Abstract
HIV-positive patients have a 60- to 200-fold increased incidence of Non-Hodgkin Lymphomas (NHL) because of their impaired cellular immunity. Some NHL are considered Acquired Immunodeficiency Syndrome (AIDS) defining conditions. Diffuse large B-cell Lymphoma (DLBC) and Burkitt Lymphoma (BL) are the most commonly observed, whereas Primary Effusion Lymphoma (PEL), Central Nervous System Lymphomas (PCNSL), Plasmablastic Lymphoma (PBL) and classic Hodgkin Lymphoma (HL) are far less frequent. Multicentric Castleman disease (MCD) is an aggressive lymphoproliferative disorder highly prevalent in HIV-positive patients and strongly associated with HHV-8 virus infection. In the pre-Combination Antiretroviral Therapy (CART) era, patients with HIV-associated lymphoma had poor outcomes with median survival of 5 to 6 months. By improving the immunological status, CART extended the therapeutic options for HIV positive patients with lymphomas, allowing them to tolerate standard chemotherapies regimen with similar outcomes to those of the general population. The combination of CART and chemotherapy/ immuno-chemotherapy treatment has resulted in a remarkable prolongation of survival among HIV-infected patients with lymphomas. In this short communication, we briefly review the problems linked with the treatment of lymphoproliferative diseases in HIV patients. Combination Antiretroviral Therapy (CART) not only reduces HIV replication and restores the immunological status improving immune function of the HIV-related lymphomas patients but allows patients to deal with standard doses of chemotherapies. The association of CART and chemotherapy allowed to obtain better results in terms of overall survival and complete responses. In the setting of HIV-associated lymphomas, many issues remain open and their treatment is complicated by the patient’s immunocompromised status and the need to treat HIV concurrently.
Keywords: Human immunodeficiency virus (HIV) lymphomas, diffuse large B-cell lymphoma (DLBCL), central nervous system lymphomas (PCNSL), burkitt lymphoma (BL), primary effusion lymphoma (PEL), plasmablastic lymphoma (PL), hodgkin lymphoma (HL), combination antiretroviral therapy (CART)
1. Introduction
Human Immunodeficiency Virus (HIV) infection results in impaired cellular immunity, thus predisposing patients to developing opportunistic infections and neoplasms. The introduction of Combination Antiretroviral Therapy (CART) has increased life expectancy in HIV-infected patients by improving performance status and immune function and declining the incidence of opportunistic infections and Acquired Immune Deficiency Syndrome (AIDS) -defining malignancies. However, HIV non-Hodgkin lymphoma (NHL) still represents the leading cause of AIDS-related deaths [1]. HIV-positive patients have a 60- to 200-fold increased incidence of Non-Hodgkin Lymphoma (NHL) especially in patients with less than 500 cells/µL. Diffuse Large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) are the most common, whereas Primary Central Nervous System lymphomas (PCNSL), Primary Effusion lymphoma (PEL), Plasmablastic Lymphoma (PL) and classic Hodgkin lymphoma (HL) are far less frequent [2-4].
Multicentric Castleman's Disease (MCD) is referred to as a group of rare and aggressive lymphoproliferative disorders more commonly observed in HIV positive patients and has been linked to human herpesvirus 8. Castleman disease is a heterogeneous cluster of disorders, with distinct unicentric CD (UCD) and multicentric CD (MCD) subtypes. Unicentric Castleman disease (UCD) is localized and carries an excellent prognosis, whereas multicentric Castleman disease (MCD) is a systemic disease occurring most commonly in the setting of HIV [4]. No correlation was observed with CD4 cell counts or the use of CART but HIV-MCD is strongly associated with HHV 8, indicating a link between Kaposi Sarcoma (KS) and MCD. In fact, KS is present in up to 70% of patients with MCD at diagnosis [5]. HIV-associated DLBCL is divided into centroblastic and immunoblastic variants. Histologically the centroblastic type is characterized by diffused sheets of large lymphoid cells with round or oval nuclei and prominent nucleoli. DLBCL express germinal center-associated markers, such as CD10 and BCL6, and are typically CD20 positive. The immunoblastic variant refers to those cases containing more than 90% immunoblasts and often exhibits features of plasmacytoid differentiation that may confound the distinction from plasmablastic lymphomas. These tumors are CD10-negative, post-germinal center derived, and frequently positive for MUM1/IRF4 and CD138/syndecan-1 markers associated with plasma cell derivation. Follicular lymphomas and peripheral T cells are rarely observed in HIV-infected patients. In HIV patients, diffuse large B-cell lymphoma (DLBCL) and primary CNS lymphoma (PCNSL) have been considered AIDS-defining events [4-6].
