Abstract
Currently, the food and agricultural sectors are concerned about environmental problems caused by raw material waste, and they are looking for strategies to reduce the growing amount of waste disposal. Now, approaches are being explored that could increment and provide value-added products from agricultural waste to contribute to the circular economy and environmental protection. Edible mushrooms have been globally appreciated for their medicinal properties and nutritional value, but during the mushroom production process nearly one-fifth of the mushroom gets wasted. Therefore, improper disposal of mushrooms and untreated residues can cause fungal disease. The residues of edible mushrooms, being rich in sterols, vitamin D2, amino acids, and polysaccharides, among others, makes it underutilized waste. Most of the published literature has primarily focused on the isolation of bioactive components of these edible mushrooms; however, utilization of waste or edible mushrooms themselves, for the production of value-added products, has remained an overlooked area. Waste of edible mushrooms also represents a disposal problem, but they are a rich source of important compounds, owing to their nutritional and functional properties. Researchers have started exploiting edible mushroom by-products/waste for value-added goods with applications in diverse fields. Bioactive compounds obtained from edible mushrooms are being used in media production and skincare formulations. Furthermore, diverse applications from edible mushrooms are also being explored, including the synthesis of biosorbent, biochar, edible films/coating, probiotics, nanoparticles and cosmetic products. The primary intent of this review is to summarize the information related to edible mushrooms and their valorization in developing value-added products with industrial applications.
Keywords: edible mushrooms, waste valorization, food products, industrial applications
1. Introduction
Mushrooms have long been stated as a gourmet food, especially for its subtle flavor and taste, and they have been regarded as a culinary wonder by humankind. There are 2000 different mushrooms, out of which 25 are usually consumed as food, and only a few are commercially grown [1]. Mushrooms are also used as nutraceutical foods for their high functional and nutritional value. Moreover, they have gained considerable attention due to their economic importance as well as organoleptic and medicinal properties [2,3]. It is not easy to differentiate between medicinal and edible mushrooms, as few medicinal mushrooms are edible, and many common edible mushrooms have therapeutic potential [4]. The most widely cultivated mushroom is Agaricus bisporus, followed by Flammulina velutipes, Lentinus edodes and Pleurotus spp. The crude protein content of edible mushrooms is usually high, but it varies greatly and is affected by factors such as species and stage of development of the mushroom [5]. The free amino acid level of mushrooms is usually low, ranging from 7.14 to 12.3 mg g−1 in dry edible mushrooms, and contributes to the main flavor properties of mushrooms [6]. The essential amino acid profiles of mushrooms reveal that the proteins are deficient in sulfur-containing amino acids, including methionine and cysteine. However, these edible mushrooms are comparatively rich in threonine and valine. Several vitamins such as folates, niacin and riboflavin are found in abundance in cultivated mushrooms. Mushrooms have a higher vitamin B2 content compared to most vegetables, making them a good vitamin source [7]. The bioavailable form of folate in mushrooms is folic acid [8]. Cultivated mushrooms also comprise vitamin B1 and vitamin C in small quantities and traces of vitamin B12 [7]. Edible mushrooms contain a low amount of total soluble sugars, whereas oligosaccharides are found abundantly [9]. The carbohydrate content of edible mushrooms ranges from 35 to 70% by dry weight and varies from species to species. The fatty acid level ranges from 2 to 8% in mushrooms. Additionally, polyunsaturated fatty acids account for ≥75% of total fatty acids, in contrast to saturated fatty acids, and palmitic acid is the major saturated fatty acid [10].
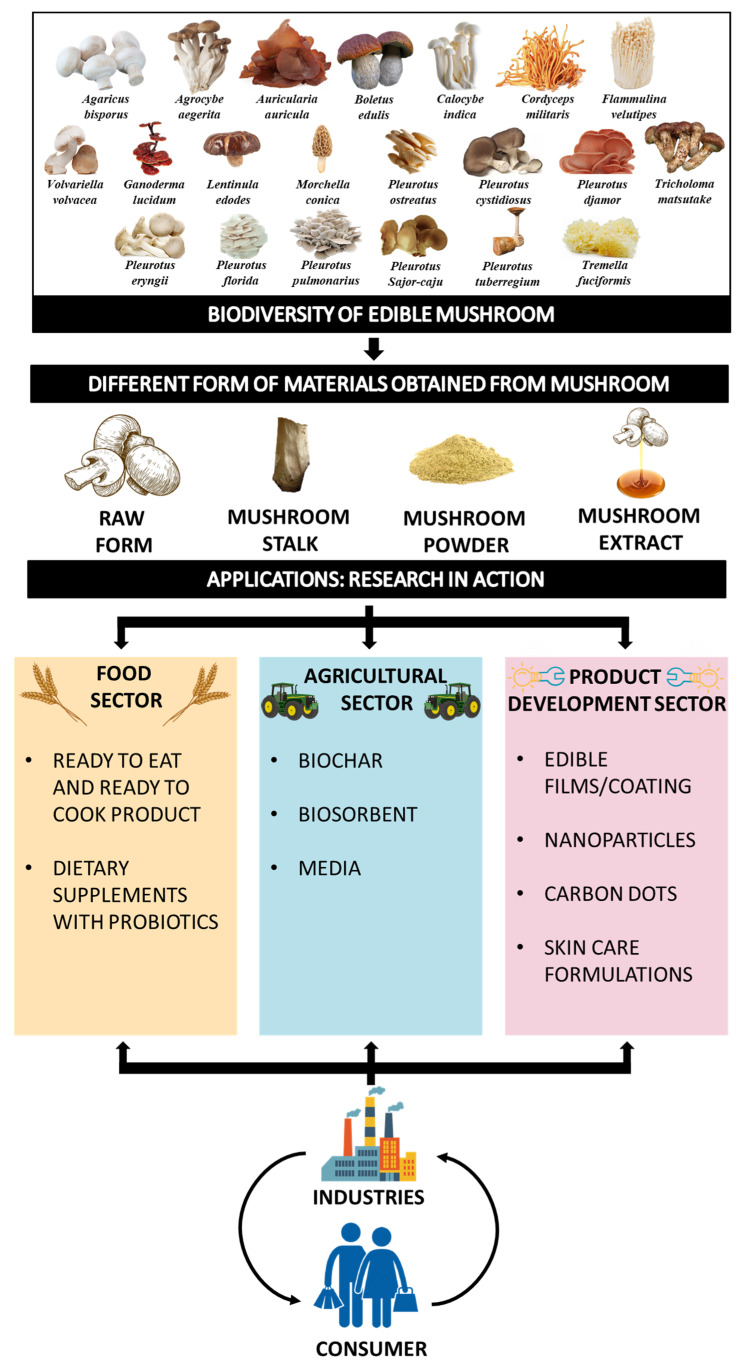
Many by-products (caps, stipes, spent mushroom substrate) are produced during mushroom production, which cause environmental pollution and increase industry management costs [11]. Spent mushroom substrate (SMS) encompasses extracellular enzymes, fungal mycelia and other substances [12]. The circular economy concept of industrial ecology is regarded as the leading principle for developing new products by using waste as a raw material [13]. From economic and environmental perspectives, the waste produced during mushroom production often leads to massive damage to valuable organic constituents and raises severe management complications. Thus, there is a need to exploit mushroom residues to extract valuable compounds that could be used in different industries, such as food, cosmetics, agricultural and textile industries, as depicted in Figure 1. The current review aims to summarize information related to edible mushrooms and discuss the utilization of edible mushrooms and their residues as a valuable good for future industrial applications.
Figure 1.
Utilization of edible mushrooms and their residues in novel industrial products.
2. Edible Mushrooms Fortified in Ready-to-Eat and Ready-to-Cook Foods
As the lifestyle of people is changing dramatically (due to liberalization policies, dual incomes, separate living of couples, innovative kitchen applications, media proliferation, etc.), the demand for convenient and health-promoting food is also increasing. Nowadays, people prefer fast and simple cooking methods instead of spending a long time in the kitchen [14]. Mushroom powder can be used in the food industry, especially in preparing baked goods (bread, biscuits and cakes) and breakfast cereals. The supplementation of mushroom powder in bakery products substantially increases crude fibers, minerals (calcium, copper, magnesium, manganese, potassium, phosphorus, iron and zinc), proteins and vitamins [15]. These components impart the abilities to fight tumors, lower blood pressure and blood sugar levels, maintain cholesterol levels and improve the immune system to fight against infection [16]. Rathore et al. [17] prepared cookies fortified with Calocybe indica mushroom, and the results depicted a decrease in starch hydrolysis and glycemic index. Wheatshiitake noodles enhanced the nutritional profile and reduced the glycemic index of foods [18]. The different food products developed by using mushrooms are listed in Table 1.
Table 1.
