Abstract
This study aims to comprehensively explore the phytoconstituents as well as investigate the different biological activities of Chasmanthe aethiopica (Iridaceae) for the first time. Metabolic profiling of the leaf methanol extract of C. aethiopica (CAL) was carried out using HPLC-PDA-ESI-MS/MS. Twenty-nine compounds were annotated belonging to various phytochemical classes including organic acids, cinnamic acid derivatives, flavonoids, isoflavonoids, and fatty acids. Myricetin-3-O-rhamnoside was the major compound identified. GLC/MS analysis of the n-hexane fraction (CAL-A) resulted in the identification of 45 compounds with palmitic acid (16.08%) and methyl hexadecanoic acid ester (11.91%) representing the major constituents. CAL-A exhibited a potent anti-allergic activity as evidenced by its potent inhibition of β-hexosaminidase release triggered by A23187 and IgE by 72.7% and 48.7%, respectively. Results were comparable to that of dexamethasone (10 nM) in the A23187 degranulation assay showing 80.7% inhibition for β-hexosaminidase release. Both the n-hexane (CAL-A) and dichloromethane (CAL-B) fractions exhibited potent anti-inflammatory activity manifested by the significant inhibition of superoxide anion generation and prohibition of elastase release. CAL showed anti-hyperglycemic activity in vivo using streptozotocin-induced diabetic rat model by reducing fasting blood glucose levels (FBG) by 53.44% as compared with STZ-treated rats along with a substantial increase in serum insulin by 22.22%. Molecular modeling studies indicated that dicaffeoylquinic acid showed the highest fitting with free binding energies (∆G) of −47.24 and −60.50 Kcal/mol for human α-amylase and α-glucosidase, respectively confirming its anti-hyperglycemic activity. Thus, C. aethiopica leaf extract could serve as an effective antioxidant natural remedy combating inflammation, allergy, and hyperglycemia.
Keywords: anti-allergic, antihyperglycemic, Chasmanthe aethiopica, anti-inflammatory, GC/MS, LC/MS
1. Introduction
Oxidative stress can be defined as the disturbance in the balance between antioxidants and free radicals within the body that leads to cell and tissue destruction. During the normal process of metabolism, the body releases free radicals with the concomitant production of natural antioxidants to antagonize and neutralize these free radicals. However, a multitude of factors results in the massive production of free radicals that exaggerates oxidative stress including the unhealthy diet and lifestyle, environmental factors such as exposure to radiation and pollution as well as the natural body immune response that temporarily contributes to oxidative stress [1]. Oxidative stress can provoke an inflammation that consequently releases excess free radicals that in turn promote more oxidative stress resulting in a vicious cycle. Chronic exposure to inflammation because of oxidative stress can elicit various human ailments such as diabetes mellitus, allergy, and cardiovascular disease in addition to cancer, and neurodegenerative disorders [2]. ROS can oxidize guanosine in DNA to 8-oxoguanosine which can lead to mutations and in consequence the genetic diseases such as cancer.
Diabetes mellitus has been recently considered the third leading cause of death following cancer and cardiovascular disorders with high prevalence all over the globe [3]. It adversely affects human health causing serious harmful changes in all parts of the body particularly the nerves and blood vessels [4]. Allergy is another global health threat that is also triggered by oxidative stress. It can threaten life in cases of severe asthma and anaphylaxis and can also interfere with the quality of life in cases of chronic allergic conditions exemplified by allergic rhinitis and eczema [5]. Despite the presence of numerous synthetic agents that could alleviate inflammation, relieve allergy, or manipulate hyperglycemia, drugs of natural origin always constitute the main key player in curing various human ailments due to their acceptable prices and safety in comparison with synthetic agents [6].
Chasmanthe aethiopica (Iridaceae) (syn. Antholyza aethiopica L.) is native to South Africa and is characterized by being a deciduous, bulbous plant that grows up to a height of 0.6 m with pale green lanceolate leaves [7]. Although members of family Iridaceae are popular by the presence of a wide array of compounds represented by flavonoids, isoflavonoids, xanthones, and quinones to which numerous biological activities including antioxidant, anti-inflammatory, antidiabetic, and phytoestrogenic effects are attributed [8,9], nothing was found in the literature regarding the phytochemical profile or the biological potential of C. aethiopica.
This study aims to comprehensively study the phytoconstituents as well as biologically investigate the different activities of the plant for the first time. Herein, we evaluated the anti-allergic and anti-inflammatory activity of the 80% methanol leaf extract of C. aethiopica (CAL) as well as its successive fractions in vitro. Additionally, the antihyperglycemic potential of CAL was assessed using streptozotocin-induced diabetes in a rat model. Moreover, metabolic profiling of CAL and its bioactive fractions was performed using LC/MS (Liquid Chromatography coupled with Mass Spectrometry) and GLC/MS (Gas–Liquid Chromatography coupled with Mass Spectrometry). To understand the reasons behind the antihyperglycemic activity, molecular modeling experiments were performed on the identified compounds from the bioactive fractions using human α-glucosidase and α-amylase, as crucial enzymes entangled in the occurrence and dissemination of diabetes.
2. Results and Discussion
2.1. Gas–Liquid Chromatography Coupled with Mass Spectroscopy (GLC-MS) Analysis
Characterization of the n-hexane fraction (CAL-A) of the leaf methanol extract (CAL) of C. aethiopica using GLC analysis (Figure 1) resulted in the tentative identification of 45 compounds belonging to sterols and fatty acids accounting for 91.62% of the total n-hexane fraction constituents as illustrated in Table 1. Palmitic acid (16.08 %) and methyl hexadecanoic acid ester (11.91%) represent the major constituents in the CAL-A fraction.
Figure 1.
GLC-chromatogram of C. aethiopica L. n-hexane fraction (CAL-A) on Rtx-5MS column.
Table 1.
Chemical profile of C. aethiopica L. n-hexane fraction (CAL-A) by GLC-MS.
| No. | Rt | Compound Name | RIExp. a | RILit b | Content% | Molecular Formula | Identification c |
|---|---|---|---|---|---|---|---|
| 1. | 31.44 | Methyl pentadecanoic acid ester | 1806 | 1812 | 0.15 | C16H32O2 | MS, RI |
| 2. | 31.68 | Neophytadiene | 1818 | 1817 | 0.50 | C20H38 | MS, RI |
| 3. | 31.85 | Hexahydrofarnesyl acetone | 1826 | 1825 | 5.30 | C18H36O | MS, RI |
| 4. | 33.47 | 7-Hexadecenoic acidmethyl ester (Z) | 1903 | 1900 | 0.68 | C17H34O2 | MS, RI |
| 5. | 33.54 | Hexadecanoic acid methyl ester | 1907 | 1907 | 11.91 | C17H34O2 | MS, RI |
| 6. | 34.45 | Palmitic acid | 1950 | 1950 | 16.08 | C16H32O2 | MS, RI |
| 7. | 34.99 | Heptadecanoic acid methyl ester | 1976 | 1978 | 0.14 | C18H36O2 | MS, RI |
| 8. | 35.55 | 15-Methyl hexadecanoic acid methyl ester | 2003 | 1996 | 0.52 | C18H36O2 | MS, RI |
| 9. | 36.77 | 16-Methyl heptadecanoic acid methyl ester | 2070 | 2077 | 0.25 | C19H38O2 | MS, RI |
| 10. | 36.90 | Linoleic acid methyl ester | 2077 | 2075 | 2.42 | C19H34O2 | MS, RI |
| 11. | 37.03 | Oleic acidmethyl ester | 2085 | 2085 | 4.10 | C19H36O2 | MS, RI |
| 12. | 37.25 | Phytol | 2096 | 2096 | 1.50 | C20H40O | MS, RI |
| 13. | 37.48 | Methyl stearate | 2109 | 2109 | 2.08 | C19H38O2 | MS, RI |
| 14. | 37.89 | Linolenic acid | 2131 | 2134 | 3.74 | C18H30O2 | MS, RI |
| 15. | 38.24 | Stearic acid | 2150 | 2155 | 0.83 | C18H36O2 | MS, RI |
| 16. | 41.08 | Eicosanoic acid methyl ester | 2306 | 2307 | 0.57 | C21H42O2 | MS, RI |
| 17. | 41.62 | 4,8,12,16-Tetramethylheptadecan-4-olide | 2336 | 2364 | 1.99 | C21H40O2 | MS |
| 18. | 42.84 | 2,2′-Methylene-bis-(6-tert butyl-4-methylphenol) | 2403 | 2398 | 0.13 | C23H32O2 | MS, RI |
| 19. | 43.86 | n-Pentacosane | 2469 | 2500 | 1.46 | C25H52 | MS |
| 20. | 44.22 | Palmitic acid β-monoglyceride | 2492 | 2498 | 0.16 | C19H38O4 | MS, RI |
| 21. | 44.41 | Docosanoic acid methyl ester Behenic acid methyl ester |
2504 | 2492 | 0.40 | C23H46O2 | MS, RI |
| 22. | 45.44 | 11-Methylpentacosane | 2570 | 2565 | 1.99 | C26H54 | MS, RI |
| 23. | 45.97 | Tricosanoic acid methyl ester | 2605 | 2615 | 0.05 | C24H48O2 | MS, RI |
| 24. | 46.95 | 2-Methylhexacosane | 2668 | 2663 | 2.41 | C27H56 | MS, RI |
| 25. | 47.49 | Tetracosanoic acid methyl ester | 2702 | 2714 | 0.14 | C25H50O2 | MS, RI |
| 26. | 48.42 | 2-Methylheptacosane | 2762 | 2762 | 2.47 | C28H58 | MS |
| 27. | 49.43 | α-Tocospiro A | 2827 | 2860 | 4.13 | C29H50O4 | MS |
| 28. | 49.74 | α-Tocospiro B | 2847 | 2881 | 4.62 | C29H50O4 | MS |
| 29. | 49.83 | 2-Methyl octacosane, | 2852 | 2857 | 3.57 | C29H60 | MS, RI |
| 30. | 50.20 | Cholesta-2,4-diene | 2876 | 2872 | 0.09 | C27H44 | MS, RI |
| 31. | 51.19 | 15-Methylnonacosane | 2940 | 2935 | 2.05 | C30H62 | MS, RI |
| 32. | 52.51 | n-Triacontane | 3025 | 3003 | 2.02 | C30H62 | MS, RI |
| 33. | 52.94 | β-Sitosterol propionate | 3053 | - | 1.77 | C32H54O2 | MS |
| 34. | 53.31 | α-Tocopherol | 3076 | 3112 | 1.76 | C29H50O2 | MS |
| 35. | 53.84 | Hentriacontane | 3110 | 3103 | 1.33 | C31H64 | MS, RI |
| 36. | 55.31 | Dotriacontane | 3205 | 3202 | 0.64 | C32H66 | MS, RI |
| 37. | 56.36 | Chondrillasterol | 3272 | 3295 | 1.54 | C29H48O | MS, RI |
| 38. | 57.16 | β-Amyrin | 3323 | 3337 | 0.42 | C30H50O | MS, RI |
| 39. | 57.43 | γ-Sitosterol | 3341 | 3351 | 0.17 | C29H50O | MS, RI |
| 40. | 58.06 | α-Amyrin | 3381 | 3376 | 1.53 | C30H50O | MS, RI |
| 41. | 58.23 | Stigmasta-3,5-dien-7-one | 3392 | - | 0.29 | C29H46O | MS |
| 42. | 59.07 | β-Amyrin acetate | 3446 | 3438 | 0.76 | C32H52O2 | MS, RI |
| 43. | 60.05 | Lupeol acetate | 3509 | 3525 | 0.64 | C32H52O2 | MS, RI |
| 44. | 60.42 | Hexadecanoic acid, 3,7,11,15-tetramethyl-2-hexadecenyl ester | 3533 | 3568 | 2.32 | C36H70O2 | MS, RI |
| Total identified (%) | 91.62 |
a Retention index determined experimentally on RTX-5MS column relative to n-alkane series (C8–C28), b Published retention indices, c Identification was based on comparison of mass spectral data (MS) and retention indices (RI) with those of NIST Mass Spectral Library (2017), Wiley Registry of Mass Spectral Data 8th edition and literature.
