Abstract
The evidence for the effects of environmental factors on COVID-19 case fatality remains controversial, and it is crucial to understand the role of preventable environmental factors in driving COVID-19 fatality. We thus conducted a nationwide cohort study to estimate the effects of environmental factors (temperature, particulate matter [PM2.5, PM10], sulfur dioxide [SO2], nitrogen dioxide [NO2], and ozone [O3]) on COVID-19 case fatality. A total of 71,808 confirmed COVID-19 cases were identified and followed up for their vital status through April 25, 2020. Exposures to ambient air pollution and temperature were estimated by linking the city- and county-level monitoring data to the residential community of each participant. For each participant, two windows were defined: the period from symptom onset to diagnosis (exposure window I) and the period from diagnosis date to date of death/recovery or end of the study period (exposure window II). Cox proportional hazards models were used to estimate the associations between these environmental factors and COVID-19 case fatality. COVID-19 case fatality increased in association with environmental factors for the two exposure windows. For example, each 10 μg/m3 increase in PM2.5, PM10, O3, and NO2 in window I was associated with a hazard ratio of 1.11 (95% CI 1.09, 1.13), 1.10 (95% CI 1.08, 1.13), 1.09 (95 CI 1.03, 1.14), and 1.27 (95% CI 1.19, 1.35) for COVID-19 fatality, respectively. A significant effect was also observed for low temperature, with a hazard ratio of 1.03 (95% CI 1.01, 1.04) for COVID-19 case fatality per 1°C decrease. Subgroup analysis indicated that these effects were stronger in the elderly, as well as in those with mild symptoms and living in Wuhan or Hubei. Overall, the sensitivity analyses also yielded consistent estimates. Short-term exposure to ambient air pollution and low temperature during the illness would play a nonnegligible part in causing case fatality due to COVID-19. Reduced exposures to high concentrations of PM2.5, PM10, O3, SO2, and NO2 and low temperature would help improve the prognosis and reduce public health burden.
Keywords: temperature, air pollution, COVID-19, fatality, cohort study
Graphical abstract
Public summary
-
•
A national retrospective cohort study was performed to quantify the effects of environmental exposure
-
•
Low temperature was observed to be associated with COVID-19 case fatality
-
•
Exposure to ambient air pollution played a nonnegligible part in COVID-19 case fatality
-
•
COVID-19 patients were more susceptible to external environmental stimulus as COVID-19 progressed from the period of symptom onset to diagnosis
Introduction
At the end of 2019, an outbreak of novel coronavirus (SARS-CoV-2) swept across the globe.1, 2, 3 Although various control measures have been implemented, such as stay-at-home orders, quarantine, social distancing and shielding, the global pandemic continues, and the numbers of cases and deaths still increase. It is therefore critical to identify key modifiable risk factors that affect the case fatality of COVID-19.4, 5, 6, 7
Environmental factors, especially air pollution and temperature, have been widely identified to be associated with increasing risk of adverse health outcomes, such as for ischemic heart disease, stroke, diabetes, chronic obstructive pulmonary disease, and respiratory infection.8, 9, 10, 11 Emerging evidence indicates potential links between exposure to polluted air and COVID-19 mortality and fatality.12,13 Recent epidemiological studies indicated that higher concentrations of pollutants were associated with worse outcome of COVID-19. For example, a multicity study from China illustrated that long-term exposure to higher levels of particulate matter was associated with increased COVID-19 case fatality.14 Wu et al. performed an ecological regression analysis and observed positive effects of higher historical PM2.5 exposures on county-level COVID-19 mortality rates in the United States.15 A hierarchical spatial analysis in England reported adverse effects of NO2 on COVID-19 mortality, but this was not found for PM2.5.16 In addition, several ecological studies have elucidated detrimental effects of NO2, with increasing COVID-19 fatality in North America, Europe, and some developed Asian cities.13,15,17 However, a systematic review of environment and COVID-19 noted some common limitations, such as ecological fallacy due to the use of an aggregated dataset without detailed personal information and adjustment for the individual-level covariates, and these limitations raise concerns about biased or spurious associations.18
Toxicological evidence suggests that short-term or long-term environmental exposures could cause pulmonary inflammation and oxide stress, altering host immune response to viral infections.19, 20, 21 It is thus reasonable to hypothesize that exposure to ambient air pollutants and unfavorable temperature could exacerbate the condition of COVID-19 and increase its case fatality. This study used a national retrospective cohort design to quantify the effects of short-term exposure to ambient low temperature and air pollution (PM2.5, PM10, SO2, NO2, and O3) on COVID-19 case fatality in China.
Results
Of the 657 cities and 2,862 counties/districts in mainland China, 321 cities and 1,676 counties/districts were affected by COVID-19 infection, accounting for about half of the geographic regions in China. A total of 71,808 confirmed COVID-19 cases were included in this nationwide retrospective cohort study. The distribution of the fatality rates across the 321 cities is illustrated in Figure S1. The average age of study participants was 51.43 years, ranging from 1 to 103 years; 49.60% of the participant were females, and 58.29% of participants were home-related workers. Among the included cases, 3,934 died of COVID-19 during the follow-up (through April 25, 2020), resulting in an overall case fatality rate of 5.48%. There was no loss to follow-up, and the average duration of the follow-up was 81.75 days (SD = 18.01).