Mushrooms fortified in ready-to-eat (RTE) and ready-to-cook (RTC) foods.
| Edible Mushroom Common Name | Scientific Name | Food Product | Beneficial Effects | Reference |
|---|---|---|---|---|
| Milky white | Calocybe indica | Cookies | Increase in protein, fiber, minerals and β-glucan, phenolic, flavonoids and antioxidants; decrease in starch, reduction in glycemic index | [17] |
| Oyster | Pleurotus sajor-caju | Biscuits | Increase in concentration of protein, dietary fiber, ash and reduction in carbohydrate | [19] |
| Shiitake | Lentinula edodes | Chips | Improvement in quality attributes (color, sensory evaluation) | [20] |
| Oyster | Pleurotus ostreatus | Biscuits | Enhancement of nutritional quality | [21] |
| White button | Agaricus bisporus | Ketchup | Increase in ash content, crude fiber, protein, total soluble solids, and reducing sugars; decrease in total sugars | [22] |
| Oyster | Pleurotus ostreatus | Jam | Increase in total soluble solids, percent acidity and reducing sugar, decrease in pH and non-reducing sugar | [23] |
| White button | Agaricus bisporus | Mushroom tikki and stuffed mushroom | Increase in protein, dietary fiber, antioxidant and phenolic components | [24] |
| Oyster | Pleurotus ostreatus | Soup | Increase in nutritional value | [25] |
| Chestnut | Agrocybe aegerita | Snacks | Manipulation of glycemic response of individuals |
[26] |
| Oyster | Pleurotus sajur-caju | Biscuit | Increase in the mineral content | [27] |
| Oyster | Pleurotus ostreatus | Vegetable mixture diets | Highly acceptable, nutritious, delicious, ready-to-eat diet | [14] |
| Oyster | Pleurotus ostreatus | Processed cheese spreads | High moisture, ash and protein content, total viable counts and spore former bacteria was lower in processed cheese supplemented with mushrooms | [28] |
| Oyster | Pleurotus ostreatus | Biscuit | Higher moisture, protein, ash content, higher hardness, darker and redder in color | [29] |
| Oyster | Pleurotus ostreatus | Spreadable processed cheese | Increase in total solids, protein, fibers and carbohydrates | [30] |
| Oyster | Pleurotus sajor-caju | chicken patty | Reduction in fat content, no change in protein and β-glucan | [31] |
| White button | Agaricus bisporus | Pasta | Improved antioxidant activity, increase moisture content, carbohydrates, decreased crude fiber, crude protein, and fat | [32] |
| Oyster | Pleurotus sajor-caju | Cookies | High protein content, low-fat content, high fiber, minerals and vitamin content | [33] |
| White button | Agaricus bisporus | Pasta | Decrease in the extent of starch degradation, increase in total phenolic content and antioxidant capacities | [34] |
| White jelly | Tremella fuciformis | Patty | Oil holding capacity of mushroom has a positive effect on cooking yield of patty as well as senses | [35] |
| Oyster | Pleurotus ostreatus | Instant noodles | Increase in protein and fiber content | [36] |
| White button | Agaricus bisporus | Beef burgers | Reduction in the fat content of beef burgers | [37] |
| Oyster | Pleurotus ostreatus | Instant soup premix | Rich in protein, crude fiber, minerals and low in fat, carbohydrate and energy value | [38] |
| White button | Agaricus bisporus | Sponge cake | Increase in apparent viscosity, volume, springiness and cohesiveness values | [39] |
| Oyster | Pleurotus sajor-caju | Biscuit | Reduction in starch pasting viscosities, starch gelatinization enthalpy value, increases in protein, crude fiber and mineral content | [16] |
| Shiitake | Lentinula edodes | Noodles | Improvement in nutritional profile and reduction in the glycemic index of foods | [18] |
| King tuber | Pleurotus tuber-regium | Cookies | Higher protein, ash, crude fiber, water-soluble vitamins and minerals | [40] |
| Oyster | Pleurotus ostreatus | Noodles | Lower level of carbohydrate, fat, and sodium | [41] |
| King trumpet | Pleurotus eryngii | Sponge cake | Increase in ash and proteincontent | [42] |
| White button, Shitake, Porcini | Agaricus bisporus, Lentinula edodes, Boletus edulis | Pasta | High firmness and tensile strength | [43] |
3. Edible Mushrooms Based Films/Coatings
Edible films/coatings are thin layers applied on the food surface to extend their shelf-life and preserve their features, functionality and properties at a low cost [44]. The mechanical strength and barrier properties of these edible films provide sufficient strength to withstand stress while handling. These films have a promising application in the agricultural, food and pharmaceutical industries. Mushrooms and their residues have many applications in food industries, but significantly fewer studies have been conducted in regards to edible film/coatings. Polysaccharides extracted/derived from edible mushrooms are extensively used in functional foods, pharmaceuticals and nutraceuticals [11]. In this regard, Bilbao-Sainzand his colleagues [45] obtained chitin from mushrooms and transformed it to chitosan.
Moreover, layer-by-layer (LbL) electrostatic deposition is used to prepare edible coatings applied to fruit bars. The application of edible mushroom coatings/films has increased the antioxidant capacity, ascorbic acid content, fungal growth prevention and firmness during storage. Additionally, Du et al. [46] developed edible films using Flammulina velutipes polysaccharides, which acted as a barrier to oxygen and water vapor, had the lowest elongation at break values and highest tensile strength for future use in food packaging industries. Table 2 lists some edible films and coatings derived from mushrooms.
Table 2.
Mushrooms and their residue-based edible film/coatings.
| Edible Mushrooms Common Name | Scientific Name | Product Used | Compounds | Key Findings | References |
|---|---|---|---|---|---|
| White button | Agaricus bisporus | Fruit bars | Chitosan | Increased antioxidant capacity, ascorbic acid content, fungal growth prevention and firmness | [45] |
| White button | Agaricus bisporus | Fresh-cut melons | Chitosan | Enhance fruit firmness, inhibit off-flavors and reduce the microbial counts (up to 4 log CFU g−1). | [47] |
| Velvet shank | Flammulina velutipes | ND | Polysaccharide | High tensile strength, barrier property to water vapor and oxygen | [46] |
| Shiitake, Velvet shank | Lentinula edodes, Flammulina velutipes | ND | Insoluble dietary fibers | Highest tensile strength and an effective barrier to water vapor | [48] |
| Indian oyster | Pleurotus pulmonarius | ND | Flour | Significant barrier properties and mechanical strength | [49] |
ND—not defined; CFU—colony-forming unit.
4. Mushrooms as a Source of Prebiotics for Food Supplementation
The consumption of high dietary fiber food has gained considerable interest owing to its ability to reduce triglycerides and blood cholesterol via the gut microbiome. A diet rich in fibers acts as a substrate for microbes and aids in their proliferation. Thus, microbial digestion products enter the systemic circulation and help in maintaining energy homeostasis [50]. Pleurotus spp. (Oyster mushroom) comprises soluble fiber compounds, particularly a small amount of glucans (chitin and galactomannans) and non-starch glucans, favoring the proliferation of lactobacilli [51]. Edible mushrooms are stated to have carbohydrates, which help them to act as prebiotics [52]. The supplementation of oyster mushroom and probiotics in poultry feed has been reported to show beneficial, synergetic effects on the immune response, performance and serum lipids in broiler chickens [53]. The blend of prebiotics and probiotics also is beneficial because of the synergistic effect between them [54]. Van Doan et al. [52] conducted a study to determine the effects of dietary supplements Pleurotus eryngii (as a prebiotic), Eryngii mushroom and Lactobacillus plantarum (as a probiotic), alone as well as in combination, on the innate immune response, growth and protection against Aeromonas hydrophila. The results showed stimulation in growth, immunity and disease resistance against Pangasius bocourti. Table 3 lists studies of different mushrooms and dietary supplementation with probiotics.
Table 3.
Applications of mushrooms as prebiotics.
| Edible Mushrooms Common Name | Scientific Name | Probiotic Used | Form of Mushroom Used | Applications | References |
|---|---|---|---|---|---|
| White button | Agaricus bisporus | Probiotics mixture (Protexin 6 × 107CFU gm−1) | Powder | Lowered total cholesterol, LDL cholesterol, triglyceride concentrations, oxidative stress and dyslipidemia in hypercholesterolemic rats | [50] |
| Wood ear/Jew’s ear | Auricularia auricula | Lactobacillus acidophilus La-5, Bifidobacterium bifidum Bb-12 | Extract | Enhancement in the survival rate of probiotics toabout 0.43 and 0.51 log CFU g−1; improved probiotic protection and functional properties of symbiotic yogurt | [55] |
| White button | Agaricus bisporus | Saccharomyces cerevisiae | Powder | Improvement in the meat quality with the incorporation of mushroom and probiotics in the broiler diet | [56] |
| Oyster | Pleurotus sajor-caju | Lactobacillus fermentum OVL | Powder | Increase in neutrophil count in rats, decrease in lymphocyte count | [57] |
| Oyster | Pleurotus ostreatus | PrimaLac (Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidium, Enterococcus faecium) | Powder | Decrease in abdominal fat on the carcass, increase in HDL concentration in plasma | [53] |
| Caterpillar | Cordyceps militaris | Lactobacillus plantarum | Spent mushroom substrate | Increase in the specific growth rate, weight gain, final weight in fish fed supplemented diets | [58] |
| Shiitake | Lentinus edodes | 1.0 ×108 CFU g−1(Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifdium, Enterococcus faecium) | Extract | No weight gain in broiler chickens | [59] |
| King oyster | Pleurotus eryngii | Lactobacillus plantarum | Powder | Growth stimulation, immunity and disease resistance | [52] |
LDL—low-density lipoproteins; HDL—high density lipoproteins.
5. Edible Mushrooms Based Media
Nowadays, mushroom processing is the primary solid-state fermentation process in fermentation industries [60]. At the commercial level, the processing occurs on a substrate made up of lignocellulose materials (corncobs, sawdust, rye, rice straw and wheat) either alone or in combination with supplements to address nutritional deficiencies [61,62]. For instance, approximately 5 kg of SMS is produced, a by-product of mushroom harvest and cultivation, from 1 kg of mushrooms [63]. The SMS comprises a high amount of residual nutrients, which pollutes the atmosphere if improperly discarded as waste [64,65]. Thus, further treatment and utilization of SMS are essential. Different types of edible mushrooms and their SMS have been used to produce low-cost growth media for various horticultural plants and microorganisms (Table 4).
Table 4.
Mushrooms and their residue-based media.
| Edible Mushrooms Common Name | Scientific Name | Media Composition | Purpose/Utilization | References |
|---|---|---|---|---|
| Velvet shank | Flammulina velutipes | Spent mushroom substrate, perlite, and vermiculite | Growing media for tomato and cucumber seedlings | [66] |
| White button, Oyster | Agaricus bisporus, Pleurotus ostreatus | Spent mushroom substrate, and Sphagnum peat | Growing media for tomato, courgette and pepper | [67] |
| Velvet shank | Flammulina velutipes | Spent mushroom substrate, and chicken manure compost | Growing media for honeydew melon | [68] |
| Velvet shank | Flammulina velutipes | Spent mushroom substrate, calcium carbonate, wheat bran, and yeast extract and inorganic salts | Production media for Bacillus thuringiensis | [69] |
| Oyster | Pleurotusf lorida | Spent mushroom substrate | Production media for lignocellulolytic enzymes | [70] |
| Oyster | Pleurotus ostreatus | Spent mushroom substrate | Production media for Lactococcus lactis | [71] |
| Oyster | Pleurotus ostreatus | Spent mushroom substrate, paddy straw, and soybean cake | Biopesticide (Trichoderma asperellum) development | [72] |
| ND | ND | Spent mushroom substrate and peat moss | Growing media for Chinese kale | [73] |
| ND | ND | Spent mushroom substrate, perlite, and vermiculite | Growing media for lettuce seedlings | [74] |
| ND | ND | Spent mushroom substrate, polished rice, full-fat soybean, and rice bran | Production media for arachidonic acid by Mortierella sp. | [75] |
| ND | ND | Spent mushroom substrate, and poultry cooked bones | Production media for solubilizationphosphate by Bacillus megaterium | [76] |
ND—not defined.
