Abstract
Background:
The-Optimal-Lymph-Flow (TOLF) intervention aims to promote lymph flow through therapeutic lymphatic exercises to relieve lymphatic pain, swelling, lymphedema symptoms, and to decrease lymph fluid levels among breast cancer survivors. To enhance the efficacy of the TOLF intervention, an innovative, intelligent, Kinect-enhanced lymphatic exercise intervention (Kinect-TOLF) was developed to teach patients to perform the lymphatic exercises correctly.
Objectives:
This feasibility trial aimed to determine the feasibility, usability, and effects of the Kinect-TOLF on lymphatic pain, swelling, lymphedema symptoms, and lymph fluid levels.
Methods:
A single-arm feasibility trial with a pre- and post-test design was employed to recruit 30 breast cancer survivors with persistent lymphatic pain or swelling. Patients received a single training session to learn how to perform the lymphatic exercises using the Kinect-TOLF program. Descriptive statistics, Wilcoxon signed-rank tests, t-test, Spearman’s rank correlation coefficients, linear regressions, and Cohen’s d were performed for data analysis. Qualitative data were assessed for common themes.
Results:
The Kinect-TOLF was effective in training patients to perform the lymphatic exercises correctly with high user satisfaction. Significant reductions were found in scores of lymphatic pain (MedΔ = −1.00, CI = [−1.5, −0.1], P = .004), arm/hand swelling (MedΔ = −1.00, CI = [−1.5, −0.5], P = .004), total swelling (MedΔ = −1.5, CI = [−2.0, −1.0], P = .003), number of lymphedema symptoms (MΔ = −3.8, CI = [−5.5, −2.1], P < .001), and lymphedema symptom severity (MΔ = −5.3, CI = [−9.5, −1.1], P = .016). A significant reduction in lymph fluid levels was found in mean L-Dex scores (MΔ = −2.68, CI = [−4.67, −0.69], P = .010). Greater decrease in mean L-Dex scores were found in patients with abnormal lymph fluid levels (L-Dex ≥ 7.1) (MΔ = −5.19, CI = [−1.75, −8.63], P = .008). Patients’ qualitative feedback supported the results of the study.
Conclusions:
The Kinect-TOLF is safe, feasible, and effective in reducing lymphatic pain, swelling, lymphedema symptoms, and in decreasing lymph fluid levels. Future research should focus on a randomized clinical trial to confirm the unique or synergistic efficacy of the Kinect-TOLF in comparison with current lymphedema treatment and other forms of exercises or movement therapy. This study was registered in ClinicalTrials.gov with US ClinicalTrials.gov Identifier: NCT03999177.
Keywords: lymphatic, pain, swelling, lymphedema, lymph fluid, breast cancer, symptoms
Introduction
Lymphatic pain, swelling, and lymphedema symptoms are ongoing debilitating adverse effects of cancer treatment that elicit distress and negative impacts on breast cancer survivors’ quality of life (QOL).1-3 Breast cancer survivors have a compromised lymphatic system due to cancer treatment,4-6 resulting in an accumulation of lymph fluid in the ipsilateral body or upper limb.7-9 The accumulation of lymph fluid leads to a chronic and co-occurring variety of pain sensations (i.e., pain/aching/soreness) in the ipsilateral upper limb or body defined as lymphatic pain and other symptoms related to fluid accumulation defined as lymphedema symptoms.7-9 Both swelling and lymphedema are caused by an abnormal accumulation of lymph fluid in the ipsilateral body or upper limb. 7 Swelling often refers to patient-reported symptom. Lymphedema is often defined as an increased limb size or girth comparing affected (or lymphedematous) and unaffected limbs.7-9
While lymphatic pain, swelling, and lymphedema symptoms are most common in patients with a diagnosis of lymphedema, 7 more than 50% of breast cancer survivors without a diagnosis of lymphedema also report daily co-occurring lymphatic pain, swelling, and lymphedema symptoms.7-9 For breast cancer survivors without a diagnosis of lymphedema, the experience of lymphatic pain, swelling, and lymphedema symptoms is a cardinal sign of an early stage of lymphedema because these symptoms often precede changes in limb size or girth and a lymphedema diagnosis.8,9 Breast cancer survivors who report pain on the affected ipsilateral upper limb or body are nearly twice as likely to develop lymphedema. 7 Without timely intervention in this early stage, lymphedema can progress into a chronic condition that no surgical or medical interventions can cure.10,11 Thus, the effective management of lymphatic pain, swelling, and lymphedema symptoms is needed for breast cancer survivors with or without a diagnosis of lymphedema.
Pharmacological interventions (eg, NSAIDs, opioids) and behavioral strategies are commonly used for pain control in cancer patients.11-16 Lymphedema treatments (e.g., manual lymph drainage, physical therapy, compression garments, upper extremity exercise, yoga) are used to control swelling. 11 Research is limited on the efficacy of interventions that target physiological factors (e.g., the compromised lymphatic drainage) to manage lymphatic pain, swelling, and lymphedema symptoms. The-Optimal-Lymph-Flow (TOLF) intervention, a patient-centered and nurse-led self-care program, aims to promote lymph flow through the performance of therapeutic lymphatic exercises and strategies to achieve nutrition-balanced, portion-appropriate diet, adequate hydration, and proper sleep to minimize the risk of lymphedema.17-19 The therapeutic lymphatic exercises include a set of muscle-tightening deep breathing and muscle-tightening pumping and shoulder exercises. Table 1 describes the physiologic rationale for each lymphatic exercise. The efficacy of the TOLF intervention to prevent lymph fluid accumulation was demonstrated in a clinical trial with 140 patients after breast cancer surgery. 17 The efficacy of TOLF for lymphatic pain was demonstrated in a 12-week pilot feasibility clinical trial that used a mobile avatar-based coaching system to deliver the TOLF intervention. 18
Table 1.
Kinect-Enhanced Lymphatic Exercise Intervention (Kinect-TOLF).
