Abstract
This study evaluated the efficacy and safety of tadalafil in pediatric patients with pulmonary arterial hypertension. This phase-3, international, randomized, multicenter (24 weeks double-blind placebo-controlled period; two-year, open-labeled extension period), add-on (patient’s current endothelin receptor antagonist therapy) study included pediatric patients aged <18 years with pulmonary arterial hypertension. Patients received tadalafil 20 mg or 40 mg based on their weight (heavy-weight: ≥40 kg; middle-weight: ≥25 to <40 kg) or placebo orally once daily for 24 weeks. Primary endpoint was change from baseline in six-minute walk distance in patients aged ≥6 years at Week 24. Sample size was amended from 134 to ≥34 patients, due to serious recruitment challenges. Therefore, statistical significance testing was not performed between treatment groups. Results showed that patient demographics and baseline characteristics (N = 35; tadalafil = 17; placebo = 18) were comparable between treatment groups; median age was 14.2 years (6.2–17.9 years) and majority (71.4%, n = 25) of patients were in the heavy-weight cohort. Least square mean (standard error) changes from baseline in six-minute walk distance at Week 24 was numerically greater with tadalafil versus placebo (60.48 (20.41) vs 36.60 (20.78) meters; placebo-adjusted mean difference (standard deviation) 23.88 (29.11)). Safety of tadalafil treatment was as expected without any new safety concerns. During study Period 1, two patients (one in each group) discontinued due to investigator’s reported clinical worsening, and no deaths were reported. In conclusion, the statistical significance testing was not performed between the treatment groups due to low sample size; however, the study results show positive trend in improvement in non-invasive measurements, commonly utilized by clinicians to evaluate the disease status for children with pulmonary arterial hypertension. Safety of tadalafil treatment was as expected without any new safety signals.
Keywords: pulmonary arterial hypertension, hypertension, pulmonary, pulmonary arterial hypertension, biomarkers, risk factors
Introduction
Pulmonary arterial hypertension (PAH) is a rare, chronic, and progressive disease characterized by elevated pulmonary artery pressure and pulmonary vascular resistance (PVR), leading to right heart failure and death. 1 , 2 PAH does occur in children with a similar, if not worse, prognosis without treatment compared with PAH in adult. The prognosis of children with PAH has improved over time due to new therapies and off-label use of adult PAH-specific therapies being administered to pediatric patients. 3
Tadalafil 40 mg once daily (QD) is a potent and selective phosphodiesterase-5 (PDE5) inhibitor approved for the treatment of PAH in adults. 4 In pediatric patients with PAH, tadalafil has been shown to be well-tolerated. 5 In a post-marketing surveillance study, Yamazaki et al. (2017) 6 demonstrated tadalafil has an acceptable safety profile in the pediatric PAH population. Furthermore, Small et al. (2019) 7 recently also reported tadalafil 40 mg QD for patients ≥40 kg, and 20 mg QD for patients <40 kg and ≥2 years old have an acceptable safety profile in pediatric patients with PAH consistent with the safety profile in adult patients with PAH. 4
There is limited data, particularly from phase 3 clinical studies, on efficacy and safety of tadalafil in pediatric patients with PAH. Therefore, the current study was conducted to evaluate the efficacy and safety of tadalafil, administered orally QD in pediatric patients with PAH. This is an ongoing study, and herein we present the results of only the double-blind placebo-controlled period (study Period 1; 24 weeks).
