Abstract
Simple Summary
This study shows how introducing milk thistle seeds into broiler chicken feed rations affects rearing results (weight gain, intake and conversion of feed), carcass composition and meat quality (pH, color, water holding capacity), the chemical composition (basic components, fatty acids) and organoleptic properties (flavor, tenderness, palatability and juiciness) of meat. Based on the results the use of milk thistle seeds in broiler chicken starter/grower diets can be recommended in the amount of 0/2% or 2/3%, respectively. However, the introduction of Silybum marianum in starter and grower rations (over the whole rearing period) made it possible to obtain the highest body weight at the lowest feed conversion per body weight gain unit, without influencing muscularity and fattening grade, at the same time improving the meat’s value for health.
Abstract
The studies aimed to evaluate the impact of milk thistle seeds in broiler chicken feed rations on rearing results, carcass composition and meat quality. The experiment involved 120 broiler chickens randomly allocated to three equinumerous groups (C, MT02, MT23). Each group was divided into five subgroups of eight chickens each. Over the first 21 days of life the birds were fed starter rations, and over the following 21 days received grower rations. Chicken starter/grower diets in groups MT02 and MT23 were supplemented with ground seeds of milk thistle in the amount of 0/2% (MT02) and 2/3% (MT23). It was demonstrated that Silybum marianum added to feed rations over the whole rearing period (group MT23) increased above 3% the birds’ body weight on rearing day 42. (p < 0.05) and decreased about 7% the feed conversion ratio (p < 0.05) in comparison to group C. No effect of feeding on the carcass composition was observed, including on muscularity and fattening grade, although diets containing milk thistle reduced (by 15% and 19% in group MT02 and MT23, respectively) the content of crude fat in chicken leg muscles (p < 0.05). The highest (p < 0.05) content of polyunsaturated fatty acids (PUFA) was determined in the breast (38.06%) and leg (37.63%) muscles of chicken receiving feed rations containing Silybum marianum throughout the rearing period. No effect of nutrition on the evaluated physical properties of muscles was observed, except on the decrease in lightness color (L*) and increase in values a* and C as well as a decrease of water holding capacity of the breast muscles. It was found that Silybum marianum in chicken diets had a positive effect on the evaluated meat flavor characteristics of the muscles. To sum up, based on the study results, including ground seeds of milk thistle in broiler chickens nutrition can be recommended in the amount of 2/3% in starter/grower diets, respectively.
Keywords: milk thistle, rations, broiler chicken, performance results, carcass value, meat quality
1. Introduction
The European Union’s prohibition of using antibiotics in feeds as growth stimulants made breeders and feed producers look for new nutritional solutions. Apart from probiotics, prebiotics, organic acids, antioxidants, and feed enzymes, various preparations of plant origin—so-called phytobiotics—have raised more and more interest [1,2,3,4,5]. Hippenstiel et al. [6] and Tavakolinasab et al. [7] report that herbs can be used in various forms: fresh or dried, infusion, brew, extract, essential oils and macerates.
Herbs and herbal preparations contain active biological ingredients, i.e.,: essential oils, tannins, glycosides, flavonoids, terpenes, mucilages, and organic acids [5,8,9,10]. Biological active components of herbal preparations display multi-directional activity: anti-stress, anti-bacterial, anti-virus, and anti-fungicidal [11,12]. In addition, they enhance the excretion of digestive enzymes increasing the appetite of animals [8,13,14]. They are believed “natural” and “safe” [12]. A positive impact of medicinal plants on the microflora of the digestive tract and productive performance of animals was demonstrated [4,15,16,17]. In the opinion of Gregačević et al. [18] phytogenic feed additives stimulate the immunological system, whereas the resistant stimulating effect of these additives is based on an increased activity of lymphocytes, macrophages, and cells.
Currently, milk thistle (Silybum marianum [L.] Gaertn) is increasingly popular in animal nutrition [9,19,20,21]. Milk thistle seeds have a different content of protein (160–300 g/kg) containing a lot of exogenous amino acids, which is confirmed by EAAI—the essential amino acid index (60.32) [22,23,24]. Crude fat from milk thistle seeds has an advantageous composition of fatty acids with oleic and linoleic acid predominating [22,23,25,26,27]. A high content of cellulose and lignin fraction in the diets of monogastric animals can pose certain limitations [28]. The main biological active component of milk thistle seeds is silymarin accounting for 1.5–3% [24,29]. Silymarin is composed of flavonolignans consisting of: silibinin (60%), isosilibinin (5%), silidianin (20%) and silicristin (10%), of which silibinin has the strongest effect [29,30,31,32]. Silymarin perfectly protects the liver from toxic agents and helps regenerate it [30,33]. In addition, it has an anti-inflammatory effect, inhibiting the migration of neutrophils and fostering the formation of prostaglandins [34,35,36]. Due to its medicinal properties, silymarin is preferred by veterinary doctors as a natural medicine and recommended for intensive animal production [7,37,38,39,40]. Therefore, milk thistle can be widely used for animal feeding and in veterinary medicine. However, the results of studies [20,41,42,43,44] differ. Šťastník et al. [43] demonstrated that using 5% or 15% of milk thistle oil cake in the diets of broiler chickens can decrease their weight gain. Similarly, Gharahveysi [44] introducing 0.3% or 3% ground milk thistle into the birds’ diets found a reduced feed intake and body weight of chickens. However, Muhammad et al. [42] and Ahmad et al. [20] showed an increase in body weight and feed conversion ratio in chickens fed diets containing 10 and 15 g/kg of milk thistle, respectively. Tedesco et al. [31] and Mojahedtalab et al. [45] showed a positive effect of supplementing broiler chicken feed rations with silymarin-phospholipid complex or silymarin on the performance results. Similarly, the post-slaughter results of birds fed diets containing Silybum marianum do not unanimously show how it affects the carcass composition and meat quality, since available literature lacks an evaluation the physico-chemical and organoleptic characteristics of meat [7,43,46,47,48,49].
The aim of the studies was to assess the effect of feeding different amounts of milk thistle seeds at different productive stages (starter and grower) on broiler productive performance, carcass composition, meat quality and sensory properties.
2. Materials and Methods
2.1. Experiment Design
The feeding experiment involved 120 Ross 308 sexed broiler chickens randomly allocated to three equinumerous groups (C, MT02, MT23). Each group was divided into five subgroups of eight chickens (4 males and 4 females) each. The birds were reared over a 42–day cycle in metal cages under standard microclimate conditions with unlimited access to water (nipple drinkers) and feed. A free feeding (ad libitum) scheme was used. Throughout the rearing period the birds were exposed to 24–hour electric lighting. In the first experimental week ambient temperature was 32 °C, and was then decreased every week (every 7 days) by 1–2 °C until it reached 21–23 °C in the last rearing week. In the first rearing period, i.e., until day 21, the birds were fed complete bulk starter feed rations and from day 22 to 42—with grower feed rations. All loose feed rations were based on wheat, soybean cake (non–GMO), soybean oil and mineral and vitamin additives. Chicken starter/grower diets in experimental groups (MT02 and MT23) were supplemented with ground seeds of milk thistle in the amount of 0/2% (MT02) and 2/3% (MT23). The nutrient content of the basal diet was calculated on the basis of the chemical composition of raw feedstuffs, and the metabolizable energy value was in line with equations from the European Tables [50]. The nutritional value of rations was calculated according to nutritional recommendations and indicated in Table 1.
Table 1.
Composition and nutritive value of rations.