Table 1 presents summary statistics for environmental factors using the two exposure windows and individual-level characteristics by survival status. Compared with those who survived, deceased individuals tended to be older (16.31% for age ≥65 years versus 0.20% for age <25 years, p < 0.001), male (6.95% versus 3.98%, p < 0.001), homemakers (7.70% versus 0.80%, p < 0.001), and migrants (6.85% versus 4.90%, p < 0.001). In critical group that was initially classified, almost half of COVID-19 deaths were identified (45.27%). For exposure window I, there were significant differences in weather conditions between the two groups, with relatively lower temperatures (5.26°C ± 2.82°C) and higher relative humidity (81.43% ± 9.41%) in the deceased group. In addition, there were significant differences in ambient air pollution for the deceased and survived groups, with more highly polluted air for the deceased group compared with the survivor group (54.01 versus 51.87 μg/m3 for PM2.5, 62.32 versus 60.81 μg/m3 for PM10, and 22.78 versus 20.34 μg/m3 for NO2). Significant disparities of environmental factors estimated using window II were also identified. To address concerns about the imbalance of sample size for deceased and survivor groups, we conducted a random resampling of the survivor group to create a subgroup with the same sample size as the deceased group (n = 3,934) and the findings of this resampling analysis were consistent with the full sample results (Table S2). Moreover, we drew maps showing the distribution of air pollution and weather during the relevant period (Figures S2–S7). Table S3 summarizes the Spearman correlation coefficients among key study variables. There are moderate- or low-correlation coefficients between air pollution and meteorological factors (0.01 ≤ |r| ≤ 0.59, except for the high correlation coefficient between PM2.5 and PM10, r = 0.96).
Table 1.
Characteristics of study participants by survival status in China
| Variable | Deceased (n = 3,934) | Alive (n = 67,874) | p valueb |
|---|---|---|---|
| Environmental factors | |||
| Exposure window Ia | |||
| Temperature (°C) | 5.26 ± 2.82 | 5.73 ± 4.11 | 0.0029 |
| Relative humidity (%) | 81.43 ± 9.41 | 79.18 ± 11.36 | <0.0001 |
| PM2.5 (μg/m3) | 54.01 ± 14.92 | 51.87 ± 20.25 | <0.0001 |
| PM10 (μg/m3) | 62.32 ± 16.33 | 60.81 ± 22.38 | <0.0001 |
| O3 (μg/m3) | 54.71 ± 7.73 | 55.84 ± 8.45 | <0.0001 |
| SO2 (μg/m3) | 7.43 ± 2.01 | 7.75 ± 3.49 | <0.0001 |
| NO2 (μg/m3) | 22.78 ± 5.81 | 20.34 ± 6.38 | <0.0001 |
| Exposure window IIa | |||
| Temperature (°C) | 7.27 ± 3.04 | 16.27 ± 2.06 | <0.0001 |
| Relative humidity (%) | 79.47 ± 6.30 | 66.44 ± 10.45 | <0.0001 |
| PM2.5 (μg/m3) | 44.71 ± 15.62 | 29.62 ± 7.23 | <0.0001 |
| PM10 (μg/m3) | 53.95 ± 16.76 | 55.57 ± 11.31 | <0.0001 |
| O3 (μg/m3) | 55. 13 ± 9.31 | 80.33 ± 9.82 | <0.0001 |
| SO2 (μg/m3) | 7.41 ± 1.80 | 9.46 ± 2.63 | <0.0001 |
| NO2 (μg/m3) | 20 .94 ± 6.17 | 28.98 ± 7.02 | <0.0001 |
| Age group (n, %) | <0.0001 | ||
| <25 years | 6 (0.15) | 3,493 (5.15) | |
| 25–64 years | 1,146 (29.13) | 50,111 (73.83) | |
| ≥65 years | 2,782 (70.72) | 14,270 (21.02) | |
| Sex (n, %) | <0.0001 | ||
| Male | 2,515 (63.93) | 33,659 (49.59) | |
| Female | 1,419 (36.07) | 34,215 (50.41) | |
| Days between symptom onset and diagnosis (n, %) | <0.0001 | ||
| <7 days | 1,362 (4.34) | 29,995 (95.66) | |
| 7–14 days | 1,557 (5.89) | 24,859 (94.11) | |
| ≥14 days | 1,015 (7.23) | 13,020 (92.77) | |
| Occupation (n, %) | <0.0001 | ||
| Medical-related | 21 (0.53) | 2,614 (3.85) | |
| Service-related | 15 (0.38) | 1,709 (2.52) | |
| Office worker | 226 (5.74) | 15,435 (22.74) | |
| Homemaker | 3,226 (82.00) | 38,632 (56.92) | |
| Others | 446 (11.34) | 9,484 (13.97) | |
| Residence (n, %) | <0.0001 | ||
| Permanent | 2,476 (62.94) | 48,055 (70.80) | |
| Temporary | 1,458 (37.06) | 19,819 (29.20) | |
| Severity (n, %) | <0.0001 | ||
| Mild | 806 (20.49) | 28,088 (41.38) | |
| Moderate | 597 (15.18) | 28,199 (41.55) | |
| Severe | 1,285 (32.66) | 10,081 (14.85) | |
| Critical | 1,246 (31.67) | 1,506 (2.22) | |
| Hospital transfer (n, %) | 0.1754 | ||
| No | 2,927 (74.40) | 49,825 (73.41) | |
| Yes | 1,007 (25.60) | 18,049 (26.59) | |
Note: PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm; SO2, sulfur dioxide; NO2, nitrogen dioxide; O3, ozone.
Exposure window I represents the mean exposure value from the date of symptom onset to the date of diagnosis; exposure window II represents the mean exposure value from the date of diagnosis to the date of death or the end of the study.
p values were calculated by t test, χ2 test or Fisher's exact test, as appropriate.
Table 2 shows the effect estimates of environmental factors on COVID-19 fatality for two exposure windows (window I and window II). Generally, it was observed that adverse environmental factors were associated with higher fatality rates for COVID-19 in crude and adjusted models. Specifically, the results of the crude model were relatively consistent with those from the adjusted model. We observed significant effects of PM2.5, PM10, and NO2, which were stronger in window II. In the adjusted model for exposure window I, each 1°C decrease in temperature and each 10 μg/m3 increase in air pollution (PM2.5, PM10, and NO2) concentrations was associated with an HR of 1.03 (95% CI 1.01, 1.04), 1.11 (95% CI 1.09, 1.13), 1.10 (95% CI 1.08, 1.13), and 1.27 (95% CI 1.19, 1.35), respectively, for fatality due to COVID-19.