| Lymphatic exercises | Physiological rationales |
|---|---|
| Muscle-tightening deep breathing | Whole body lymph fluid has to be drained through the lymphatic ducts above the heart. Muscle-tightening-deep-breathing stimulates lymphatic ducts to promote lymph fluid draining. |
| Lymph fluid drains when muscles move. Muscle-tightening-deep-breathing creates the whole body muscle movements that create muscle milking and pumping action and help drain lymph fluid. | |
| Deep breathing enhances muscle pumping in the upper body by contracting the external intercostal muscles, the accessory muscles of inspiration, and the diaphragm during inspiration and the internal intercostal muscles and abdominal muscles during expiration. | |
| Deep breathing decreases oxidative stress and oxygenates body tissues. It decreases inflammation and promotes wound healing. | |
| Muscle-tightening pumping Over-the-head pumping Horizontal pumping Push-down pumping |
Muscle-tightening pumping exercises create arm muscle milking and pumping, including the main anterior upper arm muscles (biceps brachii, brachialis, coracobrachialis), the posterior muscle of triceps brachii, and deltoid muscle (ie, the anterior deltoid, lateral deltoid, and posterior deltoid). The pumping of these muscles increases lymph fluid flow and decreases the fluid build-up in the arms. |
| Muscle-tightening pumping exercises build the arm muscles that help lymph fluid flow and drain. | |
| Shoulder exercises Shoulder rolls Clasp and spread Reach to the sky |
Improved limb mobility facilitates local muscle movements that
create muscle milking and pumping to promote lymph fluid flow
and drainage. Shoulder exercises create arm muscle milking and pumping by moving the main anterior upper arm muscles (biceps brachii, brachialis, coracobrachialis), the posterior muscle of triceps brachii, and deltoid muscle (ie, the anterior deltoid, lateral deltoid, and posterior deltoid). |
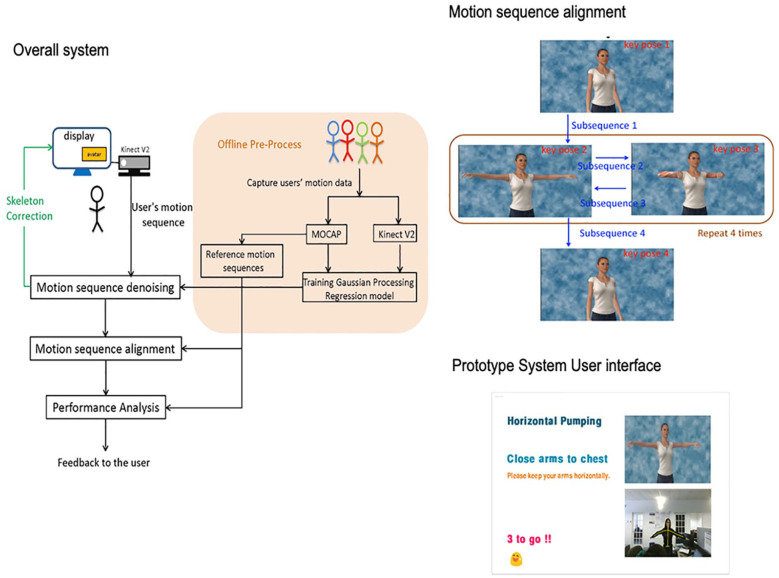
To enhance the efficacy of the TOLF intervention, we developed and tested a Kinect-enhanced lymphatic exercise intervention system (Kinect-TOLF) that uses a Microsoft Kinect sensor to capture both color and depth information while the patient is performing the lymphatic exercises following an avatar on a screen (Figure 1).20-22 The Kinect-TOLF accurately aligns a user’s motion sequence with the standard motion sequence and evaluates the “correctness” of the user’s movements in real time. This approach facilitates instantaneous feedback to the user while the user is performing each exercise.
Figure 1.
Kinect-enhanced lymphatic exercise intervention.
We conducted a single-arm feasibility clinical trial with a pre- and post-test design to assess the feasibility and usability of the Kinect-TOLF to deliver a therapeutic lymphatic exercise intervention to breast cancer survivors. The objectives of this trial were to: (1) evaluate the Kinect-TOLF in breast cancer survivors in terms of feasibility (i.e., study accrual and protocol adherence) and usability (i.e., ratings of training quality, system quality, and behavioral intention of using the Kinect-TOLF); and (2) test the efficacy of the Kinect-TOLF on reductions in lymphatic pain, swelling, lymphedema symptom occurrence (i.e., number and severity of symptoms), and lymph fluid levels. We hypothesized that a single session of Kinect-TOLF intervention would lead to significant reductions in lymphatic pain, swelling, lymphedema symptom occurrence (i.e., number and severity of symptoms), and lymph fluid levels. This study was registered in ClinicalTrials.gov with US ClinicalTrials.gov Identifier: NCT03999177.
Methods
Ethical Approval
This study (S19-00222) was approved by the Institutional Review Board of NYU Langone Health, in New York, United States.
Study Design
This study was a single-arm, single-center, feasibility clinical trial that utilized a pre- and post-test design. Feasibility of the study was defined as study accrual (N = 30) in 6 months of recruitment and >80% of protocol adherence defined as participants’ completion of the Kinect-TOLF training for lymphatic exercises. We assessed the usability of the Kinect-TOLF in terms of the extent that the user interface met Nielsen’s principles for usability (i.e., learnability, efficiency, memorability, errors, satisfaction).23-25
Setting
The study was conducted in a nursing research laboratory located in the breast cancer clinic of New York University Perlmutter Cancer Center, a National Cancer Institute designated cancer center in New York City, US.
Participants
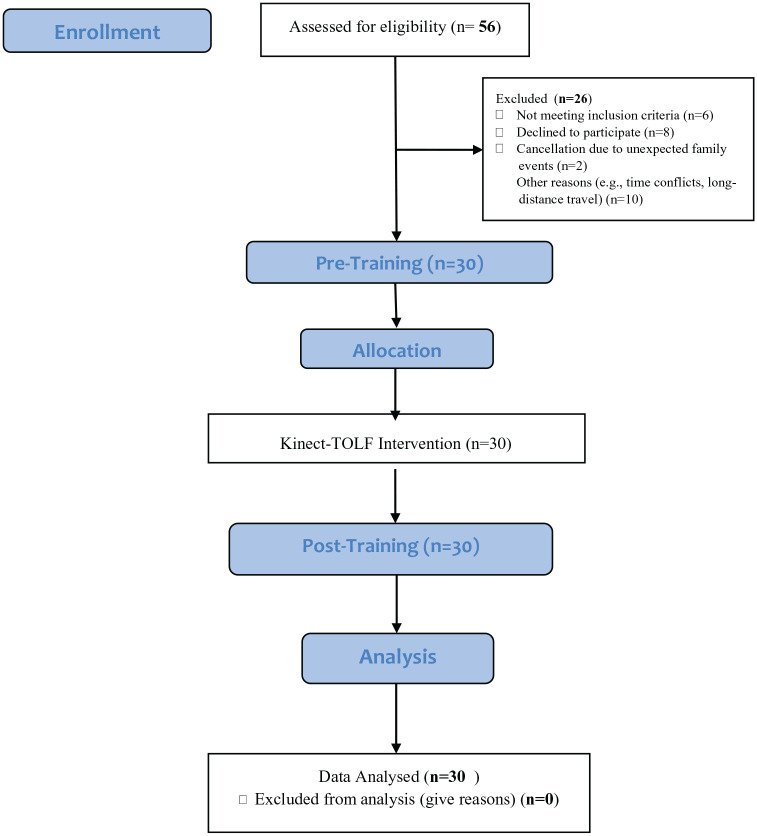
Participants were recruited from among the consecutively-identified breast cancer survivors who reported persistent lymphatic pain or swelling in the ipsilateral upper limb or body at least 12 weeks after breast cancer surgery during routine clinical visits. A total of 30 patients were recruited from July 26 to September 3, 2019. All patients signed a written consent. Figure 2 presents the Patient Flow Chart.
Figure 2.
Patient flow chart.
