Abstract
The purpose of the review is the analysis of clinical and experimental data on the etiology and pathogenesis of takotsubo syndrome (TS). TS is characterized by contractile dysfunction, which usually affects the apical region of the heart without obstruction of coronary artery, moderate increase in myocardial necrosis markers, prolonged QTc interval (in 50% of patients), sometimes elevation of ST segment (in 19% of patients), increase N-Terminal Pro-B-Type Natriuretic Peptide level, microvascular dysfunction, sometimes spasm of the epicardial coronary arteries (in 10% of patients), myocardial edema, and life-threatening ventricular arrhythmias (in 11% of patients). Stress cardiomyopathy is a rare disease, it is observed in 0.6 - 2.5% of patients with acute coronary syndrome. The occurrence of takotsubo syndrome is 9 times higher in women, who are aged 60-70 years old, than in men. The hospital mortality among patients with TS corresponds to 3.5% - 12%. Physical or emotional stress do not precede disease in all patients with TS. Most of patients with TS have neurological or mental illnesses. The level of catecholamines is increased in patients with TS, therefore, the occurrence of TS is associated with excessive activation of the adrenergic system. The negative inotropic effect of catecholamines is associated with the activation of β2 adrenergic receptors. An important role of the adrenergic system in the pathogenesis of TS is confirmed by studies which were performed using 125I-metaiodobenzylguanidine (125I -MIBG). TS causes edema and inflammation of the myocardium. The inflammatory response in TS is systemic. TS causes impaired coronary microcirculation and reduces coronary reserve. There is a reason to believe that an increase in blood viscosity may play an important role in the pathogenesis of microcirculatory dysfunction in patients with TS. Epicardial coronary artery spasm is not obligatory for the occurrence of TS. Cortisol, endothelin-1 and microRNAs are challengers for the role of TS triggers. A decrease in estrogen levels is a factor contributing to the onset of TS. The central nervous system appears to play an important role in the pathogenesis of TS.
Keywords: Etiology, pathogenesis, takotsubo syndrome, stress cardiomyopathy, endothelin-1, microRNAs
1. Introduction
In 1974, the term “stress-cardiomyopathy” regarding stress-induced injury of the heart (SIIH) in animals was first suggested by Johansson et al. [1]. In a study performed on pigs, they found that immobilization stress causes morphological changes in the myocardium, including necrosis. According to ECG data, immobilization in pigs causes an inversion of the T wave, transient displacement of the ST segment, and arrhythmias. The first report about “stress cardiomyopathy” in humans was published in 1980 by Cebelin and Hirsch [2]. It should be noted that other investigators also reported on the possibility of SIIH in humans and animals, but they did not use the term stress cardiomyopathy [3-12].
The method of quantitative evaluation of SIIH was first proposed by Miller and Mallov [9]. They found that emotional painful stress (EPS) contributes to an increase in accumulation of radioactive 99mTc-pyrophosphate in the myocardium. In addition, in experiments on the isolated perfused heart, they found that EPS contributed to the release of lactate dehydrogenase from the myocardium, which indicates necrosis of heart cells. It is established that EPS causes a decrease in pressure which is developed by the left ventricle of the isolated heart, and contributes to an increase in end-diastolic pressure [12]. Thus, it was found that stress causes necrosis of the cardiac cells and impaired pumping function of the heart.
For a long time, there were no methods for the in vivo diagnosis of the stress cardiomyopathy in humans. The situation changed in 1990 when a group of Japanese cardiologists, who used the echocardiography method, were able to identify a disease that they called “tako-tsubo syndrome” [13]. The disease was characterized by akinesia/hypokinesia of the distal left ventricle (LV) regions with basal normokinesia. There were no signs of atherosclerosis of the epicardial coronary arteries. The heart took the form of an octopus catch vessel - tako-tsubo. The authors suggested that the cause of the disease is spasm of the coronary vessels. Indeed, spasm of one of the epicardial arteries was discovered, which disappeared after the infusion of nitroglycerin, which was confirmed by an angiographic study. Eleven years later, an article appeared in an authoritative journal that confirmed the existence of takotsubo syndrome (TS) [14]. The term stress cardiomyopathy is applicable to the syndrome so it was first used by Pavin et al. [15]. Sometimes stress cardiomyopathy is called the “broken heart syndrome” [16] or “apical ballooning syndrome” [17]. Stress cardiomyopathy is a relatively rare disease, it occurs in 0.6 - 2.5% of patients with acute coronary syndrome [18-24] or 178 (0.018%) cases of TS per 1 million are registered annually in hospitalized patients [25], other authors cite a similar figure - 0.02% of the number of the hospitalized patients [26]. Today, the incidence of TTS is estimated at 2% in patients with an initial diagnosis of acute coronary syndrome (ACS) [27].
2. The main clinical manifestations of takotsubo syndrome
The incidence of takotsubo syndrome is 9 times higher in women, who are 60–70 years old, than in men [28-30]. The mortality rate with TS is 3 times higher in men [28]. The daily peak in TS incidence occurs in the morning until noon [31]. The seasonal peak in the incidence of stress cardiomyopathy in Italy and New Zealand is in summer [31, 32].
Chest pain is observed in 63% - 82% of patients with TS [33-35]. Dyspnea occurs in 27% - 50% of patients with stress cardiomyopathy [33, 36]. During the first three days after hospitalization, 39% of patients have systolic blood pressure below 90 mm Hg [37].
The main clinical manifestation of TS is contractile dysfunction (stunning), which usually affects the apical region of the heart in the absence of coronary thrombosis [13]. However, TS can be combined with coronary heart disease (CHD). A combination of CHD and TS is observed in 10-14% of patients with stressful cardiomyopathy [38, 39]. Myocardial stunning in TS usually affects the apical region of the heart, consequently, takotsubo syndrome is often called “apical ballooning syndrome” [17]. Atypical disturbance of cardiac contractility, which affects other parts of the heart, can be observed in 28-40% of patients with TS [40-44]. The left ventricular ejection fraction (LVEF) in TS is comparable to LV EF in patients with ST-segment elevation myocardial infarction (STEMI) and is 33% - 38% [45-47]. Both systolic and diastolic cardiac functions is impaired [48]. Cardiogenic shock can occur in 7% -10% of patients with stress cardiomyopathy [26, 49, 50]. According to other investigators, the incidence of cardiogenic shock in cardiomyopathy stress is 20-27% [33, 51]. The mortality of patients with TS and cardiogenic shock can be as high as 28% [52]. 45% of patients with TS develop pulmonary hypertension [53]. Contractility dysfunction in TS is persistent. An investigation by Neil et al. [54], indicates recovery of LV EF occurs only after 3 months from the onset of the disease.
Persistent ST-segment elevation can be observed in 13-19% in patients with stress cardiomyopathy [33, 55, 56], and in 60% of patients T-inversion is observed [33]. ST-segment elevation is a predictor of adverse TS events [55]. A prolongation of the QTc interval is observed in 28% - 54% of patients with TS [57, 58]. Patients with a prolonged QTc interval are more often intubated, they often develop cardiogenic shock, and arrhythmias compared with patients with a normal QTc interval [43, 44, 58]. Hospital mortality (25%) was increased in patients in whom QTc did not recover after hospitalization compared with mortality (9%) in patients who had a normal QTc interval after hospitalization [59].
It has now been documented an increased level of markers of necrosis troponin T and troponin I occurred in patients with TS [55, 60, 61]. Some investigators did not find an increase in myocardial necrosis markers in TS patients [46, 62, 63]. The level of necrosis markers in patients with TS is significantly lower than in patients with AMI. Thus, the peak creatine kinase (CK) was 50% lower in patients with TS compared to AMI patients [64]. Other data indicate that the CK peak in patients with AMI is 6 times higher than in patients with stress cardiomyopathy. Troponin T peak in patients with AMI is 6- fold higher than in patients with stress cardiomyopathy [60].
Life-threatening arrhythmias (asystole, ventricular tachycardia and fibrillation) are observed in 11% of TS patients [65]. According to other data, ventricular arrhythmias and AV block are observed in 12% of patients with TS [66].
Magnetic resonance imaging (magnetic resonance imaging) suggests that TS contributes to myocardial edema [67].
The outcome of stress cardiomyopathy can be a complete recovery or death of the patient, usually as a result of cardiogenic shock. Hospital mortality among patients with TS corresponds to 3.5% - 12% [68, 69]. Hospital mortality in patients without cardiopulmonary insufficiency is 1.4%, hospital mortality in patients with cardiopulmonary insufficiency is 18% [70]. According to Perez-Castellanos et al. [34], the hospital mortality among male patients with TS is 4.4%, and among women - 0.2%. Hospital mortality in patients with TS and life-threatening arrhythmias is 39%, in patients without arrhythmias - 9%, annual mortality among patients with arrhythmias is 48%, and in patients without arrhythmias - 14% [65]. Thus, the presence of life-threatening arrhythmias significantly worsens the prognosis of TS. Recurrence of TS occurs in 1.8-10% of patients who have experienced the takotsubo syndrome [71, 72]. Surveillance of patients who have had TS, for 5.8 ± 3.6years, indicated that 9% have re-hospitalization for heart failure [29]. Only 5% patients died from cardiac causes. Cancer is the most common cause of death for TS patients [29].
3. Problem of differential diagnosis of patients with takotsubo syndrome
The clinical manifestations of the takotsubo syndrome are similar to AMI. Patients with TS are usually diagnosed with ACS during admission [27]. The problem of differential diagnosis is aggravated by three more reasons. First of all, patients with TS may have atherosclerotic plaques in the coronary vessels [38, 39]. Secondly, in some cases, AMI can proceed without obstruction of the coronary arteries as Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA) [44, 73-75]. Myocardial infarction in the absence of obstructive coronary artery disease is observed in 5% to 6% of all patients with AMI [76]. Investigators believed that the main reasons MINOCA may be epicardial spasm, microvascular dysfunction, plaque disruption, non-angiographically obstructive coronary embolism/thrombosis, and spontaneous coronary artery dissection [44, 76]. Coronary vasospasm was diagnosed in 46% of patients with MINOCA undergoing provocative testing [77]. Some investigators suggest that patients with MINOCA have coronary microvascular dysfunction [76]. Spontaneous coronary artery dissection is a common cause of AMI among women <50 years of age [76]. Intravascular ultrasound and coherence tomography may allow one to identify plaque rupture or erosion as causative mechanisms of AMI with no obstructive coronary arteries [76]. Finally, TS can often occur atypically without apical ballooning [41, 42].
All of these reasons make it difficult to diagnose TS. An important role in the differential diagnosis of TS and AMI is played by BNP and Natriuretic Peptide N-Terminal Pro-B-Type (NT-proBNP) whose level increases in patients with TS [78-81]. The peak of NT-proBNP is noted 24 h after the onset of the disease [80]. The concentration of NT-proBNP (4702 pg/ml) is 2-fold higher in patients with TS than in AIM patients (2138 pg/ml) [81]. The ratio of NTproBNP/Troponin I and NTproBNP/CK-MB was 2235 and 678 in patients with TS and 82 and 28 in patients with AMI and STEMI (p <0.001) [81] (Table 1). Other investigators obtained similar data [82]. Thus, the ratio NTproBNP/Troponin I and NTproBNP/CK-MB can be used for the differential diagnosis of AMI and TS. Other investigators also point to the importance of identifying biomarkers in the differential diagnosis of TS [83]. Nevertheless, according to some investigators [44] these ratios present low sensitivity (50%). The ST elevation presented only in a minority of patients (12% of cases) with TS [44], that can also help in the differential diagnosis with STEMI. QTc interval prolongation is observed in 48% of patients with TS [58]. Patients with TS generally have normal epicardial coronary arteries at coronary angiography. However, TS can be combined with CHD in 10-14% of patients with TS [38, 39] (Table 1). Although according to some investigators [84], atherosclerotic plaques in coronary arteries can be detected in 70% of patients with TS. It should be noted that plaque rupture is not observed in patients with TS. Serious help in the differential diagnosis of AMI and TS can be angiography and single photon emission computed tomography with a radiopharmaceutical compound 99mTc-sestamibi, which allows one to identify a perfusion defect in AMI and show that there is no defect in perfusion at TS (Table 1).
4. Etiology of takotsubo syndrome
The most common cause of TS is physical or emotional stress. Consequently, a disease is called stress cardiomyopathy. Physical stress is observed in 39% - 55% of cases, while emotional stress has been reported in 17% - 33% of patients with stress by cardiomyopathy [85-87]. However, in 12% - 31% of cases, it is not possible to identify the cause of TS [85-87]. Serum norepinephrine levels were higher in patients with TS who experienced physical stress compared to those patients who had emotional stress or had no apparent reason for the occurrence of stress damage of the heart [69]. Five-year mortality among people undergoing physical stress is 30%, for people who had emotional stress, mortality was 9%, and for patients who could not identify the trigger, mortality over 5 years corresponded to 15% [87].
