Abstract
Background
COVID-19 results in persisting symptoms but there is little systematically collected data estimating recovery time following infection.
Methods
We followed 94% of all COVID-19 cases diagnosed in the Australian state of New South Wales between January and May 2020 using 3-4 weekly telephone interviews and linkage to hospitalisation and death data to determine if they had recovered from COVID-19 based on symptom resolution. Proportional hazards models with competing risks were used to estimate time to recovery adjusted for age and gender.
Findings
In analyses 2904 cases were followed for recovery (median follow-up time 16 days, range 1-122, IQR 11-24).There were 2572 (88.6%) who reported resolution of symptoms (262/2572 were also hospitalised), 224 (7.8%) had not recovered at last contact (28/224 were also hospitalised), 51 (1.8%) died of COVID-19, and 57 (2.0%) were hospitalised without a documented recovery date. Of those followed, 20% recovered by 10 days, 60% at 20, 80% at 30, 91% at 60, 93% at 90 and 96% at 120 days. Compared to those aged 30-49 years, those 0-29 years were more likely to recover (aHR 1.22, 95%CI 1.10-1.34) while those aged 50-69 and 70+ years were less likely to recover (aHR respectively 0.74, 95%CI 0.67-0.81 and 0.63, 95%CI 0.56-0.71). Men were faster to recover than women (aHR 1.20, 95%CI 1.11-1.29) and those with pre-existing co-morbidities took longer to recover than those without (aHR 0.90, 95%CI 0.83-0.98).
Interpretation
In a setting where most cases of COVID-19 were ascertained and followed, 80% of those with COVID-19 recover within a month, but about 5% will continue to experience symptoms 3 months later.
Funding
NSW Health Emergency Response Priority Research Projects
Keywords: COVID-19, Recovery, Cohort study
Research in context.
Evidence before this study
There is significant concern regarding the longer-term effects of COVID-19 but to date most studies have been restricted to volunteers or people hospitalised with disease which may bias estimates of recovery.
Added value of this study
In this study we followed 94% of all people with a laboratory-confirmed diagnosis of COVID-19 in the Australian state of New South Wales to determine if they had recovered from their infection. We found that 80% of those with COVID-19 recover within a month, but about 5% will continue to experience symptoms 3 months later. Older people, women and those with comorbidities were less likely to report recovery.
Implications of all the available evidence
Accurate estimates of the proportion of people diagnosed with COVID-19 who experience long-term effects are important to estimate the impact of disease at a population level. Our whole of population estimates suggest that while only a small proportion of those presenting with COVID-19 will have prolonged symptoms, given the scale of the pandemic and the number of cases diagnosed worldwide, these effects are significant and should be considered in balancing the costs and benefits of prevention strategies.
Alt-text: Unlabelled box
1. Background
COVID-19, the disease caused by SARS-CoV-2 infection was declared a global pandemic in early 2020. While substantial knowledge has now accumulated regarding the acute clinical presentation of SARS-CoV-2 infection and some of the pathophysiological changes observed, particularly in people with serious disease who are hospitalised, less is known about the clinical course, particularly in those with less severe disease. Numerous case reports from those diagnosed with COVID-19 have described a prolonged recovery with persisting symptoms including fatigue, shortness of breath, atypical chest pains or palpitations, changes in mood and sleep disturbances, and cognitive dysfunction [1], [2], [3].
There is however substantial variation in estimates of recovery time. A systematic review and meta-analysis conducted early in the pandemic, based on 7 small case series, reported mean time from symptom onset to recovery from COVID-19 was 19 days but included both hospitalised and non-hospitalised populations and there was significant heterogeneity among estimates [4]. Other studies including samples of hospitalised patients (N=143) [5], non-hospitalised adults (N=292) [6], and self-reported cases who logged symptoms into a smartphone application (N=4182) [7], have also suggested a wide spectrum of recovery times. To date, studies are limited by their unsystematic sampling frames and low response rates which means their findings may not be generalisable [8].
Australia has managed to contain COVID-19 relatively well with currently about 30,000 cases nationally in a population of over 25 million [9]. Since the beginning of the pandemic Australia has had very high SARS-CoV-2 testing rates and very low test positivity suggesting that case detection is high, at least for symptomatic infection [9]. New South Wales (NSW) is Australia's most populous state with a population of over 8 million people, and the location of most cases in the first wave of infections in Australia (January to May 2020). We used the state's comprehensive disease notification registry, follow-up questionnaires and linked health datasets to estimate the recovery time in all people diagnosed with COVID-19 in NSW.
