Abstract
Hydralazine, an arterial vasodilator, is a widely used medication for the management of hypertension and heart failure, especially for patients who cannot tolerate the use of ACEIs or ARBs. It is generally well-tolerated and has a safe profile in pregnancy. However, hydralazine can induce immune-mediated side effects, such as hydralazine-induced lupus and less commonly hydralazine-induced ANCA vasculitis. The latter most commonly affects the kidneys with or without other organ involvement. There are several cases reported in the literature of hydralazine-induced ANCA associated vasculitis (AAV) that have pulmonary manifestations, also known as hydralazine-induced pulmonary-renal syndrome (PRS), a condition with a high risk of mortality. We are reporting a case of Hydralazine-induced ANCA associated glomerulonephritis with severe diffuse alveolar hemorrhage (DAH). In addition, we will review the current literature and discuss the importance of prompt diagnosis and early management to decrease mortality and morbidity associated with this serious condition.
Keywords: Hydralazine, vasculitis, pulmonary hemorrhage, hypertension, heart failure, pulmonary-renal syndrome
1. Introduction
Hydralazine is commonly used as an antihypertensive agent as well as an afterload reducer in patients with heart failure. The management of chronic heart failure has significantly improved after hydralazine gained mainstream use, especially when combined with nitrates [1]. However, the use of hydralazine can be associated with the development of autoimmune diseases, such as lupus-like syndrome, and less commonly, AAV. When such an association is found, hydralazine should be discontinued, and appropriate immunosuppressive treatment can be considered [2, 3]. A high index of suspicion and early identification of hydralazine-associated vasculitis is essential to prevent irreversible damage [3].
2. Case Presentation
A 75-year-old female with a past medical history significant for hypertension, hyperlipidemia, biopsy-proven Membranoproliferative Glomerulonephritis (MPGN), CKD Stage III, pulmonary hypertension, aortic stenosis and paroxysmal
atrial fibrillation initially presented to the hospital with acute kidney injury, generalized volume overload, and acute hypoxemic respiratory failure requiring intubation and mechanical ventilation. Chest X-Ray (Fig. 1) was remarkable for diffuse patchy bilateral airspace opacities and interstitial prominence concerning either atypical pulmonary edema or diffuse hemorrhage. CT scan (Fig. 2) showed consolidative opacities corresponding to the X-ray related opacities. Transthoracic echocardiogram revealed normal systolic function, mild diastolic dysfunction with preserved ejection fraction. Bronchoscopy with bronchoalveolar lavage confirmed the existence of diffuse pulmonary hemorrhage. Lung involvement was so severe that the patient required several bronchoscopies to aid suctioning of blood from the airways.
Renal biopsy was performed in the setting of deteriorating renal function. While the patient had prior biopsy-proven membranoproliferative glomerulonephritis with immune-complex deposition on immunofluorescence, the new renal biopsy revealed pauci-immune focal crescentic glomerulonephritis. Immune workup showed highly elevated ANA titer (1:1280), positive anti-Histone and anti-MPO antibodies, low complements (C3 and C4), with normal serum immunofixation. Anti-ds-DNA, anti-Ro (SS-A) and anti-La (SS-B) antibodies were negative.
Of note, the patient was using hydralazine among other medicines in the setting of difficult control of her hypertension secondary to her baseline kidney disease. She used 100 mg of hydralazine TID for 4 years. Hydralazine was discontinued, and the patient was started on pulse steroids, without significant clinical response. The patient was started on hemodialysis due to anuria. She was subsequently started on plasmapheresis and Rituximab for hydralazine-induced ANCA vasculitis treatment. The patient’s condition improved and was discharged to a skilled nursing facility to wean off supplemental oxygen requirements. Subsequent chest X-Ray showed improving airspace opacities. The patient survived, however, remained on outpatient scheduled dialysis without recovery of renal function, and was declared end-stage renal disease (ESRD) after 3 months.
