Highlights
-
•
Recent advances in ultrasound combined processes are detailed.
-
•
Hybrid combination of ultrasound with conventional techniques.
-
•
Innovative combinations of ultrasound with modern techniques.
-
•
Ultrasound combination techniques as efficient tool for food processing.
Keywords: Ultrasound, Combination, Hybrid, Innovative techniques, Intensification, Mechanisms
Abstract
Ultrasound has a significant effect on the rate of various processes in food, perfume, cosmetic, pharmaceutical, bio-fuel, materials, or fine chemical industries, despite some shortcomings. Combination with other conventional or innovative techniques can overcome these limitations, enhance energy, momentum and mass transfer, and has been successfully demonstrated in many recent studies. Various ultrasound combined hybrid and innovative techniques are systematically summarized in this review for the first time. Ultrasound can be combined with diverse conventional techniques including Soxhlet, Clevenger, enzyme, hydrotropes, ionic liquids, Deep Eutectic Solvents (DES) or Natural Deep Eutectic Solvents (NADES), to enhance mixing and micro-mixing, reduced thermal and concentration gradients, and selective extraction. Moreover, combinations of ultrasound with other innovative techniques such as microwave, extrusion, supercritical fluid, subcritical and pressure liquids, Instant controlled pressure drop (DIC), Pulsed Electric Field (PEF), Ultra-Violet (UV) or Infra-Red (IR) radiations, Counter-current chromatography (CCC), or centrifugal partition chromatographs (CPC) can enable reduced equipment size, faster response to process control, faster start-up, increased production, and elimination of process steps. The theories and applications of these ultrasound combined hybrid and innovative techniques as well as their advantages and limitations are compared, and further perspectives are proposed. This review provides new insights into advances in ultrasound combined techniques and their application at research, educational, and industrial level in modern food and plant-based chemistry.
1. Introduction
Processing under extreme or non-classical conditions is currently a dynamically developing area in applied research and industry. Alternatives to conventional transformation, preservation and extraction procedures may increase production efficiency and contribute to environmental preservation by reducing the use of water, energy and generation of hazardous substances. Ultrasound is a key versatile technology in achieving principles of sustainable “green” chemistry and extraction [1], [2]. Using ultrasound, full process of extraction could be completed in minutes with high reproducibility, reducing the consumption of solvent, simplifying manipulation and work-up, eliminating post-treatment of waste water, and consuming only a fraction of the fossil energy normally needed for a conventional process or extraction method such as Soxhlet extraction or Clevenger distillation. Although ultrasound has many advantages in term of mass transfer, there are still some obvious flaws, resulting in unsatisfactory results in certain cases such as degradation of secondary and primary metabolites [3]. Ultrasound is not a heating technique, so it's not ideally suited for the heat transfer, and cannot be used for macro-mixing, therefore, is not ideally suited for the momentum transfer.
This is the first report where ultrasound combined techniques have been summarized systematically with regard to processing and extraction techniques for food and natural extracts and products. Discussions focus on development of combined hybrid and innovative techniques, type of process obtained, driving forces and working mechanisms and the improvement on the yield, selectivity and total production line have also been discussed. This review does not include the discussion on ultrasound cavitation theory [4], guidelines of good practice for ultrasound [5], and application of ultrasound in food technology [6] as excellent reviews can be found elsewhere. This review presents a complete picture of current knowledge on combined hybrid and innovative techniques for processing and extraction as success stories for research, education and at industrial scale. The readers like chemists, biochemists, chemical engineers, physicians, and food technologists even from academia or industry will find the major combined techniques obtained by (1) improving and optimization of existing processes; (2) using non-dedicated equipment; and (3) innovation in processes and procedures.
2. Towards process intensification: From individual to combined techniques
Process intensification is one of the major concerns of current industries, whatever the sector of activity may be. Nowadays, the requirement for food industry no longer only meets the economic considerations but also at the necessity of developing processes which guarantee optimal efficiency in a sustainable approach.
The development of food products makes use of different processing, extraction and preservation technologies. This is because these products are complex mixtures of a large panel of compounds including proteins, sugars, lipids, vitamins, aromas, antioxidants, etc. Before such products can be commercialized and consumed, they have to be processed, transformed and preserved for food ready meals and extracted for food ingredients.
Conventional processing and extraction techniques have long been used to obtain food ingredients and develop finished dairy products. Nevertheless, these procedures are accompanied by several drawbacks including their low performance, possible degradation of thermolabile compounds as well as their high energy consumption [7], [8], [9].
Industries are constantly searching for efficient technologies with sustainable and green values to meet consumers and regulations requirements. Process intensification can be achieved through the exploitation of existing conventional and innovative processes and the exploration of combined technologies potential. In other words, when existing technologies are not efficient enough to ensure acceptable performance, it is recommended to intensify and assist the process with innovative technologies.
Ultrasound (US) has been considered as an innovative and promising technique of the 21st century. This technique has been increasingly used in the alimentary field including processing (crystallization, drying, filtration, cutting, etc.), preservation (inactivation of microorganisms and enzymes) and extraction processes [10], [11], [12]. These applications have already been listed in many reviews and scientific articles. The significance of US is clearly depicted from the results of a simple search on Science Direct which returned 63,588 documents on this topic, where 17,852 deal with US application for the extraction of food ingredients. This number of studies proves the relevance of US in this domain, especially in the extraction of natural products and ingredients. Power US performance relies on their positive contribution in terms of extraction kinetics, selectivity, rapidity, reduced solvent consumption and waste/CO2 emissions compared to conventional techniques [13], [14], [15], [16], [17], [18], [19]. Nowadays, ultrasonic technology represents a well-established method used at both laboratory and industrial scales. The growing number of ultrasonic patents indicates the increasing interest on this technique at industrial scale.
Different other innovative technologies were developed and valorized in food industry, especially for the extraction of food ingredients. Among these techniques, we find Microwave-Assisted Extraction (MAE), Pressurized Liquid Extraction (PLE), Supercritical Fluid Extraction (SFE) and Instantaneous Controlled Pressure Drop (DIC), etc.
Table 1 summarizes the most common conventional and innovative extraction processes, details their working principle and processing mechanism and presents their main advantages and limitations. As presented in this table, each technology presents certain advantages as well as considerable limitations, which justifies the interest increasingly given to combined techniques. Coupling different techniques may offer the solution to ensure optimal performance and provide products and ingredients of higher quality and in the meantime enhance competition of industries to be more ecologic, economic and innovative.
Table 1.
The main innovative techniques used for the extraction of natural materials.
| Technology | Working principle | Processing mechanism | Advantages | Limitations |
|---|---|---|---|---|
| Pressing-extrusion | The extrusion process consists in pushing materials through a die, aided by the pressure induced by one or two rotating screw(s) (single- or twin-screw extruder). The classical extrusion process only implies thermomechanical phenomena. When chemical reactions are induced in the extruder, a reactive extrusion process takes place [20] | Intense mixing and mechanical destructuring of biomaterials provided by screws both facilitate access to inner structures and their contents. As a result, extraction efficiency is significantly increased [20]. |
|
|
| Ultrasound-Assisted Extraction (UAE) | Cavitation through ultrasonic mechanical waves which have the property to spread in elastic medium such as extraction solvents [4]. | The implosion of cavitational bubbles generates micro-jets of extreme conditions of pressure and temperature to breakdown cell membranes. This results in enhanced porosity and accelerated solvent penetration into the biological material [3], [22], [23]. | ||
| Microwave-Assisted Extraction (MAE) | Absorption of microwave energy by the treated material through molecular interaction with the electromagnetic field [32], [33]. | It consists of a non-contact energy transfer process from electromagnetic energy into thermal energy. This energy conversion is based on two mechanisms: ionic conduction and dipole rotation [34]Microwave heating of the moisture inside the treated biomaterial results in high pressure on the cell wall resulting in their mechanical rupture. This is presumed to enhance solvent penetration into the inner tissues and to improve extraction yields and selectivity [32], [33]. |
|
|
| Pressurized Liquid Extraction (PLE) | A process which employs an extraction solvent at high temperature and pressure, below their respective critical points [35], [36]. | High conditions of temperature and pressure modify solvent physicochemical properties including surface tension, density, viscosity, diffusivity, and dielectric constant. As a result, solvent solubilization and extraction abilities will be improved. Moreover, when increasing temperature, intermolecular interactions (Van der Waals forces, hydrogen bonding and dipole attraction) that bind the targeted compound to its containing structure are considerably reduced. Thus, targeted molecules become easier to extract [35], [36] |
|
|
| Supercritical Fluid Extraction (SFE) | A fluid is consideredto be in its supercritical state when it is both heated above its critical temperature (Tc) and pressurized above its critical pressure (Pc) [1]. | Interest in SFE technique relies on supercritical fluid’s interesting properties. On the one hand, these solvents have a density close to liquids, implying that they have a solubilization power close to liquids. On the other hand, their viscosity is close to gases and their diffusivity is intermediary between liquids and gases, leading to an increase of mass transfer between the targeted molecule and the supercritical fluid. Therefore, working with supercritical fluids offers the possibility of modulating solvent selectivity [1] |
|
|
| Instant controlled pressure drop (DIC) process | DIC process involves a thermomechanical processing induced by subjecting the material to a fast transition from high steam pressure to vacuum [24]. | The creation of vacuum condition represents the first step of DIC extraction, followed by injecting high pressure saturated steam into the biomaterial for few seconds. The third DIC-stage consists of a sudden pressure drop towards vacuum (about 5 kPa with at a rate higher than 0.5 MPa/s) This pressure-drop triggers:
|
|
Possible degradation of thermolabile analytes [24] |
| Pulsed Electric Field (PEF) Extraction | PEF process is the application of repetitive short pulses with high voltage into a material held between two electrodes [40], [41]. | PEF extraction efficiency relies on the mechanism of “electroporation”, called also “electro-permeabilization”. Indeed, the application of an electric field induces the formation of pores into cell membranes. Consequently, membrane permeability and diffusion efficiency of targeted compounds increase significantly [40], [42] |
|
|
| High intensity light assisted extraction | A non-thermal method that employs Ultra-Violet (UV) rays of short wave-length and high energy. The penetrating pulses rich in UV last a few hundreds of microseconds [43], [44]. | Synthesis of secondary metabolites, playing the role of defense compounds, is usually triggered by biotic and abiotic stresses. The high intensity light, particularly in the UV region, represents an abiotic stress for plant cells. [43], [45]Furthermore, these UV-rays could physically breakdown cell walls or membranes and thereby enhance the release of cells contents into the surrounding extraction solvent [45] |
|
The strong pulse light treatment induces photochemical effects including chemical modifications, DNA cleavage, protein denaturation, etc. This way, this excessive treatment prevents cells to replicate. UVB and UVC are the most damaging wavelengths of UV light, causing direct DNA damage. As for UVA, it is less effective, causing indirect damage to cells through the production of reactive oxygen species that may damage DNA, proteins and lipids [48], [49] |
Different conventional methods can be coupled to US, such as Soxhlet, Clevenger and enzymatic extraction. US can also be coupled to other innovative sustainable “green” techniques including MAE, PLE, SFE and DIC etc.
These different combinations will permit the development of high process intensification, making use of physical and/or chemical phenomena different from those involved in individual processes.
This review provides at first a complete picture of the US necessary theoretical background (theory and mode of action). Then, it presents a summary of all current knowledge on the combination of conventional and innovative techniques with US technology for the intensification of extraction from food and natural products. Some examples of successful combinations are provided. Driving forces and working mechanisms are also discussed. The last part is devoted to the most common combinations developed for food processing and preservation.
3. Ultrasound-Assisted extraction and processing: Principle and mechanisms
Ultrasound can be defined as mechanical waves which are able to spread in elastic medium such as liquids [4]. Ultrasound (US) frequency is one the most important physical characteristics of ultrasonic waves, which ranges between 20 kHz and 10 MHz, above the human hearing range (from 16 Hz to 20 kHz) [50]. Given this large range of frequencies, two zones can be distinguished:
-
(i)
Diagnostic US characterized by high frequencies (from 2 MHz to 10 MHz) and low ultrasonic power (P < 1 W). In this frequency and power range, there is no destructive effect into the medium. The desired effect is only to characterize the medium by measuring the submitted modification of the ultrasonic wave during its propagation into the medium [50], [51], [52].
-
(ii)
Power US characterized by low frequencies (from 20 kHz to 100 kHz) and high ultrasonic power (P > 10 W) [50]. Contrary to diagnostic US, high power promotes physical and chemical effects by creating sufficient interaction between the ultrasonic wave and the elastic medium. Physical impacts are essentially observed at low frequencies (from 20 kHz to 100 kHz). This frequency range is widely valorized in solid–liquid extraction. The extended range of power US frequencies (up to 2 MHz) is used in sonochemistry. Different chemical impacts can be observed in this frequency range, mainly the formation of radicals [51]. Frequencies most commonly applied in solid–liquid extraction of food ingredients are 20, 25 and 40 kHz. At these frequencies, US contribution relies on its physical effects. US impacts, behind the enhancement of extraction performances can be summarized as follows [53].
-
(i)
Increased mass transfer and improved accessibility to cells and inner structures, due to extreme conditions of temperature and pressure generated during bubble collapse events resulting in cells damage and thinning of membranes layers.
-
(ii)
Enhanced diffusion of solvent into the matrix, due to the creation of pores into membranes which gives access to the underlying tissues. Hydration and swelling of the matrix further enlarge pores and promote access.
-
(iii)
Improved diffusion of the solutes present within pores due to shear forces, microscopic turbulence and agitation as well as inter-particle collision resulting from the propagation of ultrasonic wave and the implosion of cavitation bubbles.
-
(iv)
Increased surface area of matrix as a result of shock waves and microjets directed towards the matrix surface.
Mechanisms behind those positive impacts are beginning to be unraveled owing to the growing interest in the comprehension of US-related physical impacts.
Overall, US-induced impacts can be attributed to the cavitation phenomenon referring to bubble formation, growth and implosion during the propagation of the ultrasonic wave into an elastic medium [4], [22], [30], [52]. Bubble implosion results in the creation of hot spot with extreme conditions of temperature (up to 5000 K) and pressure (up to 5000 atm), which explains their extremely high reactivity [22], [29], [52], [54], [55], [56]. Occurring at or near a solid surface, the bubble collapse is asymmetric generating micro-jets and shock waves directed towards the solid surface [57]. This results in disruption of cell walls, particle size reduction, and enhanced mass transfer across cell membranes as well as an excellent penetration of the extraction solvent [3], [9], [58], [59]. Shear forces and the resulting inter-particle collisions have also been reported to accelerate the diffusion of matrix-contained compounds [26], [51].
Shirsath et al (2012) have defined cell disruption and breaking as the major mechanism of action of US when applied on plant solid–liquid extraction. The formed cracks increase the plant tissues permeability and thus enhance the solvent penetration into the inner plant tissues as well as the release of their content [17]. Nevertheless, US mode of action appears to be a much more complicated process than that proposed by Shirsath and coworkers. It has been found that completely different physical impacts can be observed, depending on US parameters and particularly on the nature of plant matrices.
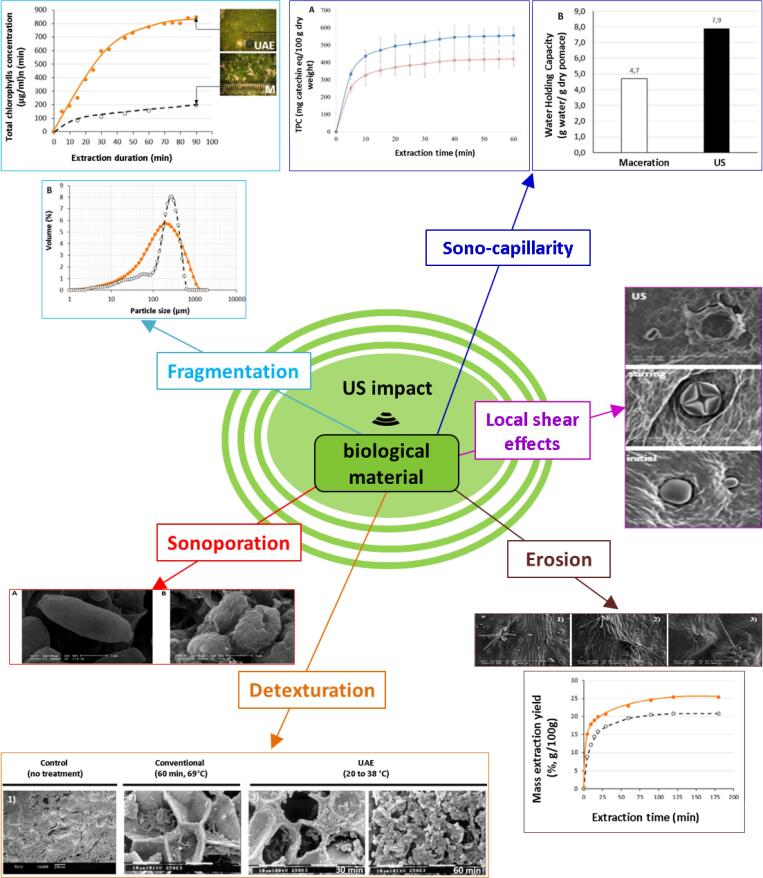
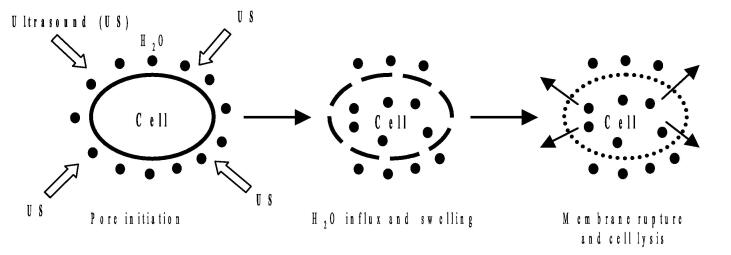
These different mechanisms were summarized in a review published by Chemat et al [1]. As explained in this review, depending on their structure, cell walls can be slightly or highly impacted by the cavitation phenomenon. This means that US-related effects can range from removing small particles or structures from the surface (erosion) to creating pores (sonoporation) or even deep fractures within the raw material (fragmentation). These effects are presumed to enhance solvent penetration into inner structures and in meantime favor the release of targeted compounds [60], [61], [62]. Furthermore, strong shear forces and turbulence, resulting from the propagation of US wave, seem to further accelerate exchange between raw material and the surrounding extraction solvent. These mechanisms result in “the increase of depth and velocity of penetration of solvent within plant inner structures”, referring to the ultrasonic capillary effect (UCE) [1]. This mechanism is associated to the increase in the swelling index and the hydration of plant material which enhance the extraction performances. A total detexturation of plant structures can be also observed due to US application [63].
