Introduction
Ibrutinib is a highly effective therapeutic agent with multiple hematologic indications, most notably chronic lymphocytic leukemia (CLL). It achieves therapeutic effects through irreversible inhibition of Bruton’s tyrosine kinase (BTK). Many patients have achieved durable progression-free and overall survival since its regulatory approval. While ibrutinib carries a favorable toxicity profile compared with prior generations of therapy, cardiac toxicities have emerged as a major clinical consideration.1, 2, 3, 4, 5
We previously reported on ibrutinib’s association with atrial fibrillation (AF) and, more recently, the incidence of ventricular arrhythmia.1,5 Occurring in 6%–16% of patients receiving ibrutinib, AF accounts for a modest degree of cardiac morbidity in this population and warrants long-term monitoring. Furthermore, a 2019 review of the World Health Organization’s (WHO) VigiBase demonstrated that ibrutinib-associated cardiovascular adverse drug reactions are more frequent in real-world practice than initially reported in clinical trial data. Specifically, the reporting odds ratio for cardiac conduction disorders (CD) in ibrutinib was 3.5 (95% confidence interval: 2.7–4.6),4 the first report of that association, to our knowledge.
Here we summarize 18 cases of high-grade heart block in individuals receiving ibrutinib. This includes 3 patients at our institution enrolled to institutional review board–approved data collection protocols and 15 unique events identified in the FDA Federal Adverse Event Reporting System (FAERS) database. We queried the database using the following 6 descriptions, reported between 2014 and 2020: cardiac arrest, AV block 2nd degree, AV block complete, conduction disorder, cardiac pacemaker insertion, and sudden cardiac death. We selected only cases with reported temporal association between ibrutinib and high-grade conduction disorder. We excluded several cases with absent clinical data. This is the first series to focus exclusively on ibrutinib-associated high-grade heart block and to discuss this conduction disorder as a rare, serious, and potentially fatal complication of ibrutinib therapy.
Case reports
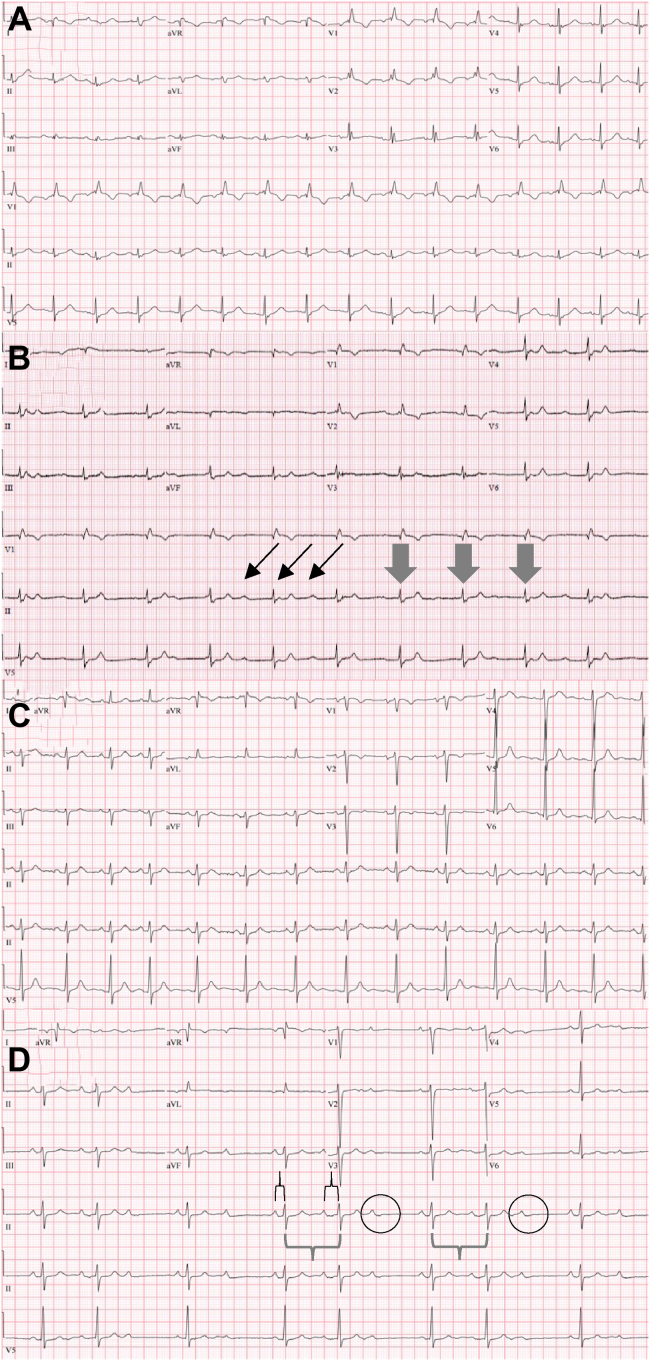