1.1. Immunological Effects of CART
The pathogenesis of these lymphomas is strictly correlated with the extent of the patient's immunodeficiency and, by restoring the patients’ immunologic status, CART has dramatically improved the outcome of HIV infected patients with NHL (Table 1). In fact, CART is significantly effective in reducing plasma HIV-RNA to undetectable levels, in returning CD4:CD8 ratio to nearly normal levels, in reducing activated cells (CD38) and in increasing naive (CD45RA+ CD45RO-) and memory (CD45RA-CD45RO+) CD4 cells. Initiating CART at the very early stages of the HIV infection results in rapid and complete normalization of T cell subsets and preservation of T cell functions. Moreover, the degree of CD4 cells depletion has a major impact on the type of lymphoma that develops.
Table 1.
Aims of antiretroviral therapy in HIV-related lymphoproliferative diseases.
| Reduces HIV-related morbidity and prolong survival Improves the quality of life Restores and preserves immunological function Durably suppresses viral load Restores the immunological status making chemotherapy treatment tolerable also in immunocompromised patients Improves bone marrow reserve by decreasing the negative impact of HIV infection on hematopoiesis Prevents opportunistic infections and HIV-associated complications Allows the use of standard doses of chemotherapy regimens |
The mechanisms of action of CART and their classification are represented in Table 2. In the case of low CD4 counts (< 200/μL), the incidence of lymphoma shifts toward subtypes such as immunoblastic diffuse large B-cell lymphoma (DLBCL-IB), Primary Effusion Lymphoma (PEL) and plasmablastic lymphoma (PBL). If the CD4 cell counts are greater than 200/μL, centroblastic diffuse large B-cell lymphoma (DLBCL-CB) and BL are the subtypes more likely to occur [6].
Table 2.
Mechanisms of action of CARTs.
|
- Nucleoside Reverse transcriptase Inhibitors (NRTIs): Block Reverse Transcriptase (RT) before HIV genetic code combines with infected cell’s code. - Protease inhibitors (PIs): Block enzymes that cut the long strands into small functional proteins and enzymes needed to assemble mature virus Prevent maturation of new viral particles -Fusion Inhibitors: Block fusion of HIV with cell membrane - CCR Antagonists: Bind to and block CCR5 co-receptor of the immune cells, thereby preventing HIV from entering and infecting the cell - Integrate inhibitors: Prevent integration of HIV DNA into the nucleus of infected cells |
1.2. HIV-Related Lymphomas Recognize an Antigen-Driven Pathogenetic Mechanism
HIV-related lymphomas share some pathogenetic features with marginal zone lymphomas, which are due to Helicobacter Pylori, Epstein Barr Virus (EBV) and Hepatitis C virus (HCV), as well as with acquired C1-inhibitor deficiency-related lymphoproliferative diseases, in which the complex antigen-antibody C1-inhibitor/C1-inhibitor antibodies might bind to BCR and continuously stimulate B cells. All of them recognize chronic antigen stimulation as their pathogenetic model [7-18]. In all these lymphoma subtypes, the mechanisms of chronic B cell antigenic proliferation represents the first step of the pathogenic process. Thus, inducing an antigen-mediated B cells expansion and probably promoting the emergence of monoclonal B cells. In the second step, B cells expansion and proliferation become antigen-independent and proliferation is due to oncogenes activation [7-18].