2. Methods
2.1. Population
Our sampling frame comprised all people diagnosed with COVID-19 in NSW. Cases were identified through the NSW Notifiable Conditions Information Management System (NCIMS) a statutory public health register that records infectious diseases legally notifiable to public health authorities. It receives electronic notifications from laboratories and health practitioners and is used for disease surveillance and outbreak management. Detailed information on the data available on COVID-19 cases in the NCIMS is available elsewhere [10]. During the study period cases of COVID-19 were considered as confirmed if they met nationally agreed case definitions, which includes having a positive test to a validated SARS-CoV-2 nucleic acid test [11]. For all cases recorded on the NCIMS database, information collected at initial case interview included the date of illness onset, reported symptoms, test date and result, as well as sociodemographic characteristics and co-morbidities. Information on hospitalisation and death from COVID-19 was entered subsequently, as it became available.
2.2. Follow-up through surveys and record linkage
Between April and July 2020 all people with confirmed COVID-19 reported in the NCIMS were contacted by telephone, on average 28 days after their initial diagnosis with COVID-19. They were asked, “Do you still have any symptoms following your COVID-19 infection?”. If yes, they were asked to report the symptoms experienced and if no, the date they last had symptoms and how long they were sick for. Interviewers were instructed to only collect symptoms that were new following the COVID-19 diagnosis. Those reporting having symptoms were interviewed at approximately 3-weekly intervals until resolution of symptoms. Contact for interview was not attempted if information provided to NCIMS indicated the person had died, or at the time of attempted contact the person was hospitalised or in a residential aged care facility. Telephone contact with eligible cases was attempted at least 5 times over a 2-week period before they were considered as non-responders.
We used methods previously described to link cases to a register of hospitalisations in NSW to determine if they had at least an overnight inpatient admission for COVID-19, to supplement the reporting to NCIMS [12]. Reports of COVID-19 related deaths were obtained from the NCIMS record. Interviews were approved by the NSW Health Department as part of routine public health surveillance and this study was approved by the UNSW Human Research Ethics Committee (HC200483).
2.3. Data Statement
Data used in these analyses are held by the NSW Ministry of Health. Data are only available on request to the Ministry and with appropriate ethical clearances regarding use.
2.4. Analysis
For our primary analysis we included all confirmed COVID-19 cases reported to the NCIMS database with an onset date from 1 January to 29 May 2020, and for which there was a follow-up record through either a completed telephone interview, or a COVID-19 hospitalisation or death record. The outcome of interest was recovery from COVID-19 defined as the absence of self-reported symptoms of COVID-19. Follow-up commenced at the reported date of onset of COVID-19 which was the date of first symptoms or the date of first positive test, whichever came first, and concluded at the first date no symptoms of COVID-19 were reported or the date of last interview (if symptoms continued), or if they had no interview record, the last date of COVID-19 related hospitalisation or date of death. Cases who had no symptoms at the time of their first positive SARS-CoV-2 test were included in analyses, as a few reported having had symptoms at the 28-day interview but for the vast majority of these cases, recovery was considered to occur one day following their positive test.
Survival analysis was used to estimate the relative risks of recovery by age, gender, socioeconomic status, symptoms reported at onset and selected pre-existing co-morbidities. As people who die from COVID-19 cannot recover, a competing-risks regression was used to allow for the competing risks of COVID-19 death [13]. All analyses were routinely adjusted for age and gender.
2.5. Role of the funding sources
The study funders had no role in study design, data analysis, data interpretation or writing of the report. The corresponding author had full access to the data in the study and had final responsibility for the decision to submit for publication.
3. Results
In total there were 3096 confirmed COVID-19 cases diagnosed in NSW during the study period. Of these cases follow-up information was available for 2904 (93.8%) who were followed in statistical analyses for a median of 16 days; range 1 to 122; interquartile [IQR] range 11 to 24. Of those with follow-up data, 2572 (88.6%) had a documented interview record that they had recovered (of which 262 were hospitalised), and 224 (7.8%) had not yet recovered at time of last telephone contact (of which 28 were hospitalised). A further 51 (1.8%) died of COVID-19 and 57 (2.0%) were hospitalised for COVID-19 without a documented interview record of a recovery date. A flow diagram describing the outcome for all cases with follow-up information is shown in Appendix 1. The mean time between onset of COVID-19 and first interview or between subsequent interviews if required, was 29 days (SD 12; median 27 days, IQR 23-33). The average age of the 2904 cases was 47 years (standard deviation [SD] 20 years), 51% were female and 65% had a reported comorbidity. Of cases analysed, 3.9% (113/2904) were asymptomatic at diagnosis of which 94% (106/113) reported having no symptoms at follow up. The characteristics of cases overall and according to whether they were hospitalised or died are shown in Appendix 2.