Patients who met the following inclusion criteria were recruited. Inclusion criteria were women who (a) had received surgical treatment for Stage I-III breast cancer, including mastectomy, lumpectomy, sentinel lymph node biopsy (SLNB), SLNB plus lymph node dissection or axillary lymph node dissection (ALND);5,6 (b) reported persistent pain or swelling in the ipsilateral upper limb or body at least 12 weeks after surgery; (c) may or may not have had neoadjuvant or adjuvant therapy of chemotherapy or radiation; (d) may or may not have had a history of or being treated for lymphedema;5,6 and (e) were able to speak and understand English. Patients were excluded if they had (a) known metastatic disease, cancer recurrence, or lymphedema due to cancer recurrence, or other bulk disease in the thoracic or cervical regions; and (b) renal or heart failure, a cardiac pacemaker or defibrillator, artificial limbs, or were pregnant.
Sample Size
The primary objective of this study was to determine the feasibility and usability of the Kinect-TOLF. The end-users of the Kinect-TOLF are breast cancer survivors with persistent lymphatic pain or swelling. A relatively small sample size of 10 to 15 end-users is recommended for usability testing.23,24 A sample of 30 patients who represented the end-users of the breast cancer survivors with persistent lymphatic pain or swelling was appropriate for the feasibility26,27 and usability testing.23,24
Study Procedures
Kinect for Windows SDK 2.0 was installed on a study laptop and the Kinect sensor was connected to the laptop. Prior to the Kinect-TOLF training session, demographic, and clinical data were collected, BMI and intervention outcomes were assessed. The intervention outcomes were assessed in the same order prior to and after training (i.e., lymphatic pain, arm/hand swelling, lymphedema symptoms, lymph fluid level). Upon the completion of the single training session, each patient was instructed to sit and rest for 5 minutes, followed by post training assessment of the intervention outcomes. In addition, patients were asked to complete the usability questionnaires and qualitative evaluation of the training. All the self-reported questionnaires were administered to the participants electronically via a study iPad connected to the study specific electronic database capture system. Two researchers who were not involved in the Kinect-TOLF training assessed patients’ lymph fluid levels using a bioimpedance device independently prior to and after the training session.
To evaluate usability of the Kinect-TOLF, we completed a heuristic evaluation with each patient. A heuristic evaluation is a usability inspection method in which a relatively small group of end-users examine the extent to which a user interface meets Nielsen's principles for usability.23,24 These principles include visibility of system status; the match between the system and the real world; user control and freedom; recognition rather than recall; and flexibility and efficiency of use.23-25 Each patient completed a set of specified tasks designed to explicate system features and freely explore the prototype. Then, patients completed a heuristic evaluation checklist that included ratings of the severity of heuristic violations (i.e., no usability problem, cosmetic problem, minor usability problem, major usability problem, usability catastrophe).
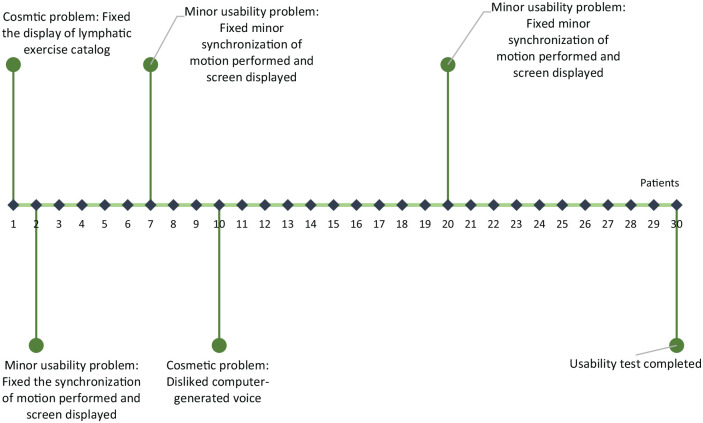
We used an iterative process to test the usability and acceptance of the Kinect-TOLF and refine the program based on patients’ “think aloud” comments during the training sessions.19,23-25 The “think aloud” method generated data on patients’ ongoing thought processes as they completed a set of training tasks designed to explicate system features.19,23-25 Only a few refinements were made based on patients’ comments, such as motion synchronization when patients performed the exercises (Figure 3).
Figure 3.
Refinement of the Kinect-enhanced lymphatic exercise training system.
The Kinect-TOLF Lymphatic Exercise Intervention
As an essential component of The-Optimal-Lymph-Flow (TOLF) intervention,17,18 the Kinect-TOLF is an innovative and intelligent Kinect-enhanced lymphatic exercise intervention that teaches patients to perform correctly the lymphatic exercises (Table 1).17-22 The Kinect-TOLF is intelligent in that it can automatically detect whether a user is performing the set of lymphatic exercises correctly in real time and provide instantaneous feedback to the user while the user is following the avatar model in the video to perform the lymphatic exercises. This approach enhances patients’ self-efficacy to perform the lymphatic exercises correctly. The therapeutic lymphatic exercises are safe and were found to be efficacious in prior studies.17-19 The Kinect-TOLF uses the Kinect v2 sensor that is commercially available and widely used in video games.20-22 No foreseeable risks are associated with the use of the Kinect-TOLF.17-22 No injuries and complaints were reported with these exercises in previous studies17-19 as well as in this study. Each patient received a single training session to learn how to perform the lymphatic exercises using the Kinect-TOLF program. The training session ended when the patient was able to perform each of the 7 lymphedema exercises correctly using the Kinect-TOLF. The duration of the training session was 10 to 15 minutes.
Measures and Outcome Variables
Clinical and Demographic Information were collected from patients’ self-reports and medical record reviews, including age, financial status, types of surgeries, time since surgery, lymph nodes procedure(s), and receipt of radiation and chemotherapy. Height and weight were measured and body mass index [BMI] calculated.
The Perceived Ease of Use and Usefulness Questionnaire is a valid and reliable 8-item instrument to evaluate users’ acceptance of a new information system.19,28,29 Items were rated on a 5-point Likert Scale that ranged from 2 (strongly agree) to −2 (strongly disagree). 19
The Post Study System Usability Questionnaire was used to assess user satisfaction with system usability on a Likert scale that ranged from 1 (strongly agree) to 7 (strongly disagree). 30 A modified 13-item tool that focused on the system’s usefulness and information quality was used in the study. 19 The questionnaire consists of 3 subscales, namely: system usefulness, information quality, and interface quality. The overall reliability of the scale was .97.19,30
Breast Cancer and Lymphedema Symptom Experience Index (BCLE-SEI) was used to assess lymphatic pain (i.e., pain/aching/soreness), arm/hand swelling, total swelling (i.e., sum of arm/hand, breast, and chest wall swelling), and lymphedema symptom occurrence (i.e., number and severity of lymphedema symptoms), including impaired limb mobility in shoulder, arm, elbow, wrist, fingers, firmness, tightness, heaviness, fibrosis (toughness or thickness of skin), stiffness, tenderness, hotness/increased temperature, redness, blistering, numbness, burning, stabbing, tingling (pain and needles), limb fatigue/weakness, and seroma (pocket of fluid developed).7-9,18,31 Adequate internal consistency was demonstrated with a Cronbach’s alpha of .92 for symptom occurrence. Discriminant validity of the instrument was supported by a significant difference in symptom occurrence (z = −6.938, P < .000) between breast cancer survivors with and without lymphedema.18,31 The occurrence and severity of each of the 24 lymphedema symptom were evaluated. Symptom severity was rated on a Likert scale that ranged from “0” (absence of a symptom) to “4” (presence of a symptom). Higher scores indicate more severe symptoms. A response frame of “now” was used when rating each symptom before and after the intervention.