2.2. HPLC-PDA-ESI-MS/MS Analysis for Characterization of C. aethiopica Methanol Extract
Metabolite profiling of C. aethiopica total leaf extract (CAL) was carried out using HPLC-PDA-ESI-MS/MS analysis. A total of 29 chromatographic peaks were annotated using simultaneously acquired HPLC–PDA, and HPLC–MS base peak chromatograms (Figure 2). Tentative metabolite assignments were achieved by comparison of mass and UV spectral data of the detected compounds in both negative and positive ionization modes with reported data alongside online public databases. The identified secondary metabolites are listed in Table 2 along with their spectroscopic data. Compounds were illustrated in Figure 3. Several classes of compounds were identified in C. aethiopica total leaf extract including organic acids, cinnamic acid derivatives, flavonoids, isoflavonoids, and fatty acids. Flavonoids represented the most abundant class of metabolites detected in CAL. This is the first comprehensive metabolites profiling of genus Chasmanthe using HPLC-ESI-MS.
Figure 2.
HPLC-ESI-MS base peak chromatogram of C. aethiopica leaf total methanol extract in the negative ion mode.
Table 2.
Metabolite profiling of C. aethiopica leaf total methanol extract via HPLC-PDA-ESI-MS/MS in the positive and negative ion mode.
| Peak No. |
tR (min) |
Name | UV λmax (nm) |
[M − H]+ (m/z) |
[M − H]− (m/z) | MS2 (m/z) |
Molecular Formula | References |
|---|---|---|---|---|---|---|---|---|
| 1. | 2.59 | Quinic acid | 274 | 191 | 173, 127, 85 | C7H12O6 | [16] | |
| 2. | 5.09 | Phenyl alanine | 252, 275 | 166 | 164 | 147, 119 | C9H11NO2 | [17] |
| 3. | 5.75 | Protocatechuic acid | 254, 286 | 153 | 109 | C7H6O4 | [18] | |
| 4. | 7.21 | Dicaffeoyl quinic acid | 269, 310 | 515 | 353, 341, 323, 191, 179 | C25H24O12 | [19] | |
| 5. | 7.54 | Dicaffeoyl quinic acid isomer | 282, 310 | 515 | 353, 341, 323, 191, 179 | C25H24O12 | [19] | |
| 6. | 7.65 | Hydroxy-methoxy acetophenone-O-hexoside | 283 | 327 | 295, 283, 179, 165,147, 119 | C15H20O8 | [20] [21] |
|
| 7. | 8.07 | Caffeoylquinic acid | 289, 322 | 353 | 191, 179, 135 | C16H18O9 | [22] | |
| 8. | 9.24 | Caffeoylquinic acid isomer | 288, 315 | 353 | 191, 179, 135 | C16H18O9 | [22] | |
| 9. | 9.88 | Myricetin-O-coumaroyl- hexoside | 270, 316 | 625 | 479, 478, 463, 317, 271, 179 | C30H26O15 | [23] | |
| 10. | 10.34 | Iristectorin A/B (3′,5,7-Trihydroxy-4′,6-dimethoxy-isoflavone-7-O-hexoside) |
265 | 491 | 473, 447, 401, 371, 343, 329, 311, 283, 241, 191, 146, | C23H24O12 | [24] [25] |
|
| 11. | 11.12 | Myricetin-O-coumaroyl-hexoside | 263, 311 | 625 | 479, 478, 463, 317, 287, 271 | C30H26O15 | [23] | |
| 12. | 11.75 | Tricin-O-rhamnoside (Trihydroxy-dimethoxy-flavone-O-rhamnoside) | 264, 310 | 477 | 475 | 329, 328, 315, 299 | C23H24O11 | [26] |
| 13. | 13.33 | Trihydroxy-dimethoxy-flavone-O-rhamnoside | 265, 310 | 477 | 475 | 457, 447, 431, 329, 328, 301, 175, 145 | C23H24O11 | [26] |
| 14. | 14.21 | Hexahydroxy-isoflavone-C-hexoside (Irigenol-C-hexoside) | 264, 322 | 479 | 461, 433, 389, 359, 317, 316, 271, 151 | C21H20O13 | [27] | |
| 15. | 14.92 | Myricetin-3-O-rhamnoside | 255, 346 | 465 | 463 | 317, 316, 287, 271, 262, 179, 151 | C21H20O12 | [23] |
| 16. | 15.36 | Myricetin | 255, 350 | 319 | 319, 301, 273, 263, 165, 179, 153, 109 | C15H10O8 | [28] | |
| 17. | 15.87 | Tetrahydroxy dimethoxy isoflavone-O-hexoside (Hydroxy-iristectrigenin-O-hexoside) |
234, 270, 311 | 509 | 507 | 489, 476, 475, 463, 345, 329, 301, 273, 191, 175 | C23H24O13 | [29] |
| 18. | 17.19 | 6-Hydroxyluteolin 7-O-rhamnoside | 316 | 447 | 301 | C21H20O11 | [30] | |
| 19. | 17.46 | 6-Hydroxy luteolin | 234, 315 | 303 | 303, 285, 257, 165, 153, 137 | C15H10O7 | [30] | |
| 20. | 17.75 | Myricetin-p-coumaroyl-rhamnosyl-hexoside | 316 | 773 | 771 | 625, 463, 317 | C36H36O19 | [31] |
| 21. | 23.20 | 5,4′-Dihydroxy-3′-methoxy-6,7-methylenedioxy isoflavone (Iriflogenin) | 276 | 327 | 309, 291, 283, 180, 165, 137 | C17H12O7 | [32] | |
| 22. | 23.73 | Dihydroxy-methoxy-6,7-methylenedioxy isoflavone | 276 | 327 | 309, 291, 283, 229, 179, 165 | C17H12O7 | [32] | |
| 23. | 29.14 | Dihydroxy-23-oxo-12-oleanen-28-oic acid-O-pentosyl dihexoside | 275 | 941 | 779, 617, 485 | C48H77O18 | [33] | |
| 24. | 29.41 | Dihydroxy-octadecadienoic acid (Dihydroxy-linoleic acid) |
274 | 311 | 293, 267, 171, 153 | C18H32O4 | [15] | |
| 25. | 35.93 | Hydroxy octadecatrienoic acid (Hydroxy-linolenic acid) |
276 | 293 | 275, 249, 211, 183, 171, 121 | C18H30O3 | [15] | |
| 26. | 38.45 | Hydroxy octadecadienoic acid (Hydroxy-linoleic acid) |
277 | 295 | 277, 251, 211, 195, 183, 171 | C18H32O3 | [15] | |
| 27. | 41.33 | Hydroxy octadecenoic acid (Hydroxy-oleic acid) |
276 | 297 | 279, 253, 171 | C18H34O3 | [15] | |
| 28. | 48.29 | Hydroxyhexadecanoic acid (Hydroxy-palmitic acid) | Nd | 271 | 253, 225, 210 | C16H32O3 | [18] | |
| 29. | 54.51 | Hydroxyoctadecanoic acid (Hydroxy-stearic acid) | Nd | 299 | 253 | C18H36O3 | [18] |
Figure 3.
