Abstract
Background
Kabasura Kudineer (KSK) is a Siddha polyherbal decoction recommended by the Ministry of AYUSH and the Tamil Nadu government to prevent and manage COVID-19 in India.
Objective(s)
We aimed to determine the outcome of integrated therapy for COVID-19 using KSK in virologic clearance and length of hospital stay.
Materials and methods
It was a single-centre, retrospective cohort study. We included the COVID-19 patients admitted to SRM Medical College Hospital and Research Centre, Chennai, during May–June 2020. KSK was administered along with the standard of care for all the patients. We collected data pertaining to demographic, clinical, and laboratory parameters and presented as frequencies and proportions.
Results
We collected 204 COVID-19 positive patients’ data. The mean (SD) age was 39.5 (13.4) years with a range of 13–79. Majority of the patients were male (n = 157; 77%), 28% (n = 58) had any co-morbidities, and 61% (n = 131) had mild symptoms. Fever (n = 57; 27.9%) and cough (n = 53; 25.9%) were the commonly reported symptoms. Paracetamol (n = 135; 66.7%) and Zincovit (n = 197, 96.6%) were the commonly administered medicines along with KSK. About 74% of asymptomatic (n = 54) and 65% of mild symptomatic (n = 85) patients turned negative for COVID-19 in RT-PCR within 4–7 days. There was a significant difference in the blood parameters (p < 0.05) after the integrated treatment.
Conclusion
The use of KSK with standard care of treatment in COVID-19 treatment had notable results in the duration taken for virologic clearance, thereby reducing the length of hospital stay and improvement in laboratory parameters.
Keywords: Siddha, Kabasura kudineer, COVID-19, AYUSH, Traditional medicine
1. Introduction
The Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) infection has become a global health threat to the community and severely affected livelihoods of many. Over 100 million confirmed cases and 2 million deaths due to coronavirus disease 2019 (COVID-19) have been reported globally since it was identified in December 2019 [1]. However, most people with COVID-19 experience mild and moderate symptoms and approximately 10–15% of cases progress to severe illness [2,3]. Currently, there are no treatments due to the novelty of the virus and its broad clinical spectrum. In the absence of effective therapies, traditional medicines were used as an integrated treatment for managing COVID-19, as happened in the past with other infectious diseases [4].
In India, Ayurveda, Yoga and Naturopathy, Unani, Siddha and Sowa-Rigpa, and Homoeopathy (AYUSH) are the traditional medicines used since ancient times. Siddha system of medicine is primarily practiced in Southern parts of India and Tamil speaking regions of the world. In response to the COVID-19 pandemic, the Ministry of AYUSH, Govt of India issued treatment guidelines for AYUSH practitioners to prevent and manage COVID-19, including the administration of Kabasura Kudineer (KSK), a polyherbal Siddha formulation [5]. The health ministry approved the decision based on the experience of administering polyherbal Siddha formulations in the management of chikungunya and dengue outbreak in the state of Tamil Nadu [6,7].
The clinical features of SARS-CoV-2 infection may be compared with Kabasuram (Iya suram) as per the Siddha literature, which is basically due to elevation of Iyam, one of the three humors of the body. Elevated Iyam, Vali, and Azhal become Thontham (all three humors mixed and elevated), which leads to sanni which is defined as a severe and fatal stage of a disease [8]. KSK has been generally prescribed and administered to manage Iyasuram, characterized with fever and flu like symptoms by Siddha medical practitioners [9]. KSK comprises of 15 herbs [10]. The Ministry of AYUSH, Govt of India has recommended in the advisory guidelines for the prophylaxis and management of COVID-19 based on the clinical experience and evidence of pharmacological and phytochemical activities of its ingredients. However, it showed antipyretic, anti-viral, and immunomodulatory properties in initial phytochemical, pharmacological, and in vitro studies [11].