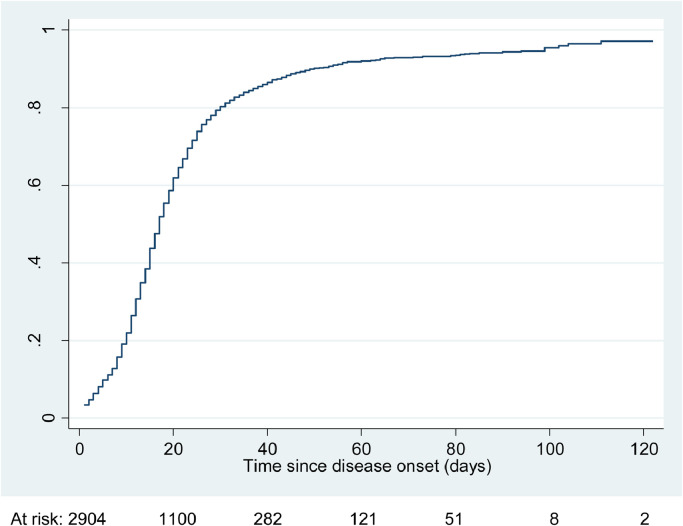
Figure 1 shows the cumulative incidence curve for recovery from COVID-19 among the 2904 cases and Appendix 3 provides the cumulative incidence data in 5-day intervals following disease onset. In the first 10 days after illness onset, only 20% of cases had recovered but by 20 days 60% had recovered; by 30 days, 80% had recovered; by 60 days 91% had recovered; by 90 days 93% had recovered, and by 120 days 96% had recovered. Of those who had not recovered at the time of their last interview, the most commonly reported residual symptoms included cough (46.7%) and fatigue (36.0%).
Figure 1.
Cumulative incidence of recovery from COVID-19 among N=2904 cases
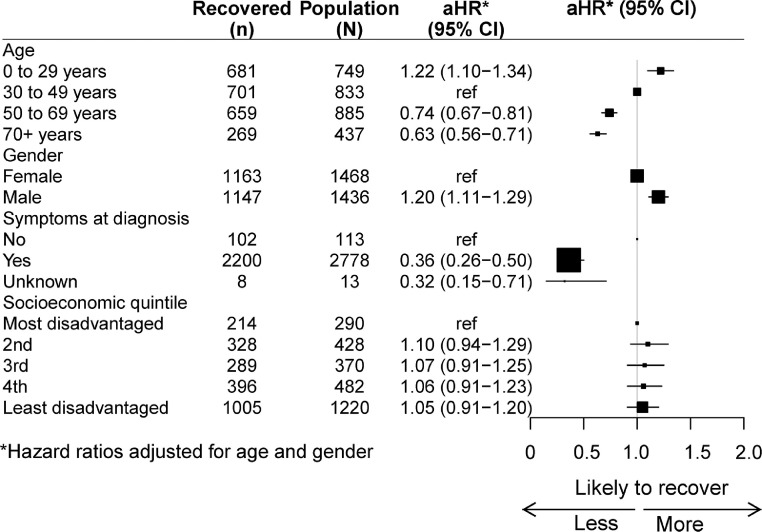
Figure 2 shows the hazard ratios for time to recovery from COVID-19 according to age, gender, presence of symptoms at diagnosis, and level of socioeconomic disadvantage and Appendix 4 shows cumulative incidence curves by age and gender and cumulative incidence tables by age group are in Appendix 5. After adjusting for gender, compared to those aged 30-49 years, those aged 0-29 years were 22% more likely to recover whilst those aged 50-69 years were 26% less likely to recover and those aged 70+ years 37% less likely to recover. After adjusting for age, men were 20% faster to recover than women. Those with symptoms at COVID-19 diagnosis were substantially slower to recover than those who were asymptomatic at diagnosis. There was no discernable difference in likelihood of recovery by socioeconomic disadvantage.
