Abstract
Left ventricular noncompaction (LVNC) is a congenital pathology that directly affects the lining walls of myocardial tissue, causing trabeculations with blood filling in the inner wall of the heart, concomitantly with the development of a mesocardial thinning. Although LVNC was described for the first time as long ago as 1984, our understanding of the disease with regard to its genetic pattern, diagnosis, clinical presentation and treatment is still scanty. LVNC can present as an isolated condition or associated with congenital heart disease, genetic syndromes or neuromuscular disease. This suggests that LVNC is not a distinct form of cardiomyopathy, but rather a morphological expression of different diseases. Recognition of the disease is of fundamental importance because its clinical manifestations are variable, ranging from the absence of any symptom to congestive heart failure, lethal arrhythmias and thromboembolic events. The study of this disease has emphasized its genetic aspects, as it may be of sporadic origin or hereditary, in which case it most commonly has an autosomal dominant inheritance or one linked to the X chromosome. Echocardiography is the gold standard for diagnosis, and magnetic resonance imaging may refine the identification of the disease, especially in those patients with non-conclusive echocardiography. This article sets out to review the main characteristics of LVNC and present updates, especially in the genetic pattern, diagnosis and treatment of the disease.
Keywords: Compacted myocardium, myocardial trabeculations, genetic heart diseases, noncompaction, left ventricular, rare heart diseases
1. INTRODUCTION
Left ventricular noncompaction (LVNC) is a rare genetic and congenital disorder characterized by the excessive formation of blood-filled trabeculae and intertrabecular recesses in the uncompressed inner endocardial wall associated with a thin, compact wall, the mesocardium [1-5]. This condition was first described by Grant in 1926 but was only classified as a primary X-linked genetic cardiomyopathy by the American Heart Association (AHA) in 2006, in contrast to the European Society of Cardiology and the International Organization for Classification of Heart Diseases, which still consider LVNC an unclassified heart disease [1, 3, 4, 6]. The most recent MOGE(S) nosology proposes a simple description of the trait in subjects with either normal left ventricular size, wall thickness and preserved systolic/diastolic function or in combination with other cardiac conditions such as hypertrophic cardiomyopathy, restrictive cardiomyopathy, dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy [7]. The pathogenic mechanisms underlying LVNC are associated with fetal age in the final phase of cardiac tissue fiber formation and compression. Evidence supporting the genetic criteria related to the syndrome identified mutations of genes encoding sarcomeric proteins that form the cytoskeleton and nuclear membranes [3, 4]. The nonembryogenic hypothesis of LVNC classifies it as a sporadic, acquired, and permanent or transient disease because acquired LVNC is presumed to be present in complex syndromes in athletes as a direct effect of cardiac remodeling, or even in response to left ventricular overload or physiological mechanisms in pregnancy. It may also be associated with certain clinical conditions. Noncompaction of the tissue occurs mostly in the apical region of the left ventricle, but in rare cases, it can affect the right ventricle or even both ventricles [3, 4, 6, 8].
The deleterious effects of LVNC on heart muscle include heart failure (HF), ventricular tachyarrhythmias, and thromboembolic events; however, severe cases lead to lethal arrhythmias and sudden death [1-3, 5, 8-9].
2. EPIDEMIOLOGY
The epidemiology of LVNC has not been fully elucidated to date. A prospective Swiss study reported 34 adult patients with LVNC aged 15 years among all patients undergoing echocardiographic assessment, accounting for 0.014% of the total sample population [10]. A prevalence of 9.2% was noted among children with previous cardiomyopathy and 18–50% among members of the same affected family [11, 12]. The prevalence of ventricular noncompaction in patients with HF was reported to be 3% [13]. However, these data may not be reliable due to the difficulty in verifying hypertrabeculation on transthoracic echocardiography, as well as the use of obsolete technology to establish the diagnosis. It is important to understand that “LVNC” describes an anatomic variant of left ventricular (LV) and does not necessarily describe a disease [14].
LVNC is not prevalent among individuals of African or Caucasian descent [15]. However, a study based on the African athletes with HF showed a high prevalence of myocardial hypertrabeculation, with about 15% of patients satisfying the echocardiographic criteria of LVNC [16]. However, it is unclear whether this cardiac morphology observed in individuals of African descent is a manifestation of LVNC or is only secondary to the increase in cardiac preload in these individuals [17, 18].
Regarding sex-based epidemiology, men are often more affected than women, with males being affected in between 52 and 86% of the cases [10, 19-21].
3. CLINICAL PRESENTATION
The clinical presentation of LVNC varies in severity, ranging from asymptomatic patients to those presenting symptoms mainly associated with cardiac changes, those with musculoskeletal symptoms, and those with symptoms possibly associated with congenital diseases, such as Ebstein’s anomaly, bicuspid aortic valve, carotid artery abnormalities, or clinical syndromes, such as Charcot-Marie-Tooth syndrome or the Melnick-Needles Syndrome [1, 3, 5].