The National Institute of Siddha (NIS) which is located in Chennai, Tamil Nadu with its excellence in research and higher education in Siddha system of medicine played a pivotal role in COVID-19 response. NIS has involved in COVID-19 response activities in collaboration with medical, academic, and research institutions in Tamil Nadu [12]. The SRM Medical College Hospital and Research Centre, a tertiary care centre in Tamil Nadu, is one such health facility in which KSK was administered along with standard care of treatment for COVID-19 patients under the supervision of Siddha faculty of NIS as a collaborative activity. This polyherbal decoction is used in general practice in the Siddha system. Though it is yet to be demonstrated by controlled clinical trials for its safety and efficacy, we got the opportunity to document the experience of administering KSK along with the routine care at SRM health facility. During the pandemic, KSK was advocated as a preventive measure and the standard care for asymptomatic, mild, and moderate symptomatic COVID-19 patients based on the evidence showing anti-viral properties in the preliminary pharmacological and phytochemical studies [11]. However, clinical documentation is highly needed to know the benefit of integrated treatment. In this context, we aimed to determine the clinical outcome of integrated therapy for COVID-19 using KSK in terms of virologic clearance for COVID-19 in RT-PCR and to estimate the length of hospital stay.
2. Materials and methods
2.1. Study setting
The study site was SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu. This is a tertiary care hospital which serves around 3, 00,000 population in the surrounding areas.
2.2. Study participants
COVID-19 positive patients who were admitted in SRM Medical College Hospital and treated with integrated therapy.
2.3. Inclusion and exclusion criteria
All patients included in this study were diagnosed for COVID-19 based on the World Health Organization (WHO) guidelines. Those who had influenza-like illness and were SARS-CoV-2 RT-PCR positive in throat swab were included. We included consecutive COVID-19 positive patients with asymptomatic and mild symptoms irrespective of age and gender who were admitted during the study period and treated with KSK along with the standard care of treatment.
2.4. Study design
This was a single-centre retrospective cohort study.
2.5. Study period
The COVID-19 patients admitted during May–June 2020 were included in this study.
2.6. Operational definitions
2.6.1. Virologic clearance
The time duration was taken from the first SARS-CoV-2 virus nucleic acid test result positive to the first negative result. Throat swabs of patients were taken to test if the patients were relieved from all the clinical symptoms for consecutive 3 days with normal oxygen saturation.
2.6.2. Length of hospital stay
The time duration was taken from hospital admission to the discharge for a patient.
2.7. Standard care of treatment
All the COVID-19 patients were managed symptomatically with the administration of antipyretics, antibiotics, expectorant, Zinc and Vitamin C, and the regular treatment related to their comorbid conditions such as anti-diabetic and anti-hypertensive drugs.
2.8. Siddha system management with KSK
KSK is a polyherbal formulation comprising of 15 medicinal herbs (Table 1). The medicine was purchased from a Good Manufacturing Practice (GMP) certified company in order to ensure good quality. The decoction was prepared by adding 10 gms of KSK powder with 240 ml of water, boiled and reduced to one-fourth (60 ml), and filtered. The decoction (60 ml) was administered orally thrice daily, 20 min before food for all the patients. Each dose of KSK decoction was administered to all the patients within 3 h of preparation. The pharmacist of NIS prepared the decoction to ensure the quality of medicine. The clinical and paramedical SRM hospital staff ascertained that the medicine was administered to the patients as per the treatment protocol prescribed. First dose of KSK was administered within 24 h of admission and continued thrice daily throughout the hospital stay. The patients stayed in the hospital until they got the virologic clearance by RT-PCR test. Further, they were advised to continue KSK twice daily for atleast a week after discharge.
Table 1.
