Abstract
Background:
Primary small cell carcinoma of the esophagus (SCCE) is a rare and extremely fatal disease. We aim to evaluate the efficacy of radical surgery for resectable SCCE and to explore potential prognostic factors.
Methods:
We retrospectively reviewed 52 consecutive SCCE patients who underwent radical surgery from February 1993 to November 2014 at a single institution. The Kaplan-Meier estimator with log-rank test was used to assess overall survival (OS), disease-free survival (DFS) and median survival time. Univariate and multivariable analyses were used to evaluate prognostic factors through Cox proportional hazard regression model.
Results:
Twenty-five (48.1%) patients were treated with surgery alone, whereas 27 (51.9%) patients underwent adjuvant therapy after surgery. The median OS time was 17.4 months (95% CI: 13.5-21.3). The median DFS time was 13.4 months (95% CI: 7.7-19.0). Patients whose tumors were located in the lower part of thoracic esophagus and the esophagogastric junction showed significantly better OS (27.0 vs. 13.2 months, P = 0.016) and DFS (27.0 vs. 11.3 months, P = 0.017) than those located in the upper and middle parts. Patients with N0 status experienced significantly better OS (21.4 vs. 11.6 months, P = 0.012) and DFS (21.4 vs. 8.6 months, P = 0.012) than those with N+ status. Patients whose tumor lengths were shorter than 5 cm had a better OS (17.4 vs. 5.7 months, P = 0.035) than those longer than 5 cm. Patients who underwent chemotherapy experienced a significantly improved OS (21.0 vs. 14.1 months, P = 0.032) compared to surgery alone. Multivariable analysis showed that lower tumor location, shorter tumor length, pN0 status and chemotherapy independently predicted better OS; lower tumor location and pN0 status independently predicted better DFS.
Conclusions:
Radical surgery in combination with chemotherapy has better outcomes than surgery alone for resectable SCCE. Higher tumor location, longer tumor length, lymph node metastasis and not undergoing chemotherapy independently predict worse prognoses.
Keywords: small cell carcinoma, esophageal neoplasm, surgery, chemotherapy, cancer therapy
Introduction
Primary small cell carcinoma of the esophagus (SCCE), which accounts for approximately 0.36%-2.8% of all esophageal neoplasms in China, 1 is a rare and extremely fatal malignant disease. SCCE was first reported in 1952 by Mckeown, 2 given its rarity and the lack of any prospective research, there are still no standard treatment recommendations available at present. Many institutions conduct surgery, radiotherapy and chemotherapy alone or in combination to treat SCCE; this approach is mostly based on the therapeutic strategies of small cell lung carcinoma (SCLC) and varies according to physicians’ preferences. Despite these efforts, the reported median survival time in SCCE is quite low, only ranging from 8-12.5 months. 1,3,4
Similar to SCLC, SCCE is highly aggressive and tends to have progressed to regional and distant metastases at the time of diagnosis. 5,6 Although surgery has been commonly used as 1 part of the multimodal treatment for resectable SCCE, the efficacy of surgical treatment is still under debate. Several studies suggested that surgery plus chemotherapy did not improve survival outcomes compared with radiotherapy in combination with chemotherapy. 7 -9 This evidence may restrict the utilization of surgical treatment for local control, considering the accompanying surgical trauma and postoperative complications. Nevertheless, some studies have suggested that surgical treatment may be beneficial. Chen at el. found that stage I-IIA patients tended to benefit from surgical treatment alone with improved survival, 10 which is partially in line with the recommendations by the latest NCCN guideline of SCLC, which state that SCLC patients with clinical stage I-IIA are likely to benefit from surgery. 11 In addition, some studies have also reported that esophagectomy with lymphadenectomy as 1 part of a multidisciplinary therapeutic strategy is associated with improved overall survival. 12,13 Thus, given the controversial conclusions reported in previous studies, the value of surgical treatment for resectable SCCE still needs further investigation.
In this study, we retrospectively collected clinicopathological and survival data of consecutive SCCE patients who underwent radical esophagectomy with lymphadenectomy from a single institution to evaluate the efficacy of surgical treatment for resectable SCCE and to explore potential relevant prognostic factors. We aimed to provide more clinical evidence for this rare malignant disease.
Methods
Patients
We retrospectively reviewed 63 consecutive patients with pathologically confirmed SCCE who were treated with surgery between February 1993 and November 2014 at Sun Yat-sen University Cancer Center. We did not include 2 patients with pathologically confirmed as the grade 2 neuroendocrine tumor of esophagus at the same period. Among 63 SCCE patients, 3 patients who did not have the required medical records were excluded, as were 3 patients with concomitant tumors of other organs, 3 patients with R2 resection, 1 patient with only exploratory thoracotomy and 1 patient who was proven to have distant metastasis during the perioperative period. A total of 52 patients were finally included in this study. Data on age, sex, tumor location, tumor length, pathological features, type of surgical approach, adjuvant/neoadjuvant therapy, pathological TNM stage (pTNM), tumor recurrence and prognosis were collected for each patient. The 8th edition of the AJCC cancer staging system for esophageal squamous cell carcinoma was applied for all patients.
Each patient was routinely evaluated through intravenous contrast-enhanced CT scans of the chest and abdomen (or PET-CT when appropriate), cervical ultrasound, endoscopic ultrasound (EUS), barium swallow and bronchoscopy before treatment. All the enrolled patients were proven to be in the clinical M0 stage. Surgical procedures included the Sweet approach, the Ivor Lewis approach and the Mckeown approach. Adjuvant therapy was mostly performed on the basis of surgeons’ preferences, including chemotherapy alone, radiotherapy alone and chemoradiotherapy. Pathological diagnosis was based on the criteria of the World Health Organization (WHO) classification, 14 where combined SCCE was defined as SCCE with an additional component of another type of carcinoma.
