Kharat and Tomasz, 2003, Lemieux et al., 2008) |
| Streptomyces avermitilis | SrtE (SrtE3) | 16 | (Das et al., 2017) |
| Streptococcus mutans | SrtA | 6 | (Lee and Boran, 2003) |
| Streptomyces coelicolor | SrtE (SrtE1 and SrtE2) | 17 | (Duong et al., 2012) |
| Streptococcus gordonii | SrtA | 7 | (Davies et al., 2009, Nobbs et al., 2007) |
| Streptococcus sanguinis | SrtA | 32 | (Yamaguchi et al., 2006, Turner et al., 2009) |
| Streptococcus uberis | SrtA | 10 | (Egan et al., 2010, Eigh et al., 2010) |
| Streptococcus thermophilus | SrtA | 2 | (Kebouchi et al., 2016) |
2. Pathogenic bacteria
2.1. Staphylococcus aureus
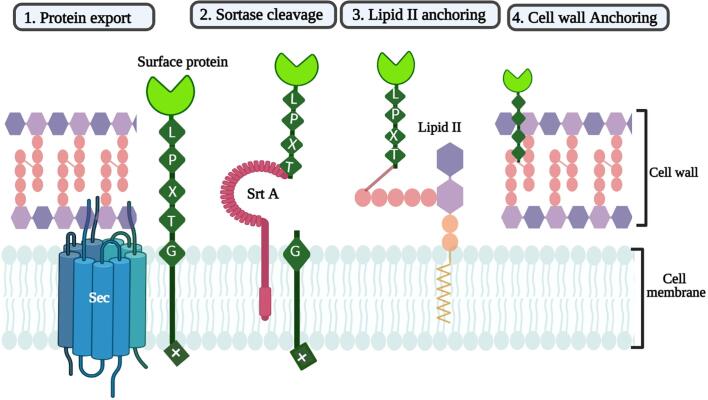
S. aureus is one of the most predominant pathogens responsible for causing mastitis, an inflammation of breast tissue (Duarte et al., 2015), skin infection, pneumonia, sepsis, and endocarditis in humans (Zhang et al., 2015). However, most of these infections are caused by the anchoring of a vast array of virulence-associated surface proteins to the cell wall, which are catalyzed by a cysteine transpeptidase enzyme called Sortase A (Mazmanian et al., 2000). S. aureus sortase A (SrtA) has been the prototype for understanding the mechanism of action of these enzymes (Mazmanian et al., 1999). The SrtA of S. aureus covalently anchors the surface proteins onto the bacterial cell wall via a C-terminal cell wall sorting signal with an LPXTG recognition motif followed by a stretch of hydrophobic amino acids and a positively charged tail (Das et al., 2017, Novick, 2000). The cell wall anchoring proteins are synthesized within the cytoplasm and translocated across the membrane through the Sec machinery. The sortase recognizes the anchoring proteins followed by a nucleophilic attack at the active site of the cysteine and cleaves the C-terminal of LPXTG motif between threonine and glycine forming a thioester intermediate complex which is then covalently anchored on the pentaglycine cross-bridge of lipid II. The lipid II protein complex then gets attached to the cell wall via transglycosylation and transpeptidation reactions (Perry et al., 2002) (Fig. 2). S. aureus attaches several surface proteins which are characterized by a C-terminal LPXTG motif, including protein A (Spa), two fibronectin-binding proteins (FnbpA and FnbpB), two clumping factors (ClfA and ClfB), a collagen-binding protein (Cna), and three serine-aspartate repeat proteins (SdrC, SdrD, and SdrE). The deletion of SrtA has led to the failure of surface protein anchoring to the cell wall (Clancy et al., 2010). The mechanism of sortase B is similar to that of sortase A, where S. aureus sortase B enzyme attaches the heme transporter IsdC protein which is a major component of the iron-regulated surface determinant system that scavenges the heme–iron from hemoglobin. The SrtB anchors IsdC to uncross-linked peptidoglycan instead of heavily cross-linked peptidoglycan. The srtB and isdC genes are located together in the isd iron-acquisition operon. However, in contrast to SrtA, SrtB recognizes NPQTN sorting signals from S. aureus (Mazmanian et al., 2002). Gene knockout studies in S. aureus revealed that the abolition of srtB gene is responsible for virulence and does not affect cell viability.
Fig. 2.
Illustration of sortase A transpeptidation reaction in S. aureus. 1) Protein synthesized from the cytosol gets translocated through the Sec machinery and gets anchored on the cell membrane. 2) Sortase recognizes the C-terminal of the LPXTG sorting motif and cleaves between threonine and glycine. 3) Sortase forms a protein complex and undergoes a nucleophilic reaction from lipid II molecule. 4) Lipid II– protein molecule gets further anchored to the cell wall via transpeptidation reaction.
2.2. Corynebacterium spp.
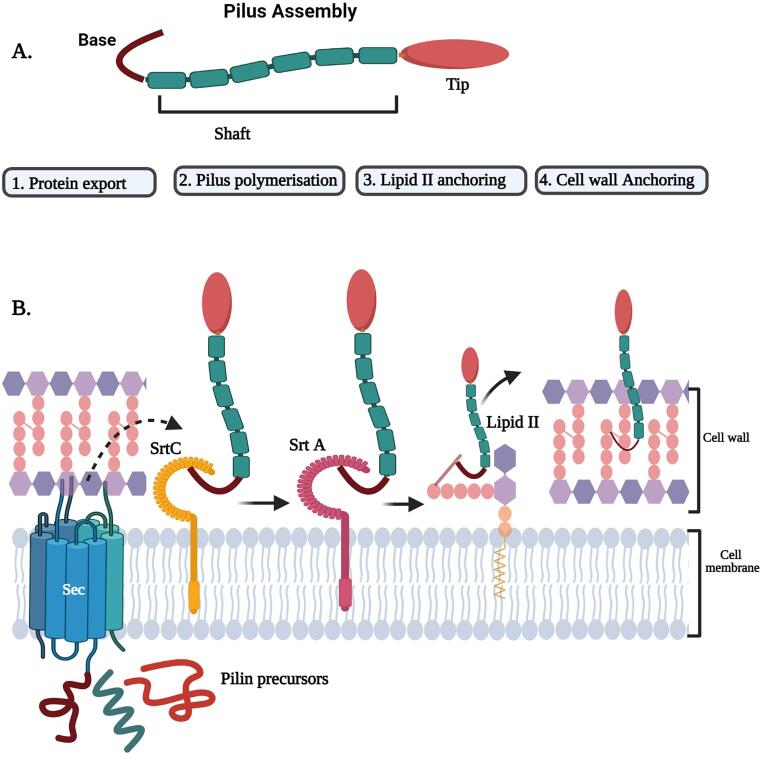
C. diphtheriae is the etiological agent of pharyngeal diphtheria in humans (Hadfield et al., 2000). The genome of C. diphtheriae NCTC13129 harbors six sortases like genes (named srtA-F), five of which presumably assembles three distinct types of pilus structures- SrtA for the SpaA-type pilus, SrtB or SrtC for the SpaD-type pilus, and SrtD or SrtE for the SpaH-type pilus (Spa for sortase-mediated pilus assembly) which are polymerized by specific Class C sortases and SrtF which belongs to class A sortase, catalyzes the anchoring of pilin monomers on the bacterial surface (Gaspar and Ton-That, 2006, Swaminathan et al., 2007, Ton-That and Schneewind, 2003). All three pilus structures share a similar architecture, a major pilin (designated as SpaA, SpaD, and SpaH) along the pilus shaft joined to the minor pilins (designated as SpaB, SpaC, SpaE, SpaF, SpaI and SpaG) located at the tip and base of the pilin (Fig. 3). To analyze the functions of sortase in pili formation, Mandlik and his coworkers constructed an isogenic mutant strain of NCTC13129 devoid of all six sortase genes (srtA–F mutant) that exhibited a severe defect in adherence to epithelial cells.
Fig. 3.
Schematic illustration of Sortase-mediated pili. A) Pilus assembly which consist of tip pilin (red), Shaft (green) and the base (brown). B. 1) The pilin precursors synthezised from the cytosol enters through the sec machinery. 2) The pilin- specific sortase SrtC (yellow) recognizes the tip pilin and cleaves at the sorting motif and forms an acyl sortase complex. The pilus specific sortase receives a nucleophilic attack from the lysine side chain from the backbone pilin to form a covalent bond between the pilins and undergoes pilin polymerization. C) The housekeeping sortase undergoes a nucleophilic attack from lipid II molecule. 4) The polymerized pilin is further anchored to the cell wall. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The single pilus-specific SrtA encoded within the spaA gene cluster specifically catalyzes the covalent crosslinking of individual pilin monomers and also anchors pili to the cell wall. To analyze the functions of SrtA, immunoelectron microscopy, and biochemical analysis showed that a strain expressing only SrtA secretes significant amounts of polymerized pilins into the culture medium, indicating that one or more sortases might be involved for efficient cell wall anchoring of pili (Mandlik et al., 2007, Ton-That et al., 2004). Indeed, the strain with the deletion of housekeeping gene srtF releases SpaA polymers into the culture medium. Thus, two sortases are involved in pilus biogenesis, a pilus-specific sortase for pilin polymerization and the housekeeping sortase for efficient anchoring of pili to the cell wall (Mandlik et al., 2010, Swaminathan et al., 2007). The deletion of srtA or spaA gene completely abrogates the assembly of SpaA pili and deletion of spaC and spaB did not abolish SpaA pilus formation. This evidence suggests that SrtA catalyzes the assembly of SpaA pilus and SpaA alone is sufficient to mediate the polymerization of a secreted protein (Marraffini et al., 2006, Ton-That and Schneewind, 2003).
Unlike the SpaA-type pili, which are assembled by a single sortase, two sortases; SrtB and SrtC catalyze the assembly of the SpaD-type pili. The deletion of srtB alone or both srtB and srtC abrogated the incorporation of SpaE into SpaDF pili. These results demonstrate that SpaDEF pilus assembly specifically requires SrtB for the incorporation of SpaE into SpaDF pili and whose assembly requires either SrtB or SrtC (Gaspar and Ton-That, 2006).
Likewise, the SpaH pilus is independently assembled and different from the other two corynebacterial pili. The SrtD specifically required for the incorporation of SpaH into SpaIG pili, whose assembly requires either SrtD or SrtE, while other remaining sortases are dispensable (Swierczynski and Ton-That, 2006). Thus, the housekeeping sortase contributes to efficient cell wall anchoring with other sortases involved in SpaD and SpaH–type pilus (Swaminathan et al., 2007). Corynebacterium spp also contains an industrially important non-pathogenic microbe, C. glutamicum which encodes a single sortase enzyme. The sortase enzyme shows high substrate specificity towards the LAXTG sorting sequence of class E sortase. The two-dimensional structure of Cg-SrtE was found to be similar to C. diphtheriae sortase. The Cg-SrtE was biochemically characterized and shows a Ca2+ independent catalytic mechanism (Susmitha et al., 2019). The high catalytic efficiency with LAXTG substrate and the Ca2+ independency, allows such non-pathogenic Cg-SrtE to be used in sortagging applications such as protein immobilization, for sortase assay kit and as a self-cleaving tag for protein purification etc., which makes the enzyme more robust than the pathogenic S. aureus SrtA variants.