Figure 2.
Relative risk of recovery by age, gender, symptoms at diagnosis and socioeconomic status
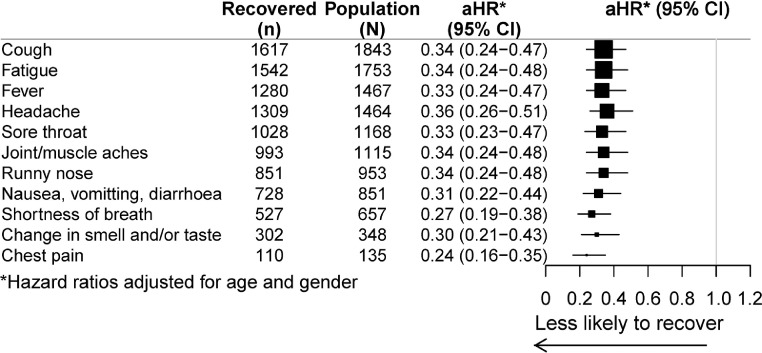
Figure 3 shows the relative risk of recovery according to symptoms reported at COVID-19 diagnosis. Compared to those without any symptoms there was a substantially lower likelihood of recovery if any symptoms were reported but this did not vary with the type of symptoms experienced.
Figure 3.
Relative risk of recovery by symptoms reported at onset (compared to those with no symptoms at all), adjusted for age and gender
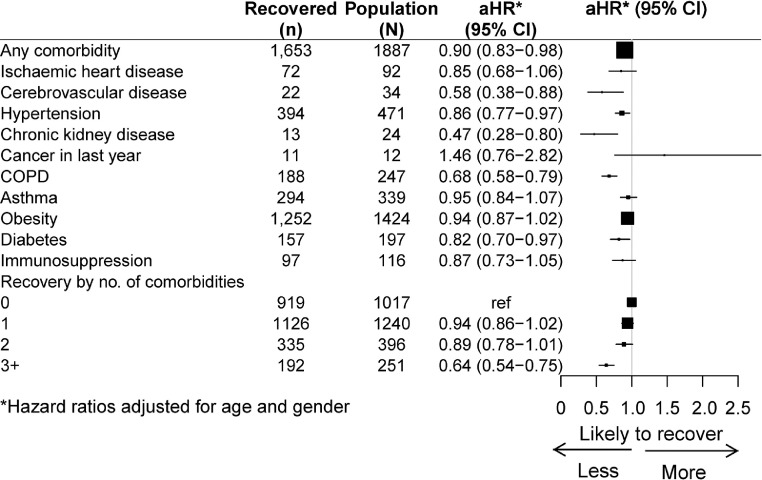
For many comorbidities examined (Figure 4), particularly among people with chronic kidney disease, cerebrovascular disease or chronic obstructive pulmonary disease (COPD), the likelihood of reporting continuing symptoms for those with the condition was lower than in those without. Those with more comorbidities were also less likely to recover than those with fewer.
Figure 4.
Relative risk of recovery by comorbidities compared to those without that comorbidity, adjusted for age and gender
4. Discussion
In this study we aimed to use a systematic and inclusive sampling frame to estimate recovery from COVID-19. In our sample of close to 3000 confirmed COVID-19 cases we found 80% of people had recovered within a month of disease onset, more than 90% recovered by 2 months and 93% by 3 months. We also found that recovery is faster in younger people, in men compared to women, and in those with no comorbidities. The major strength of our study is that we have systematically followed almost the entire population diagnosed with COVID-19 within the Australian state of NSW to ascertain recovery from infection thereby reducing potential for selection bias that may affect many other studies [8]. We had follow-up data on 2904 laboratory confirmed COVID-19 cases, which included almost 95% of all people diagnosed during the study period.