Major compounds identified in C. aethiopica leaf total methanol extract using HPLC-ESI-MS in the negative ion mode.
2.2.1. Flavonoids
Flavonols, mainly myricetin, in addition to several distinct flavones, markedly tricin and 6-hydroxyluteolin derivatives, were reported in Ixieae as important chemotaxonomic markers [10,11]. In the current study, tricin-O-rhamnoside (12), myricetin-3-O-rhamnoside (15), myricetin (16), 6-hydroxyluteolin-O-rhamnoside (18), and 6-hydroxyluteolin (19) were identified in C. aethiopica methanol extract. A mass loss of 308 amu suggested a loss of 6-rhamnosylhexose (rutinose) or a p-coumaroylhexose. However, acylation with p-coumaric acid results in a typical bathochromic shift in band I to λmax of 310–316 nm in the ultraviolet/visible (UV–VIS) spectra of flavonols and a pseudo molecular ion that is 146 amu greater than the parent glycoside. Moreover, acylation of the sugar residue causes a subsequent increase in the retention time in a chromatographic analysis [12,13]. Myricetin-O-coumaroyl-hexoside (9, 11) and myricetin-p-coumaroyl-rhamnosyl-hexoside (20) were identified herein, exhibiting λmax of 310–316 nm and pseudomolecular ions [M − H]− at m/z 625 and 771, respectively.
Several isoflavonoids were identified in C. aethiopica methanol extract including iristectorin A/B (10), irigenol-C-hexoside (14), hydroxy-iristectrigenin-O-hexoside (17), iriflogenin (21), dihydroxy-methoxy-6,7-methylenedioxy isoflavone (22). Mass spectrometry cannot generally distinguish isoflavones from flavones, where both classes follow a similar fragmentation pathway via retro Diels–Alder (RDA) fragmentation resulting in identical product ions for both classes of compounds [14]. However, conjugation between A- and B-rings is lacking in isoflavone structures. Thus, UV spectra of isoflavones could be distinguished from those of flavones by displaying a low-intensity band I absorption. Moreover, the increased A-ring oxygenation results in a bathochromic shift in band II from 249 nm in 7,4′-dihydroxyisoflavone to 261 nm in 5,7,4′-trihydroxyisoflavone and 270 nm in 5,6,7,4′-tetrahydroxyisoflavone. The spectra of isoflavones are not affected by changes in B-ring oxygenation patterns [14].
2.2.2. Fatty Acids
Several fatty acids could be annotated in the second half of the base peak chromatogram in ESI negative mode of C. aethiopica methanol extract exhibiting intense [M − H]− pseudo-molecular ions characteristic of fatty acids. Monohydroxy unsaturated fatty acids including hydroxy-octadecatrienoic acid (25), hydroxy-octadecadienoic acid (26), and hydroxy-octadecenoic acid (27) could be detected exhibiting quasi molecular ions [M − H]− at m/z 293, 295, 297, respectively. A mass difference of 2 amu between the identified fatty acids indicates an additional double bond [15]. A dihydroxy polyunsaturated fatty acid was identified as dihydroxy-octadecadienoic acid (24) exhibiting [M − H]− at m/z 311. Monohydroxy saturated fatty acids including hydroxy-hexadecanoic acid (28) and hydroxy-octadecanoic acid (29) were also assigned. Moreover, dihydroxy-23-oxo-12-oleanen-28-oic acid-O-pentosyl dihexoside (23) was identified in C. aethiopica methanol extract.
Miscellaneous compounds including quinic acid (1), phenylalanine (2), protocatechuic acid (3), dicaffeoyl quinic acid and its isomer (4–5), hydroxy-methoxy acetophenone-O-hexoside (6), caffeoyl quinic acid and its isomer (7–8) were also identified in C. aethiopica methanol extract.
2.3. In Vitro Evaluation of the Anti-Allergic Activity
C. aethiopica leaf total methanol extract (CAL), as well as its various fractions, were assessed for their anti-allergic potential using A23187- and antigen (IgE+DNP-BSA)-induced degranulation assays, and the results were illustrated in Table 3. The cytotoxic effect against rat basophilic leukemia (RBL-2H3) for all the samples was evaluated using MTT assay. All samples exhibited toxic effects to RBL-2H3 cells (viability below 80%) at concentration ≥ 10 μg/mL except for CAL-A which showed no cytotoxicity at the tested doses. Samples that were nontoxic were further examined for their anti-allergic effect. All the tested samples revealed weak inhibition percentage in both degranulation assays as illustrated in Table 3 except for CAL-A. It exhibited potent anti-allergic activity as evidenced by its pronounced inhibition percentage on β-hexosaminidase release triggered by A23187 and IgE by 72.7 and 48.7%, respectively, approaching that of dexamethasone especially in A23187 degranulation assay that revealed 80.7% inhibition for β-hexosaminidase release at 10 nM. This considerable anti-allergic activity comes following the anti-asthmatic and anti-allergic potential of previously studied Iridaceae plants such as Crocus sativus [34] and Dietes bicolor [35]. The hydroxy fatty acids and their esters, the predominant compounds in CAL-A, were previously reported to possess a significant anti-allergic activity as demonstrated in vitro and by ChemGPS-NP (chemical global positioning system for natural products) [36].
Table 3.
Anti-allergic effects of C. aethiopica leaf total methanol extract and its fractions at different concentrations using degranulation assay in RBL-2H3 cell line.
| Sample | Inhibition % of A23187-Induced β-Hexosaminidase Release a |
Inhibition % of Antigen-Induced β-Hexosaminidase Release a |
||||||
|---|---|---|---|---|---|---|---|---|
| 1 μg/mL | 10 μg/mL | 100 μg/mL | 200 μg/mL | 1 μg/mL | 10 μg/mL | 100 μg/mL | 200 μg/mL | |
| CAL | NS c | TOX d | TOX d | TOX d | NS c | TOX d | TOX d | TOX d |
| CAL-A | NS b | 8.0 ± 4.9 | 41.3 ± 5.4 *** | 72.7 ± 2.2 ***e | NS b | 4.0 ± 1.7 | 12.0 ± 5.2 | 48.7 ± 8.6 *** |
| CAL-B | NS c | TOX d | TOX d | TOX d | NS c | TOX d | TOX d | TOX d |
| CAL-C | NS c | TOX d | TOX d | TOX d | NS c | TOX d | TOX d | TOX d |
| CAL-D | NS c | TOX d | TOX d | TOX d | NS c | TOX d | TOX d | TOX d |
| CAL-E | NS c | TOX d | TOX d | TOX d | NS c | TOX d | TOX d | TOX d |
CAL: C. aethiopica leaf total methanol extract; CAL-A: C. aethiopica n-hexane fraction; CAL-B: C. aethiopica dichloromethane fraction; CAL-C: C. aethiopica ethyl acetate fraction; CAL-D: C. aethiopica n-butanol fraction; CAL-E: C. aethiopica remaining aqueous fraction. a Results are presented as mean ± S.E.M. value (n = 3). *** p < 0.001 compared with the control value (A23187 or antigen only). Dexamethasone, a positive control, inhibited 80.7 ± 3.8% (A23187-induced) and 79.7 ± 2.5% (antigen-induced) degranulation at a concentration of 10 nM. b NS: Not significant inhibition (degranulation more than 85 % of control) (n = 1). c NS: Not significant inhibition (degranulation more than 85 % of control) (n = 2). d TOX: toxic (viability less than 80% of control). e CAL-A inhibited A23187-induced β-hexosaminidase release with IC50 127.7 μg/mL.
2.4. In Vitro Evaluation of Anti-Inflammatory Activity
The anti-inflammatory activity of C. aethiopica leaf total methanol extract and its fractions on superoxide anion generation and elastase release in FMLF/CB-induced human neutrophils was evaluated. Results displayed in Table 4 showed that both n-hexane (CAL-A) and dichloromethane (CAL-B) fractions revealed potent anti-inflammatory activity manifested by significant inhibition in the generation of superoxide anion and prohibition of elastase release. CLA-A inhibited the generation of superoxide anion and elastase release by 68.68 and 65.18%, respectively at 10 μg/mL showing IC50 equal to 5.32 and 5.8 μg/mL, respectively. CAL-B exhibited 58.31 and 68.08% inhibition of superoxide anion generation and elastase release, respectively, at 10 μg/mL exhibiting IC50 values of 7.04 and 5.73 μg/mL, respectively. Many Iridaceae plants showed potent anti-inflammatory and immunomodulating effects in previous studies [35]. Noteworthy to highlight that the cellular model of isolated human neutrophils was established to elucidate the anti-inflammatory effect in this study as neutrophils are considered the first line of defense versus microbial infections in addition to being the most abundant circulating leukocytes. Moreover, they greatly contribute to the damage of tissues during several autoimmune and inflammatory diseases [37]. Elastase is a serine protease released by activated human neutrophils and can break several biomarkers comprising elastin in addition to being considered as inflammatory disorders marker. Initiation of elastase release is triggered by formyl peptides such as fMLF in neutrophils; thus, efficient elastase inhibitors can act as a logic strategy to protect tissue from excessive damage caused by inflammatory mediators [38]. The superoxide radical plays a pivotal role in the neutrophil-medicated acute inflammatory response. Superoxide radicals are produced by neutrophils to help in killing invasive microbes where superoxide evolved from actively phagocytizing neutrophils that attracts additional neutrophils via reaction and activation of latent chemotactic factors present in plasma. Hence, natural products that effectively prohibit superoxide-dependent chemotactic factor, serve as promising anti-inflammatory agents [39]. Thus, the anti-inflammatory effect is measured through the inhibition of superoxide anion generation and elastase liberation in our ongoing study.