If stress is the main cause of TS, catecholamine and cortisol levels should be elevated in patients with stress cardiomyopathy. Indeed, it turned out that the plasma epinephrine level in patients with TS in the subacute period was significantly higher (p = 0.0004) [88] than in the subsequent study [88, 89]. Performing a mental stress test resulted in a greater increase in the norepinephrine level in patients with TS than in healthy volunteers [90]. The epinephrine level in the plasma of patients with TS during hospitalization was higher than in patients with acute coronary syndrome [91]. The fact that norepinephrine and epinephrine levels increase in plasma of patients with TS has been confirmed by other investigators [92]. It has been shown that sympathetic nervous activity is enhanced in patients with TS compared with patients with chronic heart failure [93]. It should be noted that sympathetic nervous activity is enhanced in patients with chronic heart failure [94]. Apparently, if a comparison was to be made between TS patients and healthy volunteers, the difference in sympathetic activity would be even more significant. It has been shown that in patients with TS, the levels of not only catecholamines, but also cortisol were increased [95].
Consequently, physical and emotional stress is important, but apparently it is not the only etiological factor in stress cardiomyopathy. Thus, according to some data, in 37% of patients with TS, the onset of syndrome was preceded by mental illness [96]. A TS trigger may be status epilepticus [97, 98] or convulsions of a different origin [99-101]. In this regard, it should be noted that bicuculline-induced status epilepticus is accompanied by an increase in the plasma norepinephrine level in dogs [102]. It has been shown that plasma norepinephrine level was increased during the generalization of seizures in a patient [103]. Patients with TS are more likely to have anxiety disorders than patients with acute coronary syndrome [104, 105]. Depression is observed in 20% of patients with TS and anxiety is noted in 31% of patients with stress cardiomyopathy [106]. The occurrence of TS can be preceded by a stroke [36, 107, 108]. It should be noted that hemorrhagic stroke is accompanied by an increase in the plasma norepinephrine and epinephrine levels [109, 110].
Thus, mental disorders and diseases of the central nervous system can be triggers of takotsubo syndrome.
The emergence of TS is associated with over-activation of the adrenergic system [111]. Indeed, TS is often seen in patients with pheochromocytoma [112-114]. There are cases of TS after an injection of adrenergic receptor (AR) agonists [115-117]. TS has been reported after opiate withdrawal [118-120], after the administration of catecholamine re-uptake inhibitors [119]. In this regard, it should be noted that opiate withdrawal syndrome is accompanied by activation of epinephrine release from the adrenal glands [121]. TS has also been reported in people who have used methamphetamine [122, 123]. In this regard, it should be noted that amphetamine and its analogs activate α- and β-AR, which stimulate the release of norepinephrine [124]. It has been documented that thyrotoxicosis can contribute to the occurrence of TS [125-128]. The pathogenic effect of an excess of thyroid hormones appears to be related to the ability of triiodothyronine to increase β1-AR density on cell membranes [129, 130].
There is now recent evidence of a genetic predisposition to TS [131]. It is possible that this predisposition is associated with polymorphisms of genes encoding β-ARs. Consequently, a different distribution of β1-AR polymorphisms Arg389 Gly is more frequently found in TS [131].
Thus, the main causes of stress cardiomyopathy may be attributed to stress, CNS disease, and effects that increase the level of catecholamines (Fig. 1).
5. Pathogenesis of stress cardiomyopathy
The emergence of TS is associated with over-activation of the adrenergic system [23, 43, 44, 111, 132]. Indeed, as we have reported above, in patients with TS, the catecholamines level was increased and the adrenergic system was activated. In addition, it was found that as the concentration of epinephrine increases, its positive inotropic effect gives way to the negative inotropic effect due to the activation of β2-AR coupled with Gi-proteins [133], therefore the negative inotropic effect disappears after inhibition of the Gi-proteins by pertussis toxin. The β-AR agonist isoproterenol also had a biphasic effect [134]. The negative inotropic phase disappeared after the blockade of β2-AR and the use of pertussis toxin, but persisted after the application of the selective β1-AR antagonist CGP 20712A. Injection of large doses of epinephrine caused an apical disturbance of cardiac contractility, which eliminates the administration of the Gi-protein inhibitor pertussis toxin [135] (Fig. 2). The α2-AR agonist, xylazine, when administered to immobilized rats, has been shown to impair cardiac contractility, which eliminates pertussis toxin [136]. Isoproterenol (50 mg/kg intraperitoneally) was found to cause the appearance of akinetic regions in the left ventricle of rats 60 min after injection [137].
The important role of adrenergic system hyperactivation in TS is confirmed by the results of studies using AR antagonists. Thus, in a study performed on monkeys, propranolol was shown to prevent the occurrence of SIIH [10]. Propranolol had the same effect on stressed pigs [138]. The non-selective α- and β-AR antagonist labetalol prevented a disturbance of cardiac contractility in rats caused by immobilization for 30 min [139]. Metoprolol also acted [140]. We found that the selective β1-AR antagonist nebivolol prevents the occurrence of SIIH. We also found that the β2-AR antagonist ICI-118551 exacerbates stress-induced heart’s injury and that the β3-AR antagonist L748337 does not affect SIIH upon immobilization (unpublished data) (Fig. 2).
An important role of the adrenergic system in the pathogenesis of TS was confirmed by the studies which were performed using 125I-metaiodobenzylguanidine (125I -MIBG), which is an alternative to norepinephrine and, like norepinephrine, is subjected to uptake by sympathetic nerve terminals [141, 142]. Chemical sympathectomy with 6-hydroxydopamine results in impaired uptake of norepinephrine and MIBG in the heart [143]. Norepinephrine and MIBG compete with each other for the system of uptake in sympathetic nerve terminals, therefore reducing the accumulation of MIBG in the heart indicates an increase in the number of norepinephrine in the synaptic cleft, in other words, the higher the sympathetic activity, the less 125I –MIBG accumulates in the heart. Keeping volunteers in hypobaric hypoxia leads to a 2-fold increase in the plasma norepinephrine level, while the level of epinephrine does not change [144]. Twenty minutes after the cessation of hypoxia, the accumulation of 123I-MIBG in the heart was significantly reduced. The accumulation of 123I-MIBG remained reduced for at least 240 min after the cessation of the hypoxic exposure, until the norepinephrine level returned to normal [144]. Accumulation of 123I-MIBG in the heart is significantly reduced in patients with idiopathic dilated cardiomyopathy compared with volunteers without signs of heart disease [145]. In this regard, it should be noted that the plasma norepinephrine level in patients with dilated cardiomyopathy is 3-fold higher than in the control group [145].
A decrease in 125I-MIBG accumulation in the apical part of the heart has been reported in patients with TS [146]. 125I –MIBG uptake has been shown to decrease in the hypokinetic regions of the heart of patients with stress cardiomyopathy [147]. 125I –MIBG accumulation defects in the left ventricle of patients with TS have been noted by other investigators [89]. It has also been documented that the ratio of 125I –MIBG uptake in the heart to the mediastinum is lower in the TS subacute phase than with normalization of heart’s contractility 4 months after discharge from the hospital or in the control group [88]. The washout rate of 125I –MIBG from the myocardium was increased in patients with TS in the subacute period compared with the control group [88]. Similar data were obtained by Burgdorf et al. [148].
The presented data convincingly show that adrenergic system is activated in patients with TS. What are the consequences of hyperactivation of the adrenergic system? Above, we have shown that it can be a contractile dysfunction of the heart. These can be heart rhythm abnormalities which are caused by catecholamines [149]. Patients with TS may have pathological changes that can also be explained by hyperactivation of the adrenergic system, for example, inflammation and edema of the myocardium. Thus, catecholamine-induced myocarditis has been reported in patients with pheochromocytoma [150-153]. Daily administration of isoproterenol at a dose of 1.2 mg for 14 days has been shown to cause myocarditis in rats [154]. At the same time, some investigators have noted that at the time of diagnosis, many (33%) patients with TS took β-AR antagonists [26]. Among patients with recurrent TS, 51% took β-blockers [26], which contradicts the leading role of catecholamines in the pathogenesis of TS.
An endomyocardial biopsy in patients with TS showed that they had mononuclear infiltration [155] and contraction-band necrosis [155, 156]. In patients with TS, the level of C-reactive protein is increased, which reaches to its maximum 72 hours after the onset of the disease [146]. Other investigators have reported an increase in the high sensitivity of C-reactive protein level patients with TS [157, 158]. Magnetic resonance imaging (MRI) data suggests myocardial edema during stress cardiomyopathy [156, 159, 160]. There is evidence that stress cardiomyopathy leads to myocardial fibrosis [160, 161]. Myocardial edema is persistent, according to Schwarz et al. [160], in areas with normal contractility, edema disappears 4 months after disease. It was shown that the levels of tumor necrosis factor (TNF-α) and C-reactive protein in blood during hospitalization were increased compared to their levels in these patients one year after discharge. The levels of interleukin-6 (IL-6) and interleukin-10 were also increased in patients with TS with adverse events, including death [162]. The IL-6 and chemokine (C-X-C motif) ligand 1 (CXCL1) levels were increased in blood of patients with stress cardiomyopathy compared with the control group [163].
The aforementioned data strongly suggest that TS causes edema and inflammation of the myocardium. The inflammatory reaction in TS is systemic, as evidenced by an increase in C-reactive protein, TNF-α, and IL-6, CXCL1.
It is now hypothesized that coronary artery spasm plays an important role in the pathogenesis of TS [111]. In the first article on takotsubo syndrome, Sato et al. [13], presented angiographic evidence that coronary spasm plays an important role in the pathogenesis of stress cardiomyopathy. In 2002, Kurisu et al. [164], while were performing coronary angiography, documented spontaneous coronary spasm in three out of 30 patients with takotsubo syndrome. In 6 of the 14 patients which were included in the study, acetylcholine or ergonovine caused spasm of several coronary arteries. Four of these patients had spasm of single coronary arteries. These investigators hypothesized that, although the exact cause of stress for cardiomyopathy remains unclear, simultaneous multi-vascular spasm of the epicardial coronary arteries or microvascular spasm may contribute to the occurrence of TS. Other investigators also emphasize the important role of microvascular dysfunction in the pathogenesis of TS [43,44]. We would like to draw the reader’s attention to the fact that spontaneous vasospasm of the epicardial coronary arteries was detected in only 10% of patients, which does not exclude microvascular spasm. One of the causes of impaired coronary circulation may be attributed to the findings that the myocardial bridging of the left anterior descending artery was detected in 76% of TS [26].
In the last decade, thanks to the possibilities of new diagnostic technologies, in particular, ultrasound study of the coronary reserve and echo contrast myocardial perfusion, investigators have obtained results indicating that the main mechanism in the development of TS may be a microcirculatory dysfunction. Thus, in 2009, investigators [165], while were performing qualitative and quantitative myocardial contrast echocardiography at rest, revealed a disturbance of myocardial perfusion was typical for TS in the apical region of LV, in which an abnormal myocardial contractility was noted. A microcirculatory dysfunction in the acute phase of process was found. A disturbance of microcirculation was transient, followed by recovery within 6 months. Similar results were demonstrated by other investigators [47]. In 2017, Hung et al. [166], performing an echocardiographic study in a patient with TS, found indirect signs of global coronary artery vasospasm in the form of a decrease in the deformation characteristics of the left heart chambers.
Stress cardiomyopathy can adversely affect the size of the coronary reserve. Kume et al. [167], performed an invasive Doppler study of the coronary reserve with adenosine in the anterior descending, circumflex, and right coronary arteries during coronary angiography. Their study confirmed that coronary microcirculation and endothelial dysfunction were most likely the main origin of TS. Against the background of adenosine-mediated vasodilation, these investigators identified a reduction in coronary reserve in all three main coronary arteries during the acute phase of the process, followed by its recovery after 3 weeks. It was shown by utilizing stress echocardiography with adenosine in patients with TS, that there was a decrease in coronary reserve in the distal segment of the anterior descending artery [168]. At the same time, coronary reserve indicators had an inverse correlation with indicators of contractile and pumping function of LV. The authors regard these changes as manifestation of endothelial dysfunction and impaired coronary microcirculation. Similar data were obtained by other investigators with a similar interpretation [169]. In a study that was performed using dopplerography in the distal segment of the anterior descending coronary artery with contrast enhancement during stress echocardiography with β-AR agonist dobutamine, TS patients also showed a decrease in coronary reserve [170]. According to these investigators, these studies may indicate a coronary microcirculatory dysfunction in TS.
A study performed by Burgdorf [171] included two patients with TS. Evaluation of coronary perfusion was performed using 99mTc-methoxyisobutylisonitrile (99mTc-sestamibi), which accumulates in myocardial regions with normal perfusion. The study did not reveal any abnormalities. In another study by Burgdorf et al. [148], 10 patients with TS were included. Their study indicated a typical dilatation of the LV apex with hyperkinesia of the LV basal regions and akinesia of the apex of the heart. At the same time, 99mTc-sestamibi accumulation in the apical region was normal or minimally narrowed. These findings allowed the investigators to conclude that coronary spasm is rare in TS.