Table 2.
Hazard ratios and 95% CIs for case fatalities due to COVID-19 associated with environmental factors of two exposure windows in China
| Variable | Hazard ratio (95% CI) |
p valueb | |||
|---|---|---|---|---|---|
| Exposure window Ia |
Exposure window IIa |
||||
| Crudec | Adjustedd | Crudec | Adjustedd | ||
| Temperature | 1.02 (1.01, 1.03) | 1.03 (1.01, 1.04) | 1.27 (1.26, 1.28) | 3.20 (3.15, 3.25) | <0.01 |
| PM2.5 | 1.05 (1.04, 1.07) | 1.11 (1.09, 1.13) | 1.56 (1,54, 1.58) | 1.39 (1.36, 1.42) | <0.01 |
| PM10 | 1.04 (1.02, 1.05) | 1.10 (1.08, 1.13) | 1.43 (1.42, 1.44) | 1.18 (1.16, 1.21) | <0.01 |
| NO2 | 1.69 (1.93, 1.75) | 1.27 (1.19, 1.35) | 1.22 (1.14, 1.30) | 1.37 (1.29, 1.45) | 0.08 |
| SO2 | 0.70 (0.62, 0.79) | 1.10 (0.95, 1.27) | 1.13 (0.91, 1.40) | 1.51 (1.22, 1.88) | 0.20 |
| O3 | 0.84 (0.81, 0.87) | 1.09 (1.03, 1.14) | 1.27 (1.21, 1.33) | 1.25 (1.20, 1.30) | <0.01 |
Note: PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm; SO2, sulfur dioxide; NO2, nitrogen dioxide; O3, ozone.
Exposure window I represents the mean exposure value from the date of symptom onset to the date of diagnosis; exposure window II represents the mean exposure value from the date of diagnosis to the date of death or the end of the study.
Estimated using likelihood ratio test by comparing adjusted HRs for different windows.
Crude model, without any adjustment.
Multivariate model, adjusted for age, sex, occupation, residence, severity of illness, location, transfer history, temporal trend, lockdown, city-level GDP, hospital beds per 1,000 persons, temperature (only for the pollutants), and relative humidity.
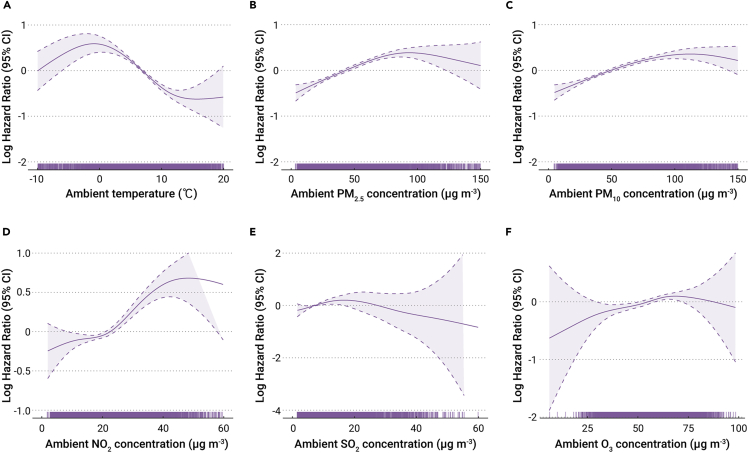
Figure 1 shows the exposure-response curves of the effects of environmental factors (exposure window I) on COVID-19 fatality. Overall, the temperature curve has a downward trend, although there is a small rise when temperature is below freezing. With the exception of SO2, all air pollutants showed curves with upward trends as the concentrations increase; these relationships were monotonic and approximately linear.
Figure 1.
Exposure-response curves for the effects of environmental factors
The exposure-response curves for exposure to ambient temperature (A), PM2.5 (B), PM10 (C), NO2 (D), SO2 (E), and O3 (F) versus case fatality due to COVID-19 (exposure window I) are shown. The solid and dashed lines represent log (hazard ratio) values and the 95% CIs, respectively. Definition of abbreviations: CI, confidence interval; PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm; SO2, sulfur dioxide; NO2, nitrogen dioxide; O3, ozone.
The associations of environmental factors with COVID-19 varied by a few characteristics (Table 3). Stronger effects occurred in the elderly, as well as those with mild symptoms and living in Wuhan or Hubei. In particular, for an increase of 10 μg/m3 in air pollutant (PM2.5, PM10, and NO2) concentration, the estimated HRs were slightly larger in those ≥65 (p < 0.01). The HRs for COVID-19 fatality related to each 10 μg/m3 increase in NO2 were higher and significant for mild patients compared with severe cases. In addition, almost all significant associations were observed across subgroups of Hubei Province and outside Hubei, although higher effects occurred in Hubei (p < 0.05). This relatively consistent pattern was also observed in Wuhan and outside Wuhan. No significant differences were observed in the subgroups before and after city lockdown.
Table 3.