For tomato seedling production, SMS-derived media from Agaricus bisporus and Pleurotus ostreatus were used. For pepper seedling production, SMS and a 50% mixture of Agaricus bisporus and Pleurotus ostreatus were used, while a low dose of SMS derived from Agaricus bisporus was reported for courgette seedling production [67]. Owing to the chemical, physical and nutritional characteristics of SMS, Sendi et al. [73] revealed that spent mushroom waste (50%) and peat moss (50%) with nitrogen, phosphorus and potassium fertilizer supplementation were the growth media components that had the greatest potential to aid in Chinese kale growth. SMS derived from Flammulina velutipes was appropriate for biopesticide (Bacillus thuringiensis) with high toxicity and long larvicidal persistence [69]. Pleurotus ostreatus derived SMS, when neutralized with calcium hydroxide, ammonium hydroxide or sodium hydroxide, may be used as a carbon source for Lactococcus lactis subsp. lactis W28 cultivation [71].
6. Edible Mushrooms Derived Biosorbents
Biosorption is a process in which a sorbate reacts with biomass or biomaterial (biosorbent), causing sorbate ions to acclimatize on the biosorbents surface and, as a result, lowering the concentration of sorbate in the solution [44]. This mechanism has gained significant attention among researchers because of its ability to immobilize heavy metals, which can contaminate the water as they are discharged untreated from electroplating, mining industries and metal processing industries. Various processes that explain the mechanism of how these biosorbents function in removing pollutants are expressed by the natural biomass complex compendium. Several functional groups (amides, amine, carboxyl, carbonyl, hydroxyl, sulfonate, sulfhydryl, phosphate and phenolic groups) are attached to these biosorbents to sequestrate contaminants [77,78]. Various studies were done to produce biosorbents from edible mushrooms to remove metal ions and dyes from an aqueous solution, as shown in Table 5.
Table 5.
Mushroom-derived biosorbents and their applications.
| Edible Mushrooms Common Name | Scientific Name | Drying Temperature/Time | Applications | References |
|---|---|---|---|---|
| Oyster | Pleurotus florida | RT/24 h | Showed 100% removal of Fe2+ from the water sample | [79] |
| White button | Agaricus bisporus | 80 °C/24 h | Successfully biosorbed Reactive Blue 49 dye (1.85 × 10−4 mol g−1) from water | [80] |
| Oyster | Pleurotus ostreatus | 40 °C/24 h | Showed greater adsorption against Pb2+(85.91 mg g−1) in water | [81] |
| Oyster | Pleurotus ostreatus | 60 °C/24 h | Biosorbed 3.8 mg g−1 of Cd2+ | [82] |
| Oyster, Black morels | Pleurotus ostreatus, Morchella conica | RT/4 days | Adsorbed methylene blue (82.81 and 38.47 mg g−1) and for malachite green (64.13 and 39.28 mg g−1) | [83] |
| Velvet shank | Flammulina velutipes | 60 °C/24 h | Maximum removal capacity against copper ions was 15.56 mg g−1 | [84] |
| Shiitake | Lentinula edodes | Freeze-dried/24 h | Maximum absorption against Congo red was 217.86 mg g−1 | [85] |
| Oyster | Pleurotus ostreatus | 78 °C/48 h | Showed maximum biosorption against uranium ion (19.95 mg g−1) | [86] |
| Oyster | Pleurotus ostreatus | 80 °C/ND | Showed maximum biosorption against Ni2+ (20.71 mg g−1) | [87] |
| King trumpet | Pleurotus eryngii | 60 °C/24 h | Showed maximum biosorption against Pb2+ (3.30 mg g−1) | [88] |
| Lingzhi | Ganoderma lucidum | 60 °C/72 h | Maximum biosorption against malachite green (40.65 mg g−1), safranine T (33.00 mg g−1), and methylene (22.37 mg g−1) | [89] |
| King trumpet | Pleurotus eryngii | 60 °C/24 h | Removed 88.38% of NO3− | [90] |
RT—room temperature; ND—not defined.
For the biosorption of lead (II) from aqueous solution, Eliescu et al. [81] compared the biosorbent activity of Pleurotus ostreatus biomass derived from spent mushroom substrate and observed Pb(II) sorption parameters. The Pleurotus ostreatus spent substrate (POBM) had a higher adsorption potential (85.91 mg g−1) than that of the Pleurotus ostreatus original spores (PO) (57.73mg g−1). Further, the presence of hydroxyl and carbonyl groups in the spent substrate of Ganodorma lucidum proved as a biosorbent for cationic dyes (malachite green, methylene blue and safranine) [89]. In another study, the hydroxyl and carboxyl groups in a Pleurotus eryngii derived biosorbent were reported to remove nitrate from an aqueous solution [90].
7. Edible Mushrooms Derived Biochar
Biochar is a stable, carbon-rich solid prepared by thermochemical decomposition or pyrolysis of organic material at high temperatures in an anaerobic environment [44]. The highly porous structure permits the extraction of humic and fluvic-like substances from biochar [91]. Furthermore, its molecular structure demonstrates high microbial and chemical stability [92], and physical and chemical properties depend on several factors such as the feedstock form, residence time, pyrolysis and furnace temperature [93,94]. A wide range of common raw materials are used as the feedstock, including wood chips, organic wastes, plant residues and poultry manure [95]. The elemental composition of biochar generally includes carbon, nitrogen, hydrogen and, to a lesser extent, K, Ca, Na and Mg [96]. Biochar is a polar or non-polar material with a high specific surface area and good affinity towards inorganic ions such as phosphate, nitrate and heavy metal ions [97,98]. Different studies have reported on biochar production from a variety of edible mushrooms and their spent substrates (Table 6).
Table 6.
Applications of biochar derived from mushrooms and their residues.
| Edible Mushrooms Common Name | Scientific Name | Process and Conditions Required for Biochar Formation | Applications | References |
|---|---|---|---|---|
| Oyster, Shiitake | Pleurotus ostreatus, Lentinula edodes | Pyrolysis at 700 °C for 2 h | Adsorbed 326mg g−1 and 398mg g−1 of lead Pb(II) from the water | [99] |
| Lingzhi | Ganoderma lucidum | Pyrolysis at 650 °C for 2 h | Showed maximal adsorption against Pb2+ (262.76 mg g−1) and Cd2+ (75.82 mg g−1) | [100] |
| White button | Agaricus bisporus | Pyrolysis at 750 °C for 3 h | Showed maximal adsorption against Cu2+(65.2 mg g−1), Cd2+(76.3 mg g−1), and Zn2+(44.4 mg g−1) in water | [101] |
| ND | ND | Pyrolysis at 300 °C for 90 min | Showed maximal adsorption against Pb2+ (21.0 mg g−1), Cu2+(18.8 mg g−1), Cd2+(11.2 mg g−1) and Ni2+(9.8 mg g−1) in water | [102] |
| ND | ND | Pyrolysis at 450 °C for 4 h | Showed maximal adsorption against crystal violet (1057mg g−1) in wastewater | [103] |
| ND | ND | Pyrolysis at 500 °C for 2 h | Showed maximal adsorption against fluoride (36.5 mg g−1) in water | [104] |
ND—not defined.
Wu et al. [99] investigated how inorganic mineral-induced alkalinity in biochars could facilitate Pb(II) removal by forming Pb(II) precipitate. The X-ray diffraction (XRD) analysis revealed that the spent mushroom substrate (SMS)-derived biochars contained Ca3(PO4)2, CaCO3 and inorganic anions (CO32−, SO42− and OH-), which might be released from the dissolved biochars and contribute to the Pb(II) precipitation process. In another study, Ganoderma lucidum derived biochars were significantly influenced by the pyrolysis temperature [100]. A reduction in biochar yield containing O/C, H/C and O functional groups and increased biochar ash, thermal stability and the specific surface were observed with temperature increase (250–650 °C). The high-temperature biochar can be an excellent adsorbent for heavy metal removal due to its wide specific surface area and mesoporous structure. Zhang et al. [101] utilized Agaricus bisporus substrate-derived biochar and used it to remove cadmium, copper and zinc from an aqueous solution. They concluded that the removal mechanism using biochar produced at 350 °C was primarily via ion exchange. In contrast, biochar was produced at a moderate temperature of 550 °C mainly via coordination with π electrons and mineral precipitation.
8. Edible Mushrooms Derived Nanoparticles (NPs)
The high concentrations of extracellular enzymes serve as bio-reducing and stabilizing agents for NP synthesis. NPs made from mushrooms are of better quality than those made from bacteria. Metal NPs synthesized using constituents such as enzymes and metabolites secreted by mushroom cells reduce the toxicity of substances [105,106]. The use of NPs is rising, especially in biomedicine and pharmaceuticals, because of their unique physicochemical properties. In the bottom-up approach, biogenic NPs are synthesized, resulting in atoms/compounds that act as the building blocks and possess the ability to self-assemble to form the final product [44]. Numerous metal oxide/noble metal NPs have been developed using extracts of edible mushrooms, as listed in Table 7.
Table 7.