The first patient was a 71-year-old woman with CLL with trisomy 12 in 82% of nuclei identified by fluorescence in situ hybridization. She had a known right bundle branch block, chronic obstructive pulmonary disease, and hypertension. A prior echocardiogram revealed a hyperdynamic left ventricle, normal systolic cardiac function, and low-grade diastolic dysfunction. She was hospitalized 3 months after starting frontline ibrutinib with lumbar back pain. A baseline electrocardiogram (ECG) is shown in Figure 1A with chronic right bundle branch block. Ibrutinib was initially continued. During the course, on day 90 of ibrutinib therapy, she was observed somnolent with profound hypotension and bradycardia to 34 beats per minute (bpm). She received atropine, and an ECG captured complete heart block, shown in Figure 1B. The patient ultimately underwent emergent semi-permanent pacemaker placement. Ibrutinib was permanently discontinued, in part owing to patient preference. Her CLL progressed, but she declined further treatment.
Figure 1.
Representative electrocardiogram (ECG) tracings of ibrutinib-associated conduction disorders. A: Patient 1: Pre-ibrutinib, showing right bundle branch block. B: Patient 1: complete heart block after ibrutinib. Nonconducting P waves marked with thin black arrows. Ventricular rate of approximately 60 beats/min shown with thick gray arrows. C: Patient 3: Pre-ibrutinib ECG suggestive of left ventricular hypertrophy. D: Patient 3: symptomatic Mobitz second-degree type 1 AV block with intermittent 2:1 AV block while on ibrutinib (increasing PR interval in brackets above lead II, unchanged QRS in brackets below lead II, and nonconducted P waves circled) before progression to complete heart block.
The second patient was an 83-year-old man with no known cardiac history. He had a long-standing low-grade lymphoproliferative disorder diagnosed 22 years prior. This was complicated by autoimmune hemolytic anemia that became refractory to rituximab. With progressive anemia he was started on single-agent ibrutinib. He tolerated monotherapy well but experienced multiple presyncopal episodes for which he did not seek medical care. Nearly 4 years after starting ibrutinib, on day 1400, he suffered a syncopal episode while exercising. His evaluation revealed intermittent bradycardia with ventricular rates 20–30 bpm. Telemetry captured multiple episodes of transient complete heart block. He underwent emergent pacemaker placement. An echocardiogram during placement showed intact systolic function, no wall motion abnormalities, and grade 1 diastolic dysfunction. He safely restarted ibrutinib within 2 weeks. Surveillance interrogation of his pacemaker was unrevealing for clinically significant arrhythmia or CD.
