Abstract
There is an urgent need for therapeutic interventions for desensitization and antibody-mediated rejection (AMR) in sensitized patients with preformed or de novo donor-specific HLA antibodies (DSA). The risk of AMR and allograft loss in sensitized patients is increased due to preformed DSA detected at time of transplant or the reactivation of HLA memory after transplantation, causing acute and chronic AMR. Alternatively, de novo DSA that develops post-transplant due to inadequate immunosuppression and again may lead to acute and chronic AMR or even allograft loss. Circulating antibody, the final product of the humoral immune response, has been the primary target of desensitization and AMR treatment. However, in many cases these protocols fail to achieve efficient removal of all DSA and long-term outcomes of patients with persistent DSA are far worse when compared to non-sensitized patients. We believe that targeting multiple components of humoral immunity will lead to improved outcomes for such patients. In this review, we will briefly discuss conventional desensitization methods targeting antibody or B cell removal and then present a mechanistically designed desensitization regimen targeting plasma cells and the humoral response.
Keywords: sensitization, desensitization, alloantibody, plasma cells, germinal center
Introduction
Desensitization treatment within the field of transplantation refers to the process of antibody removal (1), specifically preformed donor-specific HLA antibody (DSA). DSA as a barrier to successful transplantation was first described in early reports of kidney hyperacute rejection in the context of positive complement dependent cytotoxicity (CDC) by Ramon & Terasaki et al. (1), Improvements in histocompatibility testing have removed the risk of hyperacute rejection across all organs, yet acute and chronic AMR remain a major contributor to poor transplant outcomes (2, 3).
The Current Status of HLA Sensitization
For many patients awaiting transplantation, blood transfusion, prior transplantation, and pregnancy are the sources of sensitization (4, 5). There is evidence that the primary source of HLA sensitization is important. Transplantation appears consistently as the strongest sensitizing event inducing both class I and II HLA antibody (6, 7); however, there is some evidence that post-transplant, those with pregnancy induced HLA-antibody respond more rapidly (5). Waiting times for highly sensitized patients, calculated Panel Reactive Antibody (cPRA)> 80% (8) are longer, leading to increased morbidity and mortality. Compared to patients with absent or low cPRA, the highly sensitized candidates could expect to wait twice as long for a compatible transplant in both the USA (9) & UK (10). In Europe, the acceptable mismatch program has long been advocated to reduce waiting times for highly sensitized patients (11). Recently, in the USA the new kidney allocation scheme (KAS) (12), was specifically designed to improve the transplantation rates for sensitized patients by providing more allocation points and mandating regional and national sharing for those with the highest CPRA. Early KAS reports suggest that this has largely been successful (13, 14).
For sensitized patients with an incompatible living kidney donor, to whom they have DSA, the decision is whether to use a kidney paired donation (KPD) program to obtain a compatible match, await a compatible deceased donor offer, or proceed with a HLA-incompatible, positive cross match transplant (HLA-i) which requires desensitization prior to transplantation. Simulation of how KPD programs might run were initially optimistic (15), but over time many struggle with a pool enriched with highly sensitized patients unless specific matching interventions are made (16), or a combination of both desensitization and KPD is utilised (17).
The Current Standing of Desensitization
In the USA, multicenter data demonstrates a clear survival benefit (18) in proceeding with an HLAi; however, in the UK the picture is more nuanced with no survival benefit demonstrated, although for the patients awaiting a compatible transplant, around 40% remain untransplanted at 5 years post-listing (19). Desensitization then remains a guaranteed route for a highly sensitized recipient to obtain transplant, albeit with greater immunological risk. To date, desensitization therapies have largely relied upon physical methods of antibody removal in the form of repeated plasmapheresis, in conjunction with additional agents. In this review, we will outline the currently used regimen for desensitization, as well as describing new potential approaches.
The Current Desensitization Treatment Strategy
In highly sensitized patients removal of circulating anti-HLA antibody or lowering cPRA is an important and fundamental strategy for expanding donor options and successfully transplanting across DSA barriers. However, durable inhibition of HLA antibody production is the “holy grail” for successful kidney transplantation (KT) in sensitized patients.
Plasmapheresis or Immunoadsorption
Plasmapheresis (PP) has been used for several decades as a method for lowering circulating antibody in various immune diseases (20, 21). Plasmapheresis physically removes large molecular weight substances from the plasma, including antibodies, complement components immune complexes and coagulation factors (22). Using the double filtration plasmapheresis (DFPP) system, a cascade of filtration traps larger molecules, and thus allow lower molecular weight components to pass back to the patient (23).Together with IVIg, it has been used to effect successful transplantation for positive crossmatch patients, and for many units, is the mainstay of desensitization prior to transplantation (24–26). In Europe and Australia immunoadsorption (IA) using staphylococcal protein A column has been applied in eliminating antibodies (27–29). The kinetics of antibody removal by PP are predictable within limited periods compared with other treatment modalities since plasma proteins are reliably removed (30). Therefore, PP or IA can be used as an effective treatment modality in the setting of planned transplantation across a positive HLA cross-match in living donor KT. PP or IA has a limitation of antibody rebound after the completion of treatment sessions.
Intravenous Immunoglobulin
IVIG has been widely used in inflammatory and autoimmune conditions (31). IVIG also has a role in AMR treatment in kidney transplantation (32). Although widely used as part of desensitization regimens for many decades, the precise mechanism of action is unknown as a result of its broad spectrum of effects. Many potential mechanisms of action of IVIG in transplantation have been proposed. The main mechanisms are considered to be neutralization of circulating anti-HLA antibodies with anti-idiotypic antibodies (31), the inhibition of complement activation (33, 34), and binding to Fc receptors on immune cells (35, 36). It is also postulated that IvIg following plasmapheresis prevents rebound of DSA, by providing an abundant quantity of circulating IgG (37, 38). IVIG has been used in various doses according to protocol from 100mg/kg to 2.0g/kg in desensitization prior to living donor KT or for deceased donor KT of patients with high PRA.
Although various combinations of IVIG or PP with rituximab have been proposed, two protocols have been widely accepted and used (39).
PP With Low-Dose IVIG vs. High-Dose IVIG Alone
Using PP with low-dose IVIG, many centers report transplant outcomes with acute AMR rates of 12-43% when used in combination with various induction agents, anti-thymocyte globulin, anti-IL-2Rc antibody or OTK3 (40–43). The NIH IGO2 study, a controlled clinical, multi-center, double blinded trial of IVIG (2g/kg, monthly 4 times) versus placebo in sensitized patients, HLA antibody levels were reduced further, and the transplantation rate was higher in the IVIG group than in the placebo group (44). Glotz et al. reported results of high-dose IVIG desensitization with anti-thymocyte globulin induction in cross-match positive patients (45). Jordan et al. reported successful transplantation outcomes with two doses of 2mg/kg IVIG on day 0 and day 30 with rituximab in 20 patients (46). In this study, 16 patients among 20 could receive KT within 6 months. In their subsequent series, they used high-dose IVIG 2g/kg, 3 times on day 1, day 30 and at the time of transplantation with rituximab (47). Among 76 patients with PRA ≥30%, 31 patients received living donor KT, and 45 patients received deceased donor KT with reduced waiting time of 4.2 ± 4.5 months.
Anti-CD20 Antibody (Rituximab)
Rituximab is an anti-CD20 monoclonal antibody that binds to CD20 expressed on immature and mature B-lymphocytes, inducing apoptosis via antibody-dependent cytotoxicity, complement-dependent cytotoxicity or direct apoptosis. Originally, anti-CD 20 antibody was used to treat B-cell lymphoma. In transplantation, rituximab was introduced to deplete B cells with the goal of reducing donor-specific antibody (DSA) production (48). Rituximab has been used as an additional therapy as part of desensitization treatments, in conjunction with plasmapheresis & IvIg (46, 49). The half-life of rituximab in patients with end-stage renal disease is known to be 9-14 days (50). Rituximab administration can maintain durable B-cell depletion for at least six months, but rituximab does not bind to plasma cells as they do not express CD20 (51).
Unmet Need For Sensitized Patients
Because no randomized controlled clinical trial has compared the two main protocols described above, and the study populations and the criteria for transplantation vary, it is difficult to evaluate which protocol is best. Desensitization protocols using high-dose IVIG or low-dose IVIG + PP with rituximab have relative advantages and disadvantages. A PP-based protocol with low-dose IVIG, within limited periods, is more effective and predictable for lowering antibody levels. On the other hand, in spite of possible non-response, high-dose IVIG has the advantage in patients with high PRA on the waiting list of being less invasive given the unpredictable time to transplantation. However, both current desensitization protocols have limitations. Regardless of whether high-dose IVIG or low-dose IVIG with PP were used, acute AMR rate as well as acute cellular rejection rates were higher in desensitized patients than in non-sensitized patients (52). In a study that included surveillance biopsy of desensitized KT recipients, the subclinical AMR rate was 31% at 3 months post-transplantation, and patients with subclinical AMR at 3 months post-transplantation had higher C4d, ptc and arteriosclerosis scores post-transplantation at 1 year than the patients without subclinical AMR at 3 months post-transplantation (53). Transplant glomerulopathy was reported at a rate of 44% at a mean of 18 months post-transplantation (54). After desensitization, long-term outcomes of KT seems to be worse than for unsensitized patients (42).
Sensitization in Thoracic Organ Transplant Recipients: Similarities and Differences
Thoracic transplantation shares similar immunologic challenges as HLA sensitization in kidney recipients. However, no alternative organ replacement modalities support life in end-stage lung disease as dialysis in end-stage renal disease. While left ventricular assist devices (LVAD) have emerged as a viable alternative to heart transplant, it is not without significant risks and complications that limit access to therapy. As such, thoracic transplantation faces a greater urgency and waitlist mortality, and desensitization regimens must take into account these temporal challenges of sensitized patients. 1 in 7 adult heart transplant candidates are sensitized, a number that has doubled in the past two decades (55). A rising incidence is anticipated due to the expanding use of LVADs as a bridge to transplantation, advanced congenital heart disease surgery leading to more patients surviving to require transplant, and, to a smaller extent, an increase in re-transplantation (55). On the contrary, the true burden of sensitization in lung transplantation is unknown. National and international registries lack robust DSA or PRA/cPRA data for lung transplants. In the ISHLT registry, women, who are known to have greater sensitization secondary to pregnancy, comprise 60% of the waitlist but receive only 43% of the transplants, with a median time to transplantation of 233 days compared to 86 for men (56). To better understand and quantify this issue, comprehensive cPRA reporting in lung transplantation registries is required. Many lung transplant programs currently practice avoidance of DSA at the time of organ allocation, significantly limiting sensitized candidates’ access to transplant (57).
A common sequela of AMR in heart transplantation is cardiac allograft vasculopathy (CAV), and in lungs, chronic lung allograft dysfunction (CLAD), both of which result in significant mortality and morbidity within 5 years of transplantation (57–63). The primary goals of desensitization in thoracic transplantation are to increase access to transplantation through expansion of the donor organ pool and to prevent AMR and its subsequent morbidity and mortality. No approach has demonstrated significant and sustainable reductions in HLA antibody prior to transplant, and patients with elevated PRA continue to be at higher risk for rejection and reduced survival (64).
Shifting Toward Sensitization
As mentioned, use of mechanical support as bridge to transplantation has been steadily increasing, reaching 50% of patients on the waiting list for heart transplant in 2013. Particular attention to the immunologic challenges associated with LVADs to target interventions is necessary. While many studies suggest that LVAD-associated allosensitization limits sensitized candidates’ access to transplant, they fail to show that it leads to rejection or increased mortality after receiving a transplant (65). Notably, most of the evidence implicating such findings has been gathered from studies that examined pulsatile-flow LVADs and pre-dates the use of current generation continuous-flow LVAD (65–67). In a more recent study by Ko et al, 23% of patients became newly sensitized after continuous-flow LVAD implant (68). Compared with patients without new sensitization or those already sensitized at baseline, these patients had an increased risk of ACR and AMR, but comparable survival 5 years post-transplant, consistent with an earlier study (69). This suggests that even if the alloantibody levels were decreased before transplant by conventional methods, such as IVIg and plasmapheresis, maintenance immunosuppression targeting memory B cells and plasma cells is critical to prevent rebound DSA. In addition, the patients who were newly sensitized after LVAD implant and did not reach transplantation had a higher level of allosensitization (27.9% vs. 10.2%) and a high mortality of 39.5% during follow-up. This is consistent with a study by Alba et al. that also found an association between high PRA and lower transplant probability that likely drives the high mortality observed (70). A key concern with LVAD is the requirement of blood transfusions that result in the generation of new anti-HLA antibodies; however, our understanding of the mechanism by which patients on LVAD support develop allosensitization is largely unchanged since 1999 (71). It is also known that platelets and fibrinogen can adhere to the surface of LVAD coated with polyurethane membrane and form a fibrin matrix which traps other cells (72). The trapped cells could provide subsequent excessive activation signals via cytokines and costimulation to T cells. During this aberrant state of T cell activation, LVAD patients are believed to develop B cell hyper-reactivity with subsequent allosensitization. By way of a CD95-dependent pathway, these activated T cells then undergo apoptosis (73).
