Abstract
Background
To assess the prevalence, risk factors and prognostic significance of retropharyngeal lymph node (RPLN) metastasis diagnosed by magnetic resonance imaging (MRI) in patients with hypopharyngeal squamous cell carcinoma (HPSCC).
Methods
259 patients from three cancer institutions in China from Jan 2010 to Dec 2018 were analyzed, retrospectively. All the patients had been given pre-treatment magnetic resonance imaging (MRI) of head and neck and were then treated with definitive radiotherapy with or without chemotherapy. Pretreatment diagnostic MRIs were reviewed by a dedicated head and neck radiologist, for the presence or absence of radiographically positive RPLN, cervical LN and tumor invasion.Demographic variables were analysed by descriptive statistics using SPSS 20.0. Predictors of the presence of RPLN and its prognostic significance were examined.
Results
RPLN metastasis was discovered in 44 patients (17%). Logistic analysis showed that posterior pharyngeal wall (PPW) primary tumor; PPW invasion; N2-3; multiple cervical lymph node (LN) involvement (>2 LNs) were associated with RPLN metastasis, with metastasis rates 37%, 30%, 31% and 33% respectively. Patients with RPLN metastasis had a significantly reduced 5-year overall survival (OS) and disease-free survival (DFS) compared to the non-RPLN metastasis group (OS 28% vs. 48%, p=0.001; DFS 25% vs. 41%, p=0.040).
Conclusions
RPLN metastasis was not uncommon in HPSCC patients. Risk factors were: PPW primary tumor, PPW invasion and cervical LN status. RPLN metastasis is a poor prognosticator for survival.
Keywords: hypopharyngeal cancer (HPC), hypopharyngeal squamous cell carcinoma (HPSCC), retropharyngeal lymph node (RPLN), prognostic, magnetic resonance image (MRI), pyriform sinus (PS)
Introduction
Among head and neck cancers, hypopharyngeal carcinoma (HPC) is most often detected at an advanced stage with poor prognosis, having 5 year survival rates of 31-47% (1–3). Due to the abundant lymph flow from the HPC, about 70% of patients have already presented with cervical node metastasis at their initial diagnosis (3). However, retropharyngeal lymph node (RPLN) metastasis of HPC often receives less consideration than metastasis to lymph nodes in the neck. There are few published reports concerning the incidence and role of RPLN metastasis in HPC (4, 5). Selected surgical reports have shown a direct pathway of drainage for hypopharyngeal cancers to the lateral retropharyngeal nodes through the ascending pathway, which can in fact bypass the jugulodigastric nodes (4, 6). However, the prevalence of RPLN involvement in HPC ranged hugely from approximately 10% to 60%, due to relatively small sample sizes (7).
The significance in prognosis of RPLNs metastasis in HPC is poorly understood. There is no consistency regarding the clinical significance of RPLN metastasis in HPC. Several studies suggested that RPLN metastasis significantly influenced overall survival and should be appreciated as an important prognostic factor in HPC (6). There are also a few studies which found no difference in local recurrence, distant metastasis or survival rates between the RPLN metastatic and RPLN non-metastatic groups (8).
Therefore, we investigated the treatment results in HPC from three centers in China, to estimate the prevalence of RPLNs in HPC, identify risk factors associated with RPLN metastasis and determine the prognostic implications of RPLN metastasis.
Materials and Methods
Patients
We retrospectively examined patients from Jan 2010 to Dec 2018 diagnosed with non-distant metastasis hypopharyngeal squamous cell carcinoma (HPSCC), from three cancers from China: Cancer Institute/Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College; Sun Yat-sen University Cancer Center, the State Key Laboratory of Oncology in South China’s Collaborative Innovation Center of Cancer Medicine; and Harbin Medical University Cancer Hospital. Institutional Research Ethics Board approval was obtained prior to conducting the study.
