Abstract
An important component of tissue engineering (TE) is the supporting matrix upon which cells and tissues grow, also known as the scaffold. Scaffolds must easily integrate with host tissue and provide an excellent environment for cell growth and differentiation. Human amniotic membrane (hAM) is considered as a surgical waste without ethical issue, so it is a highly abundant, cost-effective, and readily available biomaterial. It has biocompatibility, low immunogenicity, adequate mechanical properties (permeability, stability, elasticity, flexibility, resorbability), and good cell adhesion. It exerts anti-inflammatory, antifibrotic, and antimutagenic properties and pain-relieving effects. It is also a source of growth factors, cytokines, and hAM cells with stem cell properties. This important source for scaffolding material has been widely studied and used in various areas of tissue repair: corneal repair, chronic wound treatment, genital reconstruction, tendon repair, microvascular reconstruction, nerve repair, and intraoral reconstruction. Depending on the targeted application, hAM has been used as a simple scaffold or seeded with various types of cells that are able to grow and differentiate. Thus, this natural biomaterial offers a wide range of applications in TE applications. Here, we review hAM properties as a biocompatible and degradable scaffold. Its use strategies (i.e., alone or combined with cells, cell seeding) and its degradation rate are also presented.
Keywords: amniotic membrane, cells, biological scaffold, tissue engineering, repair, reconstruction
1. Introduction
Tissue engineering (TE) aims to induce tissue growth by combining cells, scaffolds, and growth factors or biomolecules [1]. Hence, the cells require the development of a scaffold from native or synthetic biomaterials, or a combination of the two, that mimics the extracellular matrix (ECM) [2]. The scaffold should have tissue integration properties and should be easily colonized with cells and be able to adhere, proliferate/survive, differentiate, and replicate the cell/tissue function [3]. The ideal scaffold requires easy handling and production, with biocompatibility, biodegradability, and mechanical properties consistent with the anatomical site of implantation. Depending on the application, it could be selectively permeable (to avoid invasion by fibrous tissue [4]) or porous (to ensure cellular penetration and adequate diffusion of nutrients to the cells within the construct and to the ECM formed by these cells [3]). When required, it should create and maintain space [4].
We have developed several TE products combining cells with biocompatible scaffolds [5,6]. We have been studying the benefit of the human amniotic membrane (hAM) in bone and nerve repair and in oral and maxillofacial surgery over the last few years [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24].
The hAM derived from the placenta is considered surgical waste that can be obtained after elective cesarean surgery. It is thus a highly abundant, readily available, and cost-effective biological tissue that does not raise ethical issues. Thanks to its unique biological properties, this natural membrane has been used for over a century in medicine, especially in the field of ophthalmology and dermatology [25,26]. hAM is known to display several biological properties that are able to promote wound healing. It is a biocompatible immune-privileged tissue that exerts an anti-inflammatory, antifibrotic, antimicrobial, and antimutagenic effect [27,28]. hAM is a source of growth factors, cytokines, and hAM cells with stem cell properties [26,29,30]. Moreover, it combines adequate mechanical properties (permeability, stability, elasticity, flexibility, resorbability) [31,32] with good cell adhesion capacity, thanks to its natural ECM structural components (hyaluronic acid, collagens, laminin, fibronectin, and proteoglycans) [33].
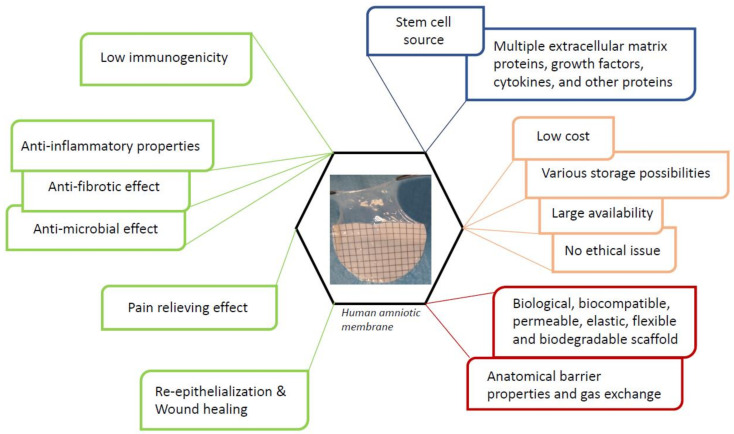
Consequently, hAM represents a “ready to use” TE product, containing inherent cells and growth factors [34,35,36] (Figure 1). In addition, it is a suitable natural scaffold for cell seeding, proliferation, and/or differentiation. Thus, hAM-based scaffolds have been developed to improve its healing capacity and, mostly, to produce a qualified TE construct.
Figure 1.
Human amniotic membrane properties as an ideal scaffold for tissue engineering.
The efficacy of hAM alone or combined with cells has been widely investigated in experimental and clinical studies. Therefore, its support function is emphasized by the development of hAM composites and commercial products [9,31,32,36]. The use of hAM cells in the TE field is more sporadic [37].
The purpose of this review is to describe hAM properties as a biocompatible and degradable scaffold for TE applications. Moreover, an overview of hAM used alone or combined with cells is presented. We also aim to explore cell seeding and the degradation rate of hAM as there is currently limited data in the literature.
2. Human Amniotic Membrane
2.1. Anatomy and Physiology
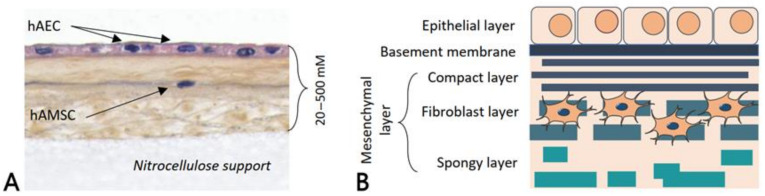
The human placenta plays a key role in the development and survival of the fetus, acting as physical and biological protection [38]. It is composed of two fetal membranes: an outer chorionic membrane and an inner hAM or amnion. hAM lines the amniotic cavity, in contact with the amniotic fluid. It contains three main layers: an epithelial monolayer that is separated from the stroma layer by a basement membrane (Figure 2) [26].
Figure 2.
(A) Histological staining of fresh human amniotic membrane. hAEC: human amniotic epithelial cell, hAMSC: human amniotic mesenchymal stromal cell. (B) Representative structure of human amniotic membrane. The epithelial side, which consists of a monolayer of hAECs, and the mesenchymal layer, composed of hAMSC. A thick basement membrane separates both sides.
The amniotic epithelium is characterized by a single layer of human amniotic epithelial cells (hAECs), which usually have a columnar or cuboidal shape. It has been reported that hAECs express stem cell markers, retain the pluripotency of the undifferentiated epiblast, and have pluripotency and the ability to differentiate toward all three germ layers [29,30,39]. hAECs are densely adherent to the basement membrane, which lies at their outer edge. These cells secrete collagen type III and IV as well as noncollagenous glycoproteins (laminins, nidogen, and fibronectin) that form the basement membrane of the hAM [40]. This basement membrane is one of the thickest found in humans, and it provides support to the fetus during gestation. The third layer, called the stroma layer, is a collagen-rich mesenchymal layer that contains three components: (i) a compact layer, which is a dense and almost acellular layer mainly composed of collagen type I and III and fibronectin; (ii) a fibroblastic layer, where fibroblast-like mesenchymal cells (human amniotic mesenchymal stromal cells (hAMSC)) and rare macrophages with a loose fibroblast network can be observed; and (iii) the outer layer, called the spongy layer because of the high quantity of proteoglycans and glycoproteins leading to a spongy appearance on histological sections [38,41,42]. This spongy layer is made of loosely arranged collagen fibers and separates the amniotic and chorionic mesoderm. Collagen types I, III, V, and VI, secreted by the hAMSC, are the major proteins of the ECM in the stroma layer [43]. Those cells meet MSC minimal criteria [29], with divergence about their pluripotency [30]: hAMSC lack markers associated with pluripotency, such as TRA-1-60 and TRA-1-81 [44], whereas pluripotency markers SSEA-3 and SSEA-4 were reported to be positive [45].
hAM is a translucent biological structure that is neither vascularized nor innervated. Nutrients and oxygen are provided by the surrounding chorionic fluid, amniotic fluid, and the fetal surface vessels through diffusion mechanisms [34].
2.2. Collection and Preservation Methods
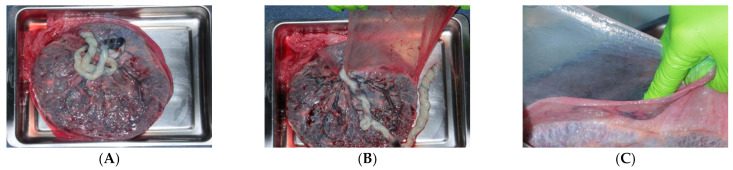
Placenta is generally obtained from healthy pregnant patients undergoing elective caesarian surgery after proper informed consent [26]. A rigorous serological screening must be performed on pregnant donors for human immunodeficiency virus-1/2, Hepatitis B, Hepatitis C, human T-cell lymphotropic virus, syphilis, cytomegalovirus, and tuberculosis. Placentas obtained from caesareans are the preferred source because placentas from vaginal deliveries can be contaminated and, therefore, unsuitable for transplantation. After the delivery, the collected placenta is placed in a sterile transport medium to avoid drying. Then the placenta is processed under aseptic conditions to obtain hAM. After repeated rinses of the placenta, the amnion is easily separated from the underlying chorion along their natural cleavage plane since the hAM spongy layer is loosely connected to the chorion (Figure 3). The placenta is routinely washed using a saline sterile solution containing antibiotics such as streptomycin, penicillin, neomycin, and amphotericin prior to storage [46].
Figure 3.
Human amniotic membrane collection. (A) Placenta. (B) Amnion and chorion. (C) Amnion detached from the chorion.
Long-time storage before use is recommended by regulatory agencies of many countries to avoid the possibility that the donor is in the “window period” of infection. Thus, several preserving methods, such as cryopreservation, freeze-drying (lyophilization), or air-drying, have been developed. Whatever the method used, the processing and preservation of hAM will affect the properties of the biological material [47].
Cryopreservation in glycerol, acting as a cryoprotectant, is the most commonly used preservation method. Several studies reported the use of dimethyl sulfoxide as an alternative solution to cryopreserve hAM [48]. The cryopreserved format is safe and efficient, as reported by many experimental and clinical studies. Cryopreservation allows better preservation of proteins and growth factors compared to lyophilization, which is especially important when the tissue contains few proteins [49]. However, cryopreservation has some limitations and impacts the viability of the hAM cells [11,14,50]. Moreover, it requires expensive and cumbersome equipment to freeze a high quantity of amniotic tissue to −80 °C. Moreover, storage cannot exceed several months. Another difficulty is the necessary respect of the cold chain, making transportation difficult [46,51].
Lyophilization or freeze-drying is a preservation technique that consists of removing water from tissue by the process of sublimation. This process induces some alterations concerning structure, biological, and physical properties [49]. However, it results in a decrease of destructive chemical reactions, avoiding tissue deterioration, and the samples can be stored safely for several years at room temperature [49,51,52]. Transport is simple, in contrast to cryopreserved hAM [52]. A pretreatment with trehalose prior to lyophilization has been proposed to improve its quality. As the water loss caused by lyophilization may affect the physical and biological structures of amnion, trehalose can replace some water content in the cells, and it might have a positive effect on the stabilization of proteins and other components [46,53].
Air-drying is another preservation technique that is low-cost, and the final product is easy to store at room temperature [47,54]. hAM is kept at room temperature under a laminar flow hood and exposed to air for different time periods.
Lyophilization and air-drying are usually followed by sterilization of the hAM by gamma-radiation [48]. Sterilization with peracetic acid has also been proposed as an alternative to gamma-radiation [55]. Both products can be easily cut to the desired size and shaped with scissors just before use [56,57]. In addition, the graft is ready to use, contrary to glycerol-preserved membranes that require thawing and rinsing for approximately 1 h.
Several enzymatic, chemical, or mechanical techniques have been developed for denuding hAM [46]. Indeed, denuded (or de-epithelized) amnion promotes better cell proliferation and differentiation, better structural integrity, and more uniform cell outgrowth compared to the intact format [58,59]. Hence, it has been the preferred choice for ocular surface reconstruction. Similarly, decellularization treatment has also been applied to hAM. It aims to remove the major immunogenic cellular components, membrane-associated antigens, and soluble proteins, thus preventing the initiation of a cell-mediated or humoral immune response and subsequent degradation and rejection after clinical implantation [60,61]. Decellularization results in a significant decrease in hAM thickness without significantly decreasing its ultimate tensile strength, extensibility, or elasticity [60]. Both de-epithelization and decellularization strategies could be applied to fresh and cryo-preserved hAM. They are mainly combined with lyophilization or air-drying.
2.3. Biological Properties
hAM is an immune-privileged tissue as it contains some immunoregulatory factors, such as HLA-G, which is an immunosuppressive factor, and the Fas ligand [28]. This effect is also supported by the low/absent level of expression of HLA class I molecules and the absence of HLA class II molecules [62], thereby avoiding allograft rejection. Several growth factors are produced by hAM cells, such as epidermal growth factor (EGF), keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and macrophage colony-stimulating factor (M-CSF) [63,64]. Moreover, hAM has an anti-inflammatory effect, driven by both hAECs and hAMSC, which express various antiangiogenic and anti-inflammatory proteins such as the interleukin (IL)-1 receptor antagonist, tissue inhibitors of metalloproteinase (TIMPs)-1, -2, -3, -4, and IL-10 [65]. It has both angiogenic and antiangiogenic properties [66]. A few studies have suggested that hAM cells may exert an anticancer effect [67,68], mainly explained by the antiangiogenic, proapoptotic, and immunoregulatory activities of amnion.
hAM is also known to induce an antiadhesive and antiscarring effect. It reduces protease activity via the secretion of tissue inhibitors of TIMPs, and downregulates the expression of transforming growth factor beta (TGF-β), which is responsible for the activation of fibroblasts, thereby inducing an antifibrotic effect [27,69]. hAM is also known to exert an antimicrobial effect and, therefore, protects the wound from infection [70]. The antibacterial effect of hAM can be illustrated by its expression of natural antimicrobial molecules such as β-defensins and elafin [71] and its inhibitory effect against several bacteria (streptococcus group A or S. aureus) [72]. It can also be explained by its close adherence to the wound surface, avoiding contamination [73]. This close adherence is also known to maintain a moist environment, which contributes to the pain-relieving effect of hAM [48]. Indeed, it can be used to reduce the pain of burn or surgical wounds, acting as a biological dressing that protects the exposed nerve [74]. Finally, several studies have highlighted its ability to enhance re-epithelization [51,75].
Biological properties have to be modulated by the variability of hAM due to inter- and intradonor variations [76,77,78], subregional differences [79], or preservations methods [80], but this is without any clinical evidence.
2.4. Mechanical Properties
The physical properties of hAM, such as elasticity, stiffness, and mechanical strength, are other key elements of its attractiveness for TE [20,46]. Amnion is one of the thickest human membranes that adhere firmly to an exposed surface, for example, osteoarthritis articular cartilage [73]. Fresh hAM is a translucent tissue, and its thickness ranges from 0.02 to 0.5 mm. Collagens, elastin, and other ECM components play an important role in its biomechanical properties [43,81]. Indeed, it has been suggested that collagen proteins play a key role in the stress tolerance of fetal membranes because it has been observed that the collagen content was reduced in pathological fetal membranes that ruptured early [43]. Moreover, it seems that collagen types I and III predominate and form parallel bundles, providing the mechanical integrity of hAM. Collagen type V and VI form filamentous connections between interstitial collagens and the epithelial basement membrane [35]. To enhance its mechanical properties or to overcome the lack of space-maintenance capabilities, the use of multi-layered hAM [82,83] has been suggested or, alternatively, to reinforce hAM with a stronger biomaterial such as the electrospun nanofibers of polymers [84,85] or viscoelastic electrospun nanofibrous silk fibroin [86].
Both physical and mechanical properties of hAM are also affected by preservation methods, sterilization, and cell removal (Figure 4) [18].
Figure 4.
Human amniotic membrane formats. (A) Fresh. (B) Cryopreserved. (C) Lyophilized. (D) Decellularized and lyophilized [18].
Cryopreservation often increases hAM’s thickness, whereas lyophilization decreases it [87]. Moreover, it was shown that cryopreservation did not affect some of hAM’s biomechanical properties [21]. Following rehydration, the lyophilized amnion returns to a layered structure; it thickens and becomes flaccid, and its transparency increases, suggesting that the membrane may have sufficient strength [56]. Recent studies have compared fresh, cryopreserved, lyophilized, and decellularized-then-lyophilized hAM [18,21]. In vivo, fresh hAM and decellularized-then-lyophilized hAM were significantly stronger than cryopreserved hAM and lyophilized hAM. Thus, the decellularization process increased the physical and mechanical properties of hAM. It made hAM significantly more stretchable than fresh hAM, significantly enhancing the tearing strength and significantly decreasing the hAM’s rate of resorption. One study also suggested that the sterilization process by gamma-radiation reduced its mechanical properties [88].
Moreover, differences in mechanical properties, thickness, and transparency have been reported depending on hAM subregions [20,76,79,89]. However, the clinical impact of such changes has not yet been evaluated.
2.5. Biocompatibility
Biocompatibility is the ability of a material to perform its desired function without causing any local or systemic adverse response in the recipient of the material [90]. As detailed before, hAM possesses a low risk of immunogenicity, which is an important criterion for a biocompatible scaffold [35]. Some authors have compared the in vivo biocompatibility of a synthetic scaffold and a biological scaffold made of hAM during the early phase of implantation in rats [91]. Histology and immunohistochemistry analyses revealed inflammatory infiltration in the synthetic-scaffold-implanted rats, but not in the hAM-implanted rats. At the same time, they demonstrated the in vivo biocompatibility of fresh hAM by complete blood count, clinical chemistry measurements, and immunohistochemical analysis.
hAM biocompatibility has also been evaluated following different preservation methods and/or osteodifferentiation [13,18,53,92,93]. It appears that fresh and preserved or osteodifferentiated hAM are biocompatible with slight variabilities, showing a slight-to-moderate inflammatory reaction compared to controls. In some applications, hAM has been used as a coating to improve the biocompatibility of other materials [32].
2.6. Cell Adhesion, Proliferation, and Differentiation
hAM has the ability to promote cell adhesion and proliferation, thanks to its ECM structural components (hyaluronic acid, collagens, laminin, fibronectin, and proteoglycans) [33]. Lyophilization improves its adhesion properties compared to fresh and cryopreserved hAM [87].
The application of hAM to the ocular surface results in an excellent substrate on which hAECs of the ocular surface can easily migrate, adhere, and grow [48]. That is why de-epithelization processes have been developed to expose the basal membrane in order to allow better cell proliferation and differentiation and the uniformity of cell outgrowth [58,59]. Zhang et al. compared the de-epithelialization of hAM by 20% ethanol, 1.2 U/mL dispase, 0.02% ethylenediaminetetraacetic (EDTA), 0.25% trypsin-EDTA, or 5 M urea, respectively, followed by gentle scraping [94]. The results indicated that urea denudation preserved basement membrane integrity, ECM, and growth factor composition and had higher cell attachment and proliferation efficiencies than the other modalities.
Four preparations were examined to determine the effect of total, partial, or non-decellularization on subsequent limbal epithelial cell expansion on hAM [55]. Complete removal of the hAECs resulted in a higher percentage of confluence of limbal epithelial cells but a lower cell density than the intact preparation. Thus, removing the hAM epithelium does not increase proliferation but, rather, facilitates migration of limbal epithelial cells that become larger in comparison with cell culture on intact amnion.
Fresh or preserved hAM, intact or denuded, and decellularized hAM have been used as biological substrates for cell culture growth with different cell types [35,87]. Moreover, the culture of human MSC on the hAM does not affect their immunophenotype or differentiation abilities [95]. Amnion was also used as a delivery system for chondrogenic MSC or adipose-derived MSC [96,97]. We investigated the capacity of fresh, cryopreserved, lyophilized, and decellularized-then-lyophilized hAM to support BM-MSC proliferation and osteodifferentiation [18,21]. We reported that decellularized format was the most suitable scaffold for BM-MSC proliferation and osteodifferentiation.
There are currently very few studies comparing the different sides of hAM to promote cell seeding. Initially, rabbit articular chondrocytes were seeded on three different hAM substrates: the epithelial side of intact hAM (IHE), basement side of denuded hAM (DHB), and stromal side of denuded hAM (DHS) [98]. While chondrocytes grew in a monolayer on the surface of the IHE and DHB substrates, the cells seeded in DHS penetrated and spread into the whole thickness of the stromal layer. The results suggested that denuded hAM was able to support chondrocyte proliferation with phenotype conservation in vitro and seemed more favorable when DHS was used. Later, Diaz et al. specified that the stromal side is more suitable than the epithelial one for human chondrocyte growth because of possible competition between chondrocytes and hAECs [73]. Both the basement membrane side and the collagenous stroma side of the acellular hAM matrix were capable of providing a preferential environment for driving the osteogenic differentiation of human dental apical papilla cells (APCs) with proven stem cell characteristics [99]. In addition, even without osteodifferentiation factors, APC cells differentiated on acellular amnion: more specifically, the collagenous stroma side was more effective than the basement membrane side.
Porcine urothelial cells were seeded on the hAM epithelium, denuded, and the stromal sides were cultured for 3 weeks [100]. The fastest growth and the highest differentiation of urothelial cells were demonstrated on the stromal version scaffold, which enabled the development of a tissue-engineered urothelium, with molecular and ultrastructural properties comparable to that of the native urothelium.
Recently, adipose-derived MSC and a human immortalized keratinocyte cell line (HaCaT) were seeded on the three different layers of the hAM and cultured for 3 weeks. Cell attachment and viability and the mechanical strengths of the basement membrane were assessed before and after cell culture [101]. All three layers supported the attachment and proliferation of cells with no visible cytotoxic effects. However, the growth and viability of both cell types cultured on the basement membrane were significantly higher than on the epithelial and stromal layers.
2.7. Biodegradation
Depending on the application/implantation site and species, hAM degradation varies from several days to several months [12,24], but the data are insufficiently reported. In ophthalmology, but also in wound healing, premature degradation may mean frequent repeat transplantations [24]. Exploiting hAM for corneal reconstruction, it has been observed occasionally that a residual subepithelial hAM may persist and inadvertently opacified the visual axis [26]. The fabrication of a composite material by adding silk to hAM in order to improve the degradation rate of hAM has been proposed [86].
Using a common murine animal model in a subcutaneous site, 8 weeks after implantation, all samples could be located. We noted a slight difference in tissue degradation between non-osteodifferentiated hAM (fresh hAM and cultured hAM) and osteodifferentiated hAM, probably due to a mineralized hAEC layer [13]. Additionally, we reported that the preservation methods of hAM may influence its degradation rate [18,21]. Decellularized-then-lyophilized hAM had the slowest rate of resorption compared to fresh, cryopreserved, and lyophilized hAM. It was the only membrane still present 8 weeks after subcutaneous implantation in rats.
3. Tissue Engineering Applications
Depending on the targeted TE application, hAM can be combined with natural or synthetic materials and/or additional cells. Here, a nonexhaustive list of publications was established for each tissue [31,32]. Examples of studies combining cells with hAM and no additional scaffold are summarized in Table 1. Clinical trials using hAM and/or hAM cells for regenerative medicine applications are reviewed in Table 2.
Table 1.
Use of amniotic membrane as a scaffold for tissue engineering.
| Authors | Tissue Engineering Applications | Amniotic Membrane Formats | Modalities of Amniotic Membrane Usage | Cells Seeded on Amniotic Membrane | Sides of Cells Seeding | Assessment |
|---|---|---|---|---|---|---|
| Shortt et al., 2009 |
Ocular surface | Cryopreserved or Decellularized + Cryopreserved | Single membrane | Human limbal epithelial stem cells | Basement membrane (?) | In vitro/Ex vivo |
| Zhang et al., 2013 | Ocular surface | Cryopreserved or De-epithelialized | Single membrane | Human limbal epithelial cells | Basement membrane | In vitro/Ex vivo |
| Che et al., 2019 | Ocular surface | De-epithelialized | Multilayer ultrathin amnion (3–4 layers) | Human corneal stromal cells Keratocytes |
Basement membrane Cells between the layers |
In vitro/Ex vivo |
| Bandeira et al., 2019 | Ocular surface | Cryopreserved + De-epithelialized | Single membrane/Cover | Human conjunctival epithelial cells | Basement membrane | Clinical study |
| Yang et al., 2006 | Skin | Cryopreserved + De-epithelialized | Single membrane/Cover | Human keratinocytes | Basement membrane | In vitro/Ex vivo + In vivo |
| Kim et al., 2008 | Skin | Cryopreserved + De-epithelialized | Single membrane/Cover | Rabbit bone marrow autologous or allologous MSC | Basement membrane | In vivo |
| Redondo et al., 2011 | Skin | Cryopreserved + De-epithelialized | Single membrane/Cover | Human melanocytes | Basement membrane | Clinical study |
| Tsai et al., 2007 | Vascular system | Cryopreserved + De-epithelialized sow amnion | Single membrane | Porcine vascular endothelial cells | Basement membrane | In vitro/Ex vivo |
| Niknejad et al., 2011 | Vascular system | Fresh or Cryopreserved or Lyophilized |
Single membrane | Rat vascular endothelial cells | Epithelial | In vitro/Ex vivo |
| Lee et al., 2012 | Vascular system | Air-dried + De-epithelialized + Glutaraldehyde |
Tube of amnion | Porcine vascular endothelial cells | NS | In vitro/Ex vivo |
| Amensag et al., 2012 | Vascular system | Two cycles of freezing and thawing + Decellularized | Tube of six-layered amnion |
Human umbilical vein endothelial cells Human vascular smooth muscle cells |
Stromal | In vitro/Ex vivo |
| Amensag et al., 2017 | Vascular system | Two cycles of freezing and thawing + Decellularized | Tube of six-layered amnion |
Human vascular smooth muscle cells | NS | In vitro/Ex vivo + In vivo |
| Swim et al., 2018 | Vascular system | Decellularized + Lyophilized | Multilayer amnion/Cover | Human thymus-derived MSC Human umbilical cord blood MSC Human umbilical vein endothelial cells Cardiac myocytes Arterial smooth muscle cells |
NS | In vitro/Ex vivo + In vivo |
| Sharifiaghdas et al., 2009 | Vaginal and bladder | Fresh + De-epithelialized | Single membrane | Human bladder smooth muscle cells | Basement membrane | In vitro/Ex vivo |
| Seyed-Forootan et al., 2018 | Vaginal and bladder | Fresh | Two layers of amnion/Cover | Autologous skin fibroblasts | NS | Clinical study |
| Sharifiaghdas et al., 2007 | Urethra | Fresh + De-epithelialized | Single membrane | Mouse urothelial cells | Basement membrane | In vitro/Ex vivo |
| Sartoneva et al., 2011 | Urethra | Fresh + De-epithelialized | Amnion attached to a membrane fixation device (cell crowns) | Human urothelial cell |
NS | In vitro/Ex vivo |
| Jerman et al., 2014 | Urethra | Cryopreserved | Single membrane | Porcine urethral cells | Epithelial or Basement membrane or Stromal | In vitro/Ex vivo |
| Wang et al., 2014 | Urethra | De-epithelialized | Single membrane/Cover | Rabbit urethral epithelial cells | NS | In vitro/Ex vivo + In vivo |
| Chen et al., 2018 | Urethra | Decellularized + Lyophilized | Tube of amnion | Allogenic canine endothelial progenitor cells +/− bone marrow MSC | NS | In vitro/Ex vivo + In vivo |
| Jin et al., 2007 | Cartilage | Cryopreserved or Cryopreserved + De-epithelialized | Single membrane/Cover | Rabbit chondrocytes | Epithelial or Basement membrane or Stromal | In vitro/Ex vivo + In vivo |
| Díaz-Prado et al., 2010 | Cartilage | Cryopreserved or Cryopreserved + De-epithelialized | Single membrane | Human chondrocytes | Epithelial or Basement membrane or Stromal | In vitro/Ex vivo |
| Krishnamurithy et al., 2011 | Cartilage | Air-dried or Lyophilized |
Single membrane | Rabbit chondrocytes | Basement membrane | In vitro/Ex vivo |
| Tan et al., 2011 | Cartilage | Air-dried or Lyophilized |
Single membrane | Rabbit bone marrow MSC | NS | In vitro/Ex vivo |
| Garcia et al., 2015 | Cartilage | Fresh or cryopreserved or and cryopreserved | Single membrane/Cover | Sheep bone marrow MSC | Stromal | In vitro/Ex vivo + In vivo |
| Tsugawa et al., 2011 | Bone | Cryopreserved + De-epithelialized | Single membrane/Cover | Mouse bone marrow-derived osteoblast cells | Stromal | In vitro/Ex vivo + In vivo |
| Chen et al., 2012 | Bone | Decellularized + Dried | Single membrane | Human dental apical papilla cells | Basement membrane or Stromal | In vitro/Ex vivo |
| Semyari et al., 2015. | Bone | Fresh decellularized rabbit amnion | Single membrane/Cover | Rabbit adipose-derived MSC | NS | In vitro/Ex vivo + In vivo |
| Akazawa et al., 2016 | Bone | Cryopreserved + Decellularized | Single membrane/Cover | Human calvaria osteoblasts Human dermal fibroblasts Human umbilical vein endothelial cells Mouse osteoblasts Human periodontal ligament stem cells |
NS | In vitro/Ex vivo + In vivo |
| Tang et al., 2017 | Bone | Fresh + De-epithelialized | Single membrane | Human umbilical vein endothelial cells Rat bone marrow MSC |
NS | In vitro/Ex vivo |
| Akhlaghi et al., 2019 | Bone | Decellularized + Lyophilized | Single membrane/Cover | Buccal fat pad-derived stem cells | NS | Clinical study |
| Ahn et al., 2006 | Oral mucosa | De-epithelialized + Lyophilized | Single membrane/Cover | Rabbit oral keratinocytes | Basement membrane | In vitro/Ex vivo + In vivo |
| Amemiya et al., 2010 | Oral mucosa | Cryopreserved + De-epithelialized | Single membrane/Cover | Human oral mucosal epithelial cells | Basement membrane | In vitro/Ex vivo + In vivo |
| Amemiya et al., 2009/2015 | Oral mucosa | Cryopreserved + De-epithelialized | Single membrane/Cover | Human oral mucosal epithelial cells | Basement membrane | Clinical study |
| Hsueh et al., 2016 | Oral mucosa | De-epithelialized + air dried | Single membrane | Human oral mucosal epithelial cells | Basement membrane | In vitro/Ex vivo |
| Amemiya et al., 2008 | Periodontal | Cryopreserved + De-epithelialized | Single membrane/Cover | Dog periodontal ligament cells | Basement membrane | In vivo |
| Iwasaki et al., 2013 | Periodontal | Decellularized + Cryopreserved | Single membrane/Cover | Human periodontal ligament stem cells | NS | In vitro/Ex vivo + In vivo |
| Amemiya et al., 2014 | Periodontal | De-epithelialized | Single membrane/Cover | Human periosteum derived stem cells | NS | In vitro/Ex vivo + In vivo |
| Wu et al., 2015 | Periodontal | De-epithelialized | Single membrane/Cover | Human adipose-derived MSC | Basement membrane | In vitro/Ex vivo + In vivo |
| Honjo et al., 2015 | Periodontal | Cryopreserved + De-epithelialized | Amnion placed on a cell culture insert | dental pulp-derived cell sheet | Basement membrane | In vitro/Ex vivo |
| Zhang et al., 2006 | Nerve | NS in the abstract/Not translated to English | A scroll/wrap of amnion | Autogenous Schwann cell | NS in the abstract/Not translated to English | In vivo |
| Li et al., 2013 | Nerve | Fresh | A scroll/wrap of amnion | Allogenic human umbilical cord MSC | NS | Clinical study |
| He et al., 2002 | Tendon | De-epithelialized + Cryopreserved | A scroll/wrap of amnion | Fetal rabbit skin fibroblasts | Attachment on ECM and proliferation on stromal layer | In vitro/Ex vivo + In vivo |
| Parveen et al., 2019 | Cardiac | Trypsinized + Cryopreserved | Single membrane | Human-induced pluripotent stem cell-derived cardiomyocytes |
Basement membrane (?) | In vitro/Ex vivo |
ECM = Extracellular Matrix; MSC = Mesenchymal Stromal Cells; NS = Not Specified; ?: information assumed by the authors from article content; De-epithelialized = amnion without AEC; Decellularized = amnion without AEC and AMSC.
Table 2.
Clinical trials using human amniotic membrane cells and/or human amniotic membrane as a scaffold for tissue engineering purposes (https://clinicaltrials.gov (accessed on 7 October 2020)).
| Conditions | Clinical Trials Id | Phase | Tissue Engineering Product Evaluated | Status | Sponsor | Results/Status or Remarks |
|---|---|---|---|---|---|---|
| OCULAR SURFACE DISEASE | NCT00348114 | 2 | Amnion + ex vivo expanded limbal epithelial stem cells | Suspended | Singapore National Eye Centre | Estimated Enrolment: 8 participants Estimated Study Completion Date: May 2006 |
| LIMBAL STEM CELL DEFICIENCY | NCT00736307 | 1 2 |
Amnion + cultured limbal epithelial stem cells | Completed | Royan Institute, Tehran, Iran | Enrolment: 10 participants Study Completion Date: October 2009 |
| UNILATERALLIMBAL STEM CELL INSUFFICIENCY | NCT01377311 | 1 | Amnion + cultured limbal stem cells | Terminated | National Taiwan University Hospital | Enrolment: 0 participants Study Completion Date: April 2010 |
| LIMBAL INSUFFICIENCY SYMBLEPHARON |
NCT00491959 | 1 | Amnion + oral mucosal epithelial cells | Terminated (Due to unstable cell sheet quality, the technique was not tested on patients) | National Taiwan University Hospital | Enrolment: 0 participants Study Completion Date: April 2010 |
| SYMBLEPHARON | NCT00799526 | 1 | Amnion + ex vivo cultivated autologous conjunctival epithelial cells | Unknown | Federal University of São Paulo | Estimated Enrolment: 10 participants Estimated Study Completion Date: November 2010 |
| EYE INJURY | NCT01123044 | 3 | Amnion + autologous limbal epithelial cells | Unknown | Ministry of Health, Malaysia | Enrolment: 42 participants Estimated Study Completion Date: September 2012 |
| EPIDERMOLYSIS BULLOSA WITH MITTEN HANDS | NCT01908088 | 1 | Amnion + autologous fibroblasts | Completed | Royan Institute | Enrolment: 6 participants Study Completion Date: July 2013 |
| CORNEAL DISEASE PTERYGIUM MYOPIA HYPEROPIA |
NCT02148016 | 1 2 |
Autologous limbal stem cell + amnion as a protective contact lens | Unknown | Sun Yat-sen University | Estimated Enrolment: 30 participants Estimated Study Completion Date: September 2014 |
| LIMBUS CORNEAE INSUFFICIENCY SYNDROME | NCT01562002 | 1 2 |
Amnion + allogenic bone marrow MSC versus amnion + allogenic limbal stem cells | Completed | Instituto Universitario de Oftalmobiología Aplicada (Institute of Applied Ophthalmobiology)—IOBA | Enrolment: 27 participants Study Completion Date: December 2014 |
| OCULAR SURFACE RECONSTRUCTION | NCT01341223 | Observational | Amnion as a carrier for ex vivo cell culture | Unknown | National Taiwan University Hospital | Estimated Enrolment: 50 participants Estimated Study Completion Date: Mars 2016 |
| LIMBAL STEM CELL DEFICIENCY | NCT03226015 | Observational | Amnion + autologous oral mucosa | Completed | Klinikum Chemnitz gGmbH | Enrolment: 27 participants Study Completion Date: May 2017 |
| LIMBAL STEM CELL DEFICIENCY | NCT01619189 | 2 | Amnion + allogeneic or autologous limbal epithelial stem cells | Completed | CHNO des quinze-vingtsParis, France | Enrollment: 14 participants Study Completion Date: 6 March 2017 |
| LIMBAL STEM CELL DEFICIENCY | NCT02579993 | Interventional | Amnion + in vitro expanded limbal stem cells | Terminated (Preliminary results not favorable) |
Instituto de Oftalmologia Conde de Valenciana | Enrolment: 10 participants Study Completion Date: March 2018 |
| LIMBAL STEM CELL DEFICIENCY | NCT02592330 | 1 | Amnion + expanded autologous limbal epithelial cells | Recruiting | Massachusetts Eye and Ear Infirmary | Estimated Enrollment: 17 participants Estimated Study Completion Date: 30 June 2023 |
| WOUNDS | NCT02314416 | 4 | Amniotic stem cells + collagen matrix | Withdrawn | Augusta University | Enrolment: 0 participant Study Completion Date: May 2015 |
| ASHERMAN’S SYNDROME | NCT03223454 | 1 | Amnion + AEC | Unknown | The Second Affiliated Hospital of Chongqing Medical University | Estimated Enrolment: 50 participants Estimated Study Completion Date: March 2021 |
| ENDOMETRIUM INFERTILE PATIENTS | NCT04676269 | 1 | Amnion + autologous endometrium cells or allogenic AEC or both type of cells | Recruiting | Indonesia University | Estimated Enrolment: 40 participants Estimated Study Completion Date: 15 December 2021 |
| ANTERIOR CRUCIATE LIGAMENT RUPTURE | NCT03294759 | Interventional | Amnion collagen matrix wrap + bone MSC | Active, not recruiting | Andrews Research & Education Foundation | Actual Enrolment: 40 participants Estimated Study Completion Date: 25 September 2021 |
| ANTERIOR CRUCIATE LIGAMENT RUPTURE | NCT03294720 | Interventional | Amnion collagen matrix wrap + bone MSC | Active, not recruiting | Andrews Research & Education Foundation | Actual Enrolment: 10 participants Estimated Study Completion Date: 20 March 2021 |
| NONUNION FRACTURE | NCT03031509 | 1 2 |
AEC | Not yet recruiting | Shanghai East Hospital | Estimated Enrollment: 36 participants Estimated Study Completion Date: December 2020 |
MSC = Mesenchymal Stromal Cells; AEC = Amniotic epithelial cells.
3.1. Eye
As mentioned earlier, hAM as a “simple scaffold” has a clinical indication in ophthalmology. Over the past two decades, excellent outcomes have been reported after transplantation of cultivated limbal stem cells on denuded hAM for limbal stem cell deficiency [102,103,104,105] or, similarly, with oral epithelium [106].
Later, different cell types were cultured on hAM to enhance its healing potential and expand its use to other indications in ophthalmology: it has resulted in several experimental and clinical studies (Table 1 and Table 2) [107].
As mentioned before, de-epithelialization and decellularization have been compared, with satisfying results, for limbal stem cell growth and/or migration [55,94].
A new method to fabricate a tissue-engineered corneal stromal in combination with keratocytes and multilayer ultrathin hAM was recently investigated [108]. A novel 3D biomimetic corneal model was developed to replicate corneal stromal organization with multilayer ultrathin hAM: it allowed the maturation of corneal stroma–like tissues in vitro.
In 2019, the first clinical study was conducted to evaluate the efficiency of using cultivated conjunctival epithelium transplantation on denuded hAM prepared using ice-cold urea as a basement membrane scaffold for cell-based tissue-engineered treatments of ocular surface disorders [109]. The protocol was applied to two patients, and the results indicated that this method could facilitate and mainstream a minimally invasive cell-based treatment for the reconstruction of extensive ocular surface wounds.
Monville et al. developed a human pluripotent stem cell retinal pigment epithelium sheet, disposed on hAM, that sustained the vision of rodents with retinal degeneration compared to the same cells injected in suspension [110]. After validation in a primate model [111], the first cell therapy for retinitis pigmentosa patients carrying retinal pigment epithelium gene mutations (LRAT, RPE65, and MERTK) was approved in 2019.
3.2. Skin
hAM has been used clinically for centuries as a biological dressing to treat acute and chronic wound injuries and burns, acting as a physical and biological barrier [112]. More than 200 clinical trials have reported its efficacy for wound healing [113].
A similarity between normal human skin and hAM layers exists. Consequently, amnion could provide a scaffold for a living-skin equivalent, greatly simplifying the procedures for making a dermal matrix and avoiding the use of animal collagen, which is costly and ethically problematic [74,114].
That is why, in addition to wound dressing, Yang et al. also suggested the use of hAM as a scaffold to create a skin substitute for wound closure. Amnion scaffolds seeded with human keratinocytes have generated living skin equivalents and have been successfully transplanted into an animal model [114]. Kim et al. recognized its added value in the management of full-thickness skin defects in rabbits [115]. Redondo et al. suggested the use of this allograft as a new strategy for inducing repigmentation in patients with vitiligo [116]. They cultured autologous melanocytes on a denuded hAM. The combined product was then implanted onto lesions of four patients with vitiligo, and the results showed a 90–95% repigmentation.
A new interesting and promising approach has been developed by Murphy et al. [113]. After grinding lyophilized hAM, they combined this solubilized allograft with hyaluronic acid, and they made a composite hydrogel delivery system. The aim was to obtain a cell-free solution while maintaining high concentrations of cell-derived cytokines and growth factors. This new amnion-derived material showed encouraging results to promote wound healing and reduce scar contraction in a full-thickness murine wound model.
3.3. Vascular System
Whereas cryopreservation is commonly used, Niknejad et al. suggested that lyophilized hAM is more suitable than the fresh and cryopreserved formats to culture endothelial cells [87].
The cell culture of porcine arterial endothelial cells on hAM has been proposed for the fabrication of tissue-engineered blood vessels [117,118]. They first demonstrated that porcine endothelial cells can successfully be seeded on sow’s hAM, with an increase in the expressions of junctional proteins while the expression of the adhesive inflammatory molecules decreases. Then, they realized tissue-engineered blood vessels made with rolled hAM, thus creating a tube of amnion that is endothelialized with porcine vascular endothelial cells [118]. The feasibility of a vein conduit fabrication from hAM and its implantation in the external jugular vein of juvenile sheep was also assessed [119].
An in vitro study reported the use of decellularized hAM seeded with human umbilical vein endothelial cells and human vascular smooth muscle cells prior to being rolled into a dense construct as an alternative strategy to develop cell-dense vascular bioscaffolds. It resulted in a mechanically stable, multilayered tissue-engineered blood vessel conduit that can be manufactured into different diameters and shapes to suit the targeted applications [120]. The acellular hAM conduits were surgically implanted as arterial interposition grafts into the carotid arteries of immunocompetent rabbits [121]. The grafts demonstrated patency over four weeks (n = 3), with no hyperacute rejection or thrombotic occlusion. Swim et al. combined decellularization and freeze-drying to produce a monolayer or a multilayer amnion-based scaffold suitable for TE constructs, designed for reconstructive heart surgery [122]. Whereas both preservation procedures enhanced the cell viability and growth of various cell types seeded on hAM in vitro, the multilayered construct displayed enhanced biomechanical properties. It was implanted in a piglet model of left pulmonary artery grafting. The results showed its in vivo suitability and biocompatibility for vascular repair, as demonstrated by the development of newly formed endothelium in the intima, a smooth muscle cell-rich medial layer and an adventia containing new vasa vasorum, an endothelial cell layer in the inner side of the graft, and a smooth muscle layer in the outer side [122].
Finally, an in vitro study evaluated the blood compatibility of the epithelial and stromal surfaces of the amnion for potential use in vascular TE [123]. These results suggested that hAM, which contains hAECs and hAMSC, has appropriate hemocompatibility to be employed in the field, especially as a vein substitute. No significant difference was seen between the epithelial and stromal sides of the amnion.
3.4. Bladder and Vagina
In the early 1980s, the first studies on animals with glutaraldehyde-stabilized or fresh hAM used for bladder reconstruction were reported [124], with fast epithelialization and improved functionality [125]. Later, it was observed that hAM may be a substitute for the transitional epithelium of the bladder in dogs [126]. Three layers of rehydrated hAM, sutured to the bladder defect, have been experimented on in vesico-vaginal fistulae, demonstrating a structured implementation of a new method for vesico-vaginal fistulae repair following IDEAL recommendations [127]. In an original way, a sandwich-structured biocomposite material was made of cryopreserved hAM, covered on both sides with two-layered membranes of electrospun poly-(l-lactide-co-ecaprolactone) for bladder augmentation in a rat model [85].
In a clinic setting, Brandt et al. explored the use of hAM grafts in 8 female patients with urological congenital defects. They reported that the procedure was quick and effective for appropriate restoration of the function and cosmetics of the lower urogenital tract [128].
Several authors have reported the use of hAM for vaginoplasty in patients suffering from congenital absence of the vagina or for gender reassignment surgery. The creation of a neovagina is, thus, associated with an amnion graft. Both fresh and preserved hAM were assessed and resulted in adequate anatomic and functional outcomes [129,130,131]. In vitro studies have investigated the growth pattern, morphology, and specific features of human bladder smooth muscle cells on two different matrixes, amnion and collagen, and showed abundant cell-to-cell adhesions with hAM [132]. Satisfactory outcomes were also obtained when autologous fibroblasts were seeded onto hAM prior to its graft to cover the neovagina [133]. The two layers of amnion and fibroblasts were more resistant to trauma and laceration than amnion without seeded cells.
3.5. Urethral
Based on the number of surveys conducted or ongoing clinical studies, urology has also played a large part in studies using hAM [84,134,135]. Shakeri et al. evaluated hAM as a xenograft for urethroplasty in rabbits [136]. They concluded that it was an inexpensive, simple, and biodegradable graft, yielding very little antigen effect, and a viable option in surgical urethroplasty. A study compared the effects of acellular hAM to synthetic poly-l-lactide-co-1-caprolactone on human urothelial cell viability, proliferation, and urothelial differentiation levels, with unfavorable results for the amnion [137]. Salehipour et al. evaluated its use in the reconstruction of long ureteral defects in dogs and speculated its efficacy as a patch graft versus a full circumferential graft in the reconstruction of ureteral defects [138].
In a clinical setting, Koziak et al. explored its use in the reconstruction of long ureteral structures in 2 and then 11 patients [139,140]. hAM was successfully used to supplement ureteral wall defects. Indications for the procedure included ureteral strictures of a 5.5 cm average (range, 3–8 cm), localized in different parts of the ureter: upper (5), middle (5), and lower (3).
The proliferation quality of mouse urothelial cells has been assessed on three natural matrixes of hAM, peritoneum, and omentum compared to collagen matrix, with promising results for the amnion [141]. As described before, the fastest growth and highest differentiations of urothelial cells were demonstrated on the hAM stromal side [100]. Denuded hAM, inoculated with primary rabbit urethral epithelial cells and applied as urethroplastic material in the rabbit models of urethral injury, displayed good biocompatibility [142]. Chen et al. seeded allogeneic BM-MSC and/or endothelial progenitor cells on decellularized amnion (with the cell-seeded surface facing the corpus spongiosum) as a treatment for urethral defects in dogs [143]. Subsequently, they concluded that hAM seeded with allogeneic endothelial progenitor cells +/− BM-MSC can more effectively repair a 3-cm circumferential urethral defect in a large animal model.
3.6. Cartilage
Similar components (hyaluronan acid, proteoglycans, and collagen) have been found between the ECM of hAM and native cartilage [33]. The potential of fresh, cryopreserved, lyophilized, or dried amnion to act as MSC [96,144] or chondrocyte [145] cell carriers and promote MSC chondrogenic differentiation was investigated with success.
As mentioned before, in vitro and in vivo studies performed on cryopreserved intact or de-epithelialized hAM have stated that its stromal side is a more suitable scaffold than its epithelial side to promote chondrocyte proliferation and to maintain their phenotype [73,98]. In vivo, denuded amnion alone was compared to denuded amnion seeded with chondrocytes to repair a rabbit osteochondral defect. The rate of regenerated cartilage was significantly higher when chondrocytes seeded on hAM were facing the defect, suggesting that denuded amnion can act as a cell carrier matrix for cartilage regeneration [98]. In vivo results suggested that fresh and cryopreserved amnions alone compared to cryopreserved amnion previously cultivated with BM-MSC showed similar regenerative properties [144].
Interestingly, hAM was combined with fibrin to develop a new 3D scaffold that was able to promote bovine chondrocytes in vitro proliferation [146]. Similarly, hAM has also been combined—as cell-free material—with a synthetic scaffold in poly-d,l-lactic-co-glycolic acid, which, once implanted in osteochondral defects, was able to promote regeneration of hyaline-like cartilage [147].
3.7. Bone
The ability of hAM to be osteodifferentiated in toto has been established in vitro [10,13,15,19,148]. However, when associated with a bone substitute and implanted in a mice subcutaneous model, fresh and in vitro osteodifferentiated hAM were not able to induce ectopic bone formation [13].
Interestingly, in an orthotopic model, fresh hAM had a periosteum-like effect when implanted over a segmental defect in rabbits [149]. Moreover, cryopreserved hAM slightly enhanced bone regeneration when used as a membrane for guided bone regeneration (GBR) in a murine calvaria model [14]. GBR function has also been explored with a decellularized–lyophilized format [21,150].
hAM showed similarities with the induced membrane (IM) [12]. The IM technique (also called the Masquelet technique) is a commonly used two-step procedure to treat segmental long bone defects. The first step allows the generation of a foreign body membrane (the IM), which protects the bone auto- or allograft from resorption by the local environment [151,152,153,154]. The similarity between these two membranes (hAM and IM) generated by the body may simplify the Masquelet technique into a single procedure, avoiding the time required for the formation of the IM and the second surgery. Accordingly, a decellularized–lyophilized hAM, as an alternative to the induced membrane technique, was used in a segmental femoral defect model [23].
Processed hAM, seeded with bone marrow (BM) or adipose-derived MSC, led to encouraging results in a calvarial bone defect animal model [155,156] and was a suitable scaffold for cell proliferation and osteogenic differentiation [18,21,150]. As shown before, even in the absence of osteoinduction, acellular hAM matrix exerted the substrate-induced effect of initiating APC differentiation [99]. In an original way, transplantation of MSC from periodontal ligaments and osteoblasts using double-layered cell transfer significantly enhanced in vivo bone formation compared to single-cell-type transplantation [157].
A clinical study reported the successful use of decellularized hAM in combination with autologous buccal-fat-pad-derived stem cells to treat large bone defects in jaws [158]. In the case series, the combination of bone substitutes (hydroxyapatite and platelet-rich fibrin) with amnion allowed periapical bone healing [159].
3.8. Oral, Periodontal, and Maxillofacial Surgery
Since its first use in 1985 by Lawson et al. for the treatment of oral mucosa defects [160], this allograft has been widely studied in the field of oral and maxillofacial surgery, and promising results exist for oral soft tissue regeneration [16]. Multilayered hAM was used to close oronasal fistula in minipigs [161] and in four patients [83]. Moreover, the amniochorionic membranes were compared to the conventional membrane already used for GBR procedures in oral surgery [162].
Several studies have reported the ability of hAM to stimulate healing and enhance epithelial regeneration of human oral mucosa defects after excision of benign and precancerous lesions [163,164]. In this context, a bioartificial mucosa using cultured oral keratinocytes on hAM was fabricated to evaluate the possibility of developing a prelaminated myomucosal flap using the fabricated bioartificial mucosa and a local muscle flap [165]. Similarly, denuded (hyper)dry or cryopreserved hAM have been used alone [56] or seeded with oral mucosal epithelial cell sheets and transferred to the mucosal defect in both preclinical [166,167] and clinical studies [168,169].
The use of hAM to treat root exposure caused by gingival recession has been successfully reported in several clinical studies. When the hAM graft was associated with a gingival flap, the root coverage and gingival thickness and biotype were improved [170,171,172].
In a combined way, autologous keratinocytes cultured on hAM combined with poly(L-lactic acid) were transplanted with success to cover intraoral fistulas and bone loss after osteoradionecrosis in 9 patients (15 procedures) [173].
Periodontal disease affects the supportive tissue of teeth, which include the periodontal ligament. Several preclinical studies have aimed to investigate the efficacy of hAM to treat periodontal disease. Amnion was seeded with periodontal ligament stem cells or periosteum-derived cells [166,174,175] or adipose-derived MSC [176]. These studies concluded that hAM could be a useful scaffold for periodontal regeneration by avoiding the proliferation of connective tissue on the denuded root surface in the periodontal defect. Finally, an in vitro study suggested the potential of dental-pulp-derived cell sheets cultured on hAM substrates for periodontal TE [177].
In maxillofacial surgery, hAM was used as an interpositional material to prevent temporomandibular joint re-ankylosis in a rabbit model [178]. Similar results were observed when cryopreserved hAM was compared to fresh hAM [179]. Improvement in chewing efficiency and the absence of pain were related in one case report [180]. Its use has also been tested with success in the treatment of two patients with bisphosphonate-related osteonecrosis of the jaw [181].
3.9. Nerve
Several studies have reported the use of fresh or preserved hAM as a scaffold for nerve regeneration, highlighting a proregenerative effect on injured peripheral nerves, thanks to its antifibrotic and antiscarring effects [17]. For example, fresh hAM was implanted in a rat model of sciatic nerve scarring to treat recurring perineural adhesions and the associated nerve scarring. Accelerated recovery of sciatic nerve function was observed when the epithelial side of hAM was applied toward the nerve [182]. To manage nerve injury, cryopreserved hAM was wrapped around the damaged nerve, and scar formation and functional recovery were assessed. Although both functional and morphological parameters were not significantly improved, the nerves wrapped with hAM had significantly fewer adhesions and less scar formation than controls [183]. For both indications, only a few studies have specified the orientation of the applied amnion [17]: stromal side against the nerve [184] or epithelial side [182,185]. In a combined way, a dehydrated amnion filled with skeletal muscle cells, harvested from neighboring tissue, showed encouraging results in humans for repairing post-traumatic nerve defects of 3 to 5 cm in length [186]. Later, this clinical proof of concept was substantiated by an experimental model [185]. Additionally, amnion tubes were manufactured to cover the gap and edges of the nerve with favorable functional in vivo results [187,188,189]. Recently, an electrospun polycaprolactone–amnion nanofibrous membrane showed satisfying results for the treatment of sciatic nerve compression in a rat model [190].
Only two articles were found with a TE purpose. The first reports the use of a scroll of amnion derivative (ZQ membrane) combined with cultured autogenous Schwann cells [191]. The second was the application of human umbilical cord MSC-loaded hAM for the repair of radial nerve injury, with functional recovery better in the transplantation group than the control group [192].
3.10. Ligament and Tendon
The interest in hAM for ligament and tendon healing has also been explored [193,194]. This tissue has the ability to prevent tendon adhesions after injury and reconstruction [195]. One study investigated the effects of fresh denuded amnion and hyaluronic acid, alone and in combination, on adhesions and healing following chicken flexor tendon repair. The prevention of adhesion formation was superior when hAM was wrapped around the repaired tendon [196]. Another study reported the effectiveness of decellularized amnion to promote endogenous healing and prevent tendon adhesion in the same model [197].
This method of tendon-wrapping, in which cryopreserved hAM is laid over the damaged tendon, has also been successfully reported in humans [198,199]. The addition of a de-epithelialized and lyophilized hAM wrap around a composite silk scaffold that included tenocytes accelerated cellular migration and angiogenesis in neotendons in rabbits [200].
We have found only one article with a TE purpose. It evaluated fetal skin fibroblast cells seeded on hAM ECM in an in vivo model of Achille tendon defect, with promising functional results [201].
3.11. Heart
In the field of cardiology, hAM patching improved ischemic heart repair in rat and mice models [202,203,204]. Similarly, an acellular hAM was explored in vivo as a pericardial substitute [205]. Results showed that hAM use increased the pericardium repair thickness, thanks to its ability to reduce the incidence of adhesions and scarring. Four weeks after surgery, host cells organized with tissue fibrils and capillaries were clearly identified in the surface (epicardial) coating of the hAM patch, indicating that its outer layer became well integrated with the host tissues.
Medical case reports have also stated that hAM patching had anti-inflammatory effects and reduced new-onset postoperative fibrillation in patients undergoing cardiac surgery [206,207].
In a combined way, a novel composite biomaterial was developed by processing human cardiac ECM into a hydrogel and combining it with cell-free hAM via a dry-coating procedure [208]. The researchers concluded that the incorporation of human cardiac ECM hydrogel shifts and enhances the bioactivity of decellularized hAM, facilitating its use in future cardiac applications. Overall, based on their results, this scaffold may be a potential platform for the epicardial delivery of cells and therapeutic agents as it possesses superior adhesion capacity, supports cell proliferation and viability, and modulates inflammatory responses.
Only one article was reported with a TE purpose. It investigated an in-house generated human-induced pluripotent-stem cell-derived cardiac progenitor seeded on trypsinized and cryopreserved hAM to construct a cardiac cell sheet [209]. The study showed that the seeded progenitor cells grafted onto the matrix of hAM differentiated in situ into functional and relatively mature cardiomyocytes. hAM slightly improved the development of cardiomyocytes compared to the control basement membrane matrix, Matrigel™.
3.12. Clinical Trials
Due to historical use and banking facilities, to date, hAM is mainly exploited under its scaffold format instead of its derived cells.
Consequently, the assessment of the overall number of clinical trials evaluating hAM as a scaffold for TE purposes revealed that ophthalmology has the biggest share, with 14 clinical trials (Table 2). In this indication, various autologous or allogenic cells have been seeded on hAM: limbal (epithelial) stem cells, cornea stem cells, oral mucosal epithelial cells, conjunctival epithelial cells, fibroblasts, and BM-MSC.
hAM-based scaffolds have been studied sporadically in Asherman’s Syndrome, endometrium infertile patients, and anterior cruciate ligament ruptures. Interestingly, in gynecology, hAM was combined with isolated hAECs. In this indication, these cells have also been explored alone in other clinical trials not included in Table 2 (NCT03207412, NCT02912104, NCT03381807, NCT03223454). Finally, both hAM cells and hAECs have been examined alone in wound healing and nonunion fracture, respectively.
4. Conclusion
Since promising results have been achieved with hAM in ophthalmology and dermatology, an increasing number of publications have suggested its potential for other TE applications. Experimental works have described promising results in vascular, bone, and cartilage repair and oral surgery, similarly to the research conducted in overall TE in recent years. From our analysis, nerve, ligament, tendon, and cardiac applications were sporadic compared to urology.
In clinics, bone, oral mucosa, and ligament repair have been investigated, and two industrial clinical trials have been conducted. Thus, the participation of the industry in the TE field is highly anticipated. Some exogenous indications in gynecology (Asherman’s Syndrome and endometrium infertile patients) that do not properly belong to the TE field have also been explored.
Looking at the TE constructs more in detail, decellularization or, mainly, the de-epithelialization process has been applied to hAM. Predominantly in the cartilage TE area, researchers have compared the efficacy of seeding the three layers (epithelial, basement membrane, and stromal). The basement membrane layer seems to be more favorable for cell seeding, proliferation, and differentiation. To date, there is no consensus on the best cells to seed on hAM, and the choice is mostly driven by the tissue to regenerate. In all cases, a good balance must be found between a noninvasive procedure for the collection of cells and their final functional capacity. That is why, for example, oral mucosa has been considered a source of epithelial cells in ophthalmology. The in vivo degradation rate of hAM is not detailed enough in the literature and should be more evaluated.
Finally, we note that clinical trials have intensively explored hAM as a scaffold compared to the use of its cells as a TE construct. Increased knowledge of hAM cells, in particular regarding their function, will encourage future clinical investigations.
Acknowledgments
The coauthors are grateful to Franck Daval (Bibliothèque Universitaire Santé, Université Bourgogne Franche-Comté, F-25000 Besançon, France) for bibliographic support. This publication is based upon work from COST Action 17116 “SPRINT”, supported by COST (European Cooperation in Science and Technology)—http://www.cost.eu (accessed on 7 October 2020).
Author Contributions
F.G. and M.F. designed the study and wrote the manuscript. S.C. revised both all the versions of the manuscript and the language format. C.M., J.-C.F. and A.L. contributed to the understanding of hAM application in Oral, Periodontal, and Maxillofacial Surgery; L.O. in bone and cartilage repair and F.A. in urethral reconstruction. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Langer R., Vacanti J. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Vacanti J.P., Langer R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354:S32–S34. doi: 10.1016/S0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14:88–95. doi: 10.1016/s1369-7021(11)70058-x. [DOI] [Google Scholar]
- 4.Naung N.Y., Shehata E., Van Sickels J.E. Resorbable Versus Nonresorbable Membranes. Dent. Clin. N. Am. 2019;63:419–431. doi: 10.1016/j.cden.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Gindraux F., Obert L., Laganier L., Barnouin L. Industrial approach in developing an advanced therapy product for bone repair. J. Tissue Eng. Regen. Med. 2010;4:194–204. doi: 10.1002/term.227. [DOI] [PubMed] [Google Scholar]
- 6.Louvrier A., Euvrard E., Nicod L.P., Rolin G., Gindraux F., Pazart L., Houdayer C., Risold P.Y., Meyer F. Odontoblastic differentiation of dental pulp stem cells from healthy and carious teeth on an original PCL-based 3D scaffold. Int. Endod. J. 2017;51:e252–e263. doi: 10.1111/iej.12746. [DOI] [PubMed] [Google Scholar]
- 7.Gindraux F., Obert L. Bioreconstruction: De l’os à la peau Tome 2. Sauramps Médical; Montpellier, France: 2010. Human amniotic membranes: Benefits for bone repair/regeneration; pp. 85–91. [Google Scholar]
- 8.Obert L., Genestier L., Froidevaux L., Averlant E., Laurent R., Wajszczak L., Zwetyenga N., Pouthier F., Malugani C., Gindraux F. Technique de Masquelet. Sauramps Médical; Montpellier, France: 2012. Amniotic membrane for bone repair? Reflection around of the Masquelet technique to one stage/Membrane amniotique pour la réparation osseuse? Réflexion autour de la simplification de la technique de Masquelet à une chirurgie. [Google Scholar]
- 9.Gindraux F., Laurent R., Nicod L., Billy B., Meyer C., Zwetyenga N., Wajszczak L., Garbuio P., Obert L. Human Amniotic Membrane: Clinical Uses, Patents And Marketed Products. Recent Pat. Regen. Med. 2013;3:193–214. doi: 10.2174/22102965113039990021. [DOI] [Google Scholar]
- 10.Laurent R., Brennan M., Renaud A., D’arros C., Obert L., Layrolle P., Gindraux F. Osteodifferentation of intact human amniotic membrane through a jet sprayed polycaprolactone nanofibre scaffold. Bone Jt. J. Orthop. Proc. Suppl. 2014;96-B:113 [Google Scholar]
- 11.Laurent R., Nallet A., Obert L., Nicod L., Gindraux F. Storage and qualification of viable intact human amniotic graft and technology transfer to a tissue bank. Cell Tissue Bank. 2014;15:267–275. doi: 10.1007/s10561-014-9437-x. [DOI] [PubMed] [Google Scholar]
- 12.Gindraux F., Rondot T., de Billy B., Zwetyenga N., Fricain J.-C., Pagnon A., Obert L. Similarities between induced membrane and amniotic membrane: Novelty for bone repair. Placenta. 2017;59:116–123. doi: 10.1016/j.placenta.2017.06.340. [DOI] [PubMed] [Google Scholar]
- 13.Laurent R., Nallet A., Obert L., Nicod L.P., Meyer C., Layrolle P., Zwetyenga N., Gindraux F., De Billy B. Fresh and in vitro osteodifferentiated human amniotic membrane, alone or associated with an additional scaffold, does not induce ectopic bone formation in Balb/c mice. Cell Tissue Bank. 2017;18:17–25. doi: 10.1007/s10561-016-9605-2. [DOI] [PubMed] [Google Scholar]
- 14.Fénelon M., Chassande O., Kalisky J., Gindraux F., Brun S., Bareille R., Ivanovic Z., Fricain J.-C., Boiziau C. Human amniotic membrane for guided bone regeneration of calvarial defects in mice. J. Mater. Sci. Mater. Med. 2018;29:78. doi: 10.1007/s10856-018-6086-9. [DOI] [PubMed] [Google Scholar]
- 15.Laurent R., Nicod L., Layrolle P., de Billy B., Obert L., Gindraux F. Osteogenic potential and immunogenicity of human amniotic membrane: In vivo and in vivo studies. Orthop. Proc. 2018;96-B:112 [Google Scholar]
- 16.Fénelon M., Catros S., Fricain J.C. What is the benefit of using amniotic membrane in oral surgery? A comprehensive review of clinical studies. Clin. Oral Investig. 2018;22:1881–1891. doi: 10.1007/s00784-018-2457-3. [DOI] [PubMed] [Google Scholar]
- 17.Bourgeois M., Loisel F., Obert L., Pluvy I., Gindraux F. Can the amniotic membrane be used to treat peripheral nerve defects? A review of literature. Hand Surg. Rehabil. 2019;38:223–232. doi: 10.1016/j.hansur.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Fenelon M., Maurel D.B., Siadous R., Gremare A., Delmond S., Durand M., Brun S., Catros S., Gindraux F., L’Heureux N., et al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications. Mater. Sci. Eng. C. 2019;104:109903. doi: 10.1016/j.msec.2019.109903. [DOI] [PubMed] [Google Scholar]
- 19.Gualdi T., Laurent R., Moutarlier V., Fenelon M., Nallet A., Pouthier F., Obert L., De Billy B., Meyer C., Gindraux F. In vitro osteodifferentiation of intact human amniotic membrane is not beneficial in the context of bone repair. Cell Tissue Bank. 2019;20:435–446. doi: 10.1007/s10561-019-09778-3. [DOI] [PubMed] [Google Scholar]
- 20.Grémare A., Jean-Gilles S., Musqui P., Magnan L., Torres Y., Fénelon M., Brun S., Fricain J.-C., L’Heureux N. Cartography of the mechanical properties of the human amniotic membrane. J. Mech. Behav. Biomed. Mater. 2019;99:18–26. doi: 10.1016/j.jmbbm.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Fenelon M., Etchebarne M., Siadous R., Grémare A., Durand M., Sentilhes L., Torres Y., Catros S., Gindraux F., L’Heureux N., et al. Assessment of fresh and preserved amniotic membrane for guided bone regeneration in mice. J. Biomed. Mater. Res. Part A. 2020;108:2044–2056. doi: 10.1002/jbm.a.36964. [DOI] [PubMed] [Google Scholar]
- 22.Etchebarne M., Fricain J.-C., Kerdjoudj H., Di Pietro R., Wolbank S., Gindraux F., Fenelon M. Use of Amniotic Membrane and Its Derived Products for Bone Regeneration: A Systematic Review. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.661332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenelon M., Etchebarne M., Siadous R., Grémare A., Durand M., Sentilhes L., Catros S., Gindraux F., L’Heureux N., Fricain J.-C. Comparison of amniotic membrane versus the induced membrane for bone regeneration in long bone segmental defects using calcium phosphate cement loaded with BMP-2. Mater. Sci. Eng. C. 2021;124:112032. doi: 10.1016/j.msec.2021.112032. [DOI] [PubMed] [Google Scholar]
- 24.Odet S., Louvrier A., Meyer C., Nicolás F.J., Hofmann N., Chatelain B., Mauprivez C., Laurence S., Kerdjoudj H., Zwetyenga N., et al. Surgical application of human amniotic membrane and amnion-chorion membrane in the oral cavity and efficacy evaluation: Corollary with ophthalmological and wound healing experiences. Front. Bioeng. Biotechnol. 2021;9:443. doi: 10.3389/fbioe.2021.685128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis J.S. Skin grafting at the johns hopkins hospital. Ann. Surg. 1909;50:542–549. doi: 10.1097/00000658-190909000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dua H.S., Gomes J.A., King A.J., Maharajan V. The amniotic membrane in ophthalmology. Surv. Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Ricci E., Vanosi G., Lindenmair A., Hennerbichler S., Peterbauer-Scherb A., Wolbank S., Cargnoni A., Signoroni P.B., Campagnol M., Gabriel C., et al. Anti-fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell Tissue Bank. 2012;14:475–488. doi: 10.1007/s10561-012-9337-x. [DOI] [PubMed] [Google Scholar]
- 28.Kubo M., Sonoda Y., Muramatsu R., Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Investig. Ophthalmol. Vis. Sci. 2001;42:1539–1546. [PubMed] [Google Scholar]
- 29.Parolini O., Alviano F., Bagnara G.P., Bilic G., Bühring H.-J., Evangelista M., Hennerbichler S., Liu B., Magatti M., Mao N., et al. Concise Review: Isolation and Characterization of Cells from Human Term Placenta: Outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 30.Silini A.R., Di Pietro R., Lang-Olip I., Alviano F., Banerjee A., Basile M., Borutinskaite V., Eissner G., Gellhaus A., Giebel B., et al. Perinatal Derivatives: Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature. Front. Bioeng. Biotechnol. 2020;8:610544. doi: 10.3389/fbioe.2020.610544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leal-Marin S., Kern T., Hofmann N., Pogozhykh O., Framme C., Börgel M., Figueiredo C., Glasmacher B., Gryshkov O. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020:1–18. doi: 10.1002/jbm.b.34782. [DOI] [PubMed] [Google Scholar]
- 32.Tehrani F.D., Firouzeh A., Shabani I., Shabani A. A Review on Modifications of Amniotic Membrane for Biomedical Applications. Front. Bioeng. Biotechnol. 2021;8:606982. doi: 10.3389/fbioe.2020.606982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilic D., Vićovac L., Nikolic M. Human amniotic membrane grafts in therapy of chronic non-healing wounds. Br. Med. Bull. 2016;117:59–67. doi: 10.1093/bmb/ldv053. [DOI] [PubMed] [Google Scholar]
- 34.Toda A., Okabe M., Yoshida T., Nikaido T. The Potential of Amniotic Membrane/Amnion-Derived Cells for Regeneration of Various Tissues. J. Pharmacol. Sci. 2007;105:215–228. doi: 10.1254/jphs.CR0070034. [DOI] [PubMed] [Google Scholar]
- 35.Niknejad H., Peirovi H., Jorjani M., Ahmadiani A., Ghanavi J., Seifalian A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008;15:88–99. doi: 10.22203/eCM.v015a07. [DOI] [PubMed] [Google Scholar]
- 36.Arrizabalaga J.H., Nollert M.U. Human Amniotic Membrane: A Versatile Scaffold for Tissue Engineering. ACS Biomater. Sci. Eng. 2018;4:2226–2236. doi: 10.1021/acsbiomaterials.8b00015. [DOI] [PubMed] [Google Scholar]
- 37.Nejad A.R., Hamidieh A.A., Amirkhani M.A., Sisakht M.M. Update review on five top clinical applications of human amniotic membrane in regenerative medicine. Placenta. 2021;103:104–119. doi: 10.1016/j.placenta.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Bourne G. The Foetal Membranes: A Review of the Anatomy of Normal Amnion and Chorion and Some Aspects of Their Function. Postgrad. Med. J. 1962;38:193–201. doi: 10.1136/pgmj.38.438.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miki T., Strom S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. Rep. 2006;2:133–141. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- 40.Pasquier J.-C., Doret M. Les membranes fœtales: Développement embryologique, structure et physiopathologie de la rupture prématurée avant terme. J. Gynécologie Obs. Biol. Reprod. 2008;37:579–588. doi: 10.1016/j.jgyn.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Mamede A.C., Carvalho M.J., Abrantes A.M., Laranjo M., Maia C.J., Botelho M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012;349:447–458. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 42.Bryant-Greenwood G.D. The extracellular matrix of the human fetal membranes: Structure and function. Placenta. 1998;19:1–11. doi: 10.1016/S0143-4004(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 43.Malak T., Ockleford C., Bell S., Dalgleish R., Bright N., MacVicar J. Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta. 1993;14:385–406. doi: 10.1016/S0143-4004(05)80460-6. [DOI] [PubMed] [Google Scholar]
- 44.Miki T., Marongiu F., Dorko K., Ellis E.C., Strom S.C. Isolation of Amniotic Epithelial Stem Cells. Curr. Protoc. Stem Cell Biol. 2010;12:1E.3.1–1E.3.10. doi: 10.1002/9780470151808.sc01e03s12. [DOI] [PubMed] [Google Scholar]
- 45.Kim J., Kang H.M., Kim H., Kim M.R., Kwon H.C., Gye M.C., Kang S.G., Yang H.S., You J. Ex Vivo Characteristics of Human Amniotic Membrane-Derived Stem Cells. Cloning Stem Cells. 2007;9:581–594. doi: 10.1089/clo.2007.0027. [DOI] [PubMed] [Google Scholar]
- 46.Riau A.K., Beuerman R.W., Lim L.S., Mehta J.S. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010;31:216–225. doi: 10.1016/j.biomaterials.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 47.Von Versen-Hoeynck F., Steinfeld A.P., Becker J., Hermel M., Rath W., Hesselbarth U. Sterilization and preservation influence the biophysical properties of human amnion grafts. Biologicals. 2008;36:248–255. doi: 10.1016/j.biologicals.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Jirsova K., Jones G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017;18:193–204. doi: 10.1007/s10561-017-9618-5. [DOI] [PubMed] [Google Scholar]
- 49.Rodríguez-Ares M.T., López-Valladares M.J., Touriño R., Vieites B., Gude F., Silva M.T., Couceiro J. Effects of lyophilization on human amniotic membrane. Acta Ophthalmol. 2009;87:396–403. doi: 10.1111/j.1755-3768.2008.01261.x. [DOI] [PubMed] [Google Scholar]
- 50.Hennerbichler S., Reichl B., Pleiner D., Gabriel C., Eibl J., Redl H. The influence of various storage conditions on cell viability in amniotic membrane. Cell Tissue Bank. 2006;8:1–8. doi: 10.1007/s10561-006-9002-3. [DOI] [PubMed] [Google Scholar]
- 51.Fairbairn N., Randolph M., Redmond R. The clinical applications of human amnion in plastic surgery. J. Plast. Reconstr. Aesthetic Surg. 2014;67:662–675. doi: 10.1016/j.bjps.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 52.Laranjo M. Preservation of Amniotic Membrane. In: Mamede A.C., Botelho M.F., editors. Amniotic Membrane: Origin, Characterization and Medical Applications. Springer; Dordrecht, The Netherlands: 2015. pp. 209–230. [Google Scholar]
- 53.Nakamura T., Sekiyama E., Takaoka M., Bentley A.J., Yokoi N., Fullwood N.J., Kinoshita S. The use of trehalose-treated freeze-dried amniotic membrane for ocular surface reconstruction. Biomaterials. 2008;29:3729–3737. doi: 10.1016/j.biomaterials.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 54.Thomasen H., Pauklin M., Steuhl K.-P., Meller D. Comparison of cryopreserved and air-dried human amniotic membrane for ophthalmologic applications. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009;247:1691–1700. doi: 10.1007/s00417-009-1162-y. [DOI] [PubMed] [Google Scholar]
- 55.Shortt A.J., Secker G.A., Lomas R.J., Wilshaw S.-P., Kearney J.N., Tuft S.J., Daniels J.T. The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials. 2009;30:1056–1065. doi: 10.1016/j.biomaterials.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 56.Arai N., Tsuno H., Okabe M., Yoshida T., Koike C., Noguchi M., Nikaido T. Clinical Application of a Hyperdry Amniotic Membrane on Surgical Defects of the Oral Mucosa. J. Oral Maxillofac. Surg. 2012;70:2221–2228. doi: 10.1016/j.joms.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 57.Koob T.J., Young C.S., Lim J.J., Chinn K., Massee M., Carter M., Spencer R., Mason D., Fetterolf D. A Primer on Amniotic Membrane Regenerative Healing. Mimedx; Marietta, GA, USA: 2016. [Google Scholar]
- 58.Koizumi N., Fullwood N.J., Bairaktaris G., Inatomi T., Kinoshita S., Quantock A.J. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2000;41:2506–2513. [PubMed] [Google Scholar]
- 59.Koizumi N., Rigby H., Fullwood N.J., Kawasaki S., Tanioka H., Koizumi K., Kociok N., Joussen A.M., Kinoshita S. Comparison of intact and denuded amniotic membrane as a substrate for cell-suspension culture of human limbal epithelial cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006;245:123–134. doi: 10.1007/s00417-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 60.Wilshaw S.P., Kearney J.N., Fisher J., Ingham E. Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng. 2006;12:2117–2129. doi: 10.1089/ten.2006.12.2117. [DOI] [PubMed] [Google Scholar]
- 61.Courtman D.W., Pereira C.A., Kashef V., McComb D., Lee J.M., Wilson G.J. Development of a pericardial acellular matrix biomaterial: Biochemical and mechanical effects of cell extraction. J. Biomed. Mater. Res. 1994;28:655–666. doi: 10.1002/jbm.820280602. [DOI] [PubMed] [Google Scholar]
- 62.Ilancheran S., Moodley Y., Manuelpillai U. Human Fetal Membranes: A Source of Stem Cells for Tissue Regeneration and Repair? Placenta. 2009;30:2–10. doi: 10.1016/j.placenta.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Grzywocz Z., Pius-Sadowska E., Klos P., Gryzik M., Wasilewska D., Aleksandrowicz B., Dworczynska M., Sabalinska S., Hoser G., Machalinski B., et al. Growth factors and their receptors derived from human amniotic cells in vitro. Folia Histochem. Cytobiol. 2014;52:163–170. doi: 10.5603/FHC.2014.0019. [DOI] [PubMed] [Google Scholar]
- 64.Koizumi N.J., Inatomi T.J., Sotozono C.J., Fullwood N.J., Quantock A.J., Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 2000;20:173–177. doi: 10.1076/0271-3683(200003)2031-9FT173. [DOI] [PubMed] [Google Scholar]
- 65.Hao Y., Ma D.H.-K., Hwang D.G., Kim W.-S., Zhang F. Identification of Antiangiogenic and Antiinflammatory Proteins in Human Amniotic Membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 66.Niknejad H., Paeini-Vayghan G., Tehrani F., Khayat-Khoei M., Peirovi H. Side dependent effects of the human amnion on angiogenesis. Placenta. 2013;34:340–345. doi: 10.1016/j.placenta.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Niknejad H., Yazdanpanah G. Anticancer effects of human amniotic membrane and its epithelial cells. Med. Hypotheses. 2014;82:488–489. doi: 10.1016/j.mehy.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 68.Niknejad H., Khayat-Khoei M., Peirovi H., Abolghasemi H. Human amniotic epithelial cells induce apoptosis of cancer cells: A new anti-tumor therapeutic strategy. Cytotherapy. 2014;16:33–40. doi: 10.1016/j.jcyt.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Lee S.B., Li D.Q., Tan D.T., Meller D.C., Tseng S.C. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr. Eye Res. 2000;20:325–334. doi: 10.1076/0271-3683(200004)2041-5FT325. [DOI] [PubMed] [Google Scholar]
- 70.Chopra A., Thomas B.S. Amniotic Membrane: A Novel Material for Regeneration and Repair. J. Biomim. Biomater. Tissue Eng. 2013;18:1–8. [Google Scholar]
- 71.King A., Paltoo A., Kelly R., Sallenave J.-M., Bocking A., Challis J. Expression of Natural Antimicrobials by Human Placenta and Fetal Membranes. Placenta. 2007;28:161–169. doi: 10.1016/j.placenta.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Kjaergaard N., Hein M., Hyttel L., Helmig R., Schønheyder H., Uldbjerg N., Madsen H. Antibacterial properties of human amnion and chorion in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001;94:224–229. doi: 10.1016/S0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 73.Díaz-Prado S., Rendal-Vázquez M.E., Muiños-López E., Hermida-Gómez T., Rodríguez-Cabarcos M., Fuentes-Boquete I., De Toro F.J., Blanco F.J. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11:183–195. doi: 10.1007/s10561-009-9144-1. [DOI] [PubMed] [Google Scholar]
- 74.Farhadihosseinabadi B., Farahani M., Tayebi T., Jafari A., Biniazan F., Modaresifar K., Moravvej H., Bahrami S., Redl H., Tayebi L., et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2018;46:431–440. doi: 10.1080/21691401.2018.1458730. [DOI] [PubMed] [Google Scholar]
- 75.Herndon D.N., Branski L.K. Contemporary Methods Allowing for Safe and Convenient Use of Amniotic Membrane as a Biologic Wound Dressing for Burns. Ann. Plast. Surg. 2017;78:S9–S10. doi: 10.1097/SAP.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 76.Hopkinson A., McIntosh R.S., Tighe P.J., James D.K., Dua H.S. Amniotic Membrane for Ocular Surface Reconstruction: Donor Variations and the Effect of Handling on TGF-β Content. Investig. Opthalmol. Vis. Sci. 2006;47:4316–4322. doi: 10.1167/iovs.05-1415. [DOI] [PubMed] [Google Scholar]
- 77.López-Valladares M.J., Rodríguez-Ares M.T., Touriño R., Gude F., Silva M.T., Couceiro J. Donor age and gestational age influence on growth factor levels in human amniotic membrane. Acta Ophthalmol. 2010;88:e211–e216. doi: 10.1111/j.1755-3768.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- 78.Rahman I., Said D.G., Maharajan V., Dua H.S. Amniotic membrane in ophthalmology: Indications and limitations. Eye. 2009;23:1954–1961. doi: 10.1038/eye.2008.410. [DOI] [PubMed] [Google Scholar]
- 79.Weidinger A., Poženel L., Wolbank S., Banerjee A. Sub-Regional Differences of the Human Amniotic Membrane and Their Potential Impact on Tissue Regeneration Application. Front. Bioeng. Biotechnol. 2021;8:613804. doi: 10.3389/fbioe.2020.613804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Russo A., Bonci P., Bonci P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane. Cell Tissue Bank. 2011;13:353–361. doi: 10.1007/s10561-011-9261-5. [DOI] [PubMed] [Google Scholar]
- 81.Jabareen M., Mallik A.S., Bilic G., Zisch A.H., Mazza E. Relation between mechanical properties and microstructure of human fetal membranes: An attempt towards a quantitative analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;144:S134–S141. doi: 10.1016/j.ejogrb.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 82.Li W., Ma G., Brazile B., Li N., Dai W., Butler J.R., Claude A.A., Wertheim J.A., Liao J., Wang B. Investigating the Potential of Amnion-Based Scaffolds as a Barrier Membrane for Guided Bone Regeneration. Langmuir. 2015;31:8642–8653. doi: 10.1021/acs.langmuir.5b02362. [DOI] [PubMed] [Google Scholar]
- 83.Rohleder N.H., Loeffelbein D.J., Feistl W., Eddicks M., Wolff K.-D., Gulati A., Steinstraesser L., Kesting M.R. Repair of Oronasal Fistulae by Interposition of Multilayered Amniotic Membrane Allograft. Plast. Reconstr. Surg. 2013;132:172–181. doi: 10.1097/PRS.0b013e3182910b50. [DOI] [PubMed] [Google Scholar]
- 84.Ramuta T.Ž., Kreft M.E. Human Amniotic Membrane and Amniotic Membrane–Derived Cells. Cell Transplant. 2018;27:77–92. doi: 10.1177/0963689717725528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adamowicz J., Pokrywczyńska M., Tworkiewicz J., Kowalczyk T., Van Breda S.V., Tyloch D., Kloskowski T., Bodnar M., Skopinska-Wisniewska J., Marszałek A., et al. New Amniotic Membrane Based Biocomposite for Future Application in Reconstructive Urology. PLoS ONE. 2016;11:e0146012. doi: 10.1371/journal.pone.0146012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gholipourmalekabadi M., Samadikuchaksaraei A., Seifalian A.M., Urbanska A.M., Ghanbarian H., Hardy J.G., Omrani M.D., Mozafari M., Reis R.L., Kundu S.C. Silk fibroin/amniotic membrane 3D bi-layered artificial skin. Biomed. Mater. 2017;13:035003. doi: 10.1088/1748-605X/aa999b. [DOI] [PubMed] [Google Scholar]
- 87.Niknejad H., Deihim T., Solati-Hashjin M., Peirovi H. The effects of preservation procedures on amniotic membrane’s ability to serve as a substrate for cultivation of endothelial cells. Cryobiology. 2011;63:145–151. doi: 10.1016/j.cryobiol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 88.Chuck R.S., Graff J.M., Bryant M.R., Sweet P.M. Biomechanical Characterization of Human Amniotic Membrane Preparations for Ocular Surface Reconstruction. Ophthalmic Res. 2004;36:341–348. doi: 10.1159/000081637. [DOI] [PubMed] [Google Scholar]
- 89.Connon C.J., Doutch J., Chen B., Hopkinson A., Mehta J.S., Nakamura T., Kinoshita S., Meek K.M. The variation in transparency of amniotic membrane used in ocular surface regeneration. Br. J. Ophthalmol. 2009;94:1057–1061. doi: 10.1136/bjo.2008.153064. [DOI] [PubMed] [Google Scholar]
- 90.Baguneid M.S., Seifalian A.M., Salacinski H.J., Murray D., Hamilton G., Walker M.G. Tissue engineering of blood vessels. BJS. 2006;93:282–290. doi: 10.1002/bjs.5256. [DOI] [PubMed] [Google Scholar]
- 91.Kamarul T., Krishnamurithy G., Salih N.D., Ibrahim N.S., Raghavendran H.R.B., Suhaeb A.R., Choon D.S.K. Biocompatibility and Toxicity of Poly(vinyl alcohol)/N,O-Carboxymethyl Chitosan Scaffold. Sci. World J. 2014;2014:905103. doi: 10.1155/2014/905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilshaw S.-P., Kearney J., Fisher J., Ingham E. Biocompatibility and Potential of Acellular Human Amniotic Membrane to Support the Attachment and Proliferation of Allogeneic Cells. Tissue Eng. Part A. 2008;14:463–472. doi: 10.1089/tea.2007.0145. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura T., Inatomi T., Sekiyama E., Ang L.P.K., Yokoi N., Kinoshita S. Novel clinical application of sterilized, freeze-dried amniotic membrane to treat patients with pterygium. Acta Ophthalmol. Scand. 2006;84:401–405. doi: 10.1111/j.1600-0420.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 94.Zhang T., Yam G.H.-F., Riau A.K., Poh R., Allen J.C., Peh G.S., Beuerman R.W., Tan D.T., Mehta J.S. The Effect of Amniotic Membrane De-Epithelialization Method on its Biological Properties and Ability to Promote Limbal Epithelial Cell Culture. Investig. Opthalmol. Vis. Sci. 2013;54:3072–3081. doi: 10.1167/iovs.12-10805. [DOI] [PubMed] [Google Scholar]
- 95.Roux S., Bodivit G., Bartis W., Lebouvier A., Chevallier N., Fialaire-Legendre A., Bierling P., Rouard H. In Vitro Characterization of Patches of Human Mesenchymal Stromal Cells. Tissue Eng. Part A. 2015;21:417–425. doi: 10.1089/ten.tea.2013.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan S.-L., Sulaiman S., Pingguan-Murphy B., Selvaratnam L., Tai C.-C., Kamarul T. Human amnion as a novel cell delivery vehicle for chondrogenic mesenchymal stem cells. Cell Tissue Bank. 2009;12:59–70. doi: 10.1007/s10561-009-9164-x. [DOI] [PubMed] [Google Scholar]
- 97.Gholipourmalekabadi M., Sameni M., Radenkovic D., Mozafari M., Mossahebi-Mohammadi M., Seifalian A. Decellularized human amniotic membrane: How viable is it as a delivery system for human adipose tissue-derived stromal cells? Cell Prolif. 2016;49:115–121. doi: 10.1111/cpr.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin C.Z., Park S.R., Choi B.H., Lee K.-Y., Kang C.K., Min B.-H. Human Amniotic Membrane as a Delivery Matrix for Articular Cartilage Repair. Tissue Eng. 2007;13:693–702. doi: 10.1089/ten.2006.0184. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y.-J., Chung M.-C., Yao C.-C.J., Huang C.-H., Chang H.-H., Jeng J.-H., Young T.-H. The effects of acellular amniotic membrane matrix on osteogenic differentiation and ERK1/2 signaling in human dental apical papilla cells. Biomaterials. 2012;33:455–463. doi: 10.1016/j.biomaterials.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 100.Jerman U.D., Veranič P., Kreft M.E. Amniotic Membrane Scaffolds Enable the Development of Tissue-Engineered Urothelium with Molecular and Ultrastructural Properties Comparable to that of Native Urothelium. Tissue Eng. Part C Methods. 2014;20:317–327. doi: 10.1089/ten.tec.2013.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iranpour S., Mahdavi-Shahri N., Miri R., Hasanzadeh H., Bidkhori H.R., Naderi-Meshkin H., Zahabi E., Matin M.M. Supportive properties of basement membrane layer of human amniotic membrane enable development of tissue engineering applications. Cell Tissue Bank. 2018;19:357–371. doi: 10.1007/s10561-017-9680-z. [DOI] [PubMed] [Google Scholar]
- 102.Schwab I.R., Reyes M., Isseroff R.R. Successful Transplantation of Bioengineered Tissue Replacements in Patients with Ocular Surface Disease. Cornea. 2000;19:421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 103.Tsai R.J.-F., Li L.-M., Chen J.-K. Reconstruction of Damaged Corneas by Transplantation of Autologous Limbal Epithelial Cells. N. Engl. J. Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 104.Koizumi N., Inatomi T., Suzuki T., Sotozono C., Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders11The authors have no financial interest in this work. Ophthalmology. 2001;108:1569–1574. doi: 10.1016/S0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- 105.Malhotra C., Jain A.K. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transplant. 2014;4:111–121. doi: 10.5500/wjt.v4.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakamura T., Inatomi T., Sotozono C., Amemiya T., Kanamura N., Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004;88:1280–1284. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walkden A. Amniotic Membrane Transplantation in Ophthalmology: An Updated Perspective. Clin. Ophthalmol. 2020;14:2057–2072. doi: 10.2147/OPTH.S208008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Che X., Wu H., Jia C., Sun H., Ou S., Wang J., Jeyalatha M.V., Yu J., Zuo C., Liu Z., et al. A Novel Tissue-Engineered Corneal Stromal Equivalent Based on Amniotic Membrane and Keratocytes. Investig. Opthalmol. Vis. Sci. 2019;60:517–527. doi: 10.1167/iovs.18-24869. [DOI] [PubMed] [Google Scholar]
- 109.Bandeira F., Yam G.H., Fuest M., Ong H.S., Liu Y., Seah X., Shen S.Y., Mehta J.S. Urea-De-Epithelialized Human Amniotic Membrane for Ocular Surface Reconstruction. Stem Cells Transl. Med. 2019;8:620–626. doi: 10.1002/sctm.18-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ben M’Barek K., Habeler W., Plancheron A., Jarraya M., Regent F., Terray A., Yang Y., Chatrousse L., Domingues S., Masson Y., et al. Human ESC–derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci. Transl. Med. 2017;9:eaai7471. doi: 10.1126/scitranslmed.aai7471. [DOI] [PubMed] [Google Scholar]
- 111.Ben M’Barek K., Bertin S., Brazhnikova E., Jaillard C., Habeler W., Plancheron A., Fovet C.-M., Demilly J., Jarraya M., Bejanariu A., et al. Clinical-grade production and safe delivery of human ESC derived RPE sheets in primates and rodents. Biomaterials. 2020;230:119603. doi: 10.1016/j.biomaterials.2019.119603. [DOI] [PubMed] [Google Scholar]
- 112.Laurent I., Astère M., Wang K.R., Cheng Q.-F., Li Q.F. Efficacy and Time Sensitivity of Amniotic Membrane treatment in Patients with Diabetic Foot Ulcers: A Systematic Review and Meta-analysis. Diabetes Ther. 2017;8:967–979. doi: 10.1007/s13300-017-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murphy S.V., Skardal A., Song L., Sutton K., Haug R., Mack D.L., Jackson J., Soker S., Atala A. Solubilized Amnion Membrane Hyaluronic Acid Hydrogel Accelerates Full-Thickness Wound Healing. Stem Cells Transl. Med. 2017;6:2020–2032. doi: 10.1002/sctm.17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang L., Shirakata Y., Shudou M., Dai X., Tokumaru S., Hirakawa S., Sayama K., Hamuro J., Hashimoto K. New skin-equivalent model from de-epithelialized amnion membrane. Cell Tissue Res. 2006;326:69–77. doi: 10.1007/s00441-006-0208-2. [DOI] [PubMed] [Google Scholar]
- 115.Kim S.S., Song C.K., Shon S.K., Lee K.Y., Kim C.H., Lee M.J., Wang L. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res. 2009;336:59–66. doi: 10.1007/s00441-009-0766-1. [DOI] [PubMed] [Google Scholar]
- 116.Redondo P., De Azcarate A.G., Marqués L., García-Guzman M., Andreu E., Prósper F. Amniotic Membrane as a Scaffold for Melanocyte Transplantation in Patients with Stable Vitiligo. Dermatol. Res. Pract. 2011;2011:1–6. doi: 10.1155/2011/532139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsai S.-H., Liu Y.-W., Tang W.-C., Zhou Z.-W., Hwang C.-Y., Hwang G.-Y., Ou B.-R., Hu C.-P., Yang V.C., Chen J.-K. Characterization of porcine arterial endothelial cells cultured on amniotic membrane, a potential matrix for vascular tissue engineering. Biochem. Biophys. Res. Commun. 2007;357:984–990. doi: 10.1016/j.bbrc.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 118.Lee P.-H., Tsai S.-H., Kuo L., Hwang C.-Y., Kuo C.-Y., Yang V.C., Chen J.-K. A prototype tissue engineered blood vessel using amniotic membrane as scaffold. Acta Biomater. 2012;8:3342–3348. doi: 10.1016/j.actbio.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 119.Peirovi H., Rezvani N., Hajinasrollah M., Mohammadi S.S., Niknejad H. Implantation of amniotic membrane as a vascular substitute in the external jugular vein of juvenile sheep. J. Vasc. Surg. 2012;56:1098–1104. doi: 10.1016/j.jvs.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 120.Amensag S., McFetridge P.S. Rolling the Human Amnion to Engineer Laminated Vascular Tissues. Tissue Eng. Part C Methods. 2012;18:903–912. doi: 10.1089/ten.tec.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amensag S., Goldberg L.A., O’Malley K.A., Rush D.S., Berceli S.A., McFetridge P.S. Pilot assessment of a human extracellular matrix-based vascular graft in a rabbit model. J. Vasc. Surg. 2017;65:839–847.e1. doi: 10.1016/j.jvs.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Swim M.M., Albertario A., Iacobazzi D., Caputo M., Ghorbel M.T. Amnion-Based Scaffold with Enhanced Strength and Biocompatibility for In Vivo Vascular Repair. Tissue Eng. Part A. 2019;25:603–619. doi: 10.1089/ten.tea.2018.0175. [DOI] [PubMed] [Google Scholar]
- 123.Kakavand M., Yazdanpanah G., Ahmadiani A., Niknejad H. Blood compatibility of human amniotic membrane compared with heparin-coated ePTFE for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2015;11:1701–1709. doi: 10.1002/term.2064. [DOI] [PubMed] [Google Scholar]
- 124.Norris M.A., Cohen M.S., Warren M.M., Becker S.N., Baur P.S., Seybold H.M. Bladder reconstruction in rabbits with glutaraldehyde-stablilized amniotic membranes. Urology. 1982;19:631–635. doi: 10.1016/0090-4295(82)90017-6. [DOI] [PubMed] [Google Scholar]
- 125.Fishman I.J., Flores F., Scott F.B., Spjut H.J., Morrow B. Use of Fresh Placental Membranes for Bladder Reconstruction. J. Urol. 1987;138:1291–1294. doi: 10.1016/S0022-5347(17)43586-5. [DOI] [PubMed] [Google Scholar]
- 126.Shakeri S., Masoudi P., Yazdani M., Monabbati A., Mehrabani D., Tanideh N. Evaluation of human amniotic membrane as a substitute for transitional epithelium of bladder in dog. J. Appl. Anim. Res. 2008;33:55–59. doi: 10.1080/09712119.2008.9706896. [DOI] [Google Scholar]
- 127.Barski D., Gerullis H., Ecke T., Varga G., Boros M., Pintelon I., Timmermans J.-P., Winter A., Bagner J.-W., Otto T. Repair of a vesico-vaginal fistula with amniotic membrane—Step 1 of the IDEAL recommendations of surgical innovation. Central Eur. J. Urol. 2015;68:459–461. doi: 10.5173/ceju.2015.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brandt F.T., Albuquerque C.D., Lorenzato F.R. Female urethral reconstruction with amnion grafts. Int. J. Surg. Investig. 2000;1:409–414. [PubMed] [Google Scholar]
- 129.Mhaskar R. Amniotic membrane for cervical reconstruction. Int. J. Gynecol. Obstet. 2005;90:123–127. doi: 10.1016/j.ijgo.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 130.Fotopoulou C., Sehouli J., Gehrmann N., Schoenborn I., Lichtenegger W. Functional and anatomic results of amnion vaginoplasty in young women with Mayer-Rokitansky-Küster-Hauser syndrome. Fertil. Steril. 2010;94:317–323. doi: 10.1016/j.fertnstert.2009.01.154. [DOI] [PubMed] [Google Scholar]
- 131.Vatsa R., Bharti J., Roy K.K., Kumar R.K., Sharma J.B., Singh N., Singhal S., Meena J. Evaluation of amnion in creation of neovagina in women with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil. Steril. 2017;108:341–345. doi: 10.1016/j.fertnstert.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 132.Sharifiaghdas F., Moghadasali R., Baharvand H., Hosseini-Moghaddam S.M., Mahmoudnejad N. Special characteristics of culturing mature human bladder smooth muscle cells on human amniotic membrane as a suitable matrix. Urol. J. 2009;6:283–288. [PubMed] [Google Scholar]
- 133.Seyed-Forootan K., Karimi H., Seyed-Forootan N.-S. Autologous Fibroblast-Seeded Amnion for Reconstruction of Neo-vagina in Male-to-Female Reassignment Surgery. Aesthetic Plast. Surg. 2018;42:491–497. doi: 10.1007/s00266-018-1088-z. [DOI] [PubMed] [Google Scholar]
- 134.Adamowicz J., Van Breda S., Tyloch D., Pokrywczynska M., Drewa T. Application of amniotic membrane in reconstructive urology; the promising biomaterial worth further investigation. Expert Opin. Biol. Ther. 2018;19:9–24. doi: 10.1080/14712598.2019.1556255. [DOI] [PubMed] [Google Scholar]
- 135.Oottamasathien S., Hotaling J.M., Craig J.R., Myers J.B., Brant W.O. Amniotic therapeutic biomaterials in urology: Current and future applications. Transl. Androl. Urol. 2017;6:943–950. doi: 10.21037/tau.2017.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shakeri S., Haghpanah A., Khezri A., Yazdani M., Monabbati A., Haghpanah S., Malekmakan L., Ayrempour S. Application of amniotic membrane as xenograft for urethroplasty in rabbit. Int. Urol. Nephrol. 2009;41:895–901. doi: 10.1007/s11255-009-9532-2. [DOI] [PubMed] [Google Scholar]
- 137.Sartoneva R., Haimi S., Miettinen S., Mannerström B., Haaparanta A.-M., Sándor G.K., Kellomäki M., Suuronen R., Lahdes-Vasama T. Comparison of a poly- l -lactide-co- ɛ -caprolactone and human amniotic membrane for urothelium tissue engineering applications. J. R. Soc. Interface. 2010;8:671–677. doi: 10.1098/rsif.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salehipour M., Mohammadian R., Jahanbini S., Emadmarvasti V., Geramizadeh B., Tanideh N. Is amniotic membrane a suitable biomaterial for reconstruction of long ureteral defects? Saudi J. Kidney Dis. Transplant. 2013;24:135–138. doi: 10.4103/1319-2442.106311. [DOI] [PubMed] [Google Scholar]
- 139.Koziak A., Marcheluk A., Dmowski T., Szcześniewski R., Kania P., Dorobek A. Reconstructive surgery of male urethra using human amnion membranes (grafts)—First announcement. Ann. Transplant. 2004;9:21–24. [PubMed] [Google Scholar]
- 140.Koziak A., Salagierski M., Marcheluk A., Szcześniewski R., Sosnowski M. Early experience in reconstruction of long ureteral strictures with allogenic amniotic membrane. Int. J. Urol. 2007;14:607–610. doi: 10.1111/j.1442-2042.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 141.Sharifiaghdas F., Hamzehiesfahani N., Moghadasali R., Ghaemimanesh F., Baharvand H. Human amniotic membrane as a suitable matrix for growth of mouse urothelial cells in comparison with human peritoneal and omentum membranes. Urol. J. 2007;4:71–78. [PubMed] [Google Scholar]
- 142.Yuan J., Wang F., Liu T., Yang L., Zhang G., Liu H., Yi X., Yang X., Lin T.-Y., Qin W. Urethral Reconstruction with Tissue-Engineered Human Amniotic Scaffold in Rabbit Urethral Injury Models. Med. Sci. Monit. 2014;20:2430–2438. doi: 10.12659/MSM.891042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen C., Zheng S., Zhang X., Dai P., Gao Y., Nan L., Zhang Y. Transplantation of Amniotic Scaffold-Seeded Mesenchymal Stem Cells and/or Endothelial Progenitor Cells From Bone Marrow to Efficiently Repair 3-cm Circumferential Urethral Defect in Model Dogs. Tissue Eng. Part A. 2018;24:47–56. doi: 10.1089/ten.tea.2016.0518. [DOI] [PubMed] [Google Scholar]
- 144.Garcia D., Longo U., Vaquero J., Forriol F., Loppini M., Khan W., Denaro V. Amniotic Membrane Transplant for Articular Cartilage Repair: An Experimental Study in Sheep. Curr. Stem Cell Res. Ther. 2014;10:77–83. doi: 10.2174/1574888X09666140710120012. [DOI] [PubMed] [Google Scholar]
- 145.Krishnamurithy G., Shilpa P.N., Ahmad R.E., Sulaiman S., Ng C.L.L., Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: In vitro study. J. Biomed. Mater. Res. Part A. 2011;99:500–506. doi: 10.1002/jbm.a.33184. [DOI] [PubMed] [Google Scholar]
- 146.Hussin I.H., Pingguan-Murphy B., Osman S.Z. The Fabrication of Human Amniotic Membrane Based Hydrogel for Cartilage Tissue Engineering Applications: A Preliminary Study; Proceedings of the 5th Kuala Lumpur International Conference on Biomedical Engineering 2011; Kuala Lumpur, Malaysia. 20–23 June 2011; Berlin/Heidelberg, Germany: Springer; 2011. pp. 841–844. [Google Scholar]
- 147.Nogami M., Kimura T., Seki S., Matsui Y., Yoshida T., Koike-Soko C., Okabe M., Motomura H., Gejo R., Nikaido T. A Human Amnion-Derived Extracellular Matrix-Coated Cell-Free Scaffold for Cartilage Repair: In Vitro and In Vivo Studies. Tissue Eng. Part A. 2016;22:680–688. doi: 10.1089/ten.tea.2015.0285. [DOI] [PubMed] [Google Scholar]
- 148.Lindenmair A., Wolbank S., Stadler G., Meinl A., Peterbauer-Scherb A., Eibl J., Polin H., Gabriel C., Van Griensven M., Redl H. Osteogenic differentiation of intact human amniotic membrane. Biomaterials. 2010;31:8659–8665. doi: 10.1016/j.biomaterials.2010.07.090. [DOI] [PubMed] [Google Scholar]
- 149.Ghanmi S., Trigui M., Baya W., Ellouz Z., Elfeki A., Charfi S., Fricain J.C., Keskes H. The periosteum-like effect of fresh human amniotic membrane on bone regeneration in a rabbit critical-sized defect model. Bone. 2018;110:392–404. doi: 10.1016/j.bone.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 150.Tang K., Wu J., Xiong Z., Ji Y., Sun T., Guo X. Human acellular amniotic membrane: A potential osteoinductive biomaterial for bone regeneration. J. Biomater. Appl. 2017;32:754–764. doi: 10.1177/0885328217739753. [DOI] [PubMed] [Google Scholar]
- 151.Bourgeois M., Loisel F., Bertrand D., Nallet J., Gindraux F., Adam A., Lepage D., Sergent P., Leclerc G., Rondot T., et al. Management of forearm bone loss with induced membrane technique. Hand Surg. Rehabil. 2020;39:171–177. doi: 10.1016/j.hansur.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 152.Gindraux F., Loisel F., Bourgeois M., Oudina K., Melin M., De Billy B., Sergent P., Leclerc G., Petite H., Auber F., et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet’s technique is performed later. Eur. J. Trauma Emerg. Surg. 2019;46:301–312. doi: 10.1007/s00068-019-01184-4. [DOI] [PubMed] [Google Scholar]
- 153.Masquelet A.C., Obert L. Induced membrane technique for bone defects in the hand and wrist. Chir. Main. 2010;29(Suppl. 1):S221–S224. doi: 10.1016/j.main.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 154.Masquelet A.-C., Fitoussi F., Begue T., Muller G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000;45:346–353. [PubMed] [Google Scholar]
- 155.Tsugawa J., Komaki M., Yoshida T., Nakahama K.-I., Amagasa T., Morita I. Cell-printing and transfer technology applications for bone defects in mice. J. Tissue Eng. Regen. Med. 2011;5:695–703. doi: 10.1002/term.366. [DOI] [PubMed] [Google Scholar]
- 156.Semyari H., Rajipour M., Sabetkish S., Sabetkish N., Abbas F.M., Kajbafzadeh A.-M. Evaluating the bone regeneration in calvarial defect using osteoblasts differentiated from adipose-derived mesenchymal stem cells on three different scaffolds: An animal study. Cell Tissue Bank. 2015;17:69–83. doi: 10.1007/s10561-015-9518-5. [DOI] [PubMed] [Google Scholar]
- 157.Akazawa K., Iwasaki K., Nagata M., Yokoyama N., Ayame H., Yamaki K., Tanaka Y., Honda I., Morioka C., Kimura T., et al. Double-layered cell transfer technology for bone regeneration. Sci. Rep. 2016;6:33286. doi: 10.1038/srep33286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Akhlaghi F., Hesami N., Rad M.R., Nazeman P., Fahimipour F., Khojasteh A. Improved bone regeneration through amniotic membrane loaded with buccal fat pad-derived MSCs as an adjuvant in maxillomandibular reconstruction. J. Cranio-Maxillofac. Surg. 2019;47:1266–1273. doi: 10.1016/j.jcms.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 159.Uppada U.K., Kalakonda B., Koppolu P., Varma N., Palakurthy K., Manchikanti V., Prasad S., Samar S., Swapna L.A. Combination of hydroxyapatite, platelet rich fibrin and amnion membrane as a novel therapeutic option in regenerative periapical endodontic surgery: Case series. Int. J. Surg. Case Rep. 2017;37:139–144. doi: 10.1016/j.ijscr.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lawson V.G. Oral Cavity Reconstruction Using Pectoralis Major Muscle and Amnion. Arch. Otolaryngol. Head Neck Surg. 1985;111:230–233. doi: 10.1001/archotol.1985.00800060054006. [DOI] [PubMed] [Google Scholar]
- 161.Kesting M.R., Loeffelbein D.J., Classen M., Slotta-Huspenina J., Hasler R.J., Jacobsen F., Kreutzer K., Al-Benna S., Wolff K.-D., Steinstraesser L. Repair of oronasal fistulas with human amniotic membrane in minipigs. Br. J. Oral Maxillofac. Surg. 2010;48:131–135. doi: 10.1016/j.bjoms.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 162.Gulameabasse S., Gindraux F., Catros S., Fricain J., Fenelon M. Chorion and amnion/chorion membranes in oral and periodontal surgery: A systematic review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020 doi: 10.1002/jbm.b.34783. [DOI] [PubMed] [Google Scholar]
- 163.Lai D.R., Chen H.R., Lin L.M., Huang Y.L., Tsai C.C. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J. Oral Pathol. Med. 1995;24:402–406. doi: 10.1111/j.1600-0714.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 164.Khademi B., Bahranifard H., Azarpira N., Behboodi E. Clinical application of amniotic membrane as a biologic dressing in oral cavity and pharyngeal defects after tumor resection. Arch. Iran. Med. 2013;16:503–506. [PubMed] [Google Scholar]
- 165.Ahn K.-M., Lee J.-H., Hwang S.-J., Choung P.-H., Kim M.-J., Park H.-J., Park J.-K., Jahng J., Yang E.-K. Fabrication of Myomucosal Flap Using Tissue-engineered Bioartificial Mucosa Constructed with Oral Keratinocytes Cultured on Amniotic Membrane. Artif. Organs. 2006;30:411–423. doi: 10.1111/j.1525-1594.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- 166.Amemiya T., Nakamura T., Yamamoto T., Kinoshita S., Kanamura N. Immunohistochemical study of oral epithelial sheets cultured on amniotic membrane for oral mucosal reconstruction. Bio-Med. Mater. Eng. 2010;20:37–45. doi: 10.3233/BME-2010-0613. [DOI] [PubMed] [Google Scholar]
- 167.Hsueh Y.-J., Huang S.-F., Lai J.-Y., Ma S.-C., Chen H.-C., Wu S.-E., Wang T.-K., Sun C.-C., Ma K.S.-K., Chen J.-K., et al. Preservation of epithelial progenitor cells from collagenase-digested oral mucosa during ex vivo cultivation. Sci. Rep. 2016;6:36266. doi: 10.1038/srep36266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Amemiya T., Nakamura T., Yamamoto T., Kinoshita S., Kanamura N. Tissue engineering by transplantation of oral epithelial sheets cultivated on amniotic membrane for oral mucosal reconstruction. Inflamm. Regen. 2010;30:176–180. doi: 10.2492/inflammregen.30.176. [DOI] [PubMed] [Google Scholar]
- 169.Amemiya T., Nakamura T., Yamamoto T., Kinoshita S., Kanamura N. Autologous Transplantation of Oral Mucosal Epithelial Cell Sheets Cultured on an Amniotic Membrane Substrate for Intraoral Mucosal Defects. PLoS ONE. 2015;10:e0125391. doi: 10.1371/journal.pone.0125391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gurinsky B. A novel dehydrated amnion allograft for use in the treatment of gingival recession: An observational case series. J. Implant Adv. Clin. Dent. 2009;1:65–73. [Google Scholar]
- 171.Shetty S.S., Chatterjee A., Bose S. Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. J. Indian Soc. Periodontol. 2014;18:102–106. doi: 10.4103/0972-124X.128261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sharma A., Yadav K. Amniotic membrane—A Novel material for the root coverage: A case series. J. Indian Soc. Periodontol. 2015;19:444–448. doi: 10.4103/0972-124X.154166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Orzechowska-Wylęgała B., Dobrowolski D., Puzzolo D., Wowra B., Niemiec W., Wylęgała A., Szczubiałka K. Use of Autologous Epithelium Transplantation on Various Scaffolds to Cover Tissue Loss in Oral Cavity: Long-Term Observation. J. Appl. Biomater. Funct. Mater. 2017;15:25–30. doi: 10.5301/jabfm.5000312. [DOI] [PubMed] [Google Scholar]
- 174.Amemiya T., Adachi K., Nishigaki M., Yamamoto T., Kanamura N. Experiences of preclinical use of periodontal ligament-derived cell sheet cultured on human amniotic membrane. J. Oral. Tissue Eng. 2008;6:106–112. [Google Scholar]
- 175.Iwasaki K., Komaki M., Yokoyama N., Tanaka Y., Taki A., Honda I., Kimura Y., Takeda M., Akazawa K., Oda S., et al. Periodontal Regeneration Using Periodontal Ligament Stem Cell-Transferred Amnion. Tissue Eng. Part A. 2013;20:693–704. doi: 10.1089/ten.tea.2013.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu P.-H., Chung H.-Y., Wang J.-H., Shih J.-C., Kuo M.Y.-P., Chang P.-C., Huang Y.-D., Wang P.-C., Chang C.-C. Amniotic membrane and adipose-derived stem cell co-culture system enhances bone regeneration in a rat periodontal defect model. J. Formos. Med Assoc. 2016;115:186–194. doi: 10.1016/j.jfma.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 177.Honjo K.-I., Yamamoto T., Adachi T., Amemiya T., Mazda O., Kanamura N., Kita M. Evaluation of a dental pulp-derived cell sheet cultured on amniotic membrane substrate. Bio-Med. Mater. Eng. 2015;25:203–212. doi: 10.3233/BME-151270. [DOI] [PubMed] [Google Scholar]
- 178.Tuncel U., Ozgenel G. Use of Human Amniotic Membrane as an Interpositional Material in Treatment of Temporomandibular Joint Ankylosis. J. Oral Maxillofac. Surg. 2011;69:e58–e66. doi: 10.1016/j.joms.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 179.Tuncel U., Kostakoglu N., Turan A., Markoc F., Gokce E., Erkorkmaz U., Turan A. The use of temporalis muscle graft, fresh and cryopreserved amniotic membrane in preventing temporomandibular joint ankylosis after discectomy in rabbits. J. Cranio-Maxillofac. Surg. 2014;42:1868–1876. doi: 10.1016/j.jcms.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 180.Guarda-Nardini L., Trojan D., Montagner G., Cogliati E., Bendini M., Manfredini D. Human Amniotic Membrane Positioning in the Surgical Treatment of Temporomandibular Joint Degenerative Disorder. Case Rep. Surg. 2019;2019:6037191. doi: 10.1155/2019/6037191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ragazzo M., Trojan D., Spagnol L., Paolin A., Nardini L.G. Use of amniotic membrane in the treatment of patients with BRONJ: Two case reports. J. Surg. Case Rep. 2018;2018:rjy073. doi: 10.1093/jscr/rjy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lemke A., Ferguson J., Gross K., Penzenstadler C., Bradl M., Mayer R., Gerner C., Redl H., Wolbank S. Transplantation of human amnion prevents recurring adhesions and ameliorates fibrosis in a rat model of sciatic nerve scarring. Acta Biomater. 2018;66:335–349. doi: 10.1016/j.actbio.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 183.Meng H., Li M., You F., Du J., Luo Z. Assessment of processed human amniotic membrane as a protective barrier in rat model of sciatic nerve injury. Neurosci. Lett. 2011;496:48–53. doi: 10.1016/j.neulet.2011.03.090. [DOI] [PubMed] [Google Scholar]
- 184.Özgenel G.Y., Filiz G. Combined Application of Human Amniotic Membrane Wrapping and Hyaluronic Acid Injection in Epineurectomized Rat Sciatic Nerve. J. Reconstr. Microsurg. 2004;20:153–157. doi: 10.1055/s-2004-820772. [DOI] [PubMed] [Google Scholar]
- 185.Marchesini A., Raimondo S., Zingaretti N., Riccio V., Battiston B., Provinciali M., Geuna S., Riccio M. The amnion muscle combined graft (AMCG) conduits in nerves repair: An anatomical and experimental study on a rat model. J. Mater. Sci. Mater. Med. 2018;29:120. doi: 10.1007/s10856-018-6126-5. [DOI] [PubMed] [Google Scholar]
- 186.Riccio E., Esposito G., Franzone A., Imbriaco M., Santangelo M., Pisani A. Renal Sympathetic-Nerve Ablation for Uncontrolled Hypertension in a Patient With Single-Kidney Autosomal Dominant Polycystic Kidney Disease. J. Clin. Hypertens. 2014;16:385–386. doi: 10.1111/jch.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ozcan G., Shenaq S., Spira M. Vascularized Nerve Tube: An Experimental Alternative for Vascularized Nerve Grafts Over Short Gaps. J. Reconstr. Microsurg. 1993;9:405–413. doi: 10.1055/s-2007-1006749. [DOI] [PubMed] [Google Scholar]
- 188.Mohammad J., Shenaq J., Rabinovsky E., Shenaq S. Modulation of Peripheral Nerve Regeneration: A Tissue-Engineering Approach. The Role of Amnion Tube Nerve Conduit across a 1-Centimeter Nerve Gap. Plast. Reconstr. Surg. 2000;105:660–666. doi: 10.1097/00006534-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 189.Mligiliche N., Endo K., Okamoto K., Fujimoto E., Ide C. Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J. Biomed. Mater. Res. 2002;63:591–600. doi: 10.1002/jbm.10349. [DOI] [PubMed] [Google Scholar]
- 190.Dong R., Liu C., Tian S., Bai J., Yu K., Liu L., Tian D. Electrospun polycaprolactone (PCL)-amnion nanofibrous membrane prevents adhesions and promotes nerve repair in a rat model of sciatic nerve compression. PLoS ONE. 2020;15:e0244301. doi: 10.1371/journal.pone.0244301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang Q., Gu X.-M., Yu G.-Y., Mao T.-Q., Zheng J.-C., Tong Q.-Y. Repair of peripheral nerve defect by a scroll of amnion derivative compound with cultured autogenous Schwann cell in a rat model. Zhonghua Kou Qiang Yi Xue Za Zhi. 2006;41:98–101. [PubMed] [Google Scholar]
- 192.Li Z., Qin H., Feng Z., Liu W., Zhou Y., Yang L., Zhao W., Li Y. Human umbilical cord mesenchymal stem cell-loaded amniotic membrane for the repair of radial nerve injury. Neural Regen. Res. 2013;8:3441–3448. doi: 10.3969/j.issn.1673-5374.2013.36.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Riboh J.C., Saltzman B.M., Yanke A.B., Cole B.J. Human Amniotic Membrane–Derived Products in Sports Medicine. Am. J. Sports Med. 2016;44:2425–2434. doi: 10.1177/0363546515612750. [DOI] [PubMed] [Google Scholar]
- 194.Heckmann N., Auran R., Mirzayan R. Application of Amniotic Tissue in Orthopedic Surgery. Am. J. Orthop. 2016;45:E421–E425. [PubMed] [Google Scholar]
- 195.Demirkan F., Colakoglu N., Herek O., Erkula G. The use of amniotic membrane in flexor tendon repair: An experimental model. Arch. Orthop. Trauma Surg. 2002;122:396–399. doi: 10.1007/s00402-002-0418-3. [DOI] [PubMed] [Google Scholar]
- 196.Özgenel G.Y. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J. Bone Jt. Surg. Br. Vol. 2004;86:301–307. doi: 10.1302/0301-620X.86B2.14435. [DOI] [PubMed] [Google Scholar]
- 197.Liu C., Yu K., Bai J., Tian D., Liu G. Experimental study of tendon sheath repair via decellularized amnion to prevent tendon adhesion. PLoS ONE. 2018;13:e0205811. doi: 10.1371/journal.pone.0205811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Warner M., LaSyone L. An Open-label, Single-center, Retrospective Study of Cryopreserved Amniotic Membrane and Umbilical Cord Tissue as an Adjunct for Foot and Ankle Surgery. Surg. Technol. Int. 2014;25:251–255. [PubMed] [Google Scholar]
- 199.DeMill S.L., Granata J.D., McAlister J., Berlet G.C., Hyer C.F. Safety analysis of cryopreserved amniotic membrane/umbilical cord tissue in foot and ankle surgery: A consecutive case series of 124 patients. Surg. Technol. Int. 2014;25:257–261. [PubMed] [Google Scholar]
- 200.Seo Y.-K., Kim J.-H., Eo S.-R. Co-effect of silk and amniotic membrane for tendon repair. J. Biomater. Sci. Polym. Ed. 2016;27:1232–1247. doi: 10.1080/09205063.2016.1188349. [DOI] [PubMed] [Google Scholar]
- 201.He Q., Li Q., Chen B., Wang Z. Repair of flexor tendon defects of rabbit with tissue engineering method. Chin. J. Traumatol. 2002;5:200–208. [PubMed] [Google Scholar]
- 202.Cargnoni A., Di Marcello M., Campagnol M., Nassuato C., Albertini A., Parolini O. Amniotic Membrane Patching Promotes Ischemic Rat Heart Repair. Cell Transplant. 2009;18:1147–1159. doi: 10.3727/096368909X12483162196764. [DOI] [PubMed] [Google Scholar]
- 203.Roy R., Haase T., Ma N., Bader A., Becker M., Seifert M., Choi Y.-H., Falk V., Stamm C. Decellularized amniotic membrane attenuates postinfarct left ventricular remodeling. J. Surg. Res. 2016;200:409–419. doi: 10.1016/j.jss.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 204.Lim J.J., Koob T.J., Fonger J., Koob T.J. Dehydrated human amnion/chorionmembrane allograft promotes cardiac repair following myocardial infarction. J. Cardiol. Cardiovasc. Ther. 2017;2:2–7. [Google Scholar]
- 205.Francisco J., Cunha R.C., Cardoso M., Simeoni R.B., Mogharbel B., Picharski G., Dziedzic D.S.M., Guarita-Souza L., Carvalho K. Decellularized Amniotic Membrane Scaffold as a Pericardial Substitute: An In Vivo Study. Transplant. Proc. 2016;48:2845–2849. doi: 10.1016/j.transproceed.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 206.Khalpey Z., Marsh K.M., Ferng A., Bin Riaz I., Friedman M., Indik J., Avery R., Jokerst C., Oliva I. First in Man: Amniotic Patch Reduces Postoperative Inflammation. Am. J. Med. 2015;128:e5–e6. doi: 10.1016/j.amjmed.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 207.Marsh K.M., Ferng A.S., Pilikian T., Desai A., