Abstract
Gray scale ultrasound has an important diagnostic role in native kidney disease. Low cost, absence of ionizing radiation and nephrotoxicity, short performance time, and repeatability even at the bedside, are the major advantages of this technique. The introduction of contrast enhancement ultrasound (CEUS) in daily clinical practice has significantly reduced the use of contrast enhancement computed tomography (CECT) and contrast enhancement magnetic resonance (CEMR), especially in patients with renal disease. Although there are many situations in which CECT and CEMRI are primarily indicated, their use may be limited by the administration of the contrast medium, which may involve a risk of renal function impairment, especially in the elderly, and in patients with acute kidney injury (AKI) and moderate to severe chronic kidney disease (CKD). In these cases, CEUS can be a valid diagnostic choice. To date, numerous publications have highlighted the role of CEUS in the study of parenchymal micro-vascularization and renal pathology by full integration with second level imaging methods (CECT and CEMRI) both in patients with normal renal function and with diseased kidneys. The aim of this review is to offer an updated overview of the limitations and potential applications of CEUS in native kidney disease.
Keywords: contrast-enhanced ultrasound, chronic kidney disease, ultrasonography, CEUS, renal disease, nephropathies, microbubbles
1. Introduction
The first guidelines on contrast-enhanced ultrasound (CEUS) date back to 2004 [1] and focused on hepatic disease, whereas non-hepatic applications were taken into account in 2008 [2]. The dramatic increase in non-hepatic applications for CEUS made it necessary to release dedicated guidelines in 2011 [3,4]. Ultrasound modes have been used as the first imaging method both for the native and transplanted kidneys, in patients with normal and with impaired renal function [5,6,7]. However, it is known that the use of B-mode and Doppler ultrasound alone represents a limitation in the study of kidney diseases, while the addition of contrast medium allows the display of vascular abnormalities unappreciated with conventional modes. CEUS of the kidney is off-label in Europe, but widely used for its effectiveness and safety, and recommended by European guidelines [3,4]. The purpose of this review is to offer an updated overview of potential applications and limitations of CEUS in native kidney disease.
2. Repeatability of CEUS
The question of the ability to perform CEUS in clinical practice is one of the most important obstacles in the application of this technique, even if the investigation is not technically difficult.
CEUS should be understood as an examination that is performed to complement the US examination in order to obtain additional data not obtainable with US methods or other imaging methods. For this reason, the execution of a CEUS requires adequate operator training. Using an expert operator, CEUS is an effective and repeatable imaging technique. Authors reported a very good inter-reader agreement in the characterization of renal cysts [8] and in the characterization of the renal infarct [9].
3. Tolerance and Safety
Echo-contrast media are normally well tolerated. To evaluate the safety of CEUS, we should consider not only the possible side effects related to the drug administration but also those caused by the interaction between microbubbles and ultrasounds. These are not excreted through the kidneys and can be safely administered to patients with renal insufficiency with no risk of contrast-related nephropathy or nephrogenic systemic fibrosis. Ultrasound contrast agents (UCAs) are the safest of the medical contrast agents. All the second-generation contrast agents, sulfur hexafluoride and perflubutane, are microbubbles composed of a low-solubility gas enveloped by a phospholipid shell.
As reported in the EFSUMB guidelines [3], UCAs have a low rate of anaphylactoid reactions compared to iodinated and gadoline contrast agents (0.014% vs. 0.035–0.095% vs. 0.001–0.01%). On the other hand, mild adverse reactions are more frequent (headache, nausea, chest pain and chest discomfort) and resolve spontaneously in a short time without consequence.
Usually, transitory pain at the injection point, altered taste or hypotension for vasovagal response are observed in less than 5% of subjects while rare major adverse reactions (ventricular arrhythmia) are related to the use of a high mechanical index and the consequent massive rupture of the bubbles in the tele-systolic phase. For this reason, the use of CEUS in cardiology requires particular attention [10]. On the other hand, the overall mortality rate for sulphur hexafluoride is 0.0006% in 14/2447083 exposed patients, confirming its safety against iodinated contrast agents (about 0.001%) and in these cases unfavourable underlying medical circumstances were found [3]. It is therefore advisable to pay attention to frail patients with unstable cardiovascular diseases in the days preceding CEUS.
The current guidelines advise to use a mechanical index lower than 0.2 kPa to minimize the rupture of the bubbles. Finally, current understanding cannot exclude subclinical damage and bio-effects at a distance from the use of the UCAs. Therefore, the use of these techniques should always be based on a correct evaluation of the clinical risk/benefit.
4. Limitations
In the study of renal pathology, the limits of CEUS are substantially superimposable to those found in ultrasound. In patients with anatomical kidney malformations, CEUS cannot always provide the desired information. Similar to MRI and CT for the detection of kidney tumors, an important limitation of CEUS is the difficulty of identification of both small lesions (<1 cm), especially if developing intra-parenchymally, and isoechoic with the surrounding parenchymal. CEUS is unable to provide a simultaneous view of both kidneys, as is the case in CT and MRI imaging.
5. Examination Technique and Normal Renal Parenchyma
To perform a CEUS examination, it is necessary to have an ultrasound system equipped with microbubble-specific technology, capable of separating the signal coming from microbubbles (non-linear) from that of the stationary tissues (linear) [11]. To minimize artifacts and tissue signal, the lowest possible mechanical index is used, which depends on the type of equipment available. The contrast dosage ranges from 1.4 to 2.4 mL or less, depending on the ultrasound scanner, the patient’s habitus, and the depth of the lesion to be examined. All the CEUS images reported in this paper were obtained using an intravenous injection of 1.4–2.4 mL of microbubbles containing sulfur hexafluoride. The kidney is a remarkably vascularized organ, receiving approximately 21% of cardiac output. Segmental arteries branch from the main renal artery, and give rise to the lobar arteries, which in turn branch into the arciform and interlobar arteries. Then the contrast reaches the medullary circulation until reaching the pyramids (Figure 1) [3,4].
