Abstract
Purpose of Review
The purpose of the current mini-review is to describe the importance of surface ECG for the diagnosis of conduction disorder.
Methods
The MEDLINE/PubMed database was used, with the keywords “ECG” and “conduction disorders”; over the past 10 years. Other documents were included because of their relevance.
Main Findings
Data on the anatomy and function of the cardiac electrical system have been described. Conduction disorders including sinus node dysfunction, atrioventricular blocks, intraventricular conduction disorders are exposed as to their epidemiology, etiology, presentation, anatomical site of impaired conduction of the electrical stimulus. The importance of ECG in patients with a cardiac implantable electronic device was also discussed, in addition to future perspectives.
Conclusion
Surface ECG allows the diagnosis of atrioventricular and intraventricular conduction disorder and its anatomical block site most of the time, without the need for invasive tests such as electrophysiological study. Dysfunctions of cardiac implantable electronic devices can be diagnosed by ECG, as well as the prediction of response to cardiac resynchronization therapy.
Keywords: Cardiac conduction system diseases, electrocardiography, heart conduction system, bundle-branch block, sinus node dysfunction, cardiac resynchronization therapy
1. INTRODUCTION
From the first record of cardiac electrical activity in human by Augustus Desiré Waller using a capillary electrometer in 1887 and the use of the term Electrocardiogram (ECG) by Willem Einthoven in 1893 [1-3], there have been many descriptions of structural and functional changes by using this test. Subsequent technological developments with the advent of intracardiac electrophysiology and the Smartphone ECG confirmed the value of surface ECG as a useful, simple, non-invasive and inexpensive tool in the present day [4, 5]. For a better understanding of ECG conduction disorders, knowledge of the cardiac electrical system should be reviewed.
2. CARDIAC CONDUCTION SYSTEM
The autonomic nervous system participates in the modulation of electrical conduction of the heart, resulting in synchronous contraction of the atria and the ventricles. The cardiac conduction system consists of modified and striated cardiac muscle cells and shows the main structures (Fig. 1) [6-11]:
Fig. (1).
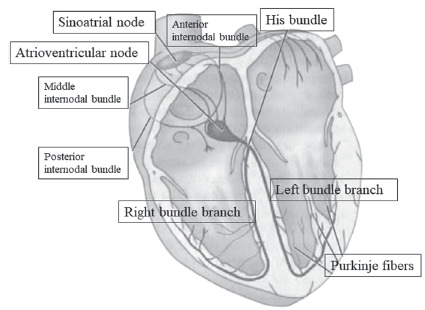
Main structures of cardiac conduction system. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Sinoatrial node or sinus node: myocyte structure described by Keith and Flack in 1907, located in the right atrium, at the junction of the superior vena cava and the right atrium, in the subepicardial region, near the terminal crest (crista terminalis). It is composed of cells in arrangement of fascicles immersed in collagen fibers. In its periphery, there are transitional cells, with intermediate morphology between cells of the sinus node and atrial contractile. It has 1.5 cm in length and 0.5 × 0.3 cm transversally in the adult heart. It is an elongated structure with thin extremities and, in a minority of normal hearts, has a horseshoe shape. It is the dominant pacemaker of the heart. From the sinus node, the electrical impulse propagates rapidly through the atrial muscle. To reach the left atrium, the impulse is conducted through the Bachman fascicle, resulting in left atrial depolarization 20 to 40 milliseconds after depolarization of the right atrium. In the right atrium, the electrical impulse is conducted to the atrioventricular (AV) node along with three internodal bundles of muscle fibers: the anterior, the middle (Wenckebach) and the posterior (Thorel).
AV node or Aschoff-Tawara node: structure about 5 mm long, 3 mm wide and 1 mm thick, located between the coronary sinus and the medial tricuspid valve leaflet, at the apex of the Koch triangle (region limited by the insertion of the tricuspid valve annulus and eustachian valve and the ostium of the coronary sinus), supported Todaro’s tendon. It is composed of a transitional zone, with posterior localization, and a compact zone, with cells grouped into bundles in the shape of the half moon. There is a delay in conduction of electrical stimulation at this node of about 90 milliseconds to complete ventricular filling before the impulse reaches the ventricular myocardium, with appropriate coupling of atrial and ventricular systole.