Methods
Study design and patient population
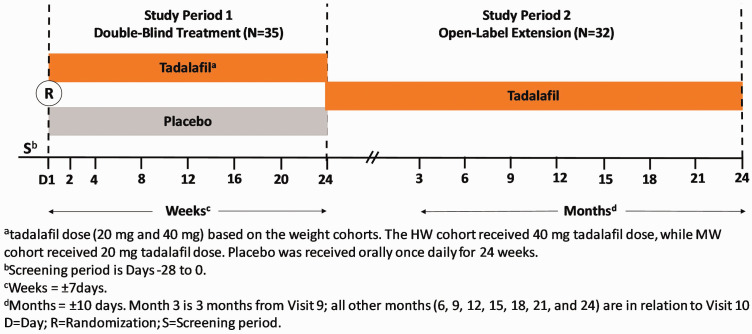
This is a phase-3, international, randomized, multicenter, two-period (Period 1: 24 weeks double-blind placebo-controlled period; and Period 2: 2-year, open-labeled extension period), add-on (i.e. in addition to the patient’s current endothelin receptor antagonist (ERA)) study (ClinicalTrials.gov Identifier: NCT01824290) (Fig. 1). Pediatric patients aged ≥6 months to <18 years with PAH (idiopathic/hereditary, related to connective tissue disease, anorexigen use, or associated with surgical repair of at least six-month duration of congenital systemic to pulmonary shunt), who had established PAH by a resting mean pulmonary artery pressure ≥25 mm Hg, pulmonary artery wedge pressure ≤15 mm Hg, and PVR ≥3 Wood units via right-heart catheterization were planned in the study. Patients enrolled had a World Health Organization (WHO) functional class value of II or III and had been receiving an ERA (such as bosentan or ambrisentan) and were on a maintenance dose with no change in dose (other than weight-based adjustments) for at least 12 weeks prior to the study. Patients with clinically significant (pulmonary artery occlusion pressure 15–18 mm Hg) aortic or mitral valve disease (i.e. aortic stenosis, aortic insufficiency, mitral stenosis, moderate or greater mitral regurgitation), unrepaired congenital heart disease, severe hepatic impairment, Child-Pugh Grade C, those who had severe hypotension or uncontrolled hypertension as determined by the investigator, and those diagnosed with Down syndrome were excluded from the study.
Fig. 1.
Study design.
MW: middle-weight; HW: heavy-weight.
Study treatment
Patients randomly (1:1) received tadalafil 20 mg or 40 mg based on their weight (heavy-weight (HW): ≥40 kg; middle-weight (MW): ≥25 to <40 kg) or placebo orally QD for 24 weeks in Period 1. The HW cohort received a 40 mg tadalafil dose, while MW cohort received a 20 mg tadalafil dose. The selected dose for each weight cohort was intended to reflect exposures comparable to the approved 40 mg dose of tadalafil in adults, and was confirmed from another tadalafil pharmacokinetic and safety study. 7 In the current study, due to recruiting challenges and study modifications, there were no patients enrolled in light-weight (LW) cohort (<25 kg).
Study assessments
The primary endpoint of the study was change from baseline in six-minute walk distance (6MWD) (meters; m) in patients aged ≥6 years at Week 24 (end of Period 1). Additionally, a supportive (sensitivity) analysis using a Bayesian mixed effects model for repeated measures (MMRM) model that leverages data from the tadalafil adult PAH study was performed to increase precision in confirming the 6MWD efficacy endpoint from this study. Secondary endpoints through Week 24 were the changes in echocardiography (tricuspid annular plane systolic excursion (TAPSE), eccentricity index (EI), pericardial effusion, maximal tricuspid regurgitant velocity), changes in N-terminal prohormone brain natriuretic peptide (NT-Pro-BNP) concentrations, Clinical Global Impression of Improvement (CGI-I), and Child Health Questionnaire Parent Form 28 (CHQ-PF28) in patients ≥5 years old and the incidence of clinical worsening (CW) and time to CW.
Patients meeting any of the following major criteria were considered to have met the definition of CW: all-cause mortality, lung or heart lung transplantation, atrial septostomy or Potts shunt, hospitalization for PAH progression, or worsening of PAH (new-onset syncope, addition of new PAH-specific concomitant therapy). Those incidences from Period 1 were adjudicated by an independent, blinded, study-specific Clinical Endpoint Committee.
Safety was evaluated in all patients who received at least one dose of study medication and safety evaluations included the recording of reported treatment-emergent adverse events (TEAEs), vital signs, and laboratory tests.
Statistical analysis
The study was initially designed to enroll 134 patients. However, due to serious challenges with patient recruitment, the study sample size was amended to recruit at least 34 patients. Moreover, the initial primary endpoint of time to CW was also changed to 6MWD. The study statistical analysis plan was amended accordingly for 6MWD and multiple analyses were switched to more descriptive versions (along with confidence intervals (CIs)) based on this smaller sample size.
As the current study had a limited number of subjects enrolled, an inspection of the numerical mean change in corresponding CIs was used instead of formal significance testing to decipher whether values were trending in a positive direction.