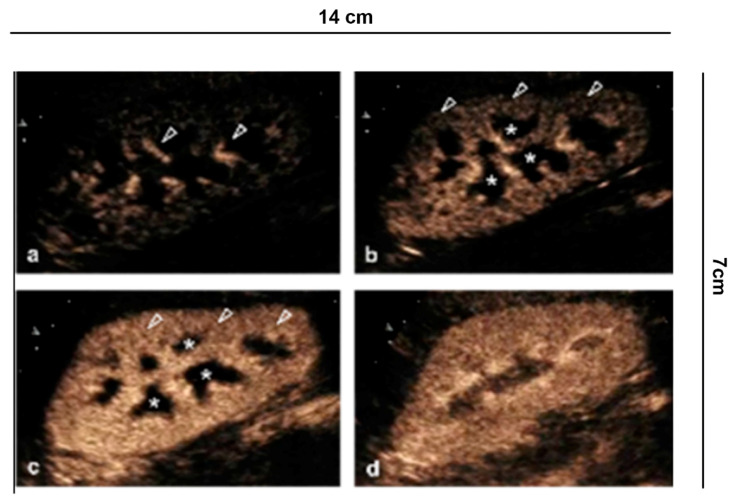
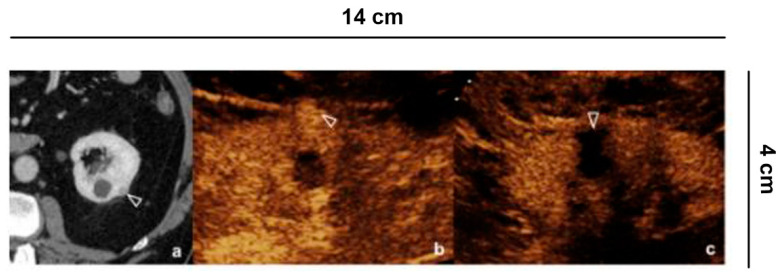
Figure 1.
Renal vascularity with CEUS. (a) A few seconds after injection of the contrast medium, the microbubbles arrive in the arterial branches of the pedicle and in the branches of the interlobar artery (arrowhead). (b) After 15 s the cortical phase begins (arrowhead) without involving the medulla (asterisk). (c) After the completion of the cortical phase (arrowhead) comes the onset of the parenchymal phase (25 s–4 min) with progressive appearance of the enhancement of the external medulla (asterisk) and (d) subsequent involvement of the pyramids.
The vascular structures are rapidly represented, appreciating a fast enhancement of the renal parenchyma from the cortical surface to the portion of the cortico-medullary junction, best visible in the slow motion video. This is different in chronic renal insufficiency, where the enhancement is less intense and fades earlier.
6. Clinical Indications in the Native Kidney
There are numerous scenarios for which CEUS is indicated [1,3,4]. Microbubble injection often complements conventional ultrasound examination, but can also be considered as a second look examination in equivocal cases investigated with other imaging techniques (CECT and CEMRI).
6.1. Characterization of Cystic Lesions
The Bosniak classification has been the most widely adopted method to characterize cystic kidney lesions with good reliability regarding their nature [12]. The classification was first introduced for CECT, which is still considered the gold standard, with probability percentages of malignancy and benignity close to surgical ones [13,14]. The introduction of modern CT technologies did not significantly affect the performance of CECT for this task [15]. The Bosniak classification has been successfully applied also with CEUS, with a similar or better performance, compared to CECT [8,16]. Regardless of the technique used, the Bosniak classification system is intrinsically subjective, a problem well acknowledged since its introduction, which led to subsequent amendments of the scoring system [17]. A considerable effort to standardize the Bosniak scoring system and to improve interobserver agreement has been recently addressed by Silverman et al. for CECT and CEMRI, and by Cantisani et al. for CEUS [18,19]. Numerous aspects should be taken into account when complex renal cysts are scored in the US [18]. A multi-parametric approach is required. Scoring is not based on the size or shape of the cyst but only on the intra-cystic content, i.e., the presence or absence of septa, the number and the thickness of the septa, the wall thickness (more or less than 3 mm), and the presence of irregularities and of nodules.
As complexity increases, the probability of being a malignant cyst grows. In the new EFSUMB classification, cystic tumors are included in category IV, or rather, probable malignant cystic tumors, according to histology. Cystic tumors comprise a diverse group of kidney lesions and can have variable biological profiles. Clearly, the US only identifies the presence of liquid and solid components, and CEUS identifies possible vascularization, referring the definition to histology (Figure 2).
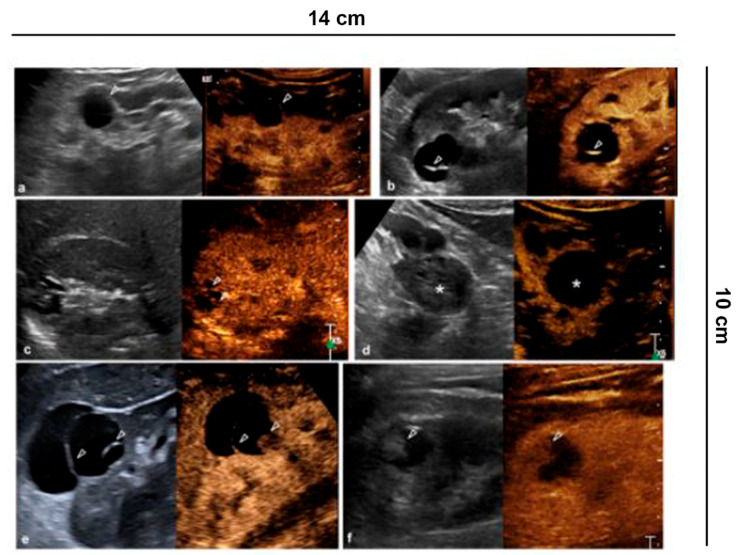
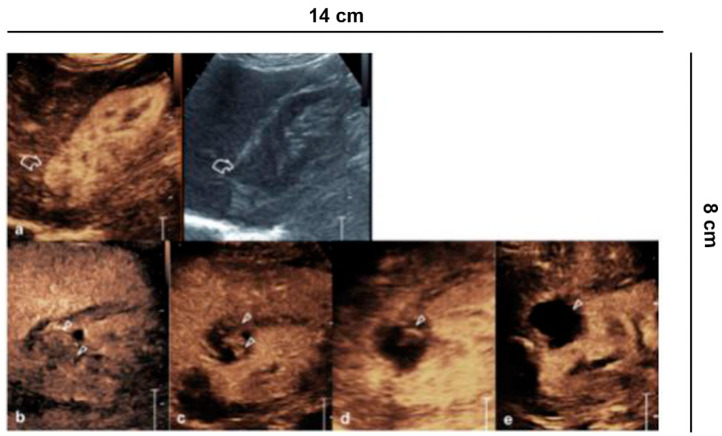
Figure 2.