2.3. Listeria monocytogenes
L. monocytogenes is a facultative intracellular food-borne Gram-positive bacterium, responsible for life-threatening infections in humans and animals. It is a causative agent of listeriosis, which is characterized by gastroenteritis, meningitis, encephalitis, bacteremia, soft tissue, and parenchymal infections, and mother-to-fetus transmission (Dussurget et al., 2004, Posfay-barbe and Wald, 2009). Among the Gram-positive bacteria, the genus Listeria contains the highest number of genes encoding surface proteins in the range of 40–45 with an LPXTG bearing motif at the C-terminal, 2 surface proteins containing an NPKSS/NAKTN motif (Lmo2185 and Lmo2186), and two sortases (SrtA and SrtB) (Boekhorst et al., 2005, Cabanes et al., 2002, Cossart, 2007, Garandeau et al., 2004). Inactivation of the srtA gene in L. monocytogenes altered the expression of specific anchored surface proteins containing the canonical LPXTG motif, ultimately decreasing the ability of the bacterial adhesion, invasion of eukaryotic cells, and affects host immune responses (Bierne et al., 2002). The RT-PCR and Western-blot data analysis demonstrates that the lack of SrtA alters the expression of LPXTG surface proteins and does not completely abolish the strong attachment of certain surface proteins to cell-wall peptidoglycan (Mariscotti et al., 2012). Therefore, as in S. aureus, the listerial SrtB represents the second class of sortase in L. monocytogenes, generally expressed in operons containing genes encoding their substrates with NPKSS/NAKTN recognition motifs. The srtB deletion mutants do not have defects in bacterial entry, growth, or motility in tissue-cultured cells and do not show attenuated virulence in mice. SrtB-mediated anchoring could therefore be required to anchor surface proteins involved in the adaptation of this microorganism to different environmental conditions (Garandeau et al., 2004).
2.4. Streptococcus spp.
Streptococcus pyogenes is a human pathogen which causes life-threatening diseases such as necrotizing facititis, septicemia and toxic shock syndrome which results in 500,000 death per year (Dekker and Boersma, 2018). S. pyogenes contains SrtA which localizes LPXTG cell surface virulent factors; M protein, GRAB, protein F, and ScpA. The other class of enzyme SrtB anchors T6 protein and its role on pathogenesis is still unclear (Barnett and Scott, 2002). The deletion of sortase A mutant leads to accumulation of surface proteins onto the cell wall, aberrant morphology, reduced growth, and increased membrane permeability (Raz et al., 2015).
Streptococcus agalactiae is known for life-threatening neonatal infections, such as pneumonia, sepsis, and meningitis (Lalioui et al., 2005). It is a commensal bacterium predominantly found in colonization with gastrointestinal and genitourinary tracts. It is a serious cause for mortality or morbidity in pregnant and non-pregnant adults suffering from significant underlying diseases (Lalioui et al., 2005, Sendi and Johansson, 2008). The genome analysis of S. agalactiae NEM316 encompasses one class A, four class C sortases (Dramsi et al., 2005), and 35 surface proteins containing a cell wall sorting signal motif (26 proteins had an LPXTG motif, 4 had an IPXTG motif, 2 had an LPXTS motif, 2 had an LPXTN motif, and 1 had an FPXTG motif (Glaser et al., 2002). S. agalactiae NEM316 strain lacking srtA gene was found defective in anchoring cell surface proteins Alp2 (GBS 0470) and ScpB (GBS1308) bearing LPXTG and LPXTN signature sequence at the C-terminal (Lalioui et al., 2005). To determine the activity of SrtA in fibronectin and fibrinogen binding, a simple binding assay (ELISA) was performed to compare the binding properties of SrtA- mutant with those of wild-type and complemented strains. This resulted in reduced binding of fibronectin and fibrinogen in SrtA- mutant strain. Thus, it is conceivable that the ScpB a fibronectin protein (Beckmann et al., 2002), and FbsA (GBS1087) a major GBS fibrinogen-binding protein is a SrtA-dependent LPXTG-containing protein (Schubert et al., 2004, Schubert et al., 2002). The inactivation of srtA in NEM316 strain decreased its adherence to human epithelial cell lines (A549, Caco-2, and HeLa) and rat cell lines (L2). Interestingly, the deletion of srtA strains did not alter the virulence in the neonatal rat sepsis model as compared to the wild-type parental strain (Lalioui et al., 2005).
Besides SrtA, four genes encoding class C sortases (SrtC) were found in NEM316, 2603 V/R, and A909 genome sequences which are arranged tandemly in two different loci, srtC1-srtC2 and srtC3-srtC4 coding for pilus biogenesis (Dramsi et al., 2006, Khare et al., 2011). Based on the electron microscopy and immunogold labeling, the NEM316 strain assembles pili from the srtC3-srtC4 locus and encodes three pilins subunits, the major pilin, and two minor pilins. Either SrtC3 or SrtC4 is required for polymerization of pili and housekeeping SrtA anchors the polymerized pili to the cell wall (Dramsi et al., 2006).
Streptococcus pneumoniae is a human pathogen responsible for multiple infections, including otitis media, meningitis, pneumonia, and septicemia (Lemieux et al., 2008). The genome of S. pneumoniae TIGR4 and R6 contains srtA gene and in addition to that TIGR4 also contains srtC-1, srtC-2, and srtC-3 for pilus assembly. The inactivation of srtA from S. pneumoniae was shown to affect the localization of β-galactosidase and neuraminidase (NanA) surface proteins and decreased bacterial adherence and invasion to human pharyngeal cells in vitro. On the other hand, srtA inactivation did not affect the virulence of capsular type III strain of S. pneumoniae in the mouse intraperitoneal model (Kharat and Tomasz, 2003, Paterson and Mitchell, 2006). The rlrA pathogenicity islet of S. pneumoniae encodes three SrtC isoforms and three structural PI-1 subunit proteins; RrgA, RrgB, and RrgC. The RrgB pilus subunit forming the major backbone of pilus which comprises a pilin motif, E box, and C-terminal cell wall sorting signal (CWSS). RrgA found at the tip of the pilus and involved in pilus adhesion. RrgC anchors assembled pilus to the cell wall in association with SrtA. The primary SrtC-1 catalyzes the polymerization of major pilin subunit RrgB. SrtC-2 binds with RrgA and attaches to other pilins. SrtC-3 preferentially binds with RrgC pilin subunit but does not have a strong affinity as SrtC-1 with RrgB (Lemieux et al., 2008, Naziga and Wereszczynski, 2017, Shaik et al., 2014).
Streptococcus mutans plays a significant role in the development of human dental cavities (Hamada and Slade, 1980). The importance of SrtA in modulating the cell-surface-related properties by surface anchoring proteins; WapA, Pac, GbpC, Dex, and FruA in S. mutans was confirmed by constructing a srtA mutant which was found to be non-adherent, non-aggregating, and less hydrophobic than the srtA complemented strain. The pathogenesis of SrtA in Streptococci was demonstrated in a rat model of infection, where the inactivation of srtA was incapable of colonizing the oral mucosa in the absence of sucrose and in the presence of sucrose colonization was found to be less effective (Lee and Boran, 2003). These phenomena provides evidence that SrtA could be a novel and attractive drug target for the prevention of cariogenicity (Lee and Boran, 2003, Murai et al., 2005).
Streptococcus gordonii is a non-cariogenic pioneer colonizer in the development of dental plaque (Kuboniwa et al., 2006). They are found at multiple sites within the oral cavity and adhere to teeth, as well as mucosa, through the interaction between macromolecules on the bacterial cell wall and proteins or glycoproteins on the oral surfaces (Aas et al., 2005, Nobbs et al., 2007). S. gordonii consists of SrtA which anchors surface adhesins (SspA/B) with an LPXTG containing recognition motifs, hydrophobic spanning regions and positively charged tail at the C-terminal. The disruption of srtA gene changes the localization and function of SspA and SspB adhesins, reduces the biofilm formation and binding to specific salivary agglutinin receptor in vitro (Davies et al., 2009).
Streptococcus sanguinis is a member of the oral mitis group of Streptococci and the initial colonizer for dental plaque formation (Kolenbrander et al., 1993). Although, during oral injuries, the harmless members of the group invade into the bloodstream causing bacteremia and infective endocarditis (Morita et al., 2014). The deficiency of srtA in S. sanguinis causes an overall reduction in virulence in association with cell surface proteins and decreased cell surface hydrophobicity. Thus, SrtA of oral streptococci is considered an important molecule for colonization on the smooth surface of the teeth and a drug target to prevent dental biofilm formation (Yamaguchi et al., 2006).
Streptococcus uberis is one of the most common pathogens associated with the lactating bovine mammary gland and impacts on animal health, welfare, and economics of milk production (Bradley et al., 2007, Egan et al., 2010, Eigh et al., 2010). S. uberis contains only a single copy of sortase A (srtA), encoding a transamidase capable of anchoring surface proteins bearing the LPXTG or LPXXXD motifs at the bacterial cell surface (Egan et al., 2010). The srtA deficient strain of S. uberis was unable to colonize the bovine mammary gland to induce clinical mastitis in dairy cattle indicating that a number of SrtA-anchored proteins are likely to be involved in the pathogenesis of this bacterium (Egan et al., 2010).
Streptococcus suis is a chain-forming Gram-positive bacteria that causes meningitis in pigs and responsible for the economic losses to the swine industry. The zoonotic pathogen also emerges to be a major risk to humans working in the pig industry (Fittipaldi et al., 2012). S. suis possess six genes (srtA, srtB, srtC, srtD, srtE and srtF) which encode proteins similar to sortase and sortase-like proteins of other streptococci. The srtA gene is linked adjacent to gyrA, the other three genes srtBCD are found in clusters sequentially within the genome and srtE and srtF located in a separate chromosome (Osaki et al., 2002). When compared to the wild-type, the deletion of srtA gene abolished the anchoring of two virulence-related proteins with LPXTG motifs, MRP (Muramidase-released protein) and Sao on the cell surface and drastically reduced its adherence to human epithelial cells (Wang et al., 2009). The enzymes of class C sortases are involved in pili assembly, of which Streptococcus suis sortase has an open-lid confirmation when the substrate binds to the enzyme (Lu et al., 2011).