3. Discussion
Hydralazine was first introduced in the market in 1951. Despite its general tolerability, it was known to be associated with immune-mediated syndromes, especially Drug-Induced Lupus Erythematosus (DILE), which classically spares the kidneys, and lacks antibodies to ds-DNA [3]. The first case of hydralazine-induced lupus was reported in 1953 [4]. This was followed by the first reports of hydralazine-induced cutaneous vasculitis [5], and renal vasculitis [6] in 1980 and 1981, respectively. The first case report of hydralazine-induced glomerulonephritis was reported in 1983 [7]. Since then, hydralazine-related renal vasculitis has been well-described in the literature and was known to be associated with extra-renal manifestations [8]. Pulmonary involvement, however, had the strongest association with mortality [9]. The association of pulmonary symptoms with hydralazine-induced glomerulonephritis was first described in 1992 in a study by Almroth et al., which investigated seventeen patients with hydralazine-associated nephritis, out of which four patients were reported to have pulmonary symptoms including hemoptysis. In that study, all patients except one had positive anti-nuclear antibody (ANA) and twelve (out of fourteen tested) were positive for anti-myeloperoxidase (Anti-MPO). Anti-ds-DNA was negative in all of them [10]. Subsequent studies by Short [11], Choi [12], and Dobre [13] also reported associated pulmonary symptoms with hydralazine-induced glomerulonephritis; however, the term “pulmonary-renal syndrome” secondary to hydralazine use was first described by Yokogawa et al. in 2009 [8]. They identified 68 patients with hydralazine-induced nephritis and reported the first case of hydralazine-induced PRS with cutaneous involvement and gangrene. Idiopathic or autoimmune pulmonary-renal syndrome has been well-known in the literature and was associated with primary vasculitides, including, but not limited to Granulomatosis with Polyangiitis (GPA), Microscopic Polyangiitis (MPA), and Eosinophilic Granulomatosis with Polyangiitis (EGPA) [14]. Drug-induced pulmonary-renal syndrome was first reported in 1982 and was related to propylthiouracil use [15]. A review of all reported cases (to the best of our knowledge) of hydralazine-induced ANCA associated Pulmonary-Renal Syndrome is summarized in Table 1.
The risks for developing hydralazine-induced ANCA vasculitis include female gender, thyroid disease [16] and human leucocyte antigen (HLA)-DR4 genotype, slow hepatic acetylation, and null gene for C4 [17]. While the risk for hydralazine-induced ANCA vasculitis seems to be dose-dependent, PRS seems to be dose-independent. Based on our review of reported cases of hydralazine-induced PRS, hydralazine dosing ranging from 50 to 300 mg per day and the duration of therapy ranged from a few weeks to more than a decade. Some reported cases developed PRS within weeks [8] or years [18] after the hydralazine dose was increased. The majority of cases of hydralazine-induced PRS underwent kidney biopsy, which classically reveals pauci-immune crescentic glomerulonephritis (although other findings exist). None of the cases we reviewed had lung biopsy performed, however.
Anti-MPO (p-ANCA) has been implicated in almost all cases of hydralazine-induced PRS to date. Anti-histone antibodies in hydralazine-induced PRS were first reported by Dobre in 2009 [13]. Weakly positive anti-PR3 was reported. The first case of hydralazine-induced PRS with predominant anti-PR3 (c-ANCA) and low titers of anti-MPO (p-ANCA) was first reported in 2014 [23].
In 2016, a study by Kumar et al. identified 323 patients diagnosed with ANCA-associated vasculitis from the beginning of 2001 till the end of 2016. Twelve patients were known to use hydralazine, out of which seven patients had bilateral pulmonary infiltrates. On our review of the supplementary material for this article, six patients had acute kidney injury at presentation (based on creatinine change from baseline), out of which three underwent kidney biopsy consistent with pauci-immune necrotizing glomerulonephritis with crescent formation (absent immune complexes). ANA and p-ANCA (anti-MPO) were positive in all seven patients, with only one patient with positive c-ANCA (anti-PR3). Anti-histone antibody was positive in six patients. Surprisingly, six patients were positive for anti-dsDNA, the youngest of which was 46 years old at the time of diagnosis. None of these seven patients had prior diagnosis of systemic lupus erythematosus or any other auto-immune condition. Anti-Smith antibody was either negative or not tested in all seven patients [30]. None of these patients was on other medications implicated in drug-induced ANCA vasculitis like propylthiouracil, minocycline, etc.