Noteworthy, almost all studies dealing with US-induced mechanisms proposed a single physical mechanism behind the ultrasound observed effects. These individual mechanisms are summarized in Fig. 1 Fig. 2.
Fig. 1.
Reported US impacts on plant tissues.
Fig. 2.
Mechanism of ultrasound-induced cell damage.
Nevertheless, a study conducted by Khadhraoui et al. (2018) showed that US impacted rosemary leaves, not by a single mechanism but by a chain detexturation mechanism where six physical impacts occurred in a special order: local erosion, shear forces, sonoporation, fragmentation, capillary effect, and detexturation [27]. Together, these different mechanisms contributed to maximize yields of extraction by increasing the surface area contact between water and leaf components. A recent study conducted by the same team [28] further pointed out the complexity of ultrasonic mode of action. Findings presented in this article demonstrated that, at the same experimental conditions, US acts through different mechanisms when applied on blackcurrant, bitter orange and artichoke leaves. The case of artichoke leaf has particularly shed the light on the complexity of plant matrices behavior in response to US treatment. For the same plant and the same specialized structure, considerable differences were noticed between upper and lower surfaces [28]. Based on these different studies, it can be concluded that US has a highly complex mode of action. This represents the main limitation of this technology, since its performance could be extremely limited by matrices resistance level. Therefore, US combination with other conventional or innovative techniques may be useful in addressing this limitation.
4. Combination of US with conventional extraction techniques: Hybrid techniques
Most common combinations of US with conventional techniques are presented and discussed in this section. Table 2 gives examples of recent applications of these hybrid combinations.
Table 2.
Examples of conventional techniques coupled to US for the extraction of natural materials.
| Technique | Matrix | Targeted compound | Device diagram | Combination impact | Ref |
|---|---|---|---|---|---|
| Ultrasound assisted Soxhlet extraction: Sono-Soxhlet | Olives | Lipids | 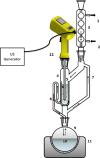 |
Improvements in extraction time over conventional Soxhlet technique. | [64] |
| Ultrasound assisted hydro-distillationSono-Clevenger | Iberis amara seeds | Volatil aromatic compounds | 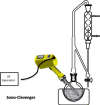 |
Higher extraction efficiency compared with traditional techniques | [67] |
| Ultrasound assisted enzymatic extraction | Sesame bran | Protein and phenolic compounds |  |
Higher protein yield and higher antioxidant capacity value compared with the combined ultrasound-assisted enzymatic extraction | [71] |
4.1. Ultrasound assisted Soxhlet extraction: Sono-Soxhlet
Fats and oils are traditionally extracted from the biomass matrix using the Soxhlet extraction. Invented in 1879, this apparatus has been widely used in various fields such as environmental applications, foodstuffs and also pharmaceuticals. Its principle is relatively easy and proceeds by an iterative percolation of condensed vapors of a boiled solvent, generally n-hexane. Nevertheless, Soxhlet extraction has some disadvantages such as a long operation time (several hours), large solvent volumes, evaporation and a concentration step needed at the end of the extraction. It is also inadequate for thermolabile analytes. There are only few processes in the literature that have reported the combination of Soxhlet extraction with innovative techniques, such as ultrasound, for the acceleration of fat and oil extraction.
The team of Luque de Castro (1998) [64] developed original Sono-Soxhlet methods. US is applied outside or inside the extraction chamber to enhance the solid–liquid extraction and migration of metabolites from solid matrix to solvent [29]. Sono-Soxhlet combines the advantages of the extraction performed with Soxhlet (extraction repeated by a fresh solvent) and enhanced mass transfer with US (reduction of extraction time). Hence, it ensures a complete, rapid, and accurate extraction of samples. This system has been successfully used for the extraction of the oil and the fatty acid from oleaginous seeds, lipids from sausage products, fat from cheese and bakery products.
4.2. Ultrasound assisted Clevenger distillation: Sono-Clevenger
The traditional method used to isolate volatile compounds such as essential oils from plant material (herbs, spices, barks, fruits…) is alembic distillation which, in chemistry laboratories, is also called Clevenger distillation. This method proceeds by the iterative distillation and boiling of the aromatic matrix in recondensed water vapor, and generally uses large quantities of water and energy. The extraction time can vary from 6 to 24 h. During distillation, fragrant plants exposed to boiling water or steam, release their essential oils through evaporation. Essential oil recovery is facilitated by distillation of two immiscible liquids, namely, water and the essential oil. This is based on the principle that, at the boiling temperature, the combined vapor pressures equal the ambient pressure. Thus, the essential oil ingredients, for which boiling points normally range from 200 to 300 °C, are evaporated at a temperature close to that of water. As steam and essential oil vapors are condensed, both are collected and separated in a vessel, traditionally called the “Florentine flask”. The essential oil, being lighter than water, floats at the top while water stays at the bottom and is separated.
Historically, there have been three types of distillation: water distillation, water-steam distillation and steam distillation. In addition, there are numerous other improved methods of producing natural fragrances and essential oils including turbo-distillation, hydro-diffusion, vacuum-distillation, continuous-distillation and dry-distillation. All these conventional extraction techniques have important drawbacks, such as low yields, formation of by-products and limited stability. The elevated temperatures and prolonged extraction time can cause chemical modifications in the essential oil components and often a loss of the most volatile molecules.
With the growing of flavor and fragrance industry and the increasing demand for more natural products, the need for novel extraction methods has become more intense. The combination of ultrasound with Clevenger or alembic distillation has attracted growing interest in the past few years. This has resulted in the development of Sono-Clevenger [65] specifically aimed for obtaining essential oils from plant materials. Sono-Clevenger is an original combination of ultrasound cavitation and Clevenger distillation at atmospheric or reduced pressure (Table 2). It provides yields comparable to those obtained by traditional hydro distillation but with reduced extraction times and enhanced quality. The thermally sensitive crude materials seem to be preserved with this method, in contrast to conventional Clevenger distillation. Ultrasound causes an internal convection movement of the solute in the solvent, which improves the mass transfer rate in the distillation process. Accelerated volatilization of plant aromatic compounds can then be observed [66].
Liu et al [67] determined the optimal condition of Sono-Clevenger for the extraction of essential oil (EO) from Iberis amara seeds using the surface response methodology. They also compared the efficiency of this treatment with traditional methods such as hydro distillation or steam distillation. It was observed that the extraction efficiency markedly increased with the assistance of ultrasound compared with traditional techniques. Oils obtained with the three techniques have slightly different but good effects: antioxidant and anti-inflammatory effects, cytotoxicity on human colon cancer, and antibacterial effects.
Few studies using ultrasound technique for the extraction of volatile substances from plants have been published. Tekin et al in 2015 [68] showed that ultrasonic bath assisted extraction can improve the EO yield of cloves. A significant effect on the amount of essential oil distilled as a function of the power of the ultrasound used can also be observed using this technique.
However, when the power used is too high, labile compounds may disintegrate due to the increase in local temperature and pressure in the solvent induced by the intensive use of ultrasound. A possible negative effect on the EO yield should therefore be considered [3]. For example, ultrasonic pre-treatment has been found to improve EO yield in mint leaves and marjoram, while no significant impact was found for chamomile flowers [69]. Therefore, the effect of ultrasound on EO extraction depends on the power of the ultrasound as well as the raw material.
4.3. Ultrasound assisted enzyme extraction
Ultrasound-assisted enzyme extraction (UAEE) is an efficient and environment friendly technique that generally gives high yields with low energy consumption. These combinations are of paramount importance in selecting the best operating conditions for scalable and cost-efficient plant material extractions to achieve excellent yields and minimize degradation. It is well known that ultrasound can either activate or denature enzymes. In each case, the parameters to be used must be optimized in order to obtain the desired results.
The increase of enzymes activity under mild ultrasonic irradiation when the shear force, temperature, pressure, and production of radicals are limited by controlling power and irradiating time, is well described in the literature [70].
In 2019 an ecofriendly process based on an enzyme-assisted extraction coupled with ultrasound extraction is proposed to obtain active compounds. Görgüç et al [71] have studied the effect of enzyme and ultrasound-assisted enzyme extraction on recovery of protein and antioxidant compounds from sesame bran using response surface methodology. Enzymatic and ultrasound assisted extraction methods increased the protein yield, total phenolic content and antioxidant capacities compared to the standard alkaline extraction method. The highest protein yield was obtained with the combined ultrasound-assisted enzymatic extraction.
5. Ultrasound-Assisted extraction (UAE) using green solvents
Most promising combinations of US with green solvents are presented in this section. Table 3 gives examples of recent applications of UAE using green solvents.
Table 3.
Recent applications of UAE using green solvents (hydrotropes, DESs and NADESs).
| Technique | Matrix | Targeted compound | Device diagram | Combination impact | Ref |
|---|---|---|---|---|---|
| Ultrasound assisted hydrotropic extraction | Leaves of palmarosa | Geraniol |  |
Increased extraction efficiency of geraniol from the leaves of palmarosa. | [73] |
| Ultrasound assisted ionic liquid extraction | Orange peel | Carotenoids |  |
Higher extraction efficiency and significantly reduced extraction time | [78] |
| Ultrasound assisted deep eutectic solvent extraction | Buckwheat sprouts | Flavonoids |  |
Higher extraction efficiency of flavonoid | [91] |
| Ultrasound assisted Natural Deep eutectic solvent extraction | Wine lees | Anthocyanins | Higher extraction efficiency of wine lees anthocyanins | [93] |
5.1. Ultrasound assisted hydrotropic extraction (UAHE)
Hydrotropy phenomenon can be defined as the increase in solubility of organic compounds that are insoluble or sparingly soluble in water in aqueous solutions in the presence of highly water-soluble organic salts, called also hydrotropes. The increase in solubility of an organic solute such as esters, alcohols, aldehydes, ketones, hydrocarbons and fats is a function of the hydrotropic concentration. This phenomenon depends not only on hydrotrope nature, but also on the solute nature [72].
Although hydrotropes were first studied in biochemistry, their role and applicability have been the subject of numerous studies in chemistry. Hydrotropes are composed of two parts: hydrophilic and hydrophobic. They don’t form micelles, but they self-aggregate gradually. They are characterized by a concentration threshold at which the solubility of hydrophobic compounds increases significantly: this threshold is called the minimum hydrotropic concentration (MHC).
Hydrotropes are widely used for the solubilization of drugs, but also as extraction agents for fragrances, for the separation of near boiling liquid mixtures by distillation or in the field of liquid–liquid extraction. The high solubilization capacity and selectivity of hydrotropic solubilization can be used for the extraction of water-insoluble bioactive compounds. In 2018, Thakker et al [73] used for the selective isolation of geraniol from the leaves of Cymbopogan martini, a newer concept of combining hydrotropic extraction with ultrasound to reduce time of extraction and hydrotropic requirement, while maintaining the quality of the product. Various parameters, such as ultrasound amplitude, cycle time and the volume and concentration of the hydrotropic solution, as well as the extraction time were studied in order to optimize the experimental conditions affecting the Ultrasound assisted hydrotropic extraction (UAHE). The results led to improvements in product yield compared to hydrotropic extraction without sonication, as well as a reduction in extraction time and a decrease in hydrotrope consumption. A synergistic effect between hydrotrope and ultrasound was thus demonstrated. This new eco-extraction method can be used to extract valuable compounds from various plants.
5.2. Ultrasound assisted ionic liquid extraction (UAILE)
Ionic liquids (ILs) are commonly defined as a class of salts, as a group of nonmolecular solvents prepared by the combination of organic cations and organic or inorganic anions showing melting points lower than their former constituents close to 100 °C [74]. ILs were first observed by Paul Walden in 1914 in the case of ethylammonium nitrate [EtNH3][NO3] which was obtained through the neutralization of ethylamine with concentrated nitric acid [75]. Among ILs properties of interest are their non– inflammability, thermal stability, low vapor pressure, and especially their impressive tunability and synthetic versatility [76]. It was only in 2007, that this solvent was used for the extraction of bioactive compounds. From this year forward, a great interest for IL as extraction medium was observed with a steady increase in the number of published manuscripts [77]. Murador et al [78] used ultrasound assisted ionic liquid extraction (UAILE) for the extraction of carotenoids from orange peel. The aim of this work was to develop an effective, rapid and environment friendly ionic liquid-based ultrasound assisted approach for the extraction of active compounds. The authors had to optimize a number of parameters such as ionic liquid concentration, soak time, solid–liquid ratio, ultrasound power, time and the number of extraction cycles. A comparison was made between extractions carried out in ionic liquid alone, in acetone and in ionic liquid combined with ultrasounds. The followed approach (UAILE) provided higher extraction efficiency and significantly reduced time and energy consumption. This environmentally friendly method can thus be applied for the extraction a wide range of active compounds.
5.3. Ultrasound assisted DES or NADES extraction (UADESE or UANADESE)
Historically, Deep Eutectic Solvents (DESs) were firstly observed by Abbott and coworkers in 2003 [79]. DESs can be prepared by mixing solid compounds which form a eutectic mixture with a melting point lower than either of the individual components melting points [80]. This is mainly due to the generation of intermolecular hydrogen bonds between hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD). Choline chloride represents the most reported HBA in scientific literature. This molecule has the ability to form hydrogen bonds with most HBD. Produced on a large scale for animal supplementation, Choline chloride is a low-cost and biodegradable derivative of vitamin B4. Moreover, its non– toxicity makes it an ideal candidate for the synthesis of green solvents. Overall, DESs share many physicochemical properties with ILs (high viscosity, low volatility, non-inflammability, chemical and thermal stability) [80]. Moreover, they present some advantages over ILs mainly the ease of their storage and synthesis as well as the low cost of their starting materials. To further meet the principles of green chemistry proposed by Anastas and Warner [2], natural sources of DESs attracted great attention in replacement of synthetic compounds [81] giving rise to a new class of DESs, namely Natural Deep Eutectic Solvents (NADESs). As in the case of DESs, NADESs are mixtures of compounds that have a much lower melting point than that of any of their individual components [82].
Besides all the advantages of DESs, NADESs are considered as environment friendly and ‘readily biodegradable’ due to the natural origin of their components [82], [83] and consequently obtained extracts can be safely used in food, pharmaceutical and cosmetics industries [84]. These new green solvents were firstly introduced by Choi and coworkers who defined them as the third liquid phase naturally occurring in all living organisms and cells [85]. According to Choi [85], this third liquid is capable of dissolving a number of natural molecules that are poorly soluble in water and lipids such as taxol and rutin as well as proteins, explaining thus many biological phenomena such as the biosynthesis of molecules that are soluble in neither water nor lipids. The compounds found to form this liquid phase are primary metabolites like organic acids (lactic, malic, citric acids, etc.), sugars (glucose, fructose, sucrose, etc.); amino acids, choline chloride, etc. [86], [87]. These natural compounds play key roles in biological processes such as drought resistance, cryoprotection and defense against external attacks [88], [89]. Noteworthy, according to the nature of their components, NADESs can be classified into four groups: (i) Derivatives from organic acids, (ii) Derivatives from choline chloride, (iii) Mixtures of sugars and (iv) other combinations [90].
Recent studies prove the effectiveness of DES and NADES in the extraction of natural products and the valorization of by-products of the food industry. For example, DES coupled with ultrasound-assisted extraction are evaluated and optimized for the extraction of major flavonoids from common buckwheat sprouts. Mansur et al [91] show that the extraction efficiency is even higher than that achieved with methanol for the extraction of these compounds. The optimized extraction procedure is reliable and efficient for the extraction of major flavonoids from common buckwheat sprouts. Chanioti et al [92] employed natural deep eutectic solvents and innovative extraction techniques for the extraction of phenolic compounds from olive pomace. The NADESs offering sustainability, biodegradability, compositional flexibility and extractability of bioactive compounds, highlight their potential to be utilized as green solvents for the extraction of phenolic compounds from olive pomace. Moreover, if they are combined with novel extraction assisted methods (e.g. microwave, ultrasound etc), high extraction efficiencies can be achieved in significantly reduced time. These studies have demonstrated the improvement in the extraction of phenolic compounds compared to conventional solvents (e.g. aqueous ethanol and water). As another example we can observe the use of NADES combined with ultrasound for the extraction of anthocyanins from wine lees [93]. A NADES based on chlorine chloride with malic acid as hydrogen bond donor is selected as the most promising, which allowed a more efficient extraction of anthocyanins from wine lees compared to a conventional solvent. Bosiljkov et al [93] combined this NADES with ultrasound and showed that this approach using green NADES solvents and ultrasound as an alternative energy source could be a good choice for the design of environmentally friendly extraction methods for the recovery of plant phenolic compounds.
6. US coupled with innovative extraction techniques
6.1. Combination of US with pressing-extrusion
Mechanical pressing is increasingly used in a great number of applications in the food domain particularly for juice and oil extraction [21]. The extrusion process consists of pushing materials through a die, aided by the pressure induced by one or two rotating screw(s) (single- or twin-screw extruder). The classical extrusion process only implies thermomechanical phenomena. When chemical reactions are induced in the extruder, a reactive extrusion process takes place [20]. This solvent-free process could be considered as an interesting alternative to solvent extraction. This is because the obtained extracts are of higher quality and do not contain any solvent traces. In order to achieve better extraction yields, research has recently focused on the combination of extrusion with other solvent extraction processes [21]. Many studies proved that US application has a significant positive effect on oil extraction in terms of extraction kinetics and yields [94], [95], [96]. Milenković et al (2018) explored the potential of US as a subsequent treatment to mechanical pressing from sunflower seeds (table 4). After pressing process, the cake contains a certain amount of residual oil. The authors claimed that US application improved extraction kinetics and oil diffusion from the mass of the cake to the extracting solvent, compared to conventional Soxhlet extraction [97].
Table 4.