Characterization of cystic lesions. (a) Bosniak category I cyst (Simple benign cysts): B-mode US and CEUS show anechoic lesion without septa, calcification and wall irregularity; (b) Bosniak category II cyst (Minimally complex benign cysts): minimally complicated cyst with single calcific septum (arrowhead) on B-mode without recognizing enhancement on CEUS; (c) Bosniak category IIF cyst (Presumably benign, imaging surveillance is advised): on US B-mode, cyst with multiple thin septa, minimally thickened (2–3 mm), which on CEUS present minimal enhancement (arrowheads), without irregularities of both the wall and the septa; (d) Bosniak category IIF cyst (Presumably benign, imaging surveillance is advised): echogenic lesion with heterogeneous content inside (asterisk) on B-mode US, which does not show wall enhancement or septa on CEUS; (e) Bosniak category III cyst (Indeterminate lesions): on B-mode, cyst with two septa about 3 mm thick which have thickened, and irregular septa (arrowhead) on CEUS; (f) Bosniak category IV cyst (Likely malignant cystic tumors): cystic lesion with a wall nodule greater than 4 mm (arrowhead).
6.2. Characterization of Indeterminate Lesions
CEUS is recommended by the EFSUMB guidelines and by the American College of Radiology (ACR) to characterize incidental renal masses [3,4] Lesions with equivocal enhancement at CECT will be characterized as solid tumors or renal cysts with CEUS. Cysts are graded according to the Bosniak criteria [20] (Figure 3).
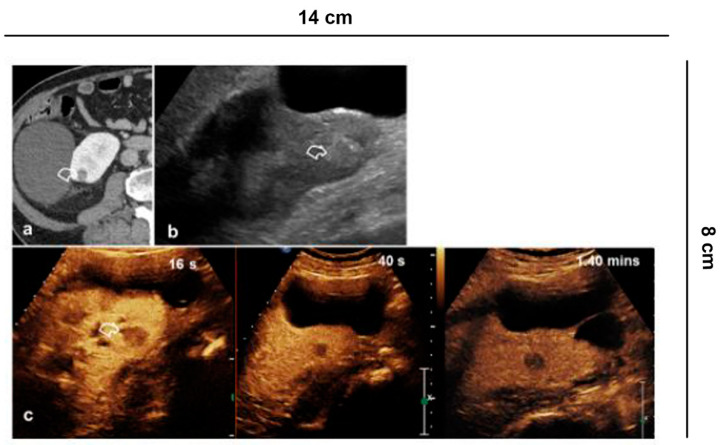
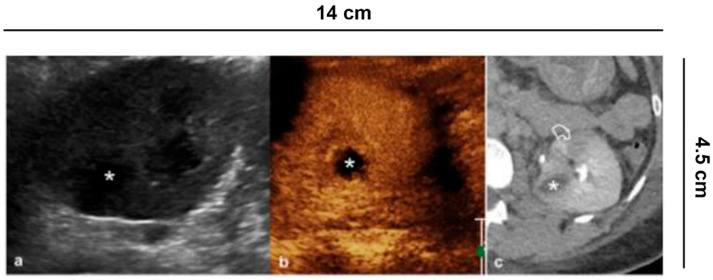
Figure 3.
Indeterminate lesion. (a) 56-year-old man in follow-up for esophageal neoplasia. CECT acquired only in the portal phase. At the lower pole of the right kidney, there is a sharp-edged formation with equivocal enhancement (curved arrow); (b) B-mode US shows a hyperechoic solid lesion (curved arrow) which appears (c) hypovascularized in all vascular phases compared to the parenchyma on CEUS (curved arrow).
6.3. Characterization of Solid Renal Lesions
With the exception of angio-myolipomas with a visible amount of fat, differentiation of the different solid renal masses is problematic even at CECT and CEMRI. CEUS is limited for this task [21] and its use is not recommended by the EFSUMB guidelines for this purpose [3,4]. Several features, however, can help guess tumor histology at multiparametric US. Clear Cell Renal Cell Carcinoma (ccRCC) is usually hyper-vascular, with heterogeneous enhancement due to necrotic areas. However, differentiation from oncocytoma and lipid-poor angio-myolipomas remains problematic. Papillary Renal Cell Carcinoma (pRCC) is often hypo-vascular [22,23].
6.4. Differential Diagnosis between Solid Renal Masses and Pseudotumors
Anatomical variations can simulate a renal mass [24]. Among pseudo-tumors in particular, hypertrophy of the Bertin columns, persistence of fetal lobatures, and presence of humps can be confused with focal lesions requiring a CECT or CEMR study for characterization. CEUS is a valid alternative for a correct differential diagnosis [25,26,27]. In fact, pseudo-tumors will present the same enhancement characteristics as the renal parenchyma, without alterations of the vascular structures (Figure 4). In lesions iso-enhancing in all vascular phases, a situation that occurs in up to 5% of cases, differentiation from pseudo-masses is also possible combining grey-scale US and CEUS, since true lesions often present with different echogenicity, and pseudo-lesions usually display a medullary component which is later enhanced compared to cortex [26].