Follow Up
Patients were regularly followed up every 3 months during the first 2 years after surgery and every 6 months thereafter. The last follow-up date was May 2019, and 5 out of 52 patients (9.6%) were lost to follow-up. The endpoints of the observation were overall survival (OS) and disease-free survival (DFS). The start date was recorded as the day of operation, while the end date was the day when endpoints were measured or the last follow-up day for censored data. All the follow-up data were collected from the Official Follow-up Department at our hospital. This study was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center.
Statistics
Mean and standard deviation (SD) as well as median and interquartile range (IQR) were used to describe continuous variables. The Kaplan-Meier estimator was used for survival analysis, and the differences were compared through the log-rank test. Sample size calculations for survival comparisons were made assuming a hazard ratio of 0.5, an allocation ratio of 1:1, with a two-sided significance level of 0.05 and a power of 80%. The estimated number of patients was 72 (36 per arm). Cox proportional hazard regression models were used to evaluate risk factors for OS and DFS by calculating the hazard ratio (HR) and 95% confidence interval (95% CI). A 2-tailed P value < 0.05 indicated a significant difference. All the statistical analyses were performed using SPSS Statistic 25 (IBM Corporation, Armonk, NY).
Ethics Statement
This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. SZR2020-054). Informed consents were omitted due to the retrospective nature.
Raw Data
We have submitted all the key raw data of this article to the Research Data Deposit public platform (www.researchdata.org.cn) with the approval RDD number: RDDA2019001117.
Results
Patient Characteristics
The detailed clinicopathological characteristics of 52 patients are listed in Table 1. Thirty-eight (73.1%) patients were males. The mean age of the patients was 57.4 (SD = 9.6) years old, and the mean body max index was 22.3 (SD = 3.7) kg/m2. The mean preoperative plasma albumin level was 41.7 (SD = 4.8) g/L, and the levels in 7 patients (13.5%) were marked as low (below 35 g/L). There were 8 patients (15.4%) with chronic diseases, including 3 cases of hypertension, 3 cases of chronic hepatitis, 1 case of rheumatic heart disease and 1 case of rheumatic arthritis. Thirty-five (63.7%) patients underwent the Sweet approach, while 32.7% underwent either the Ivor Lewis or the McKeown approach during surgery. Furthermore, 69.2% of the tumors were located in the upper and middle parts of the esophagus, and 30.8% were located in the lower part and the esophagogastric junction (EGJ). Seventeen out of 52 patients (32.7%) were diagnosed with combined SCCE, of which 13 patients were diagnosed with small cell carcinoma (SC) combined with squamous cell carcinoma (SCC), 2 patients were diagnosed with SC combined with adenocarcinoma (AC), 1 patient had concomitant large cell carcinoma (LCC), and 1 patient had both concomitant SCC and AC. The median dissected lymph nodes were 16.0 nodes (IQR: 8.0-23.0 nodes), and the median metastatic lymph nodes were 2.0 nodes (IQR: 0-5.8 nodes). Most patients had T2 (36.5%) and T3 (42.3%) status, and 32 patients (61.5%) were found to have lymph node metastases. Additionally, 8 patients were in stage I (15.4%), 13 patients were in stage II (25.0%), 18 patients were in stage III (34.6%), and 13 patients were in stage IV (25.0%).
Table 1.
Clinicopathological Characteristics of Patients.
| Variables | Patients with SCCE (n = 52) |
|---|---|
| Gender | |
| Male | 38 (73.1%) |
| Female | 14 (26.9%) |
| Age | |
| ≤60 | 29 (55.8%) |
| >60 | 23 (44.2%) |
| BMI, mean (SD) | 22.3 (3.7) |
| Chronic diseases | |
| No | 44 (84.6%) |
| Yes | 8 (15.4%) |
| Preoperative albumin level | |
| Low | 7 (13.5%) |
| Normal | 45 (86.5%) |
| Surgical approach | |
| Sweet | 35 (63.7%) |
| Ivor Lewis/McKeown | 17 (32.7%) |
| Location | |
| Upper/Middle | 36 (69.2%) |
| Lower/EGJ | 16 (30.8%) |
| Pathological features | |
| Pure SCCE | 35 (76.3%) |
| Combined SCCE | 17 (32.7%) |
| Tumor length | |
| <5cm | 46 (88.5%) |
| ≥5cm | 6 (11.5%) |
| Radical resection | |
| R0 | 47 (90.4%) |
| R1 | 5 (9.6%) |
| pT category | |
| T1 | 7 (13.5%) |
| T2 | 19 (36.5%) |
| T3 | 22 (42.3%) |
| T4 | 4 (7.7%) |
| pN category | |
| N0 | 20 (38.5%) |
| N1 | 10 (19.2%) |
| N2 | 10 (19.2%) |
| N3 | 12 (23.1%) |
| TNM stage | |
| I | 8 (15.4%) |
| II | 13 (25.0%) |
| III | 18 (34.6%) |
| IV | 13 (25.0%) |
| Adjuvant therapy | |
| No adjuvant therapy | 25 (48.1%) |
| Chemotherapy alone | 20 (38.5%) |
| Radiotherapy alone | 4 (7.7%) |
| Radiochemotherapy | 3 (5.8%) |
Abbreviations: BMI, body mass index; SCCE, small cell carcinoma of esophagus; EGJ, esophagogastric junction; SD, standard deviation.