Examples of US-innovative techniques combinations for the extraction of natural materials.
| Technique | Matrix | Targeted compound | Type of combination | Device diagram | Combination impact | Ref |
|---|---|---|---|---|---|---|
| US combined with mechanical pressing | Sunflower seed | Oil | In line | 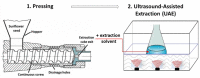 |
US application improved extraction kinetics and oil diffusion from the mass of the cake to the extracting solvent, compared to conventional Soxhlet extraction. | [97] |
| Ultrasound-Microwave Assisted Extraction (UMAE) | Sorghum husk | Natural colorants | Direct in situ (DIS) | 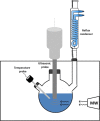 |
Significantly improved extraction yield and better dyeing properties of sorghum husk extracts. | [105] |
| Sweet potatoes (Ipomoea batatas L.) | Prebiotic oligosaccharides | Direct in situ (DIS) | Higher extraction efficiency compared to theconventional hot-water extraction, microwave-assisted extraction (MAE), and ultrasound assisted extraction (UAE) methods. | [106] | ||
| Lotus seed starch-green tea polyphenol complex | Preparation of starch-polyphenol complex | Direct in situ (DIS) | Increased extraction yield of the complex. | [107] | ||
| Potato pulp | Pectin | Direct in situ (DIS) | Increased extraction efficiency of potato pectin. | [107] | ||
| Alternate Microwave/Ultrasound with DES | Schisandra Chinensis fruit | Polysaccharides and essential oil | Alternating US/MW digestion | Higher polysaccharide and essential oils yields. | [109] | |
| Ultrasound Microwave- assisted enzymatic extraction (UMAEE) | Lentinus edodes | Polysaccharides | In line | 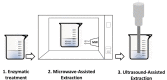 |
Higher extraction yields of polysaccharides content compared to conventional techniques. | [110] |
| Laminaria | Monoiodo-tyrosine (MIT) and Diiodo-tyrosine (DIT) | In line | 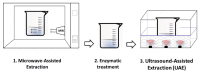 |
An efficient method to extract MIT and DIT from Laminaria. Both US and MW radiation can destroy the laminaria cell walls which improves access and extractability of targeted compounds. | [111] | |
| US combined with pressurized liquid | Sugar beet pulp | Pectin-enriched materials | In line | 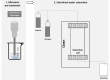 |
Higher extraction yield and improved functional properties obtained with subcritical water extraction. | [112] |
| Pomegranate peel | Phenolic compounds | Direct in situ (DIS) | 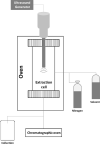 |
Higher extraction yield Reduced extraction time and temperature | [113] | |
| Fragrant oil from red pepper seed | Residual propane | In line | 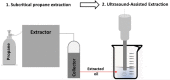 |
US was efficient in solvent residue removal. Treated oil presents good oxidation stability and quality. | [114] | |
| US combined with supercritical CO2 | Agave salmiana bagasse | Antioxidants and saponins | Direct in situ (DIS) | 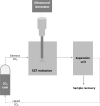 |
Increased extraction yield in terms of antioxidants and saponins contents. | [119] |
| Ginger rhizomes | Pungent compounds | Direct in situ (DIS) | Improved extraction yield of pungent compounds from ginger. | [118] | ||
| Iberis amara seeds | Cucurbitacin E (CuE) | Direct in situ (DIS) | The introduction of ultrasound to supercritical CO2 extraction enhanced CuE yield and reduced operation time as well as the amount of CO2 consumed. | [67] | ||
| Instant controlled pressure drop (DIC) combined with US | Orange peels | Antioxidants | In line |  |
The highest yield of antioxidants with best kinetics is obtained by coupling both treatments. | [39] |
| Steviarebaudiana bertoni leaves | Rebaudioside A, Vitamin B6 and vitamin B1. | In line |  |
DIC pre-treatment has a significant positive effect on the extraction of Rebaudioside A, Vitamin B6 and vitamin B1. | [122] | |
| Pulsed Electric Field (PEF) combined with US | Almond seeds | Phenolics, flavonoids, condensed tannins and anthocyanins, antioxidant activity and volatile compounds | In line | 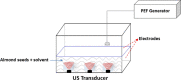 |
Combined treatment (PEF–US) has attained the highest value of total phenolics, total flavonoids, condense tannins, anthocyanin contents and antioxidant activity in DPPH, reducing power and metal chelating activity than all other treatments. | [124] |
| UV-C Radiation Combined with UAE | Cherry Tomato (Lycopersicon esculentum) | Lycopene | In line |  |
Extracted obtained from the irradiated tomatoes presented 5.8 times more lycopene content. | [126] |
| Tomatoes | Bioactive compounds | Direct in situ (DIS) | This postharvest non-thermal treatment resulted in increased lycopene, total phenols, vitamin C, hydrophilic and lipophilic antioxidant activities during storage. | [125] | ||
| US coupled with CCC and CPC | Paeonia lactiflora Pall roots | Albiflorin, benzoylpaeoniflorin, paeoniflorin, and galloylpaeoniflorin) | In line | 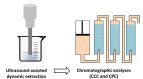 |
The four targeted compounds were successfully extracted. Compared with conventional extraction methods, this combination offers the advantages of automationand systematic extraction and isolation of natural products. | [131] |
6.2. Combination of US and MW
Microwave (MW) process relies on a non-contact energy transfer from electromagnetic energy into thermal energy. This energy conversion is based on two mechanisms: ionic conduction and dipole rotation. In other words, MW nonionizing radiation induces molecular motion through migration of ions and rotation of dipoles [34]. The overall advantages in applying MW include: faster energy absorption by the treated material, reduced thermal gradients and selective heating [98]. Furthermore, MW heating of the moisture inside the treated biomaterial causes high pressure on the cell wall resulting in their mechanical rupture. Solvent penetration into the inner tissues is therefore enhanced. Higher extraction yields and selectivity can be thus achieved [32], [33].
The combination of UAE and MW-assisted extraction (MAE) is one of the most promising combinations. These techniques can be either performed as single reactor configuration or via two separately connected reactors, which can be operated simultaneously or successively [24].
Ultrasound Microwave-Assisted Extraction (UMAE) has been successfully used for the extraction of bioactive compounds from different biomaterials [99], [100], [101], [102], [103], [104], [105], [106], [107], [108].
Some of these recent applications are presented in Table 4, giving more details concerning systems used and combination-associated impacts.
Wizi and coworkers (2018) explored simultaneous UMAE system through a direct in situ configuration (Table 4) for solid–liquid extraction from sorghum husk. Apart from a significantly improved extraction yield, the authors claimed that this combination ensured extracts with better dyeing properties [104]. The same configuration was successfully used to extract prebiotic oligosaccharides contained in sweet potatoes (Ipomoea batatas L.) [105] and to prepare starch-polyphenol complex from Lotus seed starch suspensions with added green tea polyphenols [106]. In both cases, the combined irradiation gave better extraction yields compared to conventional techniques.
UMAE combined with acid extraction increased extraction efficiency of potato pectin [107]. Significant performance gain was also noticed by Li and coworkers (2019) who have explored the potential of alternating US and MW to extract polysaccharides and essential oil from Schisandra Chinensis fruit [108]. Ultrasound Microwave-assisted enzymatic extraction (UMAEE) was used to obtain polysaccharides from Lentinus edodes. Although performed in separate steps, this combination was proved to considerably increase polysaccharides contents [109]. Wang et al (2019) applied UMAEE for Monoiodo-tyrosine (MIT) and Diiodo-tyrosine (DIT) extraction from Laminaria. This sequential treatment was proved to be an efficient method to extract MIT and DIT. US and MW positive contribution is related to the destruction of the laminaria cell walls which improves access and extractability of target compounds [110]. To summarize, all cited studies showed that US combined, simultaneously or successively, with MW radiation exhibit higher efficiency in shorter extraction time. This cost-effective combination has a great potential for application in solid–liquid extraction.
US-associated cavitation phenomenon promotes the release of targeted compounds through the physical damage of cell walls and membrane layers [98]. This results in increased mass transfer and accelerated solvent penetration. Concerning MW radiation, it is presumed to heat the biomaterial very quickly inducing the migration of dissolved molecules. Hence, simultaneous or successive US/MW treatment allows to achieve higher extraction efficiency [24], [98].
6.3. Combination of US with pressurized liquids
Pressurized-liquid extraction (PLE) is also called accelerated solvent extraction (ASE), pressurized hot solvent extraction (PHSE), high-pressure solvent extraction (HPSE), high-pressure high-temperature solvent extraction (HPHTSE), and subcritical solvent extraction (SSE). This extraction technique employs an extraction solvent at high temperature and pressure, below their respective critical points [35], [36]. At these high conditions of temperature and pressure, solvent physicochemical properties including surface tension, density, viscosity, diffusivity, and dielectric constant are significantly modified. This results in enhanced solubilization and extraction abilities. Moreover, when increasing temperature, intermolecular interactions (Van der Waals forces, hydrogen bonding and dipole attraction) that bind the targeted compound to its containing structure are drastically reduced. This makes their extraction easier [35], [36].
Even though this extraction technique improves solvent properties, accessibility to inner structures still represent a limiting factor especially when biological barriers are particularly difficult to overcome. For this, its combination with US appears of great interest since the latter has been proved to enhance accessibility through cell disruption and membranes thinning. Different solvents (water, ethanol, hydro-alcoholic mixtures, etc.) have been used, at their subcritical state, in combination with US. Chen and coworkers [111] have successfully extracted pectin-enriched material from sugar beet pulp by ultrasonic pre-treatment combined with subcritical water extraction (Table 4). These two techniques were performed in separate steps. As a result, higher extraction yields and extracts with improved functional properties (pasting temperature, gelatinization temperature and enthalpy, etc.) were obtained.
This combination was also performed for the extraction of phenolic compounds from pomegranate peel [112]. In this study, ultrasonic treatment and subcritical solvent extraction were carried out simultaneously (Table 4). Results showed higher extraction yields achieved in reduced extraction time and at lower temperature.
Liu et al (2020) have used ultrasonic treatment to remove the residual solvent from red pepper seed oil obtained by subcritical propane extraction (Table 4). US application allowed the removal of residual propane. The overall combination provided red pepper oil with good oxidation stability and high quality [113]. It can be concluded from these examples that whatever the type of combination (in line or in situ), US and subcritical solvent extraction offer great advantages in terms of extraction yields, time and energy saving.
6.4. Combination of US with supercritical fluids
Supercritical fluid extraction (SFE) is a high-pressure extraction method which employs an extraction solvent in the supercritical state [114]. A fluid is considered in its supercritical state when it is both, heated above its critical temperature (Tc) and pressurized above its critical pressure (Pc) [1]. Supercritical fluids (SCF) have particular gas-like and liquid-like properties. Their viscosity is close to gases and their diffusivity is intermediary between liquids and gases [1]. Hence, a SCF has the ability to penetrate through solid matrices like a gas, and to dissolve targeted compounds like a liquid [115]. Moreover, SCF density can be easily tuned via the variation of pressure or temperature. All these characteristics make SCF efficient solvents providing a certain degree of selectivity and a high recovery of compounds in a short time [1], [114]. Carbon dioxide (CO2) is the most commonly used SCF. Among the properties of interest of CO2 are its nontoxicity, noninflammability, chemical inertness and easily separable nature after extraction. Furthermore, SFE using CO2 is adapted to the extraction of thermolabile compounds since it employs low temperatures, compared to other conventional techniques [115]. Overall, SFE offers several advantages over conventional extraction techniques, including enhanced extraction kinetics and improved extraction selectivity as well as the absence of solvent residue [116]. This technique presents yet some disadvantages such as low extraction yields and high cost compared to some conventional processes [116]. Hence, US assistance may be useful in addressing these limitations.
Combination of US with CO2, using the system presented in Table 4, resulted in improved extraction yield of pungent compounds from ginger rhizomes. This performance gain could be explained by US-induced cellular damage, highlighted with SEM observations of treated ginger rhizomes. This results in doubled diffusivity of targeted compounds in the initial stages of extraction [117]. Higher extraction yield of antioxidants and saponins from agave bagasse was also obtained using ultrasonically-assisted supercritical CO2 extraction. In this case, the use of a multiplate ultrasound transducer allowed to avoid the compaction phenomenon within the extraction cell and to intensify the extraction process through US positive contribution [118].
The introduction of US to supercritical CO2 extraction enhanced Cucurbitacin E yield from Iberis amara seeds. Moreover, this combination allowed to reduce operation time as well as the amount of CO2 consumed [113]. To conclude, many studies proved that the combination of US and SFE is a promising alternative to conventional extraction processes. US introduction, on one hand, is of great interest since it offers many advantages, including the reduction of pressure, temperature, the flow rate of CO2 and the overall extraction time while enhancing mass transfer rate and providing higher extraction yields [53]. On the other hand, better stability of CO2, due to its inert nature and process operation at low temperature leads to minimal damage of thermally sensitive compounds [53].
6.5. Combination of US with DIC process
The Instantaneous Controlled Pressure Drop process, abbreviated as DIC according to the French expression “Détente Instantanée Contrôlée,” was developed by Allaf et al. (1993) [119]. DIC extraction consists of a thermomechanical processing which usually starts by creating a vacuum condition, followed by injecting steam into the material for several seconds, then proceeding to a sudden pressure drop towards vacuum [24], [120].
This pressure-drop triggers [3], [120]:
-
▪
A rapid auto-vaporization of the moisture inside the treated material, which results in an expansion of the treated material.
-
▪
The instantaneous cooling of the biomaterial.
-
▪
A swelling or even rupture of cell walls resulting in higher porosity and enhanced accessibility and extraction kinetics.
Noteworthy, solute diffusion in the solvent inside the pore is generally considered to be the limiting process within the expanded structure. Coupled to US, internal transfer of solute present within the pore can likewise be intensified by inducing convection transfer rather than diffusion [120].
Allaf et al (2013) [39] coupled DIC pre-treatment and UAE to intensify antioxidants extraction from orange peels (Table 4). The highest yield of antioxidants with best kinetics is obtained by coupling both treatments. DIC combined to UAE also gave satisfying amounts of rebaudioside A, vitamin B1 and vitamin B6 from Steviarebaudiana bertoni leaves. Swell drying by DIC significantly improved the yields of the three bioactive components [121].
6.6. Combination of US with pulsed electric field
Pulsed electric field (PEF) technique consists of the application of repetitive short pulses with high voltage into a material held between two electrodes [40], [41]. The driving force behind PEF efficiency in solid–liquid extraction is the electrical breakage of cellular membranes, also referred as “electroporation” or “electropermeabilization” [40]. In other words, the application of an electric field induces the formation of pores inside the membrane, thus increasing its permeability [42]. Higher extraction kinetics can be therefore reached.
PEF technique is a nonthermal method which is less destructive relative to other methods of cell damage. This means that this method allows a selective extraction while preserving the color, flavor, vitamin C content, etc. [122].
Nevertheless, PEF presents some shortcomings related to the difficulty of using it with conductive materials as well as the necessity to be combined with other techniques to recover acceptable yields of specific compounds of interest from raw material [33].
As an example of this promising combination, different bioactive compounds (phenolics, flavonoids, condense tannins and anthocyanins, antioxidant activity and volatile compounds) were successfully extracted from almond seeds using PEF coupled to US treatment. It has been demonstrated in this study that combined treatment (PEF–US) allowed to attain the highest value of total phenolics, total flavonoids, condense tannins, anthocyanin contents and antioxidant activity in DPPH, reducing power and metal chelating activity compared to other treatments [123].
6.7. Combination of US with UV-C radiation
UV-C radiation is a non-thermal method that employs Ultra-Violet (UV) rays of short wave-length and high energy. The penetrating pulses rich in UV last a few hundreds of microseconds [43], [44]. On the one hand, synthesis of secondary metabolites, playing the role of defense compounds, is usually triggered by biotic and abiotic stresses. The high intensity light, particularly in the UV region, represents an abiotic stress for plant cells [43], [45]. Consequently, the synthesis of secondary metabolites can be considerably improved when UV-C radiation is employed as a pre-treatment prior to solid–liquid extraction. On the other hand, when applied during solid–liquid extraction, these UV-rays could physically breakdown cell walls and thereby enhance the release of cells contents into the surrounding extraction solvent [45]. Coupling UV-C with US could be a very promising method for faster extraction and better efficiency. Esua et al (2019) [124] investigated the effect of simultaneous UV-C radiation and US postharvest treatment on tomato bioactive compounds during 28 days storage period. Results demonstrated that this non-thermal treatment resulted in increased lycopene, total phenols, vitamin C as well as hydrophilic and lipophilic antioxidant activities.
In a study conducted by Lima and coworkers, cherry tomatoes were first submitted to UV-C radiation [125]. The powder of irradiated tomatoes was then submitted to UAE. Interestingly, extracts obtained from the irradiated tomatoes presented 5.8 times more lycopene content compared to control samples. Therefore, combining UV-C and US simultaneously or in separate steps appears to be of great interest. It is noteworthy that, despite its great potential, this combination has not yet been adequately exploited.
6.8. Combination of US with CCC and CPC
The separation and isolation of extracted natural compounds from a complex extract is a key step which needs to be fully mastered. Different chromatographic techniques were developed and used for this purpose, including gas chromatography (GC), supercritical fluid chromatography (SFC) and liquid chromatography (LC) [126], [127]. Counter-current chromatography (CCC) belongs to the family of LC. The most important feature of CCC is that it uses a support-free liquid mobile phase. Both the mobile and the stationary phase are liquid [128], [129]. CCC offers, on one hand, a great advantage related to the elimination of irreversible adsorption of the sample onto a solid support [130]. On the other hand, the liquid nature of the stationary phase imposes the design of specific hydrodynamic and hydrostatic columns [131], [132]. It is important to note that CCC term is now being used only to refer to hydrodynamic columns. As for hydrostatic ones, they are called centrifugal partition chromatographs (CPC), coming from the patented trade name of the Japanese Sanki company [132]. Compounds separated by CCC and CPC using the same two-phase solvent system are generally different. Hence, combining CCC and CPC can improve the separation efficiency [133]. Zhang and coworkers [130] developed an ultrasound-assisted dynamic extraction system coupled with CCC and CPC for simultaneous extraction and isolation of natural compounds from white peony roots (Table 4). Results showed that targeted compounds were successfully extracted. Furthermore, compared with conventional extraction methods, this combination offers the advantages of automation and systematic extraction and isolation of natural products.
7. Ultrasound combined hybrid and innovative techniques for food processing
Ultrasound combined hybrid and innovative techniques are also use for food processing (Table 5).
Table 5.