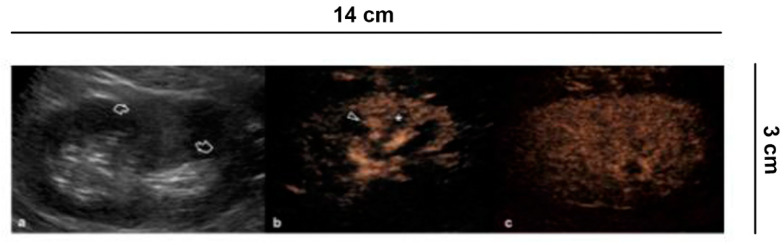
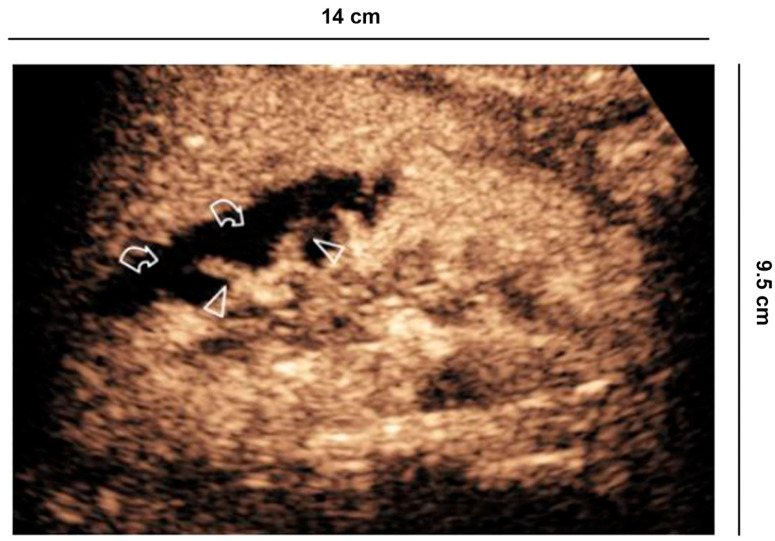
Figure 4.
Differential diagnosis between solid renal masses and pseudo-tumors. (a) B-mode US shows a hyperechoic delimited area in the middle third of the left kidney as a possible solid lesion (curved arrows). (b) CEUS demonstrates, on the other hand, a normal cortical vascularization (arrowhead) with the intact medulla (asterisk) and with (c) the parenchymal phase preserved.
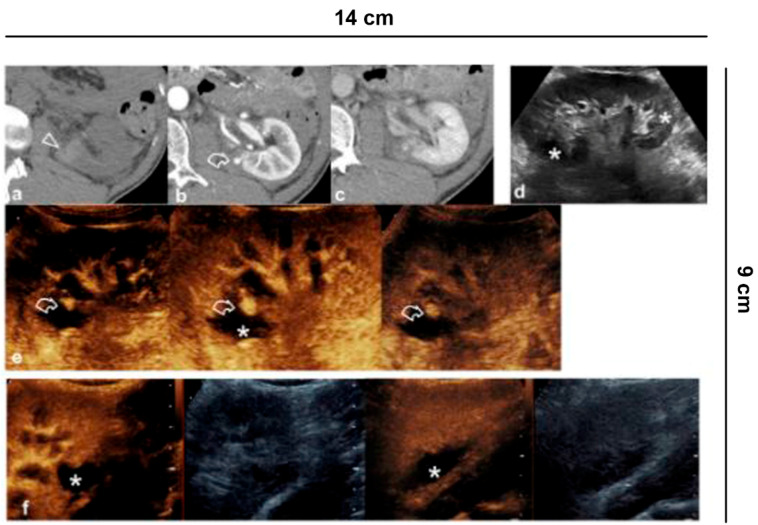
6.5. Follow-Up of Tumor Ablation
In accordance with the EAU guidelines [28], evaluation of ablative treatments of small kidney lesions requires a close monitoring of the ablated area, both to check for any complications and to assess the success of the procedure. CEUS has progressively taken a role in the follow-up, especially early after the procedure [29,30,31]. The treatment is considered successful if the lesion becomes completely avascular. This usually occurs early after radiofrequency and microwave ablation (Figure 5) [29,32], while it may take up to one month after cryoablation (Figure 6), requiring a tailored follow-up [30,31]. Therefore, an adequate knowledge of the type of ablative treatment allows a correct interpretation of the images, thus reducing the risk of misdiagnosis.
Figure 5.
Follow up of microwave tumor ablation. (a) CECT shows a small renal lesion, partially exophytic, adjacent to a simple cyst, in the middle third of the left kidney; (b) Before the microwave treatment CEUS evidences a hyper-vascular, partially exophytic lesion (arrowheads); (c) After 12 h from the microwave ablation, CEUS shows an avascular area without recognizing the lesion (arrowheads), a sign of treatment success.
Figure 6.
Ablative technique follow-up. (a) Before the cryo-ablative treatment, CEUS shows an iso-vascular, partially exophytic lesion at the upper pole of the right kidney (curved arrow). After treatment, CEUS shows diffuse and persistent intralesional vascularization (arrowheads) to 24 h (b); at 7 (c) and 14 days (d) there is a progressive reduction of vascularity; after one month (e) the lesion appears completely avascular, a sign of a successful treatment.
6.6. Complicated Pyelonephritis
Pyelonephritis is a clinical diagnosis. Ultrasound is required to rule out obstructive causes. Other imaging such as CEUS or CECT are indicated in complicated pyelonephritis, or if clinical improvement is lacking at 72 h after medical treatment with antibiotics [33] (Figure 7). Ultrasound is not specific, and can be completely negative. Conversely, CEUS can visualize hypo-perfused areas of parenchyma up to true avascular, rounded areas, with possible peripheral rim, consistent with abscesses. Inflammatory areas are more visible in the parenchymal late phase [34]. In complicated pyelonephritis, CEUS can be used as a monitoring tool for therapeutic response.
Figure 7.