The main cardiac symptoms associated with LVNC are related to HF, occurring in up to half of the patients. Atrial fibrillation can affect 25% of adult patients and ventricular tachyarrhythmias up to around 50%, in addition to clinical symptoms reported by the patients themselves, such as weakness and palpitations [3, 8]. There is a possible association between bradycardia and Wolff-Parkinson-White syndrome in pediatric patients with LVNC [22]. Other frequent manifestations are related to thromboembolic events, such as stroke, pulmonary embolism, and mesenteric ischemia [1-3,8-9]. In asymptomatic patients, LVNC is identified by echocardiography or when the patient is subjected to family screening. However, when the disease is identified during the fetal period, the presence of systemic diseases, such as mitochondrial alterations and metabolic disorders, is frequently reported [8].
4. GENETICS
Familial incidence is the most prevalent, accounting for up to 50% of cases [23]. The most common pattern of genetic inheritance is autosomal dominant, followed by X-linked [24]. Evidence from genetic studies has indicated that the main cause of LVNC is the mutation of genes encoding sarcomeric proteins representing up to 30% of all cases [25]. Other reported mutations were in genes encoding cytoskeletal, Z-line, and mitochondrial proteins [26]. The most common mutations were in the myosin heavy chain 7 (MYH7), protein-binding protein C myosin (MYBPC3), tropomyosin alfa (TPM1), myocardial actin (ACTC1), troponin T (TNNT2), and cardiac troponin I (TNNI3) genes [23, 25, 27]. Familial LVNC, when accompanied by hypertrophic or dilated cardiomyopathy, is typical of the MYH7 mutation [27]. All of this information is summarized below (Table 1).
Less common genetic disorders in LVNC patients include Z-band protein mutations, such as those of the cypher/ZASP cytoskeleton protein, alpha-distrobrevin (DTNA) [28], calcium transport proteins, calsequestrin (CASQ2), phospholamban (PLN) [23], membrane proteins (A/C lamina) [29], and mitochondrial enzymes, such as tafazzin protein G 4.5 [26]. These disorders are usually transmitted through autosomal dominant inheritance with incomplete penetration. The exception is the mutation in the TAZ, which shows an X-linked recessive inheritance pattern that results in an error in the tafazzin proteins that is responsible for the Barth syndrome [26, 30, 31]. This syndrome, characterized by dilated cardiomyopathy or LVNC at an early age in males, is often accompanied by neutropenia, lactic acidosis, and lipid metabolism abnormalities [31, 32].
Some mutations are associated with specific phenotypes, such as the pNGly482Arg mutation-bearing HCN4, associated with the bradycardia phenotype in patients with LVNC [33]. This is because potassium/sodium hyperpolarization-activated cyclic nucleotide-controlled channel 4 is a protein that is encoded by the HCN4 gene in humans. There are four HCN channels, with HCN4 being the most expressed for the regulation of the heart rate in mammals [34, 35] and is most associated with bradycardia [36, 37]. The role of HCN channels in autonomic heart rate control is not yet fully understood.
Mutations in the isolated DMD and EMD genes seem insufficient to cause LVNC, although they are associated with phenotypic variations in patients with ventricular noncompaction, such as Emery-Dreifuss muscular dystrophy [21, 38]. The L1988R mutation in the SCN5A gene does not alter SCN5A channel density and may or may not cause arrhythmias in patients with LVNC [37].
This is an online review of the Mendelian Inheritance in Man (OMIM). The other information is available on the OMIM website [39].
5. DIAGNOSIS
There is no gold standard for the diagnosis of LVNC, which should, therefore, be made based on the clinical and morphological findings compatible with the disease. The first step in the diagnosis of LVNC is transthoracic echocardiography. Other imaging examinations may also be useful for the diagnosis or confirmation of LVNC, such as cardiac resonance, computed tomography, and left ventriculography.
5.1. Echocardiography
Echocardiography is the main examination used to diagnose LVNC [17]. The morphological criteria of LVNC analyzed by transthoracic echocardiography were suggested by Chin et al. [19], Stollberger et al. [20], Jenni et al. [40, 41] and Paterick et al. [42]. Jenni et al. criteria are the ones most commonly used.
a). Criteria Proposed by Chin et al. [19]
A ratio between the distance from the epicardial surface to the trabecular recess and the distance from the epicardial surface to the trabecular peak of less 0.5 is suggestive of LVNC. These criteria are being applied for trabeculations of the left ventricular apex with four-chamber or subxiphoid apical images at the end of diastole.
b). Criteria Proposed by Stollberger et al. [20]
The presence of more than three trabeculations on the left ventricular wall with the apical location of the papillary muscles visible in a single image plane and perfused intertrabecular spaces from the ventricular cavity visualized by color Doppler imaging is suggestive of LVNC.
c). Criteria Proposed by Jenni et al. [40]
Criteria suggestive of LVNC is based on a maximum ratio between uncompressed and compacted myocardium greater than 2 at the end of the systole parasternal short axis. In addition, the middle (especially lower and lateral) and apical ventricular segments should be involved, with evidence of ventricular cavity blood flow in deep intertrabecular recesses by color Doppler (Fig. 1) [41].
d). Criteria Proposed by Paterick et al. [42]
The ratio between the thickness of the uncompressed and compacted myocardium should be greater than 2; the measurement should be taken at the end of diastole in the transverse parasternal view. The authors state that although this criterion increases the accuracy of the myocardial thickness measurement, more studies are required to validate this assertion.