Ultrasound combined hybrid and innovative techniques in Food Processing.
| Technique | Product | Condition | Salient findings | Reference |
|---|---|---|---|---|
| Frying | ||||
| US assisted osmotic dehydration (UAOD) as a pretreatment, followed by frying | Potatoes | Pretreatment conditions: 90 min Osmotic dehydration, 30 min UAOD, using 15% sodium chloride/50% sucrose solution prior to frying(170 °C) for 2–6 min | By 12.5% (db), UAOD reduced the oil content of fried potatoes, compared to untreated fried potatoes, at the end of frying. No significant difference between OD & UAOD in oil uptake reduction in fried potatoes. UAOD improved color of French fries and shortened the pretreatment duration of OD by about 67%. | [137] |
| ultrasonic-assisted frying | Meatballs | US power: 0, 200, 400, 600 and 800 WFrequency: 20 kHz applied during frying (12 min, 160 °C). | US-assisted frying was concluded as a potential approach in improving overall flavor of fried meatballs. US treatment significantly increased thiobarbituric acid reactive substances and decreased free fatty acids. US-fryingincreased the contents of 7 free amino acids including Lys, Glu, Gly, Ala, Tyr, Ser and Cys. Showed a positive impact on nucleotides formation and can enhance a more desirable flavor within 400 W. | [140] |
| US as a pretreatment before frying | Fried potatoes | Potato sticks in water treated with US. Frequency: 35 and 130 kHz) US power densities: 0, 9.5, 47.6 and 95.2 W/kg, Intensities: 10, 50 and 100% and water temperatures (30 and 42 °C). Followed by frying in refined sunflower oil (171 ± 1 °C) | At lower frequencies, US more effective in modification of weight gain, moisture and electrical conductivity during soaking, and on fried potatoes color. Soaking temperature had an impact on US effect. Treatment led to changes in total acrylamide content in fried potatoes. | [139] |
| Mushroom (Agaricus bisporus) chips | Vacuum frying (VF) Microwave vacuum frying (MVF) US assisted microwave vacuum frying (UMVF) | Microwave power: 800, 900 and 1000 WFrying temperature: 80, 85 and 90 ℃.US balancing sources: 120 W, 28 kHz. The frying temperature and vacuum pressure were set at 90 ℃ and 12 ± 1 kPa respectively | Optimum condition (1000 W, 90 ℃), gave higher moisture evaporation rate and low oil content. UMVF could reduce oil content (16–20%)compared to other treatments. UMVF chips: Better texture, most acceptable color, best matrices, accelerated frying, comparatively lower uptake of oil. | [141] |
| Fermentation | ||||
| US assisted fermentation | Lebanese apples | Microorganism: Hanseniaspora sp. yeast. US: 100 W, 40 kHz, power supply (220 V), Cyclic mode, variable periods of US pulses duration (0.5–2 s), followed by 6 s pauses. | Optimal US pulse duration on the yeast growth rate: 0.5 s followed by 6 s rest period, and during 6 h of both lag and log phases. Compared to untreated samples, US parameters resulted in faster glucose consumption in the medium during the fermentation. A significant enhancement in biomass growth and consumption of glucose, accompanied by significant decrease in the ethanol yield. | [142] |
| US assisted dough fermentation | Wheat dough | Bag with dough place in an US bath(40 kHz). Bath temperature maintained at 36–38 °C. Ultrasonic power density: from 15.38 W/L to 38.46 W/LTreatment time:20 min-50 min. Dough fermentation in tank: 40 min, 36 °C and 83% R.H. | US assisted dough fermentation improved the quality of the steamed bread. Fresh steamed bread hardness reduced by 22.4%.Specific volume enhancement: 6.7% at US power density; 23.08 W/L, 40 min. During storage, bread prepared by using US was softer compared to control. Springiness was lower when storage time under 48 h. | [144] |
| US assisted fermentation | Soyabean meal | US power density; 0.08 W/mL, Frequency:33 kHz Treatment time:1 hBacillus subtilis | Enhancement compared to control (peptide contents:31.27%; soluble protein :18.79%).Antioxidant activity and functional properties enhanced with US. | [145] |
| Freezing/crystallization | ||||
| US during immersion freezing | Broccoli | 30 kHz, 150 W; 20 kHz, 175 W for 120–180 sec. | Microstructure and textural firmness were better than the normal immersion freezing. Drip loss was noticed to be minimised. US was found to be promising. | [170] |
| US assisted immersion freezing | Fish | Immersion freezing tank (−25 ± 0.5 °C), 30 kHz, 0–175 W. US (On and off 30 s) On: 30 sec on/30 s off) 9 min cycle | Samples had smaller ice crystals compared to air freezing and immersion freezing, resulting in less deterioration of tissues of muscle during storage. During storage, compared to the other methods, lower total volatile basic nitrogen values and thiobarbituric acid reactive substance were observed. | [171] |
| US assisted immersion freezing | Mushrooms | Frequency: 20 kHz; US intensity: 0.13 W cm−2, 0.27 W cm−2 and 0.39 W cm−2 | US (20 kHz, 0.39 W cm−2) reduced freezing time by 40%.Reduction in peroxidase enzyme activities and polyphenol oxidase and drip loss. Improved whiteness index, chroma and textural hardness value. | [173] |
| US assisted immersion freezing | Potatoes | 35 kHz0.32 W/g, 8 s,-0.5, −2.0 and −3.0 ℃ | Nucleation was anticipated and freezing time was reduced. At −2.0℃, the shortest time was observed. | [174] |
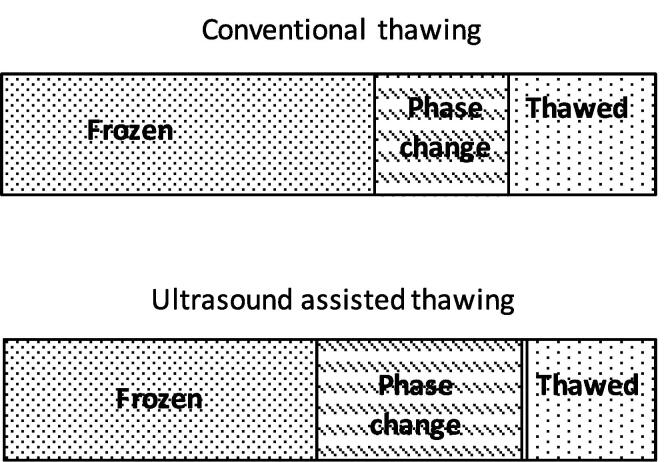
| Defrosting/ thawing | ||||
| Ultasound assisted thawing (UAT) | Bighead carp | 28 kHz, 0.135 W/mL, slow freezing(-18 °C) / fast freezing (-100 °C liquid nitrogen) frozen for 30 d at −18 °C, followed by water immersion thawing, air thawing and ultrasound assisted thawing. | Compared to water and air thawing, UAT drastically reduced thawing time. UAT was helpful in preserving the pH, color and lipid oxidation. Fast freezing UAT and Water immersion thawing, showed maximum muscle tissue destruction and water loss. UAT was found to be the best thawing method for big head carp which had undergone slow freezing. | [177] |
| UAT | Mango pulp | 28 kHz, UAT (intensities: 0.037, 0.074 and 0.123 W/mL in water bath at 4 ± 1 °C and 25 ± 1 °C, respectively | US was found to be a prospective alternative to conventional method of thawing. When compared with conventional method, a reduction in time by 16–64% was observed and more phenolic acids were obtained. US thawing (25 °C) reduced thawing time by 51–73% when compared with 4 °C. US intensities (0.074–0.123 W/mL) at 25 °C resulted in better thawing efficiency and nutritional quality. | [178] |
| Ultrasound assisted vacuum thawing (UVT) | Red seabream (Pagrus major) | UT; 200 W, 40 kHzUVT: 0.06 MPa, 0 °C, 200 W, 40 kHz | UVT samples showed poor viscoelasticity, the reason was considered to be the less denaturation of muscle proteins. Microwave assisted vacuum thawing (MVT) showed a viscoelasticity similar to fresh sample. UVT samples showed that the secondary structure was retained, while with MVT, non-uniform structure was observed. UVT and MVT were mentioned to have the potential to improve physicochemical properties of protein during thawing. | [177] |
| Low intensity ultrasound assisted thawing | Pork Longissimus dorsi muscle | 25 kHz. 0.2, 0.4, and 0.6 W/cm2 | US shortened thawing time by 87% through thawing from −5 to −1°C compared to air thawing. Ultrasound assisted thawing did not damage the textural and technological attributes. | [179] |
| Drying | ||||
| US pretreatment followed by hot air and freeze drying | Carrot discs | Probe system, 20 kHz, Amplitude: 24.4, 42.7 and 61.0 μm. Processing time: 3 and 10 min. Hot air drying (60 °C, 0.3 m/s). | Higher carotenoids and polyacetylenes retention in US pre-treated samples. US pre-treatment, a potential alternative to blanching, before drying carrots. | [180] |
| Hot air convective drying with contacting ultrasound system | Garlic slices | Sonication: 1513.5 W/m2Air velocity: 2.5 m/s Temperature: 50 °C, 60 °C and 70 °C. | Contacting US reduced drying time, enhanced water diffusion and reduced quality loss. Organosulfur compounds were better preserved, browning was minimized. | [181] |
| Airborne ultrasound assisted convective drying | Potato | 25 kHz, 100 and 200 W, air velocity 4 m/s, 50 °C | US reduced processing time from 5 to 3 h. US lowered energy consumption and helped retain quality parameter. | [182] |
| Ultrasound combined vacuum pretreatment(UVP) and convective drying | Okra | 25 kHz, P = 80, 200 and 320 W, T = 25 °C, t = 5, 10 and15 min. Optimised (250 W, 0.5 cm thickness and ultrasonic treatment for 15 min) | UVP enhanced convective drying, maintained the physicochemical properties and also reduced the energy consumption. | [183] |
| Cooking | ||||
| US assisted cooking | Spiced beef | Power levels: 0, 400, 600, 800 and 1000 W. Frequency: 20 kHzCooking time 120 min. | Permeability of NaCl enhanced due to. High power US resulted in increase in the lipid oxidation, which helped in strengthening the volatile compounds. At 800 W, US improved chemical profiles of spiced beef flavour and taste | [185] |
| US assisted cooking | Spiced beef | Power level: 0, 400, 600, 800 and 1000, Frequency: 20 kHz, Cooking time (80, 100 and 120 min) | US improved salt penetration, affected tenderness and water holding capacity of spiced beef was improved. | [186] |
| US assisted cooking | Mortadella | US 25 kHz | US improved the cooking process,accelerated increase in internal temperature and more homogeneity were observed in the mortadellas. US did not accelerate protein and lipid oxidation and no color changes were observed and did not affect the microbiological quality. Positive effects of US on gel formation were found as an increase in hardness and chewiness was reported. | [187] |
| Combination of US and temperature hydration | White kidney beans | Hydration using US (28 W/L volumetric power), 45 kHz and temperatures (25, 35, 45 and 55C). | Both temperature and US enhanced the hydration process. When in combination, ultrasound effect decreased with increasing temperature of soaking. The cooking process was not affected by both the different temperatures and ultrasound. | [188] |
| US assisted cooking | Mortadella | US 25 kHz | US improved the cooking process, accelerated increase in internal temperature and more homogeneity were observed in the mortadellas. US did not accelerate protein and lipid oxidation and no color changes were observed and did not affect the microbiological quality. Positive effects of US on gel formation were found as an increase in hardness and chewiness was reported. | [187] |
| Filtration | ||||
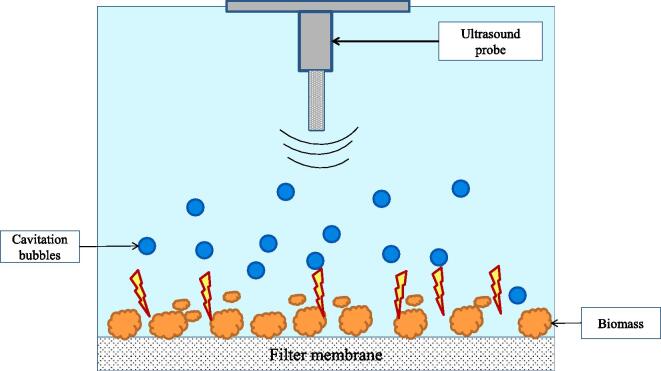
| US assisted cross flow ultrafiltration | Skim milk | Small angle X ray scattering Cross-Flow US-coupled Filtration CellUS intensity: 0.6 to 2.9 W cm−2 . | Feed milk viscosity not affected at 20 kHz, 2 W cm−2. US improved filtration. Partial disruption of concentrated layer occurred by US, accelerating permeate flux. The effect of ultrasound was found to decrease when the feed concentration increased. US was found to be promising as the formation of reversible fouling layer was limited, therefore higher permeate flux was induced. | [193] |
| US assisted cross flow ultrafiltration | Skim milk | Small angle X ray scattering Cross-Flow US-coupled Filtration CellUS intensity: 0.6 to 2.9 W cm−2 . | Feed milk viscosity not affected at 20 kHz, 2 W cm−2. US improved filtration. Partial disruption of concentrated layer occurred by US, accelerating permeate flux. The effect of ultrasound was found to decrease when the feed concentration increased. US was found to be promising as the formation of reversible fouling layer was limited, therefore higher permeate flux was induced. | [194] |
| US assisted defouling | Whey solution | 50 kHz, 300 W, 55 kPa, 20–22°C. Membrane: Cross flow UF. | 112% flux recovery. Ultrasound led to physical cleaning. Surfactant along with ultrasound showed a synergistic effect. | [195] |
| US assisted defouling | Soyabean protein | 40 kHz, (0, 1.43, 2.13, 3.57 W.cm -2) Power, (20, 30, 40, 50, 60, 70 kPa) operating pressure, | Permeate flux: 86.3 kg.m-2h−1 Frequency: 23 kHz, 3.57 Wcm−2. US leads to formation of cracks on membrane surface. Polyvinylidenefluoride (PVDF) membrane more resistant compared to other membranes. | [196] |
| US assisted defouling | Carrot juice | 20 kHz, 400, 600, 800, 1000 W, 0.2, 0.5 bar. Microfiltration system with PVDF | US enhanced the flux. US reduced diphasic nature of juice, affected fouling. @ 1000 W, 100 ml solution, 30 min sonication | [191] |
| US-assisted emulsification | ||||
| US assisted emulsification | Mustard oil in water | Ultrasonic power amplitude of 40%, 30 min, Hydrophilic lipophilic balance value of 10, Ψs of 0.08 (8%, v/v), Ψo of 0.1 (10%, v/ v) | Good emulsion stability (up to 3 months). Only physical effects of US were observed and no changes in molecular structure of oil were seen. | [198] |
| High intensity US | Whipped cream | US 20 kHz (100 and 300 W) for 0, 5, 10 and 15 min (Pulse on-time: 2 s, off-time: 4 s). | US enhanced the quality and properties of whipped cream up to a particular US input energy. Protein chains underwent denaturation, and opened to cover fat cells, enhancing the properties. | [199] |
| Pickling/brining | ||||
| US assisted brining | Chinese cabbage | 35 kHz, NaCl (10, 15, and 20% w/w), 1:10 (sample: salt solution), 25 °C(room temperature). Samples removed at 0, 30, 60, 90, 120, 150, and 180 min and wrapped in absorbent paper for 2 min to remove excess solution | US enhanced the cabbage hardness and chewiness, improved brining process, gave a homogenous salt distribution and improved Kimchi quality. | [201] |
| High intensity US | Pork meat (Longissimus dorsi) | US: 40 kHz; 37.5 W/dm3NaCl concentration :50, 100, 150, 200, 240 and 280 kg NaCl/m3Brining:15, 30, 45, 60, 90 and 120 min. Water immersion: 20 s, blotting followed by wrapping in plastic waterproof film. Storage :18 ± 0.5 ⁰C. | US significantly influenced salt gain and enhanced brining. Reduced brining time. Gave uniform salt distribution. | [202] |
| US assisted diffusion | Pork | 70 W, 20 kHz, Ultrasonic power: 9.29 and 54.9 W cm−2. | ||
| 5% NaCl/ Salt replacer, brining time 120 min. | US enhanced the salt diffusion into the matrix, compared to static brining. US brined samples, improved the texture of brined samples both with NaCl and salt replacer. | [203] | ||
| Sterilization | ||||
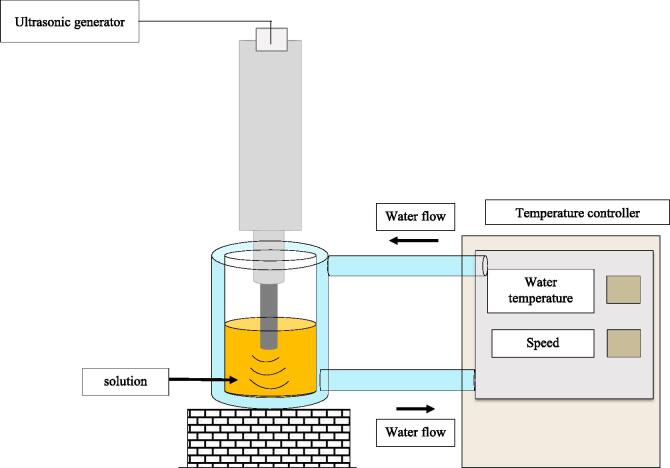
| US assisted pasteurisation | Juices | Designing of batch ultrasonic reactor. | [205] | |
| Frequency: 20 kHzAmplitude: 45%E.coli and S.aureus. | E. coli inactivation: 60 °C, Staphylococcus aureus: 62 °C. 5-log microbial reduction achieved in 0.38 min and 0.55 min, respectively. US damaged cell structure leading to inactivation of microbes. Pasteurisation time reduced. No negative impacts on organoleptic characteristics and nutritional properties. | |||
| US assisted pasteurisation | Pear juice | US assisted pasteurisation was compared with conventional method. 25, 45 and 65 ⁰C for 10 min using a 750 W probe sonicator (frequency 20 kHz and amplitude 70%). Conventional: 65 ⁰C, 10 min; 95 ⁰C, 2 min. | Both US and conventional method were able to attain the complete inactivation of microbes and enzymes. US treated samples showed better ascorbic acid and other nutritive compounds retention. | [206] |
| US assisted pasteurisation | Chocolate milk beverage | US energy densities :0.3–3.0 kJ/cm3 | ||
| Conventional HTST pasteurization: 72 °C/15 s | High intensity US was more effective than conventional. | |||
| Energy density had a direct impact on mesophilic microorganisms, log reduction, fat globule distribution and rheological behaviour, antioxidant activity, Fatty acid profile and volatile compounds. | [207] | |||
| Depolymerization | ||||
| US assisted depolymerization | Guar gum | US (20 kHz frequency), 240 w with enzyme cellulase. | US and enzyme combination, better than stirring with enzyme alone. 98% extent of depolymerization was observed. | [210] |
| High power US depolymerization | Starch paste | Corn starch pastes, US: 20 kHzPower: 13.5/ 29.9 W) Time: 20 min | Viscosity & hydrodynamic radius reduced with increase in US treatment time. High amylose content pastes were resistant to US treatment. | [211] |
7.1. Ultrasound assisted frying
US is generally employed as a pretreatment before frying. The raw material is immersed in water or hyperosmotic solution and US waves are passed through it [134]. These waves create rapid compression and expansions, which are similar to squeezing and releasing a sponge and also facilitate removal of strongly bound moisture [135], [136]. In a study carried out on kiwi fruit, the authors observed that utilizing ultrasound for osmotic dehydration led to an increase in water loss and was also able to maintain the cell structure [137]. US-assisted osmotic dehydration (UAOD) was used as a pre-treatment before deep fat frying of potatoes and at the end of frying, a reduction by 12.5% (db) in oil content of the fried potatoes was observed, compared to untreated fried potatoes. However, no significant difference in the reduction of oil uptake by the fried potatoes was noted between UAOD and osmotic dehydration without ultrasound. It was also noted that UAOD, improved the color of the French fries [136]. US as a pre-treatment also reduced acrylamide in fried potatoes [138]. Also, in another study, ultrasonic-assisted frying was concluded as a potential approach to improve the overall flavor of fried meatballs [139]. The US can also be used in combination with other technologies such as US and microwave assisted vacuum frying (USMVF) [134]. In a study, the impact of vacuum frying, combined with US and microwave (UMVF) on the quality of fried mushrooms was investigated and was compared with vacuum frying and microwave combined vacuum frying technology. It was observed that US, weakened the mushroom structure and thereby enhanced the movement of moisture from tissues to oil during frying. In UMVF, a synergistic effect was observed because of US and microwave, where the volumetric heating because of microwave and vibration caused by US worked together and significantly increased the oil temperature and enhanced the drying rate. The reason for the highest moisture removal in UMVF, was attributed to US [140].