Composition and ingredients of KSK administered with the standard care of treatment for COVID-19 during May–June 2020 at SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu.
| Name of herb in tamil | Botanical name | Quantitya |
|---|---|---|
| Chukku | Zingiber officinale | 35 g |
| Thippili | Piper longum | 35 g |
| Lavangam | Syzygium aromaticum | 35 g |
| Sirukaanjuriver | Tragia involucrata | 35 g |
| Agragaram | Anacyclus pyrethrum | 35 g |
| Karimulli | Solanum erianthum | 35 g |
| Kadukkaithol | Terminalia chebula | 35 g |
| Adathodaiilai | Justicia adhatoda | 35 g |
| Karpooravalliilai | Coleus amboinicus | 35 g |
| Koshtam | Sassure alappa | 35 g |
| Seenthil | Tinospora cordifolia | 35 g |
| Nilavembu | Andrographis paniculata | 35 g |
| Vattathiruppi | Cissampelos pareira | 35 g |
| Koraikizhangu | Cyperus rotundus | 35 g |
| Siruthekku | Clerodendrum serratum | 35 g |
Each dose is composed of a mixture of fifteen medicinal herbs in equal quantity.
2.9. Data collection methods
We used data abstraction forms to retrieve information from the medical records. The information collected included demographic details such as age, gender, hospitalization details such as date of admission and discharge, medical history for comorbid conditions, vital parameters, all clinical signs and symptoms including other than for COVID-19. Data pertaining to the laboratory parameters of blood test, RT-PCR findings (initial date of positive and the date when the patient became negative for COVID-19 in RT-PCR), medical interventions such as KSK administration, and the list of drugs administered under standard treatment of care were also collected. We included all the patients admitted and treated in the study period, irrespective of the date of laboratory confirmation and the date of admission to the hospital. Hence, there may be a time lag between the date of first RT-PCR test on which they were found positive and the date of admission in the hospital for some patients. The decoction of KSK was administered thrice daily to all the asymptomatic and patients with mild symptoms. KSK was administered irrespective of the presence of any co-morbid conditions. Frequency and dose of administration of KSK were also abstracted from the medical records. Throat swabs of patients were taken for testing if the patients were relieved from all the clinical symptoms for continuous 3 days with normal oxygen saturation.
2.10. Statistical analysis
The collected data were entered in Microsoft Excel and cleaned before analysis. Continuous variables were described as means and SD, or as median and range and the categorical variables were expressed as frequency and proportions. Paired t-test was done to find out the significant difference in any paired variables. All statistical analyses were performed using SPSS version 16.0.
2.11. Ethics approval
This study was approved by the Institutional Ethics Committee of SRM Medical College Hospital and Research Centre, Chennai (EC No. 1968/IEC/2020) to access and analyse the hospital records. Since we reviewed only the hospital records, obtaining informed consent from the participants was waived by the Institutional Ethics Committee. We did not use any personal identifiers during the analysis nor revealed the same anywhere in this report.
3. Results
3.1. Baseline profile of the study participants
We collected 204 confirmed COVID-19 patients’ data. The mean (SD) age was 39.5 (13.4) years with a range of 13–79. Majority of the patients were male (n = 157; 77%), and 28% (n = 58) had co-morbid conditions. Among the co-morbidities, diabetes (n = 35; 17.2%) and hypertension (n = 26; 12.7%) were predominantly reported. The mean value of systolic and diastolic blood pressure was 119.1 (11.2%) mm Hg and 77.9 (7.9%) mm Hg, respectively on the day of admission. The median hospital stay was 9 days (IQR 10-7) (Table 2).
Table 2.
Demographic and clinical characteristics of patients with COVID-19 treated during May–June 2020 (n = 204) in SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu.