5.2. Magnetic Resonance Imaging
Cardiac magnetic resonance imaging (CMRI) is used when the echocardiography findings are inconclusive. Echocardiography does not always completely visualize the apical region and may underestimate the degree of LVNC. Thus CMRI has become the method of choice to confirm or rule out LVNC. Trabeculations and recesses appear differently on CMRI compared with echocardiography and computed tomography (CT). CMRI provides a good spatial resolution of the left ventricular segments as a whole, covering the apex and lateral wall [43]. Quantitative criteria are fundamental because regions of apparent noncompaction are also common in patients without cardiovascular disease [44]. The best criterion for LVNC identification in CMRI was a ratio of the uncompressed to compacted myocardium of >2.3 in diastole, with 83% sensitivity and 99% specificity at diagnosis [45, 46]. Another way to diagnose this entity by CMRI is to verify whether the trabeculated left ventricular mass is more than 20% of the total mass, with a sensitivity of 91.6% and a specificity of 86.5% for LVNC [47].
Nuclear magnetic resonance imaging is also of particular value for risk stratification in patients with clinically suspected LVNC and is able to detect the presence of focal myocardial fibrosis by the late myocardial enhancement technique, usually related to ventricular arrhythmias, a lower ejection fraction, and a worse prognosis [43, 44].
5.3. Computed Tomography
CT is an alternative method for the diagnosis of uncompressed cardiomyopathy if echocardiography is inconclusive and CMRI is inconclusive or unavailable at the unit. An uncompressed to compacted myocardium ratio of > 2.3 at the end of long-axis diastole is suggestive of this cardiomyopathy [48].
6. LIMITATION OF DIAGNOSTIC CRITERIA
The limited specificity of echocardiographic criteria has been observed in subsequent studies when these criteria were applied to otherwise healthy patients and control populations, especially Afro-descendent athletes [7]. The difficulty is also encountered in making the differential diagnosis since LVNC is a precursor of changes which are common to other heart diseases [49].
Comparing the three main echocardiographic criteria [17] in a study of 199 patients and 60 healthy people in the control group, 47 patients met at least one of the criteria; approximately 79% of this sample met the diagnostic criteria of noncompaction proposed by Chin [19], approximately 64% were diagnosed by the Jenni criteria [40], and less than 54% met the Stollberger criteria [20]. Only about 30% of this sample completely met all three criteria, showing the lack of consensus among them. Five of the 47 patients diagnosed this way were part of the control group consisting of healthy individuals, of whom 4 were of African descent, corresponding to 13% of the healthy subjects of African descent [17]. In another study of 1,146 healthy athletes, 10% of blacks and approximately 8.5% of Caucasians received the diagnosis of LVNC according to the Chin and Jenni criteria [4].
As the echocardiographic images of healthy people may look similar to those of patients with LVNC [4, 17], there is a growing concern regarding overdiagnosis. Thus, Garcia-Pavia et al. [49] in an editorial proposed that the definitive diagnosis should be based on echocardiography using the Jenni criteria [40] and/or CMRI with the criterion of Jacquier [47] together with at least one of the following characteristics: complications related to LVNC; previously diagnosed family member; or a cataloged LVNC-related genetic mutation [49].
Diseases that have morphological and functional alterations similar to LVNC must be differentiated to ensure the correct diagnosis [50]. The diseases and alterations that should be highlighted due to the similarities include dilated hypertrophic cardiomyopathies (mainly apical) and restrictive (including Fabry's disease); hypertensive heart disease; endomyocardial fibrosis; aberrant tendon cords; thrombi; fibroma; obliterating process; intramyocardial hematoma; cardiac metastasis; intramyocardial abscess; and changes caused by hematological disorders [50-54].
In children, it is important to differentiate LVNC from pulmonary valve atresia with intact interventricular septum as well as diseases that obstruct left ventricular outflow [13]. Cardiac tumors that stimulate blood vessel proliferation, such as hemangiomas, may have the appearance of trabeculations characteristic of LVNC and must, therefore, be distinguished from LNVC [54].
7. MANAGEMENT
Since there is no specific therapy for LVNC, treatment is based on clinical presentation. The main complications of LVNC are thromboembolic events, arrhythmias, and progressive HF. Treatment is thus centered on the prevention of such complications.
The optimal management to prevent thromboembolic events has yet to be fully elucidated. Some studies recommend the prophylactic use of oral anticoagulants such as warfarin for all patients diagnosed with LVNC [10, 21]. However, the use of anticoagulants is currently recommended only for patients with reduced systolic function, an ejection fraction below 40% and a history of previous thromboembolism or atrial fibrillation [13, 55].