7.2. US assisted fermentation
The application of US has been investigated within the fermentation industries for many years. The high frequency US, have been mainly employed for the non-destructive analytical techniques, including monitoring the fermentation processes, such as determination of yeast and maltose concentration, phase change from liquid to gel etc, while the low frequency US is used for pasteurization purposes, improving microorganism/ enzyme performance, altering the fermentation rates, degassing and deaeration of alcoholic beverages, etc. [141]. In a study, US-assisted fermentation was carried out for cider production from Lebanese apples. The authors reported that with US, glucose was consumed more rapidly, compared to the untreated culture [142]. It was also reported that there was a significant reduction in the ethanol yield, which makes US a potential technology for reducing the ethanol content in alcoholic beverages. Also, US was employed in dough fermentation, for making bread and the authors reported that US assisted fermentation improved the quality of the steamed bread [143]. In a study US assisted fermentation was carried out using soybean meal and Bacillus subtilis and the authors reported that US enhanced the contents of peptide and soluble protein and enhanced antioxidant activity and functional properties. US assisted fermentation was reported to enhance the bioactivity and functionalities of soybean meal [144].
Several studies try to explain how ultrasound might decrease or enhance activity of microorganisms with or without heat and/or pressure. Globalisation of the results presented in all studies to propose a single mechanism is quite difficult. Microorganisms differ in their resistance to ultrasound, cells of bigger size are more sensitive to ultrasound, coccal forms are more resistant than rod shaped bacteria and aerobic more than anaerobic species. Spores are more resistant than vegetative forms. Enzymes are reported to be inactivated by ultrasound due to their depolymerisation effect. The enzymes vary in their resistance to ultrasound. In general, inactivation effect of ultrasound is attributed to intracellular cavitation that disrupt cellular structural and functional components up to the point of cell lysis (Figure X) [6].
7.3. US assisted defoaming
US as a defoaming technique, was suggested for the first time in 1944 [145] and in 1951, the first extensive research focussing this, was carried out [146]. Foams, basically are thermodynamically unstable colloidal systems wherein the liquid matrix consists of gas maintained as a distinct dispersed phase [147]. Thousands of microbubbles are generated within a liquid which rise to the surface and agglomerate together before collapsing, this leads to the foam formation. During food processing, foam formation leads to several problems including inefficient filling of product into the containers, product loss and alteration of pump capacity. Therefore in order to prevent these problems, several methods are used which include mechanical, chemical, thermal (temperature change to breakdown the bubbles) and electrical methods. US is employed as a defoaming agent, which causes cavitation and acoustic streaming, high acoustic pressures and resonance, leading to foam destruction [148]. With high frequency vibrations, streaming is induced in foam’s liquid phase and this results in decrease of apparent viscosity, which enable rapid draining of fluid from the upper layers of foam bed, merging/agglomeration of bubbles and foam collapse. Anti-foaming or defoaming in food industries are restricted by the regulations and consumer perception and it is the reason that in spite of availability of various foam suppressants, only a few chemical options are employed in the food industry [149]. There are several factors involved in the defoaming using US, such as interaction of sound with foam, velocity of sound in foam, amount of acoustic energy transmitted into the foam, wetness of the foam, acoustic intensity, foam mesostructure and bubble radius etc [150]. For several decades, the use of sound energy for defoaming purposes has been known, but without their application on the industrial scale. The commonly used aerodynamic acoustic sources (Hartmann whistle and siren), had several drawbacks such as high air flow, high energy consumption, sterilization of air flow and noise production. The high intensity ultrasonic waves were found to be a potential method of defoaming as they did not require high air flow, prevented chemical contamination, and could easily be used in a sterile contained environment, which made their use in food and pharmaceutical industries appropriate [151], [6]. The ultrasonic defoamers used in a canning line [152] can be seen in Fig. 3.
Fig. 3.
Ultrasonic defoamer used in a canning line.
7.4. US assisted drying
The study of drying via sound can be dated long back when the accelerating effects of acoustic waves were first reported in 1955 [153]. Over the last 15 years, there has been an intensive investigation of the application of US to enhance the drying process. US has been reported to show enhancement in drying of materials and has also been used along with vacuum drying, hot air, fluidized bed and freeze drying [154]. US is usually employed for drying of food in three ways, namely as US pretreatment, as airborne US assisted drying and as contacting US assisted drying [155]. The effects of the treatments are mainly dependent upon a range of factors including nature of material, ultrasonic equipment, drying conditions, and accordingly the mechanism by which US works on these materials may vary [154]. In case of US pretreatment, probe or bath systems are generally used, which involve passing the ultrasonic waves through a medium, due to which compression and expansion of samples (sponge effect) takes place. This involves the formation of microscopic channels within sample leading to release of the liquid contents. Similarly, when a high-power US is used, cavitation bubbles are formed and their collapse leads to localized high pressure and temperature zones and formation of H+ and OH – ions. Also, the collapse may lead to micro streaming that facilitates agitation and heat and mass transfer, and micro jets which break the diffusion layer and also enhance mass transfer between the solids and the liquid [155]. US as pre-treatment prior to drying was studied for various food materials. In one such study it was reported that by increasing the frequency, the drying rate of apples increased [156], while it was also reported that at lower frequency and shorter US treatment time, better retention of carotenoids was observed in carrots [157]. Also, the effect of US on water loss has also been reported which states that there was an increase in water loss with the increase in sonication time in papaya [158] and pomegranate [159]. US as a pre-treatment also reduces the drying time in carrots [160] and banana [161] etc.
Airborne US is useful for drying heat sensitive materials and can act without affecting the product quality. The application results in pressure variations, micro streaming at gas–solid interface, which enhance evaporation of moisture, enhance mass transfer and accelerate diffusion [162]. Under high intensity airborne US, the pressure oscillation takes place at the gas interface and during the negative pressure cycle, the moisture is released from the sample, which does not re-enter back into the sample, this enhances the evaporation. However? it must be mentioned that there is a high energy attenuation by the gas medium and also the mismatch of acoustic impedance occurs between the gas and the air and the applied system, which limit the use of airborne US [155], [163]. Therefore a system where the US can come in direct contact with the food is desirable. In the direct contact system, the problems of energy loss and impedance matching are solved and the sponge effect and formation of microscopic channels and enhanced drying rate are observed. Many studies have been reported which state that in airborne US assisted convective drying, US enhanced the drying and also helped retain the quality in many fruits and vegetables like apples [164] and passion fruit peels [165], while it was also reported to reduce antioxidation potential in grape skin, the reason for this, was attributed to the oxidase activation and cell degradation [166]. US assisted drying has shown different impacts on the quality of food products including color change, rehydration ability, hardness, shrinkage, density etc, chemical properties like phenolics, flavonoids and antioxidants etc. The use of US with different technologies such as US assisted convective drying, US and microwave assisted convective drying etc have been mentioned in the review by [155].
7.5. US assisted freezing
Application of US in freezing process have significant advantages such as initiation of nucleation, control of ice crystal size, acceleration of freezing rate and quality improvement of the food materials [167]. The mechanism of freezing using US involves formation of microstreaming due to acoustic cavitation, leading to collisions responsible for minimizing solid- liquid interfaces, formation of cavitation bubbles which result in ultrahigh pressures that promote instantaneous nucleation [168].
Many studies have been reported related to freezing of food products using US assisted freezing process. For example US was employed during immersion freezing of broccoli and it was reported that the freezing time and times required at pre cooling, phase change and sub cooling stages of broccoli were reduced, when 30 kHz and 20 kHz were used. The microstructure and textural firmness were found to be better than the normal immersion freezing. Also, the drip loss was noticed to be minimised. US assisted freezing was found to be of great potential [169]. When US assisted immersion was compared with air freezing and immersion freezing of common carp, it was observed that US assisted immersion samples had smaller crystals throughout the samples compared to other methods which were responsible for less muscle tissue damage and was found to be an effective method of preventing deterioration of frozen fish [170]. Several food materials have been studied for US assisted freezing, which include strawberries [171], mushrooms [172], potatoes [173] and chicken [134] etc.
7.6. US assisted thawing
Thawing as a process can bring about many changes in food and therefore it is important to use an appropriate method of thawing a frozen product. In thawing with US, phase change from ice to water is faster than the conventional thawing methods and this decreases the possibility of water loss, microbial contamination and protein denaturation [174]. By generating heat inside a frozen food material, the thawing can be initiated. Also, while exposing the food to US, there may be energy losses and heat dissipation, which can result in increase in the food temperature, for instance the surface temperature may increase rapidly while in the interior it is slow [174], [175]. Over the years, benefits of US have been reported to enhance the thawing rate of a range of frozen food products (Fig. 4). In a study done on bighead carp, the authors noted that US assisted thawing not only reduced the thawing time but was also helpful in preserving the color, pH and lipid oxidation [176]. US was found to be a potential alternative to the conventional thawing method of mango pulp [177] and it was reported that the thawing time reduced and more phenolic acids were obtained.
Fig. 4.
Effect of thawing on phase change.
7.7. US assisted cooking
Cooking process can change the physical, chemical as well as the nutritional value of any food material. When carefully done, can help enhance the quality of the final product and also lead to less deterioration. US being one such technology has been used to accelerate the processes without deteriorating the food quality [180]. In a study done on spiced beef, it was reported that US enhanced the salt penetration into the spiced beef and an increase in the lipid oxidation was observed, which further enhanced the volatile compounds leading to enhancement of taste and flavor [184]. Also in a similar study it was reported that US had an impact on the tenderness of the spiced beef and improved the water holding capacity [185]. In a study done on mortadellas, the authors reported that US enhanced the cooking process, the internal temperature increased fast and was homogenous. Also, it was noted that US did not affect the microbiological quality of the product [186]. In a study on grainss, it was observed that US enhanced the grain porosity, due to the formation of microchannels inside the grain. The reason was found that the indirect effect (microchannel formation by acoustic cavitation) and direct effect (interflow and sponge effect) were responsible for the enhanced mass transfer [187].
7.8. US assisted filtration
Filtration is an important unit operation in different food products including milk, juices and oils, etc. With continued filtration, fouling of the filters takes place, which creates hindrance for the flow of the materials and therefore the cleaning of the filters and replacing them become an an important part of the process. US as a defouling technique, generates mechanical movements which are beneficial in particle detachment from filters, however in food processing, not many studies have been reported. The mechanisms by which US works against fouling are via acoustic streaming (sweeping of particles having weak inteaction with membrane), microstreamers (dragging of deposited particles by group of bubbles), microstreaming (movement of particles in different directions due to compression and rarefaction cycles) and via microjets (high speed water jets) etc. Over the years there have been various advances in the US assisted processing, amongst which the new device configurations are US probe in dead-end, submerged crossflow device, crossflow filtration system inside US bath, US applied directly in crossflow filtration and in pilot /large-scale acoustic filtration devices etc [188]. A wide range of ultrasonic antifouling systems are used, including ex-situ ultrasonic transducers (US bath system) and in situ US homogenizers. US has been mentioned as an alternative technique of membrane cleaning, which also enhances the permeate flux (Fig. 5). The reasons attributed to this are ultrasonic cavitation and the acoustic convective currents. Localized hot spots generated in the liquid and the mechanisms like microstreaming and shock waves further disrupt the interaction between foulant and membrane, detaching the fouling layer from the membrane surface and further preventing the accumulation of the microscopic particles at the solution/ membrane interface. However, the effectiveness is affected by the US frequency, hydraulic pressure and temperature etc [189]. In a study on carrot juice, the authors reported that US when used with a membrane unit, reduced the diphasic characteristic of the juice and enhanced the permeate flux during clarification [190]. In spite of the beneficial aspect, the method does have certain drawbacks such as membrane damage and denaturation of material [191]. However, it has also been reported that US combined with filters, enhances the filter life by preventing membrane clogging and caking via continuous cavitation on the filter surface [192]. The effect of US on whey fouled membranes was studied and it was stated that US was able to enhance the flux recovery and it was not dependent on the time of sonication, however by increasing the power level, the effectiveness could be enhanced. The use of surfactant along with US showed synergistic effects, by providing better efficiency of flux recovery. It was also mentioned that US treatment did not damage the membrane structure. US was therefore found to be effective in preventing membrane fouling during whey treatment in dairy industry [194]. In another study on soybean protein, it was reported that US did affect some membranes and the treatments resulted in damage of membrane surface and formed large cracks. PVDF (polyvinylidenefluoride) film was more resistant and showed less changes compared to other films (cellulose nitrate with cellulose acetate and nylon 6). Therefore, it was concluded that in these processes, the nature of the polymeric material and the US frequency, duration and intensity should be carefully selected [195].
Fig. 5.
Microjet cleaning effect on membrane surface.
7.9. US assisted emulsification
Ultrasonic emulsification has displayed a wide range of benefits compared to the conventional emulsification methods, which include more stable emulsions, with smaller sized droplet size, reduced need of surfactants, energy efficiecny, easy operation and less production cost. The process of formation of these emulsions with US involve the cavitation process, where cavitation leads to collapse of microbubbles at the interface of the immiscible phases, leading to high velocity jets and turbulence which cause disruption of droplets and thereby mixing of the phases. During the acoustic emulsification, firstly the gas bubbles/dispersed phase droplets enter the media and then the bigger bubbles/ droplets are disrupted because of cavitation into small size. A wide range of food grade o/w nanoemulsions have been made using US, including different oil phases like olive oil, basil oil, canola oil etc and several surfactants like Tween 80, whey protein isolates etc, showing good storage stability [196]. In a study, the authors reported the use of US in formation of an emulsion with mustard oil in water. It was reported that US was found to be an efficient method of making the emulsion, the droplet size was minimum at 40% ultrasonic power and the emulsion formed was stable for up to 3 months, showing no phase separation and increase in droplet size [197]. Also, in a study done on whipped cream, it was reported that up to a particular US input energy, the US had desirable effects on whipped cream, enhanced viscosity, stability and overrun and when the energy was enhanced, negative effects were observed [198]. Similarly, the use of high intensity US in yoghurt production has shown, enhanced emulsification and homogenization processes, where gel strength is enhanced and firmness is increased by whey protein coagulation, which also reduces fermentation time. While in ice cream production the high intensity US has shown reduced ice crystal size, reduced the time of freezing and prevention of incrustation on freezing surface [199].
7.10. US assisted brining
US has been reported to enable producing pickled food products, using lower amounts of salt compared to the commercially available pickles [200]. In a study done on impact of US on brining of cabbage, the authors reported that salt concentration plays an important role in the transport of water during brining as, with a high concentration (20% w/w NaCl), minimum effect on water weight changes were observed. Also, it was mentioned that the US intensity might have not been sufficient to lead to the water transport, therefore a minimum US energy is required which can depend on the salt and water content. It was also reported that US enhanced the quality of Kimchi [201]. In a study done on pork, it was reported that US enhanced the brining kinetics, moisture and salt diffusivities. It was also reported that US reduced the brining time and gave a more uniform and fast distribution of NaCl [202]. Fast brining enables bloating control, damage to structures and enzymatic softening of brined food materials [200]. In another study Ojha, Keenan et al. [203], investigated the US assisted diffusion of a sodium salt replacer on pork meat. US enhanced the salt diffusion into the matrix compared to static brining. US brined samples improved the texture of brined samples both with NaCl and salt replacer. It was reported that ultrasonic treatment had low impact on salt mass transfer into samples. Mass transfer coefficient was higher than static brining, only at high ultrasonic power (Fig. 6).
Fig. 6.
Ultrasound probe system.
7.11. US assisted sterilization/ pasteurization
The antimicrobial effects of US on the vegetative forms, has been known since 1929, when the employment of US in food processing and preservation was proposed [204]. Over the years, the use of US in food processing industry, its effect on the microbial activity as well as the quality attributes of food have been reported widely. In a study done on pasteurization of juices, a batch ultrasonic reactor was designed and a reduction of 5 log was attained in E. coli and E. aureus in 0.38 min and 0.55 min resp. US caused lethal cell structure damages which led to the inactivation, but it was also reported that pasteurization time was reduced and no negative effects were noticed on the properties (organoleptic and nutritional) of the juices. It was also reported that in the typical ultrasonic reactors, there are certain passive zones, which limit the inactivation and this was the reason, for designing the reactor. In the new system it was observed that during the processing, the high intensity US was uniformly distributed [205]. For comparison with conventional method, in a study both conventional method and US were used to pasteurize pear juice. It was observed that in conventional method complete inactivation of enzymes and microbes took place but was accompanied by high losses of phenols, ascorbic acid, flavonoids and antioxidant capacity. Whereas the US pasteurization at 65⁰C, gave a significant reduction in enzyme activity, complete inactivation of microbes and showed better retention of ascorbic acid and phenolics. It was concluded that at lower temperatures, along with US, significant microbial and enzyme inactivation can be attained [206]. In a study done on chocolate milk beverage, conventional method of pasteurization and high intensity US were compared, and it was reported that high intensity US was more effective than conventional, and they also improved the physicochemical and microbiological quality of the beverage by preserving the bioactive compounds and nutritional quality of the product [207].