Due to the small sample size in each cohort, results are presented between treatment groups (tadalafil vs placebo) and not by weight cohort. The 6MWD analysis was performed in 6MWD population which was defined, as per the study protocol/statistical analysis plan, as patients ≥6 to <18 years of age who had taken at least one dose of study medication and could perform a 6MWD test. The change in 6MWD from baseline through 24 weeks was analyzed using a restricted maximum likelihood-based MMRM. Factors in the model included visit, baseline 6MWD, and treatment group. A treatment-by-visit interaction term was also included. ERA therapy and PAH etiology were not included in the model since neither had at least three patients in the respective subgroup per treatment arm.
The supportive (sensitivity) analysis for a Bayesian MMRM model which leverages data from the adult PAH study 4 was conducted to increase precision in confirming efficacy. The Bayesian MMRM model included the same factors as the primary analysis. The model used a mixture prior approach with a pre-specified weight of 0.8 on the adult prior distribution.
The secondary efficacy endpoint of time to first occurrence of CW was analyzed using a Cox proportional hazard model. Changes in echocardiography parameters, NT-Pro BNP, and CHQ-PF28 from baseline to endpoint were analyzed using an analysis of covariance model. Proportion of patients in each of the seven response categories of CGI-I were summarized by treatment group. Descriptive summary statistics for continuous measurements included the number of nonmissing observations, mean, standard deviation (SD) or standard error (SE), median, and minimum and maximum values by each treatment group for Period 1.
Results
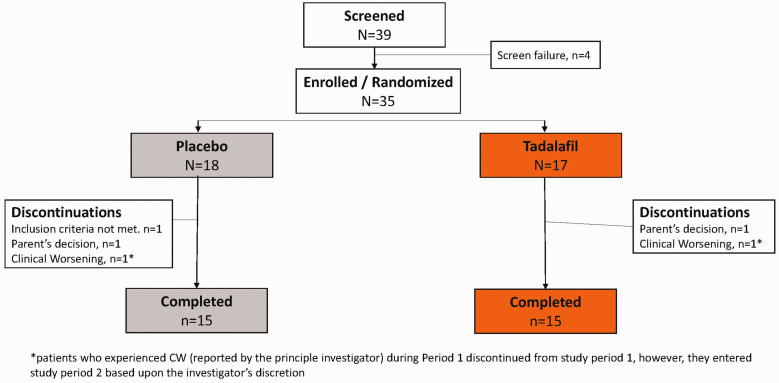
A total of 35 pediatric patients with PAH were enrolled in this study (tadalafil = 17; placebo = 18), of which 30 patients (15 in each group) completed Period 1. Five patients discontinued during Period 1, and the reasons for study discontinuation were parent’s decision (n = 2), inclusion criteria not met (n = 1), and CW reported by the principle investigator (n = 2) (Fig. 2). Per study protocol, patients who experienced CW (reported by the principle investigator) during Period 1 had to be discontinued from study Period 1; however, they entered study Period 2 based upon the investigator’s discretion. In total, 32 patients entered study Period 2. At the time of data cut-off (18 March 2019), a total of 20 patients had completed study Period 2 (10 patients in each group) and 8 patients were continuing the tadalafil treatment.
Fig. 2.
Patient disposition (Period 1 only).
Overall, the patient demographics and baseline characteristics were comparable between the treatment groups. At baseline, the median age of the study population was 14.2 years (6.2–17.9 years). The majority of patients were in the HW cohort (71.4%, n = 25) and 28.6% (n = 10) were in the MW cohort (Table 1), while none were enrolled in the LW cohort.
Table 1.
Patient demographics and baseline characteristics.