Current Desensitization Strategy in Thoracic Organ Transplantation
Despite these challenges, efforts to desensitize patients on the waitlist have generated limited success. The current research is centered around renal transplant experience with application in thoracic transplantation limited by several factors. In both heart and lung transplantation, there are requirements for donor-recipient size matching and transplant urgency is comparatively greater. Consequently, patients do not survive to begin clinical trials and the unpredictable nature of donor availability significantly limits the use of desensitization treatments prior to transplantation as prolonged period of treatment may confer more risks than benefits. Progress has been made from using IVIg and PP alone to using a variety of targeted therapies, although evidence in thoracic transplantation remains scarce. No large cohort desensitization strategy has been described in thoracic transplantation.
New Pharmacologic Strategies for Desensitization
Targeting Antibodies
IgG Endopeptidases
More recently, attempts have been made to fundamentally alter the structure of preformed antibody, using IgG endopeptidase (IdeS) which is a bacterial enzyme produced by S. pyogenes that cleaves all four human IgG subclasses into F(ab) & F (c) fragments, thus inhibiting both complement-dependent cytotoxicity and antibody-dependent cytotoxicity (74). IdeS has additional effects by cleaving the IgG present in the B-cell receptor complex (BCR), thus switching off B-cell memory as a downstream effect (75). Jordan et al. recently completed a trial of IdeS in 25 highly sensitized patients prior to HLA-incompatible kidney transplantation (76). All patients had near-complete or complete reductions of anti-HLA antibodies and donor-specific antibodies at 24 hours post-transplant, which allowed successful transplantation in 24/25 (96%). However, in 1-2 weeks the levels of these antibodies rebounded. Ultimately, one patient had graft loss from hyperacute rejection, while 10/25 (40%) had evidence of antibody-mediated rejection in the early post-transplant period. These findings suggest that IdeS has strong, albeit transient, ability to reduce DSA that may make this therapy useful in combination with strategies that allow for longer-term control of DSA rebound.
Anti-FcRn Approach
Brambell et al. identified FcRn, a neonatal IgG receptor that is closely related to the MHC Class I receptor, which is involved in a variety of critical biological and immunological functions, most notably regulating serum IgG levels and the recycling and transcytosis process that results in an increased half-life of IgG and albumin in human serum (77–80). Strategies that block the IgG-FcRn interaction are hypothesized to promote IgG degradation and decrease pathogenic autoantibodies and alloantibodies (81, 82). IVIG was one of the first therapies to decrease anti-HLA antibodies and treat antibody-mediated autoimmune diseases through blocking the IgG-FcRn pathway, leading to saturation of FcRn receptors and degradation of IgG molecules (78, 83, 84). Since then, multiple therapies targeting FcRn or the IgG-FcRn interaction have been developed as treatment for autoimmune and infectious diseases, with promising benefits as therapeutic agents in reducing AMR in transplantation. Several monoclonal antibodies against FcRn such as M281, SYNT001, Rozanolixizumab, RVT-1401, and ABY-039 are in various clinical development stages. M281, a deglycosylated IgG anti-FcRn mAb, was well tolerated and achieved reduction of serum IgG levels of 80% from baseline in a phase I clinical trial (85). Rozanolixizumab (UCB7665) is a high affinity anti-human neonatal FcRn mAb that reduced plasma IgG concentrations in cynomologus monkeys by up to 85% (86). This led to a Phase I clinical trial of Rozanolixizumab in healthy human subjects that demonstrated therapeutic potential with sustained dose-dependent reductions in serum IgG concentrations when administered IV or SC (87). Phase II clinical trials of Rozanolixizumab were recently completed in patients with immune thrombocytopenia (NCT02718716) and myasthenia gravis (NCT03052751) (86). Seijsing et al. found that an engineered alternative scaffold protein [affibody molecule (ZFcRn)] effectively blocked the IgG-FcRn interaction when repeated injections of ZFcRn and ZFcRn fused to an albumin binding domain (ABD) in mouse models led to a 40% reduction of IgG in serum (88). ABY-039 is a molecule similar to ZFcRn -ABD undergoing phase I trial (NCT03502954). Additional studies in animal models that inhibit IgG-FcRn binding include an anti-FcRn directed mAb, 1G3, that accelerated endogenous serum IgG clearance and reduced the severity of myasthenia gravis in rat models (89). Abdegs, an engineered antibody that inhibited FcRn recycling and enhanced IgG degradation, was efficacious in a murine model of arthritis (90). Synthetic FcRn-binding peptides (FcBP), small molecule FcRn antagonists, and other molecules that interact with the Fc binding site may also block IgG-FcRn interactions (78). Our group also tested anti-rhesus FcRn mAb in a skin-sensitized NHP model with kidney transplantation (manuscript in submission). Treatment with aFcRn prior to transplantation significantly reduced the levels of total and donor-specific alloantibody. However, in the context of renal transplantation, anti-FcRn treatment did not block the synthesis of DSA, such that transient reduction in DSA was followed by robust DSA increase and antibody-mediated rejection (manuscript in submission). The anti-FcRn approach demonstrated promising applications in lowering alloantibody levels in transplantation; however potential limitations and complexity of using the agent require further investigation in transplantation.
Targeting Plasma Cells
Following the discovery that alloantibody secreting cells predominantly exist as long-lived plasma cells (LLPC) in the bone marrow compartment, along with the identification of these cells as being CD138+CD20 (91), bortezomib was used to lower alloantibody (92). Bortezomib, a proteasome inhibitor (PI) which depletes non-malignant plasma cells, was proposed to reduce anti-donor HLA antibody. While some groups have demonstrated efficacy of bortezemib to desensitize transplant recipients, the drug was used in combination with conventional therapies (93). Now several biologics targeting plasma cells are available and are being considered.
Targeting Plasma cells with Proteasome Inhibition
Bortezomib (Velcade®) is a 1st generation, reversible inhibitor of the 26S proteasomal subunit. This drug is a potent inhibitor of plasma cells, which rely on rapid protein turnover to continually secrete antibodies, and succumb to oxidative stress and apoptosis when cellular recycling mechanisms are rendered nonfunctional. For this reason, bortezomib is approved for usage in multiple myeloma, a malignancy of plasma cells (94). Everly et al. first described its use as effective treatment of AMR and ACR as well as reduction in DSA in kidney transplant recipients (95), and Mulder et al. showed that proteosome inhibitors bortezomib, carfilzomib, oprozomib (ONX 0912), and immunoproteasome inhibitor ONX 0914 (previously PR-957) reduced B-cell proliferation, immunoglobulin production, and induced apoptosis of activated B-cells (96). Following some success for usage in refractory antibody-mediated rejection after kidney transplantation (97, 98), several groups have used bortezomib in the context of desensitization. Woodle et al. in the first trial with bortezomib variably combined with plasmapheresis and rituximab showed modest success with a reduction in the immunodominant DSA of 38/44 (86%) highly sensitized patients, successful transplantation of 19/44 (43.2%), and 17/19 (89.5%) of grafts functional at a median follow-up of 436 days (92). Jeong et al. used a combination of high dose IVIG, rituximab, and bortezomib and demonstrated a small reduction in the MFI value of class I PRA, and an increased rate of deceased donor kidney transplantation (8/19 or 42.1% of desensitized patients vs. 4/17 or 23.5% of controls, p = 0.004) with no graft loss in the desensitized group at a median follow-up of 23 months (93). The interpretation of these early favorable outcomes was limited by the small, non-randomized nature of the studies, and the confounding nature of its combination with conventional desensitization methods. Studies using bortezomib as monotherapy for desensitization have shown less promising results with poor reduction of anti-HLA antibodies and significant toxicity with longer courses of the drug (99, 100) that have caused enthusiasm for its use in new desensitization regimens to wane.
Carfilzomib (Kyprolis®) is a 2nd generation, irreversible inhibitor of the 20S proteasomal subunit. Studies in patients with multiple myeloma suggest that this drug may be more efficacious and better tolerated than its predecessor bortezomib (101). A current clinical trial of carfilzomib for desensitization is underway (NCT02442648). Most recently, carfilzomib was studied as desensitization monotherapy yielding 72.8% median reduction in HLA antibodies and a 69.2% reduction in bone marrow plasma cells with acceptable drug safety and toxicity (102). Another second generation PI, ixazomib, warrants further testing. Ixazomib is an oral-form peptide boronic acid proteasome inhibitor distinct from bortezomib and recently had a successful phase III trial (TOURMALINE-MM1) in multiple myeloma (103–106). Other PI’s including marizomib, delanzomib, and oprozomib are being studied as anti-cancer and autoimmune therapies. PI’s have notably been studied most recently as desensitization therapy and additional studies in utilizing PI as maintenance immunosuppressive treatment are needed.
Immunoproteasome Inhibitors
Conventional PIs are broad spectrum PIs with various dose-dependent adverse effects. An attractive alternative would be to solely target the proteasome of immune cells. Hematopoietic origin cells display proteasomes with distinct catalytic subunits and the complex is referred to as the immunoproteasome (107). Interestingly, immunoproteasome is also expressed in nonhematopoietic cells exposed to pro-inflammatory mediators such as IFN-γ and TNF-α (108). Therefore, inhibition of the immunoproteasome allows for both the targeting of immune-specific cells but also cells actively involved in the inflammatory response. In kidney transplantation, it was found that patients with chronic AMR have up-regulated immunoproteasome activity (109). Newly developed immunoproteasome inhibitors (IPI) could selectively inhibit proteasomes of cells involved in graft rejection after transplantation, such as B and T lymphocytes and APC’s, and regulate pro-inflammatory cytokines and the differentiation of helper T cells (110, 111). But similar to conventional PIs, PC population would be more sensitive on IPIs. Current work in animal models has found that IPI is superior to PI in suppressing the cellular and humoral immune response, preventing chronic AMR, and prolonging survival (110–112). ONX-0914, formerly known as PR-957, is an LMP7-selective immunoproteasome inhibitor that is undergoing clinical studies in the treatment of autoimmune diseases and has potential applications in transplantation (110, 111). ONX-0194 and bortezomib combined suppressed DSA production, B cells and plasma cells after kidney transplant, inhibited IgG, complement, and proinflammatory cytokines IFN-y and IL-17, and reduced chronic allograft nephropathy. In mismatched mouse cardiac transplantation, IPI treatment with a noncovalent LMP-7 inhibitor, DPLG3, combined with CTLA4-Ig led to decreased effector T cells and T cell exhaustion (113). Other IPI’s such as Ipsi-001 and PR-924 are currently under investigation as potential anti-cancer agents. IPI is particularly attractive due to its specificity on immune cells which shows larger safety margin compared to conventional immunoproteasome inhibitors (114, 115). This may allow the continuous (or long-term) treatment of IPI after transplantation in sensitized recipients.
Outside of multiple myeloma therapies, there is still a range of opportunities to target alloantibody reduction. Building on the success of PIs, there has been focus on inhibiting protein degradation via inhibition of initial ubiquitin binding rather than the downstream proteasome complex (116). Another promising avenue is modulating the endoplasmic reticulum (ER). Inositol-requiring enzyme 1 (IRE1) inhibitors are currently under development and may be available in the near future (117, 118).
Monoclonal Antibodies for Targeting Plasma Cells
Inhibiting proteasome activity with PI should affect more than plasma cell population since all eukaryotic cells utilize proteasome to maintain their homeostasis. Even IPI should have broad impact on immune cells. Therefore, monoclonal antibody targeting of plasma cell population is very attractive.
CD38 is expressed at high levels by B lineage progenitors in bone marrow, B-lymphocytes in germinal centers, and terminally differentiated plasma cells (119, 120). Conversely, mature naive and memory B cells express low levels of the molecule (121, 122). Plasma cells (PC) actively producing allo-antibodies should express high levels of CD38, thus resulting in a reasonable target for PC depletion in desensitization therapy (123) or deletion of plasma cells during active AMR (124). Daratumumab is a human IgGκ monoclonal antibody that targets CD38 and induces apoptosis of PC (122, 125) via Fcγ receptor-mediated cross-linking (126) and macrophage-mediated phagocytosis (127). In addition to depleting CD38+ cells, daratumumab also promotes expansion of memory and naïve T-cells (122), and is approved as monotherapy in patients with multiple myeloma (MM) (122, 125, 128, 129). Isatuximab, is an anti-CD38 mAb also used in the treatment of MM. It induces apoptosis of CD38+ cells through Fc-dependent and Fc-independent mechanisms (130), depletes B-lymphocyte precursors (131), and depletes NK cells through direct activation and crosslinking of CD38 and CD16 on NK cells (130). Elotuzumab is an IgG1 mAb that targets signaling lymphocytic activation molecule F7 (SLAMF7), also known as CD319, which is highly expressed on MM, NK and other immune cells (132). Elotuzumab was found to activate NK cells and induce apoptosis of SLAMF7+ cells via both CD16-dependent and CD16-independent mechanisms (132, 133).