Complicated pyelonephritis. 53 year-old-woman with left flank pain and fever. (a) B-mode US appreciates inhomogeneous, hypoechoic area (asterisk); (b) CEUS performed in the same session confirms the presence of an avascular lesion with a peripheral rim, consistent with abscess (asterisk); (c) Urographic phase CECT, performed for the clinical worsening of the patient, confirms the presence of abscess (asterisk) and hypo-perfused area (curved arrow) at the lower pole of the kidney.
6.7. Vascular Lesions
Renal vascular disease can involve vascular structures at all levels; in the clinical suspicion of renal infarction B-mode and Color Doppler US (CDUS) is always required, although they have limitations. In fact, this only provides in real time the average speeds coded with the color on the area of interest. The vascular representation of the more distal arterial branches is not always adequate, especially in elderly and in nephropathic patients.
Renal infarction (often in triangular form) or only cortical ischemia (absence of cortical interlobular vessels) (Figure 8) [9,35] are recognizable on the other hand with CEUS, which is able to enhance the lack of vascularization, and therefore repair the damage. The sensitivity of CEUS is quite similar to that of angiography and CT [9].
Figure 8.
Vascular lesions. 56 years old man with atrial fibrillation shows creatinine elevation and right lower back pain. CEUS demonstrates focal acute cortical ischemia (curved arrow) and patent interlobar vessels (arrowheads).
CEUS is increasingly being used for evaluation of organ perfusion and lesion characterization, as advocated by the EFSUMB guidelines. Specifically, the guidelines recommend CEUS for differentiating cortical necrosis from renal infarction. Real-time dynamic CEUS depicts microcirculation throughout the kidney and should therefore identify the reverse rim sign to diagnose acute cortical necrosis. The excellent spatial resolution of CEUS allows clear differentiation between renal infarction and cortical necrosis, which appears as non-enhancing cortical areas, and preserved hilar vascularity [35] between hypo-perfused and non-perfused areas is clear following ultrasound contrast agent administration.
CT remains the gold standard for the study of kidney trauma due to its panoramic view and its greater sensitivity compared to ultrasound. Thanks to its exclusively vascular nature, CEUS increases the sensitivity of US for the detection of traumatic lesions [36] allowing the study of all the vascular phases (arterial, venous and parenchymal) in real time, but above all it is useful in the follow-up in cases of conservative therapy (Figure 9) [37].
Figure 9.
Vascular lesions. 35-year-old male patient with abdominal trauma treated conservatively. (a) CT without contrast medium shows parenchymal hyper-density from recent hematoma (arrowhead) at the lower pole of the left kidney; (b) A pseudoaneurysm (curved arrow) is recognized in the arterial phase, while in the (c) nephrographic phase the parenchymal contusion is more clearly recognized and the pseudoaneurysm is less visible; (d) B-mode US shows inhomogeneous echogenic areas (asterisks) corresponding to the contusions; (e) CEUS adequately shows all the vascular phases of the pseudoaneurysm (curved arrow) and the perirenal hematoma (asterisk); (f) Follow-up performed on 7th day.
In addition, active bleeding can be recognized with some extravasated contrast medium bubbles and pseudoaneurysms can be detected. It should be remembered that the excretory system cannot be studied with CEUS and therefore when damage to the excretory tract is suspected, CT is mandatory.
6.7.1. Renal Artery Stenosis
CDUS is considered the first-line imaging modality for the diagnosis of renal artery stenosis. This technique presents evident advantages in patients with chronic renal failure because it does not require nephrotoxic contrast agents. UCAs increase by 30-fold the intensity of the arterial Doppler signal, improve the visualization of the renal arteries and reduce the test duration and the number of non-diagnostic tests. Authors, in a study including 120 patients with 38 stenosed renal arteries, reported surprisingly good results [38]; sensitivity, specificity, PPV, NPV and accuracy were reported as being 100%, 84%, 0%, 80% and 94%, respectively, for CDUS compared with angiography. Recently, Cui et al. [39] have shown that CEUS is accurate in grading renal artery stenosis, and may represent the method of choice in diagnosing renal artery stenosis. Nevertheless, the use of CEUS in patients with renal artery stenosis is still debated and, in the near future, the application of new parametric software will allow for a quantitative analysis of contrast enhancement curves and a more accurate definition of renal perfusion.
6.7.2. Renal Vein Thrombosis
In the differential diagnosis between renal cortical necrosis (RCN) and renal vein thrombosis (RVT), the Doppler US plays an important role. Spiesecke P. et al. [40] have shown that the resistive index (RI) is a very important distinguishing feature to separate RCN (RI not measurable due to hypoperfusion) and RVT (RI > 1). However, in unclear cases, the use of a contrast agent, which will demonstrate renal medulla enhancement only in patients with NCR and the absence of enhancement in patients with RVT, may be useful. CEUS increases diagnostic accuracy in the direct diagnosis of RVT, especially in cases of thrombosis secondary to neoplasia, providing useful information on any revascularization.
7. Potential Future Scenarios
CEUS now has an established role in renal imaging. New applications require development of innovative microbubbles. The commercially available microbubble contrast agents are characterized by a wide size distribution, typically ranging from 1–10 µm in diameter. Signal is produced by resonance, which depends on beam frequency and bubble diameter [41]. Contrast specific modes operate at a relatively narrow frequency bandwidth, which means only a small fraction of the microbubbles resonates and produce a signal. Bubble diameter also shows an inverse relationship with resonance frequency, which means that higher frequency imaging and improved spatial resolution is obtained if bubble diameter is reduced [41]. One option to improve contrast-specific imaging is the manufacturing of microbubble contrast agents with smaller diameter and narrower size distribution [42]. A dramatic increase in sensitivity has been documented in vitro for these bubbles [43].Moreover, these new agents may improve our ability to target microbubbles to specific endothelial cell ligands, transporting bioactive materials such as drugs or genes, and driving their behavior on specific targets triggering specific mechanisms, such as sonoporation [44]. This opens new perspectives in kidney diseases, especially for patients with AKI and CKD [45]. In a rat model of diabetic nephropathy, for instance, the therapeutic impact of combined cytotoxic T lymphocyte-associated antigen 4 immunoglobulin (CTLA-4-Ig) treatment and microbubble-mediated exposure has been investigated, showing that the effectiveness of this treatment improves following US microbubble exposure [46]. These applications are in an early pre-clinical phase, and future studies are required to investigate their full clinical potential.