While there have been numerous case reports and case series of people experiencing prolonged recovery from COVID-19 there are few large studies that include unselected population samples documenting recovery, particularly in people who have not been hospitalised. Thus, accurate information on what proportion of COVID-19 cases are expected to experience prolonged symptoms is limited. A US study randomly surveyed adults who were diagnosed through outpatient settings. They found among 274 symptomatic cases (56% response rate) that at an average of 16 days post-laboratory diagnosis, 35% had not recovered [6]. Similar to our analysis, they found a higher proportion of younger cases had recovered and that those with more comorbidities were less likely to have recovered. However, unlike in our report, they did not find differences between men and women. In another sample of 4182 adults from the UK, the US, and Sweden (mean age 43 years; 28.5% male) who volunteered to log their symptoms into a smartphone application and had reported testing positive for SARS-CoV-2, about 13% reported symptoms lasting longer than 28 days, 4.5% for more than 8 weeks and 2.3% for more than 12 weeks [7]. Similar to our report this study also reported a longer duration of symptoms in older adults and in women but also found longer duration of symptoms in those with high body mass index. The UK Office for National Statistics has surveyed over 8000 people who tested positive for COVID-19 and reported that 21% were symptomatic at 5 weeks post-infection and 10% at 12 weeks [14]. As our sample includes all people diagnosed with COVID-19 in NSW, including those who died or were hospitalised with COVID-19 our estimates of the proportion of people recovered over time are unlikely to be directly comparable to these other samples.
Other viral infections such as Epstein-Barr virus are known to cause prolonged illness characterised by fatigue [15] and long-term effects of COVID-19 are not unexpected with studies on people with SARS-CoV-2 infection suggesting evidence of impairment in multiple organs, predominantly the heart and lungs even in those not hospitalised with the disease [16,17]. It has been hypothesised that as the virus attaches to the ACE2 receptor, which is present on the lung as well as many other organs, that inflammatory changes leading to longer-term organ damage may occur and that this may lead to long-standing symptoms from the disease [1]. Other proposed mechanisms for persisting symptoms include that some people infected by SARS-CoV-2 continue to harbour virus or persisting viral fragments which trigger immune responses, or that in some people the longer-term effects from COVID-19 result from an overactive immune response such as observed in other post-viral syndromes [18,19].
Our findings may be explained by the different mechanisms that are thought to underlie prolonged recovery from COVID-19. It is well known that COVID-19 severity, hospitalisations and deaths increase with increasing age and with the prevalence of some comorbidities. Therefore, it is unsurprising that we found recovery was longer and was less likely in these groups. It is less clear why, after adjusting for age, women took longer to recover than men. Women are known to have higher incidence of autoimmune and inflammatory conditions than men, so our findings also support suggestions of an immune mechanism underlying prolonged symptoms. It has been reported that greater socioeconomic disadvantage may increase COVID-19 severity [20] however after adjusting for other factors we did not find significant differences in likelihood of recovery between different socioeconomic groups in our whole of population sample.
During the study period (Jan to May 2020) in NSW, SARS-CoV-2 testing was predominantly recommended for individuals who had symptoms suggestive of COVID-19 and asymptomatic screening was mostly confined to close contacts of known cases. Therefore, while we were able to follow almost all cases with COVID-19 diagnosed in the state it is likely that a proportion of mostly asymptomatic cases were not diagnosed and therefore not included in this analysis [21]. As we found that only a small proportion of people (6%) who were asymptomatic at initial diagnosis reported any symptoms at follow-up it is likely that the impact of these missed cases would be to reduce overall estimated recovery times.
Study limitations include that if at interview people reported no further symptoms, they were not re-interviewed, so it is possible if people experienced a recurrence of symptoms this would not have been captured. Furthermore, symptoms initially reported were not collected using standardised tools so they may not be comparable with other studies. Also, contact protocols meant that people hospitalised or in aged care were less likely to be interviewed. While this was a relatively small proportion of the total sample, this loss to follow-up is likely to be biased towards the older age group, leading to a potential overestimate of likelihood of recovery in this group. Also while interviewers were asked to only record symptoms related to the diagnosis of COVID-19, it is possible that those with comorbidities may have had difficulty differentiating pre-existing complaints from new ones, also creating a bias towards those with more comorbidities being less likely to be classified as recovered. Finally, no functional or diagnostic measures were conducted although this was not the aim of our study and is likely to have enabled the high follow-up rates which was the purpose of this epidemiological report.
In summary, we found that in a large whole-of-population sample of mostly symptomatic people with laboratory confirmed COVID-19, while 80% recover within 30 days of infection, at 3 months from disease onset, 7% had not. Given that 1.8% of people in our sample also died of COVID-19, our data suggests that approximately 5% of people with COVID-19 will continue to experience symptoms at 3 months post-infection. Compared to conditions such as influenza where recovery is expected within 1-2 weeks [22], our data further demonstrates the substantial direct impact of COVID-19 on population health and the need to consider not only the impact of COVID-19 on hospitalisations and deaths but also the longer-term health of those who have milder forms of the disease. With now more than 150 million cases diagnosed worldwide, the findings from this study highlight the importance of preventing infections through both non-pharmaceutical interventions and vaccination to avoid direct effects on longer-term health in the population. They also highlight the need to improve clinical services to support recovery from COVID-19.