There are a number of well-defined genetic abnormalities in HIV-associated lymphoma. Burkitt lymphoma (BL) is associated with activation of the MYC gene and, when associated with HIV infection, resembles the sporadic rather than the endemic BL. Interestingly, studies have suggested that up to 20% of HIV-positive DLBCLs also harbour an MYC translocation. Moreover, BCL6 mutations are found in 20% of centroblastic DLBCL and in 60% of PEL lymphoma cases [15-17]. In the pre-CART era, patients with HIV-associated lymphoma had poor outcomes with median survival of 5 to 6 months compared with 21 months since the introduction of CART.
By improving the immunological status, CART extended the therapeutic options for HIV positive patients with lymphomas, allowing them to tolerate standard chemotherapies regimen with similar outcomes to those of the general population. Nevertheless, in the setting of HIV-associated lymphomas, many issues remain open and their treatment is complicated by the patient’s immunocompromised status and the need to treat HIV concurrently.
1) In the recent past, doses of chemotherapies were reduced due to the immunocompromised status of these patients and due to the bone marrow reserve of HIV patients, especially those with advanced infection. Standard concomitant administration of CART may improve bone marrow reserve by decreasing the negative impact of HIV infection on hematopoiesis [18, 19].
2) Chemotherapy induces a profound decrease in CD4 predisposing to opportunistic infections. For this reason, current guidelines suggest to continue CART therapy during chemotherapy 3) CART may induce side effects overlapping to those of chemotherapy such as myelosuppression. Side effects due to interactions are more likely to occur with strong CYP3A4 inhibitors such as ritonavir and cobicistat-containing regimes. The use of integrase inhibitor-based therapy could be beneficial concerning drug interactions [18].
4) Due to similar transmission routes, coinfection with hepatitis B and C is frequent and only a few data on HIV and hepatitis C virus (HCV) or Epstein Barr virus (EBV) co-infected lymphoma patients are published. While HCV related liver failure is quite rare under chemotherapy, the situation for patients with HBV coinfection is different. HBsAg positive patients have a reactivation rate of 20%-50% possibly resulting in fulminant hepatitis [19]. During chemo-immunotherapy, a CART containing nucleoside reverse transcriptase inhibitors, which are active against HIV and HBV, is recommended [20, 21].
1.3. Diagnosing and Assessing the Prognosis of HIV-Related Lymphomas
Diagnosis of lymphomas in HIV patients as in all patients with lymphoma is generally performed with excisional biopsy followed by tissue evaluation by an expert immunopathologist. Patients should receive a complete medical history including prior opportunistic infections and history of HIV resistance, immune function, HIV viral control and antiretroviral treatment. The physical examination should include a careful assessment of lymph node regions, liver and spleen. Relevant laboratory studies include a complete blood count, chemistry profile with lactate dehydrogenase and uric acid levels, CD4 cell count and HIV viral load and Hepatitis B and C serologies. A bone marrow aspirate and biopsy should be performed at initial diagnosis and Central Nervous System (CNS) diagnostic interventions in aggressive DLBCL should be performed according to the current guidelines for non-HIV DLBCL [22]. CT scanning of the chest, abdomen and pelvis and MRI of the head should be performed. The use of the positron emission tomography (PET), which is universally used to evaluate disease burden in HIV lymphoma patients, remains controversial and in some series, this test failed to predict remission in HIV-related lymphomas.