In another study, myocardial blood flow and coronary reserve were assessed using positron emission tomography (PET) with 13N ammonium [172]. It was found that myocardial blood flow and coronary reserve were significantly reduced in the area of the apical segments, compared with the middle and basal segments. These changes completely disappeared after 3 months. However, in a study done by Hasbak et al. using PET [173], a decrease in myocardial blood flow in the apical region of the heart could not be detected. Perfusion in the apex region of the heart remained within the normal range, and increased in the basal regions [173]. Later on, the same group demonstrated in 25 patients that myocardial beds according to PET data with 13N-ammonium or 82Rb were unchanged in the apical and midventricular regions and increased in basal regions [174]. These investigators concluded that with TS, normal perfusion of certain regions of the myocardium and hyperperfusion of the basal segments takes place [174]. Somewhat different data was provided by Ghadri et al. [175] in the description of the clinical case of a patient with TS. Their studies indicated a reduction of perfusion and coronary reserve (PET data with 13N ammonium), as well as glucose metabolism (PET data from 18F-fluorodeoxyglucose) 7 days after hospitalization and full recovery of these processes after 3 months. These investigators observed changes with the development of microcirculatory dysfunction associated with the release of catecholamines into the blood. In addition, these investigators noted that the disturbance of myocardial blood flow and coronary reserve with TS are global [175].
The aforementioned studies suggested that some patients with TS have spasm of the epicardial coronary arteries. Angiographic studies indicate that in patients with stress cardiomyopathy, there is a disturbance of the coronary microcirculation and a decrease in the coronary reserve, but there is no direct evidence of spasm of the coronary arteries in the majority of patients with TS. It has been hypothesized that the over-activation of the adrenergic system plays a key role in the pathogenesis of TS [111]. In a study done by Redfors et al. [176], SIIH was induced by injection of the β-AR agonist isoproterenol. Microcirculation in the myocardium was assessed using contrast echocardiography. These investigators failed to detect microcirculation disorders. They concluded that apical perfusion is not impaired in rats with isoproterenol-induced heart injury.
Some investigators [177] have rejected the hypothesis of global vasospasm of the coronary arteries in the origin of stress cardiomyopathy. They consider inflammation, edema of the myocardium and vascular wall with the subsequent compression of small vessels and endothelial dysfunction as the main cause of microcirculatory disorders. It is believed that inflammation plays an important role in the origin of the takotsubo syndrome, which contributes to the slow coronary blood flow [178].
There is reason to believe that blood viscosity may play an important role in the pathogenesis of microcirculatory dysfunction. Increased blood viscosity is one of the factors which causes slower blood flow [179]. It was found that patients with TS, when the cold pressor test was performed, showed an increase in total blood viscosity, plasma viscosity, a decrease in red blood cell deformability compared with the control group. This study included women of similar age with the same risk factors without coronary artery stenosis [180]. In both groups, a rise in the level of catecholamines was observed after the cold test. One of factors contributing to an increase in blood viscosity is likely to be increased platelet aggregation, which persists 3 months after the onset of disease [91]. Catecholamines are believed to be triggers for increasing blood viscosity in patients with TS [180]. Indeed, the whole blood viscosity has been shown to correlate with the level of epinephrine [181]. Synchronism of the increase in the level of catecholamines and the whole blood viscosity has also been noted by other investigators [182].
The aforementioned studies present substantial evidence indicating that catecholamines may act as TS triggers. However, there is the possibility that other humoral factors, such as endothelin, are involved in the TS pathogenesis. Endothelin-1 can cause spasm of the coronary arteries [183–185]. The coronary arteries of small diameter are most sensitive to endothelin-1 [183]. It is generally accepted that endothelin has a positive inotropic effect [186, 187]. However, there is evidence that endothelin-1 may reduce contractility of isolated mouse cardiomyocytes [188]. Endothelin receptor antagonists have been reported to increase the survival rate of heart failure rats [189,190], therefore endothelin may be a potential trigger point TS. It was found that the level of endothelin-1 in plasma of patients with TS is 2-fold higher than in healthy subjects [191]. These investigators believed that their data confirm microvascular spasm hypothesis of TS. However, in another clinical study, the differences in endothelin-1 levels were not found in those patients with TS compared the control group of people of similar age, gender, cardiovascular risk factors and medications [192]. Thus, the question of the involvement of endothelin-1 in the pathogenesis of TS remains open.
We hypothesize that cortisol may be another candidate for the role of a trigger in the pathogenesis of TS. Indeed, the cortisol levels are elevated in patients with TS [95]. Immobilization stress is commonly used to model TS in rats [139, 140, 193]. Immobilization causes an increase in serum not only in the corticosterone levels, but also in cortisol in these animals [194]. Glucocorticoids are known to have a permissive effect, that is, they enhance the effect of catecholamines [195, 196]. We found that the glucocorticoid receptor antagonist mifepristone reduces heart damage during immobilization stress (unpublished data).
It has been shown that microRNAs may be involved in the pathogenesis of TS [191, 197, 198]. MicroRNAs are generally considered small noncoding RNA sequences (20-23 nucleotides) that suppress the expression of various genes in eukaryotic cells [199]. MicroRNA formation occurs as a result of RNA transcription by RNA polymerase II enzyme [200], sequential processing of primary transcripts by Drosha and DGCR8 complexes (inside the cell nucleus) [201], and migration of microRNA precursors (pre-microRNA, 70 nucleotides) from the nucleus to the cytoplasm with the exportin-5 protein [200]. In the cytoplasm, pre-microRNA is hydrolyzed with RNase III and then bound to the RISC (microRNA-induced silencing complex) complex, forming a mature microRNA (miRNA) molecule. According to some investigators [202, 203], mature miRNAs together with a complex of RISC proteins, binding to complementary sites on mRNA, blocks the translation of mRNA. Due to this ability, miRNAs participate in the regulation of a number of cellular functions and processes, such as growth, differentiation, proliferation, metabolism and apoptosis [204, 205]. It is now believed that miRNA exert their effects by migration with the blood flow to target cells inside exosomes (microvesicles) [206]. Microvesicles, in turn, can be released from cells whose tissue has undergone transient ischemia/reperfusion [206].
The aforementioned study which was performed in 2010 included patients with normal coronary arteries, stable angina STEMI, non-STEMI, takotsubo syndrome, dilated cardiomyopathy [197]. miRNA miR-1 levels were elevated only in STEMI. The miR-133a level was elevated in all patients except patients with normal coronary arteries and stable angina. These investigators tried to find out the reason for the increase. They incubated H9c2 cardiomyoblasts with calcium ionophore A23187. It turned out that A23187 leads to the appearance in the culture medium of exosomes containing miR-133a. The maximum increase in miR-133a level was at concentrations of A23187 that cause the death of cardiomyoblasts. The investigators concluded that miR-133a appears in blood of patients as a result of myocardial damage and death of cardiomyocytes [197]. The investigators of another study found that miRNA125a-5p level decreases in blood of patients with TS [191]. This miRNA inhibits the expression of endothelin-1, the level of which is elevated in patients with stress cardiomyopathy. It is possible that a decrease in the level of miRNA125a-5p is the cause of an increase in the concentration of endothelin-1 in the blood of patients with TS. The level of other miRNAs (miR-16, miR-26a, miR-1, miR-133a) was, on the contrary, increased in patients with TS compared to healthy subjects. The physiological significance of this increase in miRNA remains unclear.
Takotsubo syndrome mainly affects women who are in a state of menopause. Consequently, some investigators believe that one of the factors contributing to the onset of TS is a decrease in estrogen levels [17, 207]. Indeed, estrogen increases the tolerance of the heart to the ischemia/reperfusion [208, 209], therefore, it is likely that it can increase cardiac tolerance to stress. A study was performed on ovariectomized female rats, some of animals received estrogen [210]. Animals were subjected to immobilization stress. Chronic estrogen supplementation has been shown to improve stress-induced cardiac dysfunction and significantly increase blood pressure [210]. In addition, estrogen supplementation contributed to a threefold increase in heat shock protein 70 (HSP70). Heat shock proteins are known to increase cardiac tolerance to adverse effects, such as ischemia/reperfusion [211]. It can be assumed that such an increase in the HSP70 level contributes to increased tolerance of the heart to stress. In a study that was performed on sham-operated and the ovariectomized rats, it was shown that epinephrine resulted in more serious cardiac dysfunction and higher plasma troponin I concentration in ovariectomized rats than in the sham-operated group [212]. 17β-Estradiol eliminated hypersensitivity to epinephrine in ovariectomized rats. The selective β2-AR antagonist ICI118,551 eliminated the negative inotropic effect of epinephrine in ovariectomized rats. An increase in the cAMP level in the myocardium and the amount of phosphorylated protein kinase A (p-PKA) in myocardium after epinephrine injection was lower in ovariectomized rats than in the sham-operated rats. The administration of 17β-estradiol to ovariectomized rats promoted a rise in the cAMP level and an increase in the amount of p-PKA after administration of epinephrine [212]. Thus, the presented data indicates that ovariectomy increases the sensitivity of the heart to stress and the cardiotoxic effect of epinephrine. Estradiol eliminates these disorders. In addition, these data suggest that the negative inotropic effect of ovariectomy after epinephrine injection is associated with the activation of β2-AR. These facts suggest that a low level of estrogen may contribute to the occurrence of TS due to the activation of β2-AR. However, these data do not explain the fact why TS is less common in men. It is possible that testosterone prevents the development of TS.
The central nervous system appears to play an important role in the pathogenesis of TS. The aforementioned studies indicate that TS is common in patients with CNS diseuses (subarachnoid hemorrhage, status epilepticus, anxiety disorders, depression). According to some studies, 37% of TS patients were preceded by a mental illness [96]. In a study which was performed by Belcour et al. [98], stress cardiomyopathy was detected in 56% of patients with status epilepticus. According to Porto et al. [213], TS patients often suffered from neurological disorders. The most frequent neurological disorders were subarachnoid hemorrhage, followed by stroke/transient ischemic attack, and less often seizures. According to Wagdy and El Maghawry [26], mental and neurological disorders occur in 56% of patients with TS. Other investigators cite a similar figure [214]. Back in 1969, before the discovery of the “broken heart syndrome” in the UK, it was demonstrated that mortality among widowers is higher than that of their married peers [7]. The investigators considered that the cause of the increased mortality was a “broken heart,” that is heart disease. In 1980, autopsy results of people who died suddenly as a result of physical violence were published [2]. All victims of violence had myofibrillar degeneration of the heart, which could have occurred only as a result of psychological trauma. The authors of the discovery suggested to name this disease as stress cardiomyopathy. It was later known as takotsubo syndrome [13]. The most recent work has shown the important role of emotional stress in TS pathogenesis [106, 155, 215-217]. As we noted above, depression was observed in 20% of patients with TS, and anxiety in 31% of patients [106]. According to other data, anxiety disorders occur in 53% of TS patients [104].
The presented data show the important role of CNS in the pathogenesis of TS. In 2017, Tavazzi et al. [218] hypothesized the existence of neurogenic stress cardiomyopathy, which is similar to takotsubo syndromes. In their opinion, these are two different syndromes with common pathways. They report that abnormalities in cardiac contractility are present in 30% of patients with severe brain damage without a known history of cardiac disease. According to Tavazzi et al. [218], patients with severe brain damage experience an “autonomic storm,” which is responsible for cardiac stunning (Fig. 1). We have attempted to compare TS and neurogenic stress cardiomyopathy, as presented by the authors of the hypothesis. The only difference between these diseases was that with TS, a disturbance is local (apical, basal with mild-ventricular), and with neurogenic stress cardiomyopathy, contractility dysfunction is global. A similar view is held by Marafioti et al. [219], who hypothesized that in severe brain diseases promote Takotsubo-like cardiac pathology development. In the occurrence of this pathology, the insula cortex lesion seems to play a pivotal role. Some investigators do not share this view [220]. We also do not share this view. In our opinion, for a more feasible hypothesis of neurogenic stress cardiomyopathy, the differences between these two syndromes should be larger.
6. The problem of treatment of takotsubo syndrome
There is currently no consensus on the most appropriate TS therapy. In the absence of randomized clinical trials, there is no evidence for highly effective treatment of TS. It is believed that in most cases, this therapy should be conservative [44]. However, some investigators suggest using intra-aortic balloon pumping to treat TS [43, 221]. In the first days after hospitalization, the risk of arrhythmias and cardiogenic shock is high, consequently, treatment should be aimed at preventing these disorders. It is suggested that the following pharmacological agents should be used for TS: β-blockers, diuretics, anticoagulants, antiarrhythmics, and levosimendan [132, 221]. In our opinion, the issue of using β-blockers cannot be considered to be resolved. On the one hand, according to our unpublished data, β1-AR antagonists are able to prevent stress injury of the heart in rats. On the other hand, it is well known that β-AR antagonists have the negative inotropic effect. Consequently, one cannot rule out the possibility that they will exacerbate heart failure in patients with TS. Therefore, some investigators recommend the use of short-acting intravenous β-blockers in patients without severe heart failure and bradycardia [43, 132]. Moreover, it is now recommended to use catecholamines in patients with TS and cardiogenic shock, but keeping aware that they can exacerbate heart damage [132]. Levosimendan has been suggested as an alternative to catecholamines, but there is a lack of convincing data on its effectiveness in patients with TS [132]. It is believed that in the absence of obstruction, drugs with positive inotropic action (i.e., dobutamine, milrinone, dopamine, or levosimendan) can be used to increase cardiac output [43]. Some investigators recommend the use of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers for treatment of TS [44, 132]. Other investigators recommend that prophylactic anticoagulants should be used to prevent apical thrombus formations [132].