7.12. US assisted depolymerization
The shear forces generated by bubble implosion during US treatment are strong enough to rupture the covalent bonds between the monosaccharide units, thus facilitating a decrease in the average molecular mass of the polymer. The advantages of the ultrasonic depolymerization have several advantages over other thermal, chemical and enzymatic treatments. It does not require addition of any other substances and the polymer generally tends to break towards the middle of the chain without the formation of monomers and side reactions. As a result, same size molecules are obtained with a cheap and fast method, without the need of any procedures for subsequent purification [208] written by Dodero, Vicini et al [209]. In a study, US assisted enzymatic depolymerization was carried out of a solution of aqueous guar gum and it was reported that with optimized US and enzyme treatment, 98% of guar gum depolymerization was achieved. It was also reported that US gave rise to increase in tryptophan numbers on enzyme surface, i.e. increased activity of enzyme for the depolymerization. The combination of both enzyme and US was found to be effective [210]. A study was done to investigate the impact of US on starch pastes with different amylose content. It was reported that the efficiency of US decreased with increase in amylose content of the different types of corn starch used [211]. Also, in another study US was found to be an effective method of depolymerizing sodium alginate [209].
8. Conclusion
Ultrasound technology has been demonstrated as a promising low cost, rapid, and highly effective for mass, momentum and heat transfer due to cavitation phenomena. Nevertheless, ultrasound technique still has several shortcomings, especially for processing complex systems such as food or natural products. Advances in material sciences and other technologies has resulted in overcoming several limitations posed by US. It is evident that the combinations of US with other techniques outlined in this review has demonstrated in significant achievements and has become an important field of active research. Studies have demonstrated that combined conventional and innovative techniques with US enable to identify synergy especially for transfers modes that cannot be achieved using ultrasound alone. In conclusion, ultrasound combined techniques have greatly enhanced and promoted ultrasound use in food processing and natural products extraction, but there are many more opportunities to address and much work still to do in numerous research fields such as cosmetics, nutraceuticals, pharmaceutics, and fine chemicals [211].
CRediT authorship contribution statement
B. Khadhraoui: Conceptualization, Methodology, Software, Writing - review & editing. V. Ummat: Conceptualization, Methodology, Software, Writing - review & editing. B.K. Tiwari: Conceptualization, Methodology, Software, Writing - review & editing. A.S. Fabiano-Tixier: Conceptualization, Methodology, Software, Writing - review & editing. F. Chemat: Conceptualization, Methodology, Software, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
B.K. Tiwari, Email: Brijesh.Tiwari@teagasc.ie.
F. Chemat, Email: farid.chemat@univ-avignon.fr.
References
- 1.Chemat F., Rombaut N., Meullemiestre A., Turk M., Périno-Issartier S., Fabiano-Tixier A.-S., Abert-Vian M. Review of green food processing techniques. Preservation, transformation, and extraction. Innovative Food Sci. Emerg. Technol. 2017;41:357–377. [Google Scholar]
- 2.Anastas P.T., Warner J.C. Oxf. Univ; Press: 1998. Green chemistry: Theory and practice. [Google Scholar]
- 3.Chemat F., Rombaut N., Sicaire A.-G., Meullemiestre A., Fabiano-Tixier A.-S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 4.G. Cravotto, P. Cintas, Power ultrasound in organic synthesis: moving cavitational chemistry from academia to innovative and large-scale applications, Chem. Soc. Rev. 35 (2006) 180-196. [DOI] [PubMed]
- 5.Vinatoru M. Ultrasonically assisted extraction (UAE) of natural products some guidelines for good practice and reporting. Ultrason. Sonochem. 2015;25:94–95. doi: 10.1016/j.ultsonch.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 6.F. Chemat, Zill-e-Huma, M. Kamran Khan, Applications of ultrasound in food technology: Processing, preservation and extraction, Ultrason. Sonochem. 18 (2011) 813-835. [DOI] [PubMed]
- 7.Ollanketo M., Peltoketo A., Hartonen K., Hiltunen R., Riekkola M.L. Extraction of sage (Salvia officinalis L.) by pressurized hot water and conventional methods: antioxidant activity of the extracts. Eur. Food Res. Technol. 2002;215:158–163. [Google Scholar]
- 8.Wang L., Weller C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006;17:300–312. [Google Scholar]
- 9.Rombaut N., Fabiano Tixier A.-S., Bily A., Chemat F. Green extraction processes of natural products as tools for biorefinery Biofuels. Bioprod. Biorefin. 2014;8:530 544. [Google Scholar]
- 10.Misra N.N., Koubaa M., Roohinejad S., Juliano P., Alpas H., Inácio R.S., Saraiva J.A., Barba F.J. Landmarks in the historical development of twenty first century food processing technologies. Food Res. Int. 2017;97:318–339. doi: 10.1016/j.foodres.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Jiang T., Zhan S., Li S., Zhu Z., He J., Lorenzo J.M., Barbac F.J. From ‘green’ technologies to ‘red’ antioxidant compounds extraction of purple corn: a combined ultrasound– ultrafiltration– purification approach. J. Sci. Food Agric. 2018;98:4919–4927. doi: 10.1002/jsfa.9024. [DOI] [PubMed] [Google Scholar]
- 12.Marić M., Grassinob A.N., Zhuc Z., Barbad F.J., Brnčić M., Brnčić S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018;76:28–37. [Google Scholar]
- 13.Z-S. Zhang, L-J. Wang, D. Li, S-S. Jiao, X. Dong Chen a, Z-H. Mao.Ultrasound-assisted extraction of oil from flaxseed. Separation and Purification Technology 62 (2008) 192–198.
- 14.M. Virot, V. Tomao, C. Le Bourvellec, C.M.C.G. Renard, F. Chemat. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction Ultrasonics Sonchemistry. 17 (2010) 1066-1074. [DOI] [PubMed]
- 15.Mason T.J., Chemat F., Vinatoru M. The extraction of natural products using ultrasound or microwaves. Curr. Org. Chem. 2011;15:237–247. [Google Scholar]
- 16.Achat S., Tomao V., Madani K., Chibane M., Elmaataoui M., Dangles O., Chemat F. Direct enrichment of olive oil in oleuropein by ultrasound-assisted macerationat laboratory and pilot plant scale. Ultrason. Sonochem. 2012;19:777–786. doi: 10.1016/j.ultsonch.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Shirsath S.R., Sonawane S.H., Gogate P.R. Intensification of extraction of natural products using ultrasonic irradiations-A review of current status. Chem. Eng. Process. 2012;53:10–23. [Google Scholar]
- 18.Assami K., Pingret D., Chemat S., Meklatia B.Y., Chemat F. Ultrasound induced intensification and selective extraction of essential oil from Carum carvi L. seeds. Chem. Eng. Process, Process Intensification. 2012;62:99–105. [Google Scholar]
- 19.Majid I., Nayik G.A., Nanda V. Ultrasonication and food technology: A review. Cogent Food & Agriculture. 2015;1:107–122. [Google Scholar]
- 20.Vauchel P., Arhaliass A., Legrand J., Baron R., Kaas R. Chapter 11. Extrusion-Assisted Extraction: Alginate Extraction from Macroalgae by Extrusion Process. In: Lebovka N., Vorobiev E., Chemat F., editors. Enhancing Extraction Processes in the Food Industry. CRS Press; New York: 2012. pp. 323–340. [Google Scholar]
- 21.Chemat F., Fabiano-Tixier A.-S., Abert Vian M., Allaf T., Vorobiev E. Solvent-free extraction of food and natural products. Trends Anal. Chem. 2015;71:157–168. [Google Scholar]
- 22.D. Bermúdez-Aguirre D, T. Mobbs, G.V. Barbosa-Cánovas. chapter 3. Ultrasound Applications in Food Processing, in: H. Feng, G.V. Barbosa-Canovas, J. Weiss (Eds.), Ultrasound Technologies for Food and Bioprocessing, Springer New York Dordrecht Heidelberg London, 2011, pp. 65-106.
- 23.Awad T.S., Moharram H.A., Shaltout O.E., Asker D., Youssef M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012;48:410–427. [Google Scholar]
- 24.F. Chemat, F., G. Cravotto. Chapter 6. Combined extraction techniques, in: N. Lebovka, E. Vorobiev, F. Chemat (Eds), Enhancing extraction processes in the food industry, Contemporary Food Engineering. CRC Press (Taylor \& Francis Group), USA, 2011, pp 173-193.
- 25.K. Vilkhu K, R. Mawson, L. Simons, D. Bates. Applications and opportunities for ultrasound assisted extraction in the food industry- A review. Innov. Food Sci. Emerg. Technol. 9 (2008) 161–169.
- 26.Kentish S., Feng H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014;5:263–284. doi: 10.1146/annurev-food-030212-182537. [DOI] [PubMed] [Google Scholar]
- 27.Khadhraoui B., Turk M., Fabiano-Tixier A.-S., Petitcolas E., Robinet P., Imbert R., El Maâtaoui M., Chemat F. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound Towards chain detexturation mechanism. Ultrasonics Sonochem. 2018;24:482–492. doi: 10.1016/j.ultsonch.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Khadhraoui B., Fabiano-Tixier A.-S., Petitcolas E., Robinet P., Imbert R., El Maâtaoui M., Chemat F. Microscopic imaging as a tool to target spatial and temporal extraction of bioactive compounds through ultrasound intensification. Ultrason. Sonochem. 2019;53:214–225. doi: 10.1016/j.ultsonch.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 29.S. Vyas S and Y-P. Ting. A Review of the Application of Ultrasound in Bioleaching and Insights from Sonication in (Bio). Chemical Processes Resources. 7 (2018) 3-16.
- 30.Pingret D., Fabiano-Tixier A.-S., Chemat F. Degradation during application of ultrasound in food processing: A review. Food Control. 2013;31:593–606. [Google Scholar]
- 31.Wood R.J., Lee J., Bussemaker M.J. A parametric review of sonochemistry: Control and augmentation of sonochemical activity in aqueous solutions. Ultrason. Sonochem. 2017;38:351–370. doi: 10.1016/j.ultsonch.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Sun J., Wang W., Yue Q. Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials. 2016;9:231. doi: 10.3390/ma9040231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan M.K., Ahmad K., Hassana S., Imran M., Ahmad N., Xu C. Effect of novel technologies on polyphenols during food processing. Innovative Food Sci. Emerg. Technol. 2018;45:361–381. [Google Scholar]
- 34.Sadeghi A., Hakimzadeh V., Karimifar B. Microwave assisted extraction of bioactive compounds from food: A review. Int. J. Food Sci. Nutrition Eng. 2017;7(1):19–27. [Google Scholar]
- 35.Andreu V., Picó Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. Trends Anal. Chem. 2019;118:709–721. [Google Scholar]
- 36.G. Alvarez-Rivera, M. Bueno, D. Ballesteros-Vivas, J.A. Mendiola, E. Ibaňez. Chapter 13. Pressurized Liquid Extraction, in: C. Poole (Ed), Liquid-phase extraction, Elsevier, 2020, pp 375-398.
- 37.P. Raut, D. Bhosle, A. Janghel, S. Deo, C. Verma, S.S. Kumar, M. Agrawal, N. Amit, M. Sharma, T. Giri, D.K. Tripathi, Ajazuddin, A. Alexander. Emerging Pressurized Liquid Extraction (PLE) Techniques as an Innovative Green Technologies for the Effective Extraction of the Active Phytopharmaceuticals. Research Journal of Pharmacy and Technology; Raipur 8 (2015) 800-810.
- 38.Mustafa A., Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta. 2011;703:8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Allaf T., Tomao V., Ruiz K., Bachari K., ElMaâtaoui M., Chemat F. Deodorization by instant controlled pressure drop autovaporization of rosemary leaves prior to solvent extraction of antioxidants. LWT - Food Sci. Technol. 2013;51:111–119. [Google Scholar]
- 40.Vorobiev E., Lebovka N. Chapter 2. Pulsed Electric Field-Assisted Extraction. In: Lebovka N., Vorobiev E., Chemat F., editors. Enhancing Extraction Processes in the Food Industry. CRS Press; New York: 2012. pp. 25–84. [Google Scholar]
- 41.M. Nowacka, S. Tappi, A. Wiktor, K. Rybak, A. Miszczykowska, J. Czyzewski, K. Drozdzal, D. Witrowa-Rajchert, U. Tylewicz. The Impact of Pulsed Electric Field on the Extraction of Bioactive Compounds from Beetroot. Foods 28 (2019) 244; doi:10.3390/foods8070244. [DOI] [PMC free article] [PubMed]
- 42.Bozinou E., Karageorgou I., Batra G., Dourtoglou V.G., Lalas S.I. Pulsed electric field extraction and antioxidant activity determination of moringa oleifera dry leaves: A comparative study with other extraction techniques. Beverages. 2019;5:1–13. [Google Scholar]
- 43.Hossain M.B., Aguiló-Aguayo I., Lyng J.G., Brunton N.P., Rai D.K. Effect of pulsed electric field and pulsed light pre-treatment on the extraction of steroidal alkaloids from potato peels. Innovative Food Sci. Emerg. Technol. 2015;29:9–14. [Google Scholar]
- 44.Yasothai R., Giriprasad R. High intensity pulsed light technology in food processing. Int. J. Sci. Environment Technol. 2015;4:234–236. [Google Scholar]
- 45.Kim S.-W., Ko M.-J., Chung M.-S. Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J. Cleaner Prod. 2019;231:1192–1199. [Google Scholar]
- 46.Liu A., Cai L., Lu X., Han X., Ying T. Effect of postharvest UV-C irradiation on phenolic compound content and antioxidant activity of tomato fruit during storage. J. Integrative Agricult. 2012;11:159–165. [Google Scholar]
- 47.Majeed M., Hussain A.I., Chatha S.A.S., Khosa M.K.K., Kamal G.M., Kamal M.A., Liu M. Optimization protocol for the extraction of antioxidant components from Origanum vulgare leaves using response surface methodology. Saudi J. Biolog. Sci. 2016;23:389–396. doi: 10.1016/j.sjbs.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwaw E., Ma Y., Tchabo W., Apaliya M.T., Sackey A.S., Wu M., Xiao L. Effect of pulsed light treatment on the phytochemical, volatile and sensorial attributes of lactic-acid-fermented mulberry juice. Int. J. Food Prop. 2018;21:228–243. [Google Scholar]
- 49.Sydney T., Marshall-Thompson J.-A., Kapoore R.V., Vaidyanathan S., Pandhal J., Fairclough J.P.A. The effect of high-intensity ultraviolet light to elicit microalgal cell lysis and enhance lipid extraction. Metabolites. 2018;65:1–13. doi: 10.3390/metabo8040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.C. Pétrier, N. Gondrexon, P. Boldo. (2008). Ultrasons et sonochimie. Techniques de l’ingénieur Chimie verte : optimisation des modes de séparation, d’activation et de synthèse base documentaire TIB493DUO : 1-14.
- 51.T.J. Mason, L. Paniwnyk, F. Chemat. Ultrasound as a preservation technology. In P. Zeuthen, L. Bogh-Sorensen (Eds.). Food Preservation Techniques. New York: CRC Press, 2003, pp. 303-337.
- 52.Pingret D., Fabiano-Tixier A.S., Chemat F. Ultrasound-assisted Extraction’. In: Mauricio M.A., Rostagno M.J., editors. Natural Product Extraction: Principles and Applications. The Royal Society of Chemistry; United Kingdom: 2013. pp. 89–112. [Google Scholar]
- 53.Panda D., Manickam S. Natural products and process intensification mechanism and perspectives. Appl. Sci. 2019;9:766. doi: 10.3390/app9040766. [DOI] [Google Scholar]
- 54.F. Chemat, V. Tomao, M. Virot, M. Ultrasound-Assisted Extraction in Food Analysis. In: S. Otles, (ed.) Handbook of Food Analysis Instruments. New York: CRC press, (2008) 85-104.
- 55.Louisnard O., González-García J. Acoustic cavitation. The Physical and Chemical Effects of Ultrasound. In: Feng H., Barbosa-Canovas G., Weiss J., editors. Ultrasound Technologies for Food and Bioprocessing. Springer; New York: 2011. pp. 13–64. [Google Scholar]
- 56.T.Y. Wu, N. Guo, C.Y. Teh, J.X.W. Theory and Fundamentals of Ultrasound. In: T.Y. Wu, N. Guo, C.Y. Tech, J.X.W. Hay. Advances in Ultrasound Technology for Environmental Remediation. Briefs in Molecular Science. Dordrecht: Springer, 2013, pp. 5-12.