Mushroom-derived nanoparticles and their applications.
| Edible Mushrooms Common Name | Scientific Name | Types of Nanoparticles Synthesized | Reaction Temperature/Time | Morphology | Size | Applications | References |
|---|---|---|---|---|---|---|---|
| White button | Agaricus bisporus | Copper | RT/24 h | Spherical | 2–10 nm | Antibacterial activity against Enterobacter aerogens; Antioxidant activity using DPPH, and ABTS; Anti-cancer activity against cancer cell lines SW620 (colon cancer) | [107] |
| Brown oyster | Pleurotus cystidiosus | Gold | 29 °C/24 h | ND | ND | Antioxidant activity using DPPH, and ABTS | [108] |
| Oyster | Pleurotus florida | Gold | 70 °C/1.5 h | Spherical | 2–14 nm | Anti-cancer activity against cancer cell lines A-549 (Human lung carcinoma), K-562 (Human chronic myelogenous leukemia bone marrow), HeLa (Human cervix) and MDA-MB (Human adenocarcinoma mammary gland) | [109] |
| Oyster | Pleurotus ostreatus | Gold | 29 °C/24 h | Spherical | 22.9 nm | Antioxidant activity using DPPH, and ABTS | [108] |
| Oyster | Pleurotus sajor-caju | Gold | RT/12 h | Spherical | 16–18 nm | Anti-cancer activity against cancer cell lines HCT-116 (colon cancer) | [110] |
| King tuber | Pleurotus tuber-regium | Selenium | RT/24 h | Spherical | 91–102 nm | Anti-cancer activity against gastric adenocarcinoma AGS | [111] |
| Oyster | Pleurotus ostreatus | Silver | 25 °C/48 h | Spherical | 17.5 nm | Anti-cancer activity against cancer cell lines HepG2 (human liver) and MCF-7 (breast) | [112] |
| Lingzhi | Ganoderma lucidum | Silver | ND/ND | Spherical | 15–22 nm | Antioxidant activity using DPPH; Antibacterial activity against Staphylococcus aureus, Enterococcus hirae, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa, Legionella pneumophila subsp. Pneumophila; and antifungal activity against Candida albicans | [113] |
| Matsutake | Tricholoma matsutake | Silver | RT/30 min | Spherical | 10–70 nm | Antibacterial activity against Bacillus cereus, Escherichia coli | [114] |
| Milky white, Oyster, White button, Lingzhi | Calocybe indica, Pleurotus ostreatus, Agaricu sbisporus, Ganoderma lucidum | Silver | RT/12 h | Spherical | 80–100 nm | Antibacterial activity against Staphylococcus aureus | [115] |
| Pink oyster | Pleurotus djamor | Titanium oxide | RT/20 min | Spherical | 31 nm | Antibacterial activity against Corynebacterium diphtheria, Pseudomonas fluorescens, and Staphylococcus aureus; Anti-cancer activity against cancer cell lines A-549 (Human lung carcinoma); larval toxicity against Aedes aegypti, Culex quinquefasciatus | [116] |
| Pink oyster | Pleurotus djamor | Zinc oxide | RT/24 h | Sphere | 74.36 nm | Antioxidant activity using DPPH, ABTS, and H2O2; larval toxicity against Aedes aegypti, Culex quinquefasciatus; Antibacterial activity against Corynebacterium diphtheria, Pseudomonas fluorescens, and Staphylococcus aureus | [117] |
RT—room temperature; ND—not defined; DPPH-2,2-diphenyl-1-picrylhydrazyl-hydrate; ABTS-2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid).
CuNPs derived from an aqueous extract of Agaricus bisporus showed the highest inhibitory activity against Enterobacter aerogens with an inhibition zone of 15.00 mm [107]. Antioxidant assays showed a possible scavenging effect against the ABTS radical (82%), the NO radical (76%) and the DPPH radical (72%). AgNPs derived using Ganoderma lucidum extract showed antibacterial effects with minimum inhibitory concentration (MIC) values of 128, 16 and 64 μg L−1 against Gram-positive bacteria (B. cereus, E. hirae, S. aureus) and 16, 64 and 128 μg L−1 against Gram-negative bacteria (L. pneumophila subsp. pneumophila, E. coli, P. aeruginosa). AgNPs derived from the pine mushroom (Tricholoma matsutake) displayed a zone of inhibition of 21.00mm against E. coli [115]. The anti-cancerous effect of Ganoderma neo-japonicum derived AgNPs against MDA-MB-231 human breast cancer cells was dose-dependent, according to Gurunathan et al. [118], and cells exposed to AgNPs generated more reactive oxygen species and hydroxyl radicals. At dilutions ranging from 10 to 30 g mL−1, AuNPs derived from Pleurotus florida extract by photo-irradiation demonstrated dose-dependent antiproliferative activity against various cancer cell lines (A-549, K-562, HeLa and MDA-MB) [109]. Chaturvedi et al. [110] investigated cytotoxicity and observed that AgNPs and AuNPs formed by Pleurotus sajor-caju extract (PS) were effective against the HCT-116 cancer cell line. HCT-116 cancer cell viability was inhibited by P. sajor-caju extract, AuNPs and AgNPs, with IC50 values of 60, 80 and 50 g mL−1, respectively. Compared to other PS extracts and AuNPs, green synthesized AgNPs showed strong antiproliferative activity due to ROS generation, which led to oxidative stress and resulted in improper protein functionality and integrity.
9. Edible Mushrooms Derived Carbon Dots
Carbon dots (CDs), photoluminescent substances with a size of less than 10 nm, can be synthesized by top-down and bottom-up approaches [44]. The top-down synthetic route involves a complex and synthetic condition; a broad carbon structure is broken down using electro-oxidation, acid-assisted chemical oxidation, and laser ablation [44]. However, the bottom-up approach, which relies on plants and their by-products instead of the chemicals, is superior compared to the top-down approach. Proteins, carbohydrates, lipids, lignin and cellulose are all abundant in biological materials. Edible mushrooms are relatively inexpensive and contain various chemical constituents such as carbon, oxygen, phosphorus and nitrogen, often depicted as carboxyl and amine groups. The presence of carbohydrates, amino acids, polysaccharides, citric acid, flavonoids, lipids, vitamins and proteins make them ideal for CDs development [119]. CDs have also shown effectiveness in biomedical applications and energy storage systems, including water purification, pathogen identification, environmental research and heavy metal and additive detection in food (Table 8).
Table 8.
Mushrooms as a carbon source for preparing carbon dots.
| Edible Mushrooms Common Name | Scientific Name | Production Conditions | Applications | References |
|---|---|---|---|---|
| Oyster | Pleurotus sp. | Hydrothermal/120 °C/4 h | Selective sensitivity for Pb2+; Antibacterial activity against Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa; Anti-cancer activity against breast cancer cells (MDA-MB-231) | [119] |
| Velvet shank | Flammulina velutipes | Hydrothermal/250 °C/4 h | Sensed Cr6+ with a limit of detection 0.73 µM and volatile organic compounds | [120] |
| Oyster | Pleurotu ssp. | Hydrothermal/200 °C/25 h | Sensed nitroarenes in water samples | [121] |
| Paddy straw | Volvariella volvacea | Hydrothermal/200 °C/25 h | Sensed Pb2 with limit of detection 12 nM and for Fe3+ 16 nM | [122] |
| ND | ND | Hydrothermal/200 °C/6 h | Sensed hyaluronic acid and hyaluronidase | [123] |
ND—not defined.
Pleurotus sp. derived CDs were prepared by Boobalan et al. [119], and the authors found that the presence of hydroxyl and carboxyl groups (derived from amino acids and polysaccharides) caused them to bind with selective Pb2+ ions, forming lead hydroxide and carboxylate, which causes their quenching. Further, they observed an increase in antibacterial activity with increased CDs concentration, and they reported anti-cancer activity with a half-maximal inhibitory concentration (IC50) of 3.34 g mL−1. In addition to this, Zulfajri et al. [121] developed CDs using oyster mushroom (OM) for sensing nitroarenes (NAs). The study demonstrated that the sensing mechanisms such as fluorescence resonance energy transfer (FRET), inner filter effect (IFE) and photo-induced electron transfer (PET) quenched the fluorescence emission intensity of OM-CDs by NAs. The spiked underground water recoveries were found to be 97.20 and 102.60%, with RSDs under 2%, representing the reliability of OM-CDs as a sensor for NAs with good repeatability, precision and recovery. Yang et al. [123] developed CDs that can detect hyaluronic acid in human urine samples in concentrations ranging from 50 pM to 50 mM.
10. Edible Mushrooms Based Skin Care Formulations
Cosmetics are personal care products that are used to cleanse and beautify the skin [124]. The demand for cosmetics containing natural ingredients is increasing due to their organic, healthier and environmentally friendly characteristics [125]. Lentinan, carotenoids, ceramides, schizophyllan and ω-3, ω-6 and ω-9 fatty acids as well as resveratrol obtained from macro fungi, especially mushrooms, are now paving their way into cosmetics [126,127]. These are reported to treat beauty issues such as fine lines, wrinkles, uneven tone and texture due to the antioxidant and anti-inflammatory traits. There are few studies where edible mushrooms are used in skincare formulations, as compiled in Table 9.
Table 9.
Mushroom-based skincare formulations.
| Edible Mushrooms Common Name | Scientific Name | Product Base | Applications | References |
|---|---|---|---|---|
| White button, Oyster, Shiitake | Agaricus bisporus, Pleurotus ostreatus, Lentinula edodes | Cream | Anti-inflammatory; anti-tyrosinase; antioxidant and antibacterial activity | [128] |
| Lingzhi | Ganoderma lucidum | Cream | Anti-tyrosinase; antioxidant and antibacterial activity | [129] |
| Oyster | Pleurotus ostreatus | Cream | Skin fairness | [130] |
| Oyster | Pleurotus ostreatus | Gel | Anti-tyrosinase; antioxidant activity | [131] |
| Snow | Tremella fuciformis | Gel | Hand sanitizer | [132] |
| White button, Oyster | Agaricus bisporus, Pleurotus ostreatus | Cream | Anti-tyrosinase; antioxidant and antibacterial activity | [133] |
In a study conducted by Taofiq et al. [129], terpenoids (ganoderic acids C2, A and H) and phenolics (protocatechuic, p-hydroxybenzoic and syringic acids) were identified as essential cosmeceutical components in the G. lucidum extracts. Further, Taofiq et al. [133] reported that ethanolic extracts of Agaricus bisporus and Pleurotus ostreatus contain essential bioactive compounds, but they readily are degraded and oxidized. Thus, they developed microencapsulated extracts incorporated into a semi-solid base cream. Their efficacy was compared with those of free-forms in terms of bioactivity, in vitro release and real-time conditions (up to 6 months). Antimicrobial and anti-tyrosinase activities were also observed for the formulations prepared with the encapsulated forms, but the extract released over time was found to be insufficient to exert an impact on the antioxidant action. Lourith et al. [132] developed moisturizing hand sanitizer using snow mushroom polysaccharides. The sanitizer showed no signs of irritation when applied to the volunteers and significantly (p < 0.05) moistened the skin compared with the placebo.