Conclusion
The presented data indicate that emotional and physical stress plays a triggering role in the onset of takotsubo syndrome. Indeed, in patients with TS plasma norepinephrine and epinephrine level are increased, which proves the stressful nature of TS. However, in 12% - 31% of cases, it is not possible to identify the main cause of TS. It would be interesting to compare the level of catecholamines in those patients with TS who have undergone stress, and in patients with takotsubo syndrome, in whom the cause of cardiomyopathy has not been identified. In 37% of patients with TS, the mental illness preceded stress cardiomyopathy. The trigger for TS may be status epilepticus, stroke, pheochromocytoma, adrenergic injection, opiate withdrawal syndrome. These conditions are accompanied by an increase in the level of catecholamines, which play a trigger role in TS. Catecholamines in toxic doses have a negative inotropic effect, which confirms their role in the pathogenesis of takotsubo syndrome. It has been documented that patients with TS have signs of myocarditis and systemic inflammation, microcirculatory dysfunction, some patients have coronary spasm. Whether catecholamines can cause all these disturbances is still unknown. The mystery is the fact that countless millions of people endure stress each year, but only a few thousand develop stress cardiomyopathy. Apparently, there are the certain mechanisms in the body of most individuals that limit the stress response. In this regard, we should recall the hypothesis of Professor Meerson about the existence of stress-limiting systems to which he attributed endogenous antioxidants, GABA, and endogenous opioids [222, 223]. The ability of opioids to limit the adrenergic effect on the heart is a known fact [224]. In our opinion, a comparative determination of the level of opioid peptides in patients with TS, patients with acute coronary syndrome and healthy volunteers could help in fully understanding the pathogenesis of TS.
Fig. (1).
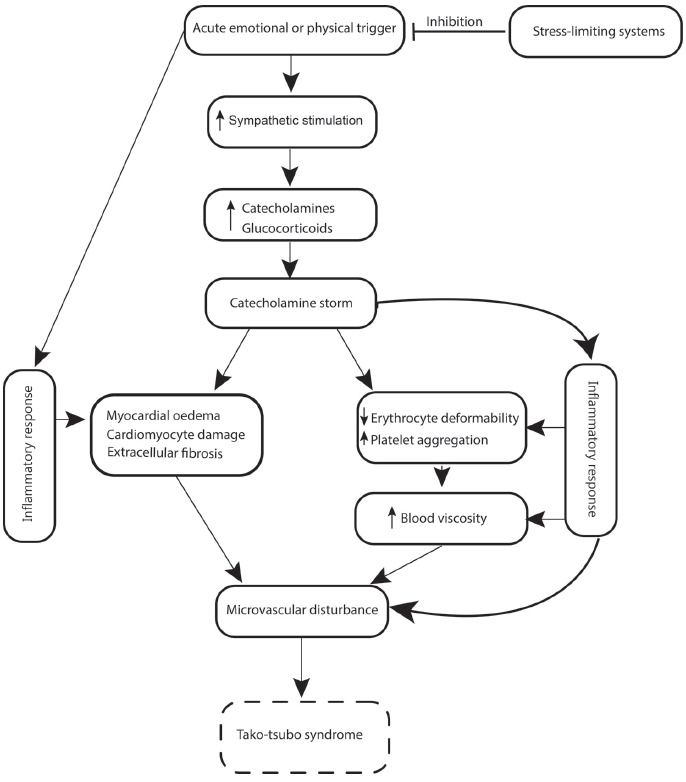
Hypothetical pathophysiologic mechanisms of takotsubo syndrome.
Fig. (2).
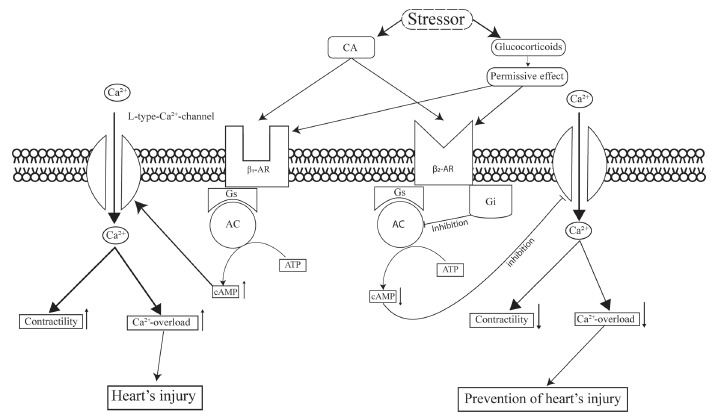
An involvement of β1 and β2 adrenergic receptors in pathogenesis takotsubo syndrome. Abbreviations. AR, adrenergic receptor, Gs, Gi, G-proteins, AC, adenylyl cyclase, cAMP, adenosine 3’,5’-cyclic monophosphate.
Table 1.
The differential diagnosis of patients with Takotsubo Syndrome (TS), Acute Myocardial Infarction (AMI) and Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA).
| Indexes | TS | AMI | MINOCA |
|---|---|---|---|
| Symptoms | Chest pain, dyspnea, arrhythmias, heart failure, cardiogenic shock sudden cardiac death. TS patients are usually women | Chest pain, dyspnea, arrhythmias, heart failure, cardiogenic shocksudden cardiac death | Chest pain, dyspnea, arrhythmias, heart failure, cardiogenic shock sudden cardiac death |
| ECG | ST-segment elevation (12% of cases) or depression, T-wave inversion, QTc prolongation (50% of cases) | ST-segment elevation (STEMI), ST-segment depression (NSTEMI), T-wave inversion | ST-segment elevation or ST-segment depression and/or T-wave inversion |
| Echocardiography | Hypokinesia or akinesia may be apical, midventricular, basal or focal | Regional wall motion abnormalities | Regional wall motion abnormalities |
| Coronary Angiography | Absence of obstructive CAD and/or intravascular imaging suggestive of acute plaque destabilization | CAD with acute plaque rupture and thrombus | Absence of angiographic obstructive CAD |
| MRI | Myocardial edema and regional wall motion abnormalities | Myocardial edema and regional wall motion abnormalities | Myocardial edema and regional wall motion abnormalities |
| Biomarkers | NTproBNP/TnI ratio is 2235, NTproBNP/CK-MB ratio is 678 The TnI and CK-MB level is lower than in patients with STEMI |
NTproBNP/TnI ratio is 82 NTproBNP/CK-MB ratio is 28 |
TnI and CK-MB is increased as in STEMI |
Note: CAD, coronary artery disease, CK-MB, creatine kinase, MRI, magnetic resonance image, NSTEM, non-ST elevated myocardial infarction, NTproBNP, Natriuretic Peptide N-Terminal Pro-B-Typ, STEMI, ST-elevated myocardial infarction, TnI, troponin I.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
This work was supported by the Russian Science Foundation Grant 16-15-10001, a section devoted to the role of the β1-adrenergic receptors in the pathogenesis of takotsubo syndrome, was performed within the framework of state task AAAA-A15-115120910024-0.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Johansson G., Jonsson L., Lannek N., Blomgren L., Lindberg P., Poupa O. Severe stress-cardiopathy in pigs. Am. Heart J. 1974;87(4):451–457. doi: 10.1016/0002-8703(74)90170-7. [DOI] [PubMed] [Google Scholar]
- 2.Cebelin M.S., Hirsch C.S. Human stress cardiomyopathy. Myocardial lesions in victims of homicidal assaults without internal injuries. Hum. Pathol. 1980;11(2):123–132. doi: 10.1016/S0046-8177(80)80129-8. [DOI] [PubMed] [Google Scholar]
- 3.Cannon W.B. “Voodoo” Death. Am. Anthropol. 1942;44(2):169–181. doi: 10.1525/aa.1942.44.2.02a00010. [DOI] [Google Scholar]
- 4.Moritz A.R., Zamcheck N. Sudden and unexpected deaths of young soldiers; diseases responsible for such deaths during World War II. Arch. Pathol. (Chic.) 1946;42(5):459–494. [PubMed] [Google Scholar]
- 5.Pruitt R.D. On sudden death. Am. Heart J. 1964;68(1):111–118. doi: 10.1016/0002-8703(64)90247-9. [DOI] [PubMed] [Google Scholar]
- 6.James T.N., Froggatt P., Marshall T.K. Sudden death in young athletes. Ann. Intern. Med. 1967;67(5):1013–1021. doi: 10.7326/0003-4819-67-5-1013. [DOI] [PubMed] [Google Scholar]
- 7.Parkes C.M., Benjamin B., Fitzgerald R.G. Broken heart: A statistical study of increased mortality among widowers. BMJ. 1969;1(5646):740–743. doi: 10.1136/bmj.1.5646.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corley K.C., Shiel F.O., Mauck H.P., Greenhoot J. Electrocardiographic and cardiac morphological changes associated with environmental stress in squirrel monkeys. Psychosom. Med. 1973;35(4):361–364. doi: 10.1097/00006842-197307000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Miller D.G., Mallov S. Quantitative determination of stress-induced myocardial damage in rats. Pharmacol. Biochem. Behav. 1977;7(2):139–145. doi: 10.1016/0091-3057(77)90198-8. [DOI] [PubMed] [Google Scholar]
- 10.Corley K.C., Mauck H.P., Shiel F.O., Barber J.H., Clark L.S., Blocher C.R. Myocardial dysfunction and pathology associated with environmental stress in squirrel monkey: effect of vagotomy and propranolol. Psychophysiology. 1979;16(6):554–560. doi: 10.1111/j.1469-8986.1979.tb01520.x. [DOI] [PubMed] [Google Scholar]
- 11.Meerson F.Z., Trikhpoeva A.M. Prevention of disturbances of myocardial contractility arising after emotional pain stress by γ-hydroxybutyric acid and the antioxidant ionol. Bull. Exp. Biol. Med. 1980;90(5):1493–1496. doi: 10.1007/BF00834074. [DOI] [Google Scholar]
- 12.Meerson F.Z., Ustinova E.E. Effect of long- and short-term stress on resistance of the heart to anoxia. Bull. Exp. Biol. Med. 1983;95(1):27–30. doi: 10.1007/BF00831215. [DOI] [PubMed] [Google Scholar]
- 13.Sato H., Tateishi H., Uchida T., et al. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. In: Eds Kodama K., Haze K., Hon M., editors. Clinical aspect of myocardial injury: From ischemia to heart failure Japanese Tokyo: Kagakuyourosha. 1990. pp. 56–64. [Google Scholar]
- 14.Tsuchihashi K., Ueshima K., Uchida T., et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J. Am. Coll. Cardiol. 2001;38(1):11–18. doi: 10.1016/S0735-1097(01)01316-X. [DOI] [PubMed] [Google Scholar]
- 15.Pavin D., Le Breton H., Daubert C. Human stress cardiomyopathy mimicking acute myocardial syndrome. Heart. 1997;78(5):509–511. doi: 10.1136/hrt.78.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lateef F. The “broken heart sydrome”: You’re likely to have it only once! Singapore Med. J. 2010;51(4):e76–e78. [PubMed] [Google Scholar]
- 17.Kuo B.T., Choubey R., Novaro G.M. Reduced estrogen in menopause may predispose women to takotsubo cardiomyopathy. Gend. Med. 2010;7(1):71–77. doi: 10.1016/j.genm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008;155(3):408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Buja P., Zuin G., Di Pede F., et al. Long-term outcome and sex distribution across ages of left ventricular apical ballooning syndrome. J. Cardiovasc. Med. (Hagerstown) 2008;9(9):905–909. doi: 10.2459/JCM.0b013e3282fec072. [DOI] [PubMed] [Google Scholar]
- 20.Mansencal N., Abbou N., N’Guetta R., Pillière R., El Mahmoud R., Dubourg O. Apical-sparing variant of Tako-Tsubo cardiomyopathy: prevalence and characteristics. Arch. Cardiovasc. Dis. 2010;103(2):75–79. doi: 10.1016/j.acvd.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Sinning C., Keller T., Abegunewardene N., Kreitner K-F.F., Münzel T., Blankenberg S. Tako-Tsubo syndrome: Dying of a broken heart? Clin. Res. Cardiol. 2010;•••:771–780. doi: 10.1007/s00392-010-0224-9. [DOI] [PubMed] [Google Scholar]
- 22.Awad H.H., McNeal A.R., Goyal H. Reverse Takotsubo cardiomyopathy: A comprehensive review. Ann. Transl. Med. 2018;6(23):460–0. doi: 10.21037/atm.2018.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Y-Hassan S Tornvall P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin. Auton. Res. 2018;28(1):53–65. doi: 10.1007/s10286-017-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamoorthy P., Garg J., Sharma A., et al. Gender differences and predictors of mortality in takotsubo cardiomyopathy: Analysis from the national inpatient sample 2009-2010 database. Cardiology. 2015;132(2):131–136. doi: 10.1159/000430782. [DOI] [PubMed] [Google Scholar]
- 25.Khera R., Light-McGroary K., Zahr F., Horwitz P.A., Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am. Heart J. 2016;172:53–63. doi: 10.1016/j.ahj.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagdy K., ElMaghawry M. Takotsubo cardiomyopathy: A potentially serious trap (Data from the International Takotsubo Cardiomyopathy Registry). Glob. Cardiol. Sci. Pract. 2015;2015(4):55. doi: 10.5339/gcsp.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlossbauer Susanne A., Jelena-Rima G., Frank S., Christian T. The challenge of takotsubo syndrome: Heterogeneity of clinical features. Swiss Med. Wkly. 2017;147:w14490. doi: 10.4414/smw.2017.14490. [DOI] [PubMed] [Google Scholar]
- 28.Lemor A., Ramos-Rodriguez A.J., De La Villa R., et al. Impact of gender on in-hospital outcomes in patients with Takotsubo syndrome: A nationwide analysis from 2006 to 2014. Clin. Cardiol. 2019;42(1):13–18. doi: 10.1002/clc.23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H., Senecal C., Lewis B., et al. Natural history and predictors of mortality of patients with Takotsubo syndrome. Int. J. Cardiol. 2018;267:22–27. doi: 10.1016/j.ijcard.2018.04.139. [DOI] [PubMed] [Google Scholar]