8. Conclusions
CEUS is increasingly used to solve interpretative doubts arising with other methods. In fact, it can be considered both as a problem-solving technique in recognizing and distinguishing cystic vs. solid lesions, and as a technique to be used during follow-up in conservative treatments. The non-exposure to radiation and high safety profile allow it to be used even in rapid succession in cases of traumatic injuries treated conservatively, or in renal lesions requiring follow-up for prolonged periods, thus reducing the need for CECT and CEMRI scanning.
Abbreviations
| AKI | acute kidney injury |
| ccRCC | clear Cell Renal Cell Carcinoma |
| CDUS | Color Doppler US |
| CECT | contrast-enhanced computed tomography |
| CEMRI | contrast-enhanced MRI |
| CEUS | contrast enhancement ultrasound |
| CKD | chronic kidney disease |
| CRF | chronic renal failure |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| pRCC | papillary Renal Cell Carcinoma |
| UCAs | ultrasound contrast agents |
| US | ultrasound |
Author Contributions
Conceptualization: A.G. and I.C.; Methodology: A.G. and M.B.; Formal Analysis: A.G., I.C.; Investigation: P.L. and M.Z.; Data Curation: A.G. and M.B.; Writing —Original Draft Preparation: I.C.; Writing—Review & Editing: I.C., M.B. and A.G.; Visualization: P.L., F.P., L.G., V.C., A.B., and M.Z.; Supervision: A.G. and M.B.; Project Administration: A.G. and M.B. All authors meet the ICMJE recommendations for authorship credit. All authors have read and approved the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have contributed equally. The authors had full access to the data and take full responsibility for its integrity. For this retrospective analysis, it was not necessary to request authorization from the ethics committee. The contents of this paper are consistent with the principles of the Declaration of Helsinki in the latest version. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Vito Cantisani lecturer fees from Bracco, Samsung, Canon.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Albrecht T., Blomley M., Bolondi L., Claudon M., Correas J.-M., Cosgrove D., Greiner L., Jäger K., Jong N., Leen E., et al. Guidelines for the Use of Contrast Agents in Ultrasound—January 2004. Ultraschall Med. 2004;25:249–256. doi: 10.1055/s-2004-813245. [DOI] [PubMed] [Google Scholar]
- 2.Claudon M., Cosgrove D., Albrecht T., Bolondi L., Bosio M., Calliada F., Correas J.-M., Darge K., Dietrich C., D’Onofrio M., et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS)—Update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 3.Sidhu P., Cantisani V., Dietrich C., Gilja O., Saftoiu A., Bartels E., Bertolotto M., Calliada F., Clevert D.-A., Cosgrove D., et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version) Ultraschall Med. 2018;39:e2–e44. doi: 10.1055/a-0586-1107. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu P., Cantisani V., Dietrich C., Gilja O., Saftoiu A., Bartels E., Bertolotto M., Calliada F., Clevert D.-A., Cosgrove D., et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version) Ultraschall Med. 2018;39:154–180. doi: 10.1055/s-0044-101254. [DOI] [PubMed] [Google Scholar]
- 5.Bertolotto M., Quaia E., Galli G., Martinoli C., Locatelli M. Color Doppler Sonographic Appearance of Renal Perforating Vessels in Subjects with Normal and Impaired Renal Function. J. Clin. Ultrasound. 2000;28:10. doi: 10.1002/1097-0096(200007/08)28:6<267::AID-JCU1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Pozzi Mucelli R., Bertolotto M., Quaia E. Imaging Techniques in Acute Renal Failure. In: Ronco C., Bellomo R., La Greca G., editors. Contributions to Nephrology. Vol. 132. KARGER; Basel, Switzerland: 2001. pp. 76–91. [DOI] [PubMed] [Google Scholar]
- 7.Quaia E., Bertolotto M. Renal Parenchymal Diseases: Is Characterization Feasible with Ultrasound? Eur. Radiol. 2002;12:2006–2020. doi: 10.1007/s00330-002-1360-z. [DOI] [PubMed] [Google Scholar]
- 8.Quaia E., Bertolotto M., Cioffi V., Rossi A., Baratella E., Pizzolato R., Cova M.A. Comparison of Contrast-Enhanced Sonography with Unenhanced Sonography and Contrast-Enhanced CT in the Diagnosis of Malignancy in Complex Cystic Renal Masses. Am. J. Roentgenol. 2008;191:1239–1249. doi: 10.2214/AJR.07.3546. [DOI] [PubMed] [Google Scholar]
- 9.Bertolotto M., Martegani A., Aiani L., Zappetti R., Cernic S., Cova M.A. Value of Contrast-Enhanced Ultrasonography for Detecting Renal Infarcts Proven by Contrast Enhanced CT. A Feasibility Study. Eur. Radiol. 2008;18:376–383. doi: 10.1007/s00330-007-0747-2. [DOI] [PubMed] [Google Scholar]
- 10.Miller D.L., Dou C., Lucchesi B.R. Are ECG Premature Complexes Induced by Ultrasonic Cavitation Electrophysiological Responses to Irreversible Cardiomyocyte Injury? Ultrasound Med. Biol. 2011;37:312–320. doi: 10.1016/j.ultrasmedbio.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granata A., Zanoli L., Insalaco M., Valentino M., Pavlica P., Di Nicolò P.P., Scuderi M., Fiorini F., Fatuzzo P., Bertolotto M. Contrast-Enhanced Ultrasound (CEUS) in Nephrology: Has the Time Come for Its Widespread Use? Clin. Exp. Nephrol. 2015;19:606–615. doi: 10.1007/s10157-014-1040-8. [DOI] [PubMed] [Google Scholar]