11. Conclusions
Edible mushrooms and their by-products are extensively involved in different fields with diverse applications. Due to their nutritional and functional values, mushrooms are taken as dietary supplements with probiotics and are fortified as RTE and RTC food. The bioactive molecules obtained from them are found to have applications in food products. Other than this, mushrooms are found to play an important role in the production of biochar, biosorbent, carbon dots, media, nanoparticles and skincare formulations. All the above-stated products synthesized or developed with the aid of mushrooms have shown effective results in invitro studies. However, edible films/coatings and skincare formulations developed from these edible mushrooms are still limited to the invitro level and are not yet being exploited at the commercial and industrial levels. Additionally, these edible mushrooms and their waste have immeasurable economic potential in different industries, and it can often lead to the synthesis of novel products. Still, these edible mushrooms remain an untapped resource with vast industrial applications. Thus, there is a dire need for sensible and responsible management within the production system to explore the potential of these edible mushrooms.
Acknowledgments
University of Hradec Kralove supported this work (Faculty of Science, VT2019-2021).
Author Contributions
Conceptualization, D.K., N.C.-M. and K.K.; writing—review, H.K., K.B. and R.S. (Ruchi Sharma); editing, D.S.D., R.S. (Reena Singh) and C.C.; critical revising, E.N., R.V., A.T. and K.A.A.-E., D.K., N.C.-M., K.M. and K.K.; funding acquisition, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the UHK (Faculty of Science, VT2019-2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valverde M.E., Hernández-Pérez T., Paredes-López O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015;2015:376387. doi: 10.1155/2015/376387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang S.T., Miles P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact. 2nd ed. CRC Press; Boca Raton, FL, USA: 2008. [Google Scholar]
- 3.Ergönül P.G., Akata I., Kalyoncu F., Ergönül B. Fatty acid compositions of six wild edible mushroom species. Sci. World J. 2013;2013:163964. doi: 10.1155/2013/163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillamón E., García-Lafuente A., Lozano M., D’Arrigo M., Rostagno M.A., Villares A., Martínez J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia. 2010;81:715–723. doi: 10.1016/j.fitote.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Longvah T., Deosthale Y.G. Composition and nutritional studies on edible wild mushroom from Northeast India. Food Chem. 1998;63:331–334. doi: 10.1016/S0308-8146(98)00026-0. [DOI] [Google Scholar]
- 6.Maga J.A. Mushroom flavor. J. Agric. Food Chem. 1981;29:1–4. doi: 10.1021/jf00103a001. [DOI] [Google Scholar]
- 7.Mattila P., Konko K., Euvola M., Pihlava J., Astola J., Vahteristo L. Contents of vitamins, mineral elements and some phenolic compound in cultivated mushrooms. J. Agric. Food Chem. 2001;42:2449–2453. doi: 10.1021/jf00047a016. [DOI] [PubMed] [Google Scholar]
- 8.Clifford A.J., Heid M.K., Peerson J.M., Bills N.D. Bioavailability of food folates and evaluation of food matrix effects with a rat bioassay. J. Nutr. 1991;121:445–453. doi: 10.1093/jn/121.4.445. [DOI] [PubMed] [Google Scholar]
- 9.Bano Z., Rajarathnam S. Pleurotus mushrooms. Part II. Chemical composition, nutritional value, post-harvest physiology, preservation, and role as human food. Crit. Rev. Food Sci. Nutr. 1988;27:87–158. doi: 10.1080/10408398809527480. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiroa B., Pinhoa P.G., Andradea P.B., Baptistab P., Valentao P. Fatty acid composition of wild edible mushrooms species: A comparative study. Microchem. J. 2009;93:29–35. doi: 10.1016/j.microc.2009.04.005. [DOI] [Google Scholar]
- 11.Antunes F., Marçal S., Taofiq O., Morais A.M.M.B., Freitas A.C., Ferreira I.C.F.R., Pintado M. Valorization of mushroom by-products as a source of value-added compounds and potential applications. Molecules. 2020;25:2672. doi: 10.3390/molecules25112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim S.-H., Lee Y.-H., Kang H.-W. Efficient recovery of lignocellulolytic enzymes of spent mushroom compost from oyster mushrooms, Pleurotus spp., and potential use in dye decolorization. Mycobiology. 2013;41:214–220. doi: 10.5941/MYCO.2013.41.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirabella N., Castellani V., Sala S. Current options for the valorization of food manufacturing waste: A review. J. Clean Prod. 2014;65:28–41. doi: 10.1016/j.jclepro.2013.10.051. [DOI] [Google Scholar]
- 14.Soliman A., Abbas M., Ahmed S. Preparation, canning and evaluation process of vegetable mixture diets (ready-to-eat) supplemented with mushroom. Suez Canal Univ. J. Food Sci. 2017;4:19–28. doi: 10.21608/scuj.2017.6654. [DOI] [Google Scholar]
- 15.Salehi F. Characterization of different mushrooms powder and its application in bakery products: A review. Int. J. Food Prop. 2019;22:1375–1385. doi: 10.1080/10942912.2019.1650765. [DOI] [Google Scholar]
- 16.Ng S.H., Robert S.D., Ahmad W.A.N.W., Ishak W.R.W. Incorporation of dietary fiber-rich oyster mushroom (Pleurotus sajor-caju) powder improves postprandial glycaemic response by interfering with starch granule structure and starch digestibility of biscuit. Food Chem. 2017;227:358–368. doi: 10.1016/j.foodchem.2017.01.108. [DOI] [PubMed] [Google Scholar]
- 17.Rathore H., Sehwag S., Prasad S., Sharma S. Technological, nutritional, functional and sensorial attributes of the cookies fortified with Calocybe indica mushroom. J. Food Meas. Charact. 2019;13:976–987. doi: 10.1007/s11694-018-0012-1. [DOI] [Google Scholar]
- 18.Wang L., Zhao H., Brennan M., Guan W., Liu J., Wang M., Brennan C. In vitro gastric digestion antioxidant and cellular radical scavenging activities of wheat-shiitake noodles. Food Chem. 2020;330:127214. doi: 10.1016/j.foodchem.2020.127214. [DOI] [PubMed] [Google Scholar]
- 19.Prodhan U.K., Linkon K.M.M.R., Al-Amin M.F., Alam M.J. Development and quality evaluation of mushroom (Pleurotus sajor-caju) enriched biscuits. Emir. J. Food Agric. 2015;27:542–547. doi: 10.9755/ejfa.2015.04.082. [DOI] [Google Scholar]
- 20.Ren A., Pan S., Li W., Chen G., Duan X. Effect of various pretreatments on quality attributes of vacuum-fried shiitake mushroom chips. J. Food Qual. 2018;2018:4510126. doi: 10.1155/2018/4510126. [DOI] [Google Scholar]
- 21.Farzana T., Mohajan S. Effect of incorporation of soy flour to wheat flour on nutritional and sensory quality of biscuits fortified with mushroom. Food Sci. Nutr. 2015;3:363–369. doi: 10.1002/fsn3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar K., Ray A.B. Development and shelf-life evaluation of tomato-mushroom mixed ketchup. J. Food Sci. Technol. 2016;53:2236–2243. doi: 10.1007/s13197-016-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan M.U., Qazi I.M., Ahmed I., Ullah S., Khan A., Jamal S. Development and quality evaluation of banana mushroom blended jam. Pak. J. Sci. Ind. Res. Ser. B. 2017;60:11–18. [Google Scholar]
- 24.Rachappa P., Sudharma D.C., Chauhan O.P., Patki P.E., Nagaraj R., Naik S., Naik R. Development and evaluation of white button mushroom based snacks. J. Food Process. Technol. 2020;11:824. [Google Scholar]
- 25.Mohajan S., Orchy T.N., Farzana T. Effect of incorporation of soy flour on functional, nutritional, and sensory properties of mushroom–moringa-supplemented healthy soup. Food Sci. Nutr. 2018;6:549–556. doi: 10.1002/fsn3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan M.A., Derbyshire E., Tiwari B.K., Brennan C.S. Enrichment of extruded snack products with coproducts from chestnut mushroom (Agrocybe aegerita) production: Interactions between dietary fiber, physicochemical characteristics, and glycemic load. J. Agric. Food Chem. 2012;60:4396–4401. doi: 10.1021/jf3008635. [DOI] [PubMed] [Google Scholar]
- 27.Bello M., Oluwamukomi M.O., Enujiugha V.N. Nutrient composition and sensory properties of biscuit from mushroom-wheat composite flours. Arch. Curr. Res. Int. 2017;9:1–11. doi: 10.9734/ACRI/2017/35686. [DOI] [Google Scholar]
- 28.Khider M., Seoudi O., Abdelaliem Y.F. Functional processed cheese spreads with high nutritional value as supplemented with fresh and dried mushrooms. Int. J. Nutr Food Sci. 2017;6:45–52. doi: 10.11648/j.ijnfs.20170601.18. [DOI] [Google Scholar]
- 29.Cornelia M., Chandra J. Utilization of white oyster mushroom powder (Pleurotus ostreatus (Jacq.) P. Kumm.) in the making of biscuit as emergency food product. Eurasia J. Biosci. 2019;13:1859–1866. [Google Scholar]
- 30.Shalaby S.M., Mohamed A.G., Farahat E.S. Preparation of functional and nutritional spreadable processed cheese fortified with vegetables and mushrooms. Int. J. Curr Res. 2018;10:74075–74082. [Google Scholar]