Adjuvant Therapy
Twenty-five (48.1%) patients were treated with surgery alone, whereas 27 (51.9%) patients underwent adjuvant therapy. Among 27 patients, 2 (7.4%) patients underwent perioperative chemotherapy, 18 (66.7%) underwent adjuvant chemotherapy, 4 (14.8%) underwent adjuvant radiotherapy, and 3 (11.1%) underwent adjuvant radiochemotherapy. In a total of 23 patients treated with chemotherapy, etoposide plus cisplatin/carboplatin (EP/EC) was the most frequently used regimen, which was administered in 12 patients (52.2%). The other regimens included cisplatin plus paclitaxel, cisplatin plus vindesine sulfate and bleomycin, cisplatin plus vindesine sulfate and fluorouracil, cisplatin plus docetaxel, cisplatin plus docetaxel and fluorouracil, cisplatin plus etoposide and capecitabine, paclitaxel plus capecitabine, gemcitabine plus capecitabine, and docetaxel plus fluorouracil. In the 4 patients with radiotherapy, the median irradiation dose of was 50 Gray (Gy) (IQR: 40-64 Gy).
Surgical Outcomes and Survival
All the patients received open operative approach. The median operating room time was 222.5 minutes (IQR: 175.0-307.5 minutes). Ten patients (19.2%) received blood transfusion during the surgery, and the median transfusion volume was 550 ml (IQR: 300-800 ml). Eight patients (15.4%) experienced surgical related complications, in which 4 patients had anastomotic fistula, 3 patients had pneumonia, 2 patient had respiratory failure and 1 patient had wound infection. The median postoperative hospital stay was 14 days (IQR: 11-21 days). No patient died during the perioperative period. One patient died (1.9%) within 90 days after the surgery.
The median follow-up time was 73.0 months (IQR: 43.5-102.1 months). The 1-, 3- and 5-year OS rates were 66.3%, 24.1% and 21.4%, respectively. The 1- and 3-year DFS rates were 56.4% and 22.1%, respectively. The median OS time was 17.4 months (95% CI: 13.5-31.3 months), and the median DFS time was 13.4 months (95% CI: 7.7-19.0 months) (Figure 1).
Figure 1.
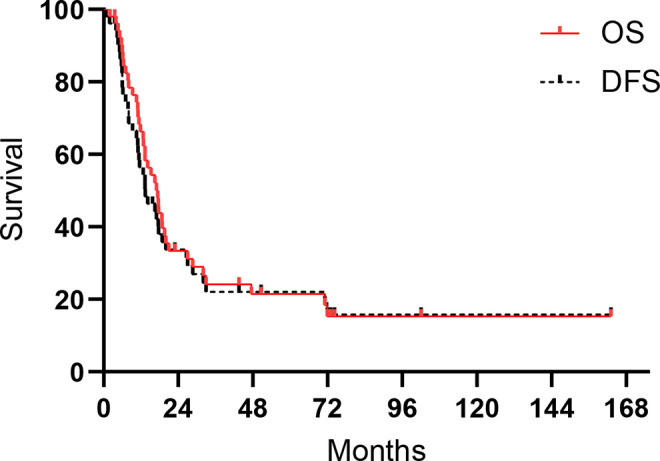
Kaplan-Meier curves for OS and DFS in SCCE.
Patients whose tumors were located in the lower part of the thoracic esophagus and in the EGJ showed significantly better OS (27.0 vs. 13.2 months, log-rank = 5.806, P = 0.016) and DFS (27.0 vs. 11.3 months, log-rank = 5.665, P = 0.017) than those whose tumors were located in the upper and middle parts (Figure 2). Figure 3 shows the association between pathological lymph node status and prognosis. Patients with N0 status experienced a significantly better OS (21.4 vs. 11.6 months, log-rank = 6.293, P = 0.012) and DFS (21.4 vs. 8.6 months, log-rank = 6.305, P = 0.012) than those with N+ status. In addition, tumor length was found to have an effect on prognosis, where patients whose tumor length was shorter than 5 cm had a better OS (17.4 vs. 5.7 months, log-rank = 4.424, P = 0.035) than those who tumor length was longer than 5 cm (Figure 4A). There were no significant differences observed in the different types of surgical approaches (log-rank = 0.747, P = 0.387 for OS; log-rank = 0.587, P = 0.444 for DFS) and in the different regimens of adjuvant therapy (log-rank = 5.655, P = 0.130 for OS; log-rank = 4.182, P = 0.242 for DFS). However, patients who underwent chemotherapy experienced a significantly improved OS (21.0 vs. 14.1 months, log-rank = 4.578, P = 0.032) compared with those who did not (Figure 4B).
Figure 2.