- 30.Scally C., Rudd A., Mezincescu A., et al. Persistent long-term structural, functional, and metabolic changes after stress-induced (takotsubo) cardiomyopathy. Circulation. 2018;137(10):1039–1048. doi: 10.1161/CIRCULATIONAHA.117.031841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manfredini R., Manfredini F., Fabbian F., et al. Chronobiology of takotsubo syndrome and myocardial infarction: analogies and differences. Heart Fail. Clin. 2016;12(4):531–542. doi: 10.1016/j.hfc.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Looi J-L., Lee M., Grey C., Webster M., To A., Kerr A.J. Seasonal variation in Takotsubo syndrome compared with myocardial infarction: ANZACS-QI 16. N. Z. Med. J. 2018;131(1471):21–29. [PubMed] [Google Scholar]
- 33.Giza D.E., Lopez-Mattei J., Vejpongsa P., et al. Stress-induced cardiomyopathy in cancer patients. Am. J. Cardiol. 2017;120(12):2284–2288. doi: 10.1016/j.amjcard.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Castellanos A., Martínez-Sellés M., Mejía-Rentería H., et al. Tako-tsubo syndrome in men: rare, but with poor prognosis. Rev. Esp. Cardiol. (Engl. Ed.) 2018;71(9):703–708. doi: 10.1016/j.rec.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Nazir S., Lohani S., Tachamo N., Ghimire S., Poudel D.R., Donato A. Takotsubo cardiomyopathy associated with epinephrine use: A systematic review and meta-analysis. Int. J. Cardiol. 2017;229:67–70. doi: 10.1016/j.ijcard.2016.11.266. [DOI] [PubMed] [Google Scholar]
- 36.Nasr D.M., Tomasini S., Prasad A., Rabinstein A.A. Acute brain diseases as triggers for stress cardiomyopathy: Clinical characteristics and outcomes. Neurocrit. Care. 2017;27(3):356–361. doi: 10.1007/s12028-017-0412-9. [DOI] [PubMed] [Google Scholar]
- 37.Chong C-R., Neil C.J., Nguyen T.H., et al. Dissociation between severity of takotsubo cardiomyopathy and presentation with shock or hypotension. Clin. Cardiol. 2013;36(7):401–406. doi: 10.1002/clc.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redfors B., Vedad R., Angerås O., et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction - A report from the SWEDEHEART registry. Int. J. Cardiol. 2015;185:282–289. doi: 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- 39.Kurisu S., Inoue I., Kawagoe T., et al. Prevalence of incidental coronary artery disease in tako-tsubo cardiomyopathy. Coron. Artery Dis. 2009;20(3):214–218. doi: 10.1097/MCA.0b013e3283299260. [DOI] [PubMed] [Google Scholar]
- 40.Kurowski V., Kaiser A., von Hof K., et al. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): Frequency, mechanisms, and prognosis. Chest. 2007;132(3):809–816. doi: 10.1378/chest.07-0608. [DOI] [PubMed] [Google Scholar]
- 41.Stiermaier T., Möller C., Graf T., et al. Prognostic usefulness of the ballooning pattern in patients with takotsubo cardiomyopathy. Am. J. Cardiol. 2016;118(11):1737–1741. doi: 10.1016/j.amjcard.2016.08.055. [DOI] [PubMed] [Google Scholar]
- 42.El-Battrawy I., Behnes M., Ansari U., et al. Comparison and outcome analysis of patients with apical and non-apical takotsubo cardiomyopathy. QJM. 2016;109(12):797–802. doi: 10.1093/qjmed/hcw092. [DOI] [PubMed] [Google Scholar]
- 43.de Chazal H.M., Buono M.G.D., Keyser-Marcus L., et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72(16):1955–1971. doi: 10.1016/j.jacc.2018.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Buono M.G., Potere N., Chiabrando J.G., Bressi E., Abbate A. Takotsubo syndrome: Diagnostic work-up and clues into differential diagnosis. Curr. Opin. Cardiol. 2019;34(6):673–686. doi: 10.1097/HCO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 45.Fitzgibbons T.P., Edwards Y.J.K., Shaw P., et al. Activation of inflammatory and pro-thrombotic pathways in acute stress cardiomyopathy. Front. Cardiovasc. Med. 2017;4(4):49. doi: 10.3389/fcvm.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dias A., Franco E., Koshkelashvili N., et al. Antiplatelet therapy in Takotsubo cardiomyopathy: Does it improve cardiovascular outcomes during index event? Heart Vessels. 2016;31(8):1285–1290. doi: 10.1007/s00380-015-0729-2. [DOI] [PubMed] [Google Scholar]
- 47.Jain M., Upadaya S., Zarich S.W. Serial evaluation of microcirculatory dysfunction in patients with Takotsubo cardiomyopathy by myocardial contrast echocardiography. Clin. Cardiol. 2013;36(9):531–534. doi: 10.1002/clc.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun T., Ming Z., Liu Z., et al. Prevalence of diastolic function and clinical impact on long-term outcome in takotsubo cardiomyopathy. Int. J. Cardiol. 2017;244:7–12. doi: 10.1016/j.ijcard.2017.06.068. [DOI] [PubMed] [Google Scholar]
- 49.Benítez S.E.P., Ramírez O.P., Bonilla C.J.S., Marín J.S. Cardiogenic shock induced by Takotsubo cardiomyopathy in early postoperative adrenalectomy period. Rev. Esp. Anestesiol. Reanim. 2019;66(5):288–291. doi: 10.1016/j.redare.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Schneider B., Athanasiadis A., Schwab J., et al. Complications in the clinical course of tako-tsubo cardiomyopathy. Int. J. Cardiol. 2014;176(1):199–205. doi: 10.1016/j.ijcard.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Pullara A., Chinaglia A., Giammaria M., et al. Takotsubo cardiomyopathy: Real life management in the intensive coronary care unit. Minerva Med. 2013;104(5):537–544. [PubMed] [Google Scholar]
- 52.Stiermaier T., Eitel C., Desch S., et al. Incidence, determinants and prognostic relevance of cardiogenic shock in patients with Takotsubo cardiomyopathy. Eur. Heart J. Acute Cardiovasc. Care. 2016;5(6):489–496. doi: 10.1177/2048872615612456. [DOI] [PubMed] [Google Scholar]
- 53.Izumo M., Shiota M., Nalawadi S., et al. Determinants of secondary pulmonary hypertension in patients with takotsubo cardiomyopathy. Echocardiography. 2015;32(11):1608–1613. doi: 10.1111/echo.12949. [DOI] [PubMed] [Google Scholar]
- 54.Neil C.J., Nguyen T.H., Singh K., et al. Relation of delayed recovery of myocardial function after takotsubo cardiomyopathy to subsequent quality of life. Am. J. Cardiol. 2015;115(8):1085–1089. doi: 10.1016/j.amjcard.2015.01.541. [DOI] [PubMed] [Google Scholar]
- 55.Santoro F., Stiermaier T., Tarantino N., et al. Impact of persistent ST elevation on outcome in patients with Takotsubo syndrome. Results from the GErman Italian STress Cardiomyopathy (GEIST) registry. Int. J. Cardiol. 2018;255:140–144. doi: 10.1016/j.ijcard.2017.11.068. [DOI] [PubMed] [Google Scholar]
- 56.Rosu D., Askandar S., Khouzam R.N. Why is reverse takotsubo “Reverse”? South. Med. J. 2017;110(5):381–385. doi: 10.14423/SMJ.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 57.Agrawal S., Shirani J., Garg L., et al. Pheochromocytoma and stress cardiomyopathy: Insight into pathogenesis. World J. Cardiol. 2017;9(3):255–260. doi: 10.4330/wjc.v9.i3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imran T.F., Rahman I., Dikdan S., et al. QT Prolongation and clinical outcomes in patients with takotsubo cardiomyopathy. Pacing Clin. Electrophysiol. 2016;39(6):607–611. doi: 10.1111/pace.12864. [DOI] [PubMed] [Google Scholar]
- 59.Lee J.H., Uhm J.S., Shin D.G., et al. Clinical significance of changes in the corrected QT interval in stress-induced cardiomyopathy. Korean J. Intern. Med. (Korean. Assoc. Intern. Med.) 2016;31(3):507–516. doi: 10.3904/kjim.2015.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharkey S.W., Lesser J.R., Menon M., Parpart M., Maron M.S., Maron B.J. Spectrum and significance of electrocardiographic patterns, troponin levels, and thrombolysis in myocardial infarction frame count in patients with stress (tako-tsubo) cardiomyopathy and comparison to those in patients with ST-elevation anterior wall myocardial infarction. Am. J. Cardiol. 2008;101(12):1723–1728. doi: 10.1016/j.amjcard.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 61.Pilgrim T.M., Wyss T.R. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: A systematic review. Int. J. Cardiol. 2008;124(3):283–292. doi: 10.1016/j.ijcard.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Akashi Y.J., Musha H., Kida K., et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur. J. Heart Fail. 2005;7(7):1171–1176. doi: 10.1016/j.ejheart.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Koike H, Sasaka K. The clinical features of takotsubo cardiomyopathy. 2003. [DOI] [PubMed]
- 64.Kurisu S., Inoue I., Kawagoe T., et al. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: Comparison with acute myocardial infarction with minimal enzymatic release. Circ. J. 2004;68(1):77–81. doi: 10.1253/circj.68.77. [DOI] [PubMed] [Google Scholar]
- 65.Jesel L., Berthon C., Messas N., et al. Ventricular arrhythmias and sudden cardiac arrest in Takotsubo cardiomyopathy: Incidence, predictive factors, and clinical implications. Heart Rhythm. 2018;15(8):1171–1178. doi: 10.1016/j.hrthm.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Stiermaier T., Rommel K.P., Eitel C., et al. Management of arrhythmias in patients with Takotsubo cardiomyopathy: Is the implantation of permanent devices necessary? Heart Rhythm. 2016;13(10):1979–1986. doi: 10.1016/j.hrthm.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 67.Abanador-Kamper N., Kamper L., Wolfertz J., Pomjanski W., Wolf-Pütz A., Seyfarth M. Evaluation of therapy management and outcome in Takotsubo syndrome. BMC Cardiovasc. Disord. 2017;17(1):225. doi: 10.1186/s12872-017-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yerasi C., Koifman E., Weissman G., et al. Impact of triggering event in outcomes of stress-induced (Takotsubo) cardiomyopathy. Eur. Heart J. Acute Cardiovasc. Care. 2017;6(3):280–286. doi: 10.1177/2048872616633881. [DOI] [PubMed] [Google Scholar]
- 69.Sobue Y., Watanabe E., Ichikawa T., et al. Physically triggered Takotsubo cardiomyopathy has a higher in-hospital mortality rate. Int. J. Cardiol. 2017;235:87–93. doi: 10.1016/j.ijcard.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 70.El-Battrawy I., Lang S., Ansari U., et al. Incidence and prognostic relevance of cardiopulmonary failure in takotsubo cardiomyopathy. Sci. Rep. 2017;7(1):14673. doi: 10.1038/s41598-017-15327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharkey S.W., Maron B.J. Epidemiology and clinical profile of Takotsubo cardiomyopathy. Circ. J. 2014;78(9):2119–2128. doi: 10.1253/circj.CJ-14-0770. [DOI] [PubMed] [Google Scholar]
- 72.Gopalakrishnan M., Hassan A., Villines D., Nasr S., Chandrasekaran M., Klein L.W. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am. J. Cardiol. 2015;116(10):1586–1590. doi: 10.1016/j.amjcard.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 73.Montenegro Sá F., Ruivo C., Santos L.G., et al. Myocardial infarction with nonobstructive coronary arteries: A single-center retrospective study. Coron. Artery Dis. 2018;29(6):511–515. doi: 10.1097/MCA.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 74.Ryabov VV, Gomboeva SB, Shelkovnikova TA, et al. Cardiac magnetic resonance imaging in differential diagnostics of acute coronary syndrome in patients with non-obstruction coronary atherosclerosis. 2017.