| Placebo(N = 18) | Tadalafil(N = 17) | Total(N = 35) | |
|---|---|---|---|
| Age (years)a | |||
| Mean (SD) | 12.8 (3.4) | 14.1 (3.5) | 13.5 (3.5) |
| Median (min–max) | 13.4 (7.3–17.7) | 15.8 (6.2–17.9) | 14.2 (6.2–17.9) |
| Weight category,b n (%) | |||
| Heavy-weight | 12 (66.7) | 13 (76.5) | 25 (71.4) |
| Middle-weight | 6 (33.3) | 4 (23.5) | 10 (28.6) |
| Gender, n (%) | |||
| Male | 9 (50.0) | 7 (41.2) | 16 (45.7) |
| Female | 9 (50.0) | 10 (58.8) | 19 (54.3) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 7 (38.9) | 8 (47.1) | 15 (42.9) |
| Not Hispanic or Latino | 6 (33.3) | 4 (23.5) | 10 (28.6) |
| Not reported | 5 (27.8) | 5 (29.4) | 10 (28.6) |
| Race, n (%) | |||
| American Indian or Alaska Native | 1 (5.6) | 3 (17.6) | 4 (11.4) |
| Asian | 1 (5.6) | 1 (5.9) | 2 (5.7) |
| Black or African American | 2 (11.1) | 1 (5.9) | 3 (8.6) |
| White | 14 (77.8) | 12 (70.6) | 26 (74.3) |
| Region, n (%) | |||
| America | 10 (55.6) | 10 (58.8) | 20 (57.1) |
| Asia | 3 (16.7) | 5 (29.4) | 8 (22.9) |
| Europe | 5 (27.8) | 2 (11.8) | 7 (20.0) |
| Height (cm), mean (SD) | 152.2 (16.8) | 153.8 (15.7) | 153.0 (16.1) |
| Weight (Kg), mean (SD) | 50.50 (15.9) | 53.27 (15.8) | 51.85 (15.7) |
| BMI (kg/m2), mean (SD) | 21.33 (4.4) | 22.00 (3.7) | 21.66 (4.0) |
| Supine systolic blood pressure (mm Hg), mean (SD) | 108.3 (12.9) | 105.6 (15.9) | 107.0 (14.3) |
| PAH etiology | |||
| Idiopathic | 11 (61.1) | 15 (88.2) | 26 (74.3) |
| Associated to surgical repair, of at least six-month duration of a congenital systemic to pulmonary shunt | 7 (38.9) | 2 (11.8) | 9 (25.7) |
| PAH duration, years, mean (SD) | 2.65 (3.2) | 4.64 (5.5) | 3.61 (4.5) |
| WHO functional class | |||
| Class II | 14 (77.8) | 14 (82.4) | 28 (80.0) |
| Class III | 4 (22.2) | 3 (17.6) | 7 (20.0) |
| 6MWD, mean (SD) | 476.7 (105.1) | 485.8 (160.2) | 481.1 (132.8) |
| CGI-S, n (%) | 18 | 16 | 34 |
| Normal, not at all ill | 3 (16.7) | 1 (6.3) | 4 (11.8) |
| Mildly ill | 7 (38.9) | 8 (50.0) | 15 (44.1) |
| Moderately ill | 8 (44.4) | 7 (43.8) | 15 (44.1) |
| Concomitant ERA therapy, N | 17 | 17 | 34 |
| Bosentan | 16 (94.1) | 16 (94.1) | 32 (94.1) |
| Macitentan | 1 (5.9) | 1 (5.9) | 2 (5.9) |
| Duration of use concomitant ERA therapy (days) mean (SD) | |||
| Bosentan | 310.2 (352.3) | 649.8 (687.9) | 480.0 (564.6) |
| Macitentan | 103.0 (0.0) | 103.0 (0.0) | 103.0 (0.0) |
aAge is calculated based on the birth date and informed consent date.
bMiddle-weight: ≥25 kg to <40 kg; heavy-weight: ≥40 kg.
6MWD: six minute walk distance; BMI: body mass index; CGI-S: clinical global impression of severity; ERA: endothelin receptor antagonist; PAH: pulmonary arterial hypertension; SD: standard deviation; WHO: World Health Organization.