Evaluation of RPLNs and Treatment
All the patients had been given pre-treatment magnetic resonance imaging (MRI) of head and neck and were then treated with definitive radiotherapy with or without chemotherapy. Patients were excluded for the following reasons: unavailable pre-treatment MRI; previous head and neck cancer; or distant metastasis. Demographic, clinical, pathologic and radiologic data were reviewed for each patient. All the initial, pretreatment diagnostic MRIs were reviewed by a dedicated head and neck radiologist, for the presence or absence of radiographically positive RPLN, cervical LN and tumor invasion.
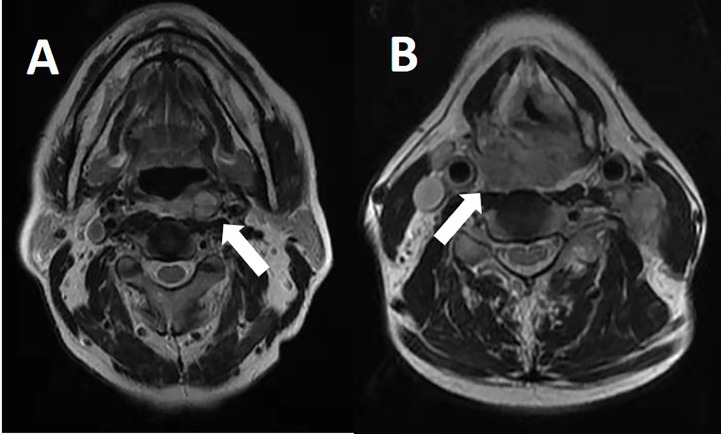
Patients who met any of the following criteria were considered as having radiographically positive RPLN: in the axial plane, the largest short diameter of the retropharyngeal node ≥5mm, any visible median RPLN ( Figure 1 ), LN with circular enhancement or central necrosis;
Figure 1.
RPLN in MRI images. (A) Left RPLN metastasis in MRI. (B) Right RPLN metastasis in MRI.
For other cervical LNs, the largest short diameter of ≥10mm or ≥11mm at level II, a 3 LN grouping, each LN having a minimal axial dimension of 8-10mm; LN with circular enhancement or central necrosis; also LN with extracapsular spread. Information, including other variables, was also collected: this encompassed extranodal extension (ENE), matted nodes, the numbers of LN and the maximum diameter of cervical LN and RPLN.
The initial treatment was radiotherapy or concurrent chemoradiotherapy. The prescribed dose to the primary hypopharyngeal lesion was 66-70 Gy in 30 to 33 fractions. The positive lymph node was irradiated to 70 Gy in 33 fractions. The high-risk lymph node area including the retropharyngeal lymph node area and the neck level of positive lymph node received 60 Gy in 33 fractions. The low-risk lymph node area was administered 50 Gy in 28 fractions with the level and the next level below positive lymph node area. Cis-platinum was mostly in concurrent chemoradiotherapy.
Locoregional, failure-free survival (LRFS) was defined as survival without either emergence of primary site tumors or recurrence in the LNs. Distant metastasis-free survival (DMFS) was defined as survival without any clinical or radiographic evidence of disease outside of the head and neck region. Disease-free survival (DFS) was defined as survival without evidence of disease at any site, where both deaths and disease recurrence represented events. Overall survival (OS) was defined as death due to any cause. Treatment finish date was used as time point zero.
Statistical Methods
Demographic variables were analysed by descriptive statistics using SPSS 20.0. Predictors of the presence of RPLN (gender, age, primary tumor invasion, N stage, ENE, matted nodes, numbers of LN, maximum diameter of LN) were examined in univariate and multivariate analyses using logistic regression.
LRFS, DMFS, OS and DFS were estimated using the Kaplan-Meier method. The significance of predictors of LRFS, DMFS, OS and DFS was assessed in univariate and multivariate analyses using COX proportional hazard models. A two-tailed p-value of less than 0.05 was considered statistically significant for all measures.
Results
Patient Demographics
A total of 259 patients diagnosed with HPC met our inclusion criteria; of these, 250 (96%) were male and 9 were female (4%). The median age was 57 years old (range: 36-85 years old). Tumor staging was reviewed according to the 7th edition of the UICC/AJCC TNM classification. Stage distributions were as follows: T1 in 32 cases, T2 in 94 cases, T3 in 95 cases and T4 in 38 cases. N stage distributions were: N0 in 75 cases, N1 in 55 cases, N2 in 119 cases and N3 in 10 cases. All patients were treated with definitive radiotherapy with or without systemic chemotherapy. Baseline clinical and treatment details can be found in Table 1 .