Lymph Fluid Level by Bioimpedance was assessed using the Imp XCA® (Impedimed, Brisbane, Australia), a bioelectrical impedance analysis (BIA) device before and after the intervention. The device measures the resistance of the extracellular fluid (i.e., the impedance) in terms of the L-Dex ratio, taking into consideration the ratio between the dominant and non-dominant arms.32,33 With accumulation of lymph fluid, the impedance of the limb decreases and the L-Dex ratio increases. The normal L-Dex ratio ranges from −10 to +10, equivalent to impedance ratios of .935 to 1.139 for the affected dominant arm and .862 to 1.066 for the non-affected non-dominant arm, respectively. A L-Dex ratio <−10 indicates a mistake in performing the measurement. A L-Dex ratio of ≥7.1 indicates that a patient has an abnormal accumulation of lymph fluid or lymphedema; the higher L-Dex ratio indicates the greater severity of abnormal accumulation of lymph fluid or lymphedema.32,33
Qualitative Data Collection At the completion of the trial, patients were asked to provide narrative responses to the open-ended questions, “what do you like about the system,” “what do you dislike about the system,” and “what can be improved?”
Data Analysis
Prior to statistical analyses, data were examined for accuracy and completeness. Kolmogorov-Smirnov test was used to identify whether the data fit a normal distribution. Descriptive statistics were used to summarize study variables, stratified by time-point of pre- and post- Kinect-TOLF intervention. Continuous variables were summarized in terms of means, medians, standard deviations (SD), ranges, and quantiles. Categorical variables were summarized using frequencies and proportions. Confidence intervals were reported to quantify the precision of estimates. The alpha level was set at .05 and 95% confidence intervals [CI] were reported for all of the statistical tests when appropriate. We used R version 3.6.2 for all of the statistical analyses.
For ordinal outcomes (i.e., lymphatic pain, hand/arm swelling) as well as for the continuous outcome of total swelling, Wilcoxon signed-rank tests were performed to test median differences in patients’ scores between pre- and post- Kinect-TOLF intervention. Spearman’s rank correlation coefficients (ρSpearman) were calculated to assess whether pre-intervention symptom levels were associated with symptom change scores for these outcomes. Effect sizes for each test were reported in the form of Wilcoxon’s r.
Changes in L-Dex values, the total number of symptoms and total symptom severity scores were estimated using a simple linear regression of the difference between the pre- and post-intervention values on the pre-intervention value (mean-centered). 34 For each linear model, diagnostic plots were evaluated to ensure statistical assumptions were met. The intercept in the model represents the mean difference in patient outcomes between the pre-intervention values and the slope. Significant intercept suggests a relationship between pre-intervention values and the amount of within-person change observed at post-intervention (e.g., patient with higher pre-intervention pain scores may experience a greater degree of relief at post-intervention than patients with lower pain scores at pre-intervention). We report Cohen’s d as an indicator of effect size for mean differences.
Qualitative data from the responses to the open-ended questions at the completion of the trial were summarized thematically.2,17-19 To ensure the credibility of qualitative data analysis, we used a modified iterative 7-step descriptive data analysis method2,17-19 to examine data, compare codes, challenge interpretations, and inductively develop themes. First, the first author and other 2 authors read the qualitative data independently several times to gain a broad understanding of the text. Second, the 3 authors met as a group to identify key quotations and discuss key codes related to the study. Third, the 3 authors combined the coded quotations into 1 file and confirmed the accuracy of the codes and quotations. Fourth, the 3 authors carefully analyzed quotation files and identifying major themes by putting key coded quotations for each theme. Fifth, the 3 authors met as a group to review major themes together and engaged in active dialogue to resolve any discrepancies. Sixth, the 3 authors validated the structure of themes by reviewing the qualitative data. Finally, the authors conducted multiple discussions until consensus was achieved about the identified themes and each aspect of the process of data analysis. Efforts were made to differentiate and compare each theme with careful selection of quotations demonstrating the essence of the study experience.17-19
Results
Characteristics of the Patients
A total of 30 patients completed the feasibility clinical trial. No injury or safety issues occurred during the trial. The patients had a mean age of 61.7 years and a mean BMI of 28.4 kg/m2. Among these patients, 86.7 % had a bachelor’s or graduate degree, 46.7% were married, 46.7% were employed, and 46.7% lived alone (Table 2).
Table 2.
Demographic Characteristics of Patients (N = 30).
| Age in years | Mean (standard deviation [SD]; range) |
| 61.7 (9.77; 42-82) | |
| Body mass index | Mean (standard deviation [SD]; range) |
| 28.4 (6.3; 21.6-42.6) | |
| Ethnicity | n (%) |
| White | 15 (50) |
| Asian | 5 (16.7) |
| Black or African-American | 5 (16.7) |
| Hispanic | 3 (10) |
| More than 1 race | 2 (6.7) |
| Highest level of education | n (%) |
| Partial college/professional school degree | 4 (13.3) |
| Bachelor’s degree | 17 (56.7) |
| Master’s/doctoral degree | 9 (30) |
| Marital status | n (%) |
| Married | 14 (46.7) |
| Divorced/separated | 6 (20) |
| Single, never married | 10 (33.3) |
| Employment | n (%) |
| Working now | 14 (46.7) |
| Retired | 14 (46.7) |
| Looking for work, unemployed | 2 (6.6) |
| Residence status | n (%) |
| Home-alone | 14 (46.7) |
| Home-family | 16 (53.3) |
| Financial status | n (%) |
| Comfortable: have more than enough to make ends meet | 11 (36.7) |
| Have enough to make ends meet | 18 (60) |
| Do not have enough to make ends meet | 1 (3.3) |
| Smoking history | n (%) |
| Never smoked | 22 (73.3) |
| Former (stopped more than 1 year before this encounter) | 8 (26.7) |
| Drinking habits | n (%) |
| Never | 4 (13.3) |
| Fewer than 1 drink per week | 14 (46.7) |
| 1 alcoholic drink per week | 3 (10) |
| 2-7 drinks per week | 8 (26.7) |
| 7 or more drinks per week | 1 (3.3) |
As shown in Table 3, 43.3% of the patients had a lumpectomy, 60% had a mastectomy, 56.7% had breast reconstruction, 83.3% had chemotherapy, and 90% had radiation therapy. While 23.3% of the patients underwent axillary lymph node dissection, 30% had only sentinel lymph node biopsy. The mean number of lymph nodes removed was 11.4. Fifty percent of the patients were diagnosed with and treated for lymphedema. The mean years elapsed since the breast cancer diagnosis was 7.54 (SD = 6.24; median = 5.16, range = 2-23 years). Among the 30 patients, 10 (33%) patients had a L-Dex ratio ≥7.1 and 20 (67%) patients had a L-Dex ratio <7.1.
Table 3.