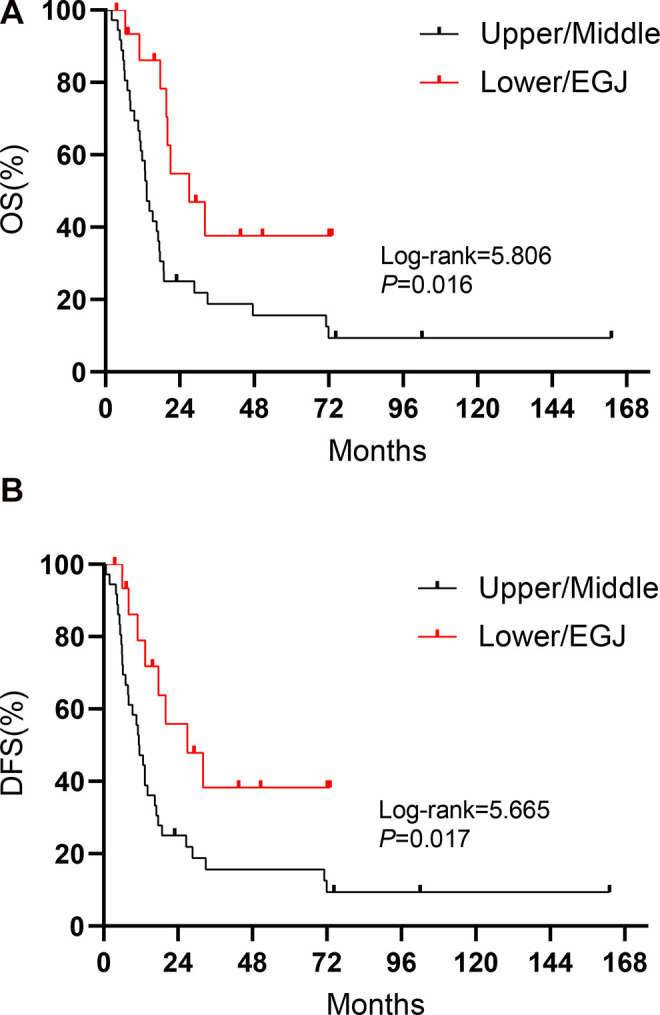
Kaplan-Meier curves for (A) OS and (B) DFS regarding tumor location in SCCE.
Figure 3.
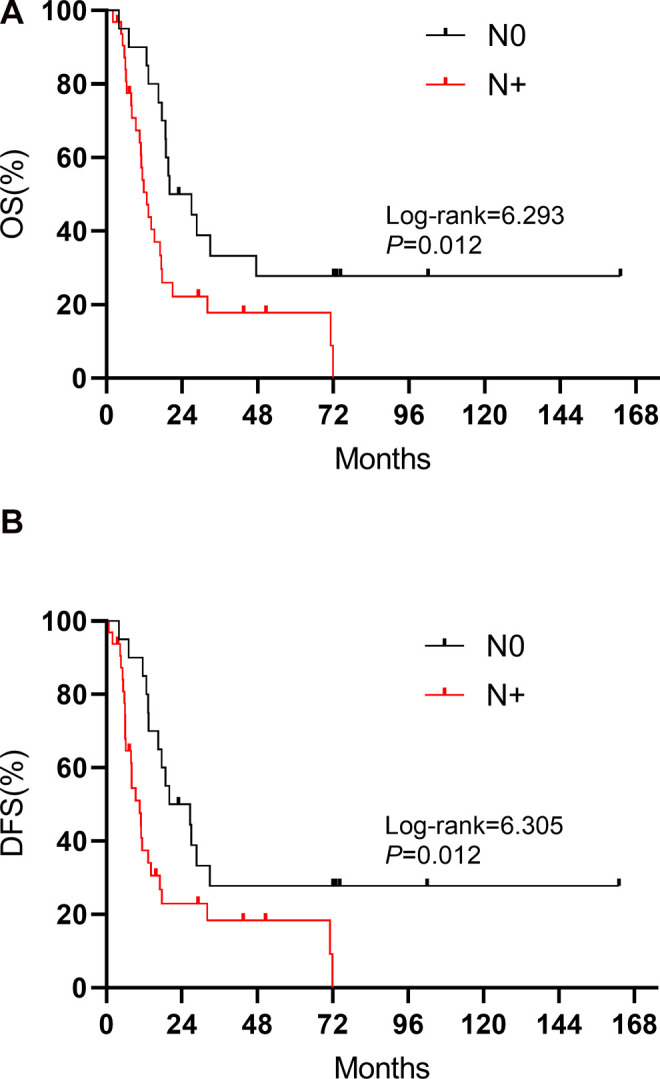
Kaplan-Meier curves for (A) OS and (B) DFS regarding pN status in SCCE.
Figure 4.
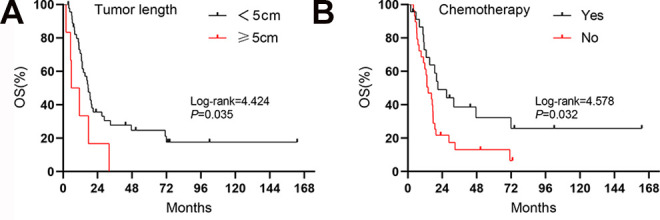
Kaplan-Meier curves for OS regarding (A) tumor length and (B) chemotherapy in SCCE.
Univariate Cox regression analysis suggested that higher BMI, lower tumor location, shorter tumor length, pN0 status and chemotherapy were associated with better OS, and higher BMI, lower tumor location and pN0 status were associated with better DFS (Table 2). Multivariable analysis showed that lower tumor location, shorter tumor length, pN0 status and chemotherapy independently predicted better OS, and lower tumor location as well as pN0 status independently predicted better DFS (Table 3).
Table 2.