Table 1.
Baseline characteristics and treatment data of all the patients and those combined with RPLN metastasis.
| Factors | Total (259) | RPLN+ (44) | Factors | Total (259) | RPLN+ (44) | ||
|---|---|---|---|---|---|---|---|
| Gender | Male | 250 | 43 (17%) | Level II | Yes | 146 | 37 (25%) |
| Female | 9 | 1 (11%) | No | 106 | 7 (7%) | ||
| Age | ≥50 | 194 | 33 (17%) | Level III | Yes | 130 | 29 (22%) |
| <50 | 65 | 11 (17%) | No | 122 | 15 (12%) | ||
| Primary site | Pyriform sinus | 206 | 26 (12%) | Level IV | Yes | 35 | 6 (17%) |
| Posterior pharyngeal wall | 49 | 18 (37%) | No | 217 | 38 (18%) | ||
| Postcricoid region | 4 | 0 | NO. of LN | ≤2 | 159 | 11 (7%) | |
| Pharyngeal wall invasion | Yes | 91 | 29 (32%) | >2 | 100 | 33 (33%) | |
| No | 155 | 15 (10%) | Matted LN | Yes | 77 | 27 (35%) | |
| T stage | Not T4 | 164 | 17 (10%) | No | 100 | 17 (17%) | |
| T4 | 95 | 27 (28%) | ENE | Yes | 107 | 31 (30%) | |
| N stage | N0-1 | 130 | 3 (3%) | No | 70 | 13 (19%) | |
| N2-3 | 129 | 40 (31%) | Concurrent chemotherapy | Yes | 139 | 24 (17%) | |
| Clinical stage | I-II | 37 | 0 | No | 82 | 15 (18%) | |
| III-IVa | 222 | 44 (20%) | Unknown | 38 | 5 (13%) |
Prevalence of RPLN Metastasis
Among the 259 patients, 44 patients (17%) presented with RPLN metastasis (the largest short diameter of the retropharyngeal node being ≥5mm in the axial plain). Among the 44 patients with RPLN metastasis, the median shortest size was 7mm (range 5-22 mm). 29 patients presented with unilateral RPLN metastasis and 15 patients with bilateral RPLN metastasis. Among the 29 patients who had unilateral positive RPLN, 25 patients demonstrated ipsilateral involvement, whereas 4 patients showed contralateral involvement. Stage distributions among the 44 patients with positive RPLN as the primary lesion were: T1 in 1 case, T2 in 12, T3 in 4 and T4 in 27 patients. Patient characteristics and treatment data are shown in Table 1 .
Risk Factors Associated With RPLN Metastasis
In univariate analysis all variables were tested, including gender, age, primary tumor status, nodal status, presence of ENE and presence of matted nodes. The relationship between RPLN metastasis and several clinical factors were analyzed. Univariate and multivariate analysis showed that independent factors associated with RPLN metastasis were: the primary tumor site being PPW vs. non-PPW (37% vs. 12%, HR=0.263, p=0.023); PPW invasion vs. no invasion (32% vs. 10%, HR=3.058, p=0.028); for N stage, N2-3 vs. N0-1 (31% vs. 3%, HR=0.106, p=0.008); and numbers of involved LN >2 vs. ≤2 (33% vs. 7%, HR=0.141, p=0.014). Univariate and multivariate analyses are shown in Table 2 .
Table 2.
Risk factors associated with RPLN metastasis in univariate and multivariate analysis.