2.5. Bacillus spp.
B. anthracis is a spore-forming, Gram-positive, soil-borne organism that causes lethal anthrax disease in humans (Mock and Fouet, 2001). B. anthracis encodes three sortase enzymes: Ba-SrtA, Ba-SrtB, and Ba-SrtC from class A, class B, and class D, respectively. Bioinformatics analysis has identified 9 to 11 putative CWSS proteins, depending on the analysed strain. B. anthracis variants lacking the srtA gene did not anchor the collagen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) BasC protein to the bacterial cell wall as opposed to its parent strain. Recombinant expressed and purified SrtA catalyzed the cleavage reaction with LPETG and LPATG peptides, consistent with the notion that B. anthracis SrtA is responsible for the cell wall anchoring of surface proteins with an LPXTG motif (Gaspar et al., 2005). GamR, a B. anthracis phage receptor, is known to be anchored by SrtA. A srtA mutant strain displays reduced susceptibility to this phage, whereas the double srtA-srtB or srtA-srtC and the triple mutant strains displayed similar susceptibility to the srtA mutant, indicating that GamR is anchored by SrtA (Davison et al., 2005). Using the GamR function of causing phage lysis, a genetic screen was performed by fusing GamR to proteins containing CWSS and analysed for in vivo anchoring to the cell wall. The strains containing the three chimeric proteins BasB, BasE, and BasJ, were resistant to lysis in the srtA mutant and complementation experiments restored phage lysis indicating they are anchored by Sortase A. Furthermore, BasA was shown to be anchored by SrtA using immunoblot analysis of peptidoglycan fractions (Aucher et al., 2011). Apart from the functional roles, Ba-srtA has been characterized structurally. The NMR-structure of Ba-SrtA shows several unique active site features that include the presence of an N-terminal extension that contacts the catalytically essential histidine which might be involved in lipid II recognition. Another feature very unique to this protein is a large structurally disordered active site loop correlated to the attachment of proteins to the m-DAP moiety of lipid II. Based on the NMR structure a lock-and-key mechanism is proposed for recognizing the sorting signal (Weiner et al., 2010)
B. anthracis srtB, which encodes sortase B, anchors IsdC to the cell wall envelopes of vegetative bacilli a heme–iron binding surface protein. Sortase B cleaves IsdC between the threonine and the glycine of its NPKTG motif sorting signal. Isogenic variants lacking either srtB or isdC display significant growth defects due to deficiencies in heme–iron scavenging, suggesting that IsdC binding to heme–iron in the cell wall envelope contributes to bacterial uptake of heme (Maresso et al., 2006). The crystal structure of B. anthracis sortase B shows β7/ β8 loop is structurally disordered similar to sortase A from B. anthracis (Zhang et al., 2004).
B. anthracis Class D SrtC anchors two substrates, BasH and BasI, to the cell wall of sporulating B. anthracis. LPNTA sorting signal of two substrates is cleaved by Sortase C (SrtC) at the C-terminal threonine (T) of the substrate to the amino group of DAP cross-bridges targeting the polypeptides to the cell wall of sporulating bacilli. Sortase C is also required for the formation of infectious spores in the tissues of animals. Where sortase C acts on two different substrates in two different sub-cellular compartments, the different surface proteins decorate different compartments of the sporulating cell, with BasI present in divisional cells and BasH present in forespores (Marraffini and Schneewind, 2007, Marraffini and Schneewind, 2006). The NMR solution structure of Sortase C shows structurally disordered surface loops (β2 − β3 and β4 − H1 loops) that surround the active site histidine, suggesting that they may play a key role in associating Ba-SrtC with its lipid II substrate similar to SrtA (Robson et al., 2012). Contradictory reports are found regarding the role of sortases in the virulence of the organism. Interestingly, the srtA, srtB, srtC mutant strains, deleted for sortase genes, are not affected in their virulence as compared to its wild-type parent strain (Fouet, 2009). It has been shown that disruption of either the srtA or srtB gene results in an inability of the bacteria to grow in J774A.1 cells and no significant difference in the growth rate was observed in BHI broth between the parent strain and mutants. However, the molecular determinants causing this effect are not known (Zink and Burns, 2005).
B. cereus is a Gram-positive spore-forming bacteria mainly associated with food poisoning and also causes opportunistic skin infections (Shinagawa, 1990). From a bioinformatics analysis, it is known that B. cereus encodes Class A sortase (SrtA), Class B sortase (SrtB), Class C sortase (SrtD), and two class D sortases (SrtC1 and SrtC2). However, the function and substrates are only characterized for the Class A and Class C (Dramsi et al., 2005). The Class C sortase is involved in pili assembly especially in the B. cereus vegetative cells. The pilus operon consists of three genes (bcpA-srtD-bcpB) and deletion of the complete operon/inactivated srtD leads to the absence of the pili (Budzik et al., 2007). BcpA is polymerized by Sortase D even in the absence of the BcpB unit. BcpB the minor pilin is cleaved by Sortase D at C-terminal threonine of IPNTG sorting signal and then amide-linked to the YPKN motif of BcpA (Budzik et al., 2009). The cleavage reaction of BcpB is very specific to Sortase D and is not cleaved by sortase A. In the case of BcpA, the LPXTG sorting signal is cleaved at the C-terminal threonine and linked to the amino group of lysine in the YPKN motif of another BcpA sub-unit by sortase D. BcpA is a substrate for both sortase D and A, and sortase A cleaves the BcpA unit at its LPXTG to terminate the pilus assembly and bond to the cell wall cross-bridge (Budzik et al., 2009). Sortase B or Sortase C are not involved in the pili assembly or attachment to the B. cereus surface. Also, the operon bcpA-srtD-bcpB is not involved in the pili formation of the spore-forming cell surfaces (Budzik et al., 2007).
B. subtilis is generally recognized as a safe (GRAS) organism. B. subtilis codes for two putative sortases (probably belonging to class D), YhcS and YwpE, and two surface proteins, YhcR and YfkN, harboring sorting motifs supposed to be recognized by the putative sortase. YfkN contains the potential sorting signal LPDTA and in YhcR the motif is LPDTS (Duc Nguyen et al., 2011). yhcS gene is expressed preferentially at the late stationary phase and anchors YhcR on the cell wall of B. subtilis cell (Duc Nguyen et al., 2011, Fasehee et al., 2011). However the role or substrate of YwpE, the second putative sortase, is yet to be determined, the genomic analysis has shown that the open reading frame for ywpE encodes a truncated sortase-like protein (Liew et al., 2012).
2.6. Propionibacterium acnes
Sortase F is the only housekeeping sortase characterized in the genome of P. acnes. Sortase F from P. acnes shows a behavior similar to sortases from class A in terms of pH dependence, recognition sequence, and catalytic activity, furthermore its activity is independent of divalent ions, which contrasts to sortase A from S. aureus. The sortase F can be used as a powerful tool alternative to sortase A for protein engineering applications (Girolamo et al., 2019).
2.7. Clostridium spp.
C. perfringens, a pathogenic bacterium causes gas gangrene and food poisoning (Adak et al., 2002). Sortase B, C, and D are identified in C. perfringens and crystal structures reported (Suryadinata et al., 2015, Tamai et al., 2019, Tamai et al., 2017). Pilin formation and its crystal structure are reported in C. perfringens. Molecular and biochemical analysis of sortase C mediated catalysis and polymerization of pilin is proposed (Tamai et al., 2019). SrtD in C. perfringens is required for effective conjugative transfer of plasmid like pCW3 carrying antibiotic-resistant genes (Revitt-Mills et al., 2021). The role of sortase is established in the pathogenesis of C. perfringens strains causing gas gangrene and necrotic enteritis (Choo et al., 2016, Lepp et al., 2021). Sortase-dependent pilus produced by the bacterium binds to collagen and causes necrotic enteritis in poultry. However, the sortase gene presenting the pilus on the cell-surface is yet to be known (Lepp et al., 2021).
C. difficile is a spore-forming and toxin-producing intestinal pathogen associated with high morbidity rates. It causes antibiotic-associated diarrhoea and severe inflammation in the intestines (Rupnik et al., 2009). C. difficile encodes for a single sortase enzyme sortase B (CD2718), belonging to the class B sortases, recognizes an entirely different sorting motif (SPxTG or PPxTG). Remarkably, unlike SrtB from other organisms, the function of this enzyme is not associated with heme or heme acquisition proteins instead possibly functions as a housekeeping sortase. The mutation of the catalytic cysteine or the addition of small inhibitors like MTSET abolishes the sortase B activity. In vitro ligation of a natural cell wall nucleophile, DAP is demonstrated (Donahue et al., 2014, Van Leeuwen et al., 2014).
Based on bioinformatics analyses, seven putative sortase substrates have been identified (Van Leeuwen et al., 2014). However, two putative substrates, CD0183 and CD2768, containing an SPXTG motif were not cleaved or anchored to the cell wall by sortase in an experimental observation (Peltier et al., 2017). Another two substrates, CD0386 and CD3392, displaying very high (94%) sequence similarity, of which CD0386 is attached to the cell wall by the sortase as demonstrated by biochemical analysis of sub-cellular fractions in a sortase knockout strain (Chambers et al., 2015). Adhesins, CD2831 and CD3246, are anchored proteins on the cell wall by SrtB activity. The functional role, particularly of CD2831 in binding to collagen, has been demonstrated in vitro through the cleaving activity of a Pro-Pro endopeptidase to release CD2831 and CD3246 from the cell surface and is regulated by C-di-GMP (Hensbergen et al., 2015, Peltier et al., 2015). The last putative substrate CD2537 contains only a weak signal peptide (Corver et al., 2017).
2.8. Actinomyces spp
S. avermitilis encodes for at least four putative class E sortase enzymes (Duong et al., 2012). Only, SrtE3 (SAV4333) has been biochemically characterized, where it recognizes a LAXTG or LPXTG motif in a calcium-independent manner (Das et al., 2017).
S. coelicolor is a soil-dwelling multi-cellular bacterium that has three differentiated states in its life cycles: vegetative hyphae, aerial hyphae, and spores. It is known to encode for seven sortase enzymes two from sortase class E and five class F enzymes (Pallen et al., 2001). Sortase class E enzymes SrtE1 and SrtE2 recognize and cleave LAXTG-containing peptides in vitro. Chaplin proteins (Chp A, B, and C) required for the aerial development of this organism contain a LAXTG-sorting signal rendering them as putative sortase substrates (Elliot et al., 2003). Sortase class E enzymes (SrtE1 and SrtE2) anchor ChpC protein to cell wall in vivo. Indeed, srtE1/srtE2 double mutants delay the formation of aerial hyphae with hindered sporulation and cease to display short Chaplin proteins (Duong et al., 2012). The crystal structure of SrtE1 has been determined at a1.93 Å resolution (Kattke et al., 2016).
A. oris is an oral bacterium, formerly known as Actinomyces naeslundii, which results in the formation of dental plaque (Persson, 2011). It consists of three cysteine transpeptidases, the housekeeping sortases (SrtA), which belongs to class E sortase (Spirig et al., 2011) and the other belongs to class C sortases, SrtC1 and SrtC2, which is involved in the assembly of two distinct forms of pili on the Actinomyces cell surface (Mishra et al., 2007). The SrtC1 is involved in the type I fimbriae formation which is further involved in the adhesion of Actinomyces to the tooth surface by recognition of proline-rich receptors (Wu et al., 2011). However, SrtC2 results in the assembly of type II fimbriae which are essential for the bacterial adhesion to the oral streptococci and host cells by recognizing the polysaccharide receptors (Wu et al., 2012).
The crystal structure of A. oris SrtA contains an open accessible active site for the attachment of surface proteins covalently bound to the cell envelope and fimbriae/pili-specific sortases (SrtC1 and SrtC2) contains a flexible lid to cover the active site which gets exposed during pili formation (Manzano et al., 2009, Persson, 2011).
The housekeeping sortases generally do not show any role in cell viability, however, SrtA of A. oris was observed to show some contradictory results. The deletion of the srtA gene was found to be lethal for the bacterial cells, which resulted in excessive membrane accumulation of the surface glycoprotein protein perturbing the cell envelope to block the growth and viability of the cells (Li et al., 2014).