| Characteristics | Values | % |
|---|---|---|
| Age (years), mean ± SD | 39.53 ± 13.4 | |
| Gender | ||
| Male | 157 | 76.9 |
| Female | 47 | 23.0 |
| Blood pressure | ||
| Systolic (mean ± SD) | 119.1 ± 11.2 | |
| Diastolic (mean ± SD) | 77.9 ± 7.9 | |
| Hospital stay, [median (IQR)] | 9 (7–10) | |
| Symptoms status | ||
| Asymptomatic | 73 | 36 |
| Mild symptomatic | 131 | 64 |
| Presence of comorbidity | 58 | 28.4 |
| Co-morbid conditions | ||
| Diabetes | 35 | 17.2 |
| Hypertension | 26 | 12.7 |
| Hypothyroidism | 5 | 2.4 |
| Renal disease | 2 | 1.0 |
| Tuberculosis | 1 | 0.5 |
| Acid peptic disease | 1 | 0.5 |
| Septic arthritis | 1 | 0.5 |
| Microcytic anaemia | 1 | 0.5 |
| Dyslipidaemia | 1 | 0.5 |
| Coronary artery disease | 1 | 0.5 |
| Bronchial asthma | 1 | 0.5 |
| Adverse drug events | ||
| Diarrhoea | 4 | 2.0 |
| Gastritis/Gastroenteritis | 3 | 1.5 |
3.2. COVID-19 related status of the included study participants
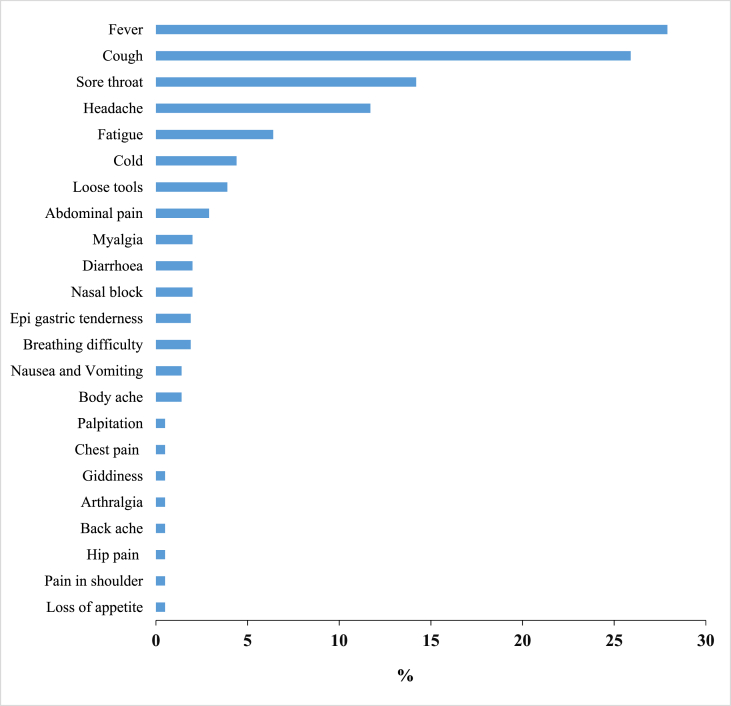
Among the total, 131 (64%) were with mild symptoms of COVID-19. The most common clinical symptoms reported by the patients were fever 57 (27.9%), cough 53 (25.9%), sore throat 29 (14.2%), headache 24 (11.7%), and fatigue 13 (6.4%) during the hospital stay (Fig. 1).
Fig. 1.
Distribution of clinical signs and symptoms among COVID-19 patients admitted during May–June 2020 (n = 204) in SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu. ∗One patient may have more than one clinical symptom.
3.3. Clinical outcomes among the study participants
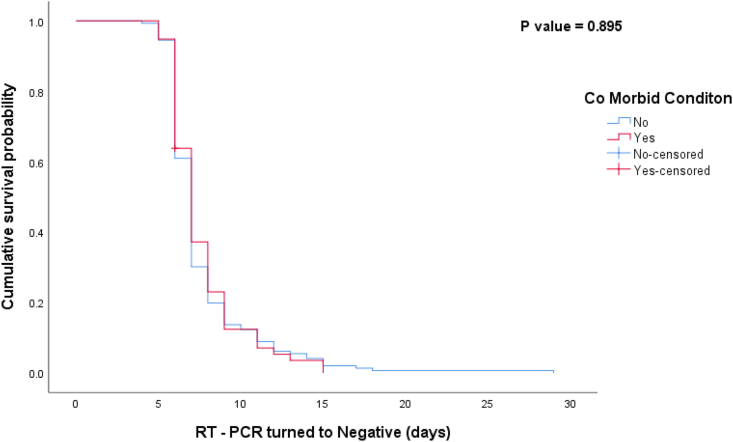
Most of the asymptomatic (n = 54; 74%) patients turned negative for COVID-19 in RT-PCR within 4–7 days and 25% (n = 18) in 8–15 days after integrated treatment with KSK. On the other hand, 65% (n = 85) and 34% (n = 44) mild asymptomatic patients turned negative in 4–7 days and 8–15 days respectively. Nearly two-thirds of hypertension patients (n = 17; 65.4%) turned negative in 4–7 days, whereas only half of the diabetes patients (n = 20; 57.1%) turned negative in 4–7 days (Table 3). Kaplan–Meier curve (Fig. 2) shows the median number of days taken for RT-PCR negative from the date of admission based on the presence and absence of comorbid condition is 7 days (CI 6.783–7.217). The log rank test showed the two curves were not significantly different (P = 0.895). However, there was a significant difference among patients in the counts of total lymphocytes, eosinophils, and basophils (p < 0.05) (Table 4) counts before and after integrated treatment. Among the total, one patient who had presented with comorbid conditions died.