| Specification | Starter | Grower | ||||
|---|---|---|---|---|---|---|
| C | MT02 | MT23 | C | MT02 | MT23 | |
| Ingredients (g/kg) | ||||||
| Wheat | 598 | 598 | 618 | 608 | 628 | 618 |
| Soybean cake | 360 | 360 | 320 | 330 | 290 | 290 |
| Milk thistle | - | - | 20.0 | - | 20.0 | 30.0 |
| Soybean oil | - | - | - | 20.0 | 20.0 | 20.0 |
| Limestone | 11.5 | 11.5 | 11.0 | 10.8 | 11.7 | 11.7 |
| NaCl | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 |
| 2–Ca phosphate | 18.5 | 18.5 | 18.0 | 18.5 | 17.3 | 17.3 |
| Premix starter/grower * | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| L–lysine (99%) | 1.00 | 1.00 | 2.00 | 1.50 | 2.00 | 2.00 |
| DL–methionine (99%) | 2.50 | 2.50 | 2.50 | 2.70 | 2.50 | 2.50 |
| Calculated nutritive value per 1 kg of diets | ||||||
| Metabolizable energy (MJ) | 13.0 | 13.0 | 13.0 | 13.3 | 13.4 | 13.4 |
| Crude protein (g) | 230 | 230 | 230 | 214 | 214 | 218 |
| Lysine (g) | 12.3 | 12.3 | 12.3 | 12.0 | 11.5 | 11.5 |
| Methionine (g) | 5.65 | 5.65 | 5.46 | 5.69 | 5.30 | 5.28 |
| Tryptophan (g) | 2.83 | 2.78 | 2.63 | 2.67 | 2.47 | 2.46 |
| Ca total (g) | 9.70 | 9.70 | 9.29 | 9.35 | 9.30 | 9.29 |
| P available (g) | 4.38 | 4.38 | 4.24 | 4.33 | 4.08 | 4.07 |
| Na (g) | 1.58 | 1.58 | 1.57 | 1.57 | 1.56 | 1.56 |
| Analyzed nutrients (%) | ||||||
| Dry matter | 89.0 | 90.0 | 90.0 | 89.3 | 89.7 | 89.7 |
| Crude ash | 5.86 | 6.11 | 5.88 | 5.66 | 5.85 | 5.94 |
| Crude protein | 23.3 | 23.0 | 23.0 | 21.3 | 21.1 | 21.8 |
| Crude fat | 3.62 | 3.47 | 3.90 | 4.19 | 4.62 | 4.95 |
| Crude fiber | 2.98 | 3.12 | 4.13 | 3.26 | 4.25 | 4.19 |
* Mineral and vitamin starter/grower premix contained, per 1 kg; mg: choline chloride 140,000/80,000, Fe 16,000/14,000, Cu 4000/2400, Co 60, Mn 24,000/20,000, Zn 22,000/12,000, I 300/200, Se 40/50, antioxidants (butylated hydroxyanisole, butylated 99 hydroxytoluene); IU:2,800,000/2,000,000 vit. A, 100,000/600,000 vit. D3; mg: 14,400/10,000 vit. E, 800/600 vit. K3, 800/400 vit. B1, 1800/1400 vit. B2, 1200/800 vit. B6, 3000/2800 pantothenic acid, 12,000/6000 nicotinic acid, 400/300 folic acid, 60/30 biotin.
During the growth experiment, the birds’ body weight was controlled on day 1, 21 and 42 along with the intake of feed in respective rearing periods. The results were used to calculate weight gain and feed conversion (FCR) per weight gain unit.
On the 42nd day of the birds’ life, ten birds (five males and five females) with a body weight representative of a specific group and sex were selected from each group and slaughtered. Next, the carcasses were cooled over 24 h at a temperature of 4 °C. To calculate the dressing percentage, the weight of cooled carcasses was determined and they were subject to simplified dissection analysis using a procedure described by Ziołecki and Doruchowski [51]. During dissection, samples of breast and leg muscles were taken for evaluating their physico-chemical and organoleptic characteristics.
2.2. Chemical Composition Evaluation of Milk Thistle and Muscles
The samples for analysis were collected according to applicable requirements [52]. The dry matter, total ash, crude protein, and crude fat contents were described by the AOAC [53] according method number: dry matter (930.15), total ash (942.05), crude protein (990.03), crude fat (991.36) and crude fiber (978.10). The gross energy of milk thistle was determined using an Oxygen Bomb Calorimeter [54]. The number of nitrogen-free extractives (NFE) was calculated from the formula:
| NFE = dry matter − (crude protein + total ash + crude fat + crude fiber) |
The fatty acid profile in milk thistle and in muscles was determined by gas chromatography [55]. Fatty acid analysis was made with gas chromatography (GC) using gas chromatograph (GCMS-QP210 Ultra, Shimadzu, Kyoto, Japan) with capillary column and flame-ionization detection and helium as the carrier gas. The initial oven temperature was 140 °C for 1 min, thereafter increased by 20 °C/min to 200 °C and held for 20 min and increased by 5 °C/min to 220 °C held for 25 min. The injector was heated to 250 °C and the detector to 270 °C. FAME standards (Supelco 37 Component FAME Mix) were used to identify the fatty acids present in the samples. Based on the percentage (% of the total) of fatty acids, we calculated the atherogenic (AI) and thrombogenic (TI) indexes, as well as the hypocholesterolemic-to-hypercholesterolemic fatty acids ratio (HH) according to Ulbricht and Southgate [56] and Santos-Silva et al. [57]:
| AI = (C12:0 + 4 × C14:0 + C16:0)/[ΣMUFA + Σ(n–6) + Σ(n–3)] |
| TI = (C14:0 + C16:0 + C18:0)/[0.5 × ΣMUFA + 0.5 × Σ(n–6) + 3 × Σ(n–3) + Σ(n–3)/Σ(n–6)] |
| HH = [(C18:1n–9 + C18:2n–6 + C20:4n–6 + C18:3n–3 + C20:5n–3 + C22:5n–3 + C22:6n–3)/(C14:0 + C16:0)] |
2.3. Physical Properties Evaluation of Muscles
The concentration of hydrogen ions (pH15 and pH24) in pectoralis maior and iliotibialis muscles was measured using a Testo 205 pH-meter with a dagger electrode. Fifteen minutes after the slaughter and after over 24 h of cooling the reaction (pH15 and pH24) was measured in muscles.
Water absorption expressed as water holding capacity (WHC) was determined by Grau and Hamm’s filter-paper press method described by Jurczak [58] based on the surface of meat juice on the filter-paper.
The color of breast muscles was determined using a Minolta Chroma Metters CR 300 (Konica Minolta Osaka, Japan) instrument according to the L, a*, b* system [59]. Two illuminant/observer combinations were applied, i.e., illuminant C (average day light) and standard observer 2° as well as illuminant D65 (day light) and standard observer 10°, recommended for measurements of meat color [60]. In the used measuring system L denotes psychometric color saturation that is a spatial vector. On the other hand, a* and b* are trichromatic coordinates, where a* as a positive value corresponds to red, and as a negative value—green; in turn, positive b* corresponds to yellow, and negative b*—blue. The color parameters a* and b* were used to calculate chroma (C) and hue (H) with formulas used by [61].
2.4. Organoleptic Properties of Muscles
The organoleptic properties of breast and thigh muscles were evaluated on a five-point scale after cooking in a 0.8% NaCl solution up to a temperature of 80 °C in the geometric center of the sample. The meat to water ratio was 1:2. The flavor of muscles in terms of palatability, flavor, juiciness and tenderness was evaluated by a group of eight trained people [62,63].
2.5. Statistical Analysis
The results were elaborated by statistical methods using one-way analysis of variance, according to the following mathematical model:
| Yik = µ + ai + eik |
where:
Yik—trait level,
µ—total mean,
ai—effect of treatment,
eik—error.
The significance of differences between mean values was verified using Tukey test at the significance level α ≤ 0.05. The results were elaborated using STATISTICA PL 13.1 software [64].
3. Results
The evaluated seeds of milk thistle (Silybum marianum [L.] Gaertn.) contained 219 g/kg of total protein and 238 g/kg of crude fat with an advantageous fatty acid composition (Table 2).
Table 2.
Basic nutrients, energy value and fatty acids profile of milk thistle seeds.
| Specification | Composition (n = 3) |
|---|---|
| Basical nutrients (g/kg) | |
| Dry matter | 886 |
| Crude ash | 31.5 |
| Crude protein | 219 |
| Crude fat | 238 |
| Crude fiber | 41.3 |
| N-free extractives | 356.2 |
| Gross energy value (kcal/kg) | 4145 |
| Fatty acids (% total FA) | |
| C12:0 | 0.010 |
| C14:0 | 0.110 |
| C16:0 | 8.52 |
| C16:1 | 0.090 |
| C18:0 | 4.78 |
| C18:1 | 23.59 |
| C18:2 | 54.64 |
| C18:3 | 0.160 |
| C20:0 | 3.25 |
| C20:1 | 0.920 |
| C22:0 | 2.41 |
| C22:4 | 0.190 |
| others | 1.33 |
| SFA | 19.08 |
| UFA | 79.59 |
| MUFA | 24.60 |
| PUFA | 54.99 |
| DFA (UFA + C18:0) | 84.37 |
| OFA (C14:0 + C16:0) | 8.63 |
| AI | 0.110 |
| TI | 0.330 |
| h/H | 9.11 |
FA—fatty acids, SFA—saturated fatty acids, UFA—unsaturated fatty acids, MUFA—monounsaturated fatty acids, PUFA—polyunsaturated fatty acids, DFA—neutral and hypocholesterolemic fatty acids, OFA—hypercholesterolemic fatty acids, AI—atherogenicity index, TI—thrombogenicity index, h/H—hypocholesterolaemic/Hypercholesterolaemic ratio.