Clinical Characteristics of Patients (N = 30).
| Location of cancer | n (%) |
| Right breast | 16 (53.3) |
| Left breast | 14 (46.7) |
| Sentinel lymph nodes biopsy | n (%) |
| Yes | 9 (30) |
| No | 21 (70) |
| Axillary lymph nodes dissection (ALND) | n (%) |
| Yes | 7 (23.3) |
| No | 23 (76.7) |
| Sentinel lymph nodes biopsy and ALND | n (%) |
| Yes | 15 (50) |
| No | 15 (50) |
| Mastectomy | n (%) |
| Yes | 18 (60) |
| No | 12 (40) |
| Lumpectomy | n (%) |
| Yes | 13 (43.3) |
| NO | 17 (56.7) |
| Breast reconstruction | n (%) |
| Yes | 17 (56.7) |
| No | 13 (43.3) |
| Being diagnosed with lymphedema | n (%) |
| Yes | 15 (50) |
| No | 15 (50) |
| Lymph fluid level by bioimpedance | n (%) |
| L-Dex ratio <7.1 | 20 (67) |
| L-Dex ratio ≥7.1 | 10 (33) |
| Chemotherapy | n (%) |
| Yes | 25 (83.3) |
| No | 5 (16.7) |
| Radiation therapy | n (%) |
| Yes | 27 (90) |
| No | 3 (10) |
| Number of lymph nodes removed | Mean (standard deviation; range) |
| 11.4 (9.1; 1 – 30) | |
| Times since breast cancer diagnosis in years | Mean/median (standard deviation; range) |
| 7.54/5.16 (6.24; 2–23) |
Feasibility
Feasibility of the study was demonstrated by timely study accrual (N = 30) in less than 3 months, ahead of the planned 6 months recruitment. The study achieved 100% protocol adherence with no patient withdrawal and protocol deviation. Of the 50 patients who were eligible for the study, only 8 patients declined the study without giving a reason. Twelve patients were eager but not able to participate in the study due to unexpected family events, time conflicts, and long-distance travel.
Usability
Patients’ responses to questions regarding Nielson's heuristic violations23-25 indicated no major usability problems or usability catastrophe associated with the Kinect-TOLF during the trial. Specifically, none of the 30 patients reported major usability problems or usability catastrophes; 24 (80%) reported no usability problem; 4 (13.3%) reported minor usability problems (ie, minor mis-synchronization of the motion performed and what the screen displayed); and 2 (6.7%) reported cosmetic problem (ie, dislike the computer-generated voice and display of the catalog of the exercises). The heuristic evaluation demonstrated that the iterative process of refinement of the Kinect-TOLF was effective. By the last 10 participants, no usability problems were reported.
Acceptance
Table 4 presents detailed information about patients’ responses to The Perceived Ease of Use and Usefulness Questionnaire.28,29 Over 90% of patients strongly agreed or agreed that the Kinect-TOLF was easy to learn, and flexible and easy to operate. Further, 93.4% of patients strongly agreed or agreed that their interactions with the system were clear and understandable. Over 80% of patients strongly agreed or agreed that the Kinect-TOLF made it easy for them to remember how to perform the lymphatic exercises. Table 5 shows patients’ responses to the Post Study System Usability Questionnaire. 30 Average ratings on all of the items ranged from 1.20 to 1.53 on a scale that ranged from 1 (strongly agree) to 7 (strongly disagree), indicating that participants strongly agreed or agreed on user satisfaction with the Kinect-TOLF.
Table 4.
The Perceived Ease of Use and Usefulness Questionnaire (N = 30).
| Strongly agree n (%) | Agree n (%) | Neutral n (%) | Disagree n (%) | Strongly disagree n (%) | |
|---|---|---|---|---|---|
| I find the system easy to use. | 18 (60) | 10 (33.3) | 1 (3.3) | 1 (3.3) | 0 (0) |
| Learning to operate or follow the system is easy for me. | 17 (56.7) | 11 (36.7) | 1 (3.3) | 1 (3.3) | 0 (0) |
| Interaction with the system is difficult. | 1 (3.3) | 2 (6.7) | 1 (3.3) | 13 (43.3) | 13 (43.3) |
| I find it easy to get the system to do what I want it to do. | 14 (46.7) | 11 (36.7) | 3 (10) | 2 (6.7) | 0 (0) |
| The system is flexible to interact with. | 14 (46.7) | 13 (43.3) | 1 (3.3) | 2 (6.7) | 0 (0) |
| It is easy for me to remember how to perform tasks using the system. | 14 (46.7) | 12 (40) | 3 (10) | 1 (3.3) | 0 (0) |
| Interacting with the system requires a lot of mental effort. | 0 (0) | 2 (6.7) | 5 (16.7) | 12 (40) | 11 (36.7) |
| My interaction with the system is clear and understandable. | 17 (56.7) | 11 (36.7) | 1 (3.3) | 1 (3.3) | 0 (0) |
Table 5.
The Post-Study System Usability Questionnaire (N = 30).
| Subscales | Items | Mean (SD) a | Median | Range b |
|---|---|---|---|---|
| System Usefulness | Overall, I am satisfied with how easy it is to use this system. | 1.4 (0.62) | 1 | 1-3 |
| It was simple to use this system. | 1.53 (0.82) | 1 | 1-4 | |
| I could effectively follow the system to learn how to perform the lymphatic exercises. | 1.23 (0.51) | 1 | 1-3 | |
| I felt comfortable using this system to learn how to perform the lymphatic exercises. | 1.33 (0.96) | 1 | 1-6 | |
| It was easy to learn the lymphatic exercises using this system. | 1.17 (0.46) | 1 | 1-3 | |
| I believe I could learn how to perform the lymphatic exercises correctly using this system. | 1.37 (0.99) | 1 | 1-6 | |
| Interface Quality | The system gave error messages that clearly told me how to fix problems. | 1.4 (0.81) | 1 | 1-5 |
| Whenever I made a mistake in performing the lymphatic exercises, the system immediately told me so. | 1.53 (0.82) | 1 | 1-4 | |
| The information (such as on-screen messages) provided with this system was clear. | 1.23 (0.5) | 1 | 1-3 | |
| Information quality | It was easy to follow the system to learn the correct way to perform the lymphatic exercises. | 1.53 (1.07) | 1 | 1-6 |
| The information provided for the system was easy to understand. | 1.33 (0.55) | 1 | 1-3 | |
| The information was effective in helping me to learn to correctly perform the lymphatic exercises. | 1.2 (0.48) | 1 | 1-3 | |
| The organization of information on the system screens was clear. | 1.2 (0.5) | 1 | 1-3 |
SD: Standard Deviation.
On a scale of 1 to 7, 1 represents strongly agree and 7 represents strongly disagree.
Effects of the Kinect-TOLF on Lymphatic Pain, Swelling, and Lymphedema Symptoms
Lymphatic pain and swelling
Table 6 provides the results of effects of the Kinect-TOLF on lymphatic pain, swelling, and lymphedema symptoms. Significant reductions were found in lymphatic pain (MedΔ = −1.00, CI = [−1.5, −0.1], P = .004), arm/hand swelling (MedΔ = −1.00, CI = [−1.5, −0.5], P = .004), and total swelling scores (MedΔ = −1.5, CI = [−2.0, −1.0], P = .003) scores. Spearman’s rank correlation coefficients suggest that patients with higher pain scores at the pre-intervention assessment had significantly greater decrease in pain (ρSpearman = .49, P = .006). Patients with greater severity of arm/hand swelling at the pre-intervention assessment had a significantly greater reduction in arm/hand swelling (ρSpearman = .66, P < .0001). Greater severity of total swelling at pre-intervention assessment was associated with greater improvement (ρSpearman = .36, P = .048).