- 12.Bosniak M.A. The current radiological approach to renal cysts. Radiology. 1986;158:1–10. doi: 10.1148/radiology.158.1.3510019. [DOI] [PubMed] [Google Scholar]
- 13.Israel G.M., Bosniak M.A. How I Do It: Evaluating Renal Masses. Radiology. 2005;236:441–450. doi: 10.1148/radiol.2362040218. [DOI] [PubMed] [Google Scholar]
- 14.Sevcenco S., Spick C., Helbich T.H., Heinz G., Shariat S.F., Klingler H.C., Rauchenwald M., Baltzer P.A. Malignancy Rates and Diagnostic Performance of the Bosniak Classification for the Diagnosis of Cystic Renal Lesions in Computed Tomography—A Systematic Review and Meta-Analysis. Eur. Radiol. 2017;27:2239–2247. doi: 10.1007/s00330-016-4631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolotto M., Zappetti R., Cavallaro M., Perrone R., Perretti L., Cova M.A. Characterization of Atypical Cystic Renal Masses With MDCT: Comparison of 5-Mm Axial Images and Thin Multiplanar Reconstructed Images. Am. J. Roentgenol. 2010;195:693–700. doi: 10.2214/AJR.09.3113. [DOI] [PubMed] [Google Scholar]
- 16.Ascenti G., Mazziotti S., Zimbaro G., Settineri N., Magno C., Melloni D., Caruso R., Scribano E. Complex Cystic Renal Masses: Characterization with Contrast-Enhanced US. Radiology. 2007;243:158–165. doi: 10.1148/radiol.2431051924. [DOI] [PubMed] [Google Scholar]
- 17.Bosniak M.A. Difficulties in Classifying Cystic Lesions of the Kidney. Urol. Radiol. 1991;13:91–93. doi: 10.1007/BF02924597. [DOI] [PubMed] [Google Scholar]
- 18.Cantisani V., Bertolotto M., Clevert D.-A., Correas J.-M., Drudi F.M., Fischer T., Gilja O.H., Granata A., Graumann O., Harvey C.J., et al. EFSUMB 2020 Proposal for a Contrast-Enhanced Ultrasound-Adapted Bosniak Cyst Categorization—Position Statement. Ultraschall Med. 2020:1300–1727. doi: 10.1055/a-1300-1727. [DOI] [Google Scholar]
- 19.Silverman S.G., Pedrosa I., Ellis J.H., Hindman N.M., Schieda N., Smith A.D., Remer E.M., Shinagare A.B., Curci N.E., Raman S.S., et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology. 2019;292:475–488. doi: 10.1148/radiol.2019182646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertolotto M., Cicero C., Perrone R., Degrassi F., Cacciato F., Cova M.A. Renal Masses with Equivocal Enhancement at CT: Characterization With Contrast-Enhanced Ultrasound. Am. J. Roentgenol. 2015;204:W557–W565. doi: 10.2214/AJR.14.13375. [DOI] [PubMed] [Google Scholar]
- 21.Bertolotto M., Derchi L.E., Cicero C., Iannelli M. Renal Masses as Characterized by Ultrasound Contrast. Ultrasound Clin. 2013;8:581–592. doi: 10.1016/j.cult.2013.07.003. [DOI] [Google Scholar]
- 22.Bertolotto M., Bucci S., Valentino M., Currò F., Sachs C., Cova M.A. Contrast-Enhanced Ultrasound for Characterizing Renal Masses. Eur. J. Radiol. 2018;105:41–48. doi: 10.1016/j.ejrad.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 23.King K.G. Use of Contrast Ultrasound for Renal Mass Evaluation. Radiol. Clin. N. Am. 2020;58:935–949. doi: 10.1016/j.rcl.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Bhatt S., MacLennan G., Dogra V. Renal Pseudotumors. Am. J. Roentgenol. 2007;188:1380–1387. doi: 10.2214/AJR.06.0920. [DOI] [PubMed] [Google Scholar]
- 25.Ascenti G., Zimbaro G., Mazziotti S., Gaeta M., Lamberto S., Scribano E. Contrast-Enhanced Power Doppler US in the Diagnosis of Renal Pseudotumors. Eur. Radiol. 2001;11:2496–2499. doi: 10.1007/s003300100861. [DOI] [PubMed] [Google Scholar]
- 26.Bertolotto M., Cicero C., Catalano O., Currò F., Derchi L.E. Solid Renal Tumors Isoenhancing to Kidneys on Contrast-Enhanced Sonography: Differentiation from Pseudomasses: Isoenhancing Tumors and Pseudomasses on Contrast-Enhanced Sonography. J. Ultrasound Med. 2018;37:233–242. doi: 10.1002/jum.14335. [DOI] [PubMed] [Google Scholar]
- 27.Granata A., Floccari F., Insalaco M., Clementi A., Di Lullo L., D’Amelio A., Zamboli P., Logias F., Romano M., Fiorini F. Ultrasound assessment in renal infections. G. Ital. Nefrol. Organo Uff. Della Soc. Ital. Nefrol. 2012;29(Suppl. S57):S47–S57. [PubMed] [Google Scholar]
- 28.Jungberg B., Albiges L., Bedke J., Bex A., Capitanio U., Giles R.H., Hora M., Klatte T., Lam T., Marconi L., et al. EAU Guidelines on Renal Cell Carcinoma 2021; Proceedings of the EAU Annual Congress; Amsterdam, The Netherlands. 8–12 July 2021; Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2021. [Google Scholar]
- 29.Meloni M.F., Bertolotto M., Alberzoni C., Lazzaroni S., Filice C., Livraghi T., Ferraioli G. Follow-Up After Percutaneous Radiofrequency Ablation of Renal Cell Carcinoma: Contrast-Enhanced Sonography Versus Contrast-Enhanced CT or MRI. Am. J. Roentgenol. 2008;191:1233–1238. doi: 10.2214/AJR.07.3238. [DOI] [PubMed] [Google Scholar]