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Gender | 0.602 (0.073-4.937) | 0.636 | ||
| Age | 0.994 (0.470-2.101) | 0.987 | ||
| Primary site | 0.394 (0.212-0.735) | 0.003 | 0.263 (0.083-0.833) | 0.023 |
| Pharyngeal wall invasion | 4.366 (2.187-8.715) | 0.000 | 3.058 (1.131-8.268) | 0.028 |
| T stage | 0.291 (0.149-0.570) | 0.000 | ||
| N stage | 0.251 (0.144-0.439) | 0.000 | 0.106 (0.020-0.564) | 0.008 |
| Level II | 4.801 (2.047-11.260) | 0.000 | 0.244 (0.063-0.947) | 0.041 |
| Level III | 2.048 (1.038-4.043) | 0.039 | 0.104 (0.025-0.443) | 0.002 |
| Level IV | 0.975 (0.378-2.510) | 0.957 | ||
| NO. of LN | 0.659 (0.562-0.773) | 0.000 | 0.141 (0.030-0.669) | 0.014 |
| Matted LN | 2.637 (1.308-5.314) | 0.007 | ||
| ENE | 1.788 (0.857-3.723) | 0.120 | ||
Prognosis Significance
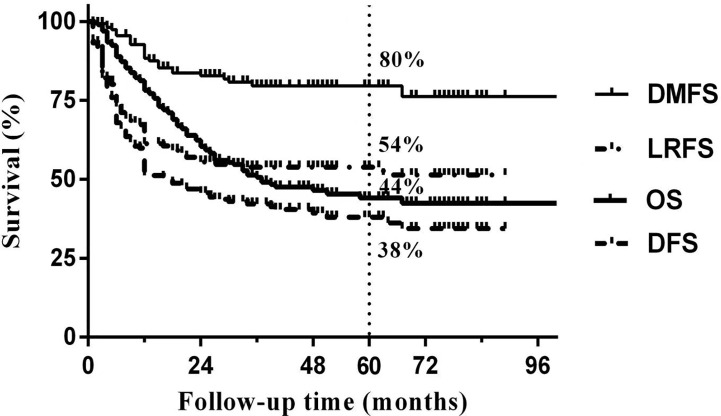
Median follow-up time was 50 months (range 3 to 103 months). The overall 5-year DFS, OS, LRFS and DMFS rates in the whole cohort were 38%, 44%, 54% and 80%, respectively. Survival curves and failure patterns are shown in Figure 2 . Patients with RPLN metastasis, when compared to the non-RPLN metastasis group, had significantly lower DFS (RPLN+ vs. RPLN- at 25% vs. 41%, p=0.040) and OS (RPLN+ vs. RPLN- at 28% vs. 48%, p=0.001). No significant difference was observed in DMFS (RPLN+ vs. RPLN- at 71% vs. 82%, p=0.097) and LRFS (RPLN+ vs. RPLN- at 44% vs. 56%, p=0.217) ( Figure 3 ). Both univariate and multivariate analyses showed that age and RPLN involvement were independent prognostic factors associated with OS and DFS. The results of univariate analyses are shown in Table 3 and multivariate analyses in Table 4 .
Figure 2.
Survival curves in the whole cohort.
Figure 3.
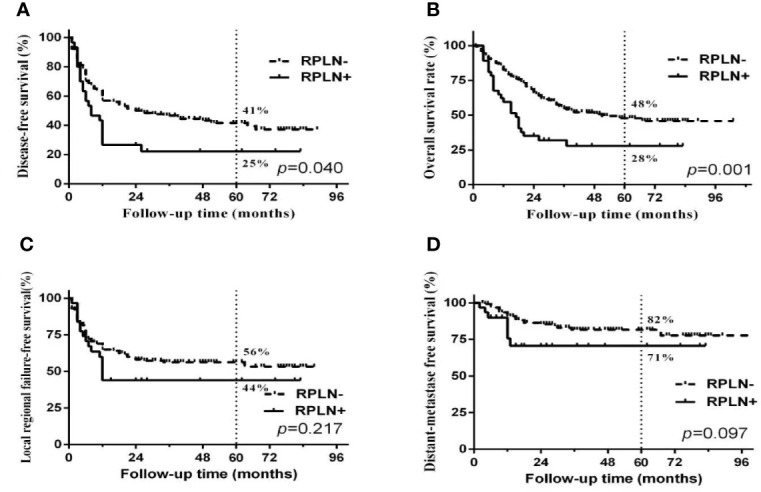
Survival curves between patients with or without RPLN metastasis. (A) disease-free survival; (B) Overall survival; (C) Locoregional failure-free survival; (D) distant-metastasis free survival.