3. Lactic acid bacteria (LAB)
Even though sortases and sortase dependent proteins (SDPs) were investigated extensively related to pathogenesis, the presence of such proteins in probiotic lactic acid bacteria provided a new avenue to look in to the role of this enzyme on probiotic attributes, such as adhesion, mucus barrier function, immune signalling and nutrient uptake. The hypothesis that sortase enzymes may play crucial roles in bacterial physiology, (as in the case of PrtP in L. lactis ssp. cremoris MG1363) as well as mediating bacterial-host interactions has accelerated the study of this enzyme in different species of LAB. Call and Klaenhammer (2013) reviewed in detail the reports on such proteins in selected species of health-promoting LAB. Recently the application of the LPXTG motif as a bio-therapeutic in Lactic acid bacteria has been reported. To date, LAB and sortase-mediated cell wall anchoring have been explored in the display of potential vaccine antigens including the tetanus toxin fragment C (Norton et al., 1996), human papillomavirus (HPV) type 16 E7 antigen (Bermúdez-Humarán et al., 2003, Cortes-Perez et al., 2005, Cortes-Perez et al., 2003), the oncofetal antigen (Fredriksen et al., 2010), and Salmonella enterica serovar typhimurium flagellin (Kajikawa et al., 2011). All these studies indicate that vaccine delivery in LAB using LPXTG or LPXTG-like cell wall anchors has great potential.
Some of the major studies summarizing the sortases in different LAB species and their roles were briefly covered in this review;
Lactobacillus rhamnosus strain GG, a well-known probiotic bacterium, also displays on its cell surface mucus binding pilus structures, along with other LPXTG surface proteins, which are processed by sortases upon specific recognition of a highly conserved LPXTG motif. Demonstration of the expression and presence of mucus binding pilin-like structures on the surface of L. rhamnosus GG has been determined (Kankainen et al., 2009) and interestingly, L. rhamnosus GG shows exemplary ability to adhere to Caco-2 cells as compared to other probiotic strains (Jacobsen et al., 1999). The genome sequence of L. rhamnosus GG revealed two potential clusters of pilus-encoding genes in tandem with a srtC gene. The first cluster identified contained genes for spaA (LGG_00442), spaB (LGG_00443), and spaC (LGG_00444) clustered with srtC1(LGG_00441), while the second cluster contained genes for spaD (LGG_02370), spaE (LGG_02371), and spaF (LGG_02372) clustered with srtC2 (LGG_02369; Bioinformatic analysis of all predicted LPXTG proteins encoded by the L. rhamnosus GG genome revealed remarkable conservation of glycine residues juxtaposed to the canonical LPXTG motif. Douillard et al (2014) investigated and defined the role of the triple glycine (TG) motif in determining sortase specificity during pilus assembly and anchoring. Mutagenesis of the TG motif resulted in a lack of an alteration of the L. rhamnosus GG pilus structures, indicating that the TG motif is critical in pilus assembly and that they govern the pilin-specific and housekeeping sortase specificity. Chaurasia et al (2016) provided new insights about pilus formation in gut-adapted L. rhamnosus GG from the crystal structure of the SpaA backbone pilin subunit. According to the paper, SpaA consists of two tandem CnaB-type domains, each with an isopeptide bond and E-box motif. Von Ossowski (2017) in his review on novel molecular insights about Lactobacillar sortase-dependent piliation described three types of lactobacillar piliation (i.e., SpaCBA, SpaFED and LrpCBA), each has been described as having the basic characteristics common to all sortase-dependent pili, but as well, certain unique properties and associated actions that are inherent to them. The authors investigated two contrasting gut-adapted species from the Lactobacillus genus, allochthonous L. rhamnosus, and autochthonous Lactobacillus ruminis.
Dieye et al (2010) identified and studied a class A sortase in Lactococcus lactis IL1403 and showed that it is responsible for the cell wall anchoring of at least five LPXTG-containing proteins. We, therefore, propose that SrtA is the housekeeping sortase in L. lactis. Surface proteins are important factors in the interaction of probiotic and pathogenic bacteria with their environment or host. The sortase mutant and one sortase-dependent protein (mucus-binding homolog) mutant showed a significant reduction in adherence to human epithelial cell lines in the case of Lactobacillus salivarius UCC118. Van Pijkeren et al (2006) identified 10 sortase-dependent surface proteins in L. salivarius UCC118, by the comparative and functional analysis of sortase-dependent proteins in the predicted secretome of L. salivarius.
As it has been emphasized, sortase, an enzyme that covalently couples a subset of extracellular proteins containing an LPXTG motif to the cell surface, is of particular interest in characterizing bacterial adherence and communication with the mucosal immune system. Lactobacillus casei BL23 harbors four sortase genes, two belonging to class A (srtA1 and srtA2) and two belonging to class C (srtC1 and srtC2). Class C sortases were clustered with genes encoding their putative substrates that were homologous to the SpaEFG and SpaCBA proteins that encode mucus adhesive pili in L. rhamnosus GG. Twenty-three genes encoding putative sortase substrates were identified in the L. casei BL23 genome with unknown (35%), enzymatic (30%), or adhesion-related (35%) functions (Muñoz-Provencio et al., 2012). In summary, in L. casei BL23, around 20 proteins are likely anchored to the cell surface by sortases. Although the specific function of most of them is unknown, most of them would account for an adaptation to persist in the gastrointestinal niche. SrtA1 is the housekeeping sortase in this strain, while SrtA2 can compensate for its absence to a certain extent.
A sortase gene, srtA, was identified in Lactobacillus acidophilus NCFM (LBA1244) and Lactobacillus gasseri ATCC 33,323 (LGAS_0825). Additionally, eight and six intact sortase-dependant proteins were predicted in L. acidophilus and L. gasseri, respectively. Inactivation of sortase did not cause significant alteration in growth or survival in simulated gastrointestinal juices. Meanwhile, both DsrtA mutants showed decreased adhesion to porcine mucin in vitro. Murine dendritic cells exposed to the DsrtA mutant of L. acidophilus or L. gasseri induced lower levels of pro-inflammatory cytokines TNF-α and IL-12, respectively, compared with the parent strains (Call et al., 2015). This study shows that sortase-dependent proteins contribute to gut retention of probiotic microbes in the gastrointestinal tract.
Streptococcus thermophilus (ST) belongs to the LAB group and is recognized as safe since it has obtained the Generally Recognized as Safe (GRAS) status. Kebouchi et al (2016) investigated, in vitro, the implication of sortase A (SrtA) and sortase-dependent proteins (SDPs) in the adhesion of ST LMD-9 strain to intestinal epithelial cells (IECs) and resistance to bile salt mixture (BSM; taurocholate, deoxycholate, and cholate). The mutation in genes srtA and mucBP leads to a significant decrease in LMD-9 adhesion capacity to Caco-2 TC7, HT29-CL.16E (mucBP gene mutation) cells. However, no difference was observed using HT29-MTX cells. The study revealed that SDPs could be involved in the LMD-9 adhesion depending on the cell lines indicating the importance of eukaryotic-cell surface components in adherence and also SDPs could contribute to resistance to bile salts probably by maintaining the cell membrane integrity.
The Lactobacillus genomes encode a single copy of the sortase (SrtA) and a variable number of LPXTG-motif-containing proteins, ranging from two proteins in Lactobacillus delbrueckii bulgaricus ATCC-BAA-365 and ATCC 11,842 and to 27 functional proteins in Lactobacillus plantarum WCFS1.
Four Lactobacillus proteins belonging to the sortase-dependent protein family have been functionally characterized. Three of the lactobacilli sortase-dependent proteins correspond to the mucus adhesins of L. reuteri 1063 Mub (Roos and Jonsson, 2002), L. plantarum WCFS1 Msa (Pretzer et al., 2005) and L. acidophilus NCFM Mub (Buck et al., 2005). The fourth characterized sortase-dependent protein is LspA of Lactobacillus salivarius UCC118 which has been reported to mediate the adhesion of this strain to human epithelial cells and mucus (Claesson et al., 2006, Van Pijkeren et al., 2006).
Similarly, bifidobacteria represent one of the dominant groups of microorganisms colonizing the human infant intestine (Turroni et al., 2014). Whole-genome transcription profiling of Bifidobacterium bifidum PRL2010, a strain isolated from an infant stool, revealed a small number of commonly expressed extracellular proteins, among which were genes that specify sortase-dependent pili modulating bacterium–host interactions. The genome of B. bifidum PRL2010 encompasses three different loci encoding predicted sortase-dependent pili, of which only pil2 and pil3 appear to be functional (Foroni et al., 2011). Similarly, the genome of B. bifidum S17 was shown to contain a large number of genes that might be involved in host colonization including Tad and sortase-dependent pili, lipoproteins, and several other genes encoding for surface proteins with domains known to mediate interaction with host structures (Westermann et al., 2012).
Adhesion of bifidobacterial cells to the mucosa of the large intestine is considered a hallmark for the persistence and colonization of these bacteria in the human gut. In this context, Milani et al (2017) analyzed the genetic diversity of the predicted arsenal of sortase-dependent pili of known and sequenced members of the Bifidobacterium genus and constructed a bifidobacterial sortase-dependent fimbriome database. Their analyses revealed considerable genetic variability of the sortase-dependent fimbriome among bifidobacterial (sub) species and they concluded that it may be due to horizontal gene transfer events. While it is SrtA reported in most bifidobacteria while searching uniprot we could see there were some genes annotated as ESN35_09070, of Bifidobacterium gallinarum, or the one in Bifidobacterium platyrrhinorum were classified as class C. However, not many studies were reported on them. Krishnan et al (2016) summarized the latest awareness about pili in probiotics with emphasis on members of lactobacilli and bifidobacteria.
Enterococci are currently leading causes of hospital-acquired infections, such as bloodstream, wound, and catheter-associated urinary tract infections (Murray, 1990). Adhesion to and biofilm formation on damaged tissue and abiotic surfaces, such as central venous and urinary catheters, are critical components of enterococcal pathogenesis that complicate successful treatments. Cell surface proteins have been shown to play significant roles in E. faecalis virulence and among these, the sortase-assembled endocarditis and biofilm-associated pilus (Ebp pilus) is important for in vitro biofilm formation and virulence in E. faecalis (Kline et al., 2009, Singh et al., 2007) and E. faecium (Sillanpää et al., 2010) and for infective endocarditis in E. faecalis (Nallapareddy et al., 2006).
Genome analysis of E. faecalis V583 revealed the presence of two class A sortases (EF_2524 and SrtA [EF_3056]), one class C sortase (SrtC [EF_0194 for biofilm and pilus-associated sortase]) (Dramsi et al., 2005, Paulsen et al., 2003), and 41 surface proteins bearing a cell wall sorting signal motif (Sillanpää et al., 2004). It is believed that atleast some of these surface proteins are microbial surface components recognizing adhesive matrix molecules that play a role in the attachment of E. faecalis to extracellular matrix proteins and thus are likely to be important for virulence. Also, two other sortases, named srt-1 and srt-2, were reported in E. faecalis strain E99 containing a bee (biofilm enhancer in enterococcus) locus; however, their occurrence was rare and they were found only in a few isolates examined from a selection of 40 E. faecalis (Telford et al., 2006). The ebp operon in E. faecalis encodes the Ebp pilus structural subunits EbpA, EbpB, and EbpC and the pilus-associated sortase SrtC. The housekeeping sortase SrtA is encoded elsewhere in the genome (Kemp et al., 2007). The ubiquitous presence of both sortase genes in E. faecalis isolates increases the likelihood that sortases in general, and srtC in particular, could be a target for disease prevention. srtC shown to be necessary for the production of the Ebp pili and important for biofilm formation and endocarditis. Nielsen et al (2013) reported that a srtA deletion mutant showed a small (5%) reduction in biofilm formation while a srtA - srtC double mutant showed a much greater reduction (74%) in comparison to a smaller reduction (44%) with a SrtC mutant. In summary, from this study, it appears likely that SrtC, presumably via SrtC-anchored surface proteins, plays an important role in both in vitro biofilm formation and in vivo murine kidney infections under the experimental conditions examined.