The last patient was a 90-year-old woman with relapsed CLL and also with trisomy 12 by fluorescence in situ hybridization. She started ibrutinib monotherapy shortly after its regulatory approval when she was in need of a viable treatment option. An echocardiogram prior to ibrutinib therapy demonstrated hyperdynamic left ventricular wall motion, left ventricular ejection fraction 75%–80%, and left atrium measuring 41 mm. On day 137 of therapy, she acutely developed palpitations and was evaluated. Telemetry showed sustained bradycardia of 38–46 bpm, and an ECG revealed new-onset 2:1 Mobitz I atrioventricular (AV) block, shown in Figure 1D (baseline 1C). Overnight telemetry uncovered several episodes of complete heart block. She underwent successful dual-chamber pacemaker placement. Repeat echocardiogram was unchanged. She restarted ibrutinib within 2 weeks after this episode, with follow-up pacemaker interrogation devoid of cardiac events.
FAERS database
We identified 15 additional cases in patients receiving ibrutinib therapy between 2014 and 2020 reported to the FAERS database. Patient and disease characteristics are described in Table 1. This query identified 5 cases of complete heart block; 9 cases of second-degree block, including multiple with Mobitz type II and high risk of progression to complete block; and 1 sinoatrial block complicated by Adams-Stokes syndrome. Although limited in clinical scope, the 15 database entries included 4 with ischemic heart disease and 2 with cardiomyopathy. Only 2 patients were taking concomitant AV nodal blocking agents prior to the incident event. The median time to onset was 11 months (range: 1–70 months) and median patient age was 79 years (range: 61–93 years). Interestingly, 1 patient was being treated with combination ibrutinib and venetoclax at the time of event.
Table 1.
FAERS heart block cases
| Number (n) | Percent (%) | |
|---|---|---|
| Sex (n = 15 cases reporting data) | ||
| Female | 7 | 47 |
| Male | 8 | 53 |
| Disease (n = 14) | ||
| CLL | 12 | 86 |
| WM | 2 | 14 |
| Conduction disorder (n = 15) | ||
| Third-degree block | 5 | 33 |
| Second-degree (Mobitz II) | 9 | 60 |
| SA block | 1 | 7 |
| Event within 1 year (n = 15) | ||
| <12 months | 11 | 73 |
| >12 months | 4 | 27 |
| Pacemaker (n = 13) | ||
| Yes | 13 | 100 |
| Restart ibrutinib (n = 13) | ||
| Yes | 8 | 62 |
| No | 5 | 38 |
Patient characteristics for 15 FAERS database cases reported between 2014 and 2020, including 5 with complete heart block. All reporting cases underwent pacemaker placement and 62% of evaluable patients restarted ibrutinib. One case did not provide ibrutinib indication and 2 each did not report pacemaker status or ibrutinib resumption.
CLL = chronic lymphocytic leukemia; SA = sinoatrial; WM = Waldenstrom macroglobulinemia.
Discussion
High-grade heart block is a rare but potentially fatal adverse event associated with ibrutinib therapy. The exact prevalence remains unclear. Each of the 17 cases reporting cardiac event outcomes described here (1 case omitted this information) underwent emergent pacemaker placement and the majority safely resumed ibrutinib. The median time to onset of high-grade AV block was 11 months (n = 18; range: 1–70 months), compared with 1 month reported in a time-to-onset analysis of the VigiBase data.4,6 Many of our cases may have other contributing factors; for example, 1 case patient had a history of nonrevascularized cardiac ischemia, and coronary artery disease was reported in 4 entries from the database query, although admittedly the breadth of clinical detail is suboptimal. Furthermore, this elderly population is certainly at risk for independent onset of advanced conduction disease. However, it is important to note that 14 of the reported cases developed high-grade AV block within 13 months of starting ibrutinib, and the VigiBase analysis showed an increased relative risk compared to patients receiving other drugs, reporting odds ratio 3.5 with 50 incident cases.4