1.4. Treatment of HIV-Related Lymphomas
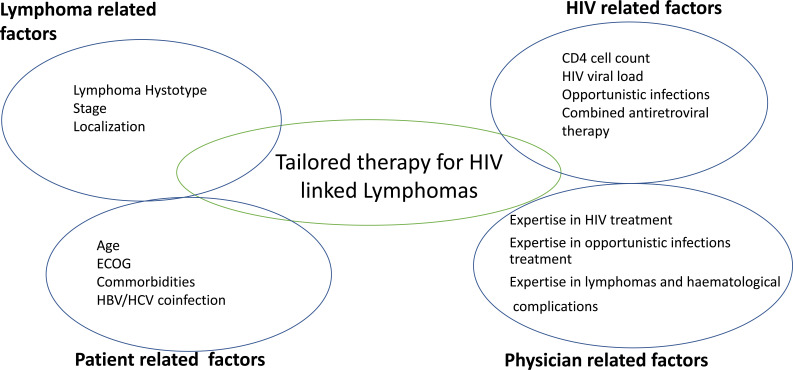
The first step in the treatment of HIV-related lymphomas consists of establishing the precise histological subtype of lymphoproliferative disease, the extent of disease, performance status and the burden of the coexisting comorbidities. The optimal initial treatment for HIV related lymphomas is not yet established. According to general consensus, the appropriate therapy should consider stage, IPI, performance status, comorbidities and subtype of lymphoma, according to guidelines established for HIV negative malignant lymphoma (Fig. 1). The discussion of treatment regimens according to histotypes in HIV-related lymphomas is beyond the scope of this paper, nevertheless, Table 3 summarizes the principal treatment regimens in HIV-related lymphomas. Dosage modifications should be considered according to immunological status and comorbidities as in non-HIV related lympho- proliferative diseases. Recent studies have shown that HIV-associated lymphomas can be treated successfully with standard doses of chemotherapy without severe toxicities, even high doses of chemotherapies and autologous stem cells transplantation can be tolerated in relapsing or nonresponding patients, as CART helps patients to better control opportunistic infections. Taken together, there are many reasons for including CART in the treatment of HIV-related lymphomas. The main reason is that CART reduces HIV virus replication, restores immunological impairment with reduced opportunistic infections and offers better high-quality responses to treatment such as overall survival (OS) and improving complete remission rates [23-25].
Fig. (1).
The choice of therapy for HIV related lymphomas depends on several factors. According to general consensus, the appropriate therapy should consider stage, IPI, performance status, comorbidities, immunological subset and previous opportunistic infections, combined antiretroviral therapy and subtypes of lymphomas. These factors also impact the outcome of HIV related lymphomas. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 3.
Standard first-line chemotherapy regimens in HIV-associated lymphoproliferative diseases.
| - | Chemotherapy Regimen |
|---|---|
| Diffuse Large B cell Lymphoma (DLBCL) | R-CHOP or R EPOCH |
| Burkit Lymphomas (BL) | B-ALL protocols HyperCVAD / HD methotrexate |
| Hodgkins Lymphomas (HL) | Early Stage: ABVD 2 or 4 cycle in early favorable stage, eventually followed by 20-30 GY involved field radiotherapy according to number of cycles Advanced stage: 6-8 cycles of ABVD 5 cycles of BEACOPP |
| Primary Effusion Lymphoma (PEL) | CHOP or CHOP-like Bortezomib Lenalidomide |
| Primary Central nervous system Lymphomas (PCNSL) | High doses Metotrexate 1g /m2 and High doses cytarabime 3 g/m2 |
| Plasmablastic Lymphomas (PBL) | B cell Acute Lymphoblastic protocols |
Abbreviations: ABVD: Doxorubicin 25mg/m2 IV Bleomycin 10,000units/m2 IV Vinblastine 6mg/m2 IV Dacarbazine 375 mg/m2 R-CHOP CHOP: ciclofosfamide 750 mg/m2, doxorubicina 50 mg/m2, vincristina 2 mg, (rituximab 375 mg/m2 al giorno 1) e prednisolone orale 100 mg nei giorni 1–5) (R)EPOCH: (Rituximab) etoposide 50 mg/m2 prednisone 60 mg/m2, vincristine 0.4 mg/m2, cyclophosphamide 750 mg/m2, and doxorubicin 10 mg/m2 HyperCVAD: Cyclophosphamide 300 mg/m2, Vincristine 1,4 mg/m2, Doxorubicin 50 mg/m2 Dexametasone 40 mg orally BEACOPP: Bleomycin 10 mg/ m2, /Etoposide 100 mg/m2; doxorubicin 25 mg/m2, Vincristi14 mg/m2 (max 2 mg) 2, cyclophosphamide 650 mg/m2, Procarbazina 100 mg/m2, Prednisone 40 mg/m2.