The indication of an implantable cardiac defibrillator (ICD) in patients with LVNC should be based on a number of considerations. ICD implantation for the primary prevention of sudden cardiac death is indicated in patients with LVNC and a left ventricular ejection fraction of up to 35% and functional class II or III HF (New York Heart Association) [46]. Patients with a history of sustained ventricular tachycardia or who recovered from cardiac arrest require ICD implantation for secondary prevention [44]. ICD is also recommended for patients who have an additional risk factor, such as a family history of sudden death, nonsustained ventricular tachycardia observed on 24-h Holter monitoring, or a history of syncope [56]. The risk of arrhythmic events and sudden death may be stratified by the presence of a fragmented QRS complex on electrocardiography in patients with LVNC, thus, also facilitating the identification of the patients most in need of ICD [57]. The treatment of HF in LVNC should be the same as in other etiologies. Thus, to control systolic or diastolic dysfunction, β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, mineralocorticoid and/or diuretic receptor antagonists may be used [58, 59]. If HF does not respond to these drugs, heart transplantation, or a left ventricular assist device (LVAD) should be considered [60-63]. The LVAD has proved to be a successful prolonged treatment, as well as being a bridge to subsequent heart transplantation [63]. All this information is summarized in (Table 2).
8. FAMILY APPROACH
The genetic form of LVNC is the most prevalent one [23]. Diagnosed patients should, therefore, be monitored along with their family members as advocated by the Heart Failure Society of America guidelines on evaluating the genetics of cardiomyopathies, considering the patient’s family history over three generations to determine the inheritance pattern and possibly diagnose the syndrome in family members [64].
First-degree relatives should undergo a screening process that is indicated from childhood or on presentation of early signs or symptoms of LVNC. In this evaluation, the patient’s history should be analyzed with emphasis laid on the search for symptoms of HF, arrhythmias, presyncope and syncope, physical examination, with attention to cardiac and skeletal muscles, electrocardiography examinations, such as 24-h Holter monitoring, exercise tests, 12-lead electrocardiography, the creatine kinase MM fraction and echocardiography. Adults with no indications after screening should be reevaluated every 3 years; children up to 3 years of age should be reevaluated annually [64].
Genetic testing may be performed, preferably for patients with more evidence of uncompressed cardiomyopathy, which may assist in the identification of other family members in the early stages of this disease [64].
9. PROGNOSIS
The prognosis of uncompressed cardiomyopathy depends on factors, such as the symptomatology, presented by the patient and the population studied. Patients with arrhythmic disorders, thromboembolism, and HF have higher morbidity and mortality rates than asymptomatic patients [65]. Probable outcomes for LVNC require further investigation, as there are few studies to date, a significant proportion of which involves samples with only a small number of cases or a short follow-up.
In a study of patients with a mean age of 41 years evaluated at approximately 3 years, asymptomatic patients had no sudden cardiovascular complications, and only 0,86% of the patients studied developed dilated cardiomyopathy during this time, while the rest maintained their previous cardiac dimensions [65]. In contrast, in symptomatic patients at diagnosis, the mortality and heart transplantation rates were around 30%. Among the patients who died, approximately 75% had limited ability or inability to perform physical activity, with a New York Heart Association functional class of III or >III [65, 66].
In a group of 241 adult patients followed for 39 months, the mortality rate was approximately 15%. Sudden death occurred in about half of these patients. HF was the precursor of death in more than 5% of patients, and only one patient died of pulmonary embolism. Among the clinical complications, arrhythmia was the most lethal [67].
Among children with a mean age of 7 years evaluated for up to 4 years, more than 5% underwent heart transplantation and approximately 13% died [68], similar to the findings in adult patients [65, 67]. The finding of arrhythmia associated with normal dimensions and functions occurred in more than 6% of children; in half of the cases, the arrhythmia was ventricular tachycardia, which is an indicator of a high risk of sudden death.
In the prenatal diagnosis, when 106 cases were studied, fewer than 50 survivors were identified aged at least 28 months and 18 were followed up for 2 months, during which one-third of the patients died or underwent heart transplantation [69].
CONCLUSION
The clinical manifestations of LVNC are variable and may range from asymptomatic presentation to severe HF or sudden death. A thorough clinical exploration is required for a correct diagnosis and prognostic assessment.
Although the clinical picture varies, due to several cardiac anomalies that are triggered by or widely associated with LVNC, the diagnosis is difficult, since no examination is considered the gold standard in the investigation of this heart disease. Although there are several criteria based on echocardiography images, they are nonspecific and often inconclusive in the vast majority of patients studied.
Despite advances in the knowledge of LVNC genetics, treatment has barely advanced, owing to pathogenic alterations, and is directed only at the control of the clinical condition.
Fig. (1).