Table 6.
Lymphatic Pain, Arm/Hand Swelling, Total Swelling, L-Dex, and Symptom Occurrence and Severity.
| Outcome variables | Pre Kinect-TOLF | Post Kinect-TOLF | Change scores (post-pre differences) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Med | IQR | Range | Med | IQR | Range | Med difference (95%CI) | Statistics | P | Effect Size (95%CI) | |
| Lymphatic limb pain | 1 | 1 | 0-4 | 0 | 2 | 0-3 | −1.0 (−1.5, −0.1) | −2.91 a | .004 | 0.53 (0.25, 0.74) |
| Arm/hand swelling | 1 | 2 | 0-4 | 0 | 1 | 0-3 | −1.0 (−1.5, −0.5) | −2.92 a | .004 | 0.54 (0.28, 0.71) |
| Total swelling | 2 | 1.75 | 0-8 | 1 | 2 | 0-9 | −1.5 (−2.0, −1.0) | −2.96 a | .003 | 0.54 (0.26, 0.76) |
| L-Dex | 12.4 | 25.4 | −7.2 to 108.1 | 9.7 | 24.6 | −9.3 to 106.0 | −2.7 (−4.7, −0.7) | −2.76 b | .010 | 0.50 d (0.11, 0.89) |
| Number of symptoms | 10.5 | 5.6 | 1-19 | 6.4 | 6.6 | 0-22 | −3.8 (−5.5, −2.1) | −4.56 b | <.001 | 0.85 d (0.43, 1.29) |
| Symptom severity score | 19.3 | 15.5 | 2-66 | 12.4 | 16.8 | 0-66 | −5.3 (−9.5, −1.1) | −2.58 b | .016 | 0.49 d (0.11, 0.88) |
Abbreviation: Med, median; IQR, inter-quartile range; SD, standard deviation.
Wilcoxon signed-rank test Z-Statistic.
Paired t-test t-statistic.
Wilcoxon’s r.
Cohen’s d.
Lymphedema symptom occurrence and severity
Table 7 presents the occurrence of the 24 lymphedema symptoms before and after the Kinect-TOLF intervention. Significant reductions were found in the total number of lymphedema symptoms (MΔ = −3.8, CI = [−5.5, −2.1], P < .001) and total symptom severity scores (MΔ = −5.3, CI = [−9.5, −1.1], P = .016, Table 6).
Table 7.
Lymphedema Symptom Occurrence Pre- and Post- Kinect-TOLF Intervention.
| Symptoms | Pre-Kinect-TOLF | Post-Kinect-TOLF |
|---|---|---|
| n (%) | n (%) | |
| Lymphatic pain (ie, pain/aching/soreness in the affected limb or body side) | 24 (80.0) | 12 (40.0) |
| Arm/hand swelling | 21 (70.0) | 14 ( 46.67) |
| Breast swelling | 11 (36.7) | 7 (23.3) |
| Chest wall swelling | 9 (30.0) | 5 (16.7) |
| Limited movement in the affected shoulder | 15 (50.0) | 12 (40.0) |
| Limited movement in the affected elbow | 8 (26.7) | 6 (20) |
| Limited movement in the affected wrist | 6 (20.0) | 7 (23.3) |
| Limited movement in the affected fingers | 11 (36.7) | 7 (23.3) |
| Limited movement in the affected arm | 21 (70.0) | 13 (43.3) |
| Arm firmness | 21 (70.0) | 8 (26.7) |
| Arm tightness | 24 (80.0) | 16 (53.3) |
| Arm heaviness | 21 (70.0) | 13 (43.3) |
| Fibrosis (ie, toughness or thickness of skin in the affected limb) | 9 (30.0) | 7 (23.3) |
| Stiffness in the affected limb | 21 (70.0) | 13 (43.3) |
| Tenderness in the affected limb | 17 (56.7) | 7 (23.3) |
| Hotness/increased temperature in the affected limb | 7 (23.3) | 3 (10.0) |
| Redness in the affected limb | 2 (6.7) | 2 (6.7) |
| Blistering in the affected limb | 1 (3.3) | 1 (3.3) |
| Numbness in the affected limb | 15 (50.0) | 12 (40.0) |
| Burning in the affected limb | 5 (16.7) | 3 (10.0) |
| Stabbing in the affected limb | 6 (20.0) | 4 (13.3) |
| Tingling (pins and needles) in the affected limb | 15 (50.0) | 9 (30.0) |
| Limb fatigue or weakness | 18 (60.0) | 14 (46.7) |
| Seroma (pocket of fluid developed) | 7 (23.3) | 4 (13.3) |
Lymph fluid level by bioimpedance
A significant reduction in lymph fluid levels was found based on mean L-Dex scores (MΔ = −2.68, CI = [−4.67, −0.69], P = .010). Higher reductions in mean L-Dex scores were observed in the 10 patients who had abnormal lymph fluid level (ie, L-Dex of ≥7.1) at pre-intervention assessment (MΔ = −5.19, CI = [−1.75, −8.63], P = .008).
Qualitative Results
Five main themes emerged from the qualitative data, namely: standardized learning for lymphatic exercises; having a control over the training; effectiveness of the training; effectiveness of the lymphatic exercises; and easy to use. Table 8 provides representative quotes for each of the theme.
Table 8.
Themes and Representative Quotes from Qualitative Evaluation (N = 30).
| Providing a standardized format for lymphatic exercises |
| • “The model is easy to follow & gives a standardized
format for proper maneuvers.” • “It is very helpful in ensuring one does the exercises properly. Additionally, the feedback is very helpful.” |
| Having a control over the training |
| • “I had control over the exercises and could repeat them if
I did not perform them well.” • “Ability to follow along independently.” |
| Effectiveness of training |
| • “It really clearly showed me how to do the exercises and
where I was making errors.” • “The exercises were thoroughly explained: Not only the how but the why. The feedback was immediate so you can clearly tell if you were doing them correctly.” • “I like the fact that it tells you if you are doing the exercises correctly.” • “I like the correction when I do the exercise. Now, I know how to do this correctly.” • “It really help me to adjust into correct position of my shoulder (a difficult task for me).” • “It is very helpful in ensuring one does the exercises properly. Additionally, the feedback is very helpful.” • “Step by step instructions on each exercise and its immediate feedback.” • “Exercises were clearly explained. Illustrations also clear. Immediate feedback helpful.” • “The interaction with the model and viewing my own body at the same time was fun.” • “I like everything about it, I think the system as a whole is quite perfunctory. I think it is a well-designed system for someone living with lymphedema such as me.” |
| Effectiveness of the lymphatic exercises |
| • “I think it’s great and effective. I can feel the positive
difference within minutes of doing the
exercises.” • “I am more than satisfied because the movements help me to stretch out tightness and relieve pain.” • “I like it. For breast cancer survivors, we can follow the program and see the videos that are easy to follow with no problem. Good for practicing exercises.” • “The exercises are very helpful.” • “Useful exercises.” |
| Ease of use |
| • “It is good and easy to follow and I like it that is shows
how you are breathing etc.” • “Easy to use and immediate feedback was given.” • “It was easy to follow.” • “I like everything, it was pretty straightforward.” |
The most salient theme was that patients felt that the Kinect-TOLF was effective in teaching them how to do the lymphatic exercises correctly by providing them with a standardized format for each exercise that was “easy to follow.” Patients appreciated that the Kinect-TOLF provided immediate feedback on “where [they were] making errors.” As 1 patient remarked, the Kinect-TOLF helped her to adjust her shoulder to the correct position for the exercises, which is a “difficult task for me.” Patients noted that the Kinect-TOLF increased their sense of self-efficacy because the program allowed them to learn and perform the exercises independently and that they could repeat them as often as they wanted to do so. Patients felt that the lymphatic exercises had immediate effects to “stretch out tightness and relieve pain,” and have a “positive difference within minutes of doing the exercises.” Finally, patients concluded that the Kinect-TOLF was “easy to use” and “straightforward.”