Univariate Cox Regression Analysis for Overall Survival and Disease-Free Survival.a
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P |
| Gender | ||||
| Female vs. Male | 1.246 (0.683-2.655) | 0.391 | 1.130 (0.573-2.228) | 0.724 |
| Age | ||||
| >60 vs. ≤60 | 0.878 (0.471-1.637) | 0.682 | 0.770 (0.413-1.436) | 0.411 |
| BMI | 0.915 (0.848-0.988) | 0.023 | 0.921 (0.853-0.993) | 0.033 |
| Chronic diseases | ||||
| Yes vs. No | 0.679 (0.265-1.742) | 0.421 | 0.643 (0.251-1.645) | 0.357 |
| Preoperative albumin level | ||||
| Low vs. Normal | 1.143 (0.446-2.929) | 0.780 | 1.025 (0.400-2.624) | 0.960 |
| Surgical approach | ||||
| Sweet | 1 (reference) | 1 (reference) | ||
| Ivor Lewis/McKeown | 0.736 (0.367-1.478) | 0.389 | 0.763 (0.380-1.531) | 0.446 |
| Location | ||||
| Upper/Middle | 1 (reference) | 1 (reference) | ||
| Lower/EGJ | 0.396 (0.181-0.863) | 0.020 | 0.401 (0.184-0.874) | 0.022 |
| Tumor length | ||||
| <5 cm vs. ≥5cm | 2.489 (1.033-5.999) | 0.042 | 2.076 (0.864-4.986) | 0.102 |
| Pathological features | ||||
| Pure SCCE | 1 (reference) | 1 (reference) | ||
| Combined SCCE | 0.867 (0.431-1.742) | 0.867 | 0.743 (0.371-1.491) | 0.404 |
| pT category | ||||
| T1-2 | 1 (reference) | 1 (reference) | ||
| T3-4 | 1.532 (0.821-2.860) | 0.180 | 1.632 (0.873-3.049) | 0.125 |
| pN category | ||||
| N0 | 1 (reference) | 1 (reference) | ||
| N+ | 2.291 (1.179-4.450) | 0.014 | 2.288 (1.178-4.441) | 0.014 |
| Chemotherapy | ||||
| Yes vs. No | 0.497 (0.258-0.954) | 0.036 | 0.563 (0.293-1.083) | 0.085 |
| Radiotherapy | ||||
| Yes vs. No | 0.693 (0.290-1.656) | 0.409 | 0.716 (0.300-1.710) | 0.452 |
Abbreviations: HR, hazard ratio; 95%CI, 95% confidence interval; vs, versus; BMI, body mass index; EGJ, esophagogastric junction.
a Bold values refer to a significant difference.
Table 3.
Multivariable Cox Regression Analysis for Overall Survival and Disease-Free Survival.a
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| Variables | HR (95%CI) | P | HR (95%CI) | P |
| Location | ||||
| Upper/Middle | 1 (reference) | 1 (reference) | ||
| Lower/EGJ | 0.254 (0.104-0.619) | 0.003 | 0.326 (0.148-0.720) | 0.006 |
| Tumor length | ||||
| ≥ 5 cm vs.<5cm | 3.900 (1.362-11.166) | 0.011 | 0.098 | |
| Chemotherapy | ||||
| Yes vs. No | 0.487 (0.247-0.960) | 0.038 | 0.177 | |
| pN category | ||||
| N+ vs. N0 | 2.344 (1.162-4.731) | 0.017 | 2.766 (1.411-5.422) | 0.003 |
| pT category | 0.593 | 0.168 | ||
| Gender | 0.309 | 0.911 | ||
| Age | 0.565 | 0.238 | ||
| BMI | 0.224 | 0.663 | ||
| Chronic diseases | 0.886 | 0.561 | ||
| Preoperative albumin level | 0.685 | 0.681 | ||
| Surgical approach | 0.729 | 0.724 | ||
| Pathological features | 0.369 | 0.203 | ||
| Radiotherapy | 0.544 | 0.518 | ||
Abbreviations: HR, hazard ratio; 95%CI, 95% confidence interval; vs, versus; EGJ, esophagogastric junction; BMI, body mass index.
a Bold values refer to a significant difference.
Discussion
As the most common small cell carcinoma of the gastrointestinal tract, SCCE is a highly aggressive tumor characterized by early metastases and poor prognosis. 15 To date, there is still no consensus on the standard treatment for SCCE, and the management of SCCE has mostly been extrapolated from the therapeutic strategies of SCLC as well as previous literature. Surgical treatment has been commonly used as a method of local-regional therapy in SCCE, with nearly 66.8% to 75.6% of patients in a limited stage undergoing surgery in China. 1,4,10 This may be because some patients were diagnosed with poorly differentiated carcinoma or other types of carcinoma (e.g., squamous cell carcinoma) for combined SCCE through gastroscopic biopsy before treatment. It is well established that radical esophagectomy is the cornerstone for treating resectable esophageal squamous cell carcinoma as well as adenocarcinoma, 16 however, the role of surgical treatment in SCCE remains largely controversial. In this study, we retrospectively reviewed data from SCCE patients who underwent radical surgical resection at our institution to provide more clinical evidence regarding the efficacy of surgical treatment and to explore relative prognostic factors.