- 31.Rosli W.I.W., Solihah M.A. Nutritional composition and sensory properties of oyster mushroom-based patties packed with biodegradable packaging. Sains Malays. 2014;43:65–71. [Google Scholar]
- 32.Chauhan N., Vaidya D., Gupta A., Pandit A. Fortification of pasta with white button mushroom: Functional and rheological properties. Int. J. Food Ferment. Technol. 2017;7:87–96. doi: 10.5958/2277-9396.2017.00008.3. [DOI] [Google Scholar]
- 33.Chaudhari P.D.N., Wandhekar S.S., Shaikh A.A., Devkatte A.N. Preparation and characterization of cookies prepared from wheat flour fortified with mushroom (Pleurotussajor-caju) and spiced with cardamom. Int. J. Res. Anal. Rev. 2018;5:386–389. [Google Scholar]
- 34.Lu X., Brennan M.A., Serventi L., Liu J., Guan W., Brennan C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glycaemic response of pasta. Food Chem. 2018;264:199–209. doi: 10.1016/j.foodchem.2018.04.130. [DOI] [PubMed] [Google Scholar]
- 35.Cha M.H., Heo J.Y., Lee C., Lo Y.M., Moon B. Quality and sensory characterization of white jelly mushroom (Tremella fuciformis) as a meat substitute in pork patty formulation. J. Food Process. Preserv. 2014;38:2014–2019. doi: 10.1111/jfpp.12178. [DOI] [Google Scholar]
- 36.Arora B., Kamal S., Sharma V.P. Nutritional and quality characteristics of instant noodles supplemented with oyster mushroom (P. ostreatus) J. Food Process Preserv. 2018;42:e13521. doi: 10.1111/jfpp.13521. [DOI] [Google Scholar]
- 37.Patinho I., Saldaña E., Selani M.M., de Camargo A.C., Merlo T.C., Menegali B.S., Contreras-Castillo C.J. Use of Agaricus bisporus mushroom in beef burgers: Antioxidant, flavor enhancer and fat replacing potential. Food Prod. Process Nutr. 2019;1:1–15. doi: 10.1186/s43014-019-0006-3. [DOI] [Google Scholar]
- 38.Srivastava A., Attri B., Verma S. Development and evaluation of instant soup premix using oyster mushroom powder. Mushroom Res. 2019;28:65–69. doi: 10.36036/MR.28.1.2019.91960. [DOI] [Google Scholar]
- 39.Salehi F., Kashaninejad M., Asadi F., Najafi A. Improvement of quality attributes of sponge cake using infrared dried button mushroom. J. Food Sci. Technol. 2016;53:1418–1423. doi: 10.1007/s13197-015-2165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolawole F.L., Akinwande B.A., Ade-Omowaye B.I.O. Physicochemical properties of novel cookies produced from orange-fleshed sweet potato cookies enriched with sclerotium of edible mushroom (Pleurotus tuber-regium) J. Saudi Soc. Agric. Sci. 2020;19:174–178. [Google Scholar]
- 41.Parvin R., Farzana T., Mohajan S., Rahman H., Rahman S.S. Quality improvement of noodles with mushroom fortified and its comparison with local branded noodles. NFS J. 2020;20:37–42. doi: 10.1016/j.nfs.2020.07.002. [DOI] [Google Scholar]
- 42.Jeong C.H., Shim K.H. Quality characteristics of sponge cakes with addition of Pleurotus eryngii mushroom powders. J. Korean Soc. Food Sci. Nutr. 2004;33:716–722. [Google Scholar]
- 43.Lu X., Brennan M.A., Serventi L., Mason S., Brennan C.S. How the inclusion of mushroom powder can affect the physicochemical characteristics of pasta. Int. J. Food Sci. Technol. 2016;51:2433–2439. doi: 10.1111/ijfs.13246. [DOI] [Google Scholar]
- 44.Kumar H., Bhardwaj K., Sharma R., Nepovimova E., Kuča K., Dhanjal D.S., Kumar D. Fruit and vegetable peels: Utilization of high value horticultural waste in novel industrial applications. Molecules. 2020;25:2812. doi: 10.3390/molecules25122812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bilbao-Sainz C., Chiou B.-S., Punotai K., Olson D., Williams T., Wood D., Rodov V., Poverenov E., McHugh T. Layer-by-layer alginate and fungal chitosan based edible coatings applied to fruit bars. J. Food Sci. 2018;83:1880–1887. doi: 10.1111/1750-3841.14186. [DOI] [PubMed] [Google Scholar]
- 46.Du H., Hu Q., Yang W., Pei F., Kimatu B.M., Ma N., Zhao L. Development, physiochemical characterization and forming mechanism of Flammulina velutipes polysaccharide-based edible films. Carbohydr. Polym. 2016;152:214–221. doi: 10.1016/j.carbpol.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 47.Poverenov E., Arnon-Rips H., Zaitsev Y., Bar V., Danay O., Horev B., Rodov V. Potential of chitosan from mushroom waste to enhance quality and storability of fresh-cut melons. Food Chem. 2018;268:233–241. doi: 10.1016/j.foodchem.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 48.Zhang K., Wang W., Zhao K., Ma Y., Cheng S., Zhou J., Wu Z. Producing a novel edible film from mushrooms (L. edodes and F. velutipes) by-products with a two-stage treatment namely grinding and bleaching. J. Food Eng. 2020;275:109862. doi: 10.1016/j.jfoodeng.2019.109862. [DOI] [Google Scholar]
- 49.Olufunmilola O.M., Shian A.J., Dooshima I.B. Effects of plasticizer concentration and mushroom (Pleurotus pulmonarius) flour inclusion on the sensory, mechanical and barrier properties of cassava starch based edible films. Eur. J. Food Sci. Technol. 2019;7:47–62. [Google Scholar]
- 50.Asad F., Anwar H., Yassine H.M., Ullah M.I., Kamran Z., Sohail M.U. White button mushroom, Agaricus bisporus (Agaricomycetes), and a probiotics mixture supplementation correct dyslipidemia without influencing the colon microbiome profile in hypercholesterolemic rats. Int. J. Med. Mushrooms. 2020;22:235–244. doi: 10.1615/IntJMedMushrooms.2020033807. [DOI] [PubMed] [Google Scholar]
- 51.Synytsya A., Míčková K., Synytsya A., Jablonský I., Spěváček J., Erban V., Čopíková J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009;76:548–556. doi: 10.1016/j.carbpol.2008.11.021. [DOI] [Google Scholar]
- 52.Van Doan H., Doolgindachbaporn S., Suksri A. Effects of Eryngii mushroom (Pleurotus eryngii) and Lactobacillus plantarum on growth performance, immunity and disease resistance of Pangasius catfish (Pangasius bocourti, Sauvage 1880) Fish Physiol. Biochem. 2016;42:1427–1440. doi: 10.1007/s10695-016-0230-6. [DOI] [PubMed] [Google Scholar]
- 53.Daneshmand A., Sadeghi G.H., Karimi A., Vaziry A. Effect of oyster mushroom (Pleurotus ostreatus) with and without probiotic on growth performance and some blood parameters of male broilers. Anim. Feed Sci. Techol. 2011;170:91–96. doi: 10.1016/j.anifeedsci.2011.08.008. [DOI] [Google Scholar]
- 54.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of probiotic. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 55.Faraki A., Noori N., Gandomi H., Banuree S.A.H., Rahmani F. Effect of Auricularia auricula aqueous extract on survival of Lactobacillus acidophilus La-5 and Bifidobacterium bifidum Bb-12 and on sensorial and functional properties of synbiotic yogurt. Food Sci. Nutr. 2020;8:1254–1263. doi: 10.1002/fsn3.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy D., Fahim A. The effect of different level of mushroom (Agaricus bisporus) and probiotics (Saccharomyces cerevisiae) on sensory evaluation of broiler meat. J. Entomol. Zool. Stud. 2019;7:347–349. [Google Scholar]
- 57.Oyetayo V.O., Oyetayo F.L. Hematological parameters of rats fed mushroom, Pleurotus sajor-caju diets and orogastrically dosed with probiotic Lactobacillus fermentum Ovl. Int. J. Probiotics Prebiotics. 2007;2:39–42. [Google Scholar]
- 58.Van Doan H., Hoseinifar S.H., Dawood M.A., Chitmanat C., Tayyamath K. Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2017;70:87–94. doi: 10.1016/j.fsi.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Willis W.L., Isikhuemhen O.S., Ibrahim S.A. Performance assessment of broiler chickens given mushroom extract alone or in combination with probiotics. Poult Sci. 2007;86:1856–1860. doi: 10.1093/ps/86.9.1856. [DOI] [PubMed] [Google Scholar]
- 60.Soccol C.R., Vandenberghe L.P.S. Overview of applied solid-state fermentation in Bazil. Biochem. Eng. J. 2008;13:205–218. doi: 10.1016/S1369-703X(02)00133-X. [DOI] [Google Scholar]
- 61.Zhang R.H., Li X.J., Fadel J.G. Oyster mushroom cultivation with rice and wheat straw. Bioresource Technol. 2002;82:277–284. doi: 10.1016/S0960-8524(01)00188-2. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez J.E., Royse D.J. Scytalidium thermophilum- colonized grain, corncobs and chopped wheat straw substrates for the production of Agaricus bisporus. Bioresour. Technol. 2009;100:1670–1674. doi: 10.1016/j.biortech.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 63.Semple K.T., Reid B.J., Fermor T.R. Impact of composting strategies on the treatment of soils contaminated with organic pollutants. Environ. Pollut. 2001;112:269–283. doi: 10.1016/S0269-7491(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 64.Fidanza M.A., Sam fond D.L., Beyen D.M., Aurentz D.J. Analysis of fresh mushroom compost. Hort. Technol. 2010;20:449–453. doi: 10.21273/HORTTECH.20.2.449. [DOI] [Google Scholar]
- 65.Rajput R., Prasad G., Chopra A.K. Scenario of solid waste management in present Indian context. Casp. J. Environ. Sci. 2009;7:45–53. [Google Scholar]