Table 3.
Frequency of patients turned negative for COVID-19 and the duration of integrated treatment using KSK during May–June 2020 (n = 204) in SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu.
| Symptomatic status | Duration of integrated treatment | Percentage of patients turned negative for RT-PCR |
|---|---|---|
| Asymptomatic | 4–7 days | 54 (74.0%) |
| 8–15 days | 18 (24.7%) | |
| >15 days | 1 (1.9%) | |
| N = 73 (100%) | ||
| Mild symptomatic | 4–7 days | 85 (64.9%) |
| 8–15 days | 44 (33.6%) | |
| >15 days | 2 (1.5%) | |
| N = 131 (100%) | ||
| Co-morbidities | ||
| Diabetes Mellitus (Type 2) | 4–7 days | 20 (57.1%) |
| 8–15 days | 16 (42.9%) | |
| Hypertension | 4–7 days | 17 (65.4%) |
| 8–15 days | 9 (34.6%) | |
Fig. 2.
Kaplan–Meier for time taken for RT-PCR test negative based on the presence of any comorbid condition during May–June 2020 in SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu.
Table 4.
Difference in the blood parameters before and after the integrated treatment during May–June 2020 (n = 204) in SRM Medical College Hospital and Research Centre, Chennai Tamil Nadu.
| Blood parameters | T | Df | Sig. (2-tailed) |
|---|---|---|---|
| Neutrophil | −1.356 | 13 | 0.198 |
| Lymphocyte | −5.479 | 10 | 0.000 |
| Eosinophil | −4.870 | 21 | 0.000 |
| Basophil | −3.138 | 49 | 0.003 |
| Monocyte | −0.692 | 1 | 0.614 |
3.4. Adverse drug reactions
No significant adverse reactions were recorded except diarrhoea (n = 4; 2%) and gastritis (n = 3; 1.5%) during the hospital stay (Table 2).
3.5. Drug profile
Antipyretics such as Paracetamol (n = 136; 66.7%), antibiotics such as Azithromycin (n = 76; 37.3%), expectorants such as syrup Ascoril (n = 36; 17.6%) and supplementary of Zincovit (n = 197; 96.6%) were the conventional drugs used during the integrated treatment (Table 5). None was withdrawn from the integrated treatment due to treatment-related discomfort or any adverse reactions.
Table 5.
Frequency of drugs used in the standard care to treat COVID-19 during May–June 2020 (n = 204) in SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu.