The seeds contained only 19.08% of SFA (saturated fatty acids). The content of MUFA (monounsaturated fatty acids) exceeded 24.60% and PUFA (polyunsaturated fatty acids) were predominant—54.99%. Among all monounsaturated acids, milk thistle contained the largest share of oleic acid—23.59%, and the polyunsaturated acids profile was dominated by linoleic acid—54.64%. As a result, nearly 85% of total fatty acids (FA) in milk thistle seeds were neutral and hypocholesterolemic acids (DFA).
The inclusion of milk thistle seeds in starter/grower diets in the amount 2/3% (MT23 group) significantly (by more than 3%) increased the body weight of broilers on the 42nd day of rearing in comparison to group C (Table 3).
Table 3.
Rearing results of broiler chickens.
| Indicators | Groups | SEM | p–Value | ||
|---|---|---|---|---|---|
| C | MT02 | MT23 | |||
| Body weight (g) | |||||
| 1 d | 39.8 | 39.3 | 39.2 | 0.083 | 0.051 |
| 21 d | 667 | 666 | 674 | 3.56 | 0.680 |
| 42 d | 2306 b | 2377 ab | 2389 a | 14.75 | <0.05 |
| Body weight gain (g) | |||||
| 1–21 d | 627 | 627 | 635 | 3.59 | 0.657 |
| 22–42 d | 1639 | 1711 | 1715 | 15.10 | 0.056 |
| 1–42 d | 2267 b | 2338 ab | 2350 a | 14.76 | <0.05 |
| Feed conversion ratio (kg) | |||||
| 1–21 d | 1.61 a | 1.57 a | 1.51 b | 0.013 | <0.05 |
| 22–42 d | 1.85 a | 1.79 b | 1.71 c | 0.016 | <0.05 |
| 1–42 d | 1.74 a | 1.69 b | 1.61 c | 0.015 | <0.05 |
C—control, MT02—milk thistle (0%/2%—starter/grower), MT23—milk thistle (2%/3%—starter/grower), SEM—standard error of mean, n = 5, chickens survivability = 100%, abc—means with different superscripts within a row are significantly different at p ≤ 0.05.
Milk thistle in starter diets (group MT23) significantly decreased feed conversion in comparison to other groups. In the second rearing period and throughout the entire rearing period—chickens fed diets with milk thistle showed more efficient (p ≤ 0.05) conversion of feed. Differences in FCR between groups MT02 and MT23 and group C were 3% and 7%, respectively, throughout the rearing period. The type of feed rations used did not affect the post-slaughter performance, except the share of drumstick muscles (Table 4).
Table 4.
Slaughter analysis of broiler chickens.
| Parameters | Group | SEM | p–Value | ||
|---|---|---|---|---|---|
| C | MT02 | MT23 | |||
| Body weight before slaughter (g) | 2330 | 2392 | 2370 | 28.4 | 0.069 |
| Cold carcasses weight (g) | 1839 | 1875 | 1856 | 28.2 | 0.882 |
| Dressing percentage (%) | 78.9 | 78.4 | 78.2 | 0.532 | 0.888 |
| Muscles total (%) | 51.4 | 51.4 | 50.1 | 0.472 | 0.466 |
| breast | 30.0 | 29.9 | 29.7 | 0.436 | 0.975 |
| thigh | 12.7 | 12.4 | 12.3 | 0.166 | 0.543 |
| drumstick | 8.74 ab | 9.03 a | 8.13 b | 0.139 | <0.05 |
| Abdominal fat (%) | 0.62 | 0.79 | 0.82 | 0.040 | 0.098 |
| Skin with subcutaneous fat (%) | 7.38 | 7.31 | 7.68 | 0.167 | 0.659 |
| Giblets total share in body weight before slaughter (%) | 3.30 | 3.20 | 3.36 | 0.029 | 0.299 |
| heart | 0.50 | 0.48 | 0.46 | 0.010 | 0.305 |
| liver | 1.79 | 1.61 | 1.71 | 0.036 | 0.124 |
| stomach | 1.01 | 1.11 | 1.18 | 0.030 | 0.056 |
C—control, MT02—milk thistle (0%/2%—starter/grower), MT23—milk thistle (2%/3%—starter/grower), SEM—standard error of mean, n = 10, ab—means with different superscripts within a row are significantly different at p ≤ 0.05.
The presence of ground seeds of Silybum marianum in starter and grower diets (MT02 group) significantly increased the share of drumstick muscles in cold carcass compared to muscles of birds from MT23 group (9.03% vs. 8.13%).
The nutrition used significantly affected the content of crude ash in breast muscles and the level of crude fat in leg muscles (Table 5).
Table 5.
Basic nutrients (g/100 g) of muscles.
| Specification | Group | SEM | p–Value | ||
|---|---|---|---|---|---|
| C | MT02 | MT23 | |||
| Breast muscles | |||||
| Dry matter | 25.4 | 25.0 | 25.0 | 0.134 | 0.292 |
| Crude ash | 1.17 a | 1.12 b | 1.16 ab | 0.007 | <0.05 |
| Crude protein | 22.3 | 22.2 | 22.0 | 0.094 | 0.576 |
| Crude fat | 1.13 | 1.10 | 0.92 | 0.639 | 0.399 |
| Leg muscles | |||||
| Dry matter | 24.5 | 24.5 | 24.1 | 0.212 | 0.726 |
| Crude ash | 1.06 | 1.07 | 1.07 | 0.004 | 0.405 |
| Crude protein | 18.8 | 19.4 | 19.5 | 0.144 | 0.102 |
| Crude fat | 4.01 a | 3.43 b | 3.25 b | 0.120 | <0.05 |
C—control, MT02—milk thistle (0%/2%—starter/grower), MT23—milk thistle (2%/3%—starter/grower), SEM—standard error of mean, n = 5 (n = ♂ + ♀), ab—means with different superscripts within a row are significantly different at p ≤ 0.05.
The breast muscles of birds from group MT02 contained significantly less crude ash than the muscles of birds from group C. Milk thistle seeds in chicken diets (groups MT02 and MT23) contributed to decreasing (p < 0.05) the content of crude fat in leg muscles.
Table 6 describes the composition and share of fatty acids in the lipid fraction of breast and leg muscles.
Table 6.
Fatty acids profile (% total FA) of muscles.
| Fatty Acids | Group | SEM | p–Value | ||
|---|---|---|---|---|---|
| C | MT02 | MT23 | |||
| Breast muscles | |||||
| C14:0 | 0.110 | 0.110 | 0.100 | 0.004 | 0.239 |
| C16:0 | 22.19 | 21.71 | 21.45 | 0.231 | 0.285 |
| C18:0 | 5.92 | 5.91 | 5.82 | 0.115 | 0.941 |
| C18:1 | 34.33 | 33.84 | 32.19 | 0.433 | 0.065 |
| C18:2 n–6 | 32.59 b | 33.87 ab | 36.00 a | 0.561 | <0.05 |
| C18:3 n–3 | 1.05 b | 1.25 ab | 1.49 a | 0.080 | <0.05 |
| C20:0 | 0.100 | 0.100 | 0.090 | 0.005 | 0.587 |
| C20:1 | 0.070 b | 0.110 a | 0.050 b | 0.010 | <0.05 |
| C20:2 | 0.080 | 0.080 | 0.070 | 0.004 | 0.743 |
| C20:3 n–3 | 0.050 | 0.050 | 0.050 | 0.001 | 0.913 |
| C20:4 n–6 | 0.600 a | 0.450 b | 0.450 b | 0.037 | <0.05 |
| SFA | 28.45 | 27.99 | 27.62 | 0.278 | 0.382 |
| UFA | 71.46 | 71.89 | 72.25 | 0.276 | 0.402 |
| MUFA | 37.09 a | 36.19 ab | 34.19 b | 0.528 | <0.05 |
| PUFA | 34.37 b | 35.70 ab | 38.06 a | 0.619 | <0.05 |
| DFA (UFA + C18:0) | 77.38 | 77.80 | 78.07 | 0.227 | 0.317 |
| OFA (C14:0 + C16:0) | 22.30 | 21.82 | 21.55 | 0.234 | 0.278 |
| n–6:n–3 | 30.17 a | 26.40 ab | 23.67 b | 0.600 | <0.05 |
| AI | 0.320 | 0.311 | 0.302 | 0.006 | 0.387 |
| TI | 0.731 | 0.712 | 0.682 | 0.018 | 0.849 |
| h/H | 3.08 | 3.18 | 3.26 | 0.108 | 0.989 |
| Leg muscles | |||||
| C14:0 | 0.110 | 0.120 | 0.110 | 0.003 | 0.268 |
| C16:0 | 21.41 | 21.66 | 21.24 | 0.091 | 0.156 |
| C18:0 | 4.72 b | 4.94 b | 5.69 a | 0.140 | <0.05 |
| C18:1 | 35.46 a | 34.60 a | 32.05 b | 0.521 | <0.05 |
| C18:2 n–6 | 32.83 b | 33.62 ab | 35.63 a | 0.476 | <0.05 |
| C18:3 n–3 | 1.65 | 1.42 | 1.61 | 0.054 | 0.174 |
| C20:0 | 0.070 b | 0.080 ab | 0.130 a | 0.009 | <0.05 |
| C20:1 | 0.100 a | 0.060 b | 0.040 b | 0.008 | <0.05 |
| C20:2 | 0.030 b | 0.030 b | 0.060 a | 0.004 | <0.05 |
| C20:3 n–3 | 0.030 | 0.020 | 0.020 | 0.002 | 0.113 |
| C20:4 n–6 | 0.320 a | 0.170 b | 0.310 a | 0.027 | <0.05 |
| SFA | 26.40 b | 26.94 ab | 27.35 a | 0.161 | <0.05 |
| UFA | 73.45 a | 72.94 ab | 72.46 b | 0.165 | <0.05 |
| MUFA | 38.59 a | 37.68 a | 34.83 b | 0.577 | <0.05 |
| PUFA | 34.86 b | 35.26 ab | 37.63 a | 0.491 | <0.05 |
| DFA (UFA + C18:0) | 78.17 | 77.88 | 78.15 | 0.083 | 0.287 |
| OFA (C14:0 + C16:0) | 21.52 | 21.78 | 21.35 | 0.093 | 0.152 |
| n–6:n–3 | 19.73 | 23.46 | 22.05 | 0.083 | 0.150 |
| AI | 0.301 | 0.300 | 0.300 | 0.006 | 0.365 |
| TI | 0.642 | 0.670 | 0.671 | 0.012 | 0.984 |
| h/H | 3.27 | 3.21 | 3.26 | 0.905 | 0.877 |
C—control, MT02—milk thistle (0%/2%—starter/grower), MT23—milk thistle (2%/3%—starter/grower), SEM—standard error of mean, n = 5, ab—means with different superscripts within a row are significantly different at p ≤ 0.05, SFA—saturated fatty acids, UFA—unsaturated fatty acids, MUFA—monounsaturated fatty acids, PUFA—polyunsaturated fatty acids, DFA—neutral and hypocholesterolemic fatty acids, OFA—hypercholesterolemic fatty acids, AI—atherogenicity index, TI—thrombogenicity index, h/H—hypocholesterolaemic/Hypercholesterolaemic ratio.