Table 3.
Results of univariate analysis in identifying factors associated with LRFS, DMFS, OS and DFS.
| Factors | 5y DFS | 5y OS | 5y LRFS | 5y DMFS | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | p | % | p | % | p | % | p | ||
| Gender | Male | 37 | 0.408 | 43 | 0.281 | 55 | 0.692 | 79 | 0.463 |
| Female | 67 | 80 | 67 | 100 | |||||
| Age | ≥50 | 46 | 0.00 | 52 | 0.003 | 63 | 0.000 | 85 | 0.032 |
| <50 | 16 | 21 | 27 | 61 | |||||
| Primary site | Posterior phayryngeal wall | 35 | 0.453 | 47 | 0.853 | 56 | 0.772 | 79 | 0.564 |
| Not PPW | 39 | 43 | 54 | 80 | |||||
| Pharyngeal wall invasion | Yes | 25 | 0.014 | 39 | 0.080 | 45 | 0.132 | 74 | 0.106 |
| No | 43 | 45 | 57 | 83 | |||||
| T stage | Not T4 | 44 | 0.040 | 44 | 0.931 | 58 | 0.124 | 83 | 0.220 |
| T4 | 29 | 46 | 47 | 74 | |||||
| N stage | N0-1 | 49 | 0.004 | 57 | 0.004 | 66 | 0.002 | 78 | 0.664 |
| N2-3 | 29 | 33 | 42 | 81 | |||||
| Level II | Yes | 28 | 0.008 | 36 | 0.038 | 45 | 0.024 | 77 | 0.632 |
| No | 53 | 56 | 64 | 82 | |||||
| Level III | Yes | 37 | 0.473 | 38 | 0.060 | 51 | 0.559 | 83 | 0.219 |
| No | 39 | 52 | 54 | 75 | |||||
| Level IV | Yes | 32 | 0.211 | 21 | 0.071 | 41 | 0.207 | 80 | 0.760 |
| No | 39 | 48 | 54 | 79 | |||||
| No. of LN | ≥2 | 43 | 0.020 | 52 | 0.007 | 60 | 0.024 | 78 | 0.812 |
| <2 | 33 | 35 | 45 | 81 | |||||
| RPLN | Yes | 25 | 0.004 | 28 | 0.001 | 44 | 0.217 | 71 | 0.097 |
| No | 41 | 48 | 56 | 82 | |||||
| Matted LN | Yes | 31 | 0.616 | 32 | 0.140 | 45 | 0.632 | 67 | 0.040 |
| No | 29 | 39 | 45 | 89 | |||||
| ENE | Yes | 37 | 0.305 | 37 | 0.612 | 50 | 0.465 | 83 | 0.129 |
| No | 16 | 35 | 38 | 65 | |||||
| Concurrent chemotherapy | Yes | 40 | 0.699 | 50 | 0.093 | 54 | 0.909 | 85 | 0.086 |
| No | 35 | 36 | 53 | 72 | |||||
Table 4.
Results of multi-variates analysis in identifying factors associated with LRFS, DMFS, OS and DFS.
| HR | 95CI | p | ||
|---|---|---|---|---|
| 5y-DFS | Age | 0.449 | 0.292-0.692 | 0.000 |
| RPLN | 0.598 | 0.394-0.907 | 0.016 | |
| Level II | 0.558 | 0.351-0.886 | 0.013 | |
| 5y-OS | Age | 0.517 | 0.337-0.793 | 0.002 |
| RPLN | 0.531 | 0.326-0.865 | 0.011 | |
| 5y-LRFS | Age | 0.414 | 0.257-0.669 | 0.000 |
| N stage | 1.992 | 1.207-3.286 | 0.007 | |
| 5y-DMFS | Matted LN | 2.726 | 0.999-7.444 | 0.050 |
Discussion
Our study, to the best of our knowledge, presents the largest cohort of patients with HPC using MRI to identify RPLN involvement. The involvement rate of RPLN was 17% in our dataset. Primary tumor status and cervical lymph nodes were closely related to RPLN metastasis. Primary tumor location at the posterior hypopharyngeal wall or tumor invading the posterior hypopharyngeal wall was associated with RPLN metastasis rates higher than 30%. Advanced N stage and multiple LN metastasis were also associated with high risk of RPLN disease. RPLN metastasis is a very poor prognosticator of DFS and OS in HPC.