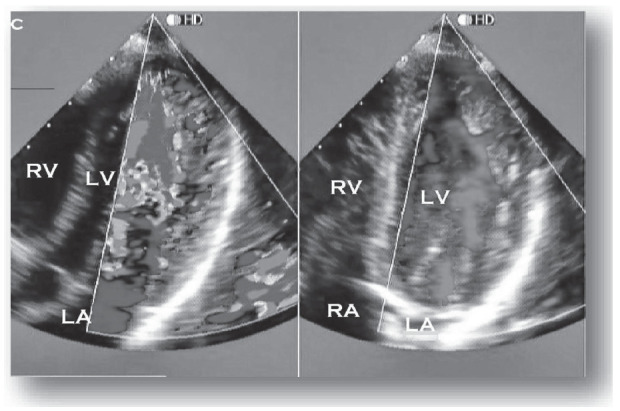
Echocardiographic 4-chamber image from a patient with LVNC. The color Doppler highlights perfusion of intertrabecular recesses from the left ventricle. RV: right ventricle; LV: left ventricle; RA: right atrium; LA: left atrium. Courtesy of Paulo G.Menge M.D. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
LVNC related genes.
| Location | Phenotype | Phenotype MIM Number | Gene/Locus |
Gene/Lócus MIM
Number |
Mode of Inheritance |
|---|---|---|---|---|---|
| 14q11.2 | Left ventricular noncompaction [5] | 613426 | MYH7 | 160760 | Autosomal dominant |
| 11p11.2 | Left ventricular noncompaction [10] | 615396 | MYBPC3 | 600958 | Autosomal dominant |
| 11p11.2 | Left ventricular noncompaction [10] | 615396 | MYBPC3 | 600958 | Autosomal dominant |
| 15q22.2 | Left ventricular noncompaction [9] | 611878 | TPM1 | 191010 | — |
| 15q14 | Left ventricular noncompaction [4] | 613424 | ACTC1 | 102540 | Autosomal dominant |
| 1q32.1 | Left ventricular noncompaction [6] | 601494 | TNNT2 | 191045 | Autosomal dominant |
| Xq28 Barth syndrome 302060 G4.5, TAZ 30039 X-linked | |||||
Abbreviations: ACTC1: actin, alpha, cardiac muscle; DTNA: dystrobrevin alpha; LVNC: left ventricular noncompaction MYBPC3: myosin-binding protein C, cardiac; MYH7: myosin heavy chain 7; TAZ ¼ tafazzin; TNNT2: troponin T2; TPM1: tropomyosin 1.
Table 2.
LVNC clinical management.
| Prophylactic Oral Anticoagulant | Use ICD for Primary Prevention | Use ICD for Secondary Prevention | Use ICD if there is an Additional Risk Factor | Treatment for HF | Heart Transplant or LVAD |
|---|---|---|---|---|---|
| EF <40%; history of ET or AF [13, 55] | EF <35% and NYHA II or III [46] | History of SVT or cardiac arrest [44] | Family history of sudden death; NSVT or history of syncope [56] | Same treatment for other etiologies [58, 59] | If HF is not responsive to drugs [60, 61, 62] |
Acknowledgements
This research was partially supported by the University of Pernambuco. We thank our colleagues from the university who provided insight and expertise that greatly assisted the research.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Dong X., Fan P., Tian T., et al. Recent advancements in the molecular genetics of left ventricular noncompaction cardiomyopathy. Clin. Chim. Acta. 2017;465(167):40–44. doi: 10.1016/j.cca.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Stämpfli S.F., Erhart L., Hagenbuch N., et al. Prognostic power of NT-proBNP in left ventricular non-compaction cardiomyopathy. Int. J. Cardiol. 2017;236:321–327. doi: 10.1016/j.ijcard.2017.02.064. [DOI] [PubMed] [Google Scholar]
- 3.Hotta V.T., Tendolo S.C., Rodrigues A.C.T., Fernandes F., Nastari L., Mady C. Limitations in the diagnosis of noncompaction cardiomyopathy by echocardiography. Arq. Bras. Cardiol. 2017;109(5):483–488. doi: 10.5935/abc.20170152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Towbin J.A., Lorts A., Jefferies J.L. Left ventricular non-compaction cardiomyopathy. Lancet. 2015;386(9995):813–825. doi: 10.1016/S0140-6736(14)61282-4. [DOI] [PubMed] [Google Scholar]
- 5.Stacey R.B., Caine A.J., Jr, Hundley W.G. Evaluation and management of left ventricular noncompaction cardiomyopathy. Curr. Heart Fail. Rep. 2015;12(1):61–67. doi: 10.1007/s11897-014-0237-1. [DOI] [PubMed] [Google Scholar]
- 6.Arbustini E., Weidemann F., Hall J.L. Left ventricular noncompaction: A distinct cardiomyopathy or a trait shared by different cardiac diseases? J. Am. Coll. Cardiol. 2014;64(17):1840–1850. doi: 10.1016/j.jacc.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doghmi N., Boulaamayl S., Kheyi J., Sabry M., Raissouni M., Asfalou I., et al. Left ventricular noncompaction—A rare form of cardiomyopathy: Revelation modes and predictors of mortality in adults through 23 cases. J. Saudi Heart Assoc. 2016;29(2):102–109. doi: 10.1016/j.jsha.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekheit S., Karam B., Daneshvar F., et al. Sudden cardiac death in isolated right ventricular hypertrabeculation/noncompaction cardiomyopathy. Ann. Noninvasive Electrocardiol. 2018;23(4):e12487. doi: 10.1111/anec.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muser D., Liang J.J., Witschey W.R., et al. Ventricular arrhythmias associated with left ventricular noncompaction: Electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2017;14(2):166–175. doi: 10.1016/j.hrthm.