Discussion
The efficacy of self-care exercise interventions relies on accurate body movements. Findings from this study demonstrated that the Kinect-TOLF intervention is feasible and efficacious in training patients to perform lymphatic exercises accurately. Feasibility of the study was supported by 100% of study accrual ahead of time (i.e., more than 3 months ahead of the planned 6 months of recruitment) and 100% of protocol adherence without any protocol deviation and patient withdrawal from the study. The majority of our patients reported no usability problems and agreed that the system was easy to learn and operate, flexible to use, clear, and understandable. As expected, some patients reported that it took a lot of mental effort to learn the lymphatic exercises. However, over 80% of them indicated that the Kinect-TOLF made it easy for them to remember how to correctly perform the exercises. Nearly every patient expressed how much they appreciated the real-time feedback, which enabled them to perform each exercise correctly. Overall, our findings provide preliminary evidence that the Kinect-TOLF is a simple and efficacious system that patients can use to learn self-care therapeutic exercises correctly.
The goal of the TOLF lymphatic exercises is to relieve lymphatic pain, swelling, and lymphedema symptoms.17,18 In face-to-face 17 and avatar-based coaching 18 studies, the TOLF intervention was safe, feasible, and efficacious in relieving lymphatic pain and reducing lymphedema symptoms as well as in decreasing the risk of objective lymph fluid accumulation by limb volume using infrared perometer. The current study extends these findings and suggests that the Kinect-TOLF is safe, feasible, and have immediate effects on lymphatic pain, swelling, lymphedema symptoms, and lymph fluid level after a single training session of the Kinect-TOLF. Significant reductions were found in lymphatic pain (P = .004), arm/hand swelling (P = .004), total swelling (P = .003), number of lymphedema symptoms (P < .001), and symptom severity scores (P = .016). In addition, patients who had the greatest severity of lymphatic pain, arm/hand swelling, and total swelling prior to intervention had the greatest improvements in these symptoms. Taken together, these findings suggest that the effects of a single session of Kinect-TOLF intervention were immediate to relieve lymphatic pain, arm/hand swelling, total swelling, and lymphedema symptoms. Patients’ immediate therapeutic responses are also supported by the qualitative data. Patients reported positive effects within minutes of performing the lymphatic exercises. These perceptions of immediate effects of the Kinect-TOLF are important because sustained behavior change is more easily maintained if patients perceive that an intervention is effective and easy to integrate into their daily lives. 35
The TOLF lymphatic exercises were designed to decrease lymph fluid levels.17,18 In a previous study, 17 97% of the 134 patients who received the face-to-face TOLF intervention maintained or decreased their preoperative limb volumes assessed using an infrared perometer at 12 months after surgery. In the current study, all of the patients had significant reductions in lymph fluid levels assessed using bioimpedance immediately after a single training session of Kinect-TOLF. In addition, greater reductions in lymph fluid levels were found in patients with abnormal lymph fluid levels (i.e., their scores decreased by 5.1 points). Given these results, it is reasonable to hypothesize that daily or weekly use of the Kinect-TOLF intervention may help patients to achieve normal lymph fluid levels.
Efforts to Prevent Bias
To prevent recruitment bias, patients were recruited from among the consecutively-identified breast cancer patients who reported persistent lymphatic pain or lymphedema symptoms in the ipsilateral upper limb or body at least 12 weeks after breast cancer surgery during routine clinical visit. In addition, the technology-enhanced Kinect-TOLF system ensured the fidelity of intervention delivery.
Strengths and Limitations of the Study
The strengths of this trial include the use of a well-designed Kinect-enhanced training system20-22 based on an evidence-based effective intervention;17-19 an adequate sample size for feasibility26,27 and usability testing;23,24 and the use of both objective and subjective measures for efficacy testing. While not a randomized trial, this study provides an effect size for each outcome variable for future trials. As Kinect-TOLF lymphatic exercises can be used alone or as an adjuvant therapy to current lymphedema treatment (e.g., compression therapy, manual lymph drainage, or other forms of exercises), a randomized clinical trial with a larger sample is warranted to confirm the unique or synergistic efficacy of the Kinect-TOLF in comparison to current lymphedema treatment and other forms of exercises or movement therapy. Future studies should also consider what constitutes a clinically meaningful difference in using the Kinect-TOLF to reduce lymphatic pain, swelling, lymphedema symptoms, and decrease lymph fluid levels. In terms of limitations, patients were highly educated and had familiarity with computers and mobile devices.
Conclusion
The Kinect-TOLF is an easy-to-use, low-cost intervention that enables patients to perform the therapeutic lymphatic exercises in a standardized way. This intelligent Kinect-enhanced lymphatic exercise intervention enhances intervention fidelity and insures that each patient receives the same quality of intervention. Importantly, findings from this study suggest that a single session of Kinect-TOLF intervention immediately reduces lymphatic pain, swelling, and lymphedema symptoms, as well as decreases lymph fluid levels. The findings of this study should be further evaluated in a large randomized clinical trial. If the results are replicated, the Kinect-TOLF intervention will be relatively easy to implement in clinical practice or at home to relieve lymphatic pain, swelling, and lymphedema symptoms, as well as to decrease lymph fluid level, ultimately to improve the QOL of breast cancer survivors.
Acknowledgments
We thank Ms. Alejandra Yancey for helping manage the trial and data collection. We thank all the patients who participated in the trial. We thank nurses, physicians, and staff at NYU Perlmutter Cancer Center for their support of the trial.