- 66.Run-Hua Z., Zeng-Qiang D., Zhi-Guo L. Use of spent mushroom substrate as growing media for tomato and cucumber seedlings. Pedosphere. 2012;22:333–342. [Google Scholar]
- 67.Medina E., Paredes C., Pérez-Murcia M.D., Bustamante M.A., Moral R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009;100:4227–4232. doi: 10.1016/j.biortech.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 68.Tam N.V., Wang C.H. Use of spent mushroom substrate and manure compost for honeydew melon seedlings. J. Plant. Growth Regul. 2015;34:417–424. [Google Scholar]
- 69.Wu S., Lan Y., Huang D., Peng Y., Huang Z., Gelbič I., Carballar-Lejarazu R., Guan X., Zhang L., Zou S. Use of spent mushroom substrate for production of Bacillus thuringiensis by solid-state fermentation. J. Econ. Entomol. 2014;107:137–143. doi: 10.1603/EC13276. [DOI] [PubMed] [Google Scholar]
- 70.Rajavat A.S., Rai S., Pandiyan K., Kushwaha P., Choudhary P., Kumar M., Chakdar H., Singh A., Karthikeyan N., Bagul S.Y., et al. Sustainable use of the spent mushroom substrate of Pleurotus florida for production of lignocellulolytic enzymes. J. Basic Microbiol. 2020;60:173–184. doi: 10.1002/jobm.201900382. [DOI] [PubMed] [Google Scholar]
- 71.Qiao J.J., Zhang Y.F., Sun L.F., Liu W.W., Zhu H.J., Zhang Z. Production of spent mushroom substrate hydrolysates useful for cultivation of Lactococcus lactis by dilute sulfuric acid, cellulase and xylanase treatment. Bioresour. Technol. 2011;102:8046–8051. doi: 10.1016/j.biortech.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 72.Singh G., Tiwari A., Rathore H., Prasad S., Hariprasad P., Sharma S. Valorization of paddy straw using de-oiled cakes for P. ostreatus cultivation and utilization of spent mushroom substrate for biopesticide development. Waste Biomass Valroi. 2021;12:333–346. doi: 10.1007/s12649-020-00957-y. [DOI] [Google Scholar]
- 73.Sendi H., Mohamed M.T.M., Anwar M.P., Saud H.M. Spent mushroom waste as a media replacement for peat moss in kai-lan (Brassica oleracea var. Alboglabra) production. Sci. World J. 2013;2013:258562. doi: 10.1155/2013/258562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu C.J., Duan Y.L., Jin R.Z., Han Y.Y., Hao J.H., Fan S.X. Spent mushroom substrates as component of growing media for lettuce seedlings; Proceedings of the 4th International Conference on Agricultural and Biological Sciences; Hangzhou, China. 26–29 June 2018; p. 012016. [Google Scholar]
- 75.Antimanon S., Chamkhuy W., Sutthiwattanakul S., Laoteng K. Efficient production of arachidonic acid of Mortierella sp. by solid-state fermentation using combinatorial medium with spent mushroom substrate. Chem. Pap. 2018;72:2899–2908. doi: 10.1007/s11696-018-0519-2. [DOI] [Google Scholar]
- 76.Wyciszkiewicz M., Saeid A., Samoraj M., Chojnacka K. Solid-state solubilization of bones by B. megaterium in spent mushroom substrate as a medium for a phosphate enriched substrate. J. Chem. Technol. Biotechnol. 2017;92:1397–1405. doi: 10.1002/jctb.5135. [DOI] [Google Scholar]
- 77.Park N., Yun Y.-S., Park J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess. Eng. 2010;15:86–102. doi: 10.1007/s12257-009-0199-4. [DOI] [Google Scholar]
- 78.Abdi O., Kazemi M. A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater. Environ. Sci. 2015;6:1386–1399. [Google Scholar]
- 79.Menaga D., Rajakumar S., Ayyasamy P.M. Spent mushroom substrate: A crucial biosorbent for the removal of ferrous iron from groundwater. SN Appl. Sci. 2021;3:32. doi: 10.1007/s42452-020-04119-6. [DOI] [Google Scholar]
- 80.Akar S.T., Gorgulu A., Kaynak Z., Anilan B., Akar T. Biosorption of reactive blue 49 dye under batch and continuous mode using a mixed biosorbent of macro-fungus Agaricus bisporus and Thuja orientalis cones. Chem. Eng. J. 2009;148:26–34. doi: 10.1016/j.cej.2008.07.027. [DOI] [Google Scholar]
- 81.Eliescu A., Georgescu A.A., Nicolescu C.M., Bumbac M., Cioateră N., Mureșeanu M., Buruleanu L.C. Biosorption of Pb(II) from aqueous solution using mushroom (Pleurotus ostreatus) biomass and spent mushroom substrate. Anal. Lett. 2020;53:2292–2319. doi: 10.1080/00032719.2020.1740722. [DOI] [Google Scholar]
- 82.Tay C.C., Liew H.H., Yin C.Y., Abdul-Talib S., Surif S., Suhaimi A.B., Yong S.K. Biosorption of cadmium ions using Pleurotus ostreatus: Growth kinetics, isotherm study and biosorption mechanism. Korean J. Chem. Eng. 2011;28:825–830. doi: 10.1007/s11814-010-0435-9. [DOI] [Google Scholar]
- 83.Yildirim A., Acay H. Biosorption studies of mushrooms for two typical dyes. JOTCSA. 2020;7:295–306. doi: 10.18596/jotcsa.581007. [DOI] [Google Scholar]
- 84.Qu J., Zang T., Gu H., Li K., Hu Y., Ren G., Xu X., Jin Y. Biosorption of copper ions from aqueous solution by Flammulina velutipes spent substrate. BioResources. 2015;10:8058–8075. doi: 10.15376/biores.10.4.8058-8075. [DOI] [Google Scholar]
- 85.Yang K., Li Y., Zheng H., Luan X., Li H., Wang Y., Du Q., Sui K., Li H., Xia Y. Adsorption of Congo red with hydrothermal treated shiitake mushroom. Mater. Res. Express. 2020;7:015103. doi: 10.1088/2053-1591/ab5ff3. [DOI] [Google Scholar]
- 86.Zhao S., Liu J., Tu H., Li F., Li X., Yang J., Liao J., Yang Y., Liu N., Sun Q. Characteristics of uranium biosorption from aqueous solutions on fungus Pleurotus ostreatus. Environ. Sci. Pollut. Res. 2016;23:24846–24856. doi: 10.1007/s11356-016-7722-x. [DOI] [PubMed] [Google Scholar]
- 87.Mahmood T., Khan A., Naeem A., Hamayun M., Muska M., Farooq M., Hussain F. Adsorption of Ni(II) ions from aqueous solution onto a fungus Pleurotus ostreatus. Desalin. Water Treat. 2016;57:7209–7218. doi: 10.1080/19443994.2015.1022802. [DOI] [Google Scholar]
- 88.Amin F., Talpur F.N., Balouch A., Afridi H.I., Khaskheli A.A. Efficient entrapping of toxic Pb(II) ions from aqueous system on a fixed-bed column of fungal biosorbent. Geol. Ecol. Landsc. 2018;2:39–44. doi: 10.1080/24749508.2018.1438746. [DOI] [Google Scholar]
- 89.Wu J., Zhang T., Chen C., Feng L., Su X., Zhou L., Chen Y., Xia A., Wang X. Spent substrate of Ganodorma lucidumas a new bio-adsorbent for adsorption of three typical dyes. Bioresour. Technol. 2018;266:134–138. doi: 10.1016/j.biortech.2018.06.078. [DOI] [PubMed] [Google Scholar]
- 90.Amin F., Talpur F.N., Balouch A., Afridi H.I., Surhio M.L. Statistical methodology for biosorption of nitrate (NO3−) ions from aqueous solution by Pleurotus eryngii fungal biomass. Model. Earth Syst. Environ. 2017;3:1101–1112. doi: 10.1007/s40808-017-0358-0. [DOI] [Google Scholar]
- 91.Lin Y., Munroe P., Joseph S., Henderson R., Ziolkowski A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere. 2012;87:151–157. doi: 10.1016/j.chemosphere.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 92.Cheng C.H., Lehmann J., Engelhard M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface change along a climosequence. Geochim. Cosmochim. Acta. 2008;72:1598–1610. doi: 10.1016/j.gca.2008.01.010. [DOI] [Google Scholar]
- 93.Joseph S.D., Camps-Arbestain M., Lin Y., Munroe P., Chia C.H., Hook J., Van Zwieten L., Kimber S., Cowie A., Singh B.P. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010;48:501–515. doi: 10.1071/SR10009. [DOI] [Google Scholar]
- 94.Bruun E.W., Hauggaard-Nielsen H., Ibrahim N., Egsgaard H., Ambus P., Jensen P.A., Dam-Johansen K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenerg. 2011;35:1182–1189. doi: 10.1016/j.biombioe.2010.12.008. [DOI] [Google Scholar]
- 95.Sohi S.P., Krull E., Lopez-Capel E., Bol R. A review of biochar and its use and function in soil. Adv. Agron. 2010;105:47–82. [Google Scholar]
- 96.Zhang H., Voroney R., Price G. Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol. Biochem. 2015;83:19–28. doi: 10.1016/j.soilbio.2015.01.006. [DOI] [Google Scholar]
- 97.Schmidt H.P., Pandit B.H., Martinsen V., Cornelissen G., Conte P., Kammann C.I. Fourfold increase in pumpkin yield in response to low-dosage root zone application of urine-enhanced biochar to a fertile tropical soil. Agriculture. 2015;5:723–741. doi: 10.3390/agriculture5030723. [DOI] [Google Scholar]
- 98.Kammann C.I., Schmidt H.P., Messerschmidt N., Linsel S., Steffens D., Müller C., Koyro H.W., Conte P., Joseph S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015;5:11080. doi: 10.1038/srep11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu Q., Xian Y., He Z., Zhang Q., Wu J., Ynag G., Zhang X., Qi H., Ma J., Xiao Y., et al. Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate. Sci Rep. 2019;9:15999. doi: 10.1038/s41598-019-52554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang J., Zhang H., Cheng H., Yan Y., Chang M., Cao Y., Huang F., Zhang G., Yan M. Spent Ganoderma lucidum substrate derived biochar as a new bio-adsorbent for Pb2+/Cd2+ removal in water. Chemosphere. 2020;241:125121. doi: 10.1016/j.chemosphere.2019.125121. [DOI] [PubMed] [Google Scholar]