Some researchers have advocated that surgical treatment is not helpful compared with less traumatic radiotherapy. These researchers found that as a strategy for local-regional therapy, surgical treatment had a similar effect to radiotherapy. 8,9 Meng et al even reported a significantly worse survival outcome of surgery plus chemotherapy, with 17.5 median survival months compared with 33 months in patients treated with radiotherapy in addition to chemotherapy. 7 Similar results were obtained by Chen and colleges. 10 In Chen’s study, the median survival time of surgery plus chemotherapy (12.5 months) was shorter than that of radiotherapy plus chemotherapy (25.7 months) in patients with stage IIB/III disease. Therefore, performing radical surgery seems unnecessary. Nevertheless, Chen also reported an opposite finding that patients with stage I/IIA disease tended to benefit from surgical treatment alone. Likewise, Zou et al concluded that radical surgical resection was sufficient for patients with stage I/IIA disease, whereas adjuvant treatment did not further improve survival. 17 These findings were inspiring; however, criticism arose when researchers recommended that stage I/IIA patients should undergo surgical procedures alone. 18 Since small cell carcinoma is inherently a systemic disease whereas chemotherapy is widely accepted as the basis of treatment, 11 critics have argued that chemotherapy should be used for all SCCE patients regardless of tumor stage, as several studies have conclusively shown benefits of this approach. 1,4,5,8 Moreover, 2 large-scale studies (583 and 387 patients) both concluded that surgical treatment could be beneficial when conducted as a part of a chemotherapy-based multidisciplinary therapeutic strategy, 12,13 and the same conclusions were found in several small-scale studies. 6,19 -21 Li et al identified 41 SCCE patients who underwent surgery from SEER database between 1975 and 2016, and they found that patients could benefit from either combined chemoradiotherapy (median OS: 19 months) or combined chemotherapy (median OS: 14 months) as compared to surgery alone (median OS: 4 months). 22 Our study also reached the same result, where surgically treated patients experienced significantly improved OS (21.0 vs. 14.1 months, P = 0.032) when they underwent further chemotherapy. The median OS time in this study was 17.4 months (95% CI: 13.5-21.3 months), which was consistent with previous studies regarding surgical treatment (range from 17.5 to 23.0 months), 7,17,21 and was longer than the reported median OS time in nonsurgical management patients (range from 8 to 16.1 months). 23 -25 Note that the OS and DFS curves were mostly overlapping in our patients, indicating patients died soon after the tumor recurrence, showing the aggressive nature of the SCCE. Hence, on the basis of previous literature as well as the results of this study, we believe that chemotherapy needs to be used for resectable SCCE patients who underwent radical esophagectomy.
Several clinical pathological factors were found to be independently related to patient prognosis in our research. Our study suggested that tumor location was an independent prognostic indicator for survival, where tumors located in the lower part of the esophagus and in the EGJ predicted a better prognosis (HR: 0.280, 95%CI: 0.116-0.678, P = 0.005 for OS; HR: 0.317, 95%CI: 0.142-0.709, P = 0.005 for DFS). A similar trend has been reported by some previous studies of SCCE. 7,26 as well as in studies of non-small-cell esophageal carcinoma. 27,28 Despite Ivor Lewis/McKeown approaches are anatomically better than the Sweet approach regarding the upper mediastinal lymph node dissection, no difference in OS and DFS between surgical approaches were found in our study. This could be caused by the unbalanced baseline characteristics between surgical approaches as well as the small sample size. The Sweet approach used to be popular in China but was not recommended by recent Chinese expert consensus in treating thoracic esophageal squamous cell carcinoma. 29,30 More evidence is needed to discuss the optimal surgical approaches in treating SCCE. Although some studies have advocated that tumor length is not associated with prognosis, 7,8,21,26 our study, as well as some previous studies, 4,17 found that a tumor length longer than 5 cm independently predicted a worse OS in SCCE (HR: 5.656, 95% CI: 2.018-15.856, P = 0.001). Based on this finding, we suggest that tumor length may serve as an effective indicator to predict prognosis in SCCE. However, considering the conflicting results regarding the predictive value of tumor length in either SCCE or non-small-cell esophageal carcinoma. 31,32 this issue still requires further verification with larger sample sizes. It has been well established that lymph node metastasis is a strong prognosis factor in non-small-cell esophageal cancer. Studies have also suggested that the same trend exists in SCCE. 10,17,21 The experience at our institution confirmed the previous literature with the results showing that patients with pN0 status experienced a significantly better OS and DFS than those with N+ status. Lymph node metastasis was also found to be independently associated with poor OS and DFS.
There are some limitations in this research. First, as with any retrospective study, selection biases existed along with data collection. In addition, due to the rarity of this disease, the sample size in this study was quite small despite the effort to prolong the observation period (from 1993 to 2014), which may have weakened the power of the current results. However, the long study period may have led to potential biases regarding the development of the therapeutic strategy as well as medical technology. For example, minimally invasive esophagectomy (such as thoraco-laparoscopic esophagectomy and robotic esophagectomy) has gradually become a standard approach in some countries, along with lower postoperative complications, faster recovery, and similar or even better survival as compared to traditional open thoracic surgery. 33 -35 Moreover, although the most commonly (52.2%) used regimen of chemotherapy in this cohort (EP/EC) was in line with the recommendations for SCLC, 11 47.8% patients still underwent various regimens according to doctors’ preferences, which may have presented an unstable effect of chemotherapy. It should be noted that in recent years immunotherapy combined with traditional treatments shows promising therapeutic efficacy in several types of tumors including advanced esophageal cancer. 36 There is an ongoing phase II clinical trial (NCT03811379) investigating the role of toripalimab, a monoclonal humanized IgG4 PD-1 antibody, in the second-line treatment of SCCE. The results of adding immunotherapy to treat SCCE are highly expected, and future studies exploring the combination of immunotherapy with surgery are needed. Despite these limitations, our study provides a comprehensive analysis for the surgical treatment of SCCE patients with detailed clinicopathological characteristics, adequate follow-up time and a relatively large study population. We believe that our experiences in the treatment of this rare disease will help doctors better understand SCCE and provide hints for treatment decisions, which may ultimately benefit SCCE patients.