- 75.Ryabov V.V., Fedorova S.B., Vyshlov E.V. Myocardial infarction with nonobstructive coronary atherosclerosis as a current problem of emergency cardiology. Sib Med J. 2019;33(4):10–18. doi: 10.29001/2073-8552-2018-33-4-10-18. [DOI] [Google Scholar]
- 76.Tamis-Holland J.E., Jneid H., Reynolds H.R., et al. American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Cont Circulation. 2019;139(18):e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 77.Montone R.A., Niccoli G., Fracassi F., et al. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018;39(2):91–98. doi: 10.1093/eurheartj/ehx667. [DOI] [PubMed] [Google Scholar]
- 78.Akashi Y.J.J., Musha H., Nakazawa K., Miyake F. Plasma brain natriuretic peptide in takotsubo cardiomyopathy. QJM. 2004;97(9):599–607. doi: 10.1093/qjmed/hch094. [DOI] [PubMed] [Google Scholar]
- 79.Stiermaier T., Santoro F., Graf T., et al. Prognostic value of N-terminal Pro-B-type natriuretic peptide in takotsubo syndrome. Clin. Res. Cardiol. 2018;107(7):597–606. doi: 10.1007/s00392-018-1227-1. [DOI] [PubMed] [Google Scholar]
- 80.Nef H.M., Möllmann H., Weber M., et al. Release pattern of cardiac biomarkers in left ventricular apical ballooning. Int. J. Cardiol. 2007;115(1):128–129. doi: 10.1016/j.ijcard.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 81.Budnik M., Kochanowski J., Piatkowski R., et al. Simple markers can distinguish Takotsubo cardiomyopathy from ST segment elevation myocardial infarction. Int. J. Cardiol. 2016;219:417–420. doi: 10.1016/j.ijcard.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 82.Randhawa M.S., Dhillon A.S., Taylor H.C., Sun Z., Desai M.Y. Diagnostic utility of cardiac biomarkers in discriminating Takotsubo cardiomyopathy from acute myocardial infarction. J. Card. Fail. 2014;20(1):2–8. doi: 10.1016/j.cardfail.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Gopalakrishnan P., Zaidi R., Sardar M.R. Takotsubo cardiomyopathy: Pathophysiology and role of cardiac biomarkers in differential diagnosis. World J. Cardiol. 2017;9(9):723–730. doi: 10.4330/wjc.v9.i9.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dawson D.K. Acute stress-induced (takotsubo) cardiomyopathy. Heart. 2018;104(2):96–102. doi: 10.1136/heartjnl-2017-311579. [DOI] [PubMed] [Google Scholar]
- 85.Konstantinos G., El-Battrawy I., Schramm K., et al. Comparison and outcome analysis of patients with takotsubo cardiomyopathy triggered by emotional stress or physical stress. Front. Psychol. 2017;8:527. doi: 10.3389/fpsyg.2017.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kagiyama N., Okura H., Matsue Y., et al. Multiple unfavorable echocardiographic findings in takotsubo cardiomyopathy are associated with increased in-hospital events and mortality. J. Am. Soc. Echocardiogr. 2016;29(12):1179–1187. doi: 10.1016/j.echo.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 87.Sharkey S.W., Maron B.J. Survival after takotsubo, revisited. J. Am. Coll. Cardiol. 2018;72(8):883–884. doi: 10.1016/j.jacc.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 88.Christensen T.E., Bang L.E., Holmvang L., et al. (123)I-MIBG scintigraphy in the subacute state of takotsubo cardiomyopathy. JACC Cardiovasc. Imaging. 2016;9(8):982–990. doi: 10.1016/j.jcmg.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 89.Marfella R., Barbieri M., Sardu C., et al. Effects of α-lipoic acid therapy on sympathetic heart innervation in patients with previous experience of transient takotsubo cardiomyopathy. J. Cardiol. 2016;67(2):153–161. doi: 10.1016/j.jjcc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 90.Smeijers L., Szabó B.M., van Dammen L., et al. Emotional, neurohormonal, and hemodynamic responses to mental stress in Tako-Tsubo cardiomyopathy. Am. J. Cardiol. 2015;115(11):1580–1586. doi: 10.1016/j.amjcard.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 91.Núñez-Gil I.J., Bernardo E., Feltes G., et al. Platelet function in Takotsubo cardiomyopathy. J. Thromb. Thrombolysis. 2015;39(4):452–458. doi: 10.1007/s11239-014-1109-y. [DOI] [PubMed] [Google Scholar]
- 92.Uchida Y., Egami H., Uchida Y., et al. Possible participation of endothelial cell apoptosis of coronary microvessels in the genesis of Takotsubo cardiomyopathy. Clin. Cardiol. 2010;33(6):371–377. doi: 10.1002/clc.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vaccaro A., Despas F., Delmas C., et al. 2014. Direct evidences for sympathetic hyperactivity and baroreflex impairment in tako tsubo cardiopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leimbach W.N., Jr, Wallin B.G., Victor R.G., Aylward P.E., Sundlöf G., Mark A.L. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73(5):913–919. doi: 10.1161/01.CIR.73.5.913. [DOI] [PubMed] [Google Scholar]
- 95.Lee S.J., Kang J.G., Ryu O.H., et al. The relationship of thyroid hormone status with myocardial function in stress cardiomyopathy. Eur. J. Endocrinol. 2009;160(5):799–806. doi: 10.1530/EJE-08-0808. [DOI] [PubMed] [Google Scholar]
- 96.Nayeri A., Rafla-Yuan E., Farber-Eger E., et al. Pre-existing psychiatric illness is associated with increased risk of recurrent takotsubo cardiomyopathy. Psychosomatics. 2017;58(5):527–532. doi: 10.1016/j.psym.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nandal S., Castles A., Asrar Ul Haq M., van Gaal W. Takotsubo cardiomyopathy triggered by status epilepticus: case report and literature review. BMJ Case Rep. 2019;12(1):e225924. doi: 10.1136/bcr-2018-225924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Belcour D., Jabot J., Grard B., et al. Prevalence and risk factors of stress cardiomyopathy after convulsive status epilepticus in ICU patients. Crit. Care Med. 2015;43(10):2164–2170. doi: 10.1097/CCM.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 99.Yamaguchi H., Nagase H., Yoshida S., et al. Acute encephalopathy with biphasic seizures and late reduced diffusion accompanied by Takotsubo cardiomyopathy. Brain Dev. 2019;41(3):305–309. doi: 10.1016/j.braindev.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 100.Finsterer J. Recurrent seizure-triggered Takotsubo associated with hypokalemia and hypomagnesemia. Seizure. 2019;65:176. doi: 10.1016/j.seizure.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Binaghi G., Congia D., Cossa S., et al. Seizures and recurrence of Takotsubo syndrome: One clinical presentation and trigger, but two different anatomical variants in the same patient. A case to meditate on. Seizure. 2018;63:37–39. doi: 10.1016/j.seizure.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 102.Young R.S.K., Osbakken M.D., Briggs R.W., Yagel S.K., Rice D.W., Goldberg S. 31P NMR study of cerebral metabolism during prolonged seizures in the neonatal dog. Ann. Neurol. 1985;18(1):14–20. doi: 10.1002/ana.410180104. [DOI] [PubMed] [Google Scholar]
- 103.Meierkord H., Shorvon S., Lightman S.L. Plasma concentrations of prolactin, noradrenaline, vasopressin and oxytocin during and after a prolonged epileptic seizure. Acta Neurol. Scand. 1994;90(2):73–77. doi: 10.1111/j.1600-0404.1994.tb02682.x. [DOI] [PubMed] [Google Scholar]
- 104.Lazzeroni D., Bini M., Castiglioni P., et al. Anxiety disorders and stressful events in Takotsubo syndrome. Cardiol. J. 2018;25(4):495–500. doi: 10.5603/CJ.a2017.0136. [DOI] [PubMed] [Google Scholar]
- 105.Goh A.C.H., Wong S., Zaroff J.G., Shafaee N., Lundstrom R.J. Comparing anxiety and depression in patients with takotsubo stress cardiomyopathy to those with acute coronary syndrome. J. Cardiopulm. Rehabil. Prev. 2016;36(2):106–111. doi: 10.1097/HCR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 106.Dias A., Franco E., Mercedes A., Hebert K., Messina D., Quevedo H.C. Clinical features of takotsubo cardiomyopathy - a single-center experience. Cardiology. 2013;126(2):126–130. doi: 10.1159/000353369. [DOI] [PubMed] [Google Scholar]
- 107.Chhabra L., Khalid N., Sareen P. Extremely low prevalence of takotsubo cardiomyopathy and transient cardiac dysfunction in stroke patients with t-wave abnormalities. Am. J. Cardiol. 2019;123(6):1009. doi: 10.1016/j.amjcard.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 108.Kumai T., Inamasu J., Watanabe E., Sugimoto K., Hirose Y. Differences between Takotsubo cardiomyopathy and reverse Takotsubo cardiomyopathy associated with subarachnoid hemorrhage. Int. J. Cardiol. Heart Vasc. 2016;11:99–103. doi: 10.1016/j.ijcha.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moussouttas M., Mearns E., Walters A., DeCaro M. Plasma catecholamine profile of subarachnoid hemorrhage patients with neurogenic cardiomyopathy. Cerebrovasc. Dis. Extra. 2015;5(2):57–67. doi: 10.1159/000431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tamaki T., Node Y., Teramoto A. Changes of the plasma ketone body level and arterial ketone body ratio at the onset of mild aneurysmal subarachnoid hemorrhage. Neurol. Res. 2008;30(9):898–902. doi: 10.1179/016164108X323708. [DOI] [PubMed] [Google Scholar]
- 111.Akashi Y.J., Nef H.M., Lyon A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015;12(7):387–397. doi: 10.1038/nrcardio.2015.39. [DOI] [PubMed] [Google Scholar]
- 112.Diaz B., Elkbuli A., Ehrhardt J.D., Jr, McKenney M., Boneva D., Hai S. Pheochromocytoma-related cardiomyopathy presenting as broken heart syndrome: Case report and literature review. Int. J. Surg. Case Rep. 2019;55:7–10. doi: 10.1016/j.ijscr.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Butt K., Ali S., Sattar Z., Ur Rahman A., Burt J.R. Funny lumps, flaming pheo, and a broken heart: A rare case of pheochromocytoma. Cureus. 2018;10(11):e3646. doi: 10.7759/cureus.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang R., Gupta D., Albert S.G. Pheochromocytoma as a reversible cause of cardiomyopathy: Analysis and review of the literature. Int. J. Cardiol. 2017;249:319–323. doi: 10.1016/j.ijcard.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 115.Bourenne J., Jeremy B., Fresco R., et al. Stress cardiomyopathy managed with extracorporeal support after self-injection of epinephrine. Case Rep. Crit. Care. 2017;2017:3731069. doi: 10.1155/2017/3731069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nassif J., Nahouli H., Khalil A., Mikhael E., Gharzeddine W., Ghaziri G. Epinephrine-induced takotsubo cardiomyopathy during laparoscopic myomectomy: Case report and review of the literature. J. Minim. Invasive Gynecol. 2017;24(6):1037–1039. doi: 10.1016/j.jmig.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 117.Vieira A., Batista B., de Abreu T.T. Iatrogenic takotsubo cardiomyopathy secondary to norepinephrine by continuous infusion for shock. Eur. J. Case Rep. Intern. Med. 2018;5(7):000894. doi: 10.12890/2018_000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Olson P.C., Agarwal V., Lafferty J.C., Bekheit S. Takotsubo Cardiomyopathy precipitated by opiate withdrawal. Heart Lung. 2018;47(1):73–75. doi: 10.1016/j.hrtlng.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 119.Kido K., Guglin M. Drug-induced takotsubo cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 2017;22(6):552–563. doi: 10.1177/1074248417708618. [DOI] [PubMed] [Google Scholar]
- 120.Sarcon A., Ghadri J.R., Wong G., Lüscher T.F., Templin C., Amsterdam E. Takotsubo cardiomyopathy associated with opiate withdrawal. QJM. 2014;107(4):301–302. doi: 10.1093/qjmed/hct219. [DOI] [PubMed] [Google Scholar]
- 121.DiStefano P.S., Brown O.M. Biochemical correlates of morphine withdrawal. 2. Effects of clonidine. J. Pharmacol. Exp. Ther. 1985;233(2):339–344. [PubMed] [Google Scholar]
- 122.Chehab O., Ioannou A., Sawhney A., Rice A., Dubrey S. Reverse takotsubo cardiomyopathy and cardiogenic shock associated with methamphetamine consumption. J. Emerg. Med. 2017;53(5):e81–e83. doi: 10.1016/j.jemermed.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 123.Voskoboinik A., Ihle J.F., Bloom J.E., Kaye D.M. Methamphetamine-associated cardiomyopathy: Patterns and predictors of recovery. Intern. Med. J. 2016;46(6):723–727. doi: 10.1111/imj.13050. [DOI] [PubMed] [Google Scholar]