Subgroup analysis for the associations of COVID-19 fatality with environmental exposure
| Variable | Temperaturea |
PM2.5a |
PM10a |
NO2a |
SO2a |
O3a |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p valueb | HR (95% CI) | p valueb | HR (95% CI) | p valueb | HR (95% CI) | p valueb | HR (95% CI) | p valueb | HR (95% CI) | p valueb | |
| Age | 0.02 | <0.01 | <0.01 | <0.01 | 0.11 | 0.58 | ||||||
| <65 | 1.00 (0.97, 1.02) | 1.06 (1.02, 1.10) | 1.05 (1.01, 1.09) | 1.11 (1.00, 1.24) | 0.89 (0.68, 1.17) | 1.07 (1.00, 1.17) | ||||||
| ≥65 | 1.04 (1.02, 1.06) | 1.14 (1.11, 1.17) | 1.13 (1.11, 1.16) | 1.34 (1.23, 1.45) | 1.16 (0.98, 1.38) | 1.10 (1.04, 1.16) | ||||||
| Sex | 0.81 | 0.64 | 0.52 | 0.15 | 0.50 | 0.32 | ||||||
| Male | 1.07 (0.98, 1.17) | 1.04 (0.96, 1.14) | 1.07 (1.00, 1.17) | 1.22 (1.13, 1.33) | 1.03 (0.86, 1.23) | 1.07 (1.01, 1.13) | ||||||
| Female | 1.09 (0.97, 1.23) | 1.07 (1.00, 1.17) | 1.12 (1.00, 1.25) | 1.35 (1.21, 1.51) | 1.14 (0.90, 1.45) | 1.12 (1.04, 1.20) | ||||||
| Severity | <0.01 | 0.09 | 0.10 | <0.01 | 0.08 | 0.39 | ||||||
| Mild | 1.13 (1.08, 1.20) | 1.18 (1.11, 1.27) | 1.16 (1.09, 1.23) | 1.62 (1.36, 1.94) | 1.91 (1.05, 3.51) | 1.18 (1.04, 1.35) | ||||||
| Severe | 1.01 (1.00, 1.03) | 1.11 (1.09, 1.13) | 1.10 (1.08, 1.12) | 1.25 (1.16, 1.34) | 1.10 (0.96, 1.27) | 1.11 (1.06, 1.17) | ||||||
| Location | <0.01 | <0.01 | <0.01 | <0.01 | 0.86 | <0.01 | ||||||
| Wuhan | 1.15 (1.12, 1.18) | 1.23 (1.19, 1.28) | 1.19 (1.15, 1.23) | 1.44 (1.33, 1.57) | 1.12 (0.69, 1.79) | 1.19 (1.11, 1.28) | ||||||
| Non-Wuhan | 1.00 (0.98, 1.02) | 1.06 (1.03, 1.09) | 1.06 (1.03, 1.09) | 1.04 (0.93, 1.16) | 1.07 (0.92, 1.25) | 1.03 (0.96, 1.09) | ||||||
| Province | <0.01 | 0.11 | 0.03 | 0.03 | <0.01 | 0.07 | ||||||
| Hubei | 1.12 (1.09, 1.15) | 1.04 (1.00, 1.05) | 1.08 (1.03, 1.13) | 1.65 (1.55, 1.76) | 1.38 (1.12, 1.71) | 1.12 (0.96, 1.32) | ||||||
| Non-Hubei | 1.02 (1.00, 1.05) | 1.09 (1.04, 1.15) | 1.02 (1.00, 1.04) | 1.33 (1.11, 1.59) | 0.71 (0.57, 0.89) | 0.96 (0.92, 1.00) | ||||||
| Lockdownc | 0.29 | 0.11 | 0.07 | 0.16 | 0.06 | <0.01 | ||||||
| After | 1.05 (1.02, 1.09) | 1.07 (1.02, 1.13) | 1.06 (1.02, 1.11) | 1.17 (1.04, 1.32) | 0.86 (0.51, 1.22) | 0.93 (0.53, 1.33) | ||||||
| Before | 1.03 (1.01, 1.04) | 1.12 (1.09, 1.14) | 1.11 (1.08, 1.14) | 1.30 (1.19, 1.42) | 1.37 (1.16, 1.62) | 1.21 (1.14, 1.29) | ||||||
Note: HR, hazard ratio; PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤10 μm; SO2, sulfur dioxide; NO2, nitrogen dioxide; O3, ozone.
All the environmental factors were estimated using exposure window I (mean exposure value from the date of symptom onset to the date of diagnosis). The effects of environmental factors were estimated using the adjusted model, adjusted for age, sex, temperature (only for the pollutants), relative humidity, occupation, residence, severity of the illness, location, transfer history, temporal trend, and lockdown.
p values were estimated using likelihood ratio test by comparing different subgroups, and p < 0.05 indicates statistical significance.
Lockdown: on January 23, 2020, the central government of China imposed a lockdown in Wuhan and other cities in Hubei.
Sensitivity analyses (Table S4) demonstrated that the effects of air pollution remained robust as the degrees of freedom of parameters changed from 2 to 6. For example, the magnitude of HRs for COVID-19 fatality associated with each 10 μg/m3 increase in PM10 remained at the level of 1.10. When the association between environmental factors and case fatality due to COVID-19 was explored using the current symptom onset date and diagnosis as well as corresponding lag days, there were significant effects of environmental factors on COVID-19 fatality across lag days (Figures S8 and S9). In addition, the estimated effects of the primary pollutant varied slightly when an adjustment was made for a second pollutant (Table S5). The exception was for NO2 and O3, whose effects were rendered nonsignificant after adjustment for PM2.5 and PM10, respectively. We also obtained similar results for combined exposure windows compared with exposure window II (Table S6).
Discussion
In this nationwide retrospective cohort study of COVID-19 patients in China, short-term exposure to ambient environmental factors during the illness course, including low temperature and PM2.5, PM10, NO2, SO2, and O3, contributed to increasing risk of death. Several exposure windows were used to examine the effects, and the estimates remained robust. To the best of our knowledge, this is the first study to explore the association of exposures to key environmental factors and COVID-19 fatality using nationwide data from low- and middle-income countries with high air pollution levels.