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann P.A., Rojas J.R., Jenni R., Oechslin E.N., Attenhofer Jost C.H. Long-term followup of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2002;36(2):493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 11.Nugent A.W., Daubeney P.E., Chondros P., et al. National Australian Childhood Cardiomyopathy Study. The epidemiology of childhood cardiomyopathy in Australia. N. Engl. J. Med. 2003;348(17):1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 12.Andrews R.E., Fenton M.J., Ridout D.A., Burch M. British Congenital Cardiac Association. New-onset heart failure due to heart muscle disease in childhood: A prospective study in the United Kingdom and Ireland. Circulation. 2008;117(1):79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- 13.Murphy R.T., Thaman R., Blanes J.G., et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur. Heart J. 2005;26(2):187–192. doi: 10.1093/eurheartj/ehi025. [DOI] [PubMed] [Google Scholar]
- 14.Kawel N., Nacif M., Arai A.E., et al. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2012;5(3):357–366. doi: 10.1161/CIRCIMAGING.111.971713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lachhab A., Doghmi N., Elfakir Y., et al. Insights from magnetic resonance imaging of left ventricular non-compaction in adults of North African descent. Int. Arch. Med. 2012;5(1):10. doi: 10.1186/1755-7682-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaye N.D., Ngaïdé A.A., Bah M.B., Babaka K., Mbaye A., Abdoul K. Non-compaction of left ventricular myocardium in sub-Saharan African adults. Heart Asia. 2017;9(2):e010884. doi: 10.1136/heartasia-2017-010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohli S.K., Pantazis A.A., Shah J.S., et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur. Heart J. 2008;29(1):89–95. doi: 10.1093/eurheartj/ehm481. [DOI] [PubMed] [Google Scholar]
- 18.Gati S., Chandra N., Bennett R.L., et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart. 2013;99(6):401–408. doi: 10.1136/heartjnl-2012-303418. [DOI] [PubMed] [Google Scholar]
- 19.Chin T.K., Perloff J.K., Williams R.G., Jue K., Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507–513. doi: 10.1161/01.CIR.82.2.507. [DOI] [PubMed] [Google Scholar]
- 20.Stöllberger C., Finsterer J., Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am. J. Cardiol. 2002;90(8):899–902. doi: 10.1016/S0002-9149(02)02723-6. [DOI] [PubMed] [Google Scholar]
- 21.Ritter M., Oechslin E., Sütsch G., Attenhofer C., Schneider J., Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin. Proc. 1997;72(1):26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- 22.Salerno J.C., Chun T.U., Rutledge J.C. Sinus bradycardia, Wolff Parkinson White, and left ventricular noncompaction: An embryologic connection? Pediatr. Cardiol. 2008;29(3):679–682. doi: 10.1007/s00246-007-9043-9. [DOI] [PubMed] [Google Scholar]
- 23.Hoedemaekers Y.M., Caliskan K., Michels M., et al. The importance of genetic counseling, DNA diagnostics, and cardiologic family screening in left ventricular noncompaction cardiomyopathy. Circ Cardiovasc Genet. 2010;3(3):232–239. doi: 10.1161/CIRCGENETICS.109.903898. [DOI] [PubMed] [Google Scholar]
- 24.Zaragoza M.V., Arbustini E., Narula J. Noncompaction of the left ventricle: Primary cardiomyopathy with an elusive genetic etiology. Curr. Opin. Pediatr. 2007;19(6):619–627. doi: 10.1097/MOP.0b013e3282f1ecbc. [DOI] [PubMed] [Google Scholar]
- 25.Probst S., Oechslin E., Schuler P., et al. Sarcomere gene mutations in isolated left ventricular noncompaction cardiomyopathy do not predict clinical phenotype. Circ Cardiovasc Genet. 2011;4(4):367–374. doi: 10.1161/CIRCGENETICS.110.959270. [DOI] [PubMed] [Google Scholar]
- 26.Ichida F., Tsubata S., Bowles K.R., et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation. 2001;103(9):1256–1263. doi: 10.1161/01.CIR.103.9.1256. [DOI] [PubMed] [Google Scholar]
- 27.Hoedemaekers Y.M., Caliskan K., Majoor-Krakauer D., et al. Cardiac β-myosin heavy chain defects in two families with non-compaction cardiomyopathy: Linking non-compaction to hypertrophic, restrictive, and dilated cardiomyopathies. Eur. Heart J. 2007;28(22):2732–2737. doi: 10.1093/eurheartj/ehm429. [DOI] [PubMed] [Google Scholar]