The mechanism of ibrutinib-mediated high-grade CD is not well understood. An in vitro study of atrial and ventricular human pluripotent stem cell–derived cardiomyocytes (CMs) demonstrated ibrutinib’s potential for direct cardiac toxicity.7 This was characterized by 2 key electrophysiologic findings associated with acquired AF: dose-dependent shortening of the action potential duration at 80% repolarization and concurrent increase of the calcium transient duration in atrial, but not ventricular, CMs. The study showed decreased cell viability of both atrial and ventricular CMs treated with ibrutinib compared with acalabrutinib, where viability remained unchanged. Separate work on cultured CMs implicated late sodium current as a tyrosine kinase inhibitor–mediated arrhythmogenic mechanism, including with chronic ibrutinib exposure.8
Growing evidence suggests that cardiac toxicities may be off-target effects. A recent chemoproteomic analysis identified C-terminal src kinase (CSK) inhibition as highly associated with increased incidence of AF. In the experimental murine model, Csk knockout led to increased AF, fibrosis, and inflammation.9 Another proposed mechanism of ibrutinib-associated AF is through downregulation of the phosphoinositide 3-kinase (PI3K)-Akt pathway. This is achieved by concurrent inhibition of BTK and TEC protein tyrosine kinases. Mouse studies with inactivated PI3K showed increased incidence of AF, depressed cardiac function, and increased cardiac fibrosis.10,11 A separate study proposed increased atrial fibrosis through development of reactive oxygen species and abnormal CM sarcoplasmic reticulum calcium release.12
It remains unclear if the mechanism that mediates ibrutinib-associated atrial arrhythmogenesis is responsible for ventricular arrhythmia and conduction disorders. These models report increased cardiac fibrosis. Tissue fibrosis and sclerosis are often present in conduction disorders, too. Ibrutinib treatment has been shown to reduce NOTCH1 activation, most pronounced at 12 months of continuous therapy.13 In the setting of cardiac ischemia, NOTCH1-activated bone marrow–derived mesenchymal stem cells promote CM survival through an antifibrotic mechanism.14 Although this hypothesis is predicated on myocardial injury, subclinical ischemia is possible in a cohort with a median age over 75.
Ibrutinib has carved a distinct role in the management of patients with hematologic malignancies. In CLL, fixed-duration combination regimens have been reported, with encouraging results.15 Next-generation BTK inhibitors are reported to carry less cardiac risk.7 These alternative strategies may reduce cardiotoxicity. Close follow-up with consideration of serial biomarkers and ECGs should be pursued for patients with pre-existing cardiac conditions including hypertension, atrial or ventricular arrhythmia, conduction disorder, or coronary ischemia. Further study may aid in developing predictive models for improved foresight into these potentially fatal adverse effects.
Conclusion
Ibrutinib-associated conduction disorders have been reported with increased frequency over the past 2 years. We report the first series of high-grade heart block, including 3 cases at our institution and 15 additional cases reported to the FAERS database. Although next-generation BTK inhibitors have been associated with decreased cardiac toxicity in development, ibrutinib continues to have a significant role in the management of multiple hematologic malignancies. Fixed-duration regimens may reduce systemic toxicity, but clinicians should maintain vigilance when treating at-risk patients with ibrutinib.
Key Teaching Points.
-
•
Ibrutinib is an irreversible inhibitor of Bruton’s tyrosine kinase and is highly effective in the management of multiple hematologic malignancies. Atrial fibrillation was the earliest and remains the most commonly reported cardiac adverse event, but recent evidence shows increased incidence of ventricular arrhythmia and conduction disorders.
-
•
The exact mechanism remains unclear, but growing evidence suggests off-target effects are implicated, with C-terminal src kinase recently suggested as a target leading to atrial fibrillation. We discuss several in vitro studies of ibrutinib’s effect on cardiomyocytes as well as multiple additional kinase pathways inhibited by ibrutinib.
-
•
In our series, 14 of 18 cases of high-degree heart block occurred within 13 months of ibrutinib initiation. All reporting cases required pacemaker placement and the majority safely resumed ibrutinib with good effect.
Acknowledgments
The authors thank the FDA Office of Regulatory Policy staff for fulfilling our query of the FAERS, providing complementary data that support the proposed toxicity association.