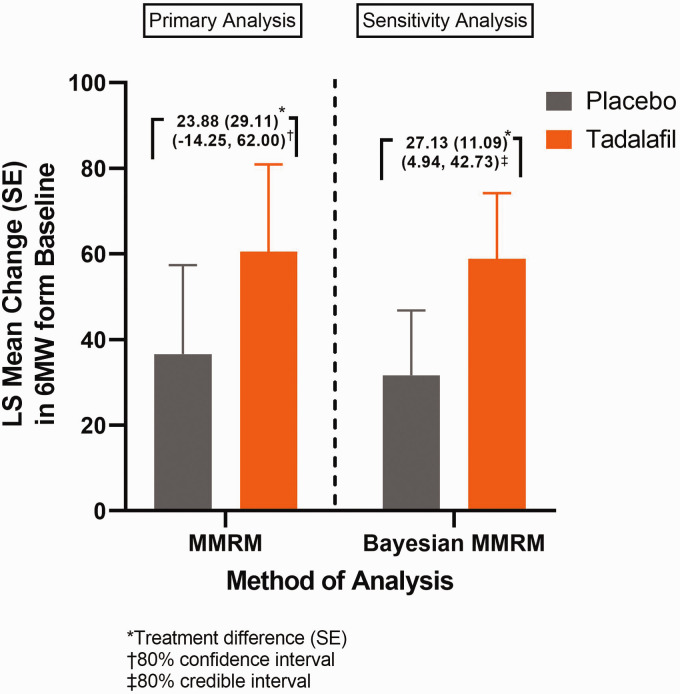
Of the 35 patients enrolled in current study, 33 patients (placebo n = 16; tadalafil n = 17) were included in the primary analysis. Two enrolled patients who were ≥6 years of age were excluded from the primary analysis due to either unmet study entry criteria or lack of post baseline 6MWD data. The least square (LS) mean (SE) changes from baseline in 6MWD at Week 24 was numerically greater with tadalafil versus placebo (60.48 (20.41) m vs 36.60 (20.78) m; placebo-adjusted mean difference (SD) 23.88 (29.11)) (Fig. 3). The posterior of mean (SD) changes from baseline in 6MWD (extrapolation data; sensitivity analysis, n = 33) at Week 24 was numerically greater with tadalafil versus placebo (58.80 (15.35) m vs 31.67 (15.14) m; placebo-adjusted mean difference (SD) 27.13 (11.09)) (Fig. 3). During Period 1 (through Week 24), two incidences of potential CW (one in each group) were reported by investigators, however, they were not confirmed by the Clinical Endpoint Committee. One CW case from placebo group was reported as worsening of PAH due to a WHO functional class increase and a 6MWD decrease, but the site did not perform a confirmative 6 MWD test in 5–10 days as described in the Protocol and another CW case from the tadalafil group was reported as new-onset syncope, but this event was revised later as presyncope, which did not meet CW definition per the study protocol. Therefore, there were no confirmed CW events during study Period 1. From baseline to Week 24, 60.0% of patients on tadalafil had no change in WHO functional class, 40.0% improved, and 0.0% worsened. Similarly, of those on placebo, 80.0% of patients had no change in WHO functional class, 20.0% improved, and 0.0% worsened. At Week 24, the LS mean (SE) change in NT-Pro-BNP concentrations were 68.26 (49.41) for placebo and –59.16 (59.64) for tadalafil (Fig. 4); the placebo-adjusted LS mean (SE) difference (95% CI) was –127.4 (56.70), (–247.05, –7.80)). The LS mean (SE) changes from baseline to Week 24 for each echocardiography parameter between tadalafil and placebo are shown in Table 2.
Fig. 3.
Least squares means (SE) change in 6MWD from baseline to Week 24 (Period 1).
LS: least square; SE: standard error; MMRM: mixed effects model for repeated measures.
Fig. 4.
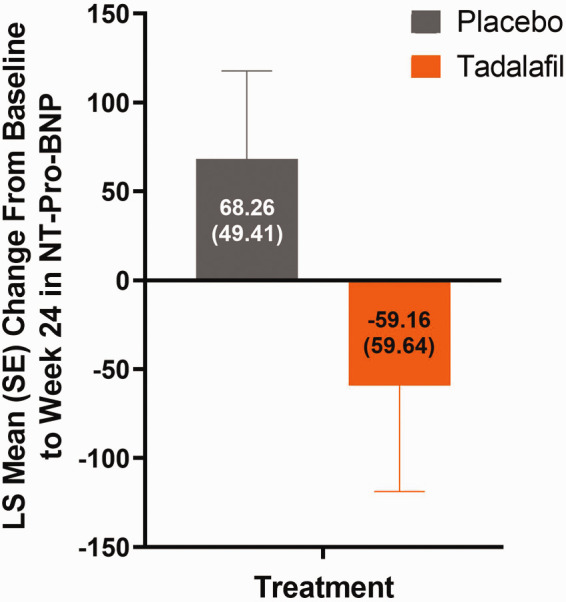
Least Squares means (SE) change in NT-Pro-BNP from baseline to Week 24 (Period 1).
LS: least square; SE: standard error; NT-Pro-BNP: N-terminal prohormone brain natriuretic peptide.
Table 2.