RPLN receive afferent lymphatic drainage from pharynx and other sites, with efferent drainage to the upper jugular lymph node chain (9). RPLN is regarded as the first lymph node station in nasopharyngeal carcinoma, with the prevalence of RPLN metastasis very high, at up to 60% (10). RPLNs are also described as a nodal bed that are at risk for spreading either oropharyngeal carcinoma or HPC (9). However, due to their deep anatomical location, surgical dissection is somewhat complicated (6). Therefore, radiological or MRI assessment is essential for diagnosis. To date, there have been few published data on prevalence of RPLN involvement in patients with non-nasopharyngeal head and neck cancer and the frequency of positive retropharyngeal nodes reported in HPC varies hugely, ranging from 10% to 62% (10–12). These highly discrepant findings across different reports were mainly ascribed to two reasons, one being that most studies included other head and neck carcinomas and had small sample sizes, and were not limited to HPC; another reason being that various imaging technologies, such as CT, MRI and (18)F-FDG PET had been used (5, 13). Actually, MRI has been shown to be superior to CT images for detecting metastatic RPLNs; MRI is considered the preferred method for assessing metastatic RPLNs, as a guide to physicians prescribing appropriate treatment (14). Considering the advantage of MRI in detection of RPLN involvement and the fact that (18)F-FDG PET is not always available, we therefore chose pre-treatment MRI as mandatory in identifying RPLN in HPC patients in our cohort.
RPLN metastasis is not uncommon in HPC, and was found in 17% of our cohort. In order to find the risk factors associated with RPLN metastasis in HPC, univariate and multivariate logistic regression analyses were conducted. Primary tumor status and LN status both significantly affected RPLN involvement. Primary tumor site location is a significant factor associated with RPLN metastasis; patients presenting with pyriform sinus (PS) showed RPLN involvement at 12%, but with posterior wall tumors, at 37%, in accordance with other studies. We also found that primary disease with PPW invasion is an independent risk factor which is associated with RPLN metastasis (PPW invasion, 32% vs no-PPW invasion at 10%, OR=3.058, p=0.028), which indicates that PPW invasion may affect LN drainage regardless of the primary tumor location. RPLN metastasis appeared to be significantly associated with N status, whether N2-3 disease (p=0.008), level II (p=0.04), or level III (p=0.002). Higher risk of RPLN involvement was also associated with LN involvement and multiple cervical LN (p=0.014). HPC at advanced N stage (N2-3) and multiple cervical LN involvement (>2 LNs) tended to develop RPLN metastases at rates higher than 30%, a figure validated by previous studies. There are differing views about the influence of cervical LNs on RPLN involvement, with some data suggesting that level V LN involvement is an independent predictor of RPLN involvement in HPC, but it needs to be interpreted cautiously (5). In our cohort, cervical LNs in level II/III instead of level V, were independent predictors for RPLN metastasis, as similarly found in a previous report (5, 15).
It has been recognized that the RPLNs are of major importance as foci of metastases in nasopharyngeal carcinoma. However, in HPC the prognostic and clinical role of RPLN disease remains controversial and improperly defined. Among head and neck cancers, HPC is most often detected at an advanced stage presenting poor prognosis, with a 5-year survival rate of 31-47% and where more than half of patients will develop disease failure (16). In this study, 5-year OS and DFS were 44% and 38%, respectively. Metastasis to RPLN is recognized as an important prognostic factor and indicates an unfavorable prognosis in head and neck cancers. Our results showed that RPLN metastasis presented with lower OS (HR 0.531, p=0.011) and DFS (HR 0.598, p=0.016). Even after multivariate analysis, RPLN metastases were still independently significant as a prognostic factor associated with poor survival. Therefore, identifying patients with high risk of RPLN metastasis is of great importance in clinical treatment decision making, especially for guiding elective LN irradiation.