The pathophysiologic mechanism by which hydralazine induces an immune response is still not completely understood. The hypothesis most agreed upon is that hydralazine accumulates in cytoplasmic granules of neutrophils, with subsequent binding to myeloperoxidase, which leads to the release of cytotoxic products and cell death. This eventually exposes normally sequestered antigens to antigen-presenting cells (APCs) with subsequent production of anti-neutrophil cytoplasmic antibodies (ANCA) and anti-nuclear antibodies (ANA) [33]. This hypothesis might be supported by the fact that ANCA antibodies are usually specific to single ANCA antigen in idiopathic ANCA vasculitis, but are usually multispecific in drug-induced ANCA vasculitis, with other antibodies present like anti-elastase and anti-lactoferrin [2]. The cross-reactivity to elastase and lactoferrin, however, was often weak, and no cross-inhibition studies were performed, making it difficult to interpret these associations. In addition, it must be noted that circulating ANCA antibodies can exist in the absence of vasculitis in drug-induced lupus.
The hallmark of treatment of drug-induced ANCA vasculitis is the discontinuation of the culprit drug (e.g. hydralazine). Since no guidelines exist for management of hydralazine-induced vasculitis, further management is mainly stemmed from the available guidelines for the management of idiopathic ANCA vasculitis, which comprises immunosuppression with corticosteroids and biologics as well as plasmapheresis in severe cases. Cyclophosphamide has been widely used in most initial cases reported of hydralazine-induced AAV with PRS (Table 1). The first successful treatment of hydralazine-induced PRS with Rituximab was reported in a severely sick patient who required dialysis and ECMO [18]. According to the 2016 European League against Rheumatism/European Renal Association-European Dialysis Transplant Association (EULAR/ERA-EDTA) guidelines for the management of idiopathic ANCA-associated vasculitis, glucocorticoids plus Cyclophosphamide or Rituximab are recommended for remission-induction in life or organ threatening AAV. This includes pulmonary hemorrhage of any severity, cardiac involvement, meningeal involvement, and acute onset mononeuritis multiplex. In non-organ threatening situations, glucocorticoids plus Methotrexate or Mycophenolate Mofetil are recommended [34]. Plasmapheresis is generally recommended in life or organ-threatening situations, creatinine greater than 5.7 mg/dL, and severe diffuse alveolar hemorrhage. Based on (Plasma Exchange and Glucocorticoids for Treatment of Anti-Neutrophil Cytoplasm Antibody (ANCA) - Associated Vasculitis) or PEXIVAS trial in 2019, it was shown that plasma exchange did not reduce the risk of end-stage renal disease or mortality in patients with ANCA-associated vasculitis [35]. It also showed that reduced-dose glucocorticoids resulted in fewer serious infections and did not substantially increase the risk for end-stage renal disease or death compared to standard-dose glucocorticoids [36]. Ultrasound-guided percutaneous renal biopsy is recommended to support the diagnosis and management of AAV and has been shown to have a low risk of complications, such as hemorrhage [37]. Increased bleeding risk has been known to be associated with patients who received plasma exchange [38].
Conclusion
Patients presenting with acute kidney injury of non-identifiable cause should raise clinical suspicion for prompt early initiation of immune workup and renal biopsy. A review of medication history is key to identifying the possible culprit drug (e.g. hydralazine) and discontinuing it. Additional treatment includes the use of immunosuppressant agents with or without plasmapheresis. Hydralazine-induced ANCA vasculitis and pulmonary–renal syndrome can be rapidly progressive and fatal. Although AAV is considered a rare condition, its actual incidence is questionable given an unknown number of unreported, missed, or undiagnosed cases in the setting of rapid progression and high mortality associated with this condition. Hydralazine has been well-implicated in AAV development as well as its life-threatening complications. Prompt diagnosis and treatment can be organ as well as life-saving.