Least squares means change secondary endpoints from baseline to Week 24 (Period 1).
| PlaceboLS mean (SE) | TadalafilLS mean (SE) | Placebo-adjusted differencesLS mean (SE); 95% CI | |
|---|---|---|---|
| Echocardiography: | |||
| TAPSE | –0.10 (0.111) | 0.33 (0.130) | 0.43 (0.136); 0.14 to 0.71 |
| Left ventricular EI-systolic | 0.11 (0.194) | –0.29 (0.218) | –0.40 (0.225); –0.87 to 0.07 |
| Left ventricular EI-diastolic | 0.08 (0.106) | –0.08 (0.122) | –0.17 (0.124); –0.43 to 0.09 |
| TRVmax | 17.01 (28.472) | 1.68 (31.066) | –15.33 (29.443); –78.48 to 47.82 |
| Pericardial effusion | Two patients in Period 1 | 0 patient in Period 1 | |
| WHO functional class | 20% improved | 40% improved | NA |
| CGI-I (over symptoms) | 46.7% improved | 64.3% improved | NA |
| CHQ-PF28 (summary score) | |||
| Physical | 9.28 (3.78) | 8.34 (4.52) | –0.94 (4.26); –9.75 to 7.86 |
| Psychosocial | 2.56 (1.06) | 0.86 (1.28) | –1.70 (1.19); –4.16 to 0.76 |
CGI-I: Clinical Global Impression of Improvement; CHQ-PF28: Child Health Questionnaire Parent Form 28; CI: confidence interval; EI: eccentricity index; NA: not applicable; SE: standard error; TAPSE: tricuspid annular plane systolic excursion; TRVmax: maximal tricuspid regurgitant velocity; WHO: World Health Organization; LS: least square.
At Week 24, the overall CGI-I was numerically greater with tadalafil versus placebo (64.3% vs 46.7%). The LS mean changes (SE) from baseline to Week 24 for all subtest and summary score on physical and psychological dimensions in CHQ-PF28 were similar between treatment groups (Table 2). During Period 1, tadalafil doses for each weight cohort were fixed (40 mg for HW and 20 mg for MW). The mean number of days of drug exposure was 158.4 (39.49) in the placebo group and 170.4 (16.00) in the tadalafil group. The mean cumulative number of doses taken during Period 1 was 153.4 for placebo and 151.0 for tadalafil.
In Period 1, 23 patients reported ≥1 TEAE (placebo, n = 8; tadalafil, n = 15) and treatment-related adverse events (AEs) were reported in nine patients (placebo, n = 1; tadalafil, n = 8). The most commonly reported TEAEs (occurring in ≥2 patients in tadalafil-treated subjects) were headache (29.4%, tadalafil; 11.1%, placebo), upper respiratory tract infection (17.6%, tadalafil; 5.6%, placebo), influenza (17.6%, tadalafil; 0.0%, placebo), arthralgia (11.8%, tadalafil; 5.6%, placebo), and epistaxis (11.8%, tadalafil; 5.6%, placebo). Two patients (one in each treatment group) discontinued the study due to CW based on investigator’s report. There were no serious adverse events (SAEs) or deaths reported in Period 1. There were two AEs of special interest that occurred in tadalafil-treated subjects: one event of spontaneous intermittent penile erection and one event of uterine bleeding.
Discussion
The present study evaluated the efficacy and safety of tadalafil in pediatric patients with PAH who were on a maintenance dose of an ERA drug treatment (bosentan or macitentan) prior to study screening and during double-blinded period. The study was initially designed to enroll 134 patients; however, there were serious challenges in recruiting study patients due to multiple forums, such as disease rarity and complexity, widespread off-label use of PAH therapies and aggressive use of combination treatments, concerns to randomize to placebo during study (parents/investigators), and multiple competing clinical trials. At the end of five years, the study enrolment was completed with 35 patients, none below 6 years old; therefore, no patient was randomized to the LW cohort. Given the small sample size, no statistical comparisons were to be made between the treatment groups.