For patients without RPLN metastasis it is still controversial as to whether radiotherapy in the RPLN area is a beneficial treatment. According to recent publications, the RPLN area is routinely defined as the radiotherapy target regardless of the clinical stage of HPC (17). However, the guidelines composed by Gregoire et al. proposed that treatment of RPLNs with prophylactic radiotherapy is not essential for HPC patients with N0 or N1 classification (16). From our results, patients with PPW disease or PS disease with PPW involvement were prone to develop RPLN metastasis, with a rate higher than 30%, and we would also have recommended that these patients be irradiated in their RPLN regions.
Our data were collected from three cancer institutions from China and included only patients with HPC; their pretreatment MRIs were used to assess the prevalence of RPLN involvement, then related risk factors and their prognostic value were identified. Several limitations should be raised. First, a caveat inherent in this study is that MRI findings may not be consistent with the original pathological examinations. Since dissection, or biopsy, of RPLN is currently difficult to perform, pathological criteria are insufficient to identify RPLN involvement, so patients may originally have been differently diagnosed (not by MRI data). Secondly, data collection was retrospective, and treatment modalities were undoubtedly different in the three institutions. Notwithstanding the above limitations, we believe the current analysis from multiple cancer centers presents a detailed description of RPLN involvement and its prognostic role in HPC, providing evidence for recommending prophylactic irradiation of RPLN in HPC. This treatment should be of great clinical value.
Conclusion
The prevalence of RPLN involvement in HPC is 17%. Risk factors for RPLN metastasis were: primary tumor located in PW, tumor with PW invasion, advanced N stage, multiple LN involvements, and level II/III LN involvement. Patients who were RPLN-positive showed significantly lower DFS and OS than those without RPLN involvement. Prophylactic treatment by irradiation of RPLN should be beneficial to all HPC patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee, Cancer Hospital, Chinese Academy of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CA, YS, and SM contributed equally to this work. CA statistical analysis and wrote paper. CA, YS, and SM collected the patient data. XY, YZ, XZ and LX statistical analysis and evaluate the MR. SL, ZL, and JY: supervision. All authors contributed to the article and approved the submitted version.
Funding
Supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Science (2019-RC-HL-004) AND Beijing Hope Run Special Fund of Cancer Foundation of China(No. LC2018L06).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Bradley PJ. Epidemiology of Hypopharyngeal Cancer. Adv Otorhinolaryngol (2019) 83:1–14. 10.1159/000492299 [DOI] [PubMed] [Google Scholar]
- 2. Eskander A, Mifsud M, Irish J, Gullane P, Gilbert R, Brown D, et al. Overview of Surgery for Laryngeal and Hypopharyngeal Cancer in Ontario, 2003-2010. Head Neck (2017) 39(8):1559–67. 10.1002/hed.24787 [DOI] [PubMed] [Google Scholar]
- 3. Kotwall C, Sako K, Razack MS, Rao U, Bakamjian V, Shedd DP. Metastatic Patterns in Squamous Cell Cancer of the Head and Neck. Am J Surg (1987) 154(4):439–42. 10.1016/0002-9610(89)90020-2 [DOI] [PubMed] [Google Scholar]
- 4. Teshima M, Otsuki N, Shinomiya H, Morita N, Furukawa T, Morimoto K, et al. Impact of Retropharyngeal Lymph Node Dissection in the Surgical Treatment of Hypopharyngeal Cancer. Head Neck (2019) 41(6):1738–44. 10.1002/hed.25608 [DOI] [PubMed] [Google Scholar]
- 5. Wu Z, Deng XY, Zeng RF, Su Y, Gu MF, Zhang Y, et al. Analysis of Risk Factors for Retropharyngeal Lymph Node Metastasis in Carcinoma of the Hypopharynx. Head Neck (2013) 35(9):1274–7. 10.1002/hed.23112 [DOI] [PubMed] [Google Scholar]
- 6. Amatsu M, Mohri M, Kinishi M. Significance of Retropharyngeal Node Dissection At Radical Surgery for Carcinoma of the Hypopharynx and Cervical Esophagus. Laryngoscope (2001) 111(6):1099–103. 10.1097/00005537-200106000-00031 [DOI] [PubMed] [Google Scholar]
- 7. Biau J, Lapeyre M, Troussier I, Budach W, Giralt J, Grau C, et al. Selection of Lymph Node Target Volumes for Definitive Head and Neck Radiation Therapy: A 2019 Update. Radiother Oncol (2019) 134:1–9. 10.1016/j.radonc.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 8. Gross ND, Ellingson TW, Wax MK, Cohen JI, Andersen PE. Impact of Retropharyngeal Lymph Node Metastasis in Head and Neck Squamous Cell Carcinoma. Arch Otolaryngol Head Neck Surg (2004) 130(2):169–73. 10.1001/archotol.130.2.169 [DOI] [PubMed] [Google Scholar]
- 9. Gunn GB, Debnam JM, Fuller CD, Morrison WH, Frank SJ, Beadle BM, et al. The Impact of Radiographic Retropharyngeal Adenopathy in Oropharyngeal Cancer. Cancer (2013) 119(17):3162–9. 10.1002/cncr.28195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang L, Zhang Y, Liu Y, Li H, Wang S, Liang S, et al. Prognostic Value of Retropharyngeal Lymph Node Metastasis Laterality in Nasopharyngeal Carcinoma and a Proposed Modification to the UICC/AJCC N Staging System. Radiother Oncol (2019) 140:90–7. 10.1016/j.radonc.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 11. Oikawa Y, Michi Y, Tsushima F, Tomioka H, Mochizuki Y, Kugimoto T, et al. Management of Retropharyngeal Lymph Node Metastasis in Oral Cancer. Oral Oncol (2019) 99:104471. 10.1016/j.oraloncology.2019.104471 [DOI] [PubMed] [Google Scholar]
- 12. Iyizoba-Ebozue Z, Murray LJ, Arunsingh M, Vaidyanathan S, Scarsbrook AF, Prestwich RJD. Incidence and Patterns of Retropharyngeal Lymph Node Involvement in Oropharyngeal Carcinoma. Radiother Oncol (2020) 142:92–9. 10.1016/j.radonc.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 13. Wu IS, Hung GU, Chang BL, Liu CK, Chang TH, Lee HS, et al. Is Unenhanced 18F-FDG-PET/CT Better Than Enhanced CT in the Detection of Retropharyngeal Lymph Node Metastasis in Nasopharyngeal Carcinoma? Ear Nose Throat J (2016) 95(4-5):178–84. 10.1177/0145561316095004-506 [DOI] [PubMed] [Google Scholar]
- 14. Kato H, Kanematsu M, Watanabe H, Mizuta K, Aoki M. Metastatic Retropharyngeal Lymph Nodes: Comparison of CT and MR Imaging for Diagnostic Accuracy. Eur J Radiol (2014) 83(7):1157–62. 10.1016/j.ejrad.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 15. Buckley JG, MacLennan K. Cervical Node Metastases in Laryngeal and Hypopharyngeal Cancer: A Prospective Analysis of Prevalence and Distribution. Head Neck. (2000) 22(4):380–5. [DOI] [PubMed] [Google Scholar]
- 16. Garneau JC, Bakst RL, Miles BA. Hypopharyngeal Cancer: A State of the Art Review. Oral Oncol (2018) 86:244–50. 10.1016/j.oraloncology.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 17. Li WJ, Zhang ZL, Yu XM, Cai XL, Pan XL, Yang XY. Expression of Claudin-1 and Its Relationship With Lymphatic Microvessel Generation in Hypopharyngeal Squamous Cell Carcinoma. Genet Mol Res (2015) 14(4):11814–26. 10.4238/2015.October.2.15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.