Non-HIV CD20+ lymphomas patients have great benefits in terms of complete responses and OS by immuno- chemotherapy regimens including Rituximab. On the other hand, Rituximab induces durable and profound CD4, CD8 and CD 19 depletion, together with potentially long-lasting
hypogammablobulinemia, that may be responsible for opportunistic infections either among non-HIV patients. In the setting of HIV-related CD20+ lymphomas, Rituximab is generally added to chemotherapy according to CD4 count. For patients with CD4 count > 50/ μL, Rituximab is generally well tolerated and should be offered in association with chemotherapy in HIV-related lymphomas adding better OS and high-quality responses [26]. The decision to include Rituximab to antineoplastic therapy should be taken with caution and individualized.
In addition, the potential tumor lysis syndrome in the setting of high proliferating lymphomas characterized by elevated Ki67 index should be taken into account.
Revaluation of the response to treatment should be considered one month after the completion of chemotherapy. Reevaluation should include, history, clinical and laboratory evaluation and reevaluation with positron emission tomography associated with computed axial tomography (PET/TAC) performed 6 to 8 weeks from the end of therapy. The previous inflammatory state of the lungs may impact on the results of scanning.
Conclusion
For relapsing/refractory patients, as in non-HIV-related aggressive lymphomas, platinum-containing regimens are generally offered with sufficient tolerability. Alternative therapies include bortezomib and autologous stem cells transplantation. A few data are available on allogeneic bone marrow transplantation in HIV patients.
Acknowledgements
None declared.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None declared.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Lewden C., May T., Rosenthal E., Burty C., Bonnet F., Costagliola D., Jougla E., Semaille C., Morlat P., Salmon D., Cacoub P., Chêne G. ANRS EN19 Mortalité Study Group and Mortavic1. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalité 2000 and 2005” surveys (ANRS EN19 and Mortavic). J. Acquir. Immune Defic. Syndr. 2008;48(5):590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 2.Beral V., Peterman T., Berkelman R., Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991;337(8745):805–809. doi: 10.1016/0140-6736(91)92513-2. [DOI] [PubMed] [Google Scholar]
- 3.Besson C., Goubar A., Gabarre J., Rozenbaum W., Pialoux G., Châtelet F.P., Katlama C., Charlotte F., Dupont B., Brousse N., Huerre M., Mikol J., Camparo P., Mokhtari K., Tulliez M., Salmon-Céron D., Boué F., Costagliola D., Raphaël M. Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood. 2001;98(8):2339–2344. doi: 10.1182/blood.V98.8.2339. [DOI] [PubMed] [Google Scholar]
- 4.Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116(22):4415–4421. doi: 10.1182/blood-2010-07-290213. [DOI] [PubMed] [Google Scholar]
- 5.Powles T., Stebbing J., Bazeos A., Hatzimichael E., Mandalia S., Nelson M., Gazzard B., Bower M. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann. Oncol. 2009;20(4):775–779. doi: 10.1093/annonc/mdn697. [DOI] [PubMed] [Google Scholar]
- 6.Little R.F., Dunleavy K. Update on the treatment of HIV-associated hematologic malignancies. Hematology (Am. Soc. Hematol. Educ. Program) 2013;2013:382–388. doi: 10.1182/asheducation-2013.1.382. [DOI] [PubMed] [Google Scholar]
