Introduction
Ebstein anomaly (EA) is a congenital heart defect characterized by the apical displacement and insertion of the septal and posterior tricuspid leaflets inside the right ventricle, which may cause different degrees of tricuspid regurgitation. The anterior leaflet usually is elongated, tethered, and fenestrated, but mobile.1,2 This tricuspid malformation is associated with right atrium and ventricle changes resulting in important electrophysiological modifications, such as prolonged intra-atrial activation, right bundle branch block, and occurrence of multiple accessory pathways.
The symptoms may appear at any age, from intrauterine life until adulthood. Early presentation of EA occurs when there is severe tricuspid regurgitation, in particular when associated with other anomalies, such as anatomical or functional right ventricular (RV) outflow tract obstruction.3 Arrhythmia is a common manifestation in adulthood. The purpose of this case report is to describe an unusual presentation of wide QRS tachycardia in a patient with EA.
Case report
A 20-year-old man with previous diagnosis of asymptomatic EA presented at the emergency department with palpitations and dizziness. Physical examination exhibited skin pallor, cyanosis, diaphoresis, and heart rate of 188 beats per minute. The electrocardiogram showed a wide QRS tachycardia (Supplementary Figure S1A), then the patient was cardioverted.
After the cardioversion, the electrocardiogram showed sinus rhythm and intraventricular delay (Supplementary Figure S1B). The transthoracic echocardiogram revealed EA with severe tricuspid regurgitation and RV dysfunction. There was significant impairment of posterior and septal tricuspid leaflets with “atrialization” of a large portion of the right ventricle.
The coronary computed tomography angiography showed no coronary obstruction. The cardiac magnetic resonance imaging revealed enlarged right atrium, septal tricuspid leaflet apical displacement of 33 mm of mitral valve plane, and elongated and redundant tricuspid valve leaflets leading to severe regurgitation. The right atrium and atrialized part of RV volume was 120 mL. The end-diastolic RV volume was 125 mL, of which 34 mL corresponded to the atrialized portion. The RV ejection fraction was 37%, with no scar detected on late gadolinium enhancement.
The patient was referred to electrophysiology study and catheter ablation. The procedure was performed with the patient under general anesthesia. The AH interval was 54 ms, HV 58 ms, and QRS 106 ms.
The presence of an accessory pathway was improbable on baseline electrophysiology (Supplementary Figure S2), with para-His pacing showing an increase in VA interval with His capture loss (Supplementary Figure S2A) and apical programmed ventricular stimulation (Supplementary Figure S2B) showing an increase in VA interval during retrograde right bundle branch block. There was no evidence of atriofascicular pathway during tricuspid annulus pacing, despite the absence of complete right bundle branch block. There was not dual AV nodal physiology.
The programmed atrial stimulation (Supplementary Figure S3) first induced both narrow QRS tachycardia and right bundle branch block tachycardia, in which V-V interval modification preceded AA modification, ruling out atrial tachycardia as the mechanism of arrhythmia. Additionally, there was no VA activation change during aberrancy recovery, and no atrial timing modification during refractory His pacing (Supplementary Figure S4) also suggested that orthodromic AV reentrant tachycardia was not the mechanism of arrhythmia. After isoproterenol effect reduction, there were some A-A changes preceded by V-V changes, dependent on AH interval change (Supplementary Figure S5), ruling out atrial tachycardia as the mechanism of tachycardia. However, after catheter manipulation on that posterior tricuspid annulus, the tachycardia was interrupted and could not be reinduced despite multiple attempts and isoproterenol infusion, so it was not possible to perform other maneuvers to confirm the exact mechanism of this tachycardia, but we interpreted that AV nodal reentrant tachycardia was the most probable mechanism of arrhythmia.
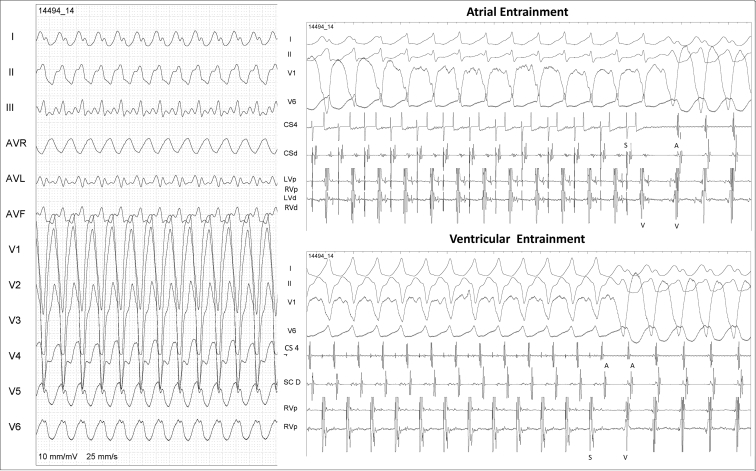
After that, atrial stimulation with isoproterenol infusion was repeated and a second wide QRS tachycardia was induced with 1:1 VA conduction, now presenting left bundle branch block morphology (Supplemental Figure S6 and Figure 1). Ventricular entrainment presented a VAV response and the atrial entrainment presented an AVVA response (Figure 1), and VA dissociation was documented with faster atrial stimulation, confirming ventricular tachycardia (VT) as the mechanism of the second arrhythmia.