Air pollutants were critical environmental drivers in triggering COVID-19 fatality. These findings are consistent with a previous study that provided evidence for an association between higher air pollution and increased risk of dying from SARS.22 In addition, emerging research has reported that chronic exposure to polluted air might result in a higher risk of dying from COVID-19.23 A recent study using county-level data for the United States found that an 8% (95% CI 2%, 15%) increase in the COVID-19 death rate was associated with an increase of 1 μg/m3 in PM2.5.24 Long-term exposure to NO2 has also been identified as an important contributor to fatality due to COVID-19.12 The significant associations between other major ambient pollutants (e.g., SO2 and O3 levels) and increased COVID-19 deaths were highlighted in a recent study in England.17 However, a cross-sectional national study in the United States did not observe significant associations of PM2.5 and O3 with COVID-19 fatality.13 In general, our evidence was derived from a nationwide cohort study with a wider exposure range, providing relatively accurate and convincing estimates.
Further, while numerous studies have focused on the link between COVID-19 transmission and temperature,25,26 the impact of temperature on COVID-19 outcomes has not been widely studied. We observed that lower temperature was associated with a greater HR of COVID-19 fatality. These findings were consistent with one recent study from Wuhan, China, which reported that higher ambient temperature was related to a lower case fatality rate due to COVID-19.27 However, the role of ambient temperature in COVID-19 fatality might be rather intricate due to the adverse effects of high and low temperatures on human health.28, 29, 30 Other seasonal data are required to determine the overall comprehensive effects of temperature.
Most COVID-19 cases were admitted in hospitals, particularly, Fangcang shelter hospitals in Wuhan, and these were large and temporary hospitals built by converting public venues.31 In the Fangcang shelter hospitals or infectious disease zones of designated hospitals, the ventilation systems maintained air exchange from outside to inside, ensuring the plausibility and accuracy of exposure assessment. Although the underlying molecular mechanisms of environmental pollutants and temperature on COVID-19 outcomes remain to be determined, prior studies offered some insight as to potential biological plausibility.19,32, 33, 34 Exposure to air pollutants can induce systemic oxidative stress and enhance inflammation, interacting with SARS-CoV-2 (the virus that causes COVID-19) to damage lymphocytes and impair the immune system.35 Elevated inflammatory factors and immune dysfunction might trigger the cytokine storm syndrome and develop into acute respiratory distress syndrome, which was associated with COVID-19 disease severity and death.36 In addition, short-term effects of cold temperatures on health mainly can result in restricted immune function and lead to changes in autonomic nervous system function, inflammatory response, and oxidative stress.37 In these situations, the symptoms of COVID-19 would progress, and the patient's condition would further deteriorate.
This study adds new evidence on the association between environmental factors and COVID-19 fatality. Comprehensive understanding of the role that external environmental factors play in triggering case fatality due to COVID-19 has valuable implications. Although we do not need another reason to reduce exposure to a poor environment, reporting these associations enriches the long list of the health effects of air pollution. More crucial is that these findings should be expanded to change clinical practice or public health programming, including identification of patients with poor prognosis at early stages, guiding resource allocation, and improvement of prognosis. Findings suggest that COVID-19 patients living in regions with high adverse environmental factors may be more vulnerable to complications from the virus, specifically higher risk for death. These environmental factors are key modifiable variables related to COVID-19 fatality. Hence, all COVID-19 patients should pay attention to self-protection to decrease their exposure to environmental stimuli, such as staying in a warmer environment and avoiding severe air pollution outside.
The results of stratified analyses indicated that those over age 65 were more susceptible to COVID-19 death, which was in line with a previous study.38 We also observed inconsistent associations across subgroups of Hubei Province and outside Hubei. The reason for this may be due to a high proportion of severe and critical cases and/or insufficient amounts of medical services in Hubei Province due to it being an epicenter. In addition, we observed a stronger effect of environmental exposures in mild cases compared with severe ones, which could be explained by the scenario that among the severe, there was a portion of COVID-19 intensive care cases (about 19.5% of severe cases) likely admitted to the intensive care unit with lower opportunity to be exposed to external environment factors.39 However, although patients were admitted to the wards, they were still affected by the external environment exposures, as there is continuous circulation between indoor and outdoor air.
Two exposure windows were used to examine the association of air pollution and temperature with COVID-19 fatality, and the estimates varied somewhat. We observed effects estimation of window II > window I, suggesting that patients were more susceptible to external environmental stimuli as COVID-19 progressed from the period of symptom onset to diagnosis. An underlying mechanism linking this phenomenon might be that the body system and internal environment experienced distinct changes in innate immune response and inflammation activation as the disease progressed, causing the body to enter a more susceptible and vulnerable state.40,41 Therefore, the cases during this sensitive period should be given higher priority and more care.
Strengths and limitations
Our study has several strengths: the large sample size of a stable target population, a longitudinal study with individual-level information, clear temporality between exposure and outcomes, the length of the follow-up from onset through vital outcomes, and adjustment for a wide range of covariates using regression models. It is recognized that the effects of environmental drivers on health outcomes mainly depend on the approaches with which they are evaluated. In this study, several exposure windows were used to evaluate environmental short-term effects, which in turn added to the robustness of the findings.
Our study also has some limitations. As this study used exposure data based on measurements from fixed air monitoring stations as the surrogate for individual exposure, exposure misclassification may be one concern. Nevertheless, this exposure assessment approach has been widely used to develop exposure estimations for large air pollution epidemiological studies, and previous studies reported that applying the monitoring values as personal exposure tended to cause underestimation of effects.42 The observed effects may still contain residual confounding due to unavailability of other individual behavioral risk factors and unmeasured time-varying confounding factors. Although some relevant individual variables, such as underlying health conditions, BMI, and smoking, were not included in the analysis due to data unavailability, we adjusted for clinical severity as one proxy for the unmeasured clinical information.43 In addition, our cohort was followed until April 25, 2020. This decision was due to data availability, although that time may not be sufficient to capture all outcomes. Moreover, although we have added one binary variable to the model to control for the effects of city lockdown, it is still insufficient to account for the lockdown effects on the associations due to lack of detailed controlled measures across different cities.