- 57.Kentish S., Ashokkumar M. The Physical and Chemical Effects of Ultrasound. In: Feng H., Barbosa-Canovas G., Weiss J., editors. Ultrasound Technologies for Food and Bioprocessing. Springer; New York: 2012. pp. 1–12. [Google Scholar]
- 58.Paniwnyk L., Cai S., Albu S., Mason T.J., Cole R. The enhancement and scale up of the extraction of antioxidants from Rosmarinus officinalis using ultrasound. Ultrason. Sonochem. 2009;16:287–292. doi: 10.1016/j.ultsonch.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Veillet S., Tomao V., Chemat F. Ultrasound assisted maceration: an original procedure for direct aromatisation of olive oil with basil. Food Chem. 2010;123:905–911. [Google Scholar]
- 60.Soria A., Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci. Technol. 2010;21:323–331. [Google Scholar]
- 61.Petigny L., Périno-Issartier S., Wajsman J., Chemat F. Batch and continuous ultrasound assisted extraction of boldo leaves (Peumus boldus Mol.) Int. J. Mol. Sci. 2013;14:5750–5764. doi: 10.3390/ijms14035750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajewska K., Mierzwa D. Influence of ultrasound on the microstructure of plant tissue. Innov. Food Sci. Emerg. Technol. 2017;43:117–129. [Google Scholar]
- 63.Chemat S., Lagha A., Ait Amar H., Bartels P.V., Chemat F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavor Fragr. J. 2004;19:188–195. [Google Scholar]
- 64.Luque de Castro M.D., García-Ayuso L.E. Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal. Chim. Acta. 1998;369:1–10. [Google Scholar]
- 65.Pingret D., Fabiano-Tixier A.-S., Chemat F. An improved ultrasound clevenger for extraction of essential oils. Food Anal. Met. 2013;7:9–12. [Google Scholar]
- 66.Vivek K., Subbarao K.V., Srivastava B. Optimization of postharvest ultrasonic treatment of kiwifruit using RSM. Ultrason. Sonochem. 2016;32:328–335. doi: 10.1016/j.ultsonch.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 67.Liu W.-Y., Ou H., Xiang Z.-B., Gregersen H. Optimization, chemical constituents and bioactivity of essential oil from Iberis amara seed extracted by ultrasound-assisted hydro-distillation compared to conventional techniques. J. Appl. Res. Med. Aromatic Plants. 2019;13:100204–100213. [Google Scholar]
- 68.Tekin K., Akalin M.K., Seker M.G. Ultrasound bath-assisted extraction of essential oils from clove using central composite design. Ind. Crops Prod. 2015;77:954–960. [Google Scholar]
- 69.Kowalski R., Kowalska G., Jamroz J., Nawrocka A., Metyk D. Effect of the ultrasound-assisted preliminary maceration on the efficiency of the essential oils distillation from selected herbal raw materials. Ultrason. Sonochem. 2015;24:214–220. doi: 10.1016/j.ultsonch.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Sinisterra J.V. Application of ultrasound to biotechnology: An overview. Ultrasonics. 1992;30:180–185. doi: 10.1016/0041-624x(92)90070-3. [DOI] [PubMed] [Google Scholar]
- 71.Görgüç A., Bircan C., Yilmaz F.M. Sesame bran as an unexploited by-product: effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019;283:637–645. doi: 10.1016/j.foodchem.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 72.Balasubramanian D., Srinivas V., Gaikar V.G., Sharma M.M. Aggregation behavior of hydrotropic compounds in aqueous solution. J. Phys. Chem. 1989;93:3865–3870. [Google Scholar]
- 73.Thakker M.R., Parikh J.K., Desai M.A. Ultrasound assisted hydrotropric extraction: A greener approach for the isolation of geraniol from leaves of Cymbopogon martinii. ACS Sustain. Chem. Eng. 2018;6:3215–3224. [Google Scholar]
- 74.Tang B., Bi W., Tian M., Row K.H. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J. Chromatogr. B. 2012;604:1–21. doi: 10.1016/j.jchromb.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 75.Plechkova N.V., Seddon K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008;37:123–150. doi: 10.1039/b006677j. [DOI] [PubMed] [Google Scholar]
- 76.Pacheco-Fernández I., Pino V. Green solvents in analytical chemistry. Curr. Opin. Green. Sustain. Chem. 2019;18:42–50. [Google Scholar]
- 77.L. Benvenuti, A. A. Ferreira Zielinski, S. R. Salvador Ferreira, Which is the best food emerging solvent: IL, DES or NADES? Trends in Food Science & Technology 90 (2019) 133-146. D.C.
- 78.Murador A.R., Braga P.L., Martins A.Z., de Rosso Mercadante V.V. Ionic liquid associated with ultrasonic-assisted extraction: A new approach to btain carotenoids from orange peel. Food Res. Int. 2019;126 doi: 10.1016/j.foodres.2019.108653. [DOI] [PubMed] [Google Scholar]
- 79.Abbott A.P., Capper G., Davies D.L., Rasheed R.K., Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003;9:70–71. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- 80.Mbous Y.P., Hayyana M., Hayyan A., Wong W.F., Hashima M.A., Looi C.Y. Applications of deep eutectic solvents in biotechnology and Bioengineering-Promises and challenges. Biotechnol. Adv. 2017;35:105–134. doi: 10.1016/j.biotechadv.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 81.Y.Dai, J.V. Spronsen, G-J. Witkamp, R. Verpoorte, Y.H. Choi. Ionic Liquids and Deep Eutectic Solvents in Natural Products. Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 76(2013) 2162−2173. [DOI] [PubMed]
- 82.Shishov A., Bulatov A., Locatelli M., Carradori S., Andruch V. Application of deep eutectic solvents in analytical chemistry. A Rev. Microchem. J. 2017;135:33–38. [Google Scholar]
- 83.Florindo C., Lima F., Ribeiro B.D., Marrucho I.M. Deep eutectic solvents: overcoming 21st century challenges. Curr. Opin Green Sustain. Chem. 2019;18:31–36. [Google Scholar]
- 84.Choi Y.H., Verpoorte R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019 doi: 10.1016/j.cofs.2019.04.003. [DOI] [Google Scholar]
- 85.Choi Y.H., Spronsen J.V., Dai Y., Verberne M., Hollmann F., Arends I.W.C.E., Witkamp G.J., Verpoorte R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011;156:1701–1705. doi: 10.1104/pp.111.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Espino M., Fernández M.Á., Gomez F.J.V., Silva M.F. Natural designer solvents for greening analytical chemistry. Trac-Trend Anal. Chem. 2016;76:126–136. [Google Scholar]
- 87.González C.G., Mustafa N.R., Wilson E.G., Verpoorte R., Choi Y.H. Application of natural deep eutectic solvents for the “green” extraction of vanillin from vanilla pods. Flavor Fragr. J. 2017:1–6. [Google Scholar]
- 88.Verpoorte R. Secondary metabolism. In: Verpoorte R., Alfermann A.W., editors. Metabolic engineering of plant secondary metabolism. Kluwer Academic Publidhers; Dordrecht, Netherlands: 2000. pp. 1–29. [Google Scholar]
- 89.Tomé L.I.N., Baião V., da Silva W., Brett C.M.A. Deep eutectic solvents for the production and application of new materials. Appl Mater Today. 2018;10:30–50. [Google Scholar]
- 90.Fernández M.Á., Boiteux J., Espino M., Gomez F.J.V., Silva M.F. Natural deep eutectic solvents- mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta. 2018;1038:1–10. doi: 10.1016/j.aca.2018.07.059. [DOI] [PubMed] [Google Scholar]
- 91.Mansur A.R., Song N.-E., Jang H.W., Lim T.-G., Yoo M., Nam T.G. Optimizing of the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019;293:438–445. doi: 10.1016/j.foodchem.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Chanioti S., Tzia C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018;48:228–239. [Google Scholar]
- 93.Bosiljkov T., Dujmić F., Bubalo M.C., Hribar J., Vidrih R., Brncić M., Zlatic E., Redovniković I.R., Jokić S. Natural deep eutectic solvents and ultrasound assisted extraction: green approaches for extraction of wine lees anthocyanins. Food Bioproduct. Process. 2017;102:195–203. [Google Scholar]
- 94.Stanisavljević T.I., Lazić L.M., Veljković B.V. Ultrasonic extraction of oil from tobacco (Nicotiana tabacum L.) seeds. Ultrason. Sonochem. 2007;14:646–652. doi: 10.1016/j.ultsonch.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Moubarik A., El-Belghiti K., Vorobiev E. Kinetic model of solute aqueous extraction from Fennel (Foeniculum vulgare) treated by pulsed electric field, electrical discharges and ultrasonic irradiations. Food Bioprod. Process. 2011;89:356–361. [Google Scholar]
- 96.Amirante R., Distaso E., Tamburrano P., Paduano A., Clodoveo L.M. Acoustic cavitation by means ultrasounds in the extra virgin olive oil extraction process. Energy Procedia. 2017;126:82–90. [Google Scholar]
- 97.Milenković D.D., Milosavljević M.M., Bojić A.L. Ultrasound-assisted extraction of sunflower oil from the cake after sunflower seed pressing. J. Agricult. Sci. 2018;63:195–204. [Google Scholar]
- 98.de Castro M.D.L., Priego-Capote F. Chapter 3. Microwave-Assisted Extraction. In: Lebovka N., Vorobiev E., Chemat F., editors. Food Processing, in: Enhancing Extraction Processes in the Food Industry. CRC Press; New York: 2012. pp. 85–122. [Google Scholar]
- 99.Hu Y., Wang T., Wang M., Han S., Wan P. Extraction of isoflavonoids from Pueraria by combining ultrasound with microwave vacuum. Chem. Eng. Process. 2008;47:2256–2261. [Google Scholar]
- 100.Z. Lianfu, L. Zelong. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrasonics Sonochemistry 15 (2008) 731–737. [DOI] [PubMed]
- 101.Trendafilova A., Todorova M. Comparison of different techniques for extraction of biologically active compounds from Achillea millefolium Proa. Nat. Prod. Com. 2008;3:1515–1518. [Google Scholar]
- 102.Lou Z., Wang H., Wang D., Zhang Y. Preparation of inulin and phenols-rich dietary fibre powder from burdock root. Carbohydr Polym. 2009;78:666–671. [Google Scholar]
- 103.Lou Z., Wang H., Zhu S., Zhang M., Gao Y., Ma C., Wang Z. Improved extraction and identification by ultra-performance liquid chromatography tandem mass spectrometry of phenolic compounds in burdock leaves. J. Chrom A. 2010;1217:2441–2446. doi: 10.1016/j.chroma.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 104.Wizi J., Wang L., Hou X., Tao Y., Ma B., Yang Y. Ultrasound-microwave assisted extraction of natural colorants from sorghum husk with different solvents. Ind. Crops Prod. 2018;120:203–213. [Google Scholar]
- 105.Z. Guo, B. Zhao, H. Li, Song Miao, B. Zheng. Optimization of ultrasound-microwave synergistic extraction of prebiotic oligosaccharides from sweet potatoes (Ipomoea batatas L.). Innovative Food Science and Emerging Technologies 54 (2019) 51–63.
- 106.A. Zhao, S. Sun, H. Lin, L. Chen, S. Qin, W. Wu, B. Zheng, Z. Guo. Physicochemical properties and digestion of the lotus seed starch-green tea polyphenol complex under ultrasound-microwave synergistic interaction. Ultrasonics Sonochemistry 52 (219) 50-61. [DOI] [PubMed]
- 107.Yang J.-S., Mu T.-H., Ma M.-M. Optimization of ultrasound-microwave assisted acid extraction of pectin from potato pulp by response surface methodology and its characterization. Food Chem. 2019;289:351–359. doi: 10.1016/j.foodchem.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 108.Li J.-H., Li W., Luo S., Ma C.-H., Liu S.-X. Alternate ultrasound/microwave digestion for deep eutectic hydro-distillation extraction of essential oil and polysaccharide from Schisandra chinensis (Turcz.) Baill. Molecules. 2019;24:1–23. doi: 10.3390/molecules24071288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yin A., Fan X., Fan Z., Shi D., Gao H. Optimization of enzymes-microwave-ultrasound assisted extraction of Lentinus edodes polysaccharides and determination of its antioxidant activity. Int. J. Biol. Macromol. 2018;111:446–454. doi: 10.1016/j.ijbiomac.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Wang X., Xu J., Wang L., Gao X., Fu X., Zhao Y. Optimization of microwave-ultrasound-assisted enzymatic hydrolysis extraction of iodine amino acids in laminaria by high performance liquid chromatography with a photodiode array detector. Algal Res. 2019;39 [Google Scholar]
- 111.Chen H.-M., Fu X., Luo Z.-G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015;168:302–310. doi: 10.1016/j.foodchem.2014.07.078. [DOI] [PubMed] [Google Scholar]
- 112.Sumere B.R., de Souza M.C., dos Santos M.P., Bezerra R.M.N., da Cunha D.T., Martinez J., Rostagno M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.) Ultrasonics – Sonochem. 2018;48:151–162. doi: 10.1016/j.ultsonch.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 113.Liu H.-M., Yao Y.-G., Ma Y.-X., Wang X.-D. Ultrasound-assisted desolventizing of fragrant oil from red pepper seed by subcritical propane extraction. Ultrasonics – Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104943. [DOI] [PubMed] [Google Scholar]
- 114.S.P. Jesus and M.A.A. Meireles. Chapter 3. Supercritical Fluid Extraction: A Global Perspective of the Fundamental Concepts of this Eco-Friendly Extraction Technique, in: F. Chemat, M. Abert Vian (Eds), Alternative Solvents for Natural Products Extraction, Springer, 2014, pp 39-72.
- 115.Singh R.K., Avula R.Y. Chapter 7. Supercritical Fluid Extraction. In: Lebovka N., Vorobiev E., Chemat F., editors. Food Processing, in: Enhancing Extraction Processes in the Food Industry. CRC Press; New York: 2012. pp. 195–222. [Google Scholar]
- 116.Dassoff E.S., Li Y.O. Mechanisms and effects of ultrasound-assisted supercritical CO2 extraction. Trends Food Sci. Technol. 2019;86:492–501. [Google Scholar]
- 117.Balachandran S., Kentish S.E., Mawson R., Ashokkumar M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006;13:471–479. doi: 10.1016/j.ultsonch.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 118.Santos-Zea L., Gutiérrez-Uribe J.A., Benedito J. Effect of ultrasound intensification on the supercritical fluid extraction of phytochemicals from Agave salmiana bagasse. J. Supercritical Fluids. 2019;144:98–107. [Google Scholar]
- 119.K. Allaf, N. Louka, J.M. Bouvier, F. Parent, M. Forget. Drying of products by controlled pressure reduction by heating and pressure increase by heat transfer gas followed by connection to vacuum. 1993, French Patent 19920004540.
- 120.T. Allaf, C. Besombes, V. Tomao, F. Chemat, K. Allaf. Coupling DIC and Ultrasound in Solvent Extraction Processes, in, T. Allaf and K. Allaf (Eds), Instant Controlled Pressure Drop (D.I.C.) in Food Processing, Springer, New York, 2014, pp 151-162.
- 121.Mannaï A., Jableoui C., Hamrouni L., Allaf K., Jamoussi B. DIC as a pretreatment prior to ultrasonic extraction for the improvement of rebaudioside A yield and preservation of vitamin B1 and B6. J. Food Meas. Charact. 2019;13:2764–2772. [Google Scholar]
- 122.N.I. Lebovka, M.I. Bazhal, E. Vorobiev. Pulsed electric field breakage of cellular tissues: vidualisation of percolative properties. Innovative Food Science & Emerging Technologies 2 (2001) 113-125.
- 123.M.F. Manzoor, X-A. Zeng, A. Rahaman, A. Siddeeg, R.M. Aadil, Z. Ahmed, J. Li1, D. Ni. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J Food Sci Technol 56 (2019) 2355–2364. [DOI] [PMC free article] [PubMed]
- 124.Esua O.J., Chin N.L., Yusof Y.A., Sukor R. Effects of simultaneous UV-C radiation and ultrasonic energy postharvest treatment on bioactive compounds and antioxidant activity of tomatoes during storage. Food Chem. 2019;270:113–122. doi: 10.1016/j.foodchem.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 125.Lima A.R., Cristofoli N.L., Veneral J.G., Fritz A.R.M., Vieira M.C. Optimization conditions of UV-C radiation combined with ultrasound-assisted extraction of cherry tomato (Lycopersicon esculentum) Lycopene Extract. Int. J. Food Stud. 2019;8:65–80. [Google Scholar]
- 126.Li S., Wang W., Tang H., Chen K., Yang J., He L., Ye H., Peng A., Chen L. Comparison of counter-current chromatography and preparative high performance liquid chromatography applied to separating minor impurities in drug preparations. J. Chromatogr. A. 2014;1344:51–58. doi: 10.1016/j.chroma.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 127.Chen T., Liu Y., Zou D., Chen C., You J., Zhou G., Sun J., Li Y. Application of an efficient strategy based on liquid–liquid extraction, high-speed countercurrent chromatography, and preparative HPLC for the rapid enrichment, separation, and purification of four anthraquinones from Rheum tanguticum. J. Sep. Sci. 2014;37:165–170. doi: 10.1002/jssc.201300648. [DOI] [PubMed] [Google Scholar]
- 128.Marston A., Slacanin I., Hostettmann K. Centrifugal partition chromatography in the separation of natural products. Phytochem. Anal. 1990;1:3–17. [Google Scholar]
- 129.Gavioli A., Maier N.M., Minguillón C., Lindner W. Preparative enantiomer separation of dichlorprop with a Cinchona-Derived chiral selector employing centrifugal partition chromatography and high-performance liquid chromatography: a comparative study. Anal. Chem. 2004;76:5837–5848. doi: 10.1021/ac040102y. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Y., Liu C., Li J., Qi Y., Li Y., Li S. Development of ‘‘ultrasound-assisted dynamic extraction’’ and its combination with CCC and CPC for simultaneous extraction and isolation of phytochemicals. Ultrason. Sonochem. 2015;26:111–118. doi: 10.1016/j.ultsonch.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 131.Armstrong D.W., Bertrand G.L., Berthod A. Study of the origin and mechanism of band broadening and pressure drop in centrifugal countercurrent chromatography. Anal. Chem. 1988;60:2513–2519. doi: 10.1021/ac00173a017. [DOI] [PubMed] [Google Scholar]
- 132.A. Berthod and K. Faure. Chapter 3. Separations with a Liquid Stationary Phase: Countercurrent Chromatography or Centrifugal Partition Chromatography. In: J.L. Anderson, A. Berthod, V. Pino Estévez, A.M. Stalcup (Eds), Analytical Separation Science, 4, Wiley-VCH Verlag GmbH & Co. KGaA., 2015, pp.1177-1206.
- 133.Friesen J.B., McAlpine J.B., Chen S.N., Pauli G.F. Countercurrent separation of natural products: An update. J Nat Prod. 2015;78:1765–1796. doi: 10.1021/np501065h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang C., Li X.-A., Wang H., Xia X., Kong B. Ultrasound-assisted immersion freezing reduces the structure and gel property deterioration of myofibrillar protein from chicken breast. Ultrason. Sonochem. 2020:105137. doi: 10.1016/j.ultsonch.2020.105137. [DOI] [PubMed] [Google Scholar]
- 135.Fernandes F.A., Gallão M.I., Rodrigues S. Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: Melon dehydration. LWT-Food Sci. Technol. 2008;41(4):604–610. [Google Scholar]
- 136.Karizaki V.M., Sahin S., Sumnu G., Mosavian M.T.H., Luca A. Effect of ultrasound-assisted osmotic dehydration as a pretreatment on deep fat frying of potatoes. Food Bioprocess Technol. 2013;6(12):3554–3563. [Google Scholar]
- 137.K., Fan, M. Zhang, W. Wang and B. Bhandari “A novel method of osmotic-dehydrofreezing with ultrasound enhancement to improve water status and physicochemical properties of kiwifruit.” International Journal of Refrigeration 113: (2020). 49-57.