Fig. (1).
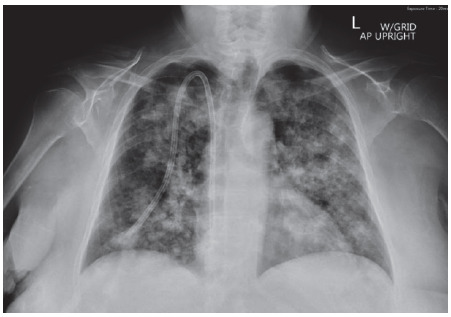
Initial Chest X-Ray showing bilateral airspace and interstitial opacities concerning atypical pulmonary edema of alveolar hemorrhage. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
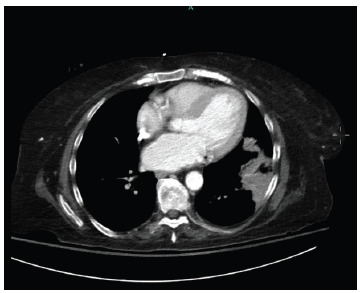
CT scan of the chest without contrast showing consolidative opacity in the lateral basal left lower lobe, as well as small portion of the lingula. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Reported cases of Pulmonary-Renal Syndrome (PRS) secondary to Hydralazine-Induced ANCA Associated Vasculitis (AAV).
| Year | Author(s) | n | HA Daily Dose (mg) | HA Duration | ANA | Anti-MPO | Anti-PR3 | AHA | Anti-DS-DNA | Anti-GBM | Low C3, C4 | Treatment* | Survived** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1992 | Almroth et al. [10] | 4 | 50-200 | 6 m -10 y | 4 | 4 | N/A | N/A | 0 | N/A | N/A | S(4), Cy (2), Az (1), P(1) | 3 |
| 1995 | Short et al. [11] | 2 | 75 | 4-5 y | 2 | 2 | N/A | N/A | 0 | N/A | N/A | S(2), Cy(1) | N/A |
| 2000 | Choi et al. [2] | 5 | 100-200 | 1-10 y | 4 or 5 | 5 | 0 | N/A | 0 | N/A | N/A | S(5), Cy(5) | 4 |
| 2009 | Dobre et al. [13] | 1 | 225 | 3 y | 1 | 1 | 1 (low) | 1 | 1 | N/A | 0 | S, Cy | 1 |
| 2009 | Yokogawa et al. [8] | 1 | 300 | 16 m | 1 | 1 | N/A | 1 | 0 | 0 | 1 | S, Cy | 1 |
| 2011 | Marina et al. [19] | 1 | 150 | 4 y | 1 | 1 | N/A | 1 | 0 | 0 | 0 | S, Cy | 0 |
| 2012 | Kalra et al. [20] | 1 | 225 | 2 y | 1 | 1 | N/A | 1 | 1 | N/A | 0 | S, Cy, MMF | 0 |
| 2013 | Kassa Y et al. [21] | 1 | N/A | N/A | 1 | N/A | N/A | N/A | 1 | N/A | 0 | S, P, R | 0 |
| 2014 | Namas et al. [22] | 1 | 150 | 3 y | 1 | 1 | 1 (low) | 1 | 0 | N/A | 0 | S, Cy, P | 1 |
| 2014 | Agarwal et al. [23] | 1 | 300 | 4.5 y | 1 | 1 | 1 (high) | 1 | 0 | 0 | 1(slight) | S, Cy | 1 |
| 2016 | Rasla et al. [24] | 1 | 50 | 6 y | N/A | 1 | N/A | 1 | N/A | 0 | 1 | S, Cy, P | 1 |
| 2016 | Babar*** et al. [25] | 1 | 50 | N/A | N/A | 1 | 0 | 1 | N/A | N/A | 0 | S, Cy, P | 0 |
| 2016 | Goehler et al. [26] | 1 | N/A | N/A | 0 | 1 | N/A | N/A | N/A | N/A | N/A | S, Cy, R | 1 |
| 2016 | Al Ahwel et al. [27] | 1 | N/A | N/A | 1 | 1 | N/A | 1 | N/A | N/A | 0 | S, P, R | 1 |
| 2017 | Patel et al. [28] | 1 | N/A | 2 y | 1 | 1 | N/A | 1 | 0 | 0 | 0 | N/A | 0 |
| 2017 | Zuckerman et al. [29] | 2 | 100-300 | 6 w – 5 y | 1 | 1 | 1 (high) | 2 | 1 | 0 | 1 | S(2), R(2), P(1) | 1 |
| 2018 | Kumar et al.**** [30] | 7 | 100-300 | 1-4 y | 7 | 7 | 1 | 6 | 6 | 0/4 | 3 | S(6), Cy(4), MMF(2)***** | 7 |
| 2018 | Aeddula et al. [31] | 1 | N/A | N/A | 0 | 1 | 0 | N/A | N/A | 0 | 1(slight) | S, R, P | 1 |
| 2019 | Nguyen et al. [32] | 1 | N/A | N/A | 1 | 1 | 1 | 1 | 1 | N/A | N/A | S, R | 1 |
| 2019 | Paley et al.[18] | 1 | 300 | 4 y | 1 | 1 | 0 | 1 | 1 | 0 | 1 | S, R | 1 |
Abbreviations: HA: Hydralazine; ANA: antinuclear antibody; MPO: myeloperoxidase; PR3: proteinase 3; AHA: anti-histone antibody; Cy: Cyclophosphamide; R: Rituximab; S: Corticosteroids; P: plasmapheresis; Az: Azathioprine; MMF: mycophenolate mofetil; N/A: not available or not reported, n = number of cases per report or study.