Conclusion
In conclusion, SCCE is a rare, highly aggressive malignant disease with a poor prognosis. There is still no consensus on the standard therapeutic strategy for SCCE. The present study suggests that radical esophagectomy in combination with chemotherapy has better outcomes than surgery alone for resectable SCCE. Higher tumor location, longer tumor length, lymph node metastasis and not undergoing chemotherapy independently predict worse prognoses.
Acknowledgments
Ningbo Fan would like to thank Guangzhou Elite Scholarship Council (GESC) for the study financial support.
Abbreviations
- SCCE
primary small cell carcinoma of the esophagus
- OS
overall survival
- DFS
disease-free survival
- SCLC
small cell lung carcinoma
- pT
pathological T status
- pN
N status
- pM
pathological M status
- pTNM
pathological TNM stage
- EUS
endoscopic ultrasound
- WHO
world health organization
- SC
small cell carcinoma
- SCC
squamous cell carcinoma
- AC
adenocarcinoma
- LCC
large cell carcinoma
- SD
standard deviation
- IQR
interquartile range
- BMI
body mass index
- Gy
Gray
- EP
etoposide + cisplatin
- EC
etoposide + carboplatin.
Authors’ Note: Ningbo Fan, MD, and Zhen Wang, MD, contributed equally to this paper. This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. SZR2020-054). Informed consents were omitted due to the retrospective nature.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Guangdong Medical Science and Technology Research Fund (grant A2017356), Guangdong Esophageal Cancer Institute Science and Technology Program (grant Q201902) and Sun Yat-sen University Young Teacher Training Project (grant KY051083).
ORCID iD: Ningbo Fan, MD  https://orcid.org/0000-0002-1622-6747
https://orcid.org/0000-0002-1622-6747
References
- 1. Lu XJ, Luo JD, Ling Y, et al. Management of small cell carcinoma of esophagus in China. J Gastrointest Surg. 2013;17(7):1181–1187. doi:10.1007/s11605-013-2204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKeown F. Oat-cell carcinoma of the oesophagus. J Pathol Bacteriol. 1952;64(4):889–891. doi:10.1002/path.1700640420 [DOI] [PubMed] [Google Scholar]
- 3. Song Y, Wang W, Tao G, Zhu W, Zhou X, Pan P. Survival benefit of radiotherapy to patients with small cell esophagus carcinoma: an analysis of Surveillance Epidemiology and End Results (SEER) data. Oncotarget. 2016;7(13):15474–15480. doi:10.18632/oncotarget.6764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lv J, Liang J, Wang J, et al. Primary small cell carcinoma of the esophagus. J Thorac Oncol. 2008;3(12):1460–1465. doi:10.1097/JTO.0b013e31818e1247 [DOI] [PubMed] [Google Scholar]
- 5. Ku GY, Minsky BD, Rusch VW, Bains M, Kelsen DP, Ilson DH. Small-cell carcinoma of the esophagus and gastroesophageal junction: review of the Memorial Sloan-Kettering experience. Ann Oncol. 2008;19(3):533–537. doi:10.1093/annonc/mdm476 [DOI] [PubMed] [Google Scholar]
- 6. Medgyesy CD, Wolff RA, Putnam JB, Jr, Ajani JA. Small cell carcinoma of the esophagus: the University of Texas M. D. Anderson Cancer Center experience and literature review. Cancer. 2000;88(2):262–267. [PubMed] [Google Scholar]
- 7. Meng MB, Zaorsky NG, Jiang C, et al. Radiotherapy and chemotherapy are associated with improved outcomes over surgery and chemotherapy in the management of limited-stage small cell esophageal carcinoma. Radiother Oncol. 2013;106(3):317–322. doi:10.1016/j.radonc.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 8. Ding J, Ji J, Zhu W, et al. A retrospective study of different treatments of limited-stage small-cell esophageal carcinoma and associated prognostic factor analysis. Dis Esophagus. 2013;26(7):696–702. doi:10.1111/dote.12017 [DOI] [PubMed] [Google Scholar]
- 9. Raja S, Rice TW, Rajeswaran J, Zhong J, Blackstone EH. Esophageal small-cell cancer: study of a rare disease. Dis Esophagus. 2013;26(7):690–695. doi:10.1111/dote.12022 [DOI] [PubMed] [Google Scholar]
- 10. Chen WW, Wang F, Chen S, et al. Detailed analysis of prognostic factors in primary esophageal small cell carcinoma. Ann Thorac Surg. 2014;97(6):1975–1981. doi:10.1016/j.athoracsur.2014.02.037 [DOI] [PubMed] [Google Scholar]
- 11. Network NCC. NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer, Version 1.2019. National Comprehensive Cancer Network; Updated April 9, 2019. Accessed April 9, 2019. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf [Google Scholar]
- 12. Wong AT, Shao M, Rineer J, Osborn V, Schwartz D, Schreiber D. Treatment and survival outcomes of small cell carcinoma of the esophagus: an analysis of the National Cancer Data Base. Dis Esophagus. 2017;30(2):1–5. doi:10.1111/dote.12487 [DOI] [PubMed] [Google Scholar]
- 13. Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol. 2013;20(13):4239–4244. doi:10.1245/s10434-013-3167-3 [DOI] [PubMed] [Google Scholar]
- 14. Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. WHO Classification of Tumours of the Digestive System. Vol. 13-14. IARC Press; 2010. [Google Scholar]
- 15. Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004;22(13):2730–2739. doi:10.1200/JCO.2004.09.075 [DOI] [PubMed] [Google Scholar]