There are only anecdotal cases evaluating monoclonal antibodies targeting PC in organ transplantation to prevent or treat antibody-mediated rejection. Daratumumab showed effective desensitization and reversed acute/chronic antibody-mediated rejection (134, 135). In our sensitized NHP model, we reported the effectiveness of daratumumab in combination with an anti-CXCR4 antagonist, plerixafor which mobilizes PC from bone marrow to peripheral blood (135). However, we also reported a possible off-target effect of daratumumab which result in depletion of other CD38 expressing regulatory cells including Treg, Breg, MDSC etc. This feature makes daratumumab attractive for multiple myeloma (122), but could trigger alloimmune responses in transplantation patients. Daratumumab and eculizumab combined therapy reduced dnDSAs, improved heart and kidney graft function, and resulted in undetectable circulating PCs. However, class II DSA returned after discontinuing daratumumab therapy (135). The second patient was a highly sensitized recipient who received daratumumab desensitization therapy prior to heart transplantation. After eight weeks, there was found to be reduction in cPRA (98% vs 62%) and class 1 anti-HLA antibodies (35 vs 14) (135). Currently, there is a phase 1 clinical trial to evaluate daratumumab in decreasing circulating antibodies in sensitized recipients awaiting heart transplantation (ClinicalTrials.gov, NCT04088903). There is also a clinical trial to evaluate the safety and efficacy of isatuximab as desensitization therapy in patients awaiting kidney transplantation (ClinicalTrials.gov, NCT04294459). If applied to transplant, these therapies from myeloma field need be carefully evaluated on their off-target effect in a transplantation setting.
Costimulation Blockade
Rebound of DSA after short-term PI has been reported (136–138). This repletion of PC and DSA would be partially due to an intra-marrow PC repopulation which might be related to PC populations resistant to PI treatment. In the meantime, PC population can expand outside of bone marrow. We observed that the depletion of PC with bortezomib initiated germinal center activation (138). This is probably due to the tightly intertwined network among humoral components. PC may provide a negative feedback loop to Tfh cells (or GC response) since these cell populations compete for similar cytokines/survival factors. Therefore, once one population, in this case PC, disappear then the other cell population (Tfh) is promoted (138). For this reason, targeting T cell help for B cell activation could be a potential strategy for desensitization, especially since the impact of costimulation blockade on humoral responses has been shown in multiple studies (139–141). We and others have reviewed this topic (142–145). It is notable that targeting PC together with costimulation signals successfully prevented the rapid rebound of DSA seen with PI monotherapy (146–149). This suggests that targeting a single humoral component might not be effective in controlling preformed or on-going allo-humoral responses.
Targeting Mediators/Survival Factor
Interleukin-6 Receptor Inhibition
Interleukin-6 (IL-6) is a pleiotropic cytokine produced by many different cell lineages. The membrane-bound IL-6 receptor (IL-6R) is expressed only on hepatocytes and some immune cells (150), but a soluble IL-6R also exists that can bind IL-6 and together this complex can signal through the transmembrane cytokine receptor gp130 (trans-signaling) expressed on nearly all cell types (151). IL-6 is critical for many inflammatory pathways and has a key role in the induction of follicular helper T cells, which direct naïve B cells in the germinal center to differentiate to memory B cells and high-affinity, IgG-secreting plasma cells (152). Accordingly, dysregulated production of IL-6 has been associated with chronic diseases such as diabetes, systemic lupus erythematosus, rheumatoid arthritis, cancer, end-stage renal disease, crescentic glomerulonephritis, and graft versus host disease (153–158). IL-6 has also been associated with deviation of T cells towards a Th17 phenotype, reduction of the proportion of Treg cells, and potentiation of allograft rejection in kidney transplantation (159).
Tocilizumab (Actemra®) is a humanized monoclonal antibody with activity against both the membrane and soluble forms of IL-6R approved to treat moderate to severe rheumatoid arthritis, systemic juvenile idiopathic arthritis, polyarticular juvenile idiopathic arthritis, and Castleman’s disease (151). Pharmacologic inhibition of IL-6 signaling is attractive in the context of desensitization strategies, as animal models have shown that this therapy reduces alloantibody responses by inhibition of bone marrow plasma cells and induction of Treg cells (160). Vo et al. recently examined the efficacy of high dose IVIG + tocilizumab in 10 highly sensitized patients who were poorly responsive to high dose IVIG + rituximab (161). This regimen was associated with reduced donor specific antibody number and strength, decreased wait list time, and increased rate of transplantation. No transplanted patients had evidence of antibody-mediated rejection on protocol biopsies. Larger, randomized control trials will be helpful in determining the ultimate value of this treatment given these promising preliminary results.
Anti-BAFF Agents
B cell activating factor (BAFF) is a homotrimer and member of the tumor necrosis factor (TNF) family that is found on the cell surface as a transmembrane protein or released in soluble form after cleavage (162). BAFF is secreted by multiple cell types, binds to three separate receptors, and is critical for the maturation of B cells (163). BAFF also acts as a potent B cell activator and is important in B cell proliferation and differentiation. Therefore, blocking this molecule may be essential when targeting allo-B cell response. A monoclonal antibody against BAFF, belimumab (Benlysta®), was the first targeted biologic approved for the treatment of systemic lupus erythematosus (164). Belimumab monotherapy was tested for desensitization in kidney transplantation (NCT01025193), but this trial was closed early due to a reported lack of efficacy. Blisibimod is a second anti-Baff agent developed for SLE. It is a fusion protein consisting of four BAFF binding domains. This anti-BAFF agent completed Phase II testing and currently being tested in a Phase 3 trial, CHABLIS-SC1 [(165), NCT01162681]. While considerable progress has been made in the field of desensitization, many potential and untested therapies remain. Other anti-BAFF agents including tabalumab, atacicept, and blisibimod have not been evaluated for desensitization in human trials.
A Multi-Modal Approach to Desensitization
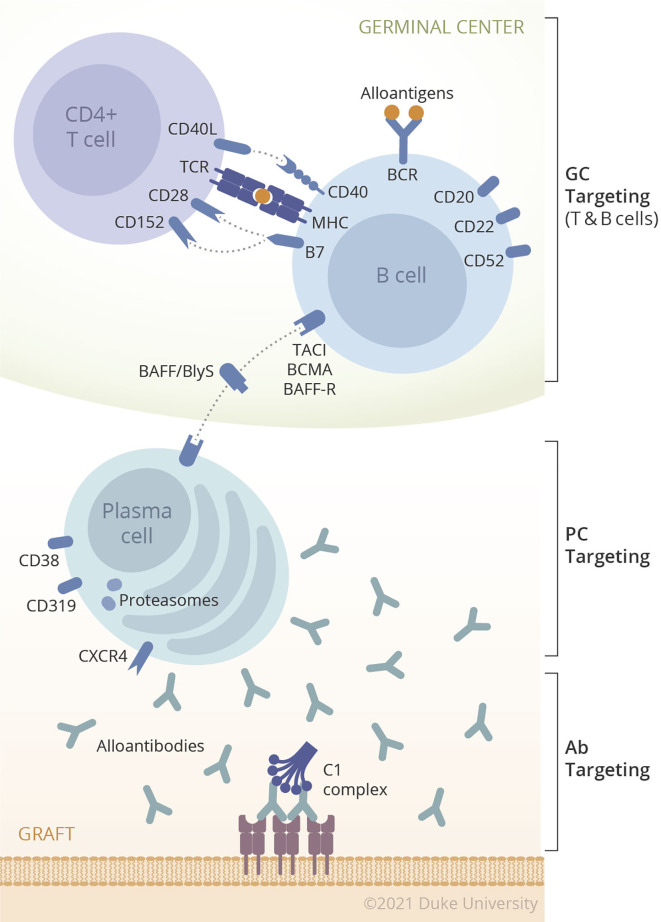
The concept of desensitization has been expanded from only targeting alloantibody (IVIG/IA/plasmapheresis) to instead targeting the upstream sources of antibody such as B cells (Rituximab) and PC (proteasome inhibitor). The conventional desensitization concept, removal of preformed antibody, may prevent hyperacute rejection or acute AMR but without long-lasting impact on humoral alloimmunity. While many desensitization therapies have been tried alone or in combination in animal models and human trials, none yet have solved the barriers to transplantation faced by highly sensitized patients with high titer HLA antibodies. The answer to desensitization may lie in novel therapies not yet tested or those outside the field of transplantation. Therefore, upcoming therapies targeting plasma cells are potentially very attractive. However, considering the previous sensitization events to HLA, allograft could trigger the memory response in sensitized patients. For this reason, targeting each component of humoral response such as alloantibody, B cells, or PC, would tentatively reduce the steady state level of DSA, this would not promote long-term control of humoral response after transplantation. Due to its compensatory mechanism, it would more logical that we develop strategies to desensitizing patients that target multiple steps of DSA production. Fortunately, there are many agents targeting each step of the humoral response as shown in Figure 1 and Table 1 . Unfortunately, there will also be too many potential combinations of biologics to permit exhaustive evaluation of each possible combination. Therefore, rational approaches merit testing in a preclinical model before being translated into the clinic.
Figure 1.
Multiple components of humoral immunity in organ transplantation.
Table 1.
Broad Overview of Possible Therapeutics for New Desensitization Regimens.
| Drug | Target | Development | Reference |
|---|---|---|---|
| B Cells | |||
| Ofatumumab | Anti-CD20 | FDA approval for CLL | (166, 167) |
| Ocrelizumab | Anti-CD20 | FDA approval for primary progressive multiple sclerosis | (168) |
| Ocaratuzumab | Anti-CD20 | Clinical trials | (169, 170) |
| Obinutuzumab | Anti-CD20 | FDA approved for CLL | (171) |
| Blisibimod | Anti-BAFF | Clinical trials | (165) |
| Tabalumab | Anti-BAFF | Clinical trials | (172–175) |
| Atacicpet | Anti-APRIL & Anti-BAFF | Clinical trials | (176) |
| BR3-Fc | Anti-BAFF | Clinical trials | (177, 178) |
| Belimumab | Anti-BAFF | FDA approval for SLE | (179) |
| hAPRIL.03A & hAPRIL.01A | Anti-APRIL | Pre-clinical | (180) |
| Epratuzumab | Anti-CD22 | Clinical trials | (181, 182) |
| Lucatumumab | Anti-CD40 | Clinical trials | (172, 183) |
| Dacetuzumab | Anti-CD40 | Clinical trials | (172, 184) |
| Galiximab | anti-CD80 | Clinical trials | (185, 186) |
| Plasma Cells | |||
| Indatuximab ravtansine | anti-CD138 | Clinical trials | (172, 187) |
| Isatuximab | Anti- CD38 | Clinical trials | (172, 188, 189) |
| Moxetumomab | anti-CD22 immunotoxin | Clinical trials | (190) |
| Siltuximab | IL-6 inhibitors | FDA approval for multicentric Castleman’s disease | (172, 191, 192) |
| Daratumumab | Anti-CD38 | FDA approval for multiple myeloma | (172, 191) |
| MOR202 | Anti-CD38 | Clinical trials | (172, 191) |
| Elotuzumab | Anti-CS1 | FDA approval for multiple myeloma | (193) |
| Milatuzumab | Anti-CD74 | Clinical trials | (172) |
| T Follicular Cells | |||
| Pembrolizumab | PD-1 inhibitor | FDA approval for unresectable or metastatic solid tumor | (191, 194) |
| Nivolumab | PD-1 inhibitor | FDA approval for inoperable or metastatic melanoma | (172, 191, 195–198) |
| Pidilizumab | PD-1 and DLL1 Inhibitor | Clinical trials | (191) |
| BGB-A317 | PD-1 inhibitor | Clinical trials | (199) |
| Durvalumab | PD-L1 | FDA approval for locally advanced or metastatic urothelial carcinoma | (200–202) |
| Ubiquitin-Proteasome Inhibitors | |||
| IPP-201101 | Spliceosomal peptide | Clinical trials | (203, 204) |
| Marizomib | Proteasome inhibitor | Clinical trials | (203, 205–209) |
| Delanzomib | Proteasome Inhibitor | Clinical trials | (203, 210) |
| Oprozomib | Proteasome Inhibitor | Clinical trials | (203) |
| IPSI-001 | Immunoproteasome | Pre-clinical | (115, 203, 211) |
| ONX-0914 | Immunoproteasome | Pre-clinical | (203, 212) |
| PR-924 | Immunoproteasome | Pre-clinical | (203, 213) |
| RO5045337 | Ubiquitin E3 ligase | Clinical trials | (203) |
| RO5503781 | Ubiquitin E3 ligase | Clinical trials | (203) |
| LCL161 | Ubiquitin E3 ligase | Clinical trials | (203, 214) |
| AEG 35156 | Ubiquitin E3 ligase | Clinical trials | (203, 215, 216) |
| Lenalidomide | Ubiquitin E3 ligase | FDA approval for multiple myeloma and myelodysplastic syndromes | (203) |
| Pomalidomide | Ubiquitin E3 ligase | FDA approval for relapsed and refractory multiple myeloma | (203) |
| Ubistatins | 19S proteasome | Pre-clinical | (203, 217) |
| b-AP15 | 19S *DUBs | Pre-clinical | (203) |
| P5091 | DUBs | Pre-clinical | (203) |
| P22077 | DUBs | Pre-clinical | (203) |
| WP-1130 | DUBs | Pre-clinical | (203) |
*DUB - Proteasome-associated deubiquitinases.