- 7.Carbone A., Gloghini A. AIDS-related lymphomas: From pathogenesis to pathology. Br. J. Haematol. 2005;130(5):662–670. doi: 10.1111/j.1365-2141.2005.05613.x. [DOI] [PubMed] [Google Scholar]
- 8.Gaidano G., Capello D., Carbone A. The molecular basis of acquired immunodeficiency syndrome-related lymphomagenesis. Semin. Oncol. 2000;27(4):431–441. [PubMed] [Google Scholar]
- 9.Barber D.L., Andrade B.B., Sereti I., Sher A. Immune reconstitution inflammatory syndrome: The trouble with immunity when you had none. Nat. Rev. Microbiol. 2012;10(2):150–156. doi: 10.1038/nrmicro2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann G.R., Perrin L., Pantaleo G., Opravil M., Furrer H., Telenti A., Hirschel B., Ledergerber B., Vernazza P., Bernasconi E., Rickenbach M., Egger M., Battegay M., Swiss HIV Cohort Study Group CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch. Intern. Med. 2003;163(18):2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 11.Rattotti S., Ferretti V.V., Rusconi C., Rossi A., Fogazzi S., Baldini L., Pioltelli P., Balzarotti M., Farina L., Ferreri A.J.M., Laszlo D., Speziale V., Varettoni M., Sciarra R., Morello L., Tedeschi A., Frigeni M., Defrancesco I., Zerbi C., Flospergher E., Nizzoli M.E., Morra E., Arcaini L. “Rete Ematologica Lombarda” (REL - Hematology Clinical Network of Lombardy - Lymphoma Workgroup). Lymphomas associated with chronic hepatitis C virus infection: A prospective multicenter cohort study from the Rete Ematologica Lombarda (REL) clinical network. Hematol. Oncol. 2019;37(2):160–167. doi: 10.1002/hon.2575. [DOI] [PubMed] [Google Scholar]
- 12.Spina V., Khiabanian H., Messina M., Monti S., Cascione L., Bruscaggin A., Spaccarotella E., Holmes A.B., Arcaini L., Lucioni M., Tabbò F., Zairis S., Diop F., Cerri M., Chiaretti S., Marasca R., Ponzoni M., Deaglio S., Ramponi A., Tiacci E., Pasqualucci L., Paulli M., Falini B., Inghirami G., Bertoni F., Foà R., Rabadan R., Gaidano G., Rossi D. The genetics of nodal marginal zone lymphoma. Blood. 2016;128(10):1362–1373. doi: 10.1182/blood-2016-02-696757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xochelli A., Bikos V., Polychronidou E., Galigalidou C., Agathangelidis A., Charlotte F., Moschonas P., Davis Z., Colombo M., Roumelioti M., Sutton L.A., Groenen P., van den Brand M., Boudjoghra M., Algara P., Traverse-Glehen A., Ferrer A., Stalika E., Karypidou M., Kanellis G., Kalpadakis C., Mollejo M., Pangalis G., Vlamos P., Amini R.M., Pospisilova S., Gonzalez D., Ponzoni M., Anagnostopoulos A., Giudicelli V., Lefranc M.P., Espinet B., Panagiotidis P., Piris M.A., Du M.Q., Rosenquist R., Papadaki T., Belessi C., Ferrarini M., Oscier D., Tzovaras D., Ghia P., Davi F., Hadzidimitriou A., Stamatopoulos K. Disease-biased and shared characteristics of the immunoglobulin gene repertoires in marginal zone B cell lymphoproliferations. J. Pathol. 2019;247(4):416–421. doi: 10.1002/path.5209. [DOI] [PubMed] [Google Scholar]
- 14.Sbattella M., Zanichelli A., Ghia P., Gattei V., Suffritti C., Teatini T., Cicardi M., Castelli R. Splenic marginal zone lymphomas in acquired C1-inhibitor deficiency: Clinical and molecular characterization. Med. Oncol. 2018;35(9):118. doi: 10.1007/s12032-018-1183-7. [DOI] [PubMed] [Google Scholar]
- 15.Castelli R., Wu M.A., Arquati M., Zanichelli A., Suffritti C., Rossi D., Cicardi M. High prevalence of splenic marginal zone lymphoma among patients with acquired C1 inhibitor deficiency. Br. J. Haematol. 2016;172(6):902–908. doi: 10.1111/bjh.13908. [DOI] [PubMed] [Google Scholar]