Conclusion
This study suggests that short-term exposure to air pollution and low temperature could increase the case fatality due to COVID-19 in China. Research on how environmental factors may drive COVID-19 case fatality is crucial to guiding clinical practice and public health programming to curb influences related to the outbreak.
Material and methods
Study population and design
This retrospective cohort study used the National Notifiable Disease Reporting System of China to enroll all confirmed COVID-19 cases from December 8, 2019, through April 15, 2020. Cases were followed up for vital status until April 25, 2020. Eligible participants included those: (1) diagnosed as confirmed COVID-19 cases with a positive viral nucleic acid test result on oropharyngeal swab samples in strict accordance with national criteria and (2) who had complete medical and epidemiological information, including severity of the illness, date of symptom onset, and date of diagnosis. A total of 1,752 cases (2.38%) were excluded from 73,560 original cases due to unavailability of environmental exposure data. The remaining 71,808 COVID-19 cases were included in this analysis. General demographic and illness-related characteristics were obtained from the National Notifiable Disease Reporting System of China, including age, sex, birth date, occupation, residential location, severity of the illness, hospital transfer history, date of symptom onset, date of diagnosis, and date of death (for the deceased).
Approval to conduct this study was obtained from the Biomedical Research Ethics Review Committee of Sun Yat-sen University School of Public Health (no. 2020016).
Estimation of environmental exposures
Daily ambient air pollution data (PM2.5, PM10, NO2, SO2, and O3) during the study period were obtained from China's National Real-Time Publishing Platform for Daily Air Quality, which included 1,256 monitors at the end of 2015, with the number in each city ranging from 1 to 17. There is a series of supporting standards and regulations for air-quality monitoring process and choice of location, which could ensure representativeness of the general background levels of air pollution for the population's daily air pollution exposure.44 Due to the unavailability of each case's detailed residential address, city-wide average data were used to represent exposure based on the exposure windows. Each participant's exposures were estimated by matching residential city with air pollution concentration in the corresponding city. Considering possible differences and susceptibility of short-term exposure to adverse environmental factors at different stages of COVID-19, we divided the exposures into different periods. Exposure window I represents the period of time from symptom onset to diagnosis, indicating the environmental exposure before hospitalization or quarantining. Exposure window II includes the time from date of diagnosis to date of death or recovery or end of the study period and represents participants' environmental exposures during treatment. In addition, we combined the two exposure windows to create one exposure window for the full course of symptomatic disease (i.e., symptom onset to death or recovery or end of the study). This complete exposure window was calculated for each participant to assess overall effects of the environmental factors. The county-level daily temperature (°C) and relative humidity (%) were obtained from the National Meteorological Monitoring Center. Mean temperature was then calculated for each case for each of the aforementioned exposure windows.
Statistical analysis
Student's t test was used to examine statistical differences for the continuous variables according to the vital status and expressed as mean ± standard deviation (SD). Categorical variables were presented with percentages, and chi-square tests were used to evaluate differences. Spearman's correlation coefficients were estimated among study variables to exclude the potential multicollinearity.
Cox proportional hazards models were applied to estimate the association between COVID-19 fatality and the two exposures of air pollution and temperature. Schoenfeld residuals were performed to evaluate the proportional hazards assumption and no violations were observed (p > 0.05). Univariate analysis was conducted, and then multivariate models were generated to control for potential confounding factors. Two criteria were used to select covariates for the final adjusted model: (1) variables known or hypothesized to be risk factors for COVID-19 fatality according to the previous study38 and (2) variables significantly associated with COVID-19 fatality in the univariate analysis (Table S1). Based on these criteria, the following variables were included in the model: sex (male or female), age (<25 years, 25–65 years, and ≥65 years), occupation (medical-related, service-related, office worker, homemaker, and others), residence (permanent or temporary), clinically diagnosed severity of the illness (mild, moderate, severe, and critical), location (Wuhan or non-Wuhan), transfer to a higher-level hospital (yes or no), and relative humidity and temperature. Nonparametric smoothing was done by way of a natural smoothing splines function for trend on days included in the model to account for the natural temporal trend of associations and other potential factors related to COVID-19 fatality. The temporal trend, temperature, and relative humidity were characterized by natural cubic splines for their potentially nonlinear relationship, with 3 degrees of freedom for each based on previous studies.45,46 In addition, to account for the effects of city lockdown, we determined whether the date of symptoms of onset for each case occurred before or after the city lockdown in each city and added one binary variable to the model. The Chinese government committed to paying for all COVID-19-related hospital bills for every patient. In theory, this would eliminate confounding by some health and medical variables related to access to care and resources. Despite this, we included city-level per-capita GDP and hospital beds per 1,000 persons (each as derived from the China Health Statistical Yearbook 2019) in the model to adjust for potential confounding bias. Details of the overall model are illustrated in the supplemental information (Text S1 and S2). We also plotted the exposure-response relationship curves between environmental factors and COVID-19 fatality with natural cubic spline functions with 3 degrees of freedom in the regression model to examine the nonlinear relationship. Hazard ratios (HRs) and 95% confidence intervals (CIs) were computed for COVID-19 fatality associated with per °C decrease in temperature or per 10 μg/m3 increase in each air pollutant.