Table 4.
Inhibitory effects of C. aethiopica leaf total methanol extract and its fractions on superoxide anion generation and elastase release in FMLF/CB-induced human neutrophils.
| Sample | Superoxide Anion | Elastase Release | ||
|---|---|---|---|---|
| IC50 (μg/mL) a | Inhibition% | IC50 (μg/mL) a | Inhibition% | |
| CAL | >10 | 36.45 ± 2.58 *** | >10 | 15.27 ± 2.13 ** |
| CAL-A | 5.32 ± 0.46 | 68.68 ± 3.87 *** | 5.80 ± 0.34 | 65.18 ± 6.81 *** |
| CAL-B | 7.04 ± 1.28 | 58.31 ± 3.76 *** | 5.73 ± 0.64 | 68.08 ± 7.25 *** |
| CAL-C | >10 | 43.84 ± 1.94 *** | >10 | 24.51 ± 4.05 ** |
| CAL-D | >10 | 22.47 ± 5.53 * | b | b |
| CAL-E | >10 | 26.49 ± 2.73 *** | >10 | 5.30 ± 2.67 |
CAL: C. aethiopica leaf total methanol extract; CAL-A: C. aethiopica n-hexane fraction; CAL-B: C aethiopica dichloromethane fraction; CAL-C: C. aethiopica ethyl acetate fraction; CAL-D: C. aethiopica n-butanol fraction; CAL-E: C. aethiopica remaining aqueous fraction. a Percentage of inhibition at 10 μg/mL concentration. Results are presented as mean ± S.E.M. (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control value (fMLF/CB). b Sample CAL-D had promoting effects (23.86 ± 3.78% in the presence of CB) on elastase release in human neutrophils.
2.5. In Vitro Antioxidant Activity Using DPPH Assay
The main mechanistic basis for most in vitro antioxidant assays takes place by either HAT (hydrogen atom transfer) or ET (election transfer) reactions. DPPH is monitored by determining the color change of the DPPH radical. The DPPH assay relied upon election transfer reaction, wherein DPPH itself reacts as a radical and a probe. The presence of antioxidants in any sample lightens the color of DPPH by donating an electron/hydrogen atom to the unpaired electron of DPPH that turns the purple color of DPPH colorless, which is measured using UV–VIS spectrophotometer at a wavelength of 517 nm [40]. The DPPH assay showed that C. aethiopica leaf total methanol extract showed substantial antioxidant potential with an IC50 equals of 0.56 mg/mL that may be attributed to the richness of the extract with phytoconstituents belonging to different classes represented mainly by flavonoids and phenolic acid as revealed from the LC/MS analysis.
2.6. In Vitro Anti-Hyperglyceamic Activity
The anti-hyperglycemic activity of the C. aethiopica leaf total methanol extract (CAL) was evaluated in vitro using 3T3-L1 adipocytes using two concentrations which are 30 and 50 μg/mL that were further compared with insulin and pioglitazone, positive controls, using the same concentrations. Results displayed in Table 5 clarified that the concentration of glucose was decreased from 23.9 and 24.1 mmol/L to 22.8 and 22.6 mmol/L after treatment with 30 and 50 μg/mL of CAL, respectively. This showed a moderate reduction in the culture medium glucose concentration estimated by 4.6 and 6.2% as compared to untreated control group. CAL approaches in this regard insulin and pioglitazone that showed 6.3 and 4.37% reduction at 30 μg/mL and 10.5% reduction at 50 μg/mL as compared to untreated control group.
Table 5.
Effect of C. aethiopica leaf total methanol extract (CAL) at concentrations of 30 and 50 μg/mL on glucose consumption in media of 3T3-L1 adipocyte cultures.
| Glucose Concentration in mmol/L | ||
|---|---|---|
| 30 μg/mL | 50 μg/mL | |
| Pioglitazone | 22.9 ± 0.7 | 21.10 ± 0.6 * |
| Insulin | 22.4 ± 0.4 * | 21.56 ± 0.7 * |
| CAL | 22.8 ± 0.9 | 22.6 ± 0.1 * |
| Control | 23.9 ± 0.7 | 24.1 ± 0.6 |
| Medium | 25.3 ± 0.6 | 25.0 ± 0.6 |
Data are measured in triplicates (n = 3) and presented as means ± SEM. * Significantly different from the untreated control group at p < 0.05.
2.7. In Vivo Assessment of the Antihyperglycemic Activity
The antihyperglycemic effect of C. aethiopica leaf total methanol extract (CAL) was evaluated in STZ-induced diabetic rats. The injection of STZ into animals triggered a considerable increase in FBG level by 278% when compared to the normal control group (Figure 4). This change was concomitant with a significant reduction in the serum insulin level by 52.63%, with regard to the normal group. Meanwhile when GLB was administered to STZ-diabetic rats it caused a notable decrease in FBG level by 28.57% accompanied by a marked increase in serum insulin level by 22.22% in comparison with STZ-treated rats. However, the administration of the CAL to STZ-treated rats elicited a significant reduction in FBG level by 53.44%, as compared with the STZ-treated rats with concomitant elevation in serum insulin estimated by 22.22%. The results of CAL suggested a potent antihyperglycemic effect approaching that of GLB. The effectiveness of CAL as a promising antihyperglycemic is attributed to its richness by flavonoids and phenolic acids. These secondary metabolites were previously showed to possess antihyperglycemic activity via acting as potent α-glucosidase inhibitors [41,42]. It is noteworthy to mention that myricetin derivatives exhibited considerable α-amylase as well as α-glucosidase inhibitory activity. They also displayed an insulin-like action via stimulating glucose and lipid uptake as well as enhancing adiponectin production by stimulating the insulin signaling pathway in a manner similar to insulin. Myricetin derivatives trigger Akt1, protein kinase B, peroxisome proliferator-activated receptor gamma, and Slc2a4, glucose transporter, genes up-regulation resulting in antihyperglycemic activity [43]. Moreover, quinic acid and its derivatives effectively stimulate Ca2+-dependent mitochondrial function and increase insulin production from β-cells of the pancreas [44]. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cell [44].
Figure 4.
Effect of intraperitoneal injection of 20 mg/kg/day of C. aethiopica leaf total methanol extract (CAL) and 600 μg/kg/day glibenclamide (GLB) on FBG (A) and serum insulin (B) levels in STZ-induced diabetic rats. Data are measured in triplicates (n = 3) and presented as means ± S.E.M. * Significantly different from the STZ-treated group at p < 0.05; FBG was assessed using a glucose oxidase kit; serum insulin was determined by an immunoassay kit.
2.8. Molecular Modeling
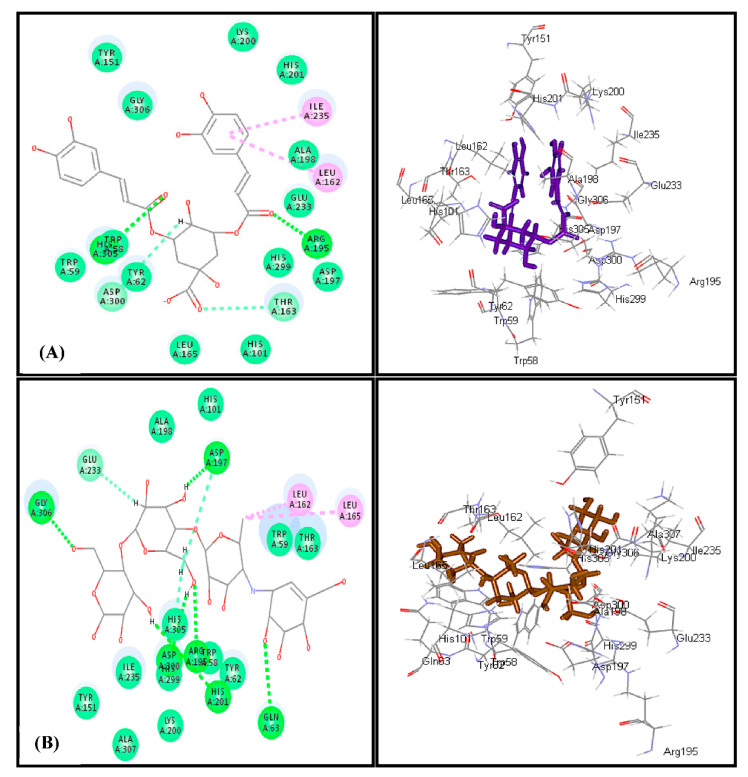
Docking of most of the major compounds identified from C. aethiopica leaf total methanol extract inside the active centers of human α-amylase (HA) and human α-glucosidase (HG) was performed to further ascertain the results of the in vitro and in vivo study and provides an outline for the probable mechanism of action. Most of the docked compounds showed certain fitting within the active sites of both examined enzymes. Dicaffeoylquinic acid revealed the highest fitting with free binding energies (∆G) equals to −47.24 and −60.50 Kcal/mol for human α-amylase (HG) and human α-glucosidase (HG), respectively (Table 6). The highest fitting of dicaffeoylquinic acid at human α-amylase (HA) is attributed to the formation of two conventional H-bonds with His 305 and Arg 195, two π-alkyl interactions with Ile 235 and Leu 162, two C-H bonds with Asp 300 and Thr 163 in addition to the formation of many Van der Waals interactions. Regarding acarbose, it forms six conventional H- bonds with Gly 306, Asp197, Gln 63, His 291, Arg 195 and Asp 300, two alkyl interactions with Leu 162 and Leu 165, two C-H bonds with Glu 233 and His 305 in addition to Van der Waals interactions with many amino acid residues at the active site (Figure 5). The firm binding of dicaffeoylquinic acid at the active site of human α-glucosidase (HG) can be interpreted by the formation of multiple tight bonds at the active sites, namely, seven conventional H-bonds with Gln 1561, His 1584, Asp 1279, Lys 1460, and Asp 1157; two π–π interactions with Phe 1560 and Tyr 1251; and hydrophobic C-H bonds with Pro 1159 and Tyr 125 in addition to the formation of many Van der Waals interactions with most of the amino acid moieties present at the active site of the enzyme together with an attractive charged ionic bond formation between the carboxylic acid moiety of the quinic acid and Lys 1460 residue in the active site (Figure 6). These results for dicaffeoylquinic acid approached that of acarbose, the standard antihyperglycemic agent that displayed ∆G of −76.29 and −89.19 Kcal/mol for human α-amylase (HG) and human α-glucosidase (HG), respectively. Acarbose formed eleven H-bonds with Gln 1158, Lys 1460, Lys 1164, Asp 1157, Arg 1510, and Asp 1420, in addition to two C-H bonds with Trp 1369 and Asp 1526 at the human α-glucosidase (HG) active center.