Figure 1.
Second wide QRS tachycardia atrial and ventricular pacing maneuvers. Top: Atrial entrainment maneuver showing QRS fusion and an AVVA response; this maneuver confirms ventricular tachycardia as the mechanism of the second tachycardia. Bottom: Apical right ventricular entrainment, showing a VAV response.
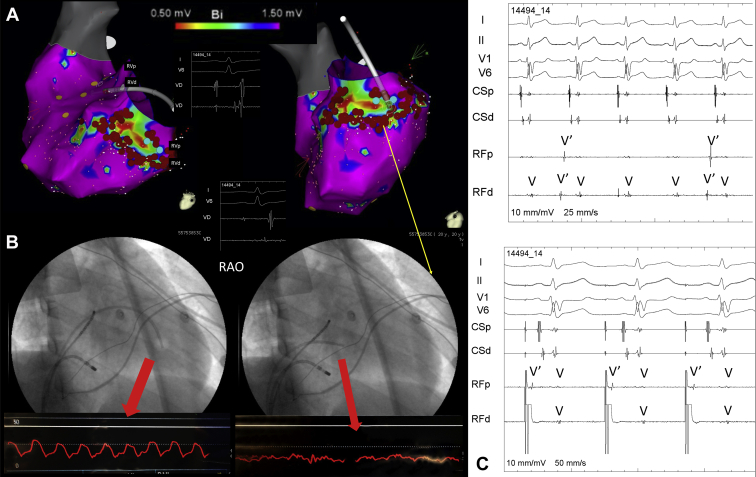
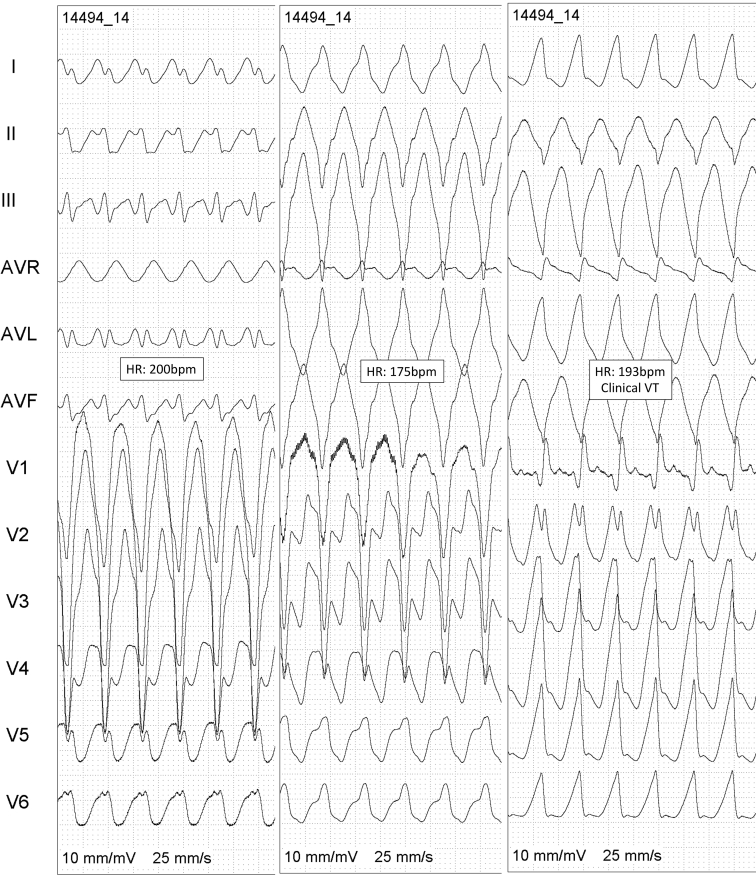
The CARTO voltage map (Figure 2A) demonstrated a posterior-lateral-basal RV scar with abnormal fragmented and late potentials in that part that corresponded to the atrialized part of the ventricle that was confirmed by Swan-Ganz pressure and coronary angiography (Figure 2B). After radiofrequency applications on the border of the scar, there was a dissociation of the potentials inside that area (Figure 2C). During VT ablation a total of 3 different VT morphologies were induced (Figure 3), VT#3 being identical to the clinical VT.