Subgroup analysis
Stratified analyses by sex (male and female), age group (<65 years and ≥65 years), location (Wuhan and non-Wuhan), and the clinically diagnosed severity of illness (mild [including the original mild and moderate] and severe [including the original severe and critical]) were performed to examine potential effect modifiers. In addition, considering the potential disparities in medical resources, treatment supplies, and public health interventions by region and time, study participants were divided into those living outside of Hubei Province and within Hubei, before lockdown and after lockdown. Separate Cox models for each subgroup described above were applied to obtain corresponding HRs for environmental factors. The statistical difference between subgroups was calculated as follows: . , , , and presented the effect estimates and corresponding SDs in two subgroups.47 Considering the possible type I errors introduced by multiple runs of the likelihood ratio test, we conducted the Bonferroni correction of likelihood ratio test for testing multiple hypotheses to avoid potential type I errors.48
Sensitivity analysis
Several sensitivity analyses were performed to examine the robustness of our estimates. First, we conducted two-pollutant models with adjustment for a second air pollutant to assess the independent effect of the primary one. Second, different degrees of freedom from 2 to 6 for temperature and relative humidity were specified to examine the impact of alternative degrees of freedom on the estimated effects of environmental factors. Finally, other exposure windows were assigned to the participants, including single-day exposure prior to symptom onset date and diagnosis date (lag0 to lag3 for air pollution, lag0 to lag10 for temperature). Considering the accumulative effects of environmental factors, the associations between the exposures and COVID-19 were examined using multiday lag structures: a moving average of the current day and the previous 1, 2, and 3 or more days (lag01, lag02, and lag03 for air pollution; lag03, lag05, lag07, and lag010 for temperature).
Statistical analyses were performed by R software. A value of two-tailed p < 0.05 was considered statistically significant.
Acknowledgments
We would like to extend our sincere gratitude and admiration to academician Jianguo Xu at State Key Laboratory of Infectious Disease Prevention and Control, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, for his guidance, support, numerous helpful suggestions and continuous encouragement on this study. We also thank all the staff for their comments on a draft version of the manuscript. This study was supported by the National Natural Science Foundation of China (82041021 and 42041001), the Bill & Melinda Gates Foundation (INV-006371), and the General Program of the State Key Laboratory of Infectious Disease Prevention and Control of China (2020SKLID201).
Author contributions
H.L., and Q.L. had the idea for and designed the study and had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. F.T. and X.L. drafted the paper. F.T., Z.Q., and A.L. contributed to the exposure assessment. F.T., H.L., S.Z., Y.N., and L.Q. did the analysis. X.L., Y.N., L.Q., Q.C., and S.Z. collected the data. All authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published.
Declaration of interests
The authors declare they have no competing interests.
Published Online: June 18, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xinn.2021.100139.
Contributor Information
Hualiang Lin, Email: linhualiang@mail.sysu.edu.cn.
Qiyong Liu, Email: liuqiyong@icdc.cn.
Lead contact website
The website of the lead contact is at http://sph.sysu.edu.cn/teacher/361.
Supplemental information
References
- 1.Wang Y., Liu Y., Liu L., et al. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J. Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslam A., Singh J., Robilotti E., et al. Severe acute respiratory syndrome coronavirus 2 surveillance and exposure in the perioperative setting with universal testing and personal protective equipment policies. Clin. Infect Dis. 2020 doi: 10.1093/cid/ciaa1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kar S., Leszczynski J. From animal to human: interspecies analysis provides a novel way of ascertaining and fighting COVID-19. Innovation. 2020;1:100021. doi: 10.1016/j.xinn.2020.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng X.-y., Guan W.-j., Zhong N.-s. Clinical characteristics of COVID-19 in developing countries of western pacific: low case-fatality rate unraveled. Lancet Reg. Health West. Pac. 2021;6:100073. doi: 10.1016/j.lanwpc.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai C.L., Kornilov S.A., Roper R.T., et al. Characteristics and factors associated with coronavirus disease 2019 infection, hospitalization, and mortality across race and ethnicity. Clin. Infect Dis. 2021 doi: 10.1093/cid/ciab154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh K., Kim Y.-E., Ji W., et al. Decrease in hospital admissions for respiratory diseases during the COVID-19 pandemic: a nationwide claims study. Thorax. 2021 doi: 10.1136/thoraxjnl-2020-216526. thoraxjnl-2020-216526. [DOI] [PubMed] [Google Scholar]
- 7.Ra S.H., Lim J.S., Kim G.-u., et al. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax. 2021;76:61–63. doi: 10.1136/thoraxjnl-2020-215042. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., Qi J., Ruan Z., et al. Changes in life expectancy of respiratory diseases from attaining daily PM2.5 standard in China: a nationwide observational study. Innovation. 2020;1:100064. doi: 10.1016/j.xinn.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu W., Guo Y., Shi L., et al. The association between long-term exposure to low-level PM2.5 and mortality in the state of Queensland, Australia: a modelling study with the difference-in-differences approach. PLoS Med. 2020;17:e1003141. doi: 10.1371/journal.pmed.1003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q., Li S., Coelho M., et al. The association between heatwaves and risk of hospitalization in Brazil: a nationwide time series study between 2000 and 2015. PLoS Med. 2019;16:e1002753. doi: 10.1371/journal.pmed.1002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan Z., Qi J., Qian Z., et al. Disease burden and attributable risk factors of respiratory infections in China from 1990 to 2019. Lancet Reg. Health West. Pac. 2021;11:100153. doi: 10.1016/j.lanwpc.2021.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang D., Shi L., Zhao J., et al. Urban air pollution may enhance COVID-19 case-fatality and mortality rates in the United States. Innovation. 2020;1:100047. doi: 10.1016/j.xinn.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y., Pan J., Wang W., et al. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total Environ. 