* Number in brackets represents the number of patients per study who received that treatment. No brackets if only one patient per report.
** Survived acute setting and discharged (not to hospice) but might have died within months from other complications.
*** Babar’s case did not clearly state pulmonary involvement but reported respiratory symptoms and multi-organ failure, which might have involved the lungs.
**** Kumar’s study reported 8 cases with pulmonary involvement. We excluded one case as it had pleural effusion, but no pulmonary infiltrate.
***** One of the two patients on MMF was already on baseline immunosuppression with MMF due to renal transplant.
Acknowledgements
Declared none.
Consent for Publication
Verbal consent was obtained.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Farag M., Mabote T., Shoaib A., et al. Hydralazine and nitrates alone or combined for the management of chronic heart failure: A systematic review. Int. J. Cardiol. 2015;196:61–69. doi: 10.1016/j.ijcard.2015.05.160. [DOI] [PubMed] [Google Scholar]
- 2.Timlin H., Liebowitz J.E., Jaggi K., Geetha D. Outcomes of hydralazine induced renal vasculitis. Eur. J. Rheumatol. 2018;5(1):5–8. doi: 10.5152/eurjrheum.2017.17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ihle B.U., Whitworth J.A., Dowling J.P., Kincaid-Smith P. Hydralazine and lupus nephritis. Clin. Nephrol. 1984;22(5):230–238. [PubMed] [Google Scholar]
- 4.Morrow J.D., Schroeder H.A., Perry H.M., Jr Studies on the control of hypertension by hyphex. II. Toxic reactions and side effects. Circulation. 1953;8(6):829–839. doi: 10.1161/01.CIR.8.6.829. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein R.M., Egerton-Vernon J., Webster J. Hydralazine-induced cutaneous vasculitis. BMJ. 1980;280(6208):156–157. doi: 10.1136/bmj.280.6208.156-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludmerer K. Renal failure, dyspnea and anemia in a 57 year old woman. Clinicopathologic conference. Am. J. Med. 1981;71(5):876–886. doi: 10.1016/0002-9343(81)90385-5. [DOI] [PubMed] [Google Scholar]
- 7.Björck S., Westberg G., Svalander C., Mulec H. Rapidly progressive glomerulonephritis after hydralazine. Lancet. 1983;2(8340):42. doi: 10.1016/S0140-6736(83)90021-1. [DOI] [PubMed] [Google Scholar]
- 8.Yokogawa N., Vivino F.B. Hydralazine-induced autoimmune disease: Comparison to idiopathic lupus and ANCA-positive vasculitis. Mod. Rheumatol. 2009;19(3):338–347. doi: 10.3109/s10165-009-0168-y. [DOI] [PubMed] [Google Scholar]
- 9.Hogan S.L., Nachman P.H., Wilkman A.S., Jennette J.C., Falk R.J. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J. Am. Soc. Nephrol. 1996;7(1):23–32. doi: 10.1681/ASN.V7123. [DOI] [PubMed] [Google Scholar]