Author Contributions
AC, MM, and DO participated in literature search and wrote the manuscript. BE, JP, and KF participated in writing the manuscript. AJ critically reviewed the manuscript. JK and SK participated in writing and reviewing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health as part of the Nonhuman Primate Transplantation Tolerance Cooperative Study Group under U19AI131471 (awarded to SK) and Opportunities Pool Round 13 (awarded to JK).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Patel R, Terasaki PI. Significance of the Positive Crossmatch Test in Kidney Transplantation. New Engl J Med (1969) 280:735–9. 10.1056/NEJM196904032801401 [DOI] [PubMed] [Google Scholar]
- 2. Loupy A, Lefaucheur C. Antibody-Mediated Rejection of Solid-Organ Allografts. N Engl J Med (2018) 379:1150–60. 10.1056/NEJMra1802677 [DOI] [PubMed] [Google Scholar]
- 3. Valenzuela NM, Reed EF. Antibody-Mediated Rejection Across Solid Organ Transplants: Manifestations, Mechanisms, and Therapies. J Clin Invest (2017) 127:2492–504. 10.1172/JCI90597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yabu JM, Anderson MW, Kim D, Bradbury BD, Lou CD, Petersen J, et al. Sensitization From Transfusion in Patients Awaiting Primary Kidney Transplant. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association - European Renal Association. Nephrol Dialysis Transpl (2013) 28:2908–18. 10.1093/ndt/gft362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgins R, Lowe D, Daga S, Hathaway M, Williams C, Lam FT, et al. Pregnancy-Induced HLA Antibodies Respond More Vigorously After Renal Transplantation Than Antibodies Induced by Prior Transplantation. Hum Immunol (2015) 76:546–52. 10.1016/j.humimm.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 6. Picascia A, Grimaldi V, Sabia C, Napoli C. Comprehensive Assessment of Sensitizing Events and Anti-HLA Antibody Development in Women Awaiting Kidney Transplantation. Transpl Immunol (2016) 36:14–9. 10.1016/j.trim.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 7. El-Awar N, Terasaki P, Lazda V, Nikaein A, Manning C, Arnold AN. Almost All Patients Who Are Waiting for a Regraft of a Kidney Transplant Have Anti-HLA Antibodies. Transplant Proc (2002) 34:2531–2. 10.1016/S0041-1345(02)03520-0 [DOI] [PubMed] [Google Scholar]
- 8. Cecka JM, Calculated PRA. (CPRA): The New Measure of Sensitization for Transplant Candidates. Am J Transplant Off J Am Soc Transplantationand Am Soc Transplant Surgeons (2010) 10:26–9. 10.1111/j.1600-6143.2009.02927.x [DOI] [PubMed] [Google Scholar]
- 9. Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, et al. Optn/Srtr 2013 Annual Data Report: Kidney. Am J Transplant (2015) 15(Suppl 2):1–34. 10.1111/ajt.13195 [DOI] [PubMed] [Google Scholar]
- 10. Pruthi R, Hilton R, Pankhurst L, Mamode N, Hudson A, Roderick P, et al. Uk Renal Registry 16th Annual Report: Chapter 4 Demography of Patients Waitlisted for Renal Transplantation in the UK: National and Centre-Specific Analyses. Nephron Clin Pract (2013) 125:81–98. 10.1159/000360023 [DOI] [PubMed] [Google Scholar]
- 11. Claas FH, Witvliet MD, Duquesnoy RJ, Persijn GG, Doxiadis II. The Acceptable Mismatch Program as a Fast Tool for Highly Sensitized Patients Awaiting a Cadaveric Kidney Transplantation: Short Waiting Time and Excellent Graft Outcome. Transplantation (2004) 78:190–3. 10.1097/01.TP.0000129260.86766.67 [DOI] [PubMed] [Google Scholar]
- 12. Bray RA, Gebel HM. The New Kidney Allocation System (KAS) and the Highly Sensitized Patient: Expect the Unexpected. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surgeons (2014) 14:2917. 10.1111/ajt.12974 [DOI] [PubMed] [Google Scholar]
- 13. Parsons RF, Locke JE, Redfield RR, 3rd, Roll GR, Levine MH. Kidney Transplantation of Highly Sensitized Recipients Under the New Kidney Allocation System: A Reflection From Five Different Transplant Centers Across the United States. Hum Immunol (2017) 78:30–6. 10.1016/j.humimm.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gebel HM, Kasiske BL, Gustafson SK, Pyke J, Shteyn E, Israni AK, et al. Allocating Deceased Donor Kidneys to Candidates With High Panel-Reactive Antibodies. Clin J Am Soc Nephrol (2016) 11:505–11. 10.2215/CJN.07720715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gentry SE, Segev DL, Montgomery RA. A Comparison of Populations Served by Kidney Paired Donation and List Paired Donation. Am J Transplant (2005) 5:1914–21. 10.1111/j.1600-6143.2005.00964.x [DOI] [PubMed] [Google Scholar]
- 16. Ferrari P, Fidler S, Holdsworth R, Woodroffe C, Tassone G, Watson N, et al. High Transplant Rates of Highly Sensitized Recipients With Virtual Crossmatching in Kidney Paired Donation. Transplantation (2012) 94:744–9. 10.1097/TP.0b013e3182612967 [DOI] [PubMed] [Google Scholar]
- 17. Montgomery RA, Lonze BE, Jackson AM. Using Donor Exchange Paradigms With Desensitization to Enhance Transplant Rates Among Highly Sensitized Patients. Curr Opin Organ Transplant (2011) 16:439–43. 10.1097/MOT.0b013e32834897c1 [DOI] [PubMed] [Google Scholar]
- 18. Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, et al. Survival Benefit With Kidney Transplants From HLA-Incompatible Live Donors. New Engl J Med (2016) 374:940–50. 10.1056/nejmoa1508380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manook M, Koeser L, Ahmed Z, Robb M, Johnson R, Shaw O, et al. Post-Listing Survival for Highly Sensitised Patients on the UK Kidney Transplant Waiting List: A Matched Cohort Analysis. Lancet (2017) 389(10070):727–34. 10.1016/S0140-6736(16)31595-1 [DOI] [PubMed] [Google Scholar]
- 20. Yazdi MF, Baghianimoghadam M, Nazmiyeh H, Ahmadabadi AD, Adabi MA. Response to Plasmapheresis in Myasthenia Gravis Patients: 22 Cases Report. Rom J Intern Med (2012) 50:245–7. [PubMed] [Google Scholar]
- 21. Batocchi AP, Evoli A, Di Schino C, Tonali P. Therapeutic Apheresis in Myasthenia Gravis. Ther Apher (2000) 4:275–9. 10.1046/j.1526-0968.2000.004004275.x [DOI] [PubMed] [Google Scholar]
- 22. Pusey CD, Levy JB. Plasmapheresis in Immunologic Renal Disease. Blood Purif (2012) 33:190–8. 10.1159/000334155 [DOI] [PubMed] [Google Scholar]
- 23. Higgins R, Lowe D, Hathaway M, Lam FT, Kashi H, Tan LC, et al. Double Filtration Plasmapheresis in Antibody-Incompatible Kidney TransplantationTherapeutic Apheresis and Dialysis : Official Peer-Reviewed Journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. Ther Apher Dialysis (2010) 14:392–9. 10.1111/j.1744-9987.2010.00821.x [DOI] [PubMed] [Google Scholar]
- 24. Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, et al. Plasmapheresis and Intravenous Immune Globulin Provides Effective Rescue Therapy for Refractory Humoral Rejection and Allows Kidneys to be Successfully Transplanted Into Cross-Match-Positive Recipients. Transplantation (2000) 70:887–95. 10.1097/00007890-200009270-00006 [DOI] [PubMed] [Google Scholar]
- 25. Thielke J, DeChristopher PJ, Sankary H, Oberholzer J, Testa G, Benedetti E. Highly Successful Living Donor Kidney Transplantation After Conversion to Negative of a Previously Positive Flow-Cytometry Cross-Match by Pretransplant Plasmapheresis. Transplant Proc (2005) 37:643–4. 10.1016/j.transproceed.2004.12.063 [DOI] [PubMed] [Google Scholar]
- 26. Warren DS, Simpkins CE, Cooper M, Montgomery RA. Modulating Alloimmune Responses With Plasmapheresis and IVIG. Curr Drug Targets Cardiovasc Haematol Disord (2005) 5:215–22. 10.2174/1568006054064735 [DOI] [PubMed] [Google Scholar]
- 27. Lorenz M, Regele H, Schillinger M, Kletzmayr J, Haidbauer B, Derfler K, et al. Peritransplant Immunoadsorption: A Strategy Enabling Transplantation in Highly Sensitized Crossmatch-Positive Cadaveric Kidney Allograft Recipients. Transplantation (2005) 79:696–701. 10.1097/01.TP.0000148732.26761.FA [DOI] [PubMed] [Google Scholar]
- 28. Bartel G, Wahrmann M, Regele H, Kikić Z, Fischer G, Druml W, et al. Peritransplant Immunoadsorption for Positive Crossmatch Deceased Donor Kidney Transplantation. Am J Transplant (2010) 10:2033–42. 10.1111/j.1600-6143.2010.03226.x [DOI] [PubMed] [Google Scholar]
- 29. Morath C, Beimler J, Opelz G, Scherer S, Schmidt J, Macher-Goeppinger S, et al. Living Donor Kidney Transplantation in Crossmatch-Positive Patients Enabled by Peritransplant Immunoadsorption and Anti-CD20 Therapy. Transpl Int (2012) 25:506–17. 10.1111/j.1432-2277.2012.01447.x [DOI] [PubMed] [Google Scholar]
- 30. Hakim RM, Milford E, Himmelfarb J, Wingard R, Lazarus JM, Watt RM. Extracorporeal Removal of Anti-HLA Antibodies in Transplant Candidates. Am J Kidney Dis (1990) 16:423–31. 10.1016/S0272-6386(12)80054-0 [DOI] [PubMed] [Google Scholar]
- 31. Kazatchkine MD, Kaveri SV. Immunomodulation of Autoimmune and Inflammatory Diseases With Intravenous Immune Globulin. N Engl J Med (2001) 345:747–55. 10.1056/NEJMra993360 [DOI] [PubMed] [Google Scholar]
- 32. Jordan SC, Quartel AW, Czer LS, Admon D, Chen G, Fishbein MC, et al. Posttransplant Therapy Using High-Dose Human Immunoglobulin (Intravenous Gammaglobulin) to Control Acute Humoral Rejection in Renal and Cardiac Allograft Recipients and Potential Mechanism of Action. Transplantation (1998) 66:800–5. 10.1097/00007890-199809270-00017 [DOI] [PubMed] [Google Scholar]
- 33. Basta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, et al. F(Ab)’2-Mediated Neutralization of C3a and C5a Anaphylatoxins: A Novel Effector Function of Immunoglobulins. Nat Med (2003) 9:431–8. 10.1038/nm836 [DOI] [PubMed] [Google Scholar]
- 34. Lutz HU, Stammler P, Bianchi V, Trüeb RM, Hunziker T, Burger R, et al. Intravenously Applied IgG Stimulates Complement Attenuation in a Complement Dependent Autoimmune Disease at the Amplifying C3 Convertase Level. Blood (2004) 103:465–72. 10.1182/blood-2003-05-1530 [DOI] [PubMed] [Google Scholar]
- 35. Dalakas MC. The Use of Intravenous Immunoglobulin in the Treatment of Autoimmune Neuromuscular Diseases: Evidence-Based Indications and Safety Profile. Pharmacol Ther (2004) 102:177–93. 10.1016/j.pharmthera.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 36. Samuelsson A, Towers TL, Ravetch JV. Anti-Inflammatory Activity of IVIG Mediated Through the Inhibitory Fc Receptor. Science (2001) 291:484–6. 10.1126/science.291.5503.484 [DOI] [PubMed] [Google Scholar]
- 37. Ahmadi F, Dashti-Khavidaki S, Khatami MR, Gatmiri M, Ahmadi F, Mahdavi-Mazdeh M, et al. Comparing Plasmapheresis Plus IVIg With Plasmapheresis Plus IVIg Plus Rituximab on the Management of Suspicious Antibody-Mediated Acute Rejection in Kidney Transplant Recipients. Int J Organ Transplant Med (2019) 10:127–36. [PMC free article] [PubMed] [Google Scholar]
- 38. Rocha PN, Butterly DW, Greenberg A, Reddan DN, Tuttle-Newhall J, Collins BH, et al. Beneficial Effect of Plasmapheresis and Intravenous Immunoglobulin on Renal Allograft Survival of Patients With Acute Humoral Rejection. Transplantation (2003) 75:1490–5. 10.1097/01.TP.0000060252.57111.AC [DOI] [PubMed] [Google Scholar]
- 39. Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, et al. National Conference to Assess Antibody-Mediated Rejection in Solid Organ Transplantation. Am J Transplant (2004) 4:1033–41. 10.1111/j.1600-6143.2004.00500.x [DOI] [PubMed] [Google Scholar]
- 40. Gloor JM, DeGoey SR, Pineda AA, Moore SB, Prieto M, Nyberg SL, et al. Overcoming a Positive Crossmatch in Living-Donor Kidney Transplantation. Am J Transplant (2003) 3:1017–23. 10.1034/j.1600-6143.2003.00180.x [DOI] [PubMed] [Google Scholar]
- 41. Magee CC, Felgueiras J, Tinckam K, Malek S, Mah H, Tullius S. Renal Transplantation in Patients With Positive Lymphocytotoxicity Crossmatches: One Center’s Experience. Transplantation (2008) 86:96–103. 10.1097/TP.0b013e318176ae2c [DOI] [PubMed] [Google Scholar]