Feeding chickens with feed rations containing 2% (starter) and 3% (grower) of Silybum marianum significantly increased the share of stearic acid C18:0 (classified as neutral and hypocholesterolemic acid) in leg muscles only. Milk thistle introduced into chicken diets increased the content of linoleic acid (C18:2) in both evaluated muscle types, but a higher content of this acid was found in the muscles of chickens from group MT23 in comparison to the control group. Breast muscles of birds receiving feed rations with milk thistle in both rearing periods (MT23 group) contained by about 42% more linoleic acid than in group C (p < 0.05). A high (p < 0.05) content of PUFA was determined in the breast (38.06%) and leg (37.63%) muscles of chicken receiving feed rations containing ground seeds of Silybum marianum throughout the birds’ rearing period in comparison to group C. In addition, the breast muscles of birds from group MT23 featured a lower (p < 0.05) ratio of n–6:n–3 fatty acids in comparison to chickens fed rations without the phytobiotic.
The introduction of Silybum marianum into chicken diets did not affect the evaluated physical properties (pH, color, WHC) of thigh muscles, but it changed the color and WHC of breast muscles (Table 7).
Table 7.
Physical parameters of muscles.
| Parameters | Group | SEM | p–Value | ||
|---|---|---|---|---|---|
| C | MT02 | MT23 | |||
| Breast muscles | |||||
| pH15 | 6.27 | 6.17 | 6.30 | 0.315 | 0.218 |
| pH24 | 5.60 | 5.92 | 5.85 | 0.060 | 0.058 |
| L* | 53.4 a | 49.4 b | 50.8 ab | 0.589 | <0.05 |
| a* | 2.90 b | 5.17 a | 4.46 a | 0.284 | <0.05 |
| b* | 0.810 | 0.790 | 1.19 | 0.168 | 0.579 |
| C = [(a*)2 + (b*)2]0.5 | 3.08 b | 5.29 a | 4.71 a | 0.280 | <0.05 |
| H = log(b*/a*) | 0.290 | 0.140 | 0.300 | 0.046 | 0.327 |
| WHC (%) | 7.46 b | 11.0 a | 12.7 a | 0.970 | <0.05 |
| Thigh muscles | |||||
| pH15 | 6.02 | 6.20 | 6.05 | 0.038 | 0.114 |
| pH24 | 6.00 | 5.93 | 5.98 | 0.033 | 0.659 |
| L* | 50.7 | 51.5 | 53.2 | 0.583 | 0.190 |
| a* | 3.34 | 2.64 | 2.72 | 0.290 | 0.591 |
| b* | 0.860 | 0.390 | 0.510 | 0.248 | 0.740 |
| C = [(a*)2 + (b*)2]0.5 | 3.80 | 2.99 | 2.86 | 0.278 | 0.342 |
| H = log(b*/a*) | 0.320 | 0.135 | 0.184 | 0.106 | 0.773 |
| WHC (%) | 5.50 | 5.56 | 6.97 | 0.637 | 0.644 |
C—control, MT02—milk thistle (0%/2%—starter/grower), MT23—milk thistle (2%/3%–starter/grower), SEM—standard error of mean, n = 10, ab—means with different superscripts within a row are significantly different at p ≤ 0.05, L*—lightness, a*—redness, b*—yellowness, C—chroma, H—hue, WHC—water holding capacity.
Breast muscles of chickens from group MT02—fed with rations containing the evaluated phytobiotic at the grower stage only—were darker (p < 0.05). In addition, the use of milk thistle in the diets of birds intensified meat color toward red (a*) and increased chroma (C) of breast muscles compared to the C group. The water holding capacity (WHC) was lower (p < 0.05) in the breast muscles of chickens receiving diets containing Silybum marianum.
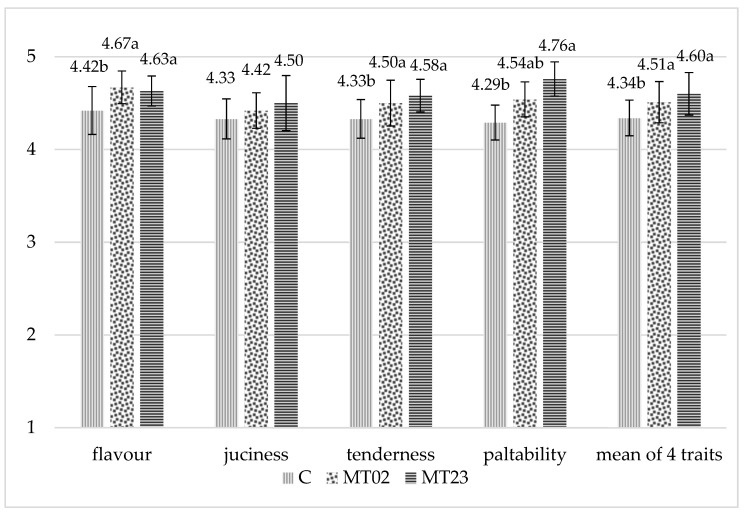
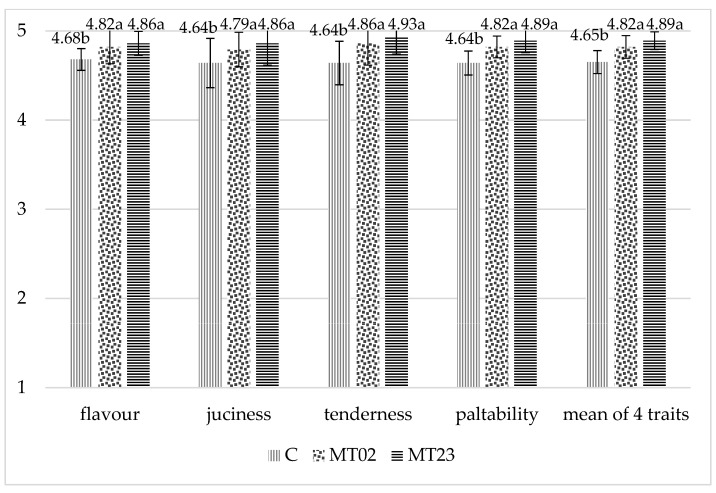
Adding ground seeds of milk thistle both in starter diets (20 g/kg) and grower diets (30 g/kg) and only in grower diets (20 g/kg) had a significant (p < 0.05) impact on the evaluated taste characteristics (flavor, tenderness, palatability, juiciness) of breast and thigh muscles, except on juiciness in breast muscles (p > 0.05) (Figure 1 and Figure 2).