Table 6.
Free binding energies (∆G) of the major identified compounds with human α-amylase (HA) and human α-glucosidase (HG) enzymes active centers using molecular docking with pH- and rule-based methods and expressed in kcal/mol.
| Compound | Human α-Amylase (HA) | Human α-Glucosidase (HG) | ||
|---|---|---|---|---|
| pH-Based | Rule-Based | pH-Based | Rule-Based | |
| Quinic acid | −15.49 | −15.49 | −15.23 | −15.23 |
| Phenyl alanine | −32.81 | −32.81 | −28.53 | −28.53 |
| Protocatecheuic acid | −31.44 | −31.40 | −31.84 | −31.80 |
| Dicaffeoylquinic acid | −47.24 | −47.24 | −60.50 | −60.50 |
| Caffeoyl quinic acid | −38.43 | −38.43 | −37.68 | −37.68 |
| Iristectorin | −4.17 | −4.17 | −14.84 | −14.84 |
| Myricetin-3-O-rhamnoside | −26.44 | −21.14 | −27.30 | −22.92 |
| Myricetin | −44.05 | −41.05 | −45.83 | −41.82 |
| 6-Hydroxyluteolin 7-O-rhamnoside | −39.66 | −20.66 | −45.68 | −23.64 |
| 6-Hydroxyluteolin | −33.45 | −21.66 | −36.10 | −23.82 |
| Iriflogenin | −11.57 | −11.57 | −13.41 | −13.41 |
| Dihydroxy-linoleic acid | −23.28 | −23.28 | −29.60 | −29.60 |
| Hydroxy-linolenic acid | −3.89 | −3.69 | 0.17 | 0.12 |
| Hydroxy-oleic acid | −32.61 | −32.61 | −35.86 | −35.86 |
| Hydroxy-stearic acid | −44.62 | −44.62 | −52.17 | −52.17 |
| Hydroxy-palmitic acid | −44.62 | −44.62 | −48.43 | −48.43 |
| Acarbose | −76.29 | −76.29 | −89.19 | −89.19 |
Figure 5.
2D and 3Dbinding modes of dicaffeoylquinic acid (A) and acarbose (B) within human amylase (HA) active centers employing C-docker protocol.
Figure 6.
2D and 3D binding modes of dicaffeoylquinic acid (A) and acarbose (B) within human glucosidase (HG) active centers employing C-docker protocol.
3. Materials and Methods
3.1. Chemical Reagents and Kits
Glibenclamide (GLB), streptozotocin (STZ), [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT), Dulbecco’s modified Eagle’s medium-high glucose powder (DMEM), streptomycin, calcium ionophore A23187, o-phthalaldehyde, p-nitrophenyl-N-acetyl-d-glucosaminide (p-NAG), dexamethasone, and penicillin were bought from Sigma-Aldrich (St. Louis, MO, USA). Roche Diagnostics Germany (Mannheim, Germany) kindly supplied glucotest strips. The radioimmunoassay kit used for determination of insulin level and the glucose oxidase kit were purchased from Amersham Biosciences; (Piscataway, NJ, USA) and Siemens Healthcare Diagnostics (Deerfield, IL, USA), respectively. DNP-BSA (dinitrophenyl-conjugated bovine serum albumin) and FBS (fetal bovine serum) were obtained from Merck (Kenilworth, NJ, USA) and Hyclone (Logan, UT, USA), respectively. Other kits used for assessment of LPO (lipid peroxidation) and MDA (malondialdehyde) were obtained from Fluka (Buchs, Switzerland). Dr. Daniel H. Conrad (Virginia Commonwealth University, Richmond, VA, USA) generously supplied Mouse anti-DNP IgE antibody. All other solvents for LC/MS as well as GLC/MS analyses were of analytical grade.
3.2. Plant Material
C. aethiopica (Iridaceae) leaves were collected from El-Rai botanical garden, El Kanater El Khayreya, Qalyubiya, Egypt. The plants were kindly identified and authenticated by Mrs. Therese Labib, Consultant of Plant Taxonomy at the Ministry of Agriculture and former Director of Orman Botanical Garden, Giza, Egypt, and Dr. Mohammed El-Gebaly, Department of Botany, National Research Centre (NRC), Giza, Egypt. Voucher specimens were deposited in the herbarium of Pharmacognosy Department, Faculty of Pharmacy, Ain Shams University (voucher specimen number: PHG-P-CA-261).
3.3. Preparation of the Plant Extract and Its Fractions
Air-dried leaves of C. aethiopica L. (100 g) were powdered and percolated in 80% methanol (1 L × 3) followed by its filtration. The obtained filtrate was concomitantly evaporated under reduced pressure and temperature (45 °C) until dryness and lyophilized to give 25.50 g of the total methanol extract (CAL). The solid residue (20 g) was dissolved in water: methanol (1: 4) and then partitioned successively with n-hexane, dichloromethane, ethyl acetate, and n-butanol to give CAL-A (2.67 g), CAL-B (3.74 g), CAL-C (2.34 g), and CAL-D (1.16 g), respectively. The remaining aqueous fraction CAL-E was 9.58 g.
3.4. Gas–Liquid Chromatography Coupled with Mass Spectroscopy (GLC-MS) Analysis
GLC-MS analysis was achieved on Shimadzu GCMS-QP 2010 (Shimadzu Corporation, Koyoto, Japan) equipped with Rtx-5MS (30 m × 0.25 mm i.d. × 0.25 µm film thickness) capillary column (Restek, PA, USA) and coupled to a Shimadzu mass spectrometer. Initial column temperature was set at 50 °C for 3 min; 50–300 °C at a rate of 5 °C/min and then 10 min isothermal at 300 °C. The injector temperature was 280 °C. The carrier gas (helium) flow rate was set at 1.37 mL/min. The interface and ion source temperatures were adjusted to 280 and 220 °C, respectively. Diluted samples (1% v/v) were injected in split mode with split ratio 15:1. The injection volume was 1 μL. Mass spectra were recorded in EI mode of 70 eV, scanning from m/z 35 to 500.
Compound Identification
The tentative identification of the compounds found by GLC-MS was performed based on their retention indices relative to a homologous series of n-alkanes (C8–C28) injected under the same conditions and comparing their mass spectra with those in the National Institute of Standards and Technology (NIST) and Wiley library database in addition to literature [45,46,47,48,49,50,51,52].
3.5. HPLC-PDA-ESI-MS/MS Analysis
HPLC-PDA-ESI-MS/MS analysis was carried out on a Finnigan LCQ-Duo ion trap mass spectrometer (Thermo Quest, San Jose, CA, USA) with an ESI source coupled to a Finnigan Surveyor HPLC system (Accela autosampler, MS pump plus, and PDA detector plus) (Thermo, San Jose, CA, USA) with an EC 150/3 Nucleodur 100-3 C18ec column (Macherey-Nagel, Düren, Germany). A gradient of water and acetonitrile (ACN) (with 0.1% formic acid for ESI+ mode and without formic acid for ESI− mode) was applied from 2% to 100% ACN in 60 min at 30 °C at a flow rate of 0.5 mL/min. The injection volume was 20 µL. All samples were measured in the positive and negative ion mode. The MS was operated at +10 V capillary voltage for ESI+ and −10 V for ESI−, 240 °C source temperature, and high purity nitrogen as a sheath and auxiliary gas at a flow rate of 80 and 40 (arbitrary units), respectively. The ions were detected in a mass range of 50–2000 m/z. A collision energy of 35 eV was used in MS/MS fragmentation. Data acquisitions and analyses were executed by XcaliburTM 2.0.7 software (Thermo Scientific, Karlsruhe, Germany). Tentative metabolite assignments were achieved by comparison of mass and UV spectral data of the detected compounds in both negative and positive ionization modes with reported data alongside online public databases where references are added Table 2.
3.6. In Vitro Biological Evaluation
3.6.1. In Vitro Assessment of the Anti-Allergic Activity
Cell Culture and Cell Viability Assay
The mucosal mast cell-derived rat basophilic leukemia (RBL-2H3) cell line was obtained from the American Type Culture Collection. The growth of RBL-2H3 was carried out in a DMEM medium containing 10% FBS in addition to 100 U/mL penicillin and 100 μg/mL streptomycin. The culturing of cells was done in 10 cm cell culture dishes (Cellstar) using a humidified chamber containing 5% CO2 in the air and were kept at 37 °C. A methyl thiazole tetrazolium (MTT) assay was performed to assess the probable toxic effects of the tested samples concerning RBL-2H3 cells [53] as previously described [54,55,56,57].