- 42. Haririan A, Nogueira J, Kukuruga D, Schweitzer E, Hess J, Gurk-Turner C, et al. Positive Cross-Match Living Donor Kidney Transplantation: Longer-Term Outcomes. Am J Transplant (2009) 9:536–42. 10.1111/j.1600-6143.2008.02524.x [DOI] [PubMed] [Google Scholar]
- 43. Thielke JJ, West-Thielke PM, Herren HL, Bareato U, Ommert T, Vidanovic V, et al. Living Donor Kidney Transplantation Across Positive Crossmatch: The University of Illinois at Chicago Experience. Transplantation (2009) 87:268–73. 10.1097/TP.0b013e3181919a16 [DOI] [PubMed] [Google Scholar]
- 44. Jordan SC, Tyan D, Stablein D, McIntosh M, Rose S, Vo A, et al. Evaluation of Intravenous Immunoglobulin as an Agent to Lower Allosensitization and Improve Transplantation in Highly Sensitized Adult Patients With End-Stage Renal Disease: Report of the NIH IG02 Trial. J Am Soc Nephrol (2004) 15:3256–62. 10.1016/j.trre.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 45. Glotz D, Antoine C, Julia P, Suberbielle-Boissel C, Boudjeltia S, Fraoui R, et al. Desensitization and Subsequent Kidney Transplantation of Patients Using Intravenous Immunoglobulins (Ivig). Am J Transplant (2002) 2:758–60. 10.1034/j.1600-6143.2002.20809.x [DOI] [PubMed] [Google Scholar]
- 46. Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and Intravenous Immune Globulin for Desensitization During Renal Transplantation. N Engl J Med (2008) 359:242–51. 10.1056/NEJMoa0707894 [DOI] [PubMed] [Google Scholar]
- 47. Vo AA, Peng A, Toyoda M, Kahwaji J, Cao K, Lai CH, et al. Use of Intravenous Immune Globulin and Rituximab for Desensitization of Highly HLA-sensitized Patients Awaiting Kidney Transplantation. Transplantation (2010) 89:1095–102. 10.1097/TP.0b013e3181d21e7f [DOI] [PubMed] [Google Scholar]
- 48. Clatworthy MR. Targeting B Cells and Antibody in Transplantation. Am J Transplant (2011) 11:1359–67. 10.1111/j.1600-6143.2011.03554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loupy A, Suberbielle-Boissel C, Zuber J, Anglicheau D, Timsit MO, Martinez F, et al. Combined Posttransplant Prophylactic IVIg/Anti-CD 20/Plasmapheresis in Kidney Recipients With Preformed Donor-Specific Antibodies: A Pilot Study. Transplantation (2010) 89:1403–10. 10.1097/TP.0b013e3181da1cc3 [DOI] [PubMed] [Google Scholar]
- 50. Vieira CA, Agarwal A, Book BK, Sidner RA, Bearden CM, Gebel HM, et al. Rituximab for Reduction of Anti-HLA Antibodies in Patients Awaiting .Renal Transplantation: 1. Safety, Pharmacodynamics, and Pharmacokinetics. Transplantation (2004) 77:542–8. 10.1097/01.TP.0000112934.12622.2B [DOI] [PubMed] [Google Scholar]
- 51. Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of Rituximab in Kidney Allotransplantation. Am J Transplant (2006) 6:2418–28. 10.1111/j.1600-6143.2006.01497.x [DOI] [PubMed] [Google Scholar]
- 52. Susal C, Dohler B, Opelz G. Presensitized Kidney Graft Recipients With HLA Class I and II Antibodies are at Increased Risk for Graft Failure: A Collaborative Transplant Study Report. Hum Immunol (2009) 70:569–73. 10.1016/j.humimm.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 53. Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, et al. Outcome of Subclinical Antibody-Mediated Rejection in Kidney Transplant Recipients With Preformed Donor-Specific Antibodies. Am J Transplant (2009) 9:2561–70. 10.1111/j.1600-6143.2009.02813.x [DOI] [PubMed] [Google Scholar]
- 54. Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, et al. Baseline Donor-Specific Antibody Levels and Outcomes in Positive Crossmatch Kidney Transplantation. Am J Transplant (2010) 10:582–9. 10.1111/j.1600-6143.2009.02985.x [DOI] [PubMed] [Google Scholar]
- 55. Chih S, Patel J. Desensitization Strategies in Adult Heart transplantation-Will Persistence Pay Off? J Heart Lung Transplant (2016) 35:962–72. 10.1016/j.healun.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 56. Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th Adult Lung and Heart-Lung Transplant Report-2012. J Heart Lung Transplant (2012) 31:1073–86. 10.1016/j.healun.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 57. Tinckam KJ, Keshavjee S, Chaparro C, Barth D, Azad S, Binnie M, et al. Survival in Sensitized Lung Transplant Recipients With Perioperative Desensitization. Am J Transplant (2015) 15:417–26. 10.1111/ajt.13076 [DOI] [PubMed] [Google Scholar]
- 58. Verleden SE, Todd JL, Sato M, Palmer SM, Martinu T, Pavlisko EN, et al. Impact of CLAD Phenotype on Survival After Lung Retransplantation: A Multicenter Study. Am J Transplant (2015) 15:2223–30. 10.1111/ajt.13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thabut G, Mal H. Outcomes After Lung Transplantation. J Thorac Dis (2017) 9:2684–91. 10.21037/jtd.2017.07.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Todd JL, Finlen Copeland CA, Neely M, Palmer SM. (604) - FVC and FEV1 Decline in Chronic Lung Allograft Dysfunction (Clad) Phenotypes. J Heart Lung Transplant (2016) 35:S223–4. 10.1016/j.healun.2016.01.633 [DOI] [Google Scholar]
- 61. Schmauss D, Weis M. Cardiac Allograft Vasculopathy: Recent Developments. Circulation (2008) 117:2131–41. 10.1161/CIRCULATIONAHA.107.711911 [DOI] [PubMed] [Google Scholar]
- 62. Lund LH, Edwards LB, Dipchand AI, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant (2016) 35:1158–69. 10.1016/j.healun.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 63. Tremblay-Gravel M, Racine N, Ducharme A, Pelletier GB, Giraldeau G, Liszkowski M, et al. Changes in Prevalence, Progression and Outcomes of Coronary Allograft Vasculopathy Over 25 Years Following Cardiac Transplantation: A Single Center Experience. J Am Coll Cardiol (2017) 69:939. 10.1016/S0735-1097(17)34328-0 28231946 [DOI] [Google Scholar]
- 64. Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of Pretransplant Panel-Reactive Antibody on Outcomes in 8,160 Heart Transplant Recipients in Recent Era. Ann Thorac Surg (2007) 84:1556–1562; discussion 1562-1553. 10.1016/j.athoracsur.2007.05.095 [DOI] [PubMed] [Google Scholar]
- 65. Shankar N, Daly R, Geske J, Kushwaha SK, Timmons M, Joyce L, et al. LVAD Implant as a Bridge to Heart Transplantation is Associated With Allosensitization as Measured by Single Antigen Bead Assay. Transplantation (2013) 96:324–30. 10.1097/TP.0b013e3182985371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Joyce DL, Southard RE, Torre-Amione G, Noon GP, Land GA, Loebe M. Impact of Left Ventricular Assist Device (LVAD)-Mediated Humoral Sensitization on Post-Transplant Outcomes. J Heart Lung Transplant (2005) 24:2054–9. 10.1016/j.healun.2005.06.028 [DOI] [PubMed] [Google Scholar]
- 67. Pamboukian SV, Costanzo MR, Dunlap S, Rayburn B, Westfall AO, You ZY, et al. Relationship Between Bridging With Ventricular Assist Device on Rejection After Heart Transplantation. J Heart Lung Transplant (2005) 24:310–5. 10.1016/j.healun.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 68. Ko BS, Drakos S, Kfoury AG, Hurst D, Stoddard GJ, Willis CA, et al. Immunologic Effects of Continuous-Flow Left Ventricular Assist Devices Before and After Heart Transplant. J Heart Lung Transplant (2016) 35:1024–30. 10.1016/j.healun.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 69. John R, Lietz K, Schuster M, Naka Y, Rao V, Mancini DM, et al. Immunologic Sensitization in Recipients of Left Ventricular Assist Devices. J Thorac Cardiovasc Surg (2003) 125:578–91. 10.1067/mtc.2003.30 [DOI] [PubMed] [Google Scholar]
- 70. Alba AC, Tinckam K, Foroutan F, Nelson LM, Gustafsson F, Sander K, et al. Factors Associated With Anti-Human Leukocyte Antigen Antibodies in Patients Supported With Continuous-Flow Devices and Effect on Probability of Transplant and Posttransplant Outcomes. J Heart Lung Transplant (2015) 34:685–92. 10.1016/j.healun.2014.11.024 [DOI] [PubMed] [Google Scholar]
- 71. Ankersmit HJ, Edwards NM, Schuster M, John R, Kocher A, Rose EA, et al. Quantitative Changes in T-cell Populations After Left Ventricular Assist Device Implantation: Relationship to T-cell Apoptosis and Soluble CD95. Circulation (1999) 100:Ii211–215. 10.1161/01.CIR.100.suppl_2.II-211 [DOI] [PubMed] [Google Scholar]
- 72. Thyagarajan B, Kumar MP, Sikachi RR, Agrawal A. Endocarditis in Left Ventricular Assist Device. Intractable Rare Dis Res (2016) 5:177–84. 10.5582/irdr.2016.01049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ankersmit HJ, Tugulea S, Spanier T, Weinberg AD, Artrip JH, Burke EM, et al. Activation-Induced T-cell Death and Immune Dysfunction After Implantation of Left-Ventricular Assist Device. Lancet (1999) 354:550–5. 10.1016/S0140-6736(98)10359-8 [DOI] [PubMed] [Google Scholar]
- 74. Johansson BP, Shannon O, Bjorck L. IdeS: A Bacterial Proteolytic Enzyme With Therapeutic Potential. PloS One (2008) 3:e1692. 10.1371/journal.pone.0001692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jarnum S, Bockermann R, Runstrom A, Winstedt L, Kjellman C. The Bacterial Enzyme IdeS Cleaves the IgG-Type of B Cell Receptor (Bcr), Abolishes Bcr-Mediated Cell Signaling, and Inhibits Memory B Cell Activation. J Immunol (Baltimore Md. 1950) (2015) 195:5592–601. 10.4049/jimmunol.1501929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jordan SC, Lorant T, Choi J. Igg Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. New Engl J Med (2017) 377:442–53. 10.1056/NEJMoa1612567 [DOI] [PubMed] [Google Scholar]
- 77. Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, et al. Functional Expression of the MHC Class I-related Receptor, FcRn, in Endothelial Cells of Mice. Int Immunol (1998) 10:1289–98. 10.1093/intimm/10.9.1289 [DOI] [PubMed] [Google Scholar]
- 78. Sockolosky JT, Szoka FC. The Neonatal Fc Receptor, FcRn, as a Target for Drug Delivery and Therapy. Adv Drug Delivery Rev (2015) 91:109–24. 10.1016/j.addr.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Raghavan M, Chen MY, Gastinel LN, Bjorkman PJ. Investigation of the Interaction Between the Class I MHC-Related Fc Receptor and its Immunoglobulin G Ligand. Immunity (1994) 1:303–15. 10.1016/1074-7613(94)90082-5 [DOI] [PubMed] [Google Scholar]
- 80. Brambell FW. The Transmission of Immune Globulins From the Mother to the Foetal and Newborn Young. Proc Nutr Soc (1969) 28:35–41. 10.1079/PNS19690007 [DOI] [PubMed] [Google Scholar]
- 81. Jordan SC, Ammerman N, Vo A. Implications of Fc Neonatal Receptor (Fcrn) Manipulations for Transplant Immunotherapeutics. Transplantation (2020) 104:17–23. 10.1097/TP.0000000000002912 [DOI] [PubMed] [Google Scholar]
- 82. Getman KE, Balthasar JP. Pharmacokinetic Effects of 4C9, an Anti-FcRn Antibody, in Rats: Implications for the Use of FcRn Inhibitors for the Treatment of Humoral Autoimmune and Alloimmune Conditions. J Pharm Sci (2005) 94:718–29. 10.1002/jps.20297 [DOI] [PubMed] [Google Scholar]
- 83. Ward ES, Devanaboyina SC, Ober RJ. Targeting FcRn for the Modulation of Antibody Dynamics. Mol Immunol (2015) 67:131–41. 10.1016/j.molimm.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bleeker WK, Teeling JL, Hack CE. Accelerated Autoantibody Clearance by Intravenous Immunoglobulin Therapy: Studies in Experimental Models to Determine the Magnitude and Time Course of the Effect. Blood (2001) 98:3136–42. 10.1182/blood.V98.10.3136 [DOI] [PubMed] [Google Scholar]