- 30.Bertolotto M., Siracusano S., Cicero C., Iannelli M., Silvestri T., Celia A., Guarise A., Stacul F. Cryotherapy of Renal Lesions: Enhancement on Contrast-Enhanced Sonography on Postoperative Day 1 Does Not Imply Viable Tissue Persistence: Early Contrast-Enhanced Sonography After Renal Tumor Cryoablation. J. Ultrasound Med. 2017;36:301–310. doi: 10.7863/ultra.16.02061. [DOI] [PubMed] [Google Scholar]
- 31.Bertolotto M., Campo I., Sachs C., Ciabattoni R., Cicero C., Drudi F.M., Derchi L.E., Cova M.A. Contrast-Enhanced Ultrasound after Successful Cryoablation of Benign and Malignant Renal Tumours: How Long Does Tumour Enhancement Persist? J. Med. Imaging Radiat. Oncol. 2021 doi: 10.1111/1754-9485.13149. [DOI] [PubMed] [Google Scholar]
- 32.Hoeffel C., Pousset M., Timsit M.-O., Elie C., Méjean A., Merran S., Tranquart F., Khairoune A., Joly D., Richard S., et al. Radiofrequency Ablation of Renal Tumours: Diagnostic Accuracy of Contrast-Enhanced Ultrasound for Early Detection of Residual Tumour. Eur. Radiol. 2010;20:1812–1821. doi: 10.1007/s00330-010-1742-6. [DOI] [PubMed] [Google Scholar]
- 33.Bonkat G., Bartoletti R., Bruyère F., Cai T., Geerlings S.E., Köves B., Schubert S., Wagenlehner F., Devlies W., Horváth J., et al. EAU Guidelines on Urological-Infections-2021; Proceedings of the EAU Annual Congress; Amsterdam, The Netherlands. 8–12 July 2021; Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2021. [Google Scholar]
- 34.Fontanilla T., Minaya J., Cortés C., Hernando C.G., Arangüena R.P., Arriaga J., Carmona M.S., Alcolado A. Acute Complicated Pyelonephritis: Contrast-Enhanced Ultrasound. Abdom. Imaging. 2012;37:639–646. doi: 10.1007/s00261-011-9781-2. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf G.T., Sellars M.E., Huang D.Y., Deganello A., Sidhu P.S. Cortical Necrosis Secondary to Trauma in a Child: Contrast-Enhanced Ultrasound Comparable to Magnetic Resonance Imaging. Pediatr. Radiol. 2014;44:484–487. doi: 10.1007/s00247-013-2818-7. [DOI] [PubMed] [Google Scholar]
- 36.Valentino M., Serra C., Zironi G., De Luca C., Pavlica P., Barozzi L. Blunt Abdominal Trauma: Emergency Contrast-Enhanced Sonography for Detection of Solid Organ Injuries. Am. J. Roentgenol. 2006;186:1361–1367. doi: 10.2214/AJR.05.0027. [DOI] [PubMed] [Google Scholar]
- 37.Valentino M., Ansaloni L., Catena F., Pavlica P., Pinna A.D., Barozzi L. Contrast-Enhanced Ultrasonography in Blunt Abdominal Trauma: Considerations after 5 Years of Experience. Radiol. Med. 2009;114:1080–1093. doi: 10.1007/s11547-009-0444-0. [DOI] [PubMed] [Google Scholar]
- 38.Ciccone M.M., Cortese F., Fiorella A., Scicchitano P., Cito F., Quistelli G., Pertosa G., D’Agostino R., Guida P., Favale S. The clinical role of contrast-enhanced ultrasound in the evaluation of renal artery stenosis and diagnostic superiority as compared to traditional echo-color-Doppler flow imaging. Int. Angiol. 2011;30:135–139. [PubMed] [Google Scholar]
- 39.Cui Y., Zhang Q., Yan J., Wu J. The Value of Contrast-Enhanced Ultrasound versus Doppler Ultrasound in Grading Renal Artery Stenosis. BioMed Res. Int. 2020:1–10. doi: 10.1155/2020/7145728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiesecke P., Münch F., Fischer T., Hamm B., Lerchbaumer M.H. Multiparametric Ultrasound Findings in Acute Kidney Failure Due to Rare Renal Cortical Necrosis. Sci. Rep. 2021;11:2060. doi: 10.1038/s41598-021-81690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalla Palma L., Bertolotto M. Introduction to Ultrasound Contrast Agents: Physics Overview. Eur. Radiol. 1999;9:S338–S342. doi: 10.1007/PL00014069. [DOI] [PubMed] [Google Scholar]
- 42.Frinking P., Segers T., Luan Y., Tranquart F. Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol. 2020;46:892–908. doi: 10.1016/j.ultrasmedbio.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Segers T., Kruizinga P., Kok M.P., Lajoinie G., de Jong N., Versluis M. Monodisperse Versus Polydisperse Ultrasound Contrast Agents: Non-Linear Response, Sensitivity, and Deep Tissue Imaging Potential. Ultrasound Med. Biol. 2018;44:1482–1492. doi: 10.1016/j.ultrasmedbio.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Deelman L.E., Declèves A.-E., Rychak J.J., Sharma K. Targeted Renal Therapies through Microbubbles and Ultrasound. Adv. Drug Deliv. Rev. 2010;62:1369–1377. doi: 10.1016/j.addr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hull T.D., Agarwal A., Hoyt K. New Ultrasound Techniques Promise Further Advances in AKI and CKD. J. Am. Soc. Nephrol. 2017;28:3452–3460. doi: 10.1681/ASN.2017060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Wang P., Li X., Dong Y., Wu S., Xu M., Chen X., Wang S., Zheng C., Zou C. Combination CTLA-4 Immunoglobulin Treatment and Ultrasound Microbubble-Mediated Exposure Improve Renal Function in a Rat Model of Diabetic Nephropathy. Aging. 2021;13:8524–8540. doi: 10.18632/aging.202664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.