Figure 1.
Sensory evaluation of breast muscles (point). C—control, MT02—milk thistle (0%/2%—starter/grower), MT23—milk thistle (2%/3%—starter/grower), SEM—standard error of mean, n = 8, a,b—means with different superscripts within a row are significantly different at p ≤ 0.05.
Figure 2.
Sensory evaluation of thigh muscles (point). C—control, MT02—milk thistle (0%/2%—starter/grower), MT23—milk thistle (2%/3%—starter/grower), SEM—standard error of mean, n = 8, a,b—means with different superscripts within a row are significantly different at p ≤ 0.05.
Breast and thigh muscles of chickens fed with rations containing milk thistle in both rearing periods (group MT23) scored the highest.
4. Discussion
The content of total protein in the evaluated seeds of milk thistle (Silybum marianum [L.] Gaertn.) ranged from 161 to 250 g/kg as reported by many researchers [21,22,28,65]. In turn, a higher content of protein in milk thistle seeds (up to 30.09%), depending on the variety and origin, was found by Aziz et al. [24]. In the opinion of Grela et al. [21] and Aziz et al. [24] milk thistle seeds are not only a source of protein but also energy, which is corroborated by the level (up to 24.8%) of crude fat determining the energy value of the raw material. A lower (17.5–21.6%) content of fat in milk thistle seeds was demonstrated by Růžičková et al. [66], and a higher content (26.05–30.5%) of this component was found in the experiments by [25,26]. According to [25,26,67,68], the content of crude fat in milk thistle seeds depends on many factors such as: agricultural engineering, the environment, variety, and year of harvest. The determined content of crude fiber in the evaluated seeds amounted to 41.3 g/kg, which was below the range of 45.6–54.6 g/kg reported by Grela et al. [23].
An analysis of the fatty acids profile of Silybum marianum seeds showed a high level of unsaturated fatty acids, including linoleic acid (54.64% FA) and oleic acid (23.59% FA). Similar content of fatty acids was measured by Garaev et al. [69], Růžičková et al. [66] and Harrabi et al. [26], who in the evaluated samples of milk thistle seeds determined 50.58–66.4% of linoleic acid, 16.26–25.44% of oleic acid, 7.24–9.20% of palmitic acid and 3.56–5.92% of stearic acid. The above-mentioned higher shares of linoleic acid (64.4%) and oleic acid (26.38%) in the lipids of Silybum marianum seeds were found by Khan et al. [25], whereas a lower (45.36% and 39%) share of linoleic acid but a higher (31.58% and 36%) share of oleic acid was determined by Hasanloo et al. [70] and Majidi et al. [27], respectively.
Grela at al. [23] demonstrated a share of MUFA (24.98% vs. 24.60% FA) and that of PUFA (55.56% vs. 54.99% FA), which is similar to the level determined by the present authors. Wierzbowska et al. [68] found that the share of polyunsaturated fatty acids (PUFA), including linoleic acid, was lower than in own studies. In turn, Kralik et al. [47] noted a higher content (63.11%) of PUFA in milk thistle seeds. Big differences in the share of respective fatty acids in the seeds of Silybum marianum depending on the year of harvest were revealed by Sadowska et al. [28].
A positive impact on weight gain and feed conversion after introducing milk thistle into the feed rations for broiler chickens was observed by Tedesco et al. [31] and Muhammad et al. [42]. Mojahedtalab et al. [45] noted a linear improvement in body weight gain with a decreasing conversion of feed and an increasing content of silymarin in feed rations for broiler chickens. Similarly, Ahmad et al. [20] showed that overall body weight and feed conversion ratio were significantly (p < 0.5) higher for group MT–15 (rations 15 g/kg of milk thistle) compared with other experimental groups. In turn, Gharahveysi [44] found that the feed intake and body weight of chickens decreased as the share of milk thistle in their diet increased. Feed intake in diets containing 0.3% or 3% of Silybum marianum was 3.440 and 3.407 kg, respectively, and body weight was 1.810 and 1.793 kg, respectively. Other authors who demonstrated a reduction in the productivity of broiler chickens after including Silybum marianum in their diets were Suchý et al. [41], Kalantar et al. [71] and Šťastník et al. [43]. Suchý et al. [41] found that the use of 0.2% or 1% milk thistle seed expeller in feed rations for Ross 308 broilers resulted in decreasing weight gain and impaired feed conversion. Similarly, Kalantar et al. [71] showed that 0.5% Silybum marianum introduced into feed rations for broiler chickens decreased (by about 10%) daily weight gain and increased (by 4.5%) the feed conversion rate (FCR) in comparison to birds fed diets without milk thistle. The weight gain of chickens decreased after using 5% or 15% of milk thistle (Silybum marianum) oil cake in feed rations, as noted by Šťastník et al. [43].
A decreased carcass yield after adding Silybum marianum to broiler diets was observed by Schiavone et al. [46] and Šťastník et al. [43]. Šťastník et al. [43] using 5% or 15% of milk thistle seeds in feed rations observed a decrease in carcass yield by 3.86% and 4.22% of carcass yield compared to control chickens. A linear decrease in carcass yield accompanied by an increase in the share of milk thistle in the diets of birds was also obtained by Schiavone et al. [46]. On the other hand, an increase (p < 0.05) in the dressing percentage of chickens after adding Silybum marianum in the amount of 15 g/kg of feed rations was observed by Ahmad et al. [20].
In the production of broilers breast muscle weight in relation to carcass weight is of economic significance. Breast muscles account for about 30% of edible meat in the whole carcass [72], which is corroborated by the results of own studies. The absence of any impact of Silybum marianum in broiler chicken diets on the share of breast muscles in the carcass is consistent with the findings of Šťastník et al. [43] and Rashidi et al. [49]. Rashidi et al. [49] also demonstrated that a share (0.5%, 1%, 1.5% and 2%) of Silybum marianum in diets had no impact on the share of abdominal fat. A significant decrease in the share of abdominal fat in the carcasses of birds fed with rations containing milk thistle extract (250 mg/kg) was found by Tavakolinasab et al. [7].
Šťastník et al. [43] reports that broilers receiving feed rations with a higher (15%) content of milk thistle had a higher (2.69% vs. 2.3%) share of liver than those fed with diets free from and containing 5% of Silybum marianum. Similarly, Tavakolinasab et al. [7] report that introducing milk thistle extract in the amount of 250 mg/kg of chicken diet leads to an increase (by 0.21%) in the share of liver.
Poultry meat available on the market should be of proper quality, perceived by consumers as a collection of many features, including nutritional value as one of the most important characteristics [73,74,75]. Poultry meat is a source of complete animal protein that—according to FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization)—is equivalent to milk protein. In addition, its energy value is lower [76,77].
The absence of an impact of milk thistle in broiler chicken feed on the total protein content in breast and leg muscles corroborates the results obtained by Šťastník et al. [48]. However, own studies showed a significant reduction in the amount of crude fat contained in the leg muscles of birds receiving feed rations with milk thistle in comparison to the control group. Schiavone et al. [46] studied chemical properties of breast meat and showed that the application of 40 and 80 ppm dried extract of milk thistle fruit in broiler feed (1.19% and 1.74% in fresh weight, respectively) significantly reduced fat content (2.15%). Thigh fat decreased in birds fed 40 ppm dried Silybum marianum extract (3.81% live weight) compared to a control group (4.79% live weight) and birds fed 80 ppm dried SM fruit extract (4.22% live weight). Dry matter, protein and ash content of breast and thigh meat did not significantly differ between the control and dried Silybum marianum extract fed groups. In turn, Grela et al. [21] analyzing the content of crude fat in the longissimus lumborum muscle of fatteners fed with rations containing 3% or 6% of milk thistle seeds noted a significant reduction in the content of this ingredient only when the content of milk thistle in the diet was reduced.
Own studies showed an increased share of stearic and linoleic acids in the muscles of chickens receiving feed rations containing milk thistle seeds. In turn, Kralik et al. [47], using feed rations with 3% of milk thistle oil for slaughter chickens, noted a decreased share of those acids in the breast and leg muscles. On the other hand, Grela et al. [21], having introduced 3% or 6% of milk thistle seeds in the diets of fatteners, noted a significant decrease in the share of stearic acid and an increased content of linoleic acid in the longissimus lumborum muscle. The increased share of PUFA in the muscles of birds fed with diets containing Silybum marianum is contrary to the findings of Kralik et al. [47] but consistent with those of Grela et al. [21] in studies involving fatteners. In addition, the values of atherogenicity index (AI), thrombogenicity index (TI) and hypo- and hypercholesterolemic ratio (h/H) in the breast muscles of chickens receiving experimental feed rations corroborated the tendency noted in the studies by Grela et al. [21].