- 16. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. The Lancet. 2017;390(10110):2383–2396. doi:10.1016/s0140-6736(17)31462-9 [DOI] [PubMed] [Google Scholar]
- 17. Zou B, Li T, Zhou Q, et al. Adjuvant therapeutic modalities in primary small cell carcinoma of esophagus patients: a retrospective cohort study of multicenter clinical outcomes. Medicine (Baltimore). 2016;95(17):e3507. doi:10.1097/MD.0000000000003507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Purwar P, Jiwnani S, Karimundackal G, Pramesh CS. Management of esophageal small cell carcinoma. Ann Thorac Surg. 2015;99(4):1488. doi:10.1016/j.athoracsur.2014.11.029 [DOI] [PubMed] [Google Scholar]
- 19. Kuo CH, Hsieh CC, Chan ML, et al. Small cell carcinoma of the esophagus: a report of 16 cases from a single institution and literature review. Ann Thorac Surg. 2011;91(2):373–378. doi:10.1016/j.athoracsur.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 20. Chen SB, Yang JS, Yang WP, et al. Treatment and prognosis of limited disease primary small cell carcinoma of esophagus. Dis Esophagus. 2011;24(2):114–119. doi:10.1111/j.1442-2050.2010.01112.x [DOI] [PubMed] [Google Scholar]
- 21. Xie MR, Xu SB, Sun XH, et al. Role of surgery in the management and prognosis of limited-stage small cell carcinoma of the esophagus. Dis Esophagus. 2015;28(5):476–482. doi:10.1111/dote.12230 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Ma J, Wang H, Niu J, Zhou L. Population-based analysis of small cell carcinoma of the esophagus using the SEER database. J Thorac Dis. 2020;12(7):3529–3538. doi:10.21037/jtd-20-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakajima Y, Zenda S, Minashi K, et al. Non-surgical approach to small cell carcinoma of the esophagus: does this rare disease have the same tumor behavior as SCLC? Int J Clin Oncol. 2012;17(6):610–615. doi:10.1007/s10147-011-0332-1 [DOI] [PubMed] [Google Scholar]
- 24. Yau KK, Siu WT, Wong DC, et al. Non-operative management of small cell carcinoma of esophagus. Dis Esophagus. 2007;20(6):487–490. doi:10.1111/j.1442-2050.2007.00635.x [DOI] [PubMed] [Google Scholar]
- 25. Atsumi K, Shioyama Y, Nomoto S, et al. Chemoradiation for small cell esophageal carcinoma: report of 11 cases from multi-institution experience. J Radiat Res. 2010;51(1):15–20. doi:10.1269/jrr.09074 [DOI] [PubMed] [Google Scholar]
- 26. Wang HH, Zaorsky NG, Meng MB, et al. Multimodality therapy is recommended for limited-stage combined small cell esophageal carcinoma. Onco Targets Ther. 2015;8:437–444. doi:10.2147/OTT.S76048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Emi M, Hihara J, Hamai Y, Furukawa T, Ibuki Y, Okada M. Clinicopathologic features of submucosal esophageal squamous cell carcinoma. Ann Thorac Surg. 2017;104(6):1858–1864. doi:10.1016/j.athoracsur.2017.06.037 [DOI] [PubMed] [Google Scholar]
- 28. Yamada M, Oda I, Tanaka H, et al. Tumor location is a risk factor for lymph node metastasis in superficial Barrett’s adenocarcinoma. Endosc Int Open. 2017;5(9):E868–E874. doi:10.1055/s-0043-115388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Fang W, Yu Z, et al. Chinese expert consensus on mediastinal lymph node dissection in esophagectomy for esophageal cancer (2017 edition). J Thorac Dis. 2018;10(4):2481–2489. doi:10.21037/jtd.2018.03.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mao Y, Yu Z, You B, et al. Society for translational medicine expert consensus on the selection of surgical approaches in the management of thoracic esophageal carcinoma. J Thorac Dis. 2019;11(1):319–328. doi:10.21037/jtd.2018.12.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haisley KR, Hart KD, Fischer LE, et al. Increasing tumor length is associated with regional lymph node metastases and decreased survival in esophageal cancer. Am J Surg. 2016;211(5):860–866. doi:10.1016/j.amjsurg.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 32. Bollschweiler E, Baldus SE, Schroder W, Schneider PM, Holscher AH. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94(5):355–363. doi:10.1002/jso.20569 [DOI] [PubMed] [Google Scholar]
- 33. Gisbertz SS, Hagens ERC, Ruurda JP, et al. The evolution of surgical approach for esophageal cancer. Ann N Y Acad Sci. 2018;1434(1):149–155. doi:10.1111/nyas.13957 [DOI] [PubMed] [Google Scholar]
- 34. National Health Commission of the People’s Republic of China. Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version). Chin J Cancer Res. 2019;31(2):223–258. doi:10.21147/j.issn.1000-9604.2019.02.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gottlieb-Vedi E, Kauppila JH, Malietzis G, Nilsson M, Markar SR, Lagergren J. Long-term survival in esophageal cancer after minimally invasive compared to open esophagectomy: a systematic review and meta-analysis. Ann Surg. 2019;270(6):1005–1017. doi:10.1097/SLA.0000000000003252 [DOI] [PubMed] [Google Scholar]
- 36. Vivaldi C, Catanese S, Massa V, et al. Immune checkpoint inhibitors in esophageal cancers: are we finally finding the right path in the mist? Int J Mol Sci. 2020;21(5):1658. doi:10.3390/ijms21051658 [DOI] [PMC free article] [PubMed] [Google Scholar]