- 28.Vatta M., Mohapatra B., Jimenez S., et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J. Am. Coll. Cardiol. 2003;42(11):2014–2027. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Hermida-Prieto M., Monserrat L., Castro-Beiras A., et al. Familial dilated cardiomyopathy and isolated left ventricular noncompaction associated with lamin A/C gene mutations. Am. J. Cardiol. 2004;94(1):50–54. doi: 10.1016/j.amjcard.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Bleyl S.B., Mumford B.R., Thompson V., et al. Neonatal, lethal noncompaction of the left ventricular myocardium is allelic with Barth syndrome. Am. J. Hum. Genet. 1997;61(4):868–872. doi: 10.1086/514879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing Y., Ichida F., Matsuoka T., et al. Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol. Genet. Metab. 2006;88(1):71–77. doi: 10.1016/j.ymgme.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Millat G., Janin A., de Tauriac O., Roux A., Dauphin C. HCN4 mutation as a molecular explanation on patients with bradycardia and non-compaction cardiomyopathy. Eur. J. Med. Genet. 2015;58(9):439–442. doi: 10.1016/j.ejmg.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig A., Zong X., Stieber J., Hullin R., Hofmann F., Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 1999;18(9):2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann F., Biel M., Kaupp U.B. International Union of Pharmacology. LI. Nomenclature and structure-function relationships of cyclic nucleotide-regulated channels. Pharmacol. Rev. 2005;57(4):455–462. doi: 10.1124/pr.57.4.8. [DOI] [PubMed] [Google Scholar]
- 35.Laish-Farkash A., Glikson M., Brass D., et al. A novel mutation in the HCN4 gene causes symptomatic sinus bradycardia in Moroccan Jews. J. Cardiovasc. Electrophysiol. 2010;21(12):1365–1372. doi: 10.1111/j.1540-8167.2010.01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama R., Kinoshita K., Hata Y., et al. A mutant HCN4 channel in a family with bradycardia, left bundle branch block, and left ventricular noncompaction. Heart Vessels. 2018;33(7):802–819. doi: 10.1007/s00380-018-1116-6. [DOI] [PubMed] [Google Scholar]
- 37.Meinke P., Nguyen T.D., Wehnert M.S. The LINC complex and human disease. Biochem. Soc. Trans. 2011;39(6):1693–1697. doi: 10.1042/BST20110658. [DOI] [PubMed] [Google Scholar]
- 38.Yuan J., Xue B. Role of structural flexibility in the evolution of emerin. J. Theor. Biol. 2015;385:102–111. doi: 10.1016/j.jtbi.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 39.McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University 2014 http://omim.org/
- 40.Jenni R., Oechslin E., Schneider J., Attenhofer Jost C., Kaufmann P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86(6):666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frischknecht B.S., Attenhofer Jost C.H., Oechslin E.N., et al. Validation of noncompaction criteria in dilated cardiomyopathy, and valvular and hypertensive heart disease. J. Am. Soc. Echocardiogr. 2005;18(8):865–872. doi: 10.1016/j.echo.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Paterick T.E., Umland M.M., Jan M.F., et al. Left ventricular noncompaction: a 25-year odyssey. J. Am. Soc. Echocardiogr. 2012;25(4):363–375. doi: 10.1016/j.echo.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Thuny F., Jacquier A., Jop B., et al. Assessment of left ventricular non-compaction in adults: Side-by-side comparison of cardiac magnetic resonance imaging with echocardiography. Arch. Cardiovasc. Dis. 2010;103(3):150–159. doi: 10.1016/j.acvd.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Petersen S.E., Selvanayagam J.B., Wiesmann F., et al. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005;46(1):101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 45.Daimon Y., Watanabe S., Takeda S., Hijikata Y., Komuro I. Two-layered appearance of noncompaction of the ventricular myocardium on magnetic resonance imaging. Circ. J. 2002;66(6):619–621. doi: 10.1253/circj.66.619. [DOI] [PubMed] [Google Scholar]
- 46.Elliott P.M. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Russ J Cardiol. 2015;121(5):7–57. doi: 10.15829/1560-4071-2015-5-7-57. [DOI] [Google Scholar]
- 47.Jacquier A., Thuny F., Jop B., et al. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010;31(9):1098–1104. doi: 10.1093/eurheartj/ehp595. [DOI] [PubMed] [Google Scholar]
- 48.Sidhu M.S., Uthamalingam S., Ahmed W., et al. Defining left ventricular noncompaction using cardiac computed tomography. J. Thorac. Imaging. 2014;29(1):60. doi: 10.1097/RTI.0b013e31828e9b3d. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed15&NEWS=N [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Pavia P., de la Pompa J.L. Left ventricular noncompaction. J. Am. Coll. Cardiol. 2014;64(19):1981–1983. doi: 10.1016/j.jacc.2014.08.034. https://linkinghub.elsevier.com/retrieve/pii/S073510971406152X [DOI] [PubMed] [Google Scholar]
- 50.Azevedo O., Gaspar P., Sá Miranda C., Cunha D., Medeiros R., Lourenço A. Left ventricular noncompaction in a patient with fabry disease: Overdiagnosis, morphological manifestation of fabry disease or two unrelated rare conditions in the same patient? Cardiology. 2011;119(3):155–159. doi: 10.1159/000330924. [DOI] [PubMed] [Google Scholar]