Degranulation Assays in Mast Cells
The extent of A23187-induced degranulation in RBL-2H3 cells was evaluated using a β-hexosaminidase activity assay as formerly described [54,58]. The release of β-hexosaminidase from activated RBL-2H3 cells induced by IgE was assessed following the method described earlier [59]. Dexamethasone (10 nM) was used as a positive control. Absorbance was determined using a microplate reader at λ = 405 nm. The percentage of inhibition of β-hexosaminidase release from RBL-2H3 cells was computed using the following equation, calculated as a percentage of the control value (untreated stimulated cells:
3.6.2. In Vitro Assessment of Anti-Inflammatory Activity
The assessment of anti-inflammatory activity was performed using human neutrophils in which blood was withdrawn from healthy volunteers (20–35 years old) following a protocol approved by the institutional review board at Chang Gung Memorial Hospital adopting a standard method as formerly described [60]. However, the inhibition of superoxide generation was assessed using the assay based on the reduction of ferricytochrome c as previously reported [60]. Meanwhile, the release of elastase from activated neutrophils was measured using the elastase substrate, N-methoxysuccinyl-Ala-Pro-Val-p-nitroanilide, following the previously described procedure [60].
3.6.3. In Vitro Antioxidant Activity Evaluation Using DPPH Assay
The ability of CAL to donate hydrogen atom or an electron was determined via bleaching the purple color of DPPH• methanol solution 0.2, 0.4, 0.6, and 1.0 mg/mL that was performed in triplicate. Briefly, 50 μL of each concentration of the tested sample was added to 5 mL of 0.004% methanol solution of DPPH. After 30 min incubation at room temperature, the absorbance was measured versus a blank at λ = 517 nm using a Shimadzu UV-1601 spectrophotometer (Kyoto, Japan). The percentage of inhibition of DPPH• was calculated using the following equation: Free radical scavenging activity = [Ac − As/Ac] × 100, where Ac: absorbance of control and As: absorbance of sample. The IC50 of ascorbic acid, positive control, was 1.6 μg/mL [61].
3.6.4. In Vitro Anti-Hyperglycemic Evaluation Using 3T3-L1 Adipocyte Culture
Stock solutions of the tested sample at 30 and 50 μg/mL in DMSO were used in which DMSO concentration does not exceed 0.1% in the medium of working solutions. However, the stock solution of insulin was prepared by dissolving 102 M of insulin in 0.01 M of acetic acid; pH 3.0 [62]. 5 × 105 cells/mL of mouse pre-adipocytes 3T3-L1 cells obtained from the American Type Culture Collection (ATCC) were used and cultured in DMEM medium supplemented with 5.56 mmol/L D-glucose, 10% FBS, and 1% PS (penicillin–streptomycin) solutions. The cells were kept at 37 °C in a humidified condition composing of 95% air and 5% CO2. After achieving 100% confluence, the cells were treated with 25 mmol/L D-glucose, 0.32 μM insulin, 0.5 mM 3-isobutyl-1-methylxanthine, and 1 μM dexamethasone for 48 h to improve their differentiation. Cells treated with CAL as well as control untreated cells were kept in DMEM media with 25 mmol/L D-glucose for 24 h. Pioglitazone and insulin were employed as a positive control meanwhile the concentrations of glucose in the medium were employed as a tool to assess anti-hyperglycemic effectiveness of the tested sample [62].
3.7. In Vivo Biological Evaluation
3.7.1. Animals and Animal Treatment
Rats (Wister male) of 150–250 g, were provided by King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia, animal facility. The study protocol received approval from the local ethics committee for animal care, Taibah University, Saudi Arabia (Approval number TUCDREC/20160131). The animals were kept under standard conditions of temperature, relative humidity, as well as day and light cycle. Animals were allowed free access to standard laboratory food as previously reported [62] and as indicated by the WHO (World Health Organization) [62].
3.7.2. Diabetes Induction in Rats
The induction of diabetes in rats was achieved by the intraperitoneal administration of streptozotocin (mg/kg) as a single dose as previously described [63]. Enzymatic strips were used to examine the existence of glucose in the urine on the third day. Glibenclamide was utilized as a positive control.
3.7.3. Experimental Protocol
The animals were randomly distributed into four groups, 8 animals per group. The experimental protocol was done following what was previously reported in which groups 1, 2, and 3 represented the normal, streptozotocin (STZ), and glibenclamide (GLB) administered groups, respectively [62]. The fourth group orally received 20 mg/kg body weight of the leaf methanol extract of C. aethiopica L. (CAL) for ten successive days. Scarification was done on the 11th day of treatment by cervical dislocation after light ether anesthesia. The separated serum was subjected to centrifugation and used for the determination of glucose and insulin levels.
3.7.4. Assessment of the Biochemical Parameters
Serum glucose and insulin were assessed using the glucose oxidase assay and Amersham insulin immune reactive kit, respectively [62,64], as listed by the manufacturer [65].
3.8. Statistical Analysis
Statistical analysis was done using one-way ANOVA followed by Dunnet’s test which was done using Sigma Plot (Systat software, San Jose, CA, USA) in case of anti-allergic evaluation; student’s t-test (SigmaPlot) for anti-inflammatory assay; however, for the antihyperglycemic determination, ANOVA was followed by Tukey–Kramer multiple comparison test (p < 0.05). Results were given as means ± S.E.M. Graphs were plotted using GraphPad Prism 6 software (GraphPad Inc., La Jolla, CA, USA).
3.9. Molecular Modeling
Molecular docking of the identified compounds from the total methanol leaf extract (CAL) was performed using Discovery Studio 2.5 (Accelrys Inc., San Diego, CA, USA) implementing C-docker protocol on human α-amylase (HA) (PDB ID 1B2Y) and humanα-glucosidase (HG) (PDB ID 3TOP), retrieved from the protein data bank (www.pdb.org, accessed on 28 May 2021) using both pH based and rule-based methods. This was done following the previously reported method [3,35,66].
4. Conclusions
This study comprehensively studies the phytoconstituents as well as the different biological activities of the C. aethiopica for the first time. GC analyses revealed that palmitic acid and methyl hexadecanoic acid ester are the predominant compounds in C. aethiopica n-hexane fraction of the leaf extract. Meanwhile, myricetin-3-O-rhamnoside was the major compound in C. aethiopica total methanol extract as displayed in HPLC-PDA-ESI-MS/MS analysis. C. aethiopica n-hexane exhibited the highest anti-allergic and anti-inflammatory potential whereas C. aethiopica total methanol extract showed a potent antihyperglycemic activity in streptozotocin-induced diabetes in an animal model that was further confirmed using in silico studies. C. aethiopica could serve as an effective antioxidant natural remedy combating inflammation, allergy, and hyperglycemia that will be welcomed by a large category of patients worldwide. Further studies are recommended for the isolation and structural elucidation of the compounds existing in the extract with subsequent assessment of their biological activities.
Acknowledgments
I. M. A. acknowledges Science and Technology Development Fund in Egypt (STDF, project ID 25448) for funding the postdoctoral fellowship in Heidelberg, Germany.
Author Contributions
Conceptualization, methodology, software, writing—original draft preparation, I.M.A. and F.S.Y.; methodology, resources, writing—review and editing; M.K., H.A.E.-B., B.W., T.-L.H., and B.-H.C.; supervision, funding acquisition, writing—review and editing, F.-R.C.; supervision and writing—review and editing, M.E.-S., M.W., and A.N.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology of Taiwan (Grant number: MOST 106-2320-B-037-008-MY2, MOST 108-2320-B-037-022-MY3, 108-2811-B-037-511, and 109-2927-I-037-502, awarded to F.-R. Chang, and MOST 108-2320-B-037-004, awarded to B.-H. Chen). In addition, this research was funded by the Drug Development and Value Creation Research Center, Kaohsiung Medical University & Department of Medical Research, Kaohsiung Medical University Hospital (Grant number: KMU-TC108A03-11, KMU-TC109A03-2) and Chang Gung Memorial Hospital (CMRPF1G0241-3; BMRP450).