In general, Class A sortases, which appear to be ubiquitous in many Gram-positive bacteria, anchor a large number and broad range of surface proteins (Marraffini et al., 2006). The sortase C class of enzymes is predicted to anchor a much smaller set of substrates, and the genes coding for these are typically clustered with the substrate genes and involved in pilus biogenesis in addition to surface anchoring (Scott and Zähner, 2006, Telford et al., 2006). Pansegrau and Bagnoli (2015) reviewed the pilus assembly in Gram-positive bacteria. They illustrated the operon structure of selected pilus islands.
4. Concluding remarks
To conclude, sortases are either involved in anchoring proteins to the cell wall (the so-called housekeeping sortases) or in polymerizing pilin proteins (pilin-specific sortases). A significant proportion of the work carried out on sortases so far has focused on SrtA from S. aureus, which is of great industrial benefit and representing an important therapeutic target. SrtA of S. aureus has been significantly exploited for a variety of industrial purposes which includes, enzyme immobilization, cell surface labeling, antibody-drug conjugates, dimerization and cyclization of proteins. It is also likely that sortases may soon become drug targets for the treatment of a wide range of conditions but, some of which are potentially yet to be conceived. Remarkable progress has been made in the last decade in obtaining the information about the classes of sortases A, B, C, and D from Gram‐positive bacteria. As a result, novel information about the class E sortase of Gram‐positive bacteria has been determined, which includes its function, the substrate motif, and structurally three-dimensional folds. The class F sortase was recently reported in P. acnes with a substrate-specificity similar to class A sortase. Sortases are not essential for bacterial cell survival but do significantly impact the binding to host tissues, signaling to the host, or escaping the host immune response, and thus they are equally crucial for nonpathogenic gastrointestinal bacteria as well. Besides, sortase expression signals in lactobacilli have been exploited as a means to develop oral vaccines targeted to the gastro-intestinal tract. The future is not certainly to look in to the primary sequence homology and classification but rather to focus more on structure–function studies that determine the substrate specificity and also the interaction of sortases with other membrane bound enzymes to study better cell wall assembly, such as pili biogenesis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Council of Scientific and Industrial Research (CSIR) New Delhi for the fellowship (JRF and SRF) to AS and acknowledges the supported by the ‘Innovative Young Biotechnologist Award’ by the Department of Biotechnology, Government of India (BT/11/IYBA/2018/09) to HB. The figures created with Biorender.com.
References
- Aas J.A., Paster B.J., Stokes L.N., Olsen I., Dewhirst F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adak G.K., Long S.M., O’Brien S.J. Trends in indigenous foodborne disease and deaths, England and Wales: 1992 to 2000. Gut. 2002;51:832–841. doi: 10.1136/gut.51.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucher W., Davison S., Fouet A. Characterization of the Sortase Repertoire in Bacillus anthracis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T.C., Scott J.R. Differential Recognition of Surface Proteins in Streptococcus pyogenes by Two Sortase Gene Homologs. J. Bacteriol. 2002;184:2181–2191. doi: 10.1128/JB.184.8.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C., Waggoner J.D., Harris T.O., Tamura G.S., Rubens C.E. Identification of Novel Adhesins from Group B Streptococci by Use of Phage Display Reveals that C5a Peptidase Mediates Fibronectin Binding. Infect. Immun. 2002;70:2869–2876. doi: 10.1128/IAI.70.6.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez-Humarán L.G., Cortes-Perez N.G., Le Loir Y., Gruss A., Rodriguez-Padilla C., Saucedo-Cardenas O., Langella P., De Oca-Luna R.M. Fusion to a carrier protein and a synthetic propeptide enhances E7 HPV-16 production and secretion in Lactococcus lactis. Biotechnol. Prog. 2003;19:1101–1104. doi: 10.1021/bp0340077. [DOI] [PubMed] [Google Scholar]
- Bierne H., Mazmanian S.K., Trost M., Pucciarelli M.G., Liu G., Dehoux P., Jänsch L., Garcia-del Portillo F., Schneewind O., Cossart P. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 2002;43:869–881. doi: 10.1046/j.1365-2958.2002.02798.x. [DOI] [PubMed] [Google Scholar]
- Boekhorst J., Been M.W.H.J.D., Kleerebezem M., Siezen R.J. Genome-Wide Detection and Analysis of Cell Wall-Bound Proteins with LPxTG-Like Sorting Motifs. J. Bacteriol. 2005;187:4928–4934. doi: 10.1128/JB.187.14.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A.J., Leach K.A., Breen J.E., Green L.E., Green M.J. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet. Rec. 2007;160:253–258. doi: 10.1136/vr.160.8.253. [DOI] [PubMed] [Google Scholar]
- Buck L.B., Altermann E., Svingerud T., Klaenhammer T.R. Functional analysis of adhesion factors and signaling mechanisms in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 2005;71:8344–8351. doi: 10.1128/AEM.71.12.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzik J.M., Marraffini L.A., Schneewind O. Assembly of pili on the surface of Bacillus cereus vegetative cells. Mol. Microbiol. 2007;66:495–510. doi: 10.1111/j.1365-2958.2007.05939.x. [DOI] [PubMed] [Google Scholar]
- Budzik, J.M., Oh, S.-Y., Schneewind, O., 2009. Sortase D forms the covalent bond that links BcpB to the tip of Bacillus cereus pili. J. Biol. Chem. 284, 12989–97. 10.1074/jbc.M900927200. [DOI] [PMC free article] [PubMed]
- Cabanes D., Dehoux P., Dussurget O., Frangeul L., Cossart P. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 2002;10:238–245. doi: 10.1016/S0966-842X(02)02342-9. [DOI] [PubMed] [Google Scholar]
- Call E.K., Goh Y.J., Selle K., Klaenhammer T.R., O’Flaherty S. Sortase-deficient lactobacilli: Effect on immunomodulation and gut retention. Microbiol. 2015;161:311–321. doi: 10.1099/mic.0.000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call E.K., Klaenhammer T.R. Relevance and application of sortase and sortase-dependent proteins in lactic acid bacteria. Front. Microbiol. 2013;4:1–10. doi: 10.3389/fmicb.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.J., Roberts A.K., Shone C.C., Acharya K.R. Structure and function of a Clostridium difficile sortase enzyme. Sci. Rep. 2015;5:1–11. doi: 10.1038/srep09449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia P., Pratap S., Von Ossowski I., Palva A., Krishnan V. New insights about pilus formation in gut-adapted Lactobacillus rhamnosus GG from the crystal structure of the SpaA backbone-pilin subunit. Sci. Rep. 2016;6:1–17. doi: 10.1038/srep28664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A.G., Kim H.K., Burts M.L., Krausz T., Schneewind O., Missiakas D.M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo J.M., Cheung J.K., Wisniewski J.A., Steer D.L., Bulach D.M., Hiscox T.J., Chakravorty A., Smith A.I., Gell D.A., Rood J.I., Awad M.M. The NEAT Domain-Containing Proteins of Clostridium perfringens Bind Heme. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson M.J., Li Y., Leahy S., Canchaya C., Van Pijkeren J.P., Cerdeño-Tárraga A.M., Parkhill J., Flynn S., O’Sullivan G.C., Kevin Collins J., Higgins D., Shanahan F., Fitzgerald G.F., Van Sinderen D., O’Toole P.W. Multireplicon genome architecture of Lactobacillus salivarius. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6718–6723. doi: 10.1073/pnas.0511060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy K.W., Melvin J.A., McCafferty D.G. Sortase transpeptidases: insights into mechanism, substrate specificity, and inhibition. Biopolymers. 2010;94:385–396. doi: 10.1002/bip.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort D., Clubb R.T. A Comparative Genome Analysis Identifies Distinct Sorting Pathways in Gram-Positive Bacteria. Infect. Immun. 2004;72:2710–2722. doi: 10.1128/IAI.72.5.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Perez N.G., Azevedo V., Alcocer-González J.M., Rodriguez-Padilla C., Tamez-Guerra R.S., Corthier G., Gruss A., Langella P., Bermúdez-Humarán L.G. Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. J. Drug Target. 2005;13:89–98. doi: 10.1080/10611860400024219. [DOI] [PubMed] [Google Scholar]
- Cortes-Perez N.G., Bermúdez-Humarán L.G., Le Loir Y., Rodriguez-Padilla C., Gruss A., Saucedo-Cárdenas O., Langella P., Montes-De-Oca-Luna R. Mice immunization with live lactococci displaying a surface anchored HPV-16 E7 oncoprotein. FEMS Microbiol. Lett. 2003;229:37–42. doi: 10.1016/S0378-1097(03)00778-X. [DOI] [PubMed] [Google Scholar]
- Corver, J., Cordo’, V., van Leeuwen, H.C., Klychnikov, O.I., Hensbergen, P.J., 2017. Covalent attachment and Pro-Pro endopeptidase (PPEP-1)-mediated release of Clostridium difficile cell surface proteins involved in adhesion. Mol. Microbiol. 105, 663–673. 10.1111/mmi.13736. [DOI] [PubMed]
- Cossart P. Listeria monocytogenes Surface Proteins : from Genome Predictions to Function. Microbiol. Mol. Biol. Rev. 2007;71:377–397. doi: 10.1128/MMBR.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Pawale V.S., Dadireddy V., Singh A.K., Ramakumar S., Roy R.P. Structure and specificity of a new class of Ca2+-independent housekeeping sortase from Streptomyces avermitilis provide insights into its non-canonical substrate preference. J. Biol. Chem. 2017;292:7244–7257. doi: 10.1074/jbc.M117.782037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J.R., Svensa G., Herzberg M.C. Identification of novel LPXTG-linked surface proteins from Streptococcus gordonii. Microbiology. 2009;155:1977–1988. doi: 10.1099/mic.0.027854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison, S., Couture-Tosi, E., Candela, T., Mock, M., Fouet, A., 2005. Identification of the Bacillus anthracis (gamma) phage receptor. J. Bacteriol. 187, 6742–9. 10.1128/JB.187.19.6742-6749.2005. [DOI] [PMC free article] [PubMed]