His bundle with a structure 20 mm long and 4 mm thick in its initial portion is located at the base of the interatrial septum in subendocardial position. It crosses the right fibrous trigone (central structure of the connective tissue of the heart), towards the ventricles, and following the membranous portion of the interventricular septum.
Bundle branches: His bundle has a bifurcation in the membranous part of the interventricular septum, composed by the left and right bundle branches. The left bundle branch is divided into fascicles (anterior, septal and posterior), toward the interventricular septum to the apex of the heart and then to the base of the posterior and anterior papillary muscles. The right bundle branch proceeds anteriorly into the interventricular septum and then passes behind the septal papillary muscle, reaching the anterior papillary muscle through the marginal septum trabeculae (between the right ventricle inlet and outflow tract). Some of its fibers are directed to the cardiac musculature and the other part follows in the backward direction by the inner wall for the base of the heart. The left bundle branch is thicker, as is its posterior fascicle, while the right bundle branch is thinner. The electrical stimulus reaches the left bundle branch before the right bundle, with a time of approximately 0.013 seconds.
Purkinje fibers: They were described by Purkinje in 1845. They are terminal subendocardial branches of the electrical system of the heart, thick and with its connective tissue wrap, passing through the interior of the heart muscle.
3. METHODS
A systematic review of the conduction disorders diagnosed by the ECG was made based on articles and documents of relevance on the topic.
4. SINUS NODE DYSFUNCTION
This bradyarrhythmia encompasses the following types of electrophysiological behavior: sinoatrial disease (or sick sinus syndrome) from intrinsic or extrinsic causes, sinoatrial block and tachycardia-bradycardia syndrome. Its prevalence increases with age, reaching 1 in 600 individuals over 65 years of age. Its main causes are fibrosis (in the 7th or 8th decade of life), atherosclerosis, familial, medications, inflammatory diseases. The progression of bradycardia is slow, with a time interval of 7 to 29 years, resulting in cardiovascular events and indication for cardiac pacemaker implantation [12, 13]. The diagnosis is mainly based on clinical and electrocardiographic evaluation, either by surface ECG, exercise stress testing or Holter system. Electrophysiology testing for sinus node dysfunction is not recommended [9, 13, 14]. Electrocardiographic presentations may be sinus bradycardia at rest or chronotropic incompetence on exertion, sinus pause, and periods of tachyarrhythmias (flutter or atrial fibrillation with high ventricular response) followed by periods of bradycardia (with sinus pause). The latter is the most frequent presentation and sinus pause is a consequence of an automatic depression imposed by overstimulation mechanisms due to tachyarrhythmia.
Sinus arrest is the sudden interruption of sinus automatism, with sinus pause with no mathematical relationship to previous cycles (Fig. 2). There may be the recovery of the sinus node. Otherwise, an escape beat or escape rhythm may occur.
Fig. (2).

Electrocardiographic tracing of leads V1, V5 and D3 showing ventricular pause of 4.2 seconds in a 75-year-old patient with syncope. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In sinoatrial block, there is a delay or interruption of electrical stimulation due to changes in the sinoatrial junction. The 1st degree cannot be recognized by the surface ECG; the second degree is a consequence of an exit blockade of sinus depolarization, classified as type I and type II. Type I is characterized by progressive shortening of the P-P intervals, ending with a pause with no mathematical relationship to the preceding cycle. Differential diagnosis should be made with sinus arrhythmia and atrial extrasystoles. Type II is characterized by sinus pauses that have a mathematical relationship with the basic cycle, i.e., they are multiple of the basic cycles, without previous alteration of the P-P interval. In 3rd degree sinoatrial block, the passage of the stimulus through the sinoatrial junction is interrupted, manifesting as a sinus pause, if transient, or as a generally junctional escape rhythm.