- 85. Ling LE, Hillson JL, Tiessen RG, Bosje T, van Iersel MP, Nix DJ, et al. M281, an Anti-FcRn Antibody: Pharmacodynamics, Pharmacokinetics, and Safety Across the Full Range of IgG Reduction in a First-in-Human Study. Clin Pharmacol Ther (2019) 105:1031–9. 10.1002/cpt.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Smith B, Kiessling A, Lledo-Garcia R, Dixon KL, Christodoulou L, Catley MC, et al. Generation and Characterization of a High Affinity Anti-Human FcRn Antibody, Rozanolixizumab, and the Effects of Different Molecular Formats on the Reduction of Plasma IgG Concentration. MAbs (2018) 10:1111–30. 10.1080/19420862.2018.1505464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kiessling P, Lledo-Garcia R, Watanabe S, Langdon G, Tran D, Bari M, et al. The FcRn Inhibitor Rozanolixizumab Reduces Human Serum IgG Concentration: A Randomized Phase 1 Study. Sci Transl Med (2017) 9. 10.1126/scitranslmed.aan1208 [DOI] [PubMed] [Google Scholar]
- 88. Seijsing J, Yu S, Frejd FY, Höiden-Guthenberg I, Gräslund T. In Vivo Depletion of Serum IgG by an Affibody Molecule Binding the Neonatal Fc Receptor. Sci Rep (2018) 8:5141. 10.1038/s41598-018-23481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu L, Garcia AM, Santoro H, Zhang Y, McDonnell K, Dumont J, et al. Amelioration of Experimental Autoimmune Myasthenia Gravis in Rats by Neonatal FcR Blockade. J Immunol (2007) 178:5390–8. 10.4049/jimmunol.178.8.5390 [DOI] [PubMed] [Google Scholar]
- 90. Patel DA, Puig-Canto A, Challa DK, Perez Montoyo H, Ober RJ, Ward ES. Neonatal Fc Receptor Blockade by Fc Engineering Ameliorates Arthritis in a Murine Model. J Immunol (2011) 187:1015–22. 10.4049/jimmunol.1003780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-Lived Plasma Cells Are Contained Within the CD19(-)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity (2015) 43:132–45. 10.1016/j.immuni.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC, et al. Prospective Iterative Trial of Proteasome Inhibitor-Based Desensitization. Am J Transplant (2015) 15:101–18. 10.1111/ajt.13050 [DOI] [PubMed] [Google Scholar]
- 93. Jeong JC, Jambaldorj E, Kwon HY, Kim MG, Im HJ, Jeon HJ, et al. Desensitization Using Bortezomib and High-dose Immunoglobulin Increases Rate of Deceased Donor Kidney Transplantation. Med (Baltimore) (2016) 95:e2635. 10.1097/MD.0000000000002635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kouroukis TC, Baldassarre FG, Haynes AE, Imrie K, Reece DE, Cheung MC. Bortezomib in Multiple Myeloma: Systematic Review and Clinical Considerations. Curr Oncol (Toronto Ont.) (2014) 21:e573–603. 10.3747/co.21.1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, et al. Bortezomib Provides Effective Therapy for Antibody- and Cell-Mediated Acute Rejection. Transplantation (2008) 86:1754–61. 10.1097/TP.0b013e318190af83 [DOI] [PubMed] [Google Scholar]
- 96. Mulder A, Heidt S, Vergunst M, Roelen DL, Claas FH. Proteasome Inhibition Profoundly Affects Activated Human B Cells. Transplantation (2013) 95:1331–7. 10.1097/TP.0b013e3182911739 [DOI] [PubMed] [Google Scholar]
- 97. De Sousa-Amorim E, Revuelta I, Diekmann F, Cofan F, Lozano M, Cid J, et al. Bortezomib for Refractory Acute Antibody-Mediated Rejection in Kidney Transplant Recipients: A Single-Centre Case Series. Nephrol (Carlton Vic.) (2016) 21:700–4. 10.1111/nep.12659 [DOI] [PubMed] [Google Scholar]
- 98. Tzvetanov I, Spaggiari M, Joseph J, Jeon H, Thielke J, Oberholzer J, et al. The Use of Bortezomib as a Rescue Treatment for Acute Antibody-Mediated Rejection: Report of Three Cases and Review of Literature. Transplant Proc (2012) 44:2971–5. 10.1016/j.transproceed.2012.02.037 [DOI] [PubMed] [Google Scholar]
- 99. Diwan TS, Raghavaiah S, Burns JM, Kremers WK, Gloor JM, Stegall MD. The Impact of Proteasome Inhibition on Alloantibody-Producing Plasma Cells In Vivo. Transplantation (2011) 91:536–41. 10.1097/TP.0b013e3182081333 [DOI] [PubMed] [Google Scholar]
- 100. Moreno Gonzales MA, Gandhi MJ, Schinstock CA, Moore NA, Smith BH, Braaten NY, et al. 32 Doses of Bortezomib for Desensitization is Not Well Tolerated and Is Associated With Only Modest Reductions in Anti-HLA Antibody. Transplantation (2017) 101:1222–7. 10.1097/TP.0000000000001330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A Phase 2 Study of Single-Agent Carfilzomib (PX-171-003-A1) in Patients With Relapsed and Refractory Multiple Myeloma. Blood (2012) 120:2817–25. 10.1182/blood-2012-05-425934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tremblay S, Driscoll JJ, Rike-Shields A, Hildeman DA, Alloway RR, Girnita AL, et al. A Prospective, Iterative, Adaptive Trial of Carfilzomib-Based Desensitization. Am J Transplant (2020) 20:411–21. 10.1111/ajt.15613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee EC, Fitzgerald M, Bannerman B, Donelan J, Bano K, Terkelsen J, et al. Antitumor Activity of the Investigational Proteasome Inhibitor MLN9708 in Mouse Models of B-cell and Plasma Cell Malignancies. Clin Cancer Res (2011) 17:7313–23. 10.1158/1078-0432.CCR-11-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kupperman E, Lee EC, Cao Y, Bannerman B, Fitzgerald M, Berger A, et al. Evaluation of the Proteasome Inhibitor MLN9708 in Preclinical Models of Human Cancer. Cancer Res (2010) 70:1970–80. 10.1158/0008-5472.CAN-09-2766 [DOI] [PubMed] [Google Scholar]
- 105. Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, et al. Safety and Tolerability of Ixazomib, an Oral Proteasome Inhibitor, in Combination With Lenalidomide and Dexamethasone in Patients With Previously Untreated Multiple Myeloma: An Open-Label Phase 1/2 Study. Lancet Oncol (2014) 15:1503–12. 10.1016/S1470-2045(14)71125-8 [DOI] [PubMed] [Google Scholar]
- 106. Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med (2016) 374:1621–34. 10.1056/NEJMoa1516282 [DOI] [PubMed] [Google Scholar]
- 107. Johnson H, Anderl JL, Bradley EK, Bui J, Jones J, Arastu-Kapur S, et al. Discovery of Highly Selective Inhibitors of the Immunoproteasome Low Molecular Mass Polypeptide 2 (Lmp2) Subunit. ACS Med Chem Lett (2017) 8:413–7. 10.1021/acsmedchemlett.6b00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McCarthy MK, Weinberg JB. The Immunoproteasome and Viral Infection: A Complexregulator of Inflammation. Front Microbiol (2015) 6:21. 10.3389/fmicb.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ashton-Chess J, Mai HL, Jovanovic V, Renaudin K, Foucher Y, Giral M, et al. Immunoproteasome Beta Subunit 10 is Increased in Chronic Antibody Mediated Rejection. Kidney Int (2010) 77:880–90. 10.1038/ki.2010.15 [DOI] [PubMed] [Google Scholar]
- 110. Li J, Basler M, Alvarez G, Brunner T, Kirk CJ, Groettrup M. Immunoproteasome Inhibition Prevents Chronic Antibody-Mediated Allograft Rejection in Renal Transplantation. Kidney Int (2018) 93:670–80. 10.1016/j.kint.2017.09.023 [DOI] [PubMed] [Google Scholar]
- 111. Li J, Koerner J, Basler M, Brunner T, Kirk CJ, Groettrup M. Immunoproteasome Inhibition Induces Plasma Cell Apoptosis and Preserves Kidney Allografts by Activating the Unfolded Protein Response and Suppressing Plasma Cell Survival Factors. Kidney Int (2019) 95:611–23. 10.1016/j.kint.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 112. Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I. A Comparative Analysis Between Proteasome and Immunoproteasome Inhibition in Cellular and Humoral Alloimmunity. Int Immunopharmacol (2017) 50:48–54. 10.1016/j.intimp.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 113. Sula Karreci E, Fan H, Uehara M, Mihali AB, Singh PK, Kurdi AT, et al. Brief Treatment With a Highly Selective Immunoproteasome Inhibitor Promotes Long-Term Cardiac Allograft Acceptance in Mice. Proc Natl Acad Sci USA (2016) 113:E8425–e8432. 10.1073/pnas.1618548114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wehenkel M, Ban JO, Ho YK, Carmony KC, Hong JT. Kim, K. B. A Selective Inhibitor of the Immunoproteasome Subunit LMP2 Induces Apoptosis in PC-3 Cells and Suppresses Tumour Growth in Nude Mice. Br J Cancer (2012) 107:53–62. 10.1038/bjc.2012.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kuhn DJ, Hunsucker SA, Chen Q, Voorhees PM, Orlowski M, Orlowski RZ. Targeted Inhibition of the Immunoproteasome is a Potent Strategy Against Models of Multiple Myeloma That Overcomes Resistance to Conventional Drugs and Nonspecific Proteasome Inhibitors. Blood (2009) 113:4667–76. 10.1182/blood-2008-07-171637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Anchoori RK, Karanam B, Peng S, Wang JW, Jiang R, Tanno T, et al. A Bis-Benzylidine Piperidone Targeting Proteasome Ubiquitin Receptor RPN13/ADRM1 as a Therapy for Cancer. Cancer Cell (2013) 24:791–805. 10.1016/j.ccr.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Volkmann K, Lucas JL, Vuga D, Wang X, Brumm D, Stiles C, et al. Potent and Selective Inhibitors of the Inositol-Requiring Enzyme 1 Endoribonuclease. J Biol Chem (2011) 286:12743–55. 10.1074/jbc.M110.199737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Woodle ES, Tremblay S, Driscoll J. Targeting Plasma Cells With Proteasome Inhibitors: Principles From Primates. J Am Soc Nephrol (2017) 28:1951–3. 10.1681/ASN.2017040443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lin P, Owens R, Tricot G, Wilson CS. Flow Cytometric Immunophenotypic Analysis of 306 Cases of Multiple Myeloma. Am J Clin Pathol (2004) 121:482–8. 10.1309/74R4TB90BUWH27JX [DOI] [PubMed] [Google Scholar]
- 120. Santonocito AM, Consoli U, Bagnato S, Milone G, Palumbo GA, Di Raimondo F, et al. Flow Cytometric Detection of Aneuploid CD38(++) Plasmacells and CD19(+) B-Lymphocytes in Bone Marrow, Peripheral Blood and PBSC Harvest in Multiple Myeloma Patients. Leuk Res (2004) 28:469–77. 10.1016/j.leukres.2003.09.015 [DOI] [PubMed] [Google Scholar]
- 121. Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol Rev (2008) 88:841–86. 10.1152/physrev.00035.2007 [DOI] [PubMed] [Google Scholar]
- 122. Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab Depletes CD38+ Immune Regulatory Cells, Promotes T-cell Expansion, and Skews T-cell Repertoire in Multiple Myeloma. Blood (2016) 128:384–94. 10.1182/blood-2015-12-687749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Martin TG, Corzo K, Chiron M, Velde HV, Abbadessa G, Campana F, et al. Therapeutic Opportunities With Pharmacological Inhibition of CD38 With Isatuximab. Cells (2019) 8:1522. 10.3390/cells8121522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jordan SC, Choi J, Vo A. Achieving Incompatible Transplantation Through Desensitization: Current Perspectives and Future Directions. Immunotherapy (2015) 7:377–98. 10.2217/imt.15.10 [DOI] [PubMed] [Google Scholar]
- 125. Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab Plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med (2019) 380:2104–15. 10.1056/NEJMoa1817249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The Therapeutic Cd38 Monoclonal Antibody Daratumumab Induces Programmed Cell Death Via Fcγ Receptor-Mediated Cross-Linking. J Immunol (2016) 197:807–13. 10.4049/jimmunol.1501351 [DOI] [PubMed] [Google Scholar]
- 127. Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, et al. Antibody-Mediated Phagocytosis Contributes to the Anti-Tumor Activity of the Therapeutic Antibody Daratumumab in Lymphoma and Multiple Myeloma. MAbs (2015) 7:311–21. 10.1080/19420862.2015.1007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 With Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med (2015) 373:1207–19. 10.1056/NEJMoa1506348 [DOI] [PubMed] [Google Scholar]
- 129. Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med (2016) 375:754–66. 10.1056/NEJMoa1606038 [DOI] [PubMed] [Google Scholar]
- 130. Zhu C, Song Z, Wang A, Srinivasan S, Yang G, Greco R, et al. Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front Immunol (2020) 11:1771. 10.3389/fimmu.2020.01771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Moreno L, Perez C, Zabaleta A, Manrique I, Alignani D, Ajona D, et al. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin Cancer Res (2019) 25:3176–87. 10.1158/1078-0432.CCR-18-1597 [DOI] [PubMed] [Google Scholar]