- Dekker, F.J., Boersma, Y.L., 2018. Identification of potential antivirulence agents by substitution-oriented screening for inhibitors of Streptococcus pyogenes sortase A. Eur. J. Med. Chem. 10.1016/j.ejmech.2018.10.027. [DOI] [PubMed]
- Dieye Y., Oxaran V., Ledue-Clier F., Alkhalaf W., Buist G., Juillard V., Lee C.W., Piard J.C. Functionality of sortase a in Lactococcus lactis. Appl. Environ. Microbiol. 2010;76:7332–7337. doi: 10.1128/AEM.00928-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue E.H., Dawson L.F., Valiente E., Firth-Clark S., Major M.R., Littler E., Perrior T.R., Wren B.W. Clostridium difficile has a single sortase, SrtB, that can be inhibited by small-molecule inhibitors. BMC Microbiol. 2014;14:1–14. doi: 10.1186/s12866-014-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard F.P., Rasinkangas P., Von Ossowski I., Reunanen J., Palva A., De Vos W.M. Functional identification of conserved residues involved in Lactobacillus rhamnosus strain GG sortase specificity and pilus biogenesis. J. Biol. Chem. 2014;289:15764–15775. doi: 10.1074/jbc.M113.542332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dramsi S., Caliot E., Bonne I., Guadagnini S., Prévost M., Kojadinovic M., Lalioui L., Poyart C., Trieu-cuot P. Assembly and role of pili in group B streptococci. Mol. Microbiol. 2006;60:1401–1413. doi: 10.1111/j.1365-2958.2006.05190.x. [DOI] [PubMed] [Google Scholar]
- Dramsi S., Trieu-Cuot P., Bierne H. Sorting sortases: A nomenclature proposal for the various sortases of Gram-positive bacteria. Res. Microbiol. 2005;156:289–297. doi: 10.1016/j.resmic.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Duarte C.M., Freitas P.P., Bexiga R. Technological advances in bovine mastitis diagnosis: an overview. J. Vet. Diagnostic Investig. 2015;27:665–672. doi: 10.1177/1040638715603087. [DOI] [PubMed] [Google Scholar]
- Duc Nguyen H., Thi T., Phan P., Schumann W. Analysis and application of Bacillus subtilis sortases to anchor recombinant proteins on the cell wall. AMB Express. 2011;1:22. doi: 10.1186/2191-0855-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong A., Capstick D.S., Berardo C. Di, Findlay K.C., Hesketh A., Hong H., Elliot M.A. Aerial development in Streptomyces coelicolor requires sortase activity. Mol. Microbiol. 2012;83:992–1005. doi: 10.1111/j.1365-2958.2012.07983.x. [DOI] [PubMed] [Google Scholar]
- Dussurget O., Pizarro-cerda J., Cossart P. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 2004;58:587–610. doi: 10.1146/annurev.micro.57.030502.090934. [DOI] [PubMed] [Google Scholar]
- Egan S.A., Kurian D., Ward P.N., Hunt L., Leigh J.A. Identification of Sortase A (SrtA) Substrates in Streptococcus uberis : Evidence for an Additional Hexapeptide (LPXXXD) Sorting Motif. J. Proteome. Res. 2010;9:1088–1095. doi: 10.1021/pr901025w. [DOI] [PubMed] [Google Scholar]
- Eigh J.A.L., Gan S.A.E., Ard P.N.W., Ield T.R.F., Offey T.J.C. Original article Sortase anchored proteins of Streptococcus uberis play major roles in the pathogenesis of bovine mastitis in dairy cattle. Vet. Res. 2010;41:63. doi: 10.1051/vetres/2010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasehee H., Westers H., Bolhuis A., Antelmann H., Hecker M., Quax W.J., Mirlohi A.F., Van Dijl J.M., Ahmadian G. Functional analysis of the sortase YhcS in Bacillus subtilis. Proteomics. 2011;11:3905–3913. doi: 10.1002/pmic.201100174. [DOI] [PubMed] [Google Scholar]
- Fittipaldi N., Segura M., Grenier D., Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012;7:259–279. doi: 10.2217/fmb.11.149. [DOI] [PubMed] [Google Scholar]
- Foroni E., Serafini F., Amidani D., Turroni F., He F., Bottacini F., O’Connell Motherway M., Viappiani A., Zhang Z., Rivetti C., Van Sinderen D., Ventura M. Genetic analysis and morphological identification of pilus-like structures in members of the genus Bifidobacterium. Microb. Cell Fact. 2011;10:1–13. doi: 10.1186/1475-2859-10-S1-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouet A. The surface of Bacillus anthracis. Mol. Aspects Med. 2009;30:374–385. doi: 10.1016/J.MAM.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Frankel B.A., Kruger R.G., Robinson D.E., Kelleher N.L., McCafferty D.G. Staphylococcus aureus sortase transpeptidase SrtA: Insight into the kinetic mechanism and evidence for a reverse protonation catalytic mechanism. Biochemistry. 2005;44:11188–11200. doi: 10.1021/bi050141j. [DOI] [PubMed] [Google Scholar]
- Frankel, B.B. a, Tong, Y., Bentley, M.M.L., Fitzgerald, M.C., McCafferty, D.G., 2007. Mutational analysis of active site residues in the Staphylococcus aureus transpeptidase SrtA. Biochemistry 46, 7269–7278. 10.1021/bi700448e. [DOI] [PubMed]
- Fredriksen L., Mathiesen G., Sioud M., Eijsink V.G.H. Cell wall anchoring of the 37-kilodalton oncofetal antigen by Lactobacillus plantarum for mucosal cancer vaccine delivery. Appl. Environ. Microbiol. 2010;76:7359–7362. doi: 10.1128/AEM.01031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garandeau C., Pucciarelli M.G., Sabet C., Newton S., Portillo F.G., Cossart P., Charbit A. Sortase B, a New Class of Sortase in Listeria monocytogenes. J. Bacteriol. 2004;186:1972–1982. doi: 10.1128/JB.186.7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar A.H., Marraffini L.A., Glass E.M., DeBord K.L., Ton-That H., Schneewind O. Bacillus anthracis Sortase A (SrtA) Anchors LPXTG Motif-Containing Surface Proteins to the Cell Wall Envelope. J. Bacteriol. 2005;187:4646–4655. doi: 10.1128/JB.187.13.4646-4655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar A.H., Ton-That H. Assembly of Distinct Pilus Structures on the Surface of Corynebacterium diphtheriae. J. Bacteriol. 2006;188:1526–1533. doi: 10.1128/JB.188.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolamo S. Di, Puorger C., Castiglione M., Vogel M., Gébleux R., Briendl M., Hell T., Beerli R.R., Grawunder U., Lipps G. Characterization of the housekeeping sortase from the human pathogen Propionibacterium acnes: First investigation of a class F sortase. Biochem. J. 2019;476:665–682. doi: 10.1042/BCJ20180885. [DOI] [PubMed] [Google Scholar]
- Glaser P., Rusniok C., Buchrieser C., Chevalier F., Frangeul L., Msadek T., Zouine M., Couvé E., Lalioui L., Poyart C., Trieu-cuot P., Kunst F. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 2002;45:1499–1513. doi: 10.1046/j.1365-2958.2002.03126.x. [DOI] [PubMed] [Google Scholar]
- Hadfield T.L., McEvoy P., Polotsky Y., Tzinserling V.A., Yakovlev A.A. The pathology of diphtheria. J. Infect. Dis. 2000;181:3–7. doi: 10.1086/315551. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx A.P.A., Budzik J.M., Oh S.Y., Schneewind O. Architects at the bacterial surface-sortases and the assembly of pili with isopeptide bonds. Nat. Rev. Microbiol. 2011;9:166–176. doi: 10.1038/nrmicro2520. [DOI] [PubMed] [Google Scholar]
- Hensbergen P.J., Klychnikov O.I., Bakker D., Dragan I., Kelly M.L., Minton N.P., Corver J., Kuijper E.J., Drijfhout J.W., Van Leeuwen H.C. Clostridium difficile secreted Pro-Pro endopeptidase PPEP-1 (ZMP1/CD2830) modulates adhesion through cleavage of the collagen binding protein CD2831. FEBS Lett. 2015;589:3952–3958. doi: 10.1016/j.febslet.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Jacobsen C.N., Nielsen V.R., Hayford A.E., Møller P.L., Michaelsen K.F., Pærregaard A., Sandström B., Tvede M., Jakobsen M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999;65:4949–4956. doi: 10.1128/aem.65.11.4949-4956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa A., Nordone S.K., Zhang L., Stoeker L.L., LaVoy A.S., Klaenhammer T.R., Dean G.A. Dissimilar properties of two recombinant Lactobacillus acidophilus strains displaying Salmonella FliC with different anchoring motifs. Appl. Environ. Microbiol. 2011;77:6587–6596. doi: 10.1128/AEM.05153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankainen M., Paulin L., Tynkkynen S., Von Ossowski I., Reunanen J., Partanen P., Satokari R., Vesterlund S., Hendrickx A.P.A., Lebeer S., De Keersmaecker S.C.J., Vanderleyden J., Hämäläinen T., Laukkanen S., Salovuori N., Ritari J., Alatalo E., Korpela R., Mattila-Sandholm T., Lassig A., Hatakka K., Kinnunen K.T., Karjalainen H., Saxelin M., Laakso K., Surakka A., Palva A., Salusjärvi T., Auvinen P., De Vos W.M. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17193–17198. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebouchi M., Galia W., Genay M., Soligot C., Lecomte X., Awussi A.A., Perrin C., Roux E., Dary-Mourot A., Le Roux Y. Implication of sortase-dependent proteins of Streptococcus thermophilus in adhesion to human intestinal epithelial cell lines and bile salt tolerance. Appl. Microbiol. Biotechnol. 2016;100:3667–3679. doi: 10.1007/s00253-016-7322-1. [DOI] [PubMed] [Google Scholar]
- Kemp K.D., Singh K.V., Nallapareddy S.R., Murray B.E. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (SrtC), to biofilm formation and a murine model of urinary tract infection. Infect. Immun. 2007;75:5399–5404. doi: 10.1128/IAI.00663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharat A.S., Tomasz A. Inactivation of the srtA gene affects localization of surface proteins and decreases adhesion of Streptococcus pneumoniae to human pharyngeal cells in vitro. Infect. Immun. 2003;71:2758–2765. doi: 10.1128/IAI.71.5.2758-2765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare B., Krishnan V., Rajashankar K.R., Xin M., Narayana S.V. Structural Differences between the Streptococcus agalactiae Housekeeping and Pilus-Specific Sortases : SrtA and SrtC1. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline K.A., Kau A.L., Chen S.L., Lim A., Pinkner J.S., Rosch J., Nallapareddy S.R., Murray B.E., Henriques-Normark B., Beatty W., Caparon M.G., Hultgren S.J. Mechanism for sortase localization and the role of sortase localization in efficient pilus assembly in Enterococcus faecalis. J. Bacteriol. 2009;191:3237–3247. doi: 10.1128/JB.01837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P.E., Ganeshkumar N., Cassels F.J., Hughes C.V. Coaggregation : specific adherence among human oral plaque bacteria. FASEB J. 1993;7:406–413. doi: 10.1096/fasebj.7.5.8462782. [DOI] [PubMed] [Google Scholar]
- Krishnan, V., Chaurasia, P., Kant, A., 2016. Pili in Probiotic Bacteria. Probiotics Prebiotics Hum. Nutr. Heal. 10.5772/6308787.