- 101.Zhang G., Liu N., Luo Y., Zhang H., Su L., Oh K., Cheng H. Efficient removal of Cu(II), Zn(II), and Cd(II) from aqueous solutions by a mineral-rich biochar derived from a spent mushroom (Agaricus bisporus) substrate. Materials. 2020;14:35. doi: 10.3390/ma14010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang X., Li X., Liu G., He Y., Chen C., Liu X., Li G., Gu Y., Zhao Y. Mixed heavy metals removal from wastewater by discarded mushroom-stick biochar: Adsorption properties and mechanisms. Environ. Sci. Process. Impacts. 2019;21:584–592. doi: 10.1039/C8EM00457A. [DOI] [PubMed] [Google Scholar]
- 103.Sewu D.D., Jung H., Kim S.S., Lee D.S., Woo S.H. Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate. Bioresour. Technol. 2019;277:77–86. doi: 10.1016/j.biortech.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 104.Chen G.J., Peng C.Y., Fang J.Y., Dong Y.Y., Zhu X.H., Cai H.M. Biosorption of fluoride from drinking water using spent mushroom compost biochar coated with aluminum hydroxide. Desalin Water Treat. 2016;57:12385–12395. doi: 10.1080/19443994.2015.1049959. [DOI] [Google Scholar]
- 105.Bhardwaj K., Sharma A., Tejwan N., Bhardwaj S., Bhardwaj P., Nepovimova E., Shami A., Kalia A., Kumar A., Abd-Elsalam K.A., et al. Pleurotus macro fungi-assisted nanoparticle synthesis and its potential applications: A review. J. Fungi. 2020;6:351. doi: 10.3390/jof6040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Owaid M.N., Ibraheem I.J. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017;9:5–23. doi: 10.1515/ejnm-2016-0016. [DOI] [Google Scholar]
- 107.Sriramulu M., Shanmugam S., Ponnusamy V.K. Agaricus bisporus mediated biosynthesis of copper nanoparticles and its biological effects: An in-vitro study. Colloid Interface Sci. Commun. 2020;35:100254. doi: 10.1016/j.colcom.2020.100254. [DOI] [Google Scholar]
- 108.Madhanraj R., Eyini M., Balaji P. Antioxidant assay of gold and silver nanoparticles from edible Basidiomycetes mushroom fungi. Free Radic. Antioxid. 2017;7:137–142. doi: 10.5530/fra.2017.2.20. [DOI] [Google Scholar]
- 109.Bhat R., Sharanabasava V.G., Deshpande R., Shetti U., Sanjeev G., Venkataraman A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus florida and its anti-cancer evaluation. J. Photochem. B Biol. 2013;125:63–69. doi: 10.1016/j.jphotobiol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 110.Chaturvedi V.K., Yadav N., Rai N.K., Abd Ellah N.H., Bohara R.A., Rehan I.F., Marraiki N., Batiha G.E.S., Hetta H.F., Singh M.P. Pleurotus sajor-caju-mediated synthesis of silver and gold nanoparticles active against colon cancer cell lines: A new era of Herbonanoceutics. Molecules. 2020;25:3091. doi: 10.3390/molecules25133091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeng D., Zhao J., Luk K.H., Cheung S.T., Wong K.H., Chen T. Potentiation of in vivo anti-cancer efficacy of selenium nanoparticles by mushroom polysaccharides surface decoration. J. Agric. Food Chem. 2019;67:2865–2876. doi: 10.1021/acs.jafc.9b00193. [DOI] [PubMed] [Google Scholar]
- 112.Ismail A.F.M., Ahmed M.M., Salem A.A.M. Biosynthesis of silver nanoparticles using mushroom extracts: Induction of apoptosis in HepG2 and MCF-7 cells via caspases stimulation and regulation of BAX and Bcl-2 gene expressions. J. Pharm. Biomed. Sci. 2015;5:1–9. [Google Scholar]
- 113.Aygün A., Özdemir S., Gülcan M., Cellat K. Synthesis and Characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Biomed. Anal. 2020;178:112970. doi: 10.1016/j.jpba.2019.112970. [DOI] [PubMed] [Google Scholar]
- 114.Anthony K.J.P., Murugan M., Jeyaraj M., Rathinam N.K., Sangiliyandi G. Synthesis of silver nanoparticles using pine mushroom extract: A potential antimicrobial agent against E. coli and B. subtilis. J. Ind. Eng. Chem. 2014;20:2325–2331. doi: 10.1016/j.jiec.2013.10.008. [DOI] [Google Scholar]
- 115.Mirunalini S., Arulmozhi V., Deepalakshmi K., Krishnaveni M. Intracellular biosynthesis and antibacterial activity of silver nanoparticles using edible mushrooms. Not. Sci. Biol. 2012;4:55–61. doi: 10.15835/nsb448051. [DOI] [Google Scholar]
- 116.Manimaran K., Murugesan S., Ragavendran C., Balasubramani G., Natarajan D., Ganesan A., Seedevi P. Biosynthesis of TiO2 nanoparticles using edible mushroom (Pleurotus djamor) extract: Mosquito larvicidal, histopathological, antibacterial and anti-cancer effect. J. Clust Sci. 2020:1–12. doi: 10.1007/s10876-020-01888-3. [DOI] [Google Scholar]
- 117.Manimaran K., Balasubramani G., Ragavendran C., Natarajan D., Murugesan S. Biological applications of synthesized ZnO nanoparticles using Pleurotus djamor against mosquito larvicidal, histopathology, antibacterial, antioxidant and anti-cancer effect. J. Clust. Sci. 2020:1–13. doi: 10.1007/s10876-020-01927-z. [DOI] [Google Scholar]
- 118.Gurunathan S., Raman J., Malek S.N.A., John P.A., Vikineswary S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: A potential cytotoxic agent against breast cancer cells. Int J. Nanomed. 2013;8:4399–4413. doi: 10.2147/IJN.S51881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boobalan T., Sethupathi M., Sengottuvelan N., Kumar P., Balaji P., Gulyás B.Z., Padmanabhan P., Selvan S.T., Arun A. Mushroom-derived carbon dots for toxic metal ion detection and as antibacterial and anti-cancer agents. ACS Appl. Nano Mater. 2020;3:5910–5919. doi: 10.1021/acsanm.0c01058. [DOI] [Google Scholar]
- 120.Pacquiao M.R., de Luna M.D.G., Thongsai N., Kladsomboon S., Paoprasert P. Highly fluorescent carbon dots from enokitake mushroom as multi-faceted optical nanomaterials for Cr6+ and VOC detection and imaging applications. Appl. Surf. Sci. 2018;453:192–203. doi: 10.1016/j.apsusc.2018.04.199. [DOI] [Google Scholar]
- 121.Zulfajri M., Rasool A., Huang G.G. A fluorescent sensor from oyster mushroom-carbon dots for sensing nitroarenes in aqueous solutions. New J. Chem. 2020;44:10525–10535. doi: 10.1039/D0NJ02134B. [DOI] [Google Scholar]
- 122.Zulfajri M., Liu K.C., Pu Y.H., Rasool A., Dayalan S., Huang G.G. Utilization of carbon dots derived from Volvariella volvacea mushroom for a highly sensitive detection of Fe3+ and Pb2+ ions in aqueous solutions. Chemosensors. 2020;8:47. doi: 10.3390/chemosensors8030047. [DOI] [Google Scholar]
- 123.Yang Y., Liu M., Wang Y., Wang S., Miao H., Yang L. Carbon dots derived from fungus for sensing hyaluronic acid and hyaluronidase. Sens. Actuators B Chem. 2017;251:503–508. doi: 10.1016/j.snb.2017.05.086. [DOI] [Google Scholar]
- 124.Millikan L.E. Cosmetology, cosmetics, cosmeceuticals: Definitions and regulations. Clin. Dermatol. 2001;19:371–374. doi: 10.1016/S0738-081X(01)00195-X. [DOI] [PubMed] [Google Scholar]
- 125.Antignac E., Nohynek G.J., Re T., Clouzeau J., Toutain H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem. Toxicol. 2011;49:324–341. doi: 10.1016/j.fct.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 126.Hyde K.D., Bahkali A.H., Moslem M.A. Fungi-an unusual source for cosmetics. Fungal Divers. 2010;43:1–9. doi: 10.1007/s13225-010-0043-3. [DOI] [Google Scholar]
- 127.Camassola M. Mushrooms-the incredible factory for enzymes and metabolites productions. Ferment. Technol. 2013:2. doi: 10.4172/2167-7972.1000e117. [DOI] [Google Scholar]
- 128.Taofiq O., Heleno S.A., Calhelha R.C., Alves M.J., Barros L., Barreiro M.F., González-Paramás A.M., Ferreira I.C.F.R. Development of mushroom-based cosmeceutical formulations with anti-inflammatory, anti-tyrosinase, antioxidant, and antibacterial properties. Molecules. 2016;21:1372. doi: 10.3390/molecules21101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taofiq O., Heleno S.A., Calhelha R.C., Alves M.J., Barros L., González-Paramás A.M., Ferreira I.C.F.R. The potential of Ganoderma lucidum extracts as bioactive ingredients in topical formulations, beyond its nutritional benefits. Food Chem. Toxicol. 2017;108:139–147. doi: 10.1016/j.fct.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 130.Gupta N., Dubey A., Prasad P., Roy M. Formulation and evaluation of herbal fairness cream comprising hydroalcoholic extracts of Pleurotus ostreatus, Glycyrrhiza glabra and Camellia sinensis. UK J. Pharm. Biosci. 2015;3:41. doi: 10.20510/ukjpb/3/i3/89410. [DOI] [Google Scholar]
- 131.Hapsari R., Elya B., Amin J. Formulation and evaluation of antioxidant and tyrosinase inhibitory effect from gel containing the 70% ethanolic Pleurotus ostreatus extract. Int. J. Med. Arom. Plants. 2012;2:135–140. [Google Scholar]
- 132.Lourith N., Pungprom S., Kanlayavattanakul M. Formulation and efficacy evaluation of the safe and efficient moisturizing snow mushroom hand sanitizer. J. Cosmet. Dermatol. 2021;20:554–560. doi: 10.1111/jocd.13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taofiq O., Heleno S.A., Calhelha R.C., Fernandes I.P., Alves M.J., Barros L., González-Paramás A.M., Ferreira I.C.F.R. Mushroom-based cosmeceutical ingredients: Microencapsulation and in vitro release profile. Ind. Crops Prod. 2018;124:44–52. doi: 10.1016/j.indcrop.2018.07.057. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.