Institutional Review Board Statement
The study protocol received approval from the local ethics committee for animal care, Taibah University, Saudi Arabia (Approval number TUCDREC/20160131).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the first author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Youssef F.S., Labib R.M., Eldahshan O.A. Synergistic hepatoprotective and antioxidant effect of artichoke, fig, mulberry herbal mixture on HepG2 cells and their metabolic profiling using NMR coupled with chemometrics. Chem. Biodivers. 2017;14:e1700206. doi: 10.1002/cbdv.201700206. [DOI] [PubMed] [Google Scholar]
- 2.Ashour M.L., Youssef F.S., Gad H.A., El-Readi M.Z., Bouzabata A., Abuzeid R.M., Sobeh M., Wink M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018;70:952–963. doi: 10.1111/jphp.12904. [DOI] [PubMed] [Google Scholar]
- 3.Janibekov A.A., Youssef F.S., Ashour M.L., Mamadalieva N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crops Prod. 2018;118:142–148. doi: 10.1016/j.indcrop.2018.03.034. [DOI] [Google Scholar]
- 4.Salib J.Y., Michael H.N., Eskande E.F. Anti-diabetic properties of flavonoid compounds isolated from Hyphaene thebaica epicarp on alloxan induced diabetic rats. Pharmacogn. Res. 2013;5:22–29. doi: 10.4103/0974-8490.105644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broide D.H. Immunomodulation of allergic disease. Annu. Rev. Med. 2009;60:279–291. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youssef F.S., Hamoud R., Ashour M.L., Singab A.N., Wink M. Volatile oils from the aerial parts of Eremophila maculata and their antimicrobial activity. Chem. Biodivers. 2014;11:831–841. doi: 10.1002/cbdv.201300366. [DOI] [PubMed] [Google Scholar]
- 7.Goldblatt P., Manning J., Dunlop G. Crocosmia and Chasmanthe. Timber Press; Portland, OR, USA: 2004. [Google Scholar]
- 8.Singab A.N.B., Ayoub I.M., El-Shazly M., Korinek M., Wu T.-Y., Cheng Y.-B., Chang F.-R., Wu Y.-C. Shedding the light on Iridaceae: Ethnobotany, phytochemistry and biological activity. Ind. Crops Prod. 2016;92:308–335. doi: 10.1016/j.indcrop.2016.07.040. [DOI] [Google Scholar]
- 9.Divya G., Albert A., Singab A.N.B., Ayoub I.M., Al-Sayed E., Paul E., Manoharan K., Saso L., Selvam G.S. Renoprotective effect of tectorigenin glycosides isolated from Iris spuria L. (Zeal) against hyperoxaluria and hyperglycemia in NRK-49Fcells. Nat. Prod. Res. 2019:1–6. doi: 10.1080/14786419.2019.1613396. [DOI] [PubMed] [Google Scholar]
- 10.Harborne J., Williams C. The phytochemical richness of the Iridaceae and its systematic significance. Ann. Bot. 2000;58 doi: 10.4462/annbotrm-9061. [DOI] [Google Scholar]
- 11.Williams C.A., Harborne J.B., Goldblatt P. Correlations between phenolic patterns and tribal classification in the family Iridaceae. Phytochemistry. 1986;25:2135–2154. doi: 10.1016/0031-9422(86)80079-6. [DOI] [Google Scholar]
- 12.Rezende F.M., Ferreira M.J.P., Clausen M.H., Rossi M., Furlan C.M. Acylated flavonoid glycosides are the main pigments that determine the flower colour of the Brazilian native tree Tibouchina pulchra (Cham.) Cogn. Molecules. 2019;24:718. doi: 10.3390/molecules24040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L.-Z., Chen P., Harnly J.M. New phenolic components and chromatographic profiles of green and fermented teas. J. Agr. Food Chem. 2008;56:8130–8140. doi: 10.1021/jf800986s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markham K., Geiger H., Harborne J. The flavonoids: Advances in research since 1986. Chap. 1994;10:441–493. [Google Scholar]
- 15.Farag M.A., Gad H.A., Heiss A.G., Wessjohann L.A. Metabolomics driven analysis of six Nigella species seeds via UPLC-qTOF-MS and GC-MS coupled to chemometrics. Food Chem. 2014;151:333–342. doi: 10.1016/j.foodchem.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Farrag A.R.H., Abdallah H.M.I., Khattab A.R., Elshamy A.I., Gendy A., Mohamed T.A., Farag M.A., Efferth T., Hegazy M.F. Antiulcer activity of Cyperus alternifolius in relation to its UPLC-MS metabolite fingerprint: A mechanistic study. Phytomedicine. 2019;62:152970. doi: 10.1016/j.phymed.2019.152970. [DOI] [PubMed] [Google Scholar]
- 17.Liu M.H., Zhang Q., Zhang Y.H., Lu X.Y., Fu W.M., He J.Y. Chemical analysis of dietary constituents in Rosa roxburghii and Rosa sterilis Fruits. Molecules. 2016;21:1204. doi: 10.3390/molecules21091204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otify A.M., El-Sayed A.M., Michel C.G., Farag M.A. Metabolites profiling of date palm (Phoenix dactylifera L.) commercial by-products (pits and pollen) in relation to its antioxidant effect: A multiplex approach of MS and NMR metabolomics. Metabolomics. 2019;15:119. doi: 10.1007/s11306-019-1581-7. [DOI] [PubMed] [Google Scholar]
- 19.Olennikov D.N., Kashchenko N.I., Chirikova N.K., Vasil’eva A.G., Gadimli A.I., Isaev J.I., Vennos C. Caffeoylquinic acids and flavonoids of fringed sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS profile, HPLC-DAD quantification, in vitro digestion stability, and antioxidant capacity. Antioxidants. 2019;8:307. doi: 10.3390/antiox8080307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alperth F., Mitić B., Mayer S., Maleš Ž., Kunert O., Hruševar D., Bucar F. Metabolic profiling of rhizomes of native populations of the strictly endemic Croatian species Iris adriatica. Plant Biosyst. 2019;153:317–324. doi: 10.1080/11263504.2018.1478906. [DOI] [Google Scholar]
- 21.Ibrahim S.R., Mohamed G.A., Al-Musayeib N.M. New constituents from the rhizomes of Egyptian Iris germanica L. Molecules. 2012;17:2587–2598. doi: 10.3390/molecules17032587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elshamy A.I., Farrag A.R.H., Ayoub I.M., Mahdy K.A., Taher R.F., Gendy A.E.-N.G.E., Mohamed T.A., Al-Rejaie S.S., EI-Amier Y.A., Abd-EIGawad A.M., et al. UPLC-qTOF-MS phytochemical profile and antiulcer potential of Cyperus conglomeratus Rottb. alcoholic extract. Molecules. 2020;25:4234. doi: 10.3390/molecules25184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rached W., Bennaceur M., Barros L., Calhelha R.C., Heleno S., Alves M.J., Carvalho A.M., Marouf A., Ferreira I.C. Detailed phytochemical characterization and bioactive properties of Myrtus nivelii Batt & Trab. Food Funct. 2017;8:3111–3119. doi: 10.1039/c7fo00744b. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y.-Y., Wang Q., Qi L.-W., Qin X.-Y., Qin M.-J. Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC–DAD–ESI-MSn. J. Pharm. Biomed. Anal. 2011;56:304–314. doi: 10.1016/j.jpba.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Li W.Z., Huang W., Cheung A.W., Bi C.W., Duan R., Guo A.J., Dong T.T., Tsim K.W. Quality evaluation of Rhizoma Belamcandae (Belamcanda chinensis (L.) DC.) by using high-performance liquid chromatography coupled with diode array detector and mass spectrometry. J. Chromatogr. A. 2009;1216:2071–2078. doi: 10.1016/j.chroma.2008.05.082. [DOI] [PubMed] [Google Scholar]
- 26.Ma C., Hu L., Kou X., Lv W., Lou Z., Wang H. Rapid screening of potential α-amylase inhibitors from Rhodiola rosea by UPLC-DAD-TOF-MS/MS-based metabolomic method. J. Funct. Foods. 2017;36:144–149. doi: 10.1016/j.jff.2017.06.060. [DOI] [Google Scholar]
- 27.Buckingham J., Munasinghe V.R.N. Dictionary of Flavonoids with CD-ROM. CRC Press; Boca Raton, FL, USA: 2015. [Google Scholar]
- 28.Faheem S.A., Saeed N.M., El-Naga R.N., Ayoub I.M., Azab S.S. Hepatoprotective effect of cranberry nutraceutical extract in non-alcoholic fatty liver model in rats: Impact on insulin resistance and Nrf-2 expression. Front. Pharmacol. 2020;11:218. doi: 10.3389/fphar.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moriyasu M., Igi Y., Ichimaru M., Iwasa K., Kobayakawa J., Sato-Nishimori F., Matsukawa Y., Nagase C. New isoflavones from Belamcandae Rhizoma. J. Nat. Med. 2007;61:329–333. doi: 10.1007/s11418-007-0147-6. [DOI] [Google Scholar]
- 30.Es-Safi N.E., Kerhoas L., Ducrot P.H. Application of positive and negative electrospray ionization, collision-induced dissociation and tandem mass spectrometry to a study of the fragmentation of 6-hydroxyluteolin 7-O-glucoside and 7-O-glucosyl-(1→ 3)-glucoside. Rapid Commun. Mass Spectr. 2005;19:2734–2742. doi: 10.1002/rcm.2122. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., De Stefano R., Robine M., Butelli E., Bulling K., Hill L., Rejzek M., Martin C., Schoonbeek H.-j. Different reactive oxygen species scavenging properties of flavonoids determine their abilities to extend the shelf life of tomato. Plant. Physiol. 2015;169:1568–1583. doi: 10.1104/pp.15.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wollenweber E., Stevens J.F., Klimo K., Knauft J., Frank N., Gerhauser C. Cancer chemopreventive in vitro activities of isoflavones isolated from Iris germanica. Planta Med. 2003;69:15–20. doi: 10.1055/s-2003-37030. [DOI] [PubMed] [Google Scholar]