2020;741:140396. doi: 10.1016/j.scitotenv.2020.140396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X., Nethery R.C., Sabath M.B., et al. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konstantinoudis G., Padellini T., Bennett J., et al. Long-term exposure to air-pollution and COVID-19 mortality in England: a hierarchical spatial analysis. Environ. Int. 2021;146:106316. doi: 10.1016/j.envint.2020.106316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travaglio M., Yu Y., Popovic R., et al. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021;268:115859. doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villeneuve P.J., Goldberg M.S. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ. Health Perspect. 2020;128:95001. doi: 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S., Ni Y., Li H., et al. Short-term exposure to high ambient air pollution increases airway inflammation and respiratory symptoms in chronic obstructive pulmonary disease patients in Beijing, China. Environ. Int. 2016;94:76–82. doi: 10.1016/j.envint.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Ciencewicki J., Jaspers I. Air pollution and respiratory viral infection. Inhal. Toxicol. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Wang T., Xu M., et al. Independent effect of main components in particulate matter on DNA methylation and DNA methyltransferase: a molecular epidemiology study. Environ. Int. 2020;134:105296. doi: 10.1016/j.envint.2019.105296. [DOI] [PubMed] [Google Scholar]
- 22.Kan H.D., Chen B.H., Fu C.F., et al. Relationship between ambient air pollution and daily mortality of SARS in Beijing. Biomed. Environ. Sci. 2005;18:1–4. [PubMed] [Google Scholar]
- 23.Pansini R., Fornacca D. COVID-19 higher mortality in Chinese regions with chronic exposure to lower air quality. Front. Public Health. 2020;8:597753. doi: 10.3389/fpubh.2020.597753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X., Nethery R.C., Sabath B.M., et al. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. medRxiv. 2020 doi: 10.1101/2020.04.05.20054502. 2020.2004.2005.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Y., Pan J., Liu Z., et al. No Association of COVID-19 transmission with temperature or UV radiation in Chinese cities. Eur. Respir. J. 2020 doi: 10.1183/13993003.00517-2020. 2000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Notari A. Temperature dependence of COVID-19 transmission. Sci. Total Environ. 2021;763:144390. doi: 10.1016/j.scitotenv.2020.144390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y., Zhao Y., Liu J., et al. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724:138226. doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen R., Yin P., Wang L., et al. Association between ambient temperature and mortality risk and burden: time series study in 272 main Chinese cities. BMJ. 2018;363:k4306. doi: 10.1136/bmj.k4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martens D.S., Plusquin M., Cox B., et al. Early biological aging and fetal exposure to high and low ambient temperature: a birth cohort study. Environ. Health Perspect. 2019;127:117001. doi: 10.1289/EHP5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam H.C.Y., Chan J.C.N., Luk A.O.Y., et al. Short-term association between ambient temperature and acute myocardial infarction hospitalizations for diabetes mellitus patients: a time series study. PLoS Med. 2018;15:e1002612. doi: 10.1371/journal.pmed.1002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S., Zhang Z., Yang J., et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S.Y., Chan C.C., Su T.C. Particulate and gaseous pollutants on inflammation, thrombosis, and autonomic imbalance in subjects at risk for cardiovascular disease. Environ. Pollut. 2017;223:403–408. doi: 10.1016/j.envpol.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 33.Hassanvand M.S., Naddafi K., Kashani H., et al. Short-term effects of particle size fractions on circulating biomarkers of inflammation in a panel of elderly subjects and healthy young adults. Environ. Pollut. 2017;223:695–704. doi: 10.1016/j.envpol.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Hajat A., Allison M., Diez-Roux A.V., et al. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015;26:310–320. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D., Chen Y., Liu H., et al. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study. Signal. Transduct. Target Ther. 2020;5:62. doi: 10.1038/s41392-020-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adar S.D., Filigrana P.A., Clements N., et al. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Curr. Environ. Health Rep. 2014;1:258–274. doi: 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai J., Meng X., Wang C., et al. The cold effects on circulatory inflammation, thrombosis and vasoconstriction in type 2 diabetic patients. Sci. Total Environ. 2016;568:271–277. doi: 10.1016/j.scitotenv.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 38.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Immovilli P., Morelli N., Antonucci E., et al. COVID-19 mortality and ICU admission: the Italian experience. Crit. Care. 2020;24:228. doi: 10.1186/s13054-020-02957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., Saguner A.M., An J., et al. Dysfunctional coagulation in COVID-19: from cell to bedside. Adv. Ther. 2020;37:3033–3039. doi: 10.1007/s12325-020-01399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz J., Sarnat J.A., Coull B.A., et al. Effects of exposure measurement error on particle matter epidemiology: a simulation using data from a panel study in Baltimore, MD. J. Expo. Sci. Environ. Epidemiol. 2007;17(Suppl 2):S2–S10. doi: 10.1038/sj.jes.7500619. [DOI] [PubMed] [Google Scholar]
- 43.VanderWeele T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019;34:211–219. doi: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B.T., Su Y.X., He S.S., et al. Evolution and comparative assessment of ambient air quality standards in China. J. Integr. Environ. Sci. 2016;13:85–102. doi: 10.1080/1943815x.2016.1150301. [DOI] [Google Scholar]
- 45.Qi J., Ruan Z., Qian Z.M., et al. Potential gains in life expectancy by attaining daily ambient fine particulate matter pollution standards in mainland China: a modeling study based on nationwide data. PLoS Med. 2020;17:e1003027. doi: 10.1371/journal.pmed.1003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang Z., Yang Y., Qian Z., et al. Ambient PM2.5 and birth outcomes: estimating the association and attributable risk using a birth cohort study in nine Chinese cities. Environ. Int. 2019;126:329–335. doi: 10.1016/j.envint.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Altman D.G., Bland J.M. Statistics Notes - interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curtin F., Schulz P. Multiple correlations and Bonferroni's correction. Biol. Psychiatry. 1998;44:775–777. doi: 10.1016/s0006-3223(98)00043-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.