- 138.Antunes-Rohling A., Ciudad-Hidalgo S., Mir-Bel J., Raso J., Cebrián G., Álvarez I. Ultrasound as a pretreatment to reduce acrylamide formation in fried potatoes. Innovative Food Sci. Emerg. Technol. 2018;49:158–169. [Google Scholar]
- 139.J. Zhang, Y. Zhang, Y. Wang, L. Xing and W. Zhang “Influences of ultrasonic-assisted frying on the flavor characteristics of fried meatballs.” Innovative Food Science & Emerging Technologies: (2020(b)). 102365.
- 140.Devi S., Zhang M., Law C.L. Effect of ultrasound and microwave assisted vacuum frying on mushroom (Agaricus bisporus) chips quality. Food Biosci. 2018;25:111–117. [Google Scholar]
- 141.Ojha K.S., Mason T.J., O’Donnell C.P., Kerry J.P., Tiwari B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017;34:410–417. doi: 10.1016/j.ultsonch.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 142.Al Daccache M., Koubaa M., Salameh D., Maroun R.G., Louka N., Vorobiev E. Ultrasound-assisted fermentation for cider production from Lebanese apples. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104952. [DOI] [PubMed] [Google Scholar]
- 143.Luo D., Wu R., Zhang J., Zhang K., Xu B., Li P., Yuan Y., Li X. Effects of ultrasound assisted dough fermentation on the quality of steamed bread. J. Cereal Sci. 2018;83:147–152. [Google Scholar]
- 144.RuanY S., Li Y., Wang S., Huang J. Luo, Ma H. Analysis in protein profile, antioxidant activity and structure-activity relationship based on ultrasound-assisted liquid-state fermentation of soybean meal with Bacillus subtilis. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2019.104846. [DOI] [PubMed] [Google Scholar]
- 145.Ross S., McBain J. Inhibition of foaming in solvents containing known foamers. Ind. Eng. Chem. 1944;36(6):570–573. [Google Scholar]
- 146.Sun S.C. Destruction of flotation froth with intense high frequency sound. Min. Eng. 1951;3:865–867. [Google Scholar]
- 147.Dedhia A.C., Ambulgekar P.V., Pandit A.B. Static foam destruction: role of ultrasound. Ultrason. Sonochem. 2004;11(2):67–75. doi: 10.1016/S1350-4177(03)00134-2. [DOI] [PubMed] [Google Scholar]
- 148.Charoux C.M., Ojha K.S., O'Donnell C.P., Cardoni A., Tiwari B.K. Applications of airborne ultrasonic technology in the food industry. J. Food Eng. 2017;208:28–36. [Google Scholar]
- 149.R. Mawson, J. Tongaonkar, S. Bhagwat and A. Pandit Airborne ultrasound for enhanced defoaming applications. Innovative Food Processing Technologies, Elsevier: (2016). 347-359.
- 150.Garrett P.R. Defoaming: Antifoams and mechanical methods. Curr. Opin. Colloid Interface Sci. 2015;20(2):81–91. [Google Scholar]
- 151.J. A., Gallego JuárezG. Rodríguez Corral, V. M. Acosta Aparicio, E. Andrés Gallego, A. Blanco Blanco and F. Montoya Vitini “Procedimiento y sistema ultrasónico de desespumación mediante emisores con placa vibrante escalonada.” Sp. Pat., (2005). 2002 02113, 2002.
- 152.J. Gallego-Juárez, G. Rodríguez, E. Riera and A. Cardoni Ultrasonic defoaming and debubbling in food processing and other applications. Power Ultrasonics, Elsevier: (2015). 793-814.
- 153.Morse R. Sonic energy in granular solid fluidization. Ind. Eng. Chem. 1955;47(6):1170–1175. [Google Scholar]
- 154.Zhang Y., Abatzoglou N. Fundamentals, applications and potentials of ultrasound-assisted drying. Chem. Eng. Res. Des. 2020;154:21–46. [Google Scholar]
- 155.Huang D., Men K., Li D., Wen T., Gong Z., Sunden B., Wu Z. Application of ultrasound technology in the drying of food products. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104950. [DOI] [PubMed] [Google Scholar]
- 156.Fijalkowska A., Nowacka M., Wiktor A., Sledz M., Witrowa-Rajchert D. Ultrasound as a pretreatment method to improve drying kinetics and sensory properties of dried apple. J. Food Process Eng. 2016;39(3):256–265. [Google Scholar]
- 157.Nowacka M., Wedzik M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016;103:163–171. [Google Scholar]
- 158.Rodrigues S., Oliveira F.I., Gallão M.I., Fernandes F.A. Effect of immersion time in osmosis and ultrasound on papaya cell structure during dehydration. Drying Technol. 2009;27(2):220–225. [Google Scholar]
- 159.Allahdad Z., Nasiri M., Varidi M., Varidi M.J. Effect of sonication on osmotic dehydration and subsequent air-drying of pomegranate arils. J. Food Eng. 2019;244:202–211. [Google Scholar]
- 160.Kowalski S., Szadzińska J., Pawłowski A. Ultrasonic-assisted osmotic dehydration of carrot followed by convective drying with continuous and intermittent heating. Drying Technol. 2015;33(13):1570–1580. [Google Scholar]
- 161.P. M. Azoubel, M. d. A. M. Baima, M. da Rocha Amorim and S. S. B. Oliveira “Effect of ultrasound on banana cv Pacovan drying kinetics.” Journal of Food Engineering 97(2): (2010). 194-198.
- 162.Fan K., Zhang M., Mujumdar A.S. Application of airborne ultrasound in the convective drying of fruits and vegetables: A review. Ultrason. Sonochem. 2017;39:47–57. doi: 10.1016/j.ultsonch.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 163.J. García-PérezJ. Cárcel, S. De la Fuente-Blanco and E. R.-F. De Sarabia “Ultrasonic drying of foodstuff in a fluidized bed: parametric study.” Ultrasonics 44: (2006). e539-e543. [DOI] [PubMed]
- 164.Ó. Rodríguez, J. V. Santacatalina, S. Simal, J. V. Garcia-Perez, A. Femenia and C. Rosselló “Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties.” Journal of Food Engineering 129(2014).: 21-29.
- 165.Do Nascimento E.M., Mulet A., Ascheri J.L.R., de Carvalho C.W.P., Cárcel J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2016;170:108–118. [Google Scholar]
- 166.Cruz L., Clemente G., Mulet A., Ahmad-Qasem M., Barrajón-Catalán E., García-Pérez J.V. Air-borne ultrasonic application in the drying of grape skin: Kinetic and quality considerations. J. Food Eng. 2016;168:251–258. [Google Scholar]
- 167.Cheng X., Zhang M., Xu B., Adhikari B., Sun J. The principles of ultrasound and its application in freezing related processes of food materials: A review. Ultrason. Sonochem. 2015;27:576–585. doi: 10.1016/j.ultsonch.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 168.Qiu L., Zhang M., Chitrakar B., Bhandari B. Application of power ultrasound in freezing and thawing processes: Effect on process efficiency and product quality. Ultrason. Sonochem. 2020:105230. doi: 10.1016/j.ultsonch.2020.105230. [DOI] [PubMed] [Google Scholar]
- 169.Xin Y., Zhang M., Adhikari B. The effects of ultrasound-assisted freezing on the freezing time and quality of broccoli (Brassica oleracea L. var. botrytis L.) during immersion freezing. Int. J. Refrig. 2014;41:82–91. doi: 10.1016/j.ultsonch.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 170.Sun Q., Sun F., Xia X., Xu H., Kong B. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason. Sonochem. 2019;51:281–291. doi: 10.1016/j.ultsonch.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 171.Cheng X.-F., Zhang M., Adhikari B., Islam M.N., Xu B.-G. Effect of ultrasound irradiation on some freezing parameters of ultrasound-assisted immersion freezing of strawberries. Int. J. Refrig. 2014;44:49–55. [Google Scholar]
- 172.Islam M.N., Zhang M., Adhikari B., Xinfeng C., Xu B.-G. The effect of ultrasound-assisted immersion freezing on selected physicochemical properties of mushrooms. Int. J. Refrig. 2014;42:121–133. [Google Scholar]
- 173.Comandini P., Blanda G., Soto-Caballero M., Sala V., Tylewicz U., Mujica-Paz H., Fragoso A.V., Toschi T.G. Effects of power ultrasound on immersion freezing parameters of potatoes. Innovative Food Sci. Emerg. Technol. 2013;18:120–125. [Google Scholar]
- 174.Wu X.-F., Zhang M., Adhikari B., Sun J. Recent developments in novel freezing and thawing technologies applied to foods. Crit. Rev. Food Sci. Nutr. 2017;57(17):3620–3631. doi: 10.1080/10408398.2015.1132670. [DOI] [PubMed] [Google Scholar]
- 175.Chandrapala J., Oliver C.M., Kentish S., Ashokkumar M. Use of power ultrasound to improve extraction and modify phase transitions in food processing. Food Rev. Int. 2013;29(1):67–91. [Google Scholar]
- 176.D. Li, H. Zhao, A. I. Muhammad, L. Song, M. Guo and D. Liu “The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets.” Food Chemistry: (2020). 126614. [DOI] [PubMed]
- 177.Y., LiuS. Chen, Y. Pu, A. I. Muhammad, M. Hang, D. Liu and T. Ye “Ultrasound-assisted thawing of mango pulp: Effect on thawing rate, sensory, and nutritional properties.” Food chemistry 286: (2019). 576-583. [DOI] [PubMed]
- 178.Gambuteanu C., Alexe P. Comparison of thawing assisted by low-intensity ultrasound on technological properties of pork Longissimus dorsi muscle. J. Food Sci. Technol. 2015;52(4):2130–2138. doi: 10.1007/s13197-013-1204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rawson A., Tiwari B., Tuohy M., O’Donnell C., Brunton N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason. Sonochem. 2011;18(5):1172–1179. doi: 10.1016/j.ultsonch.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 180.Tao Y., Zhang J., Jiang S., Xu Y., Show P.-L., Han Y., Ye X., Ye M. Contacting ultrasound enhanced hot-air convective drying of garlic slices: Mass transfer modeling and quality evaluation. J. Food Eng. 2018;235:79–88. [Google Scholar]
- 181.J. Kroehnke, G. Musielak and A. BORATYŃSKA “Convective drying of potato assisted by ultrasound.” PhD Interdisciplinary Journal 1: (2014). 57-65.
- 182.Wang H., Zhao Q.-S., Wang X.-D., Hong Z.-D., Zhao B. Pretreatment of ultrasound combined vacuum enhances the convective drying efficiency and physicochemical properties of okra (Abelmoschus esculentus) Lwt. 2019;112 [Google Scholar]
- 183.Troy D.J., Ojha K.S., Kerry J.P., Tiwari B.K. Sustainable and consumer-friendly emerging technologies for application within the meat industry: An overview. Meat Sci. 2016;120:2–9. doi: 10.1016/j.meatsci.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 184.Y. Zou, D. Kang, R. Liu, J. Qi, G. Zhou and W. Zhang “Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef.” Ultrasonics sonochemistry 46: (2018(a)). 36-45. [DOI] [PubMed]
- 185.Y, Zou, W. Zhang, D. Kang and G. Zhou “Improvement of tenderness and water holding capacity of spiced beef by the application of ultrasound during cooking.” International Journal of Food Science & Technology 53(3) (2018(b)) 828-836.
- 186.J. S., Da SilvaM. Voss, C. R. de Menezes, J. S. Barin, R. Wagner, P. C. B. Campagnol and A. J. Cichoski “Is it possible to reduce the cooking time of mortadellas using ultrasound without affecting their oxidative and microbiological quality?” Meat science 159: (2020). 107947. [DOI] [PubMed]
- 187.Miano A.C., Ibarz A., Augusto P.E.D. Mechanisms for improving mass transfer in food with ultrasound technology: Describing the phenomena in two model cases. Ultrason. Sonochem. 2016;29:413–419. doi: 10.1016/j.ultsonch.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 188.A. Córdova, C. Astudillo-Castro, R. Ruby-Figueroa, P. Valencia and C. Soto “Recent advances and perspectives of ultrasound assisted membrane food processing.” Food Research International: (2020). 109163. [DOI] [PubMed]
- 189.Aktij S.A., Taghipour A., Rahimpour A., Mollahosseini A., Tiraferri A. A critical review on ultrasonic-assisted fouling control and cleaning of fouled membranes. Ultrasonics. 2020;106228 doi: 10.1016/j.ultras.2020.106228. [DOI] [PubMed] [Google Scholar]
- 190.Hemmati A., Mirsaeedghazi H., Aboonajmi M. The effect of ultrasound on permeation flux and changes in blocking mechanisms during dead-end microfiltration of carrot juice. Int. J. Nutrit. Food Eng. 2017;11(8):594–598. [Google Scholar]
- 191.Masselin I., Chasseray X., Durand-Bourlier L., Lainé J.-M., Syzaret P.-Y., Lemordant D. Effect of sonication on polymeric membranes. J. Membr. Sci. 2001;181(2):213–220. [Google Scholar]
- 192.Grossner M.T., Belovich J.M., Feke D.L. Transport analysis and model for the performance of an ultrasonically enhanced filtration process. Chem. Eng. Sci. 2005;60(12):3233–3238. [Google Scholar]
- 193.Jin Y., Hengl N., Baup S., Pignon F., Gondrexon N., Sztucki M., Gesan-Guiziou G., Magnin A., Abyan M., Karrouch M. Effects of ultrasound on cross-flow ultrafiltration of skim milk: Characterization from macro-scale to nano-scale. J. Membr. Sci. 2014;470:205–218. [Google Scholar]
- 194.S. MuthukumaranK. Yang, A. Seuren, S. Kentish, M. Ashokkumar, G. W. Stevens and F. Grieser “The use of ultrasonic cleaning for ultrafiltration membranes in the dairy industry.” Separation and Purification Technology 39(1-2): (2004). 99-107.
- 195.Wang X.-L., Li X.-F., Fu X.-Q., Chen R., Gao B. Effect of ultrasound irradiation on polymeric microfiltration membranes. Desalination. 2005;175(2):187–196. [Google Scholar]
- 196.AbbasK S., Hayat E., Karangwa M.B., Zhang X. An overview of ultrasound-assisted food-grade nanoemulsions. Food Eng. Rev. 2013;5(3):139–157. [Google Scholar]
- 197.JCarpenter, and V. K. Saharan “Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: Effect of process parameters and their optimization.” Ultrasonics Sonochemistry 35: (2017). 422-430. [DOI] [PubMed]
- 198.AmiriA A., Mousakhani-Ganjeh S., Torbati G.G., Kenari R.E. Impact of high-intensity ultrasound duration and intensity on the structural properties of whipped cream. Int. Dairy J. 2018;78:152–158. [Google Scholar]
- 199.Akdeniz V., Akalın A.S. New approach for yoghurt and ice cream production: High-intensity ultrasound. Trends Food Sci. Technol. 2019;86:392–398. [Google Scholar]
- 200.N. Bhargava, R. S. Mor, K. Kumar and V. S. Sharanagat “Advances in application of ultrasound in food processing: A review.” Ultrasonics Sonochemistry: (2020). 105293. [DOI] [PMC free article] [PubMed]
- 201.Zhao C.-C., Eun J.-B. Influence of ultrasound application and NaCl concentrations on brining kinetics and textural properties of Chinese cabbage. Ultrason. Sonochem. 2018;49:137–144. doi: 10.1016/j.ultsonch.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 202.Ozuna C., Puig A., García-Pérez J.V., Mulet A., Cárcel J.A. Influence of high intensity ultrasound application on mass transport, microstructure and textural properties of pork meat (Longissimus dorsi) brined at different NaCl concentrations. J. Food Eng. 2013;119(1):84–93. [Google Scholar]
- 203.Ojha K.S., Keenan D.F., Bright A., Kerry J.P., Tiwari B.K. Ultrasound assisted diffusion of sodium salt replacer and effect on physicochemical properties of pork meat. Int. J. Food Sci. Technol. 2016;51(1):37–45. [Google Scholar]
- 204.Harvey E.N., Loomis A.L. The destruction of luminous bacteria by high frequency sound waves. J. Bacteriol. 1929;17(5):373. doi: 10.1128/jb.17.5.373-376.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Baboli Z.M., Williams L., Chen G. Design of a batch ultrasonic reactor for rapid pasteurization of juices. J. Food Eng. 2020;268 doi: 10.3390/foods9060801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Saeeduddin M., Abid M., Jabbar S., Wu T., Hashim M.M., Awad F.N., Hu B., Lei S., Zeng X. Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT-Food Sci. Technol. 2015;64(1):452–458. [Google Scholar]
- 207.MonteiroE S.H., Silva K., Alvarenga V.O., Moraes J., Freitas M.Q., Silva M.C., Raices R.S., Sant'Ana A.S., Meireles M.A.A., Cruz A.G. Effects of ultrasound energy density on the non-thermal pasteurization of chocolate milk beverage. Ultrason. Sonochem. 2018;42:1–10. doi: 10.1016/j.ultsonch.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 208.Schittenhelm N., Kulicke W.M. Producing homologous series of molar masses for establishing structure-property relationships with the aid of ultrasonic degradation. Macromol. Chem. Phys. 2000;201(15):1976–1984. [Google Scholar]
- 209.A. Dodero, S. Vicini and M. Castellano “Depolymerization of sodium alginate in saline solutions via ultrasonic treatments: A rheological characterization.” Food Hydrocolloids: (2020). 106128.
- 210.Prajapat A.L., Subhedar P.B., Gogate P.R. Ultrasound assisted enzymatic depolymerization of aqueous guar gum solution. Ultrason. Sonochem. 2016;29:84–92. doi: 10.1016/j.ultsonch.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 211.Kang N., Zuo Y., Hilliou L., Ashokkumar M., Hemar Y. Viscosity and hydrodynamic radius relationship of high-power ultrasound depolymerised starch pastes with different amylose content. Food Hydrocolloids. 2016;52:183–191. [Google Scholar]