- 10.Almroth G., Eneström S., Hed J., Samuelsson I., Sjöström P. Autoantibodies to leucocyte antigens in hydralazine-associated nephritis. J. Intern. Med. 1992;231(1):37–42. doi: 10.1111/j.1365-2796.1992.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 11.Short A.K., Lockwood C.M. Antigen specificity in hydralazine associated ANCA positive systemic vasculitis. QJM. 1995;88(11):775–783. [PubMed] [Google Scholar]
- 12.Choi H.K., Merkel P.A., Walker A.M., Niles J.L. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: Prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum. 2000;43(2):405–413. doi: 10.1002/1529-0131(200002)43:2<405:AID-ANR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Dobre M., Wish J., Negrea L. Hydralazine-induced ANCA-positive pauci-immune glomerulonephritis: A case report and literature review. Ren. Fail. 2009;31(8):745–748. doi: 10.3109/08860220903118590. [DOI] [PubMed] [Google Scholar]
- 14.Chung S.A., Seo P. Microscopic polyangiitis. Rheum. Dis. Clin. North Am. 2010;36(3):545–558. doi: 10.1016/j.rdc.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reidy T.J., Upshaw J.D., Jr, Chesney T.M. Propylthiouracil-induced vasculitis: A fatal case. South. Med. J. 1982;75(10):1297–1298. doi: 10.1097/00007611-198210000-00044. [DOI] [PubMed] [Google Scholar]
- 16.Herman L.L., Tivakaran V.S. Hydralazine. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 17.Lionaki S., Hogan S.L., Falk R.J., et al. Association between thyroid disease and its treatment with ANCA small-vessel vasculitis: A case-control study. Nephrol. Dial. Transplant. 2007;22(12):3508–3515. doi: 10.1093/ndt/gfm493. [DOI] [PubMed] [Google Scholar]
- 18.McKinnon R.A., Nebert D.W. Possible role of cytochromes P450 in lupus erythematosus and related disorders. Lupus. 1994;3(6):473–478. doi: 10.1177/096120339400300608. [DOI] [PubMed] [Google Scholar]
- 19.Paley M.A., Edrees F., Kudose S., Gaut J.P., Ranganathan P., Vijayan A. Successful use of rituximab for hydralazine-induced anti-neutrophil cytoplasmic antibodies-associated vasculitis. Saudi J. Kidney Dis. Transpl. 2019;30(1):226–230. doi: 10.4103/1319-2442.252915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marina V.P., Malhotra D., Kaw D. Hydralazine-induced ANCA vasculitis with pulmonary renal syndrome: a rare clinical presentation. Int. Urol. Nephrol. 2012;44(6):1907–1909. doi: 10.1007/s11255-011-9989-7. [DOI] [PubMed] [Google Scholar]
- 21.Kalra A., Yokogawa N., Raja H., et al. Hydralazine-associated vasculitis: Overlapping features of drug-induced lupus and vasculitis. Semin. Arthritis Rheum. 2012;48(2):283–287. doi: 10.1016/j.semarthrit.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Kassa Y. Hydralazine (HY) induced antineutrophile cytoplasmic (ANCA) and antinuclear (ANA) positive pulmonary-renal syndrome (PRS). Am. J. Kidney Dis. 2013;61(4):B52. [Google Scholar]
- 23.Namas R., Rubin B., Adwar W., Meysami A. A challenging twist in pulmonary renal syndrome. Case Rep. Rheumatol. 2014;2014:516362. doi: 10.1155/2014/516362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal G., Sultan G., Werner S.L., Hura C. Hydralazine induces myeloperoxidase and proteinase 3 anti-neutrophil cytoplasmic antibody vasculitis and leads to pulmonary renal syndrome. Case Rep. Nephrol. 2014;2014:868590. doi: 10.1155/2014/868590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasla S, El Meligy A, Cucu DF. 2016. [PubMed]