Footnotes
Author Contributions: The “Author Contributions” section should be completed as follow: (1) Conception and design: Mei R. Fu, Yao Wang; (2) Administrative support: Mei R. Fu, Yao Wang, Deborah Axelrod, Amber A. Guth, Zhipeng Fan; (3) Provision of study material or patients: Mei R Fu, Deborah Axelrod, Amber A. Guth; (4) Collection and assembly of data: Mei R. Fu, Eunjung Ko, Simay Yazicioglu, Jeanna M. Qiu, Anna Sang; (5) Data analysis and interpretation: Mei R. Fu, Melissa McTernan, Jeanna M. Qiu; (6) Manuscript Drafting: Mei R Fu, Jeanna M. Qiu, and Melissa McTernan; (7) Manuscript revision and editing: All authors. (8) Final approval of manuscript: All authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institute of Health /National Science Foundation /National Cancer Institute (1R01CA214085-01) with Mei R Fu and Yao Wang as the multiple principal investigators. This study was also supported by a research grant from Judges and Lawyers Breast Cancer Alert with Mei R Fu as the principal investigator. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Human and Animal Rights and Informed Consent: This study (IRB # (S19-00222) was approved by the Institutional Review Board of a metropolitan cancer center in New York of the United States. All the human participants signed the informed consent. This study does not contain any animal subjects.
ORCID iDs: Mei R. Fu  https://orcid.org/0000-0003-3891-0109
https://orcid.org/0000-0003-3891-0109
Melissa L. McTernan  https://orcid.org/0000-0002-5151-8307
https://orcid.org/0000-0002-5151-8307
References
- 1. Burckhardt CS, Jones KD. Effects of chronic widespread pain on the health status and quality of life of women after breast cancer surgery. Health Qual Life Outcomes. 2005;3(30):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Degnim AC, Miller J, Hoskin TL, et al. A prospective study of breast lymphedema: frequency, symptoms, and quality of life. Breast Cancer Res Treat. 2012;134:915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eaton LH, Narkthong N, Hulett JM. Psychosocial issues associated with breast cancer-related lymphedema: a literature review. Curr Breast Cancer Rep. 2020:1-9. doi:10.1007/s12609-020-00376-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanton AW, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol. 2009;7:29-45. [DOI] [PubMed] [Google Scholar]
- 5. Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16:48-54. [DOI] [PubMed] [Google Scholar]
- 6. Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959-1972. [DOI] [PubMed] [Google Scholar]
- 7. Fu MR, Axelrod D, Cleland CM, et al. Symptom reporting in detecting breast cancer-related lymphedema. Breast Cancer (Dove Med Press). 2015;7:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu MR, Aouizerat BE, Yu G, et al. Model-based patterns of lymphedema symptomology: phenotypic and biomarker characterization. Curr Breast Cancer Rep. 2021;13:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fu MR, Conley YP, Axelrod D, et al. Precision assessment of heterogeneity of lymphedema phenotype, genotypes and risk prediction. Breast. 2016;29:231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douglass J, Graves P, Gordon S. Self-care for management of secondary lymphedema: a systematic review. PLoS Negl Trop Dis. 2016;10:e0004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armer JA, Ostby P, Ginex P, et al. ONS Guidelines for Cancer treatment-related lymphedema. Oncol Nurs Forum. 2020;47:518-538. [DOI] [PubMed] [Google Scholar]
- 12. Bennett MI, Bagnall AM, José Closs J. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain. 2009;143:192-199. [DOI] [PubMed] [Google Scholar]
- 13. Somers TJ, Kelleher SA, Dorfman CS, et al. An mhealth pain coping skills training intervention for hematopoietic stem cell transplantation patients: development and pilot randomized controlled trial. JMIR Mhealth Uhealth. 2018;6:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Groef A, Van Kampen M, Dieltjens E, et al. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Arch Phys Med Rehabil. 2015;96:1140-1153. [DOI] [PubMed] [Google Scholar]
- 15. Zengin Alpozgen A, Razak Ozdincler A, Karanlik H, Yaman Agaoglu F, Narin AN. Effectiveness of pilates-based exercises on upper extremity disorders related with breast cancer treatment. Eur J Cancer Care (Engl). 2017;26:1-8. [DOI] [PubMed] [Google Scholar]
- 16. Galiano-Castillo N, Cantarero-Villanueva I, Fernández-Lao C, et al. Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122:3166-3174. [DOI] [PubMed] [Google Scholar]
- 17. Fu MR, Axelrod D, Guth AA, et al. Proactive approach to lymphedema risk reduction: a prospective study. Ann Surg Oncol. 2014;21:3481-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu MR, Axelrod D, Guth AA, et al. mHealth self-care interventions: managing symptoms following breast cancer treatment. Mhealth. 2016;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu MR, Axelrod D, Guth AA, et al. Usability and feasibility of health IT interventions to enhance self-care for lymphedema symptom management in breast cancer survivors. Internet Interv. 2016;5:56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiang AT, Chen Q, Wang Y, Fu MR. Kinect-based in-home exercise system for lymphatic health and lymphedema intervention. IEEE J Transl Eng Health Med. 2018;6:4100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiang AT, Chen Q, Wang Y, Fu MR. Motion sequence alignment for a Kinect based in-home exercise system for lymphatic health and lymphedema intervention. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:2072-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiang AT, Chen Q, Li S, Wang Y, Fu M. Denoising of joint tracking data by Kinect sensors using clustered Gaussian process regression. MMHealth17. 2017;2017:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreuter M, Farrell D, Olevitch L, Brennan L. Tailoring Health Messages: Customizing Communication with Computer Technology. 1st ed. Lawrence Erlbaum Associates, Inc.; 2000. [Google Scholar]
- 24. Nielsen J, Mack RL. Usability Inspection Methods. 1st ed. Wiley; 1994. [Google Scholar]
- 25. Bright TJ, Bakken S, Johnson SB. Heuristic evaluation of eNote: an electronic notes system. AMIA Annu Symp Proc. 2006;2006:864. [PMC free article] [PubMed] [Google Scholar]
- 26. Browne RH. On the use of a pilot sample for sample size determination. Stat Med. 1995;14:1933-1940. [DOI] [PubMed] [Google Scholar]
- 27. Billingham AAM, Whitehead AL, Julious SA. An audit of sample size for pilot and feasibility trials being undertaking in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol. 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dillon TW, McDowell D, Salimian F, Conklin D. Perceived ease of use and usefulness of bedside-computer systems. Comput Nurs. 1998;16:151-156. [PubMed] [Google Scholar]
- 29. Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly. 1989:13:319-340. [Google Scholar]
- 30. Lewis JR. IBM computer usability satisfaction questionnaires: psychometric evaluation and instructions for use. Int J Hum Comput Interact. 1995;7:57-78. [Google Scholar]
- 31. Shi S, Lu Q, Fu MR, et al. Psychometric properties of the breast cancer and lymphedema symptom experience index: the Chinese version. Eur J Oncol Nurs. 2015;20:10-16. [DOI] [PubMed] [Google Scholar]
- 32. Fu MR, Cleland CM, Guth AA, et al. L-Dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. Lymphology. 2013;46:85-96. [PMC free article] [PubMed] [Google Scholar]
- 33. Ridner SH, Dietrich MS, Spotanski K, et al. A prospective study of L-Dex values in breast cancer patients pretreamtent and through 12 months postoperatively. Lymphat Res Biol. 2018;16:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hedberg EC, Ayers S. The power of a paired t-test with a covariate. Soc Sci Res. 2015;50:277-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hasenoehrl T, Keilani M, Palma S, Crevenna R. Resistance exercise and breast cancer related lymphedema - a systematic review update. Disabil Rehabil. 2020;42:26-35. [DOI] [PubMed] [Google Scholar]