- Kuboniwa M., Tribble G.D., James C.E., Kilic A.O., Tao L., Herzberg M.C., Shizukuishi S., Lamont R.J. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol. Microbiol. 2006;60:121–139. doi: 10.1111/j.1365-2958.2006.05099.x. [DOI] [PubMed] [Google Scholar]
- Lalioui L., Pellegrini E., Dramsi S., Baptista M., Bourgeois N., Doucet-populaire F., Rusniok C., Zouine M., Glaser P., Kunst F., Poyart C., Trieu-cuot P. The SrtA Sortase of Streptococcus agalactiae Is Required for Cell Wall Anchoring of Proteins Containing the LPXTG Motif, for Adhesion to Epithelial Cells, and for Colonization of the Mouse Intestine. Infect. Immun. 2005;73:3342–3350. doi: 10.1128/IAI.73.6.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.F., Boran T.L. Roles of sortase in surface expression of the major protein adhesin P1, saliva-induced aggregation and adherence, and cariogenicity of Streptococcus mutans. Infect. Immun. 2003;71:676–681. doi: 10.1128/IAI.71.2.676-681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux J., Woody S., Camilli A. Roles of the sortases of Streptococcus pneumoniae in assembly of the RlrA pilus. J. Bacteriol. 2008;190:6002–6013. doi: 10.1128/JB.00379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepp D., Zhou Y., Ojha S., Mehdizadeh Gohari I., Carere J., Yang C., Prescott J.F., Gong J. Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J. Bacteriol. 2021;203:e005578–20. doi: 10.1128/jb.00578-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.H., Miao J., Wu J.L., Chen S.F., Zhang Q.Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014;37:166–173. doi: 10.1016/j.foodhyd.2013.10.015. [DOI] [Google Scholar]
- Liew P.X., Wang C.L.C., Wong S.-L. Functional Characterization and Localization of a Bacillus subtilis Sortase and Its Substrate and Use of This Sortase System To Covalently Anchor a Heterologous Protein to the B. subtilis Cell Wall for Surface Display. J. Bacteriol. 2012;194:161–175. doi: 10.1128/JB.05711-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes M.J., Secrist H., Benson D.R., Jen S., Shanebeck K.D., Guderian J., Maisonneuve J.F., Bhatia A., Persing D., Patrick S., Skeiky Y.A.W. Variable expression of immunoreactive surface proteins of Propionibacterium acnes. Microbiology. 2006;152:3667–3681. doi: 10.1099/mic.0.29219-0. [DOI] [PubMed] [Google Scholar]
- Lu G., Qi J., Gao F., Yan J., Tang J., Gao G.F. A novel ‘“open-form”’ structure of sortaseC from Streptococcus suis. Proteins. 2011;79:2764–2769. doi: 10.1002/prot.23093. [DOI] [PubMed] [Google Scholar]
- Mandlik A., Swierczynski A., Das A. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends. Microbiol. 2010;16:33–40. doi: 10.1016/j.tim.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandlik A., Swierczynski A., Das A., Ton-That H. Corynebacterium diphtheriae employs specific minor pilins to target human pharyngeal epithelial cells. Mol. Microbiol. 2007;64:111–124. doi: 10.1111/j.1365-2958.2007.05630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano C., Izoré T., Job V., Di Guilmi A.M., Dessen A. Sortase activity is controlled by a flexible lid in the pilus biogenesis mechanism of Gram-positive pathogens. Biochemistry. 2009;48:10549–10557. doi: 10.1021/bi901261y. [DOI] [PubMed] [Google Scholar]
- Maresso A.W., Chapa T.J., Schneewind O. Surface protein IsdC and sortase B are required for heme-iron scavenging of Bacillus anthracis. J. Bacteriol. 2006;188:8145–8152. doi: 10.1128/JB.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariscotti J.F., Quereda J.J., Pucciarelli M.G. Contribution of sortase A to the regulation of Listeria monocytogenes LPXTG surface proteins. Int. Microbiol. 2012;15:43–51. doi: 10.2436/20.1501.01.157. [DOI] [PubMed] [Google Scholar]
- Marraffini L.A., DeDent A.C., Schneewind O. Sortases and the Art of Anchoring Proteins to the Envelopes of Gram-Positive Bacteria. Microbiol. Mol. Biol. Rev. 2006;70:192–221. doi: 10.1128/mmbr.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini L.A., Schneewind O. Sortase C-mediated anchoring of BasI to the cell wall envelope of Bacillus anthracis. J. Bacteriol. 2007;189:6425–6436. doi: 10.1128/JB.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini L.A., Schneewind O. Targeting proteins to the cell wall of sporulating Bacillus anthracis. Mol. Microbiol. 2006;62:1402–1417. doi: 10.1111/j.1365-2958.2006.05469.x. [DOI] [PubMed] [Google Scholar]
- Mazmanian S.K., Liu G., Jensen E.R., Lenoy E., Schneewind O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. 2000;97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian S.K., Liu G., Ton-That H., Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- Mazmanian S.K., Ton-That H., Su K., Schneewind O. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. 2002;99:2293–2298. doi: 10.1073/pnas.032523999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani, C., Mangifesta, M., Mancabelli, L., Lugli, G.A., Mancino, W., Viappiani, A., Faccini, A., 2017. The Sortase-Dependent Fimbriome of the Genus Bifidobacterium : Extracellular Structures with Potential To Modulate Microbe-Host Dialogue. 83, e01295-17. https://dx.doi.org/10.1128%2FAEM.01295-17. [DOI] [PMC free article] [PubMed]
- Mishra A., Das A., Cisar J.O., Ton-That H. Sortase-catalyzed assembly of distinct heteromeric fimbriae in Actinomyces naeslundii. J. Bacteriol. 2007;189:3156–3165. doi: 10.1128/JB.01952-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock M., Fouet A. Anthrax. Annu. Rev. Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- Morita C., Sumioka R., Nakata M., Okahashi N., Wada S. Cell Wall-Anchored Nuclease of Streptococcus sanguinis Contributes to Escape from Neutrophil Extracellular Trap-Mediated Bacteriocidal Activity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Provencio D., Rodríguez-Díaz J., Collado M.C., Langella P., Bermúdez-Humarán L.G., Monedero V. Functional analysis of the Lactobacillus casei BL23 sortases. Appl. Environ. Microbiol. 2012;78:8684–8693. doi: 10.1128/AEM.02287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai C., Igarashi T., Inoue M., Sasa R. Streptococcus mutans sortase catalyzes cell wall anchoring of WapA and FruA. Pediatr. Dent. J. 2005;15:127–133. doi: 10.1016/S0917-2394(05)70041-0. [DOI] [Google Scholar]
- Murray B. The Life and Times of Enterococcus. Clin. Microbiol. Rev. 1990;3:46–65. doi: 10.1128/CMR.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy S.R., Singh K.V., Sillanpää J., Garsin D.A., Höök M., Erlandsen S.L., Murray B.E. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Invest. 2006;116:2799–2807. doi: 10.1172/JCI29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziga E.B., Wereszczynski J. Molecular Mechanisms of the Binding and Specificity of Streptococcus pneumoniae Sortase C Enzymes for Pilin Subunits. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-13135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H.V., Flores-Mireles A.L., Kau A.L., Kline K.A., Pinkner J.S., Neiers F., Normark S., Henriques-Normark B., Caparon M.G., Hultgren S.J. Pilin and sortase residues critical for endocarditis- and biofilm-associated pilus biogenesis in Enterococcus faecalis. J. Bacteriol. 2013;195:4484–4495. doi: 10.1128/JB.00451-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobbs, A.H., Vajna, R.M., Johnson, J.R., Zhang, Y., Erlandsen, S.L., Oli, M.W., Kreth, J., Brady, L.J., Herzberg, M.C., 2007. Consequences of a sortase A mutation in Streptococcus gordonii 4088–4097. 10.1099/mic.0.2007/007252-0. [DOI] [PubMed]
- Norton P.M., Brown H.W.G., Wells J.M., Macpherson A.M., Wilson P.W., Le Page R.W.F. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol. Med. Microbiol. 1996;14:167–177. doi: 10.1016/0928-8244(96)00028-4. [DOI] [PubMed] [Google Scholar]
- Novick R.P. Sortase: The surface protein anchoring transpeptidase and the LPXTG motif. Trends Microbiol. 2000;8:148–151. doi: 10.1016/S0966-842X(00)01741-8. [DOI] [PubMed] [Google Scholar]
- Osaki M., Takamatsu D., Shimoji Y., Sekizaki T. Characterization of Streptococcus suis genes encoding proteins homologous to sortase of gram-positive bacteria. J. Bacteriol. 2002;184:971–982. doi: 10.1128/jb.184.4.971-982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansegrau W., Bagnoli F. Pilus Assembly in Gram-Positive Bacteria. Curr. Top Microbiol. Immunol. 2015;404:203–233. doi: 10.1007/82_2015_5016. [DOI] [PubMed] [Google Scholar]
- Paterson G.K., Mitchell T.J. The role of Streptococcus pneumoniae sortase A in colonisation and pathogenesis. Microbes Infect. 2006;8:145–153. doi: 10.1016/j.micinf.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Paterson G.K., Mitchell T.J. The biology of Gram-positive sortase enzymes. Trends Microbiol. 2004;12:89–95. doi: 10.1016/j.tim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Paulsen I.T., Banerjei L., Hyers G.S.A., Nelson K.E., Seshadri R., Read T.D., Fouts D.E., Eisen J.A., Gill S.R., Heidelberg J.F., Tettelin H., Dodson R.J., Umayam L., Brinkac L., Beanan M., Daugherty S., DeBoy R.T., Durkin S., Kolonay J., Madupu R., Nelson W., Vamathevan J., Tran B., Upton J., Hansen T., Shetty J., Khouri H., Utterback T., Radune D., Ketchum K.A., Dougherty B.A., Fraser C.M. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science (80-. 2003;). 299:2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- Peltier J., Shaw H.A., Couchman E.C., Dawson L.F., Yu L., Choudhary J.S., Kaever V., Wren B.W., Fairweather N.F. Cyclic diGMP Regulates Production of Sortase Substrates of Clostridium difficile and Their Surface Exposure through ZmpI Protease-mediated Cleavage. J. Biol. Chem. 2015;290:24453–24469. doi: 10.1074/jbc.M115.665091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J., Shaw H.A., Wren B.W., Fairweather N.F. Disparate subcellular location of putative sortase substrates in Clostridium difficile. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-08322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A.M., Ton-That H., Mazmanian S.K., Schneewind O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. III. Lipid II is an in vivo peptidoglycan substrate for sortase-catalyzed surface protein anchoring. J. Biol. Chem. 2002;277:16241–16248. doi: 10.1074/jbc.M109194200. [DOI] [PubMed] [Google Scholar]
- Persson K. Structure of the sortase AcSrtC-1 from Actinomyces oris. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011;67:212–217. doi: 10.1107/S0907444911004215. [DOI] [PubMed] [Google Scholar]