The primary analysis evaluated the numerical mean change (with corresponding CIs) in 6MWD instead of statistical significance to determine if the data were trending in the direction for improving the 6MWD. The LS mean change from baseline in 6MWD to Week 24 was numerically higher with tadalafil than placebo (60.48 vs 36.60 m), with a placebo-adjusted LS mean treatment difference of 23.88 m (80% CI: –14.25 to 62.00). The trend of positive treatment difference at Week 24 demonstrated by the primary analysis was consistent with the Bayesian MMRM using a pre-specified weight of 0.8. The model resulted in the posterior mean difference of 27.13 m and 80% credible interval (4.94–42.73) which supports the positive trend suggested by the primary analysis and indicates tadalafil treatment improves the 6MWD compared to placebo at Week 24 in this pediatric patient population.
There was also a positive trend of potential efficacy in the majority of the measurements from this study, such as NT-Pro-BNP, WHO functional class for Period 1, echocardiographic parameters (such as TAPSE, left ventricular EI-systolic, left ventricular EI-diastolic), and CGI-I. All subtest domains and summary scores on physical and psychological dimensions in CHQ-PF28 were similar between tadalafil and placebo treatment group. Two patients (one in each treatment group) reported potential CWs by investigators; however, neither case was confirmed by the Clinical Endpoint Committee.
Various therapies have been used to treat PAH in children. 8 Most of these are based on prior studies in adults and evidence-based trials in adults.9–11 In the Tracking-Outcomes-and-Practice-in-Pediatric-Pulmonary-Hypertension registry, the most common initial therapy in PAH was a PDE5 inhibitor. 12 Similar studies have suggested this class of therapy is commonly used initially or in combination with an ERA. 13 Recent pharmacokinetic studies have shown adult exposure levels can be obtained in children and tadalafil levels are lower in combination with bosentan, but remain in the expected range. 7
The overall safety profile of tadalafil, including most common TEAEs, observed in the current study was consistent with previous knowledge. The overall incidence of AEs was higher in the tadalafil group compared with placebo. All TEAEs in Period 1 (24 weeks of treatment) were mild or moderate in severity. The TEAEs considered by the investigator to be related to study medication were reported in eight subjects in the tadalafil group during Period 1. Headache was the most commonly reported TEAE considered by the investigator to be related to the study medication. There were no clinically meaningful trends or changes from baseline in laboratory parameters or vital signs, there were no SAEs, deaths, or discontinuations due to AEs during Period 1.
One limitation of the current study was small sample size due to significant challenges in the study recruitment. Primary and other additional efficacy analyses showed a positive trend supporting the efficacy of tadalafil versus placebo in the study population; however, due to the small sample size of 35 participants in Period 1, no clear conclusions can be derived from this analysis.
This study was primarily descriptive in a small number of children with PAH. Although statistical significance testing was not performed between the two treatment groups due to the low sample size, the results show a positive trend in improvement in non-invasive measurements commonly utilized by clinicians to evaluate the disease status for children with PAH. Analyses of the efficacy in the different subgroups of PAH could not be conducted, and there was no subject in the LW cohort. Tadalafil had an acceptable safety profile in this pediatric population with PAH. The overall safety profile of the study population was generally consistent with the known safety profile of tadalafil and events associated with the underlying disease state. No new safety signals were identified in the pediatric PAH population.
Acknowledgements
The authors would like to thank Kalyan Pulipaka, an employee of Eli Lilly India Services Pvt. Ltd. for providing writing support.
Contributorship: Conception and design and drafting: S.B. and B.L.; acquisition of data: D.I., D.B., R.B., and G.M.B.M.; analysis and interpretation of data and critical revision: D.I., D.B., R.B., S.B., B.L., and G.M.B.M.
Data sharing statement: Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available on request six months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Ethical approval information: Ethics and Ethics committee approval: This study was conducted in accordance with consensus ethics principles derived from international ethics guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines, the International Council for Harmonisation (ICH) Good Clinical Practices (GCP) Guideline (E6), and applicable laws and regulations. The study protocol and informed consent form were approved by an Institutional Review Board at each site. The parent/legal representative signed the informed consent form, and the patient signed the assent document (if applicable) prior to initiation of any study-related procedures. Names and locations of the approving ethics committee are given below.
Conflict of interest: The conflicts of interest of all authors on the manuscript are as follows. Simin Baygani and Baohui Li are employees of Eli Lilly and Company and own Lilly stocks. The University of Colorado receives fees for Dunbar Ivy to be a consultant and to perform research for Actelion-Janssen, Bayer, GSK, Lilly, and United Therapeutics. Gisela M.B. Meyer has nothing to disclose. Damien Bonnet is a Steering Committee member for Eli Lilly and company, and steering committee and advisory board member for Actelion Pharmaceuticals, Bayer, and Novartis. The University Medical Center Groningen contracts with Eli Lilly and Company, Actelion, and GSK for advisory board or steering committee activities of Rolf Berger.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Eli Lilly and Company.