- 124.Fitzgerald K.T., Bronstein A.C. Adderall® (amphetamine-dextroamphetamine) toxicity. Top. Companion Anim. Med. 2013;28(1):2–7. doi: 10.1053/j.tcam.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 125.Rueda D, Aguirre R, Contardo D, Finocchietto P, Hernández S, di Fonzo H. Takotsubo myocardiopathy and hyperthyroidism: A case report and literature review. 2017. [DOI] [PMC free article] [PubMed]
- 126.Murdoch D., O’Callaghan W., Reda E., Niranjan S. Takotsubo cardiomyopathy associated with primary hyperthyroidism secondary to toxic multinodular goiter. Int. J. Angiol. 2016;25(5):e121–e122. doi: 10.1055/s-0035-1548548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Omar S., Ali E., Mazek H., Mahmoud T., Soontrapa S., Suarez J. Takotsubo cardiomyopathy associated with hyperthyroidism treated with thyroidectomy. Proc. Bayl. Univ. Med. Cent. 2015;28(2):194–195. doi: 10.1080/08998280.2015.11929227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eliades M., El-Maouche D., Choudhary C., Zinsmeister B., Burman K.D. Takotsubo cardiomyopathy associated with thyrotoxicosis: A case report and review of the literature. Thyroid. 2014;24(2):383–389. doi: 10.1089/thy.2012.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rubio A., Raasmaja A., Silva J.E. Thyroid hormone and norepinephrine signaling in brown adipose tissue. II: Differential effects of thyroid hormone on beta 3-adrenergic receptors in brown and white adipose tissue. Endocrinology. 1995;136(8):3277–3284. doi: 10.1210/endo.136.8.7628361. [DOI] [PubMed] [Google Scholar]
- 130.Rubio A., Raasmaja A., Maia A.L., Kim K.R., Silva J.E. Effects of thyroid hormone on norepinephrine signaling in brown adipose tissue. I. Beta 1- and beta 2-adrenergic receptors and cyclic adenosine 3′,5′-monophosphate generation. Endocrinology. 1995;136(8):3267–3276. doi: 10.1210/endo.136.8.7628360. [DOI] [PubMed] [Google Scholar]
- 131.Ghadri J.R., Wittstein I.S., Prasad A., et al. International expert consensus document on takotsubo syndrome (Part II): Diagnostic workup, outcome, and Management. Eur. Heart J. 2018;31(22):2047–2062. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kato K., Lyon A.R., Ghadri J-R., Templin C. Takotsubo syndrome: Aetiology, presentation and treatment. Heart. 2017;103(18):1461–1469. doi: 10.1136/heartjnl-2016-309783. [DOI] [PubMed] [Google Scholar]
- 133.Heubach J.F., Ravens U., Kaumann A.J. Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human beta2-adrenoceptors overexpressed in mouse heart. Mol. Pharmacol. 2004;65(5):1313–1322. doi: 10.1124/mol.65.5.1313. [DOI] [PubMed] [Google Scholar]
- 134.Heubach J.F., Blaschke M., Harding S.E., Ravens U., Kaumann A.J. Cardiostimulant and cardiodepressant effects through overexpressed human β2-adrenoceptors in murine heart: Regional differences and functional role of β1-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 2003;367(4):380–390. doi: 10.1007/s00210-002-0681-4. [DOI] [PubMed] [Google Scholar]
- 135.Paur H., Wright P.T., Sikkel M.B., et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697–706. doi: 10.1161/CIRCULATIONAHA.112.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuroda R., Shintani-Ishida K., Unuma K., Yoshida K. Immobilization stress with α2-adrenergic stimulation induces regional and transient reduction of cardiac contraction through Gi coupling in rats. Int. Heart J. 2015;56(5):537–543. doi: 10.1536/ihj.15-034. [DOI] [PubMed] [Google Scholar]
- 137.Shao Y., Redfors B., Scharin Täng M., et al. Novel rat model reveals important roles of β-adrenoreceptors in stress-induced cardiomyopathy. Int. J. Cardiol. 2013;168(3):1943–1950. doi: 10.1016/j.ijcard.2012.12.092. [DOI] [PubMed] [Google Scholar]
- 138.Häggendal J., Johansson G., Jönsson L., Thorén-Tolling K. Effect of propranolol on myocardial cell necroses and blood levels of catecholamines in pigs subjected to stress. Acta Pharmacol. Toxicol. (Copenh.) 1982;50(1):58–66. doi: 10.1111/j.1600-0773.1982.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 139.Takano Y., Ueyama T., Ishikura F. Azelnidipine, unique calcium channel blocker could prevent stress-induced cardiac dysfunction like α·β blocker. J. Cardiol. 2012;60(1):18–22. doi: 10.1016/j.jjcc.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 140.Ishikura F., Takano Y., Ueyama T. Acute effects of beta-blocker with intrinsic sympathomimetic activity on stress-induced cardiac dysfunction in rats. J. Cardiol. 2012;60(6):470–474. doi: 10.1016/j.jjcc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 141.Sisson J.C., Wieland D.M., Sherman P., Mangner T.J., Tobes M.C., Jacques S., Jr Metaiodobenzylguanidine as an index of the adrenergic nervous system integrity and function. J. Nucl. Med. 1987;28(10):1620–1624. [PubMed] [Google Scholar]
- 142.Degrado T.R., Zalutsky M.R., Vaidyanathan G. Uptake mechanisms of meta-[123I]iodobenzylguanidine in isolated rat heart. Nucl. Med. Biol. 1995;22(1):1–12. doi: 10.1016/0969-8051(94)00084-W. [DOI] [PubMed] [Google Scholar]
- 143.Guilloteau D., Chalon S., Baulieu J.L., et al. Comparison of MIBG and monoamines uptake mechanisms: pharmacological animal and blood platelets studies. Eur. J. Nucl. Med. 1988;14(7-8):341–344. doi: 10.1007/BF00254380. [DOI] [PubMed] [Google Scholar]
- 144.Richalet J.P., Merlet P., Bourguignon M., et al. MIBG scintigraphic assessment of cardiac adrenergic activity in response to altitude hypoxia. J. Nucl. Med. 1990;31(1):34–37. [PubMed] [Google Scholar]
- 145.Merlet P., Dubois-Randé J.L., Adnot S., et al. Myocardial β-adrenergic desensitization and neuronal norepinephrine uptake function in idiopathic dilated cardiomyopathy. J. Cardiovasc. Pharmacol. 1992;19(1):10–16. doi: 10.1097/00005344-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 146.Morel O., Sauer F., Imperiale A., et al. Importance of inflammation and neurohumoral activation in Takotsubo cardiomyopathy. J. Card. Fail. 2009;15(3):206–213. doi: 10.1016/j.cardfail.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 147.Humbert O., Stamboul K., Gudjoncik A., et al. Dual Diagnostic Role of 123I-MIBG Scintigraphy in Inverted-Takotsubo Pattern Cardiomyopathy. Clin. Nucl. Med. 2015;40(10):816–818. doi: 10.1097/RLU.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 148.Burgdorf C., von Hof K., Schunkert H., Kurowski V. Regional alterations in myocardial sympathetic innervation in patients with transient left-ventricular apical ballooning (Tako-Tsubo cardiomyopathy). J. Nucl. Cardiol. 2008;15(1):65–72. doi: 10.1016/j.nuclcard.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 149.Tan A.Y., Verrier R.L. The role of the autonomic nervous system in cardiac arrhythmias. Handb. Clin. Neurol. 2013;•••:135–145. doi: 10.1016/B978-0-444-53491-0.00012-2. [DOI] [PubMed] [Google Scholar]
- 150.Roghi A., Pedrotti P., Milazzo A., Bonacina E., Bucciarelli-Ducci C. Adrenergic myocarditis in pheochromocytoma. J. Cardiovasc. Magn. Reson. 2011;13(1):4. doi: 10.1186/1532-429X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.De Miguel V, Arias A, Paissan A, et al. Catecholamine-induced myocarditis in pheochromocytoma. 2014. [DOI] [PubMed]
- 152.Begieneman M.P.V., Emmens R.W., Rijvers L., et al. Ventricular myocarditis coincides with atrial myocarditis in patients. Cardiovasc. Pathol. 2016;25(2):141–148. doi: 10.1016/j.carpath.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 153.Muratori D., Pedrotti P., Baroni M., et al. Catecholamine-induced myocarditis in pheochromocytoma. G. Ital. Cardiol. (Rome) 2017;18(2):164–168. doi: 10.1714/2663.27302. [DOI] [PubMed] [Google Scholar]
- 154.Noda M., Kawano O., Uchida O., Sawabe T., Saito G. Myocarditis induced by sympathomimetic amines. I. Jpn. Circ. J. 1970;34(1):7–12. doi: 10.1253/jcj.34.7. [DOI] [PubMed] [Google Scholar]
- 155.Wittstein I.S., Thiemann D.R., Lima J.A.C., et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005;352(6):539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 156.Iacucci I., Carbone I., Cannavale G., et al. Myocardial oedema as the sole marker of acute injury in Takotsubo cardiomyopathy: a cardiovascular magnetic resonance (CMR) study. Radiol. Med. (Torino) 2013;118(8):1309–1323. doi: 10.1007/s11547-013-0931-1. [DOI] [PubMed] [Google Scholar]
- 157.Madhavan M., Borlaug B.A., Lerman A., Rihal C.S., Prasad A. Stress hormone and circulating biomarker profile of apical ballooning syndrome (Takotsubo cardiomyopathy): Insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart. 2009;95(17):1436–1441. doi: 10.1136/hrt.2009.170399. [DOI] [PubMed] [Google Scholar]
- 158.Song B.G., Yang H.S., Hwang H.K., et al. The impact of stressor patterns on clinical features in patients with tako-tsubo cardiomyopathy: Experiences of two tertiary cardiovascular centers. Clin. Cardiol. 2012;35(11):E6–E13. doi: 10.1002/clc.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Eitel I., Lücke C., Grothoff M., et al. Inflammation in takotsubo cardiomyopathy: Insights from cardiovascular magnetic resonance imaging. Eur. Radiol. 2010;20(2):422–431. doi: 10.1007/s00330-009-1549-5. [DOI] [PubMed] [Google Scholar]
- 160.Schwarz K., Ahearn T., Srinivasan J., et al. Alterations in cardiac deformation, timing of contraction and relaxation, and early myocardial fibrosis accompany the apparent recovery of acute stress-induced (Takotsubo) cardiomyopathy: An End to the Concept of transience. J. Am. Soc. Echocardiogr. 2017;30(8):745–755. doi: 10.1016/j.echo.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 161.Parkkonen O., Nieminen M.T., Vesterinen P., et al. 2017. [Google Scholar]
- 162.Santoro F., Tarantino N., Ferraretti A., et al. Serum interleukin 6 and 10 levels in Takotsubo cardiomyopathy: Increased admission levels may predict adverse events at follow-up. Atherosclerosis. 2016;254:28–34. doi: 10.1016/j.atherosclerosis.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 163.Scally C., Abbas H., Ahearn T., et al. Myocardial and systemic inflammation in acute stress-induced (Takotsubo) cardiomyopathy. Circulation. 2019;139(13):1581–1592. doi: 10.1161/CIRCULATIONAHA.118.037975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kurisu S., Sato H., Kawagoe T., et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: A novel cardiac syndrome mimicking acute myocardial infarction. Am. Heart J. 2002;143(3):448–455. doi: 10.1067/mhj.2002.120403. [DOI] [PubMed] [Google Scholar]
- 165.Abdelmoneim S.S., Mankad S.V., Bernier M., et al. Microvascular function in takotsubo cardiomyopathy with contrast echocardiography: Prospective evaluation and review of literature. J. Am. Soc. Echocardiogr. 2009;22(11):1249–1255. doi: 10.1016/j.echo.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 166.Hung M.J., Ko T., Liang C.Y., Kao Y.C. Two-dimensional myocardial deformation in coronary vasospasm-related Takotsubo cardiomyopathy: A case report of a serial echocardiographic study. Medicine (Baltimore) 2017;96(40):e8232. doi: 10.1097/MD.0000000000008232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kume T., Akasaka T., Kawamoto T., et al. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ. J. 2005;69(8):934–939. doi: 10.1253/circj.69.934. [DOI] [PubMed] [Google Scholar]
- 168.Meimoun P., Malaquin D., Benali T., Boulanger J., Zemir H., Tribouilloy C. Transient impairment of coronary flow reserve in tako-tsubo cardiomyopathy is related to left ventricular systolic parameters. Eur. J. Echocardiogr. 2009;10(2):265–270. doi: 10.1093/ejechocard/jen222. [DOI] [PubMed] [Google Scholar]
- 169.Sganzerla P., Alioto G., Funaro A., Passaretti B., Borghini E., Guglielmetto S. Transthoracic Doppler ultrasound coronary flow reserve evaluation: Preliminary insights into pathophysiology of Takotsubo cardiomyopathy. J. Cardiovasc. Med. (Hagerstown) 2008;9(12):1229–1234. doi: 10.2459/JCM.0b013e328313e890. [DOI] [PubMed] [Google Scholar]