5. INTRAVENTRICULAR CONDUCTION DISORDERS
Intraventricular blocks are disorders with delayed or interrupted conduction of cardiac electrical stimulation, resulting in change in the duration and shape of the QRS complex on the ECG and asynchrony of the corresponding ventricular activation. They may be intermittent or permanent, frequency-dependent as well as functional or structural. These conduction disorders include the right bundle branch block, left bundle branch block, and fascicular blocks [15].
5.1. Bundle Branch Blocks
In 1985, the electrocardiographic criteria for bundle branch block were reviewed for standardization in complete and incomplete [15]. Thus, the criteria from the Mexican (1st, 2nd and 3rd grade), Hispanic (advanced, non-advanced or topographic - proximal and peripheral) criteria, previously used, were replaced by those criteria.
The prevalence of right bundle branch block increases with age and occurs in up to 1.6% of women and 4.1% of men [16], three times more often in men, reaching 14.3% of men older than 80 years [17]. It may be a finding in healthy individuals with no adverse prognosis. However, it may be associated with systemic arterial hypertension, coronary artery disease, diabetes mellitus, cardiomyopathies, myocarditis, cor pulmonale, pulmonary thromboembolism and congenital heart disease. Among those with this complete bundle branch block and heart disease, there is an increased cardiovascular mortality for both sexes. In contrast, incomplete right bundle branch block has not been associated with poor prognosis [17].
The prevalence of left bundle branch block in the general population is less than 1%, reaching 5% in those 80 years old. Risk factors associated with this conduction disorder are hypertension, coronary artery disease, aortic valve disease, myocardial diseases, or conduction system degeneration. Among patients with heart failure, 30% may have left bundle branch block (Fig. 3). This conduction disorder causes left ventricular dyssynchrony, with decreased cardiac output, increased residual systolic volume, resulting in ventricular remodeling, changes in regional perfusion, glucose absorption and calcium transport, promoting a proarrhythmic state [14, 18]. There is also evidence of the association of genetic variants and left bundle branch block. Another etiology of this conduction disorder is a complication of transcatheter aortic valve replacement. The mean incidence of this complication is between 14.0% and 45.2% [19]. Therefore, the left bundle branch block is associated with increased mortality, with major implications that the right bundle branch block.
Fig. (3).
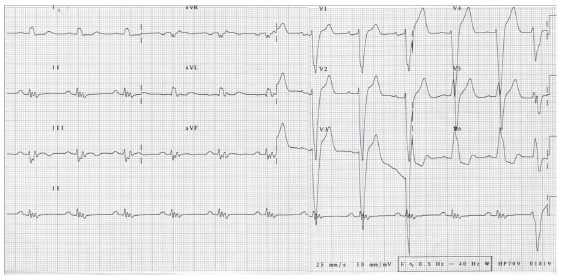
12-lead electrocardiographic tracing demonstrating sinus rhythm with complete left bundle branch block. There is also QRS fragmentation in the inferior leads. This fragmentation represents conduction delay due to myocardial scarring and is a marker of cardiovascular disease risk [22]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The diagnosis of left bundle branch block is electrocardiographic and the electrophysiological study is a Class IIa recommendation for approaching patients with this conduction disorder [14]. For the selection of patients for cardiac resynchronization therapy, the diagnosis of left bundle branch block with QRS duration > 150 ms at sinus rhythm is a recommendation of class I level of evidence A without the need for assessment of ventricular dyssynchrony by echocardiography. The guidelines recommend that this conduction disorder be associated with clinical conditions of heart failure (with left ventricular ejection fraction ≤ 35%) in NYHA functional class II or more advanced under appropriate treatment [20]. This demonstrates the importance of ECG for an indication of therapy with a positive impact on the evolution of heart failure patients. About 30% of patients do not achieve clinical improvement with cardiac resynchronization therapy and are considered non-responders. There are several factors that interfere with this response, such as patient selection, clinical parameters and comorbidities, type and location of left ventricular lead, and device programming. However, electrocardiographic algorithms such as fusion-optimized intervals to achieve the shortest possible QRS are used to improve patients' hemodynamic response [21], reaffirming the importance of ECG.