- 132. Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab Plus Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med (2018) 379:1811–22. 10.1056/NEJMoa1805762 [DOI] [PubMed] [Google Scholar]
- 133. Pazina T, James AM, Colby KB, Yang Y, Gale A, Jhatakia A, et al. Enhanced SLAMF7 Homotypic Interactions by Elotuzumab Improves NK Cell Killing of Multiple Myeloma. Cancer Immunol Res (2019) 7:1633–46. 10.1158/2326-6066.CIR-18-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Doberer K, Kläger J, Gualdoni GA, Mayer KA, Eskandary F, Farkash EA, et al. Cd38 Antibody Daratumumab for the Treatment of Chronic Active Antibody-mediated Kidney Allograft Rejection. Transplantation (2021) 105:451–7. 10.1097/TP.0000000000003247 [DOI] [PubMed] [Google Scholar]
- 135. Kwun J, Matignon M, Manook M, Guendouz S, Audard V, Kheav D, et al. Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use. J Am Soc Nephrol (2019) 30:1206–19. 10.1681/ASN.2018121254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Woodle ES, Tremblay S, Rossi A, Rojas CC, Alloway R, Roskin K, et al. Plasma Cell Targeting to Prevent Antibody-Mediated Rejection. Am J Transplant (2020) 20(Suppl 4):33–41. 10.1111/ajt.15889 [DOI] [PubMed] [Google Scholar]
- 137. Woodle ES, Tremblay S, Brailey P, Girnita A, Alloway RR, Aronow B, et al. Proteasomal Adaptations Underlying Carfilzomib-Resistance in Human Bonemarrow Plasma Cells. Am J Transplant (2020) 20:399–410. 10.1111/ajt.15634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kwun J, Burghuber C, Manook M, Iwakoshi N, Gibby A, Hong JJ, et al. Humoral Compensation After Bortezomib Treatment of Allosensitized Recipients. J Am Soc Nephrol (2017) 28:1991–6. 10.1681/ASN.2016070727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Durrbach A, Pestana JM, Florman S, Del Carmen Rial M, Rostaing L, Kuypers D, et al. Long-Term Outcomes in Belatacept- Versus Cyclosporine-Treated Recipients of Extended Criteria Donor Kidneys: Final Results From Benefit-EXT, a Phase Iii Randomized Study. Am J Transplant (2016) 16:3192–201. 10.1111/ajt.13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med (2016) 374:333–43. 10.1056/NEJMoa1506027 [DOI] [PubMed] [Google Scholar]
- 141. Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB, Iwakoshi NN, et al. Costimulation Blockade Alters Germinal Center Responses and Prevents Antibody-Mediated Rejection. Am J Transplant (2014) 14:59–69. 10.1111/ajt.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Thaunat O, Koenig A, Leibler C, Grimbert P. Effect of Immunosuppressive Drugs on Humoral Allosensitization After Kidney Transplant. J Am Soc Nephrol (2016) 27:1890–900. 10.1681/ASN.2015070781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kwun J, Manook M, Page E, Burghuber C, Hong J, Knechtle S. J. Crosstalk Between T and B Cells in the Germinal Center After Transplantation. Transplantation (2017) 101:704–12. 10.1097/TP.0000000000001588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Schroder PM, Fitch ZW, Schmitz R, Choi AY, Kwun J, Knechtle SJ. The Past, Present, and Future of Costimulation Blockade in Organ Transplantation. Curr Opin Organ Transplant (2019) 24:391–401. 10.1097/MOT.0000000000000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Leibler C, Matignon M, Moktefi A, Samson C, Zarour A, Malard S, et al. Belatacept in Renal Transplant Recipient With Mild Immunologic Risk Factor: A Pilot Prospective Study (BELACOR). Am J Transplant (2019) 19:894–906. 10.1111/ajt.15229 [DOI] [PubMed] [Google Scholar]
- 146. Burghuber CK, Manook M, Ezekian B, Gibby AC, Leopardi FV, Song M, et al. Dual Targeting: Combining Costimulation Blockade and Bortezomib to Permit Kidney Transplantation in Sensitized Recipients. Am J Transplant (2019) 19:724–36. 10.1111/ajt.15067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kwun J, Burghuber C, Manook M, Ezekian B, Park J, Yoon J, et al. Successful Desensitization With Proteasome Inhibition and Costimulation Blockade in Sensitized Nonhuman Primates. Blood Adv (2017) 1:2115–9. 10.1182/bloodadvances.2017010991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ezekian B, Schroder PM, Mulvihill MS, Barbas A, Collins B, Freischlag K, et al. Pretransplant Desensitization With Costimulation Blockade and Proteasome Inhibitor Reduces DSA and Delays Antibody-Mediated Rejection in Highly Sensitized Nonhuman Primate Kidney Transplant Recipients. J Am Soc Nephrol (2019) 30:2399–411. 10.1681/ASN.2019030304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Schroder PM, Schmitz R, Fitch ZW, Ezekian B, Yoon J, Choi AY, et al. Preoperative Carfilzomib and Lulizumab Based Desensitization Prolongs Graft Survival in a Sensitized Non-Human Primate Model. Kidney Int (2021) 99:161–72. 10.1016/j.kint.2020.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kishimoto T, Hibi M, Murakami M, Narazaki M, Saito M, Taga T. The Molecular Biology of Interleukin 6 and its Receptor. Ciba Foundation symposium (1992) 167:5–16; discussion 16-23. 10.1002/9780470514269.ch2 [DOI] [PubMed] [Google Scholar]
- 151. Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a Therapeutic Target. Clin Cancer Res an Off J Am Assoc Cancer Res (2015) 21:1248–57. 10.1158/1078-0432.CCR-14-2291 [DOI] [PubMed] [Google Scholar]
- 152. Chavele KM, Merry E, Ehrenstein MR. Cutting Edge: Circulating Plasmablasts Induce the Differentiation of Human T Follicular Helper Cells Via IL-6 Production. J Immunol (2015) 194:2482–5. 10.4049/jimmunol.1401190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Braun GS, Nagayama Y, Maruta Y, Heymann F, van Roeyen CR, Klinkhammer BM, et al. Il-6 Trans-Signaling Drives Murine Crescentic Gn. J Am Soc Nephrol JASN (2016) 27:132–42. 10.1681/ASN.2014111147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kennedy GA, Varelias A, Vuckovic S, Le Texier L, Gartlan KH, Zhang P, et al. Addition of Interleukin-6 Inhibition With Tocilizumab to Standard Graft-Versus-Host Disease Prophylaxis After Allogeneic Stem-Cell Transplantation: A Phase 1/2 Trial. Lancet Oncol (2014) 15:1451–9. 10.1016/S1470-2045(14)71017-4 [DOI] [PubMed] [Google Scholar]
- 155. Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and Diabetes: The Good, the Bad, or the Indifferent? Diabetes (2005) 54(Suppl 2):S114–124. 10.2337/diabetes.54.suppl_2.S114 [DOI] [PubMed] [Google Scholar]
- 156. Tackey E, Lipsky PE, Illei GG. Rationale for Interleukin-6 Blockade in Systemic Lupus Erythematosus. Lupus (2004) 13:339–43. 10.1191/0961203304lu1023oa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and Prostate Cancer Progression. Cytokine Growth factor Rev (2001) 12:33–40. 10.1016/S1359-6101(00)00021-6 [DOI] [PubMed] [Google Scholar]
- 158. Nishimoto N. Interleukin-6 in Rheumatoid Arthritis. Curr Opin Rheumatol (2006) 18:277–81. 10.1097/01.bor.0000218949.19860.d1 [DOI] [PubMed] [Google Scholar]
- 159. Chung BH, Kim KW, Kim BM, Doh KC, Cho ML, Yang CW. Increase of Th17 Cell Phenotype in Kidney Transplant Recipients With Chronic Allograft Dysfunction. PloS One (2015) 10:e0145258. 10.1371/journal.pone.0145258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kim I, Wu G, Chai NN, Klein AS, Jordan S. Anti-Interleukin 6 Receptor Antibodies Attenuate Antibody Recall Responses in a Mouse Model of Allosensitization. Transplantation (2014) 98:1262–70. 10.1097/TP.0000000000000437 [DOI] [PubMed] [Google Scholar]
- 161. Vo AA, Choi J, Kim I, Louie S, Cisneros K, Kahwaji J, et al. A Phase I/Ii Trial of the Interleukin-6 Receptor-Specific Humanized Monoclonal (Tocilizumab) + Intravenous Immunoglobulin in Difficult to Desensitize Patients. Transplantation (2015) 99:2356–63. 10.1097/TP.0000000000000741 [DOI] [PubMed] [Google Scholar]
- 162. Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al. BAFF, a Novel Ligand of the Tumor Necrosis Factor Family, Stimulates B Cell Growth. J Exp Med (1999) 189:1747–56. 10.1084/jem.189.11.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Mackay F, Browning JL. BAFF: A Fundamental Survival Factor for B Cells. Nature Reviews. Immunology (2002) 2:465–75. 10.1038/nri844 [DOI] [PubMed] [Google Scholar]
- 164. Dubey AK, Handu SS, Dubey S, Sharma P, Sharma KK, Ahmed QM. Belimumab: First Targeted Biological Treatment for Systemic Lupus Erythematosus. J Pharmacol Pharmacotherapeutics (2011) 2:317–9. 10.4103/0976-500X.85930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Furie RA, Leon G, Thomas M, Petri MA, Chu AD, Hislop C, et al. A Phase 2, Randomised, Placebo-Controlled Clinical Trial of Blisibimod, an Inhibitor of B Cell Activating Factor, in Patients With Moderate-to-Severe Systemic Lupus Erythematosus, the PEARL-SC Study. Ann Rheum Dis (2015) 74:1667–75. 10.1136/annrheumdis-2013-205144 [DOI] [PubMed] [Google Scholar]
- 166. Lin TS. Ofatumumab: A Novel Monoclonal Anti-CD20 Antibody. Pharmgenomics Pers Med (2010) 3:51–9. 10.2147/PGPM.S6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hagenbeek A, Gadeberg O, Johnson P, Pedersen LM, Walewski J, Hellmann A, et al. First Clinical Use of Ofatumumab, a Novel Fully Human Anti-CD20 Monoclonal Antibody in Relapsed or Refractory Follicular Lymphoma: Results of a Phase 1/2 Trial. Blood (2008) 111:5486–95. 10.1182/blood-2007-10-117671 [DOI] [PubMed] [Google Scholar]
- 168. Hutas G. Ocrelizumab, a Humanized Monoclonal Antibody Against CD20 for Inflammatory Disorders and B-cell Malignancies. Curr Opin Investig Drugs (2008) 9:1206–15. [PubMed] [Google Scholar]
- 169. Ganjoo KN, de Vos S, Pohlman BL, Flinn IW, Forero-Torres A, Enas NH, et al. Phase 1/2 Study of Ocaratuzumab, an Fc-engineered Humanized Anti-CD20 Monoclonal Antibody, in Low-Affinity FcgammaRIIIa Patients With Previously Treated Follicular Lymphoma. Leuk Lymphoma (2015) 56:42–8. 10.3109/10428194.2014.911859 [DOI] [PubMed] [Google Scholar]
- 170. Cheney CM, Stephens DM, Mo X, Rafiq S, Butchar J, Flynn JM, et al. Ocaratuzumab, an Fc-engineered Antibody Demonstrates Enhanced Antibody-Dependent Cell-Mediated Cytotoxicity in Chronic Lymphocytic Leukemia. MAbs (2014) 6:749–55. 10.4161/mabs.28282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Shah A. Obinutuzumab: A Novel Anti-CD20 Monoclonal Antibody for Previously Untreated Chronic Lymphocytic Leukemia. Ann Pharmacother (2014) 48:1356–61. 10.1177/1060028014543271 [DOI] [PubMed] [Google Scholar]
- 172. Lonial S, Durie B, Palumbo A, San-Miguel J. Monoclonal Antibodies in the Treatment of Multiple Myeloma: Current Status and Future Perspectives. Leukemia (2016) 30:526–35. 10.1038/leu.2015.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Raje NS, Moreau P, Terpos E, Benboubker L, Grząśko N, Holstein SA, et al. Phase 2 Study of Tabalumab, a Human Anti-B-cell Activating Factor Antibody, With Bortezomib and Dexamethasone in Patients With Previously Treated Multiple Myeloma. Br J Haematol (2017) 176:783–95. 10.1111/bjh.14483 [DOI] [PubMed] [Google Scholar]
- 174. Rovin BH, Dooley MA, Radhakrishnan J, Ginzler EM, Forrester TD, Anderson P. The Impact of Tabalumab on the Kidney in Systemic Lupus Erythematosus: Results From Two Phase 3 Randomized, Clinical Trials. Lupus (2016) 25:1597–601. 10.1177/0961203316650734 [DOI] [PubMed] [Google Scholar]
- 175. Raje NS, Faber , E. A JR, Richardson PG, Schiller G, Hohl RJ, Cohen AD, et al. Phase 1 Study of Tabalumab, a Human Anti-B-Cell Activating Factor Antibody, and Bortezomib in Patients With Relapsed/Refractory Multiple Myeloma. Clin Cancer Res (2016) 22:5688–95. 10.1158/1078-0432.CCR-16-0201 [DOI] [PubMed] [Google Scholar]