The studies and experiments carried out by Kralik et al. [47] and Šťastník et al. [48] showed different impacts of diets containing milk thistle on the acidity of broiler chickens’ meat. The results did not corroborate an impact of the nutrition used on the reaction of breast and leg muscles, while Kralik et al. [47] found a decrease in the initial pH and an increase in the final pH of breast muscles after adding 3% of oil to the diet. In turn, Šťastník et al. [48] noted an increased pH of breast muscles after adding 5% or 15% (of Silybum marianum) to broiler chicken feed rations.
Color is an important attribute taken into account by consumers when buying meat, and an important element of evaluating meat dishes during their consumption [78]. Meat of darker color due to a higher share of oxidized myoglobin, is less desired by consumers [75]. Broiler meat color depends on the genotype and age [79], animal technology conditions, and feeding regime [80]. Numerous studies [75,81,82] showed that the higher the pH of meat is, the darker its color and vice versa. Extremely high pH leads to DFD and low to PSE meat defect [83].
The obtained L color values in the evaluated muscles were characteristic of normal muscles, since Van Laack et al. [84] classified breast muscle tissue as normal (CIE L* ≤ 55.0) and lighter than normal (CIE L* > 60.0). A lighter L* color (61.25 and 62.58) and the yellow saturation (13.19 and 13.51) of breast muscles immediately after using 5% and 15% of Silybum marianum was observed by Šťastník et al. [48]. In turn, red saturation (4.86 and 5.16) corresponded to own results for breast muscles of birds fed with rations containing ground milk thistle seeds. In turn, Kralik et al. [47], having added milk thistle oil to the feed rations, demonstrated a lower (1.66) value for parameter a* and a higher (12.01) value for parameter b* in the breast muscles compared to the results of own studies.
The WHC results obtained for muscles of chickens fed with rations containing Silybum marianum point to an impairment of the water holding capacity, but Kralik et al. [47] noted a positive impact of milk thistle seeds on drip loss.
In these studies, a positive impact of diets containing milk thistle seeds on the organoleptic (sensory) characteristics of muscles was noted. However, Šťastník et al. [48], having introduced 5% or 15% of milk thistle into chicken diets, demonstrated a deteriorated flavor score for both types of muscles, although the difference was confirmed to be statistically significant only in the breast muscles of chickens receiving 5% Silybum marianum in diets as compared to other groups.
Higher notes for respective sensory characteristics of thigh muscles should be associated with a higher content of fat in comparison to breast muscles. Komprda et al. [85] underlined that leg meat contains more fat and flavour substances, and is thus a preferred consumer choice. According to Nowak and Trziszka [73], in selecting meat its palatability and nutritional value are equally important.
5. Conclusions
Based on the results of studies, the use of milk thistle seeds in broiler chicken starter/grower diets can be recommended in the amount of 2/3%. The introduction of Silybum marianum in both types of feed rations made it possible to obtain the highest body weight at the lowest feed conversion per body weight gain unit, without any influence on muscularity and fattening grade. At the same time, muscles of chickens fed diets containing the phytobiotic featured the healthiest fatty acid profile and good taste characteristics.
Author Contributions
Conceptualization, A.J. and D.P.; methodology, A.J. and A.M.; software, A.M.; validation, A.J.; formal analysis, A.M.; investigation, D.P. and A.J.; resources, A.J. and D.P.; data curation, A.J.; writing—original draft preparation, A.J., D.P. and A.M.; writing—review and editing, A.J. and A.M.; visualization, A.M.; funding acquisition, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Poland.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the slaughter of birds was carried out in accordance with the applicable rules on the handling of animals at the time of slaughter, including humane treatment. Additionally, the methods used in the meat quality tests were carried out in accordance with the current and commonly used methodology de-scribed in the Material and methods section. Accordingly to directive no. 2010/63/EU the approval of the Ethics Committee was not required.
Data Availability Statement
The data are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alçiçek A., Bozkurt M., Çabuk M. The effect of mixture of herbal essential oils, an organic acid or a probiotic on broiler performance. S. Afr. J. Anim. Sci. 2004;34:217–222. [Google Scholar]
- 2.Skomorucha I., Sosnówka-Czajka E. Effect of water supplementation with herbal extracts on broiler chicken welfare. Ann. Anim. Sci. 2013;13:849–857. doi: 10.2478/aoas-2013-0057. [DOI] [Google Scholar]
- 3.Radulović N.S., Mladenović M.Z., Randjelovic P.J., Stojanović N.M., Dekić M.S., Blagojević P.D. Toxic essential oils. Part IV: The essential oil of Achillea falcata L. as a source of biologically/pharmacologically active trans-sabinyl esters. Food Chem. Toxicol. 2015;80:114–129. doi: 10.1016/j.fct.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Laskowski S., Banaszkiewicz T., Milczarek A. Influence of oregano added to diets on performance, selected organs as well as morphometric traits and pH of digestive tract of broiler chickens. Med. Weter. 2017;73:781–785. doi: 10.21521/mw.5820. [DOI] [Google Scholar]
- 5.Zhang Z.S., Wang S., Liu H., Li B.Z. Constituents and thermal properties of milk thistle seed oils extracted with three methods. LWT Food Sci. Technol. 2020;126:109282. doi: 10.1016/j.lwt.2020.109282. [DOI] [Google Scholar]
- 6.Hippenstiel F., Abdel-Wareth A.A.A., Kehraus S., Südekum K.-H. Effect of selected herbs and essential oils, and their active components on feed intake and performance of broilers—A review. Arch. Geflugelkd. 2011;75:226–234. [Google Scholar]
- 7.Tavakolinasab F., Khosravinia H., Masouri B. Effects of Milk Thistle, Artichoke and Olive Extracts in Comparison with Atorvastatin and Gemfibrozil on Liver Function in Broiler Chicken. Poult. Sci. J. 2020;8:109–117. doi: 10.36478/javaa.2020.18.25. [DOI] [Google Scholar]
- 8.Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- 9.Stastnik O., Pavlata L., Mrkvicova E. The Milk Thistle Seed Cakes and Hempseed Cakes are Potential Feed for Poultry. Animals. 2020;10:1384. doi: 10.3390/ani10081384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak A., Florkowska K., Zielonka-Brzezicka J., Duchnik W., Muzykiewicz A., Klimowicz A. The effects of extraction techniques on the antioxidant potential of extracts of different parts of milk thistle (Silybum marianum L.) Acta Sci. Pol. Technol. Aliment. 2021;20:37–46. doi: 10.17306/J.AFS.0817. [DOI] [PubMed] [Google Scholar]
- 11.Brzóska F., Śliwiński B., Michalik-Rutkowska O. Effect of herb mixture on productivity, mortality, carcass quality and blood parameters of broiler chickens. Ann. Anim. Sci. 2010;10:157–165. [Google Scholar]
- 12.Wallace R.J., Oleszek W., Franz C., Hahn I., Baser K.H.C., Mathe A., Teichmann K. Dietary plant bioactives for poultry health and productivity. Brit. Poultry Sci. 2010;51:461–487. doi: 10.1080/00071668.2010.506908. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed A., Mangaiyarkarasi R., Shahid N., Umar M., Shahina N., Rahmanullah S., Zahra Y. Effect of black tea extract (polyphenols) on performance of broilers. Int. J. Adv. Res. 2013;1:563–566. [Google Scholar]
- 14.Khan S.H. The use of green tea (Camellia sinensis) as a phytogenic substance in poultry diets. Onderstepoort J. Vet. Res. 2014;81:1–8. doi: 10.4102/ojvr.v81i1.706. [DOI] [PubMed] [Google Scholar]