Figure 2.
A: Electroanatomical voltage map of the right ventricle (RV) showing an inferobasal scar with abnormal potentials inside the scar. B: Swan-Ganz catheter positioned on the outflow tract (left) and on the basal portion of the RV (right) showing atrial pressure on that position, confirming that the scar on electroanatomical mapping was related to the atrialized portion of the RV. Ablation was performed in the border of the scar, leading to isolation of the scar confirmed by an entrance block (C, top) and exit block (C, bottom) after pacing maneuvers.
Figure 3.
Three different ventricular tachycardia (VT) morphologies induced during the procedure. After ablation, no VT was induced anymore.
At the end of ablation, no supraventricular or ventricular arrhythmias were induced after atrial and ventricular stimulation, even during isoproterenol infusion.
In a 4-year follow-up, the patient remained with no medication and asymptomatic, without syncope or palpitations.
Discussion
EA occurs in less than 1% of congenital defects, with an incidence of approximately 1–5 per 200,000 births.4 One-half of the patients with this disease have another abnormality, such as interatrial or interventricular communication, patent foramen ovale, RV outflow obstruction, and others.5 EA clinical manifestation is very variable. While some patients are asymptomatic, others may have dyspnea, palpitations, and syncope. Echocardiography remains the gold standard for the diagnosis.3 Even so, magnetic resonance imaging may be useful.
The EA abnormalities, in addition to different degrees of RV dysfunction and a high accessory pathway prevalence, lead to an elevated rate of tachyarrhythmias. The incidence of accessory pathways in EA patients has been estimated at 10%–38%. Atriofascicular fibers can be found in 5% of this population and AV nodal reentry in 8%–13%.2 In a series of 297 patients with EA, 6% presented Wolff-Parkinson-White syndrome, 10% paroxysmal supraventricular tachycardia, and 13% atrial fibrillation, tachycardia, or flutter.6 The incidence of ventricular tachycardia in patients with EA is about 2% and of sudden cardiac death is 3%–6%.7,8 In a series of 964 patients with EA, 24 (2.5%) had an implantable cardioverter-defibrillator, most owing to secondary prevention.9 At the emergency room, owing to hemodynamic instability and indispensable electrical cardioversion, this differentiation is often not possible. In some cases, the posterior electrocardiogram analysis may not be elucidative either.10,11 Therefore, the electrophysiological study is useful in order to explain the arrhythmia pathophysiological mechanism.
However, the differential diagnosis of tachyarrhythmia in this group of patients may be challenging, especially on wide QRS tachycardias, which can be 1 of 3 possibilities: supraventricular tachycardia with aberrant conduction, antidromic atrioventricular reentrant tachycardia, or ventricular tachycardia.12 Some conditions increase the risk of an unsuccessful ablation in EA, like a severely dilated right atrium, which interferes with catheter stabilization; low-voltage and fractioned electrograms due to the atrialized ventricle, complicating tricuspid ring electrogram identification and precise accessory pathway localization; and the presence of multiple accessory pathways, that occurs in up 50% of the patients. Moreover, on individuals with EA, the radiofrequency application carries a higher risk of coronary damage, since the atrialized right ventricle is adjacent to atrioventricular ring. Therefore, proceeding with a right coronary angiography during the mapping makes the ablation safer. The series of patients with congenital heart defects submitted to ventricular tachycardia ablation, especially people with EA, are still limited and have recurrence rates of at least 20%.
On electrophysiological study, atrial and ventricular activation variations, as well as maneuvers during sinus rhythm and tachycardia, may clarify the diagnosis and electrophysiological mechanism of supraventricular arrhythmias. However, for a conclusion, the electrophysiological maneuvers should be consistent and reproducible. In the present case, the spontaneous changes in V-V leading to A-A changes in the first tachycardia ruled out atrial tachycardia. The classical maneuvers of para-Hisian pacing and retrograde right bundle block do not demonstrate the presence of retrograde pathway, so AV nodal reentrant tachycardia was the most probable mechanism of arrhythmia. During the second 1:1 wide complex tachycardia, VT was documented using atrial pacing showing QRS narrowing and an AVVA response.13
After the ablation of the scar and isolation of that portion of the ventricle, the supraventricular tachycardia was also not induced.
