Introduction
Merkel cell carcinoma (MCC) is a rare aggressive cutaneous neuroendocrine tumor with a high risk of locoregional recurrence and distant metastasis. Immune checkpoint inhibitors (ICIs), avelumab and pembrolizumab, have been approved by the US Food and Drug Administration for the treatment of advanced MCC.1
Talimogene laherparepvec (T-VEC) is a genetically modified type 1 oncolytic herpes simplex virus encoding granulocyte-macrophage colony-stimulating factor. It has been approved by the US Food and Drug Administration for the local treatment of unresectable cutaneous, subcutaneous, and nodal melanoma lesions; however, the clinical benefits of T-VEC in immunosuppressed patients with MCC, including solid organ transplant recipients, are not yet known. We report a case of surgically unresectable stage IIIB MCC in a patient with a history of heart transplantation and prior radiation, who had a complete response to intralesional T-VEC.
Case report
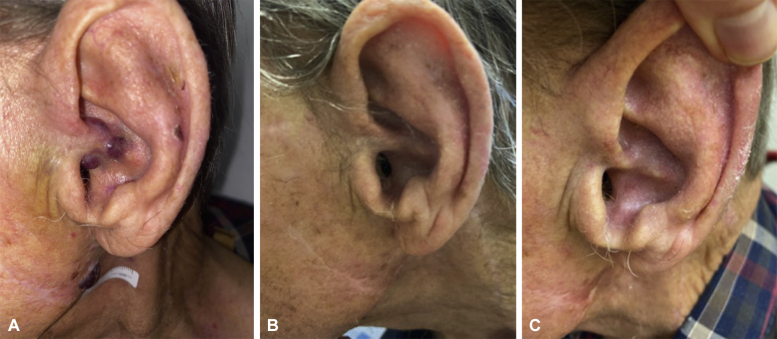
A 78-year-old Caucasian man presented with a history of heart transplantation performed 18 years ago; on chronic immunosuppression with cyclosporine (50 mg twice a day); and with metastatic cutaneous squamous cell carcinoma of the left parotid gland, status post excision, lymph node dissection, and 66 Gy of adjuvant radiation 1 year prior. He presented to our multidisciplinary MCC clinic with a primary MCC lesion on the left conchal bowl (Fig 1, A) and in-transit MCC metastases throughout the prior radiation field, including the left lateral neck, left lower neck, and the left clavicle. The positron emission tomography and computed tomography scan was unremarkable for distant disease and serology was negative for Merkel cell polyomavirus oncoprotein antibodies. A multidisciplinary tumor board recommended treatment with T-VEC as the patient was not a candidate for ICI given his cardiac transplant history, and a reirradiation in the prior radiation field was not advised. He received T-VEC injections into the left neck per protocol with an initial test dosage of 2 mL of 106 plaque-forming units/mL, followed by injections every 2 weeks into all lesions (total dosage per treatment cycle of 1-3 mL of 108 plaque-forming units/mL). Clinical disease at all sites regressed after the second round of 108 plaque-forming units/mL injections (Fig 1, B) and the complete pathologic response after 6 cycles of T-VEC was confirmed by skin biopsy. Furthermore, there was no evidence of the clinical disease at the 5-month follow-up (Fig 1, C). Toxicity was limited to grade 1 fatigue, and there was no evidence of cardiac rejection.
Fig 1.
A, Merkel cell carcinoma of the left conchal bowl and the left jawline, prior to T-VEC injections. B, Complete clinical response of the left conchal bowl and left jawline lesions following 2 T-VEC injections to each site, 2 weeks apart. C, 5-month follow-up with no evidence of clinical disease. T-VEC, Talimogene laherparepvec.