Each of the local patients discussed has provided written, informed consent for participation in DF/HCC IRB-approved protocol 99-224 (NCT04028531) and/or 01-206 permitting longitudinal abstraction of clinical data while being managed for a known lymphoproliferative disorder.
Footnotes
Conflicts of Interest: ARV, BLL, EDJ, and EPA have no competing financial interests. JJM has served on advisory boards for Bristol Myers Squibb, Takeda, Audentes, Deciphera, Janssen, ImmunoCore, Boston Biomedical, Amgen, Myovant, Cytokinetics, and AstraZeneca and is supported by NIH grants (R01HL141466, R01HL155990, R01HL156021). JRB has served as a consultant for Abbvie, Acerta, Astra-Zeneca, Beigene, Catapult, Dynamo Therapeutics, Eli Lilly, Juno/Celgene/Bristol Myers Squibb, Kite, MEI Pharma, Nextcea, Novartis, Octapharma, Pfizer, Rigel, Sunesis, TG Therapeutics, and Verastem; received research funding from Gilead, Loxo, Sun, TG Therapeutics, and Verastem; served on data safety monitoring committees for Invectys; and is supported by NIH R01 CA 213442.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Brown J.R., Moslehi J., O’Brien S. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796–1805. doi: 10.3324/haematol.2017.171041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd J.C., Brown J.R., O’Brien S. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leong D.P., Caron F., Hillis C. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128:138–140. doi: 10.1182/blood-2016-05-712828. [DOI] [PubMed] [Google Scholar]
- 4.Salem J.-E., Manouchehri A., Bretagne M. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74:1667–1678. doi: 10.1016/j.jacc.2019.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Lampson B.L., Yu L., Glynn R.J. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581–2584. doi: 10.1182/blood-2016-10-742437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratain M.J., Moslehi J.J., Lichter A.S. Ibrutinib’s cardiotoxicity-an opportunity for postmarketing regulation. JAMA Oncol. 2021;7:177–178. doi: 10.1001/jamaoncol.2020.5742. [DOI] [PubMed] [Google Scholar]
- 7.Shafaattalab S., Lin E., Christidi E. Ibrutinib displays atrial-specific toxicity in human stem cell-derived cardiomyocytes. Stem Cell Rep. 2019;12:996–1006. doi: 10.1016/j.stemcr.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang T., Moslehi J.J., Roden D.M. Proarrhythmic effects of ibrutinib, a clinically approved inhibitor of Bruton’s tyrosine kinase (BTK) used in cancer therapy [abstract] Circulation. 2015;132(Suppl 3):A14587. Abstract. [Google Scholar]
- 9.Xiao L., Salem J.E., Clauss S. Ibrutinib-mediated atrial fibrillation attributable to inhibition of CSK. Circulation. 2020;142:2443–2455. doi: 10.1161/CIRCULATIONAHA.120.049210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMullen J.R., Boey E.J.H., Ooi J.Y.Y., Seymour J.F., Keating M.J., Tam C.S. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829–3830. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L., Li L., Ruan Y. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16:1374–1382. doi: 10.1016/j.hrthm.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Yang X., An N., Zhong C. Enhanced cardiomyocyte reactive oxygen species signaling promotes ibrutinib-induced atrial fibrillation. Redox Biol. 2020;30:101432. doi: 10.1016/j.redox.2020.101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Papa B., Baldoni S., Dorillo E. Decreased NOTCH1 activation correlates with response to ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2019;25:7540–7553. doi: 10.1158/1078-0432.CCR-19-1009. [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Hiroi Y., Ngoy S. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011;123:866–876. doi: 10.1161/CIRCULATIONAHA.110.947531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davids M.S., Brander D.M., Kim H.T. Ibrutinib plus fludarabine, cyclophosphamide, and rituximab as initial treatment for younger patients with chronic lymphocytic leukaemia: a single-arm, multicentre, phase 2 trial. Lancet Haematol. 2019;6:e419–e428. doi: 10.1016/S2352-3026(19)30104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]