- 176. Morand EF MJ, Kao AH, Vazquez-Mateo C, Wax S, Chang P, Pudota K, et al. Attainment of Low Disease Activity by Patients With Systemic Lupus Erythematosus Starting With High Disease Activity in a 24-Week, Randomized, Placebo-Controlled, Phase Iib Study of Atacicept (Address II) [Abstract]. In: Arthritis & Rheumatology, vol. 69. San Diego, CA: Wiley. (2017). [Google Scholar]
- 177. Page EK, Dar WA, Knechtle SJ. Tolerogenic Therapies in Transplantation. Front Immunol (2012) 3:198. 10.3389/fimmu.2012.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Vugmeyster Y, Seshasayee D, Chang W, Storn A, Howell K, Sa S, et al. A Soluble BAFF Antagonist, BR3-Fc, Decreases Peripheral Blood B Cells and Lymphoid Tissue Marginal Zone and Follicular B Cells in Cynomolgus Monkeys. Am J Pathol (2006) 168:476–89. 10.2353/ajpath.2006.050600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al. Randomized, Placebo-Controlled Study of Belimumab, a Monoclonal Antibody That Inhibits B Lymphocyte Stimulator, in Patients With Systemic Lupus Erythematosus. Arthritis Rheum (2011) 63:3918–30. 10.1002/art.30613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Guadagnoli M, Kimberley FC, Phan U, Cameron K, Vink PM, Rodermond H, et al. Development and Characterization of APRIL Antagonistic Monoclonal Antibodies for Treatment of B-cell Lymphomas. Blood (2011) 117:6856–65. 10.1182/blood-2011-01-330852 [DOI] [PubMed] [Google Scholar]
- 181. Micallef IN, Maurer MJ, Wiseman GA, Nikcevich DA, Kurtin PJ, Cannon MW, et al. Epratuzumab With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Chemotherapy in Patients With Previously Untreated Diffuse Large B-cell Lymphoma. Blood (2011) 118:4053–61. 10.1182/blood-2011-02-336990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Micallef IN, Kahl BS, Maurer MJ, Dogan A, Ansell SM, Colgan JP, et al. A Pilot Study of Epratuzumab and Rituximab in Combination With Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Chemotherapy in Patients With Previously Untreated, Diffuse Large B-cell Lymphoma. Cancer (2006) 107:2826–32. 10.1002/cncr.22342 [DOI] [PubMed] [Google Scholar]
- 183. Hassan SB, Sorensen JF, Olsen BN, Pedersen AE. Anti-CD40-mediated Cancer Immunotherapy: An Update of Recent and Ongoing Clinical Trials. Immunopharmacol Immunotoxicol (2014) 36:96–104. 10.3109/08923973.2014.890626 [DOI] [PubMed] [Google Scholar]
- 184. Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Ren H, et al. Phase I Study of the Humanized Anti-CD40 Monoclonal Antibody Dacetuzumab in Refractory or Recurrent Non-Hodgkin’s Lymphoma. J Clin Oncol (2009) 27:4371–7. 10.1200/JCO.2008.21.3017 [DOI] [PubMed] [Google Scholar]
- 185. Leonard JP, Friedberg JW, Younes A, Fisher D, Gordon LI, Moore J, et al. A Phase I/II Study of Galiximab (An Anti-CD80 Monoclonal Antibody) in Combination With Rituximab for Relapsed or Refractory, Follicular Lymphoma. Ann Oncol (2007) 18:1216–23. 10.1093/annonc/mdm114 [DOI] [PubMed] [Google Scholar]
- 186. Czuczman MS, Thall A, Witzig TE, Vose JM, Younes A, Emmanouilides C, et al. Phase I/II Study of Galiximab, An Anti-CD80 Antibody, for Relapsed or Refractory Follicular Lymphoma. J Clin Oncol (2005) 23:4390–8. 10.1200/JCO.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 187. Robak T, Robak E. Current Phase II Antibody-Drug Conjugates for the Treatment of Lymphoid Malignancies. Expert Opin Investig Drugs (2014) 23:911–24. 10.1517/13543784.2014.908184 [DOI] [PubMed] [Google Scholar]
- 188. Jiang H, Acharya C, An G, Zhong M, Feng X, Wang L, et al. SAR650984 Directly Induces Multiple Myeloma Cell Death Via Lysosomal-Associated and Apoptotic Pathways, Which is Further Enhanced by Pomalidomide. Leukemia (2016) 30:399–408. 10.1038/leu.2015.240 [DOI] [PubMed] [Google Scholar]
- 189. Deckert J, Wetzel MC, Bartle LM, Skaletskaya A, Goldmacher VS, Vallée F, et al. SAR650984, a Novel Humanized CD38-targeting Antibody, Demonstrates Potent Antitumor Activity in Models of Multiple Myeloma and Other CD38+ Hematologic Malignancies. Clin Cancer Res (2014) 20:4574–83. 10.1158/1078-0432.CCR-14-0695 [DOI] [PubMed] [Google Scholar]
- 190. Wayne AS, Shah NN, Bhojwani D, Silverman LB, Whitlock JA, Stetler-Stevenson M, et al. Phase 1 Study of the Anti-CD22 Immunotoxin Moxetumomab Pasudotox for Childhood Acute Lymphoblastic Leukemia. Blood (2017) 130:1620–7. 10.1182/blood-2017-02-749101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Jelinek T, Hajek R. Monoclonal Antibodies - A New Era in the Treatment of Multiple Myeloma. Blood Rev (2016) 30:101–10. 10.1016/j.blre.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 192. Orlowski RZ, Gercheva L, Williams C, Sutherland H, Robak T, Masszi T, et al. A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study of Siltuximab (Anti-IL-6 mAb) and Bortezomib Versus Bortezomib Alone in Patients With Relapsed or Refractory Multiple Myeloma. Am J Hematol (2015) 90:42–9. 10.1002/ajh.23868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med (2015) 373:621–31. 10.1056/NEJMoa1505654 [DOI] [PubMed] [Google Scholar]
- 194. Suen H, Brown R, Yang S, Ho PJ, Gibson J, Joshua D. The Failure of Immune Checkpoint Blockade in Multiple Myeloma With PD-1 Inhibitors in a Phase 1 Study. Leukemia (2015) 29:1621–2. 10.1038/leu.2015.104 [DOI] [PubMed] [Google Scholar]
- 195. Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, Bms-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol (2015) 33:2004–12. 10.1200/jco.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Rajan A, Gulley JL. Nivolumab (Anti-PD-1, BMS-936558, Ono-4538) in Patients With Advanced Non-Small Cell Lung Cancer. Transl Lung Cancer Res (2014) 3:403–5. 10.3978/j.issn.2218-6751.2014.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, et al. In Vitro Characterization of the Anti-PD-1 Antibody Nivolumab, BMS-936558, and In Vivo Toxicology in Non-Human Primates. Cancer Immunol Res (2014) 2:846–56. 10.1158/2326-6066.CIR-14-0040 [DOI] [PubMed] [Google Scholar]
- 198. Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, et al. A Randomized, Double-Blind, Placebo-Controlled Assessment of BMS-936558, a Fully Human Monoclonal Antibody to Programmed Death-1 (PD-1), in Patients With Chronic Hepatitis C Virus Infection. PloS One (2013) 8:e63818. 10.1371/journal.pone.0063818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Desai J, Markman B, Sandhu SK, Gan HK, Friedlander M, Tran B, et al. A Phase I Dose-Escalation Study of BGB-A317, an Anti-Programmed Death-1 (PD-1) mAb in Patients With Advanced Solid Tumors. J Clin Oncol (2017) 34. 10.1200/JCO.2016.34.15_suppl.3066 29131699 [DOI] [Google Scholar]
- 200. Venkatesan P. Durvalumab Lengthens Survival in Patients With NSCLC. Lancet Respir Med (2017) 5:850. 10.1016/S2213-2600(17)30353-3 [DOI] [PubMed] [Google Scholar]
- 201. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage Iii Non-Small-Cell Lung Cancer. N Engl J Med (2017). 377. 10.1056/nejmoa1709937 [DOI] [PubMed] [Google Scholar]
- 202. Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol (2017) 3:e172411. 10.1001/jamaoncol.2017.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Dou QP, Zonder JA. Overview of Proteasome Inhibitor-Based Anti-Cancer Therapies: Perspective on Bortezomib and Second Generation Proteasome Inhibitors Versus Future Generation Inhibitors of Ubiquitin-Proteasome System. Curr Cancer Drug Targets (2014) 14:517–36. 10.2174/1568009614666140804154511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Muller S, Monneaux F, Schall N, Rashkov RK, Oparanov BA, Wiesel P, et al. Spliceosomal Peptide P140 for Immunotherapy of Systemic Lupus Erythematosus: Results of an Early Phase II Clinical Trial. Arthritis Rheum (2008) 58:3873–83. 10.1002/art.24027 [DOI] [PubMed] [Google Scholar]
- 205. Spencer A, Harrison S, Zonder J, Badros A, Laubach J, Bergin K, et al. A Phase 1 Clinical Trial Evaluating Marizomib, Pomalidomide and Low-Dose Dexamethasone in Relapsed and Refractory Multiple Myeloma (NPI-0052-107): Final Study Results. Br J Haematol (2017) 180. 10.1111/bjh.14987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Badros A, Singh Z, Dhakal B, Kwok Y, MacLaren A, Richardson P, et al. Marizomib for Central Nervous System-Multiple Myeloma. Br J Haematol (2017) 177:221–5. 10.1111/bjh.14498 [DOI] [PubMed] [Google Scholar]
- 207. Harrison SJ, Mainwaring P, Price T, Millward MJ, Padrik P, Underhill CR, et al. Phase I Clinical Trial of Marizomib (Npi-0052) in Patients With Advanced Malignancies Including Multiple Myeloma: Study NPI-0052-102 Final Results. Clin Cancer Res (2016) 22:4559–66. 10.1158/1078-0432.CCR-15-2616 [DOI] [PubMed] [Google Scholar]
- 208. Richardson PG, Zimmerman TM, Hofmeister CC, Talpaz M, Chanan-Khan AA, Kaufman JL, et al. Phase 1 Study of Marizomib in Relapsed or Relapsed and Refractory Multiple Myeloma: NPI-0052-101 Part 1. Blood (2016) 127:2693–700. 10.1182/blood-2015-12-686378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Millward M, Price T, Townsend A, Sweeney C, Spencer A, Sukumaran S, et al. Phase 1 Clinical Trial of the Novel Proteasome Inhibitor Marizomib With the Histone Deacetylase Inhibitor Vorinostat in Patients With Melanoma, Pancreatic and Lung Cancer Based on In Vitro Assessments of the Combination. Invest New Drugs (2012) 30:2303–17. 10.1007/s10637-011-9766-6 [DOI] [PubMed] [Google Scholar]
- 210. Vogl DT, Martin TG, Vij R, Hari P, Mikhael JR, Siegel D, et al. Phase I/II Study of the Novel Proteasome Inhibitor Delanzomib (CEP-18770) for Relapsed and Refractory Multiple Myeloma. Leuk Lymphoma (2017) 58:1872–9. 10.1080/10428194.2016.1263842 [DOI] [PubMed] [Google Scholar]
- 211. Kuhn DJ, Orlowski RZ, Bjorklund CC. Second Generation Proteasome Inhibitors: Carfilzomib and Immunoproteasome-Specific Inhibitors (Ipsis). Curr Cancer Drug Targets (2011) 11:285–95. 10.2174/156800911794519725 [DOI] [PubMed] [Google Scholar]
- 212. Liu RT, Zhang P, Yang CL, Pang Y, Zhang M, Zhang N, et al. Onx-0914, a Selective Inhibitor of Immunoproteasome, Ameliorates Experimental Autoimmune Myasthenia Gravis by Modulating Humoral Response. J Neuroimmunol (2017) 311:71–8. 10.1016/j.jneuroim.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 213. Singh AV, Bandi M, Aujay MA, Kirk CJ, Hark DE, Raje N, et al. Pr-924, a Selective Inhibitor of the Immunoproteasome Subunit LMP-7, Blocks Multiple Myeloma Cell Growth Both In Vitro and In Vivo. Br J Haematol (2011) 152:155–63. 10.1111/j.1365-2141.2010.08491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. West AC, Martin BP, Andrews DA, Hogg SJ, Banerjee A, Grigoriadis G, et al. The SMAC Mimetic, LCL-161, Reduces Survival in Aggressive MYC-driven Lymphoma While Promoting Susceptibility to Endotoxic Shock. Oncogenesis (2016) 5:e216. 10.1038/oncsis.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mahadevan D, Chalasani P, Rensvold D, Kurtin S, Pretzinger C, Jolivet J, et al. Phase I Trial of AEG35156 an Antisense Oligonucleotide to XIAP Plus Gemcitabine in Patients With Metastatic Pancreatic Ductal Adenocarcinoma. Am J Clin Oncol (2013) 36:239–43. 10.1097/COC.0b013e3182467a13 [DOI] [PubMed] [Google Scholar]
- 216. Carter BZ, Mak DH, Morris SJ, Borthakur G, Estey E, Byrd AL, et al. XIAP Antisense Oligonucleotide (AEG35156) Achieves Target Knockdown and Induces Apoptosis Preferentially in CD34+38- Cells in a Phase 1/2 Study of Patients With Relapsed/Refractory AML. Apoptosis (2011) 16:67–74. 10.1007/s10495-010-0545-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Verma R, Peters NR, D’Onofrio M, Tochtrop GP, Sakamoto KM, Varadan R, et al. Ubistatins Inhibit Proteasome-Dependent Degradation by Binding the Ubiquitin Chain. Science (2004) 306:117–20. 10.1126/science.1100946 [DOI] [PubMed] [Google Scholar]