Discussion
T-VEC is a live, attenuated virus; therefore, its administration in immunocompromised patients has been limited because of the concerns for disseminated viral infection. Additionally, organ transplant recipients were excluded from immunotherapy clinical trials because of the concerns for transplant rejection, given that some recipients of PD-1 inhibitors had kidney and heart rejections.2,3 Notably, T-VEC activates antitumor innate immunity through granulocyte-macrophage colony-stimulating factor-mediated dendritic cell maturation and an increased tumor antigen presentation in the skin, whereas ICI activates a systemic, less specific adaptive immune response that may undo the tolerance to the donor organ.
To date, there have been 5 patients with MCC who were reportedly treated with T-VEC because of poor surgical candidacy or unresectable recurrent disease, and 4 of these patients had a complete durable clinical response. There have been 3 patients with MCC who were reportedly treated with T-VEC in combination with PD-1/PD-L1 ICI, and all patients achieved a clinical response.4, 5, 6, 7 T-VEC has also been used safely in a solid organ transplant patient with melanoma.8 To our knowledge, this is the first case of successful treatment of MCC in a patient with a history of solid organ transplant using oncolytic virus therapy.
Phase I and II trials for examining T-VEC therapy in MCC and other cutaneous malignancies are currently underway. Although these prospective data will be informative, these are unlikely to guide the management for transplant or immunocompromised patients who are excluded from clinical trials and for whom certain treatment options, such as ICIs, are contraindicated. Our case demonstrates T-VEC as a successful treatment option in a patient with locally advanced MCC who had limited therapeutic options because of his solid organ transplant and radiation history.
Conflicts of interest
Dr Kwong is a consultant for Genentech, Oncoderm, and Happy 2nd Birthday and is on the Advisory Board for Kyowa Kirin. Drs Hirotsu, Reddy, and Zaba and authors Hua, Tran, and Morris have no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: The Stanford University's institutional review board has approved this study.
References
- 1.Nghiem P., Bhatia S., Lipson E.J. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019;37(9):693–702. doi: 10.1200/JCO.18.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatachalam K., Malone A.F., Heady B., Santos R.D., Alhamad T. Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients. Transplantation. 2020;104(5):1041–1047. doi: 10.1097/TP.0000000000002914. [DOI] [PubMed] [Google Scholar]
- 3.Owonikoko T.K., Kumar M., Yang S. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: a case report. Cancer Immunol Immunother. 2017;66(1):45–50. doi: 10.1007/s00262-016-1918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen M.H.K., Leong S.P., Abendroth R., Kashani-Sabet M., Kim K.B. Complete clinical response to intralesional talimogene laherparepvec injection in a patient with recurrent, regionally advanced Merkel cell carcinoma. JAAD Case Rep. 2019;5(10):849–851. doi: 10.1016/j.jdcr.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westbrook B.C., Norwood T.G., Terry N.L.J., McKee S.B., Conry R.M. Talimogene laherparepvec induces durable response of regionally advanced Merkel cell carcinoma in 4 consecutive patients. JAAD Case Rep. 2019;5(9):782–786. doi: 10.1016/j.jdcr.2019.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lara K.M., In G.K., Matcuk G.R., Jr., Mehta A., Hu J.S. Talimogene laherparepvec in combination with pembrolizumab leads to a complete response in a patient with refractory Merkel cell carcinoma. JAAD Case Rep. 2018;4(10):1004–1006. doi: 10.1016/j.jdcr.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knackstedt R., Sussman T.A., McCahon L., Song J.M., Funchain P., Gastman B. Pre-treated anti-PD-1 refractory Merkel cell carcinoma successfully treated with the combination of PD-1/PD-L1 axis inhibitors and TVEC: a report of two cases. Ann Oncol. 2019;30(8):1399–1400. doi: 10.1093/annonc/mdz187. [DOI] [PubMed] [Google Scholar]
- 8.Ressler J., Silmbrod R., Stepan A. Talimogene laherparepvec (T-VEC) in advanced melanoma: complete response in a heart and kidney transplant patient. A case report. Br J Dermatol. 2019;181(1):186–189. doi: 10.1111/bjd.17783. [DOI] [PMC free article] [PubMed] [Google Scholar]