| Nature of the drug | Medicine name | Number | % |
|---|---|---|---|
| Antipyretic | Paracetamol | 136 | 66.7 |
| Antibiotics | Azithromycin | 76 | 37.3 |
| Cefoperazone | 1 | 0.5 | |
| Ofloxacin | 1 | 0.5 | |
| Inj. Cefoperazone sulbactam | 1 | 0.5 | |
| Vancomycin | 1 | 0.5 | |
| Ciprofloxacin | 1 | 0.5 | |
| Supplementary | Zincovit | 197 | 96.6 |
| Limcee | 132 | 64.7 | |
| Vit-c | 85 | 41.7 | |
| Becosules | 56 | 27.5 | |
| Bifilac | 11 | 5.4 | |
| Calcium | 1 | 0.5 | |
| Iron | 1 | 0.5 | |
| Expectorants and antihistamines | Ascoril syrup | 36 | 17.6 |
| Lecope | 5 | 2.5 | |
| Cetirizine/levocetirizine | 7 | 3.4 |
4. Discussion
In 2020, the WHO declared the ongoing COVID-19 outbreak as a Public Health Emergency of International Concern [13]. There are no specific anti-viral drugs that are effective against COVID-19 at present [14]. COVID-19 caused by SARS-CoV-2 has spread rapidly, with the characteristics of high infectivity and high susceptibility [15]. In the early phases of COVID-19 pandemic, management using Siddha herbal formulations and the routine care of treatment was tried in the South Indian setting. The NIS, an academic and research institution, had an opportunity to collaborate with the existing state government health facilities and other academic and research institutions. It paved the way to administer selected polyherbal formulations as per the treatment guidelines proposed by the Ministry of AYUSH, Govt of India and observe the outcomes. Hence, we were able to document the clinical experience in the management of COVID-19 using KSK combined with the routine care of treatment.
COVID-19 patients often carry SARS-CoV-2 for a longer time; hence, prolonged anti-viral treatment is needed to become negative in virology test. This duration is a concern in the prognosis of disease, especially among the elderly and patients with severe symptoms. Patients with chronic SARS-CoV-2 infection may develop severe lung infections and acute respiratory distress syndrome, leading to hospitalization. The COVID-19 pandemic has put unprecedented pressure on the health care system. Hence, it is crucial to shorten the duration of hospital stay of the patients. As per the revised discharge policy for COVID-19 issued by the Ministry of Health and Family Welfare (MoHFW), Govt of India, May 2020, the length of hospital stay in standard allopathy care of mild cases was 10 days [16]. In the current observation, around two-thirds of the mild symptomatic patients were relieved from symptoms, became negative for RT-PCR test, and discharged from the hospital in 10 days, which is as mentioned in the discharge policy issued by the MoHFW. The course of integrated treatment with KSK ranged from 4 to 18 days irrespective of the symptom status, which is shorter than the studies reported earlier from China (4–53 days) and from other countries (4–21 days) outside China [17].
The integrated treatment shortens the time taken to become negative for COVID-19 thereby reducing the length of hospital stay. With these observations, it may be postulated that if KSK is administered with the routine treatment, it will benefit the effective management of COVID-19. People with known diabetes and hypertension and older age groups are at a greater risk of contracting infection, worse prognosis, and mortality with respect to COVID-19 infection [18,19]. On the contrary, the time taken for virologic clearance was 5–15 days for both the groups who had either diabetes or hypertension in the current observation.
Lymphopenia is reported in severe cases and the patients with poor prognosis [20,21]. During the current integrated therapy with KSK, the identified patients with low count of lymphocytes, eosinophils, and basophils at the time of admission showed significant improvement at discharge. It is notable to mention that many of the phytoconstituents of KSK formulation are studied for their immunomodulatory potential and activities [22,23]. The ingredients in the selected Siddha formulation KSK, has antipyretic, anti-inflammatory, and immunomodulatory activities, which can be appropriately suitable for the management of COVID-19 [11,[22], [23], [24], [25], [26], [27], [28], [29], [30]]. Research studies are conducted to analyse phytochemicals from KSK against the SARS-CoV-2 through structure-based in silico molecular docking and identified potent anti-COVID-19 natural compounds [31,32]. Furthermore, clinical trials are also implemented to determine the efficacy of KSK in comparison with other standard treatment protocol [33].
5. Limitations
This study has few limitations: (i) No randomization involved, thereby leading to selection bias. However, we have included all the patients admitted during the study period. (ii) There was no comparison group in this study due to ethical consideration in treating the novel infection in the absence of standard treatment or cure. It resulted in limiting the scope of establishing analytical evidence of the integrated treatment.