- 33.Maamoun A.A., El-akkad R.H., Farag M.A. Mapping metabolome changes in Luffa aegyptiaca Mill fruits at different maturation stages via MS-based metabolomics and chemometrics. J. Adv. Res. 2019 doi: 10.1016/j.jare.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boskabady M., Tabatabaee A., Byrami G. The effect of the extract of Crocus sativus and its constituent safranal, on lung pathology and lung inflammation of ovalbumin sensitized guinea-pigs. Phytomedicine. 2012;19:904–911. doi: 10.1016/j.phymed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Ayoub I.M., Korinek M., Hwang T.-L., Chen B.-H., Chang F.-R., El-Shazly M., Singab A.N.B. Probing the antiallergic and anti-inflammatory activity of biflavonoids and dihydroflavonols from Dietes bicolor. J. Nat. Prod. 2018;81:243–253. doi: 10.1021/acs.jnatprod.7b00476. [DOI] [PubMed] [Google Scholar]
- 36.Korinek M., Tsai Y.-H., El-Shazly M., Lai K.-H., Backlund A., Wu S.-F., Lai W.-C., Wu T.-Y., Chen S.-L., Wu Y.-C. Anti-allergic hydroxy fatty acids from Typhonium blumei explored through ChemGPS-NP. Front. Pharmacol. 2017;8:356. doi: 10.3389/fphar.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang T.-L., Wang C.-C., Kuo Y.-H., Huang H.-C., Wu Y.-C., Kuo L.-M., Wu Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010;80:1190–1200. doi: 10.1016/j.bcp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 38.Braga P.C., Dal Sasso M., Culici M., Bianchi T., Bordoni L., Marabini L. Anti-inflammatory activity of thymol: Inhibitory effect on the release of human neutrophil elastase. Pharmacology. 2006;77:130–136. doi: 10.1159/000093790. [DOI] [PubMed] [Google Scholar]
- 39.McCord J.M., Roy R.S. The pathophysiology of superoxide: Roles in inflammation and ischemia. Can. J. Physiol. Pharmacol. 1982;60:1346–1352. doi: 10.1139/y82-201. [DOI] [PubMed] [Google Scholar]
- 40.Roy M.K., Koide M., Rao T.P., Okubo T., Ogasawara Y., Juneja L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010;61:109–124. doi: 10.3109/09637480903292601. [DOI] [PubMed] [Google Scholar]
- 41.Chen J., Wu Y., Zou J., Gao K. α-Glucosidase inhibition and antihyperglycemic activity of flavonoids from Ampelopsis grossedentata and the flavonoid derivatives. Bioorg. Med. Chem. 2016;24:1488–1494. doi: 10.1016/j.bmc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Rauter A.P., Martins A., Borges C., Mota-Filipe H., Pinto R., Sepodes B., Justino J. Antihyperglycaemic and protective effects of flavonoids on streptozotocin–induced diabetic rats. Phytother. Res. 2010;24:S133–S138. doi: 10.1002/ptr.3017. [DOI] [PubMed] [Google Scholar]
- 43.Arumugam B., Palanisamy U.D., Chua K.H., Kuppusamy U.R. Potential antihyperglycaemic effect of myricetin derivatives from Syzygium malaccense. J. Funct. Foods. 2016;22:325–336. doi: 10.1016/j.jff.2016.01.038. [DOI] [Google Scholar]
- 44.Heikkilä E., Hermant A., Thevenet J., Bermont F., Kulkarni S.S., Ratajczak J., Santo-Domingo J., Dioum E.H., Canto C., Barron D. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br. J. Pharmacol. 2019;176:3250–3263. doi: 10.1111/bph.14757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. 3rd ed. Allured Pub Corp; Carol Stream, IL, USA: 2004. [Google Scholar]
- 46.Ayoub I.M., Youssef F.S., El-Shazly M., Ashour M.L., Singab A.N., Wink M. Volatile constituents of Dietes bicolor (Iridaceae) and their antimicrobial activity. Z. Nat. C. 2015;70:217–225. doi: 10.1515/znc-2015-0164. [DOI] [PubMed] [Google Scholar]
- 47.Elkady W.M., Ayoub I.M. Chemical profiling and antiproliferative effect of essential oils of two Araucaria species cultivated in Egypt. Ind. Crops Prod. 2018;118:188–195. doi: 10.1016/j.indcrop.2018.03.051. [DOI] [Google Scholar]
- 48.Gad H.A., Ayoub I.M., Wink M. Phytochemical profiling and seasonal variation of essential oils of three Callistemon species cultivated in Egypt. PLoS ONE. 2019;14:e0219571. doi: 10.1371/journal.pone.0219571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Labib R.M., Ayoub I.M., Michel H.E., Mehanny M., Kamil V., Hany M., Magdy M., Moataz A., Maged B., Mohamed A. Appraisal on the wound healing potential of Melaleuca alternifolia and Rosmarinus officinalis L. essential oil-loaded chitosan topical preparations. PLoS ONE. 2019;14:e0219561. doi: 10.1371/journal.pone.0219561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saeed Kotb S., Ayoub I.M., El-Moghazy S.A., Singab A.N.B. Profiling the lipophilic fractions of Pithecellobium dulce bark and leaves using GC/MS and evaluation of their antioxidant, antimicrobial and cytotoxic activities. Chem. Biodivers. 2020;17:e2000048. doi: 10.1002/cbdv.202000048. [DOI] [PubMed] [Google Scholar]
- 51.Ashmawy A.M., Ayoub I.M., Eldahshan O.A. Chemical composition, cytotoxicity and molecular profiling of Cordia africana Lam. on human breast cancer cell line. Nat. Prod. Res. 2020:1–6. doi: 10.1080/14786419.2020.1736064. [DOI] [PubMed] [Google Scholar]
- 52.Gad H., Al-Sayed E., Ayoub I. Phytochemical discrimination of Pinus species based on GC–MS and ATR-IR analyses and their impact on Helicobacter pylori. Phytochem. Anal. 2021 doi: 10.1002/pca.3028. [DOI] [PubMed] [Google Scholar]
- 53.Chen B.-H., Wu P.-Y., Chen K.-M., Fu T.-F., Wang H.-M., Chen C.-Y. Antiallergic potential on RBL-2H3 cells of some phenolic constituents of Zingiber officinale (Ginger) J. Nat. Prod. 2009;72:950–953. doi: 10.1021/np800555y. [DOI] [PubMed] [Google Scholar]
- 54.Thabet A.A., Youssef F.S., Korinek M., Chang F.-R., Wu Y.-C., Chen B.-H., El-Shazly M., Singab A.N.B., Hwang T.-L. Study of the anti-allergic and anti-inflammatory activity of Brachychiton rupestris and Brachychiton discolor leaves (Malvaceae) using in vitro models. BMC Complement. Altern. Med. 2018;18:299. doi: 10.1186/s12906-018-2359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samir N., George R.F., Elrazaz E.Z., Ayoub I.M., Shalaby E.M., Plaisier J.R., Demitri N., Wink M. Synthesis of some tropane-based compounds targeting colon cancer. Future Med. Chem. 2020;12:2123–2140. doi: 10.4155/fmc-2020-0097. [DOI] [PubMed] [Google Scholar]
- 56.George R.F., Samir N., Ayoub I.M., Shalaby E.M., Demitri N., Wink M. Synthesis, antiproliferative activity and 2D-QSAR study of some 8-alkyl-2,4-bisbenzylidene-3-nortropinones. Future Med. Chem. 2018;10:2815–2833. doi: 10.4155/fmc-2018-0205. [DOI] [PubMed] [Google Scholar]
- 57.Ayoub I., El-Shazly M., Lu M.-C., Singab A. Antimicrobial and cytotoxic activities of the crude extracts of Dietes bicolor leaves, flowers and rhizomes. S. Afr. J. Bot. 2014;95:97–101. doi: 10.1016/j.sajb.2014.08.012. [DOI] [Google Scholar]
- 58.Matsuda H., Tewtrakul S., Morikawa T., Nakamura A., Yoshikawa M. Anti-allergic principles from Thai zedoary: Structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. 2004;12:5891–5898. doi: 10.1016/j.bmc.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 59.Chen B.-H., Hung M.-H., Chen J.Y.-F., Chang H.-W., Yu M.-L., Wan L., Tsai F.J., Wang T.-P., Fu T.-F., Chiu C.-C. Anti-allergic activity of grapeseed extract (GSE) on RBL-2H3 mast cells. Food Chem. 2012;132:968–974. doi: 10.1016/j.foodchem.2011.11.079. [DOI] [Google Scholar]
- 60.Chung Y.-M., Chang F.-R., Tseng T.-F., Hwang T.-L., Chen L.-C., Wu S.-F., Lee C.-L., Lin Z.-Y., Chuang L.-Y., Su J.-H. A novel alkaloid, aristopyridinone A and anti-inflammatory phenanthrenes isolated from Aristolochia manshuriensis. Bioorg. Med. Chem. Lett. 2011;21:1792–1794. doi: 10.1016/j.bmcl.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 61.Youssef F., Ashour M., Sobeh M., El-Beshbishy H., Singab A., Wink M. Eremophila maculata Isolation of a rare naturally-occurring lignan glycoside and the hepatoprotective activity of the leaf extract. Phytomedicine. 2016;23:1484–1493. doi: 10.1016/j.phymed.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Youssef F.S., Ashour M.L., Ebada S.S., Sobeh M., El-Beshbishy H.A., Singab A.N., Wink M. Antihyperglycaemic activity of the methanol extract from leaves of Eremophila maculata (Scrophulariaceae) in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2017;69:733–742. doi: 10.1111/jphp.12690. [DOI] [PubMed] [Google Scholar]
- 63.Thabet A.A., Youssef F.S., El-Shazly M., El-Beshbishy H.A., Singab A.N.B. Validation of the antihyperglycaemic and hepatoprotective activity of the flavonoid rich fraction of Brachychiton rupestris using in vivo experimental models and molecular modelling. Food Chem. Toxicol. 2018;114:302–310. doi: 10.1016/j.fct.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 64.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969;6:24–27. doi: 10.1177/000456326900600108. [DOI] [Google Scholar]
- 65.Gordon C., Yates A., Davies D. Evidence for a direct action of exogenous insulin on the pancreatic islets of diabetic mice: Islet response to insulin pre-incubation. Diabetologia. 1985;28:291–294. doi: 10.1007/BF00271688. [DOI] [PubMed] [Google Scholar]
- 66.Elkady M.W., Ayoub M.I., Abdel-Mottaleb Y., ElShafie M.F., Wink M. Euryops pectinatus L. flower extract inhibits P-glycoprotein and reverses multi-drug resistance in cancer cells: A mechanistic study. Molecules. 2020;25:647. doi: 10.3390/molecules25030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the first author.