- 170.Collste O., Tornvall P., Alam M., Frick M. Coronary flow reserve during dobutamine stress in Takotsubo stress cardiomyopathy. BMJ Open. 2015;5(7):e007671. doi: 10.1136/bmjopen-2015-007671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burgdorf C., Bonnemeier H., von Hof K., Schunkert H., Kurowski V. Coronary artery vasospasm or true transient left ventricular apical ballooning? Differentiation by nuclear imaging. J. Nucl. Cardiol. 2008;15(4):599–603. doi: 10.1016/j.nuclcard.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 172.Feola M., Chauvie S., Rosso G.L., Biggi A., Ribichini F., Bobbio M. Reversible impairment of coronary flow reserve in takotsubo cardiomyopathy: A myocardial PET study. J. Nucl. Cardiol. 2008;15(6):811–817. doi: 10.1007/BF03007363. [DOI] [PubMed] [Google Scholar]
- 173.Hasbak P., Kjær A., Skovgaard D., Bang L.E., Grande P., Holmvang L. Preserved myocardial blood flow in the apical region involved in takotsubo cardiomyopathy by quantitative cardiac PET assessment. J. Nucl. Cardiol. 2012;19(1):169–171. doi: 10.1007/s12350-011-9451-3. [DOI] [PubMed] [Google Scholar]
- 174.Christensen T.E., Ahtarovski K.A., Bang L.E., et al. Basal hyperaemia is the primary abnormality of perfusion in Takotsubo cardiomyopathy: a quantitative cardiac perfusion positron emission tomography study. Eur. Heart J. Cardiovasc. Imaging. 2015;16(10):1162–1169. doi: 10.1093/ehjci/jev065. [DOI] [PubMed] [Google Scholar]
- 175.Ghadri J.R., Dougoud S., Maier W., et al. A PET/CT-follow-up imaging study to differentiate takotsubo cardiomyopathy from acute myocardial infarction. Int. J. Cardiovasc. Imaging. 2014;30(1):207–209. doi: 10.1007/s10554-013-0311-x. [DOI] [PubMed] [Google Scholar]
- 176.Redfors B., Shao Y., Wikström J., et al. Contrast echocardiography reveals apparently normal coronary perfusion in a rat model of stress-induced (Takotsubo) cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging. 2014;15(2):152–157. doi: 10.1093/ehjci/jet079. [DOI] [PubMed] [Google Scholar]
- 177.Madias J.E. Takotsubo syndrome and coronary microcirculation dysfunction: Vasospasm or damage due to adjacent cardiomyocyte injury and/or myocardial edema? Int. J. Cardiol. 2016;215:90–91. doi: 10.1016/j.ijcard.2016.04.117. [DOI] [PubMed] [Google Scholar]
- 178.Yalta K., Yalta T. Physically triggered takotsubo cardiomyopathy has a worse prognosis: Potential roles of systemic inflammation and coronary slow flow phenomenon. Int. J. Cardiol. 2017;242:31–32. doi: 10.1016/j.ijcard.2017.03.082. [DOI] [PubMed] [Google Scholar]
- 179.Cho Y.I., Cho D.J. Hemorheology and microvascular disorders. Korean Circ. J. 2011;41(6):287–295. doi: 10.4070/kcj.2011.41.6.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cecchi E., Parodi G., Giglioli C., et al. Stress-induced hyperviscosity in the pathophysiology of takotsubo cardiomyopathy. Am. J. Cardiol. 2013;111(10):1523–1529. doi: 10.1016/j.amjcard.2013.01.304. [DOI] [PubMed] [Google Scholar]
- 181.Høieggen A., Fossum E., Nesbitt S.D., Palmieri V., Kjeldsen S.E. Blood viscosity, plasma adrenaline and fasting insulin in hypertensive patients with left ventricular hypertrophy. ICARUS, a LIFE Substudy. Insulin CARotids US Scandinavica. Blood Press. 2000;9(2-3):83–90. doi: 10.1080/08037050050151771. [DOI] [PubMed] [Google Scholar]
- 182.Ross A.E., Flaa A., Høieggen A., Reims H., Eide I.K., Kjeldsen S.E. Gender specific sympathetic and hemorrheological responses to mental stress in healthy young subjects. Scand. Cardiovasc. J. 2001;35(5):307–312. doi: 10.1080/140174301317116271. [DOI] [PubMed] [Google Scholar]
- 183.Tippins J.R., Antoniw J.W., Maseri A. Endothelin-1 is a potent constrictor in conductive and resistive coronary arteries. J. Cardiovasc. Pharmacol. 1989;13(Suppl. 5):S177–S179. doi: 10.1097/00005344-198900135-00048. [DOI] [PubMed] [Google Scholar]
- 184.Kurihara H., Yoshizumi M., Sugiyama T., et al. The possible role of endothelin-1 in the pathogenesis of coronary vasospasm. J. Cardiovasc. Pharmacol. 1989;13(Suppl. 5):S132–S137. doi: 10.1097/00005344-198900135-00033. [DOI] [PubMed] [Google Scholar]
- 185.Matsuyama K., Yasue H., Okumura K., et al. Increased plasma level of endothelin-1-like immunoreactivity during coronary spasm in patients with coronary spastic angina. Am. J. Cardiol. 1991;68(10):991–995. doi: 10.1016/0002-9149(91)90484-3. [DOI] [PubMed] [Google Scholar]
- 186.McClellan G, Weisberg A, Winegrad S. Endothelin regulation of cardiac contractility in absence of added endothelin. 1995. [DOI] [PubMed]
- 187.Miyauchi T., Goto K. Heart failure and endothelin receptor antagonists. Trends Pharmacol. Sci. 1999;20(5):210–217. doi: 10.1016/S0165-6147(99)01297-3. [DOI] [PubMed] [Google Scholar]
- 188.Nishimaru K., Miura Y., Endoh M. Mechanisms of endothelin-1-induced decrease in contractility in adult mouse ventricular myocytes. Br. J. Pharmacol. 2007;152(4):456–463. doi: 10.1038/sj.bjp.0707392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sakai S., Miyauchi T., Kobayashi M., Yamaguchi I., Goto K., Sugishita Y. Inhibition of myocardial endothelin pathway improves long-term survival in heart failure. Nature. 1996;384(6607):353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]
- 190.Mulder P., Richard V., Derumeaux G., et al. Role of endogenous endothelin in chronic heart failure: Effect of long-term treatment with an endothelin antagonist on survival, hemodynamics, and cardiac remodeling. Circulation. 1997;96(6):1976–1982. doi: 10.1161/01.CIR.96.6.1976. [DOI] [PubMed] [Google Scholar]
- 191.Jaguszewski M., Osipova J., Ghadri J.R., et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 2014;35(15):999–1006. doi: 10.1093/eurheartj/eht392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Naegele M., Flammer A.J., Enseleit F., et al. Endothelial function and sympathetic nervous system activity in patients with Takotsubo syndrome. Int. J. Cardiol. 2016;224:226–230. doi: 10.1016/j.ijcard.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 193.Prokudina E.S., Maslov L.N., Naryzhnaya N.V., et al. Cardioprotective properties of opioid receptor agonists in rats with stress-induced cardiac injury. Physiol. Res. 2019;68(3):375–384. doi: 10.33549/physiolres.933946. [DOI] [PubMed] [Google Scholar]
- 194.Naryzhnaya N.V., Maslov L.N., Vychuzhanova E.A., et al. Effect of hypoxic preconditioning on stress reaction in rats. Bull. Exp. Biol. Med. 2015;159(4):450–452. doi: 10.1007/s10517-015-2988-4. [DOI] [PubMed] [Google Scholar]
- 195.Wang C., Qiu W., Zheng Y., et al. 2013. [Google Scholar]
- 196.Lacasa D., Agli B., Giudicelli Y. Permissive action of glucocorticoids on catecholamine-induced lipolysis: direct “in vitro” effects on the fat cell β-adrenoreceptor-coupled-adenylate cyclase system. Biochem. Biophys. Res. Commun. 1988;153(2):489–497. doi: 10.1016/S0006-291X(88)81121-5. [DOI] [PubMed] [Google Scholar]
- 197.Kuwabara Y., Ono K., Horie T., et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4(4):446–454. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 198.d’Avenia M., Citro R., De Marco M., et al. A novel miR-371a-5p-mediated pathway, leading to BAG3 upregulation in cardiomyocytes in response to epinephrine, is lost in Takotsubo cardiomyopathy. Cell Death Dis. 2015;6(10):e1948–e8. doi: 10.1038/cddis.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Salloum F.N., Yin C., Kukreja R.C. Role of microRNAs in cardiac preconditioning. J. Cardiovasc. Pharmacol. 2010;56(6):581–588. doi: 10.1097/FJC.0b013e3181f581ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee Y., Kim M., Han J., et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee Y., Ahn C., Han J., et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 202.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 203.Eulalio A., Huntzinger E., Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132(1):9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 204.Bushati N., Cohen S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23(1):175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 205.Chang T-C., Mendell J.T. microRNAs in vertebrate physiology and human disease. Annu. Rev. Genomics Hum. Genet. 2007;8(1):215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 206.Barile L., Moccetti T., Marbán E., Vassalli G. Roles of exosomes in cardioprotection. Eur. Heart J. 2017;38(18):1372–1379. doi: 10.1093/eurheartj/ehw304. [DOI] [PubMed] [Google Scholar]
- 207.Ueyama T., Kasamatsu K., Hano T., Tsuruo Y., Ishikura F. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann. N. Y. Acad. Sci. 2008;1148(1):479–485. doi: 10.1196/annals.1410.079. [DOI] [PubMed] [Google Scholar]
- 208.Feng Y., Madungwe N.B., da Cruz Junho C.V., Bopassa J.C. Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br. J. Pharmacol. 2017;174(23):4329–4344. doi: 10.1111/bph.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Menazza S., Sun J., Appachi S., et al. Non-nuclear estrogen receptor alpha activation in endothelium reduces cardiac ischemia-reperfusion injury in mice. J. Mol. Cell. Cardiol. 2017;107:41–51. doi: 10.1016/j.yjmcc.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ueyama T., Ishikura F., Matsuda A., et al. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ. J. 2007;71(4):565–573. doi: 10.1253/circj.71.565. [DOI] [PubMed] [Google Scholar]
- 211.Yellon D.M., Downey J.M. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 212.Cao X., Zhou C., Chong J., et al. Estrogen resisted stress-induced cardiomyopathy through increasing the activity of β2AR-Gαs signal pathway in female rats. Int. J. Cardiol. 2015;187(1):377–386. doi: 10.1016/j.ijcard.2015.02.113. [DOI] [PubMed] [Google Scholar]
- 213.Porto I., Della Bona R., Leo A., et al. Stress cardiomyopathy (tako-tsubo) triggered by nervous system diseases: A systematic review of the reported cases. Int. J. Cardiol. 2013;167(6):2441–2448. doi: 10.1016/j.ijcard.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 214.Templin C., Ghadri J.R., Diekmann J., et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 215.Sharkey S.W., Lesser J.R., Zenovich A.G., et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111(4):472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 216.Sharkey S.W., Windenburg D.C., Lesser J.R., et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J. Am. Coll. Cardiol. 2010;55(4):333–341. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 217.Behrens C.B., Nef H.M., Hilpert P., et al. Major depression as a potential trigger for Tako Tsubo cardiomyopathy. Int. J. Cardiol. 2010;140(2):e40–e42. doi: 10.1016/j.ijcard.2008.11.075. [DOI] [PubMed] [Google Scholar]
- 218.Tavazzi G., Zanierato M., Via G., Iotti G.A., Procaccio F. Are neurogenic stress cardiomyopathy and takotsubo different syndromes with common pathways?: Etiopathological insights on dysfunctional hearts. JACC: Heart Failure Elsevier Inc. 2017;5:940–942. doi: 10.1016/j.jchf.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 219.Marafioti V., Turri G., Carbone V., Monaco S. Association of prolonged QTc interval with Takotsubo cardiomyopathy: A neurocardiac syndrome inside the mystery of the insula of Reil. Clin. Cardiol. 2018;41(4):551–555. doi: 10.1002/clc.22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Finsterer J. Insular cortex lesions are not the only culprit in Takotsubo syndrome. Clin. Cardiol. 2018;41(11):1407–1408. doi: 10.1002/clc.23044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Brunetti N.D., Santoro F., De Gennaro L., et al. Therapy of stress (takotsubo) cardiomyopathy: Present shortcomings and future perspectives. Future Cardiol. 2016;12(5):563–572. doi: 10.2217/fca-2016-0014. [DOI] [PubMed] [Google Scholar]
- 222.Meerson F.Z. Stress-limiting systems and the problem of the prevention of arrhythmia. Kardiologiia. 1987;27(7):5–12. [PubMed] [Google Scholar]
- 223.Meerson FZ, Phennikova MG. Adaptation to stress and physical loads. 1988.
- 224.Maslov LN, Khaliulin I, Oeltgen PR, et al. Prospects for creation of cardioprotective and antiarrhythmic drugs based on opioid receptor agonists. 2016. [DOI] [PMC free article] [PubMed]