- 15.Abdullah A.Y., Mahmoud K.Z., Nusairat B.M., Qudsieh R.I. Small intestinal histology, production parameters, and meat quality as influenced by dietary supplementation of garlic (Allium sativum) in broiler chicks. Ital. J. Anim. Sci. 2010;9:414–419. doi: 10.4081/ijas.2010.e80. [DOI] [Google Scholar]
- 16.Jahanian E., Mahdavi A.H., Asgary S., Jahanian R. Effects of dietary inclusion of silymarin on performance, intestinal morphology and ileal bacterial count in aflatoxin-challenged broiler chicks. J. Anim. Physiol. Anim. Nutr. 2016;101:e43–e54. doi: 10.1111/jpn.12556. [DOI] [PubMed] [Google Scholar]
- 17.Zaker-Esteghamati H., Seidavi A.R., Bouyeh M. A review on the effect of Silybum marianum and its derivatives on broilers under healthy and aflatoxicosis conditions: Part 1: Performance, carcass and meat characteristics, and intestinal microflora. World Poult. Sci. J. 2020;76:318–327. doi: 10.1080/00439339.2020.1740068. [DOI] [Google Scholar]
- 18.Gregačević L., Klarić I., Domaćinović M., Galović D., Ronta M. Fitogeni aditivi u hranidbi domaćih životinja. Krmiva. 2014;56:117–123. [Google Scholar]
- 19.Stopyra A., Kuleta Z., Tomczyński R., Sobiech P., Kędzierska K. Sylibum marianum in horses feeding. Ann. UMCS Lub. Polonia. Sect. DD. 2006;LXI:95–102. [Google Scholar]
- 20.Ahmad M., Chand N., Khan R.U., Ahmad N., Khattak I., Naz S. Dietary supplementation of milk thistle (Silybum marianum): Growth performance, oxidative stress, and immune response in natural summer stressed broilers. Trop. Anim. Health Pro. 2020;52:711–715. doi: 10.1007/s11250-019-02060-4. [DOI] [PubMed] [Google Scholar]
- 21.Grela E.R., Świątkiewicz M., Florek M., Wojtaszewska I. Impact of milk thistle (Silybum marianum L.) seeds in fattener diets on pig performance and carcass traits and fatty acid profile and cholesterol of meat, backfat and liver. Livest. Sci. 2020;239:10418. doi: 10.1016/j.livsci.2020.104180. [DOI] [Google Scholar]
- 22.Sadowska K. Fruits of milk thistle as a health-enhancing additive to bakery products. Zywn Nauk. Technol. Ja. 2006;2:290–296. [Google Scholar]
- 23.Grela E.R., Pietrzak K., Pecka S., Sobolewska S., Krasucki W. Ostropest plamisty w żywieniu zwierząt. Przegląd Hod. 2014;5:38–40. [Google Scholar]
- 24.Aziz M., Saeed F., Ahmad N., Ahmad A., Afzaal M., Hussain S., Mohamed A.A., Alamri M.S., Anjum F.M. Biochemical profile of milk thistle (Silybum Marianum L.) with special reference to silymarin content. Food Sci. Nutr. 2021;9:244–250. doi: 10.1002/fsn3.1990. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Khan I., Khattak H.U., Ullah I., Bangash F.K. Study of the physicochemical properties of Silybum marianum seeds oil. J. Chem. Soc. Pak. 2007;29:545–548. [Google Scholar]
- 26.Harrabi S., Romdhane H., Daassa M., Fellah H. Fatty acid and triacylglycerol compositions of milk thistle seeds growing wild in Tunisia (Silybum marianum L.) Acta Aliment. 2015;44:304–310. doi: 10.1556/066.2015.44.0007. [DOI] [Google Scholar]
- 27.Majidi M.M., Shafiei-Koij F., Pirnajmedin F., Jami M., Radan Z. Fatty acid profile, silymarin content and production properties of milk thistle (Silybum marianum) germplasm under different water environments. Crop. Pasture Sci. 2021;72:302–310. doi: 10.1071/CP20489. [DOI] [Google Scholar]
- 28.Sadowska K., Andrzejewska J., Woropaj-Janczak M. Effect of weather and agrotechnical conditions on the content of nutrients in the fruits of milk thistle (Silybum marianum L. Gaertn) Acta Sci. Pol. Hortorum Cultus. 2011;10:197–207. [Google Scholar]
- 29.Kazazis C.E., Evangelopoulos A.A., Kollas A., Vallianou N.G. The therapeutic potential of milk thistle in diabetes. Rev. Diabet. Stud. 2014;11:167–174. doi: 10.1900/RDS.2014.11.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szczucińska A., Lipkowski A.W., Baranowska B., Walisiewicz-Niedbalska W., Różycki K., Maciuszczak-Kotlarek H. Utilisation of milk thistle seed waste. I. Milk thistle oil as antioxidant. Rośliny Oleiste Oilseed Crop. 2003;XXIV:717–724. [Google Scholar]
- 31.Tedesco D., Steidler S., Galletti S., Tameni M., Sonzogni O., Ravarotto L. Efficacy of silymarin-phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poult. Sci. 2004;83:1839–1843. doi: 10.1093/ps/83.11.1839. [DOI] [PubMed] [Google Scholar]
- 32.Bijak M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—chemistry, bioavailability, and metabolism. Molecules. 2017;22:1942. doi: 10.3390/molecules22111942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhidary I.A., Rehman Z., Khan R.U., Tahir M. Anti-aflatoxin activities of milk thistle (Silybum marianum) in broiler. World Poult. Sci. J. 2017;73:559–566. doi: 10.1017/S0043933917000514. [DOI] [Google Scholar]
- 34.Ptasznik A. The role of silymarin in prevention and treatment of liver diseases. Post. Fitoter. 2004;4:189–190. [Google Scholar]
- 35.Fanoudi S., Sadat Alavi M., Karimi G., Hosseinzadeh H. Milk thistle (Silybum Marianum) as an antidote or a protective agent against natural or chemical toxicities: A review. Drug Chem. Toxicol. 2020;43:240–254. doi: 10.1080/01480545.2018.1485687. [DOI] [PubMed] [Google Scholar]
- 36.Valkova V., Duranova H., Bilcikova J., Haban M. Milk thistle (Silybum marianum): A valuable medicinal plant with several therapeutic purposes. J. Microbiol. Biotechnol. Food Sci. 2020;9:836–843. doi: 10.15414/jmbfs.2020.9.4.836-843. [DOI] [Google Scholar]
- 37.Radko L., Cybulski W. Application of silymarin in human and animal medicine. J. Pre. Clin. Clin. Res. 2007;1:22–26. [Google Scholar]
- 38.Geberemeskel G.A., Debebe Y.G., Nguse N.A. Antidiabetic effect of fenugreek seed powder solution (Trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. J. Diabetes Res. 2019;5:8507453. doi: 10.1155/2019/8507453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagy J., Such N., Koltay I.A., Molnar A., Farkas V., Dublecz K., Rozsa L., Pal L. Health protecting effects of milk thistle (Silybum marianum) Literature review. Magy. Allatorvosok. 2020;142:229–240. [Google Scholar]
- 40.Saadh M.J. Hypoglycemic and hypolipidemic activity of combined milk thistle and fenugreek seeds in alloxan-induced diabetic albino rats. Vet. World. 2020;13:1732–1736. doi: 10.14202/vetworld.2020.1732-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suchý P., Straková E., Kummer V., Herzig I., Písaříková V., Blechová R., Mašková J. Hepatoprotective Effects of Milk Thistle (Silybum marianum) Seed Cakes during the Chicken Broiler Fattening. Acta Vet. Brno. 2008;77:31–38. doi: 10.2754/avb200877010031. [DOI] [Google Scholar]
- 42.Muhammad D., Chand N., Khan S., Sultan A., Mushtaq M. Hepatoprotective role of milk thistle (Silybum marianum) in meat type chicken fed aflatoxin B1 contaminated feed. Pak. Vet. J. 2012;32:443–446. [Google Scholar]
- 43.Šťastník O., Detvanova L., Karásek F., Štenclová H., Kalhotka L., Pavlata L., Mrkvicová E. The influence of milk thistle seed cakes on broiler chickens performance parameters. Mendel Net. 2015;11:152–156. [Google Scholar]
- 44.Gharahveysi S.H. Effects of Milk Thistle Powder on Performance, Blood Parameters and Liver Enzymes of Broiler Chickens. J. Anim. Prod. 2017;19:879–889. [Google Scholar]
- 45.Mojahedtalab A., Mohammadi M., Roostaei Ali Mehr M., Asadi M. Effect of silymarin on performance and immune responses of broilers. J. Anim. Physiol. Anim. Nutr. 2013;2:49–58. doi: 10.1111/jpn.12431. [DOI] [PubMed] [Google Scholar]
- 46.Schiavone A., Righi F., Quarantelli A., Bruni R., Serventi P., Fusari A. Use of Silybum marianum fruit extract in broiler chicken nutrition: Influence on performance and meat quality. J. Anim. Physiol. Anim. Nutr. 2007;91:256–262. doi: 10.1111/j.1439-0396.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- 47.Kralik G., Kralik Z., Straková E., Šperanda M., Kralik I., Strelec I. Influence of dietary replacement of sunflower oil with milk thistle (Silybum marianum) oil on chicken meat quality and antioxidant status of liver. Acta Vet. Brno. 2015;84:373–382. doi: 10.2754/avb201584040373. [DOI] [Google Scholar]