In addition, to bundle branch blocks such as intraventricular conduction disorders, there are fascicular blocks [15, 14, 23]. The causes of these fascicular blocks are similar to those of bundle branch blocks. In relation to the left branch, there are the anterior, septal and posterior fascicles. Regarding the blockade of the left anterior fascicular block, its prevalence in the general population is 0.9 to 6.2% and is not a risk factor for cardiovascular morbidity or mortality. However, it may occur in the presence of acute myocardial infarction with adverse prognostic significance [23, 24]. Differential diagnosis of fascicular block is important. Should be considered individual's biotype, heart chambers overload and pathologic Q waves on ECG [23].
There are other cardiac conduction disorders that affect two or more fascicles. Thus, the combination of right bundle branch block with left anterior fascicular block or with left posterior fascicular block is called bifascicular block. The most common combination is the first, with a prevalence of up to 1.5% in adults. There is slow progression to total AV block unless associated with syncope [25, 26]. Another presentation of bifascicular block is the left bundle branch block, as there is impairment of conduction in the left anterior and posterior fascicles [27]. In trifascicular block, there are left bundle branch block and AV conduction delay. The association of bifascicular block with prolonged PR interval is not defined as trifascicular block [25, 27] (Fig. 4). AV conduction delay may manifest on ECG by right or left bundle branch block with total AV block or with bifascicular block and total AV block [25]. Trifascicular block may manifest with delayed conduction of both branches, with electrocardiographic pattern of branch block with greater degree of delay (Fig. 5). This delay in AV conduction is observed by prolonging the H-V interval in the intracardiac record most of the time [27].
Fig. (4).
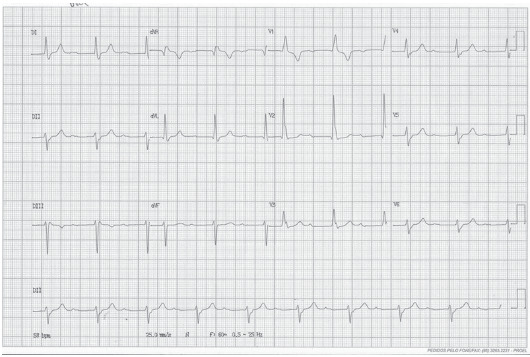
12-lead electrocardiographic tracing demonstrating sinus rhythm with bifascicular block (right bundle branch block and left anterior fascicular block) with prolonged PR. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (5).
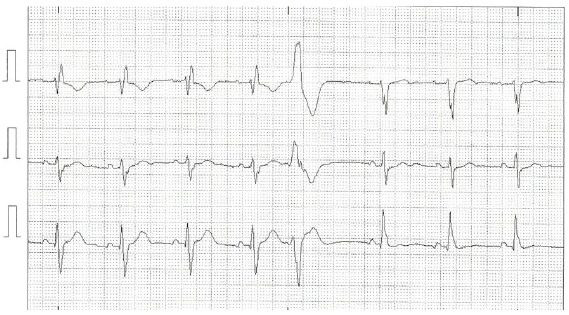
Electrocardiographic tracing of modified V1, V5, and D3 leads demonstrating sinus rhythm with alternating bundle branch block, right bundle branch block at the beginning of the ECG, followed by ventricular extrasystole and left bundle branch block. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
There are intermittent conduction disorders, which may be related to the duration of the cardiac cycle. The main causes are acceleration (tachycardia)-dependent, deceleration (bradycardia)-dependent, Ashman phenomenon, preexcitation syndromes, concealed conduction and drug effects and electrolyte disturbances [27].
5.2. Atrioventricular Blocks
AV blocks are conduction disorders that occur due to prolonged, intermittent or absent AV conduction. The classification of AV blocks is shown in Table 1.
Table 1.
Classification of atrioventricular blocks.
| AV Block Types | Definition |
|---|---|
| First-degree atrioventricular block | PR interval> 200 ms (in adults) with 1: 1 atrioventricular conduction. |
| Second-degree atrioventricular block Mobitz type 1 Mobitz type 2 |
Constant atrial frequency (<100 bpm) with a progressive prolongation of the PR interval until a P wave is not conducted, culminating in a pause. The PR interval following the pause is shorter than the one preceding the pause. There are intermittent non-conducting P waves without progressive PR interval prolongation; PR interval remains constant as well as P-P intervals. |
| 2:1 atrioventricular block | 2:1 AV conduction with atrial frequency <100 bpm and constant PR interval. |
| Advanced atrioventricular block | AV conduction 3:1 or higher. |
| Third-degree atrioventricular block (complete heart block) | Absence of AV nodal conduction (atrioventricular dissociation), with atrial frequency higher than ventricular rate. QRS complexes with intrinsic AV node frequency of 40 to 55 bpm, or wide QRS complexes with frequency of 20 to 40 bpm. |
First-degree AV block is observed in up to 7.8% of healthy individuals with a predominance of black men. The anatomic site of this block may be in the atrium, AV node (most frequent level), intra or infra-His bundle. Second-degree AV block can affect 1-2% of healthy young people, especially during sleep (if Mobitz type 1), or 0.003% (if Mobitz type 2). Mobitz type 1 is usually located on AV node with a narrow QRS complex. If the QRS complex is large (≥120 ms), second-degree AV block Mobitz type 1 may be nodal in 30% to 40% of patients and His-Purkinje system in 60% to 70% of cases. The level of conduction delay on Mobitz type 2 is below the level of the AV node (in the bundle of His and the bundle branches). In 2:1 AV block the conduction system impairment is in the AV or intra-His node if the PR interval and QRS complex are of normal duration. If the QRS complex duration is increased, i.e., there is branch block, the block level is infra-His (Fig. 6). Advanced and third-degree AV blocks occur mainly in the infranodal specialized conduction system (Fig. 7). The prevalence of third-degree AV block is 0.04% in the adult population, reaching 0.6% in hypertensive patients. In congenital etiology, its incidence is 2.1 per 100 000 live births [9, 28 - 33]. To approach AV block patients, if symptoms are present and/or the block level is infranodal, cardiac pacemaker is indicated. The electrophysiology study is a recommendation class IIb for patients without symptoms and with unclear site of AV block [14, 34]. Therefore, the surface ECG allows to estimate the anatomical site of AV block guiding the patient approach.
Fig. (6).
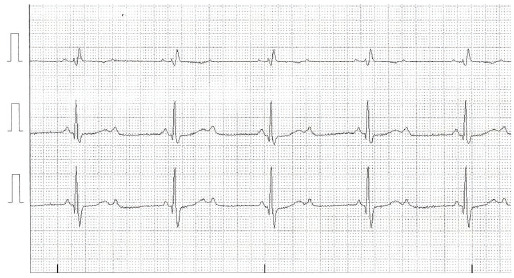
Electrocardiographic tracing of modified V1 and V5 leads and D3, demonstrating 2:1 AV block with right bundle branch block. Thus, the anatomical site of the block is infra-His. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (7).
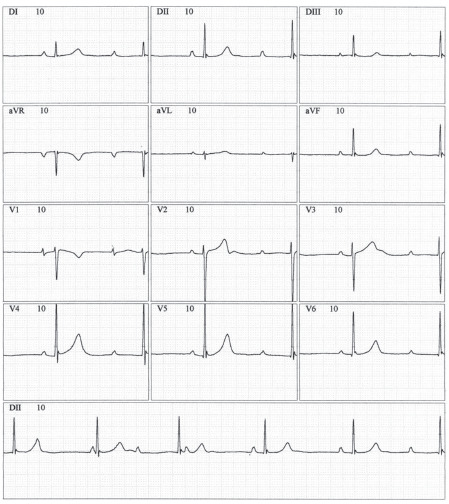
12-lead electrocardiographic tracing demonstrating sinus atrial rhythm, total AV block with narrow QRS complex, with two sinus capture beats (4th and 5th QRS complexes of the D2 lead tracing at the bottom). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
6. SURFACE ECG IN PATIENTS WITH CARDIAC IMPLANTABLE ELECTRONIC DEVICES
Cardiac implantable electronic device malfunctions occur with an annual replacement rate of 1.4 to 9.0 per 1000 implants for pacemakers and 7.9 to 38.6 per 1000 implants for cardioverter-defibrillator. The main malfunctions are hardware abnormalities in 79.8% of cases, such as battery/capacitor abnormalities and electrical issues [35]. Radiation from cardiac imaging such as tomography that directly radiates cardiac implantable electronic devices may cause electronic interference, but with rare adverse clinical events [36]. These malfunctions can be verified through the ECG.
The ECG analysis after cardiac resynchronization therapy allows to evaluate the response to that therapy (Fig. 8). The narrowing of the QRS complex after the biventricular pacing is associated with clinical and echocardiographic improvement [37, 38]. This demonstrates the prognostic significance attributed to the ECG in patients with these devices.
Fig. (8).
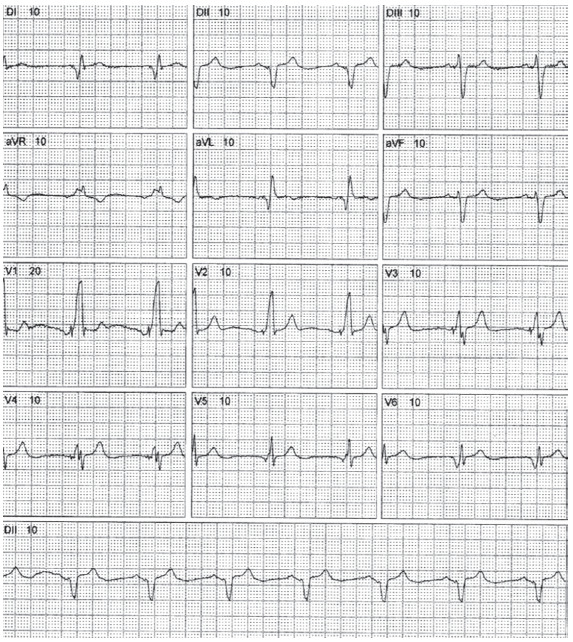
12-lead ECG of 72-year-old male patients with response to cardiac resynchronization therapy. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
7. FUTURE PERSPECTIVES
Despite this centuries-old complementary exam, there are still gaps regarding new applications and the importance of ECG. Large prospective studies to assess the prognostic value of the surface ECG in relation to cardiac resynchronization therapy are needed. Real-time electrocardiographic analysis can be expanded to provide remote monitoring of patients from distant areas with great socioeconomic impact. Artificial intelligence as an ECG interpretation tool is still under development and can assist in decision making.
CONCLUSION
Surface ECG allows the diagnosis of atrioventricular and intraventricular conduction disorder and its anatomical block site most of the time, without the need for invasive tests such as electrophysiological study. Dysfunctions of cardiac implantable electronic devices can be diagnosed by ECG, as well as the prediction of response to cardiac resynchronization therapy.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- AV
Atrioventricular
- ECG
Electrocardiogram
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Fye W.B. A history of the origin, evolution, and impact of electrocardiography. Am. J. Cardiol. 1994;73(13):937–949. doi: 10.1016/0002-9149(94)90135-X. [DOI] [PubMed] [Google Scholar]
- 2.AlGhatrif M., Lindsay J. A brief review: History to understand fundamentals of electrocardiography. J. Community Hosp. Intern. Med. Perspect. 2012;2(1) doi: 10.3402/jchimp.v2i1.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Luna A.B. Willem Einthoven and the ECG. Eur. Heart J. 2019;40(41):3381–3383. doi: 10.1093/eurheartj/ehz721. [DOI] [PubMed] [Google Scholar]
- 4.Haberman Z.C., Jahn R.T., Bose R., et al. Wireless smartphone ECG enables large-scale screening in diverse populations. J. Cardiovasc. Electrophysiol. 2015;26(5):520–526. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 5.Costantini O. Basic principles of cardiac electrophysiology. Med. Clin. North Am. 2019;103(5):767–774. doi: 10.1016/j.mcna.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Christoffels V.M., Moorman A.F. Development of the cardiac conduction system: Why are some regions of the heart more arrhythmogenic than others? Circ Arrhythm Electrophysiol. 2009;2(2):195–207. doi: 10.1161/CIRCEP.108.829341. [DOI] [PubMed] [Google Scholar]
- 7.Monfredi O., Dobrzynski H., Mondal T., Boyett M.R., Morris G.M. The anatomy and physiology of the sinoatrial node-a contemporary review. Pacing Clin. Electrophysiol. 2010;33(11):1392–1406. doi: 10.1111/j.1540-8159.2010.02838.x. [DOI] [PubMed] [Google Scholar]
- 8.McManus B.M., Wood S.M. Morphological features of normal and abnormal conduction system: Essentials for electrophysiologists. Nonpharmacological therapy of arrhythmias for the 21st century: the state of the art. Armonk, New York: Futura Publishing Company; 1998. pp. 27–56. [Google Scholar]
- 9.Josephson M.E. Clinical cardiac electrophysiology: Techniques and interpretations. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 10.Sánchez-Quintana D., Yen Ho S. Anatomy of cardiac nodes and atrioventricular specialized conduction system. Rev. Esp. Cardiol. 2003;56(11):1085–1092. doi: 10.1016/s0300-8932(03)77019-5. [DOI] [PubMed] [Google Scholar]
- 11.Anderson R.H., Boyett M.R., Dobrzynski H., Moorman A.F. The anatomy of the conduction system: implications for the clinical cardiologist. J. Cardiovasc. Transl. Res. 2013;6(2):187–196. doi: 10.1007/s12265-012-9433-0. [DOI] [PubMed] [Google Scholar]
- 12.Dobrzynski H., Boyett M.R., Anderson R.H. New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation. 2007;115(14):1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 13.John R.M., Kumar S. Sinus node and atrial arrhythmias. Circulation. 2016;133(19):1892–1900. doi: 10.1161/CIRCULATIONAHA.116.018011. [DOI] [PubMed] [Google Scholar]
- 14.Kusumoto F.M., Schoenfeld M.H., Barrett C., et al. 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American college of Cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2019;74(7):e51–e156. doi: 10.1016/j.jacc.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Surawicz B., Childers R., Deal B.J., et al. American Heart Association Electrocardiography and Arrhythmias Committee Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society; Endorsed by the International Society for Computerized Electrocardiology. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2009;53(11):976–981. doi: 10.1016/j.jacc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Thrainsdottir I.S., Hardarson T., Thorgeirsson G., Sigvaldason H., Sigfusson N. The epidemiology of right bundle branch block and its association with cardiovascular morbidity-the Reykjavik Study. Eur. Heart J. 1993;14(12):1590–1596. doi: 10.1093/eurheartj/14.12.1590. [DOI] [PubMed] [Google Scholar]
- 17.Bussink B.E., Holst A.G., Jespersen L., Deckers J.W., Jensen G.B., Prescott E. Right bundle branch block: Prevalence, risk factors, and outcome in the general population: Results from the Copenhagen City Heart Study. Eur. Heart J. 2013;34(2):138–146. doi: 10.1093/eurheartj/ehs291. [DOI] [PubMed] [Google Scholar]
- 18.Kanawati J., Sy R.W. Contemporary review of left bundle branch block in the failing heart - pathogenesis, prognosis, and therapy. Heart Lung Circ. 2018;27(3):291–300. doi: 10.1016/j.hlc.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Surkova E., Badano L.P., Bellu R., et al. Left bundle branch block: From cardiac mechanics to clinical and diagnostic challenges. Europace. 2017;19(8):1251–1271. doi: 10.1093/europace/eux061. [DOI] [PubMed] [Google Scholar]
- 20.Boriani G., Nesti M., Ziacchi M., Padeletti L. Cardiac resynchronization therapy: an overview on guidelines. Heart Fail. Clin. 2017;13(1):117–137. doi: 10.1016/j.hfc.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Tolosana J.M., Mont L. Cardiac resynchronization therapy: How to decrease nonresponders. Heart Fail. Clin. 2017;13(1):233–240. doi: 10.1016/j.hfc.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Jain R., Singh R., Yamini S., Das M.K. Fragmented ECG as a risk marker in cardiovascular diseases. Curr. Cardiol. Rev. 2014;10(3):277–286. doi: 10.2174/1573403X10666140514103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elizari M.V., Acunzo R.S., Ferreiro M. Hemiblocks revisited. Circulation. 2007;115(9):1154–1163. doi: 10.1161/CIRCULATIONAHA.106.637389. [DOI] [PubMed] [Google Scholar]
- 24.Lévy S. Bundle branch blocks and/or hemiblocks complicating acute myocardial ischemia or infarction. J. Interv. Card. Electrophysiol. 2018;52(3):287–292. doi: 10.1007/s10840-018-0430-3. [DOI] [PubMed] [Google Scholar]
- 25.Rogers RL, Mitarai M, Mattu A. Intraventricular conduction abnormalities. 2006. [DOI] [PubMed]
- 26.Pham T.H., Amsterdam E., Glassy M.S. Syncope with bifascicular block due to infra-hisian wenckebach conduction abnormality. JAMA Intern. Med. 2018;178(10):1408–1410. doi: 10.1001/jamainternmed.2018.3951. [DOI] [PubMed] [Google Scholar]
- 27.Mirvis D.M., Goldberger A.L. Electrocardiography. In: Zipes D.L., Libby P., Bonow R.O., Mann D.L., Tomaselli G.F., editors. Braunwald’s Heart Disease. 11th ed. Philadelphia: Saunders Elsevier; 2019. pp. 117–153. [Google Scholar]
- 28.Virani S.S., Alonso A., Benjamin E.J., et al. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. heart disease and stroke statistics-2020 update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 29.Pellegrini C.N., Scheinman M.M. Bradycardia: Sinus and AV node dysfunction. Herzschrittmacherther. Elektrophysiol. 2015;26(3):175–191. doi: 10.1007/s00399-015-0389-z. [DOI] [PubMed] [Google Scholar]
- 30.Nelson W.P. Diagnostic and prognostic implications of surface recordings from patients with atrioventricular block. Card. Electrophysiol. Clin. 2016;8(1):25–35. doi: 10.1016/j.ccep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 31.Barold S.S., Type I., Type I. Wenckebach second-degree AV block: A matter of definition. Clin. Cardiol. 2018;41(3):282–284. doi: 10.1002/clc.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoniadis A.P., Fragakis N.K., Maligkos G.C., Katsaris G.A. Infra-Hisian block as cause of Wenckebach’s phenomenon in an asymptomatic middle-aged man. Europace. 2010;12(6):898–902. doi: 10.1093/europace/eup450. [DOI] [PubMed] [Google Scholar]
- 33.Kashou A.H., Goyal A., Nguyen T., Chhabra L. Atrioventricular block. 2019 https://www.ncbi.nlm.nih.gov/books/NBK459147/
- 34.Katritsis D.G., Josephson M.E. Electrophysiological testing for the investigation of bradycardias. Arrhythm. Electrophysiol. Rev. 2017;6(1):24–28. doi: 10.15420/aer.2016:34:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maisel W.H., Moynahan M., Zuckerman B.D., et al. Pacemaker and ICD generator malfunctions: Analysis of food and drug administration annual reports. JAMA. 2006;295(16):1901–1906. doi: 10.1001/jama.295.16.1901. [DOI] [PubMed] [Google Scholar]
- 36.U.S. FDA https://www.fda.gov/radiation-emitting-products/electromagnetic-compatibility-emc/effects-x-ray-irradiation-ct-imaging-pacemakers-and-implantable-cardioverter-defibrillators-icd
- 37.Bazoukis G., Naka K.K., Alsheikh-Ali A., et al. Association of QRS narrowing with response to cardiac resynchronization therapy-a systematic review and meta-analysis of observational studies. Heart Fail. Rev. 2020;25(5):745–756. doi: 10.1007/s10741-019-09839-5. [DOI] [PubMed] [Google Scholar]
- 38.Ma J., Liu Y., Dong Y., Chen M., Xia L., Xu M. Association between changes in QRS width and echocardiographic responses to cardiac resynchronization therapy: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99(2):e18684. doi: 10.1097/MD.0000000000018684. [DOI] [PMC free article] [PubMed] [Google Scholar]