- 26.Babar F., Posner J.N., Obah E.A. Hydralazine-induced pauci-immune glomerulonephritis: Intriguing case series with misleading diagnoses. J. Community Hosp. Intern. Med. Perspect. 2016;6(2):30632. doi: 10.3402/jchimp.v6.30632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manne-Goehler J., Berwick J. Case of hydralazine-induced anca vasculitis.; 2016. [Google Scholar]
- 28.Al Ahwel Y.A., Ali A., Kubbara A., et al. Hydralazine induced hemoptysis. Am. J. Respir. Crit. Care Med. 2016;193:A1656. [Google Scholar]
- 29.Patel N.R., Castelino P.M., Ramasra E., Al-Khalisy H., Shingala H. Fatal autoimmune complication of hydralazine. Am. J. Respir. Crit. Care Med. 2017;195:A1468. [Google Scholar]
- 30.Zuckerman R., Patel M., Costanzo E.J., et al. Hydralazine-associated adverse events: A report of two cases of hydralazine-induced ANCA vasculitis. J. Bras. Nefrol. 2018;40(2):193–197. doi: 10.1590/2175-8239-jbn-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar B., Strouse J., Swee M., Lenert P., Suneja M. Hydralazine-associated vasculitis: Overlapping features of drug-induced lupus and vasculitis. Semin. Arthritis Rheum. 2018;48(2):283–287. doi: 10.1016/j.semarthrit.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Aeddula NR, Pathireddy S, Ansari A, Juran PJ. Hydralazine-associated antineutrophil cytoplasmic antibody vasculitis with pulmonary-renal syndrome. 2018. [DOI] [PMC free article] [PubMed]
- 33.Tsai-Nguyen G., Modrykamien A.M., Bredeweg A. Hereditary afibrinogenemia and pulmonary-renal hydralazine-induced vasculitis. Proc. Bayl. Univ. Med. Cent. 2019;32(3):397–398. doi: 10.1080/08998280.2019.1619397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan J.J., Markowitz G.S., Radhakrishnan J. Drug-induced glomerular disease: Immune-mediated injury. Clin. J. Am. Soc. Nephrol. 2015;10(7):1300–1310. doi: 10.2215/CJN.01910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yates M., Watts R.A., Bajema I.M., et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 2016;75(9):1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 36.Derebail V K, Falk R J. ANCA-Associated Vasculitis-Refining Therapy with Plasma Exchange and Glucocorticoids. 2020. [DOI] [PubMed]
- 37.Prasad N., Kumar S., Manjunath R., et al. Real-time ultrasound-guided percutaneous renal biopsy with needle guide by nephrologists decreases post-biopsy complications. Clin. Kidney J. 2015;8(2):151–156. doi: 10.1093/ckj/sfv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basic-Jukic N., Kes P., Glavas-Boras S., Brunetta B., Bubic-Filipi L., Puretic Z. Complications of therapeutic plasma exchange: Experience with 4857 treatments. Ther. Apher. Dial. 2005;9(5):391–395. doi: 10.1111/j.1744-9987.2005.00319.x. [DOI] [PubMed] [Google Scholar]