- 51.Stöllberger C., Wegner C., Finsterer J. CHADS2- and CHA2DS2VASc scores and embolic risk in left ventricular hypertrabeculation/noncompaction. J. Stroke Cerebrovasc. Dis. 2013;22(6):709–712. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Stöllberger C., Finsterer J. Pitfalls in the diagnosis of left ventricular hypertrabeculation/non-compaction. Postgrad. Med. J. 2006;82(972):679–683. doi: 10.1136/pgmj.2006.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alhabshan F., Smallhorn J.F., Golding F., Musewe N., Freedom R.M., Yoo S.J. Extent of myocardial non-compaction: comparison between MRI and echocardiographic evaluation. Pediatr. Radiol. 2005;35(11):1147–1151. doi: 10.1007/s00247-005-1551-2. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Pavia P., de la Pompa J.L. Left ventricular noncompaction: A genetic cardiomyopathy looking for diagnostic criteria. J. Am. Coll. Cardiol. 2014;64(19):1981–1983. doi: 10.1016/j.jacc.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 55.Bocchi E.A., Braga F.G.M., Ferreira S.M.A., et al. Sociedade Brasileira de Cardiologia. III Diretriz brasileira de insuficiência cardíaca crônica. Arq. Bras. Cardiol. 2009;93(1) Suppl. 1:1–71. doi: 10.1590/S0066-782X2012001000001. [DOI] [PubMed] [Google Scholar]
- 56.Caliskan K., Szili-Torok T., Theuns D.A.M.J., et al. Indications and outcome of implantable cardioverter-defibrillators for primary and secondary prophylaxis in patients with noncompaction cardiomyopathy. J. Cardiovasc. Electrophysiol. 2011;22(8):898–904. doi: 10.1111/j.1540-8167.2011.02015.x. [DOI] [PubMed] [Google Scholar]
- 57.Cetin M.S., Ozcan Cetin E.H., Canpolat U., et al. Usefulness of fragmented qrs complex to predict arrhythmic events and cardiovascular mortality in patients with noncompaction cardiomyopathy. Am. J. Cardiol. 2016;117(9):1516–1523. doi: 10.1016/j.amjcard.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 58.Weiford B.C., Subbarao V.D., Mulhern K.M. Noncompaction of the ventricular myocardium. Circulation. 2004;109(24):2965–2971. doi: 10.1161/01.CIR.0000132478.60674.D0. [DOI] [PubMed] [Google Scholar]
- 59.Goud A., Padmanabhan S. A rare form of cardiomyopathy: Left ventricular non-compaction cardiomyopathy. J. Community Hosp. Intern. Med. Perspect. 2016;6(1):29888. doi: 10.3402/jchimp.v6.29888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finsterer J., Stöllberger C., Towbin J.A. Left ventricular noncompaction cardiomyopathy: Cardiac, neuromuscular, and genetic factors. Nat. Rev. Cardiol. 2017;14(4):224–237. doi: 10.1038/nrcardio.2016.207. [DOI] [PubMed] [Google Scholar]
- 61.Uribarri A., Rojas S.V., Avsar M., et al. First series of mechanical circulatory support in non-compaction cardiomyopathy: Is LVAD implantation a safe alternative? Int. J. Cardiol. 2015;197:128–132. doi: 10.1016/j.ijcard.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 62.Cerar A, Ksela J, Poglajen G, Vrtovec B, Knezevic I. LVAD as a bridge to heart transplantation in a patient with left ventricular noncompaction cardiomyopathy and advanced heart failure. 2016. [DOI] [PubMed]
- 63.Hashemi H., Raza F.S., Harmon D.M., Alias T., Felius J., Sherwood M.J. Usefulness of a left ventricular assist device in patients with left ventricular noncompaction. Proc. Bayl. Univ. Med. Cent. 2018;31(1):61–63. doi: 10.1080/08998280.2017.1401342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hershberger R.E., Lindenfeld J., Mestroni L., Seidman C.E., Taylor M.R.G., Towbin J.A. Heart Failure Society of America. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J. Card. Fail. 2009;15(2):83–97. doi: 10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Greutmann M., Mah M.L., Silversides C.K., et al. Predictors of adverse outcome in adolescents and adults with isolated left ventricular noncompaction. Am. J. Cardiol. 2012;109(2):276–281. doi: 10.1016/j.amjcard.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 66.Elliott P.M. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur. Heart J. 2015;121(5):7–57. [Google Scholar]
- 67.Bhatia N.L., Tajik A.J., Wilansky S., Steidley D.E., Mookadam F. Isolated noncompaction of the left ventricular myocardium in adults: A systematic overview. J. Card. Fail. 2011;17(9):771–778. doi: 10.1016/j.cardfail.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Brescia S.T., Rossano J.W., Pignatelli R., et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation. 2013;127(22):2202–2208. doi: 10.1161/CIRCULATIONAHA.113.002511. [DOI] [PubMed] [Google Scholar]
- 69.Stöllberger C., Wegner C., Benatar A., et al. Postnatal outcome of fetal left ventricular hypertrabeculation/noncompaction. Pediatr. Cardiol. 2016;37(5):919–924. doi: 10.1007/s00246-016-1369-8. [DOI] [PubMed] [Google Scholar]