Author Contributors
BL, PS, DJ, VP, JK conceived the study and conducted the study. BL, DJ, TD contributed to directing and analysing the data. BL wrote the first draft. All authors reviewed drafts of the paper, interpreted findings and approved the final version.
Data sharing statement
Data used in these analyses are held by the NSW Ministry of Health. Data are only available on request to the Ministry and access requires appropriate ethical and governance clearances regarding use.
Declaration of Competing Interest
The authors have no relevant conflicts of interest to declare.
Acknowledgments
Acknowledgements
We thank the team at NSW Health who conducted the recovery interviews, the NSW Public Health Network who contribute data to NCIMS, and Wenqiang He for drawing the figures.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100193.
Appendix. Supplementary materials
References
- 1.Huang C, Huang L, Wang Y. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 3.Yelin D, Wirtheim E, Vetter P. Long-term consequences of COVID-19: research needs. The Lancet Infectious diseases. 2020;20:1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalili M, Karamouzian M, Nasiri N, Javadi S, Mirzazadeh A, Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiology and infection. 2020;148:e130. doi: 10.1017/S0950268820001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. Jama. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde M, Kim S, Lindsell C. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network — United States. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. March–June 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudre CH, Murray B, Varsavsky T. 2020. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv. [Google Scholar]
- 8.Griffith G, Morris T, Tudball M. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nature Communications. 2020;11:5729. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-19 National Incident Room Surveillance Team. COVID-19 Australia: Epidemiology Report. Comm Dis Intell. 2021;33 45:https://doi.org/10.321/cdi.2021.45.3. [Google Scholar]
- 10.NSW Centre for Health Record Linkage. https://www.cherel.org.au/data-dictionaries#section8.
- 11.Australian Government Department of Health. Coronavirus Disease 2019 (COVID-19) Series of National Guidelines. 2021 https://www1.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-novel-coronavirus.htm [Google Scholar]
- 12.Liu B, Spokes P, Alfaro-Ramirez M, Ward K, Kaldor J. Hospital outcomes after a COVID-19 diagnosis from. New South Wales Australia. Comm Dis Intell. January to May 2020 doi: 10.33321/cdi.2020.44.97. in. 2020;Online first 24/12/2020. [DOI] [PubMed] [Google Scholar]
- 13.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.UK Office for National Statistics. https://www.ons.gov.uk/news/statementsandletters/theprevalenceoflongcovidsymptomsandcovid19complicationshttps://www.ons.gov.uk/news/statementsandletters/theprevalenceoflongcovidsymptomsandcovid19complications.
- 15.White P, Thomas J, Kangro H. Predictions and associations of fatigue syndromes and mood disorders that occur after infectious mononucleosis. Lancet (London, England) 2001;358:1946–1954. doi: 10.1016/S0140-6736(01)06961-6. [DOI] [PubMed] [Google Scholar]
- 16.Dennis A, Wamil M, Kapur S. 2020. Multi-organ impairment in low-risk individuals with long COVID. https://www.medrxivorg/content/101101/2020101420212555v1fullpdf. [Google Scholar]
- 17.Puntmann VO, Carerj ML, Wieters I. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.3557. online July 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.British Society for Immunology . 2020. Long-term immunological health consequences of COVID-19. https://www.immunologyorg/sites/default/files/BSI_Briefing_Note_August_2020_FINALpdf. [Google Scholar]
- 19.Gorna R, MacDermott N, Rayner C. Long COVID guidelines need to reflect lived experience. Lanet. 2021;397:455–457. doi: 10.1016/S0140-6736(20)32705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson EJ, Walker AJ, Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gidding H, Machalek D, Hendry A. Seroprevalence of SARS-CoV-2-specific antibodies in Sydney, Australia following the first epidemic wave in 2020. Med J Aust 2020;Preprint. 2020 doi: 10.5694/mja2.50940. https://www.mja.com.au/journal/2020/seroprevalence-sars-cov-2-specific-antibodies-sydney-australia-following-first 2 November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ (Clinical research ed) 2016;355:i6258. doi: 10.1136/bmj.i6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.