ORCID iD: Baohui Li https://orcid.org/0000-0002-6451-1825
Ethics Review Board's Name and Address
1. EK der Medizinischen Universität Wien Borschkegasse 8B/E06, Wien, Wien, 1090, Austria.
2. CEP do Instituto Dante Pazzanese de CardiologiaAv. Dante Pazzanese, 500, Ibirapuera, Sao Paulo, Sao Paulo, 04012-900, Brazil.
3. CEP Irmandade de Santa Casa de Miser de Porto Alegre/ISCMPA Rua Prof. Annes Dias, 295 - 6o.Andar - Hospital Dom Vicente Scherer, Porto Alegre, RS, 90020-090, Brazil.
4. Comite de Etica em Pesquisa Complexo Hospitalar Rua Arnobio Marques, 310, Pavilhao Ovidio Montenegro, 1° andar,Santo Amaro, Recife, PE, 50100-130, Brazil.
5. “CPP”“Ile-de-France II” 149 rue de Sèvres Carré Necker Porte N2 1er étage Centre universitaire, Paris Cedex 15, 75743, France.
6. Ethik-Kommission des FB Medizin d. Justus-Liebig-Universität Frankfurter Straße 51-53 Poststelle des Klinikums, Gießen, Hessen, 35392, Germany.
7. Schneider Medical Center, Institute of Diabetes and Endocrinology, 14 Kaplan Street, Petah Tiqva, 4920235, Israel.
8. Sheba Medical Center Tel Hashomer, Tel Hashomer, Ramat Gan, 5265601, Israel.
9. National Center For Child Health And Development, 2-10-1 Ohkura, Setagaya-ku, Tokyo, 157-8535, Japan.
10. Instituto Nacional de Cardiologia Ignacio Chavez Juan Badiano No.1, Col. Seccion XVI, Mexico, DF, 14080, Mexico.
11. Komisja Bioetyczna przy “Pomnik-Centrum Zdrowia Dziecka” Al. Dzieci Polskich 20, Warszawa, 04-736, Poland.
12. Hacettepe University Faculty of Medicine Sihhiye, Ankara, 6100, Turkey.
References
- 1.Rich S. Clinical insights into the pathogenesis of primary pulmonary hypertension. Chest 1998; 114: 237S–241S. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ. Evaluation and treatment for angina in pulmonary arterial hypertension. Am J Med 2004; 116: 427–428. [DOI] [PubMed] [Google Scholar]
- 3.Vorhies EE, Ivy DD. Drug treatment of pulmonary hypertension in children. Paediatr Drugs 2014; 16: 43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 5.Takatsuki S, Calderbank M, Ivy DD. Initial experience with tadalafil in pediatric pulmonary arterial hypertension. Pediatr Cardiol 2012; 33: 683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki H, Kobayashi N, Taketsuna M, et al. Safety and effectiveness of tadalafil in pediatric patients with pulmonary arterial hypertension: a sub-group analysis based on Japan post-marketing surveillance. Curr Med Res Opin 2017; 33: 2241–2249. [DOI] [PubMed] [Google Scholar]
- 7.Small D, Ferguson-Sells L, Dahdah N, et al. Pharmacokinetics and safety of tadalafil in a paediatric population with pulmonary arterial hypertension: a multiple ascending-dose study. Br J Clin Pharmacol 2019; 85: 2302–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 2019; 53: 1801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 10.Galie N, Manes A, Negro L, et al. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J 2009; 30: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai Y, Sun L, Hu S, et al. Combination therapy in pulmonary arterial hypertension: a meta-analysis. Cardiology 2011; 120: 157–165. [DOI] [PubMed] [Google Scholar]
- 12.Humpl T, Berger RMF, Austin ED, et al. Treatment initiation in paediatric pulmonary hypertension: insights from a multinational registry. Cardiol Young 2017; 27: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JL, Nees SN, Valencia GA, et al. Sildenafil use in children with pulmonary hypertension. J Pediatr 2019; 205: 29–34.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]