- 48.Šťastník O., Jůzl M., Karásek F., Štenclová H., Nedomová Š., Pavlata L., Mrkvicová E., Doležal P., Jarošová A. The effect of feeding milk thistle seed cakes on quality indicators of broiler chickens meat. Potr. Slovak J. Food Ind. 2016;10:248–254. doi: 10.5219/579. [DOI] [Google Scholar]
- 49.Rashidi N., Bujarpoor M., Chaji M., Aghaei A. Effect of Silybum marianum seed on performance, carcass characteristics and blood parameters of broiler chickens. Anim. Prod. Res. 2015;3:11–21. [Google Scholar]
- 50.Janssen W.M.M.A. European Table of Energy Values for Poultry Feedstuffs. 3rd ed. Working Group No. 2 of the European Federation of BranchEs of the World’s Poultry Science Association; Beekbergen, The Netherlands: 1989. [Google Scholar]
- 51.Ziołecki J., Doruchowski W. The Method of Estimation of Poultry Slaughter Analysis. COBRD; Poznań, Poland: 1989. p. 22. [Google Scholar]
- 52.Vassiliou A. Commission Regulation (EC) No 152/2009 of 27 January 2009 Laying Down the Methods of Sampling and Analysis for the Official Control of Feed. Official Journal of the European Union L 54/1; Brussels, Belgium: Feb 26, 2009. [Google Scholar]
- 53.AOAC . Official Methods of Analysis of the Association of Official Analytical Chemists. 15th ed. Association of Official Analytical Chemists, Inc.; Washington, DC, USA: 1990. Chapter 32. [Google Scholar]
- 54.ISO 9831 . Animal Feeding Stuffs, Animal Products, and Faeces or Urine—Determination of Gross Calorific Value—Bomb Calorimeter Method. International Organization for Standardization; Geneva, Switzerland: 2005. [Google Scholar]
- 55.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 56.Ulbricht T.L.V., Southgate D.A.T. Coronary heart disease: Seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-M. [DOI] [PubMed] [Google Scholar]
- 57.Santos-Silva J., Bessa R.J.B., Santos-Silva F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2002;77:187–194. doi: 10.1016/S0301-6226(02)00059-3. [DOI] [Google Scholar]
- 58.Jurczak M.E. Towaroznawstwo Produktów Zwierzęcych: Ocena Jakości Mięsa. SGGW; Warszawa, Poland: 2005. pp. 117–119. [Google Scholar]
- 59.CIE . Draft Standard 014-4.3/E: Colorimetry—Part. 4: CIE 1976 L*a*b* Colour Space. CIE Central Bureau; Vienna, Austria: 2007. p. 8. [Google Scholar]
- 60.Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/S0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 61.Milczarek A., Osek M. Effectiveness evaluation of use of various protein feeds for broiler chicken feeding. Ann. Anim. Sci. 2019;19:1063–1081. doi: 10.2478/aoas-2019-0056. [DOI] [Google Scholar]
- 62.Baryłko-Pikielna N. Zarys Analizy Sensorycznej Żywności. WNT; Warszawa, Poland: 1975. pp. 183–188. [Google Scholar]
- 63.Baryłko-Pikielna N., Matuszewska I. Sensory Analysis of Food. Basics-Methods-Applications. 2nd ed. Scientific Publishing PSFT; Kraków, Poland: 2014. pp. 166–180. [Google Scholar]
- 64.StatSoft Inc. Statistica (Data Analysis Software System), Version 13.1. StatSoft Inc.; Tulsa, OK, USA: 2019. [Google Scholar]
- 65.Hermenean A., Stan M., Ardelean A., Pilat L., Mihali C.V., Popescu C., Nagy L., Deák G., Zsuga M., Kéki S., et al. Antioxidant and hepatoprotective activity of milk thistle (Silybum marianum L. Gaertn.) seed oil. Open Life Sci. 2015;10:225–236. doi: 10.1515/biol-2015-0017. [DOI] [Google Scholar]
- 66.Růžičková G., Fojtová J., Součková M. Výnos a kvalita oleje plodů ostropestřce mariánského (Silybum marianum (L.)) z pohledu prostředí a genotypu—Pilotní studie (The yield and quality of milk thistle (Silybum marianum (L). Gaertn.) seed oil from the perspective of environment and genotype—A pilot study) Acta Fytotech. Zootech. 2011;1:9–12. [Google Scholar]
- 67.Andrzejewska J., Sadowska K., Mielcarek S. Effect of sowing date and rate on the yield and flavonolignan content of the fruits of milk thistle (Silybum marianum L. Gaertn.) grown on light soil in a moderate climate. Ind. Crop. Prod. 2011;33:462–468. doi: 10.1016/j.indcrop.2010.10.027. [DOI] [Google Scholar]
- 68.Wierzbowska J., Bowszys T., Sternik P. Effect of mineral fertilization on the content and quality of fat in the achenes of milk thistle (Sylibum marianum L. Gaertner) Rośliny Oleiste Oilseed Crops. 2012;XXXIII:99–111. [Google Scholar]
- 69.Garaev E.A., Movsumov I.S., Gazizov F.Y. Neutral lipids from Silybum marianum seeds. Chem. Nat. Compd. 2010;46:629–630. doi: 10.1007/s10600-010-9694-2. [DOI] [Google Scholar]
- 70.Hasanloo T., Bahmanei M., Sepehrifar R., Kalantari F. Determination of tocopherols and fatty acids in seeds of Silybum marianum (L.) Gaerth. J. Med. Plants. 2008;7:69–75. [Google Scholar]
- 71.Kalantar M., Salary J., Nouri Sanami M., Khojastekey M., Hemati Matin H.R. Dietary supplementation of Silybum marianum or Curcuma spp. on health characteristics and broiler chicken performance. Glob. J. Anim. Sci. Res. 2014;2:58–63. [Google Scholar]
- 72.Śliwa J., Brzóska F. Effect of diets with non-GM soybean expeller on body weight, carcass quality and amino aciddigestibility in broiler chickens. Rocz. Nauk. Zootech. 2018;45:59–87. [Google Scholar]
- 73.Nowak M., Trziszka T. Consumer behavior on the poultry meat market. Zywn Nauk. Technol. Ja. 2010;1:114–120. [Google Scholar]
- 74.Tougan P.U., Dahouda M., Salifou C.F., Ahounou S.G., Kpodekon M.T., Mensah G.A., Thewis A., Karim I.Y. Conversion of chicken muscle to meat and factors affecting chicken meat quality: A review. Int. J. Agron. Agric. Res. 2013;3:1–20. [Google Scholar]
- 75.Zdanowska-Sąsiadek Ż., Michalczuk M., Marcinkowska-Lesiak M., Damaziak K. Factors determining the sensory quality of poultry meat. Bromatol. Chem. Toksyk. 2013;LVI:344–353. [Google Scholar]
- 76.Rycielska J., Jarosiewicz K., Słowiński M. Influence of selected pre-slaughter factors on chicken meat quality. Med. Weter. 2010;66:770–773. [Google Scholar]
- 77.Kunachowicz H., Nadolna I., Przygoda B., Iwanow K. Food Composition Tables. PZWL; Warszawa, Poland: 2017. [Google Scholar]
- 78.Magdelaine P., Spiess M.P., Valceschini E. Poultry meat consumption trends in Europe. World Poult. Sci. J. 2008;64:53–63. doi: 10.1017/S0043933907001717. [DOI] [Google Scholar]
- 79.Mehaffey J.M., Pradhan S.P., Meullenet J.F., Emmert J.L., McKee S.R., Owens C.M. Meat quality evaluation of minimally aged broiler breast fillets from five commercial genetic strains. Poult. Sci. 2006;85:902–908. doi: 10.1093/ps/85.5.902. [DOI] [PubMed] [Google Scholar]
- 80.Janocha A., Milczarek A., Pietrusiak D., Łaski K. The effect of rations containing hulled or hull-less barley on the slaughter parameters and the quality of broiler chicken meat. J. Cent. Eur. Agric. 2020;21:508–516. doi: 10.5513/JCEA01/21.3.2699. [DOI] [Google Scholar]
- 81.Gornowicz E., Pietrzak M. Carcasse yield and breast muscles quality as affected by broiler chicken origin. Rocz. Inst. Przem. Mięs. Tł. 2008;46:95–104. [Google Scholar]
- 82.Milan R., Klaus D. The meaning of pH—value for the meat quality of broilers—Influence of breed lines. Tehnologija Mesa. 2010;51:120–123. [Google Scholar]
- 83.Milan R., Hansgeorg H., Klaus D. Meaning of the pH value for the meat quality of broilers. Fleischwirtschaft. 2011;91:89–93. [Google Scholar]
- 84.Van Laack R.L.J.M., Liu C.H., Smith M.O., Loveday H.D. Characteristics of pale, soft, exudative broiler breast meat. Poult. Sci. 2000;79:1057–1061. doi: 10.1093/ps/79.7.1057. [DOI] [PubMed] [Google Scholar]
- 85.Komprda T., Zelenka J., Drobná Z., Jarošová A., Fajmonová E. Sensory quality of meat of turkeys fed the diet with sunflower, linseed or fish oil. Arch. Geflugelkd. 2003;67:225–230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on request from the corresponding author.