Electroanatomical mapping in these individuals has an important value because of the anatomical distortion, in order to raise the procedure’s success. Furthermore, in the present patient, the voltage and activation mapping delimited the right ventricle’s scar, assisting in the ventricular tachycardia circuit identification.
Conclusion
EA patients have a range of modifications that predispose to tachyarrhythmias. Although atrioventricular reentrant tachycardia is the most common arrhythmia, the presence of ventricular dysfunction and scar may lead to ventricular tachycardias. Therefore, the differential diagnosis of wide QRS tachycardias in EA patients is essential, and electrophysiological study is essential to define the mechanism of arrhythmia, it being possible to eliminate the mechanisms of arrhythmia by ablation.
Footnotes
The authors declare that there is no conflict of interest.
Key Teaching Points.
-
•Atrioventricular reentrant tachycardia is the most common arrhythmia in Ebstein anomaly (EA) patients. However, the presence of ventricular dysfunction and scar may lead to ventricular tachycardias.
-
•Preoperative electrophysiological study in EA patients undergoing to tricuspid valve repair is valuable. It has a high diagnostic and therapeutic yield in these patients.
-
•Electrophysiological study is essential for differential diagnosis of wide QRS tachycardias in EA patients.
-
•Electroanatomical mapping in these individuals has an important value because of the anatomical distortion.
Appendix. Supplementary data
References
- 1.Correa-Villasenor A., Ferencz C., Neill C.A., Wilson P.D., Boughman J.A. Ebstein's malformation of the tricuspid valve: genetic and environmental factors. The Baltimore-Washington Infant Study Group. Teratology. 1994;50:137–147. doi: 10.1002/tera.1420500208. [DOI] [PubMed] [Google Scholar]
- 2.Walsh E.P. Ebstein's anomaly of the tricuspid valve: a natural laboratory for re-entrant tachycardias. JACC Clin Electrophysiol. 2018;4:1271–1288. doi: 10.1016/j.jacep.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer D.S., Bull C., Till J.A. Ebstein's anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol. 1994;23:170–176. doi: 10.1016/0735-1097(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 4.Attenhofer Jost C.H., Connolly H.M., Dearani J.A., Edwards W.D., Danielson G.K. Ebstein's anomaly. Circulation. 2007;115:277–285. doi: 10.1161/CIRCULATIONAHA.106.619338. [DOI] [PubMed] [Google Scholar]
- 5.Frescura C., Angelini A., Daliento L., Thiene G. Morphological aspects of Ebstein's anomaly in adults. Thorac Cardiovasc Surg. 2000;48:203–208. doi: 10.1055/s-2000-6893. [DOI] [PubMed] [Google Scholar]
- 6.Hou Y., Fang P.H., Li H.J. Clinical analysis of arrhythmia in 297 Ebstein's anomaly patients. Chin Med J (Engl) 2012;125:3587–3588. [PubMed] [Google Scholar]
- 7.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 8.Baztarrica G.E., Sereno G.G., Villecco S.A., Porcile R. Ventricular tachycardia an atypical presentation of Ebstein's disease. Saudi Med J. 2014;35:1510–1512. [PMC free article] [PubMed] [Google Scholar]
- 9.Attenhofer Jost C.H., Tan N.Y., Hassan A. Sudden death in patients with Ebstein anomaly. Eur Heart J. 2018;39:1970–1977a. doi: 10.1093/eurheartj/ehx794. [DOI] [PubMed] [Google Scholar]
- 10.Mark D.G., Brady W.J., Pines J.M. Preexcitation syndromes: diagnostic consideration in the ED. Am J Emerg Med. 2009;27:878–888. doi: 10.1016/j.ajem.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Rao M.P., Panduranga P., Al-Mukhaini M., Al-Jufaili M. Ebstein anomaly in an adult presenting with wide QRS tachycardia: diagnostic and therapeutic dilemmas. Am J Emerg Med. 2012;30 doi: 10.1016/j.ajem.2011.03.001. 834 e1–4. [DOI] [PubMed] [Google Scholar]
- 12.Iturralde P., Nava S., Salica G. Electrocardiographic characteristics of patients with Ebstein's anomaly before and after ablation of an accessory atrioventricular pathway. J Cardiovasc Electrophysiol. 2006;17:1332–1336. doi: 10.1111/j.1540-8167.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 13.Abdelwahab A., Gardner M.J., Basta M.N., Parkash R., Khan A., Sapp J.L. A technique for the rapid diagnosis of wide complex tachycardia with 1:1 AV relationship in the electrophysiology laboratory. Pacing Clin Electrophysiol. 2009;32:475–483. doi: 10.1111/j.1540-8159.2009.02308.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.