6. Conclusion
In conclusion, the integrated management of COVD-19 with KSK and standard care of treatment has shown notable results in virologic clearance, thereby reducing hospital stay length than that mentioned in the discharge policy issued by health authorities. Moreover, there were no significant adverse reactions concerning the administration of KSK.
Recommendations
We recommend conducting studies with a comparator group to determine the significant difference in the outcome and randomized controlled trials to evaluate the safety and efficacy of KSK.
Author contributions
Ramaswamy Meenakumari: Conception, design of study. Karuppiah Thangaraj: Conception, design of study. Arunachalam Sundaram: Conception, design of study. Malayappan Meenakshi Sundaram: Conception, design of study. Ponnappan Shanmugapriya: Data collection, Data management, Supervision. Andi Mariappan: Methodology, Data Collection. Melvin George: Data Collection, Data management. Venkatesan Suba: Drafting the manuscript, Analysis of data. Elumalai Rajalakshmi: Drafting the manuscript, Analysis of data, Reviewing and Editing. Muthappan Sendhilkumar: Drafting the manuscript, Analysis of data.
Source of funding
None.
Conflict of interest
None.
Acknowledgements
Our sincere thanks to the ministry of AYUSH for supporting clinical research projects of Siddha system of medicine for COVID-19. We thank the frontline medical staff of SRM Medical College and Research Institute for their involvement and contribution in the study.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.World Health Organization WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/ Available from:
- 2.World Health Organization What we know about Long-term effects of COVID-19. https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update-36-long-term-symptoms.pdf?sfvrsn=5d3789a6_2 Available from:
- 3.Alschuler L., Chiasson A.M., Horwitz R., Sternberg E., Crocker R., Weil A., et al. 2020 Dec 23. Integrative medicine considerations for convalescence from mild-to-moderate COVID-19 disease. Explore (NY) S1550-8307(20)30417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M.S., Song E. Integrative medicine for COVID-19: researches and evidence. Integr Med Res. 2020;9(3) doi: 10.1016/j.imr.2020.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of AYUSH. https://www.ayush.gov.in/ayush-guidelines.html Available from:
- 6.Government of Tamil Nadu Directorate of public health & preventive medicine. https://www.tnhealth.org/dphfacts/chikungunya.htm Available from:
- 7.Government of Tamil Nadu Health & family Welfare department. Available from: https://tnhealth.tn.gov.in/tngovin/dph/dphdbdengue.php.
- 8.Thiyagarajan R. 2nd ed. Palani Thandayuthapani Devasthanam publications, Directorate of Indian systems of Medicine; Chennai, Tamil Nadu: 1976. Yugimunivar vaidhyachinthamani (perunool 800, Tamil) [Google Scholar]
- 9.Kuppusamy Mudaliar K.N. Govt. Of Tamil Nadu; 1987. Siddha maruthuvam (Tamil). Chennai: the directorate of Indian medicine and Homoeopathy. [Google Scholar]
- 10.Kuppusamy Mudaliar K.N., Uthamarayan K.S. Govt. Of Tamil Nadu; 2009. Siddha vaidhya thirattu Chennai: the directorate of Indian medicine and Homoeopathy; pp. 293–294. [Google Scholar]
- 11.Government of Tamil Nadu The Tamil Nadu Dr. M.G.R. Medical university. http://repository-tnmgrmu.ac.in/5357/ Available from:
- 12.National Institute of Siddha https://nischennai.org/index.html Available from:
- 13.World Health Organization . 28 January 2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance.https://apps.who.int/iris/handle/10665/330893 Available from: [Google Scholar]
- 14.World Health Organization Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov Available from:
- 15.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: a review of the 2019 novel corona virus (COVID-19) Int J Surg. 2020 Apr 1;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health and Family welfare Revised discharge policy for COVID-19. https://www.mohfw.gov.in/pdf/ReviseddischargePolicyforCOVID19.pdf Available from:
- 17.Rees E.M., Nightingale E.S., Jafari Y., Waterlow N.R., Clifford S., Pearson C.A., et al. CMMID Working Group COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020 Dec;18(1):1–22. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Molinero A., Gálvez-Barrón C., Miñarro A., Macho O., López G.F., Robles M.T., et al. Association between COVID-19 prognosis and disease presentation, comorbidities and chronic treatment of hospitalized patients. PloS One. 2020 Oct 15;15(10) doi: 10.1371/journal.pone.0239571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluge H. World Health Organization for Europe; 2020. Older people are at highest risk from COVID-19, but all must act to prevent community spread. [Google Scholar]
- 20.Fathi N., Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol Int. 2020 Sep;44(9):1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020 Dec;8 doi: 10.1186/s40560-020-00453-4. 1-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dwivedi S.K., Enespa A. Tinospora cordifolia with reference to biological and microbial properties. Int J Curr Microbiol Appl Sci. 2016;5(6):446–465. [Google Scholar]
- 23.Hossain M.D., Urbi Z., Sule A., Rahman K.M. Andrographis paniculata (Burm. f.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci World J. 2014;2014:1. doi: 10.1155/2014/274905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soltan M.M., Zaki A.K. Antiviral screening of forty-two Egyptian medicinal plants. J Ethnopharmacol. 2009 Oct 29;126(1):102–107. doi: 10.1016/j.jep.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Pawar V.A., Pawar P.R. Costus speciosus: an important medicinal plant. Int J Sci Res. 2014 Jul;3(7):28–33. [Google Scholar]
- 26.Gurgel A.P., da Silva J.G., Grangeiro A.R., Oliveira D.C., Lima C.M., da Silva A.C., et al. In vivo study of the anti-inflammatory and antitumor activities of leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae) J Ethnopharmacol. 2009 Sep 7;125(2):361–363. doi: 10.1016/j.jep.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Batiha G.E., Alkazmi L.M., Wasef L.G., Beshbishy A.M., Nadwa E.H., Rashwan E.K. Syzygium aromaticum L.(Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020 Feb;10(2) doi: 10.3390/biom10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manouze H., Bouchatta O., Gadhi A.C., Bennis M., Sokar Z., Ba-M’hamed S. Anti-inflammatory, antinociceptive, and antioxidant activities of methanol and aqueous extracts of Anacyclus pyrethrum roots. Front Pharmacol. 2017 Sep 5;8:598. doi: 10.3389/fphar.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesharwani A., Polachira S.K., Nair R., Agarwal A., Mishra N.N., Gupta S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Compl Alternative Med. 2017 Feb 14;17(1):110. doi: 10.1186/s12906-017-1620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sood R., Raut R., Tyagi P., Pareek P.K., Barman T.K., Singhal S., et al. Cissampelos pareira Linn: natural source of potent antiviral activity against all four dengue virus serotypes. PLoS Neglected Trop Dis. 2015 Dec 28;9(12) doi: 10.1371/journal.pntd.0004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent S., Arokiyaraj S., Saravanan M., Dhanraj M. Molecular docking studies on the anti-viral effects of compounds from Kabasura Kudineer on SARS-CoV-2 3CLpro. Front Mol Biosci. 2020;7:434. doi: 10.3389/fmolb.2020.613401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiran G., Karthik L., Devi M.S., Sathiyarajeswaran P., Kanakavalli K., Kumar K.M., et al. In silico computational screening of Kabasura Kudineer-official Siddha formulation and JACOM against SARS-CoV-2 spike protein. J Ayurveda Integr Med. 2020;S0975-9476(20)30024-3. doi: 10.1016/j.jaim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natarajan S., Anbarasi C., Sathiyarajeswaran P., Manickam P., Geetha S., Kathiravan R., et al. The efficacy of Siddha Medicine, Kabasura Kudineer (KSK) compared to Vitamin C & Zinc (CZ) supplementation in the management of asymptomatic COVID-19 cases: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020 Dec;21(1):1–2. doi: 10.1186/s13063-020-04823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]