- Posfay-barbe K.M., Wald E.R. Listeriosis. Semin. Fetal Neonatal Med. 2009;14:228–233. doi: 10.1016/j.siny.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Pretzer G., Snel J., Molenaar D., Wiersma A., Bron P.A., Lambert J., De Vos W.M., Van Der Meer R., Smits M.A., Kleerebezem M. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J. Bacteriol. 2005;187:6128–6136. doi: 10.1128/JB.187.17.6128-6136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Tanasescu A., Zhao A.M., Serrano A., Alston T. Streptococcus pyogenes Sortase Mutants Are Highly Susceptible to Killing by Host Factors Due to Aberrant Envelope Physiology. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revitt-Mills S.A., Watts T.D., Lyras D., Adams V., Rood J.I. The ever-expanding tcp conjugation locus of pCW3 from Clostridium perfringens. Plasmid. 2021;113 doi: 10.1016/j.plasmid.2020.102516. [DOI] [PubMed] [Google Scholar]
- Robson S.A., Jacobitz A.W., Phillips M.L., Clubb R.T. Solution Structure of the Sortase Required for Efficient Production of Infectious Bacillus anthracis Spores. Biochemistry. 2012;51:7953–7963. doi: 10.1021/bi300867t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos S., Jonsson H. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology. 2002;148:433–442. doi: 10.1099/00221287-148-2-433. [DOI] [PubMed] [Google Scholar]
- Rupnik M., Wilcox M.H., Gerding D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- Schneewind O., Model P., Fischetti V.A. Sorting of protein a to the staphylococcal cell wall. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-H. [DOI] [PubMed] [Google Scholar]
- Schubert, A., Zakikhany, K., Pietrocola, G., Meinke, A., Speziale, P., Eikmanns, B.J., Reinscheid, D.J., 2004. The Fibrinogen Receptor FbsA Promotes Adherence of Streptococcus agalactiae to Human Epithelial Cells. Infect. Immun. 72, 6197–6205. 10.1128/IAI.72.11.6197. [DOI] [PMC free article] [PubMed]
- Schubert, A., Zakikhany, K., Schreiner, M., Frank, R., Spellerberg, B., Eikmanns, B.J., Reinscheid, D.J., 2002. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 46, 557–569. doi.org/10.1046/j.1365-2958.2002.03177.x. [DOI] [PubMed]
- Scott J.R., Zähner D. Pili with strong attachments: Gram-positive bacteria do it differently. Mol. Microbiol. 2006;62:320–330. doi: 10.1111/j.1365-2958.2006.05279.x. [DOI] [PubMed] [Google Scholar]
- Sendi P., Johansson L. Invasive Group B Streptococcal Disease in Non-pregnant Adults. Infection. 2008;36:100–111. doi: 10.1007/s15010-007-7251-0. [DOI] [PubMed] [Google Scholar]
- Shaik M.M., Maccagni A., Tourcier G., Di Guilmi A.M., Dessen A. Structural basis of pilus anchoring by the ancillary pilin RrgC of Streptococcus pneumoniae. J. Biol. Chem. 2014;289:16988–16997. doi: 10.1074/jbc.M114.555854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa K. Analytical methods for Bacillus cereus and other Bacillus species. Int. J. Food Microbiol. 1990;10:125–141. doi: 10.1016/0168-1605(90)90061-9. [DOI] [PubMed] [Google Scholar]
- Sillanpää J., Nallapareddy S.R., Singh K.V., Prakash V.P., Fothergill T., Ton-That H., Murray B.E. Characterization of the ebpfm pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection. Virulence. 2010;1:236–246. doi: 10.4161/viru.1.4.11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpää J., Xu Y., Nallapareddy S.R., Murray B.E., Höök M. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology. 2004;150:2069–2078. doi: 10.1099/mic.0.27074-0. [DOI] [PubMed] [Google Scholar]
- Singh K.V., Nallapareddy S.R., Murray B.E. Importance of the ebp (endocarditis-and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J. Infect. Dis. 2007;195:1671–1677. doi: 10.1086/517524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirig T., Weiner E.M., Clubb R.T. Sortase enzymes in Gram-positive bacteria. Mol. Microbiol. 2011;82:1044–1059. doi: 10.1111/j.1365-2958.2011.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadinata R., Seabrook S.A., Adams T.E., Nuttall S.D., Peat T.S. Structural and biochemical analyses of a Clostridium perfringens sortase D transpeptidase. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015;71:1505–1513. doi: 10.1107/S1399004715009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmitha A., Nampoothiri K.M., Bajaj H. Insights into the biochemical and functional characterization of sortase E transpeptidase of Corynebacterium glutamicum. Biochem. J. 2019;476:3835–3847. doi: 10.1042/BCJ20190812. [DOI] [PubMed] [Google Scholar]
- Swaminathan A., Mandlik A., Swierczynski A., Gaspar A., Das A., Ton-That H. Housekeeping sortase facilitates the cell wall anchoring of pilus polymers in Corynebacterium diphtheriae. Mol. Microbiol. 2007;66:961–974. doi: 10.1111/j.1365-2958.2007.05968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierczynski A., Ton-That H. Type III Pilus of Corynebacteria : Pilus Length Is Determined by the Level of Its Major Pilin Subunit. J. Bacteriol. 2006;188:6318–6325. doi: 10.1128/JB.00606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai E., Katayama S., Sekiya H., Nariya H., Kamitori S. Structures of major pilins in Clostridium perfringens demonstrate dynamic conformational change. Acta Crystallogr. Sect. D Struct. Biol. 2019;75:718–732. doi: 10.1107/S2059798319009689. [DOI] [PubMed] [Google Scholar]
- Tamai E., Sekiya H., Maki J., Nariya H., Yoshida H., Kamitori S. X-ray structure of Clostridium perfringens sortase B cysteine transpeptidase. Biochem. Biophys. Res. Commun. 2017;493:1267–1272. doi: 10.1016/j.bbrc.2017.09.144. [DOI] [PubMed] [Google Scholar]
- Telford J.L., Barocchi M.A., Margarit I., Rappuoli R., Grandi G. Pili in Gram-positive pathogens. Nat. Rev. Microbiol. 2006;4:509–519. doi: 10.1038/nrmicro1443. [DOI] [PubMed] [Google Scholar]
- Ton-That H., Marraffini L.A., Schneewind O. Sortases and pilin elements involved in pilus assemly of Corynebacterium diphtheriae. Mol. Microbiol. 2004;53:251–261. doi: 10.1111/j.1365-2958.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- Ton-That H., Schneewind O. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol. Microbiol. 2003;50:1429–1438. doi: 10.1046/j.1365-2958.2003.03782.x. [DOI] [PubMed] [Google Scholar]
- Turner L.S., Kanamoto T., Unoki T., Munro C.L., Wu H., Kitten T. Comprehensive Evaluation of Streptococcus sanguinis Cell Wall-Anchored Proteins in Early Infective Endocarditis. Infect. Immun. 2009;77:4966–4975. doi: 10.1128/IAI.00760-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni F., Duranti S., Bottacini F., Guglielmetti S., Sinderen D. Van, Ventura M. Bifidobacterium bifidum as an example of a specialized human gut commensal. Front. Microbiol. 2014;5:1–9. doi: 10.3389/fmicb.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen H.C., Klychnikov O.I., Menks M.A.C., Kuijper E.J., Drijfhout J.W., Hensbergen P.J. Clostridium difficile sortase recognizes a (S/P)PXTG sequence motif and can accommodate diaminopimelic acid as a substrate for transpeptidation. FEBS Lett. 2014;588:4325–4333. doi: 10.1016/J.FEBSLET.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Van Pijkeren J.P., Canchaya C., Ryan K.A., Li Y., Claesson M.J., Sheil B., Steidler L., O’Mahony L., Fitzgerald G.F., Van Sinderen D., O’Toole P.W. Comparative and functional analysis of sortase-dependent proteins in the predicted secretome of Lactobacillus salivarius UCC118. Appl. Environ. Microbiol. 2006;72:4143–4153. doi: 10.1128/AEM.03023-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Ossowski I. Novel molecular insights about lactobacillar sortase-dependent piliation. Int. J. Mol. Sci. 2017;18:1551. doi: 10.3390/ijms18071551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li M., Feng Y., Zheng F., Dong Y., Pan X., Cheng G., Dong R., Hu D., Feng X., Ge J., Liu D., Wang J., Cao M., Hu F., Tang J. The involvement of sortase A in high virulence of STSS-causing Streptococcus suis serotype 2. Arch. Microbiol. 2009;191:23–33. doi: 10.1007/s00203-008-0425-z. [DOI] [PubMed] [Google Scholar]
- Wei X., Wang S., Zhao X., Wang X., Li H., Lin W., Lu J., Zhurina D., Li B., Riedel C.U., Sun Y., Yuan J. Proteomic profiling of Bifidobacterium bifidum S17 cultivated under in vitro conditions. Front. Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner E.M., Robson S., Marohn M., Clubb R.T. The sortase A enzyme that attaches proteins to the cell wall of Bacillus anthracis contains an unusual active site architecture. J. Biol. Chem. 2010;285:23433–23443. doi: 10.1074/jbc.M110.135434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W.J., Lenoy E., Murphy T., Tardio L.A., Burgio P., Projan S.J., Schneewind O., Alksne L. Effect of srtA and srtB gene expression on the virulence of Staphylococcus aureus in animal models of infection. J. Antimicrob. Chemother. 2004;53:480–486. doi: 10.1093/jac/dkh078. [DOI] [PubMed] [Google Scholar]
- Westermann C., Zhurina D.S., Baur A., Shang W., Yuan J., Riedel C.U. Exploring the genome sequence of Bifidobacterium bifidum S17 for potential players in host-microbe interactions. Symbiosis. 2012;58:191–200. doi: 10.1007/s13199-012-0205-z. [DOI] [Google Scholar]
- Wu C., Mishra A., Reardon M.E., Huang I.H., Counts S.C., Das A., Ton-That H. Structural determinants of actinomyces sortase SrtC2 required for membrane localization and assembly of type 2 fimbriae for interbacterial coaggregation and oral biofilm formation. J. Bacteriol. 2012;194:2531–2539. doi: 10.1128/JB.00093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Mishra A., Yang J., Cisar J.O., Das A., Ton-That H. Dual function of a tip fimbrillin of Actinomyces in fimbrial assembly and receptor binding. J. Bacteriol. 2011;193:3197–3206. doi: 10.1128/JB.00173-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Terao Y., Ogawa T., Takahashi T., Hamada S., Kawabata S. Role of Streptococcus sanguinis sortase A in bacterial colonization. Microbes Infect. 2006;8:2791–2796. doi: 10.1016/j.micinf.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yang F., Zhang X., Jing H., Ren C., Cai C., Dong Y., Zhang Y., Zou Q., Zeng H. Protective Efficacy and Mechanism of Passive Immunization with Polyclonal Antibodies in a Sepsis Model of Staphylococcus aureus Infection. Sci. Rep. 2015;5:1–12. doi: 10.1038/srep15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Wu R., Joachimiak G., Mazmanian S.K., Missiakas D.M., Gornicki P., Schneewind O., Joachimiak A. Structures of Sortase B from Staphylococcus aureus and Bacillus anthracis Reveal Catalytic Amino Acid Triad in the Active Site. Structure. 2004;12:1147–1156. doi: 10.1016/J.STR.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink S.D., Burns D.L. Importance of srtA and srtB for growth of Bacillus anthracis in macrophages. Infect. Immun. 2005;73:5222–5228. doi: 10.1128/IAI.73.8.5222-5228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]