- 16.Gaidano G., Lo Coco F., Ye B.H., Shibata D., Levine A.M., Knowles D.M., Dalla-Favera R. Rearrangements of the BCL-6 gene in acquired immunodeficiency syndrome-associated non-Hodgkin’s lymphoma: Association with diffuse large-cell subtype. Blood. 1994;84(2):397–402. doi: 10.1182/blood.V84.2.397.397. [DOI] [PubMed] [Google Scholar]
- 17.Gaidano G., Carbone A., Pastore C., Capello D., Migliazza A., Gloghini A., Roncella S., Ferrarini M., Saglio G., Dalla-Favera R. Frequent mutation of the 5′ noncoding region of the BCL-6 gene in acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood. 1997;89(10):3755–3762. [PubMed] [Google Scholar]
- 18.Noy A. Optimizing treatment of HIV-associated lymphoma. Blood. 2019;134(17):1385–1394. doi: 10.1182/blood-2018-01-791400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castelli R., Ferraris L., Pantaleo G., Lambertenghi Deliliers G., Cicardi M. High rate of hepatitis B viral breakthrough in elderly non-Hodgkin lymphomas patients treated with Rituximab based chemotherapy. Dig. Liver Dis. 2016;48(11):1394–1397. doi: 10.1016/j.dld.2016.08.113. [DOI] [PubMed] [Google Scholar]
- 20.Cruciani M., Gatti G., Vaccher E., Di Gennaro G., Cinelli R., Bassetti M., Tirelli U., Bassetti D. Pharmacokinetic interaction between chemotherapy for non-Hodgkin’s lymphoma and protease inhibitors in HIV-1-infected patients. J. Antimicrob. Chemother. 2005;55(4):546–549. doi: 10.1093/jac/dki050. [DOI] [PubMed] [Google Scholar]
- 21.Sandherr M., Hentrich M., von Lilienfeld-Toal M., Massenkeil G., Neumann S., Penack O., Biehl L., Cornely O.A. Antiviral prophylaxis in patients with solid tumours and haematological malignancies--update of the Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO). Ann. Hematol. 2015;94(9):1441–1450. doi: 10.1007/s00277-015-2447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz N., Zeynalova S., Nickelsen M. CNS International Prognostic Index: A Risk Model CT scanning of the chest, abdomen and pelvis and MRI of the head should be performed. Oncol. 2016;34:3150–3156. doi: 10.1200/JCO.2015.65.6520. [DOI] [PubMed] [Google Scholar]
- 23.Kasamon Y.L., Jones R.J., Piantadosi S., Ambinder R.F., Abrams R.A., Borowitz M.J., Morrison C., Smith B.D., Flinn I.W. High-dose therapy and blood or marrow transplantation for non-Hodgkin lymphoma with central nervous system involvement. Biol. Blood Marrow Transplant. 2005;11(2):93–100. doi: 10.1016/j.bbmt.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Barta S.K., Xue X., Wang D., Tamari R., Lee J.Y., Mounier N., Kaplan L.D., Ribera J.M., Spina M., Tirelli U., Weiss R., Galicier L., Boue F., Wilson W.H., Wyen C., Oriol A., Navarro J.T., Dunleavy K., Little R.F., Ratner L., Garcia O., Morgades M., Remick S.C., Noy A., Sparano J.A. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood. 2013;122(19):3251–3262. doi: 10.1182/blood-2013-04-498964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boué F., Gabarre J., Gisselbrecht C., Reynes J., Cheret A., Bonnet F., Billaud E., Raphael M., Lancar R., Costagliola D. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin’s lymphoma. J. Clin. Oncol. 2006;24(25):4123–4128. doi: 10.1200/JCO.2005.05.4684. [DOI] [PubMed] [Google Scholar]
- 26.Wyen C., Jensen B., Hentrich M., Siehl J., Sabranski M., Esser S., Gillor D., Müller M., Van Lunzen J., Wolf T., Bogner J.R., Wasmuth J.C., Christ H., Fätkenheuer G., Hoffmann C. Treatment of AIDS-related lymphomas: rituximab is beneficial even in severely immunosuppressed patients. AIDS. 2012;26(4):457–464. doi: 10.1097/QAD.0b013e32834f30fa. [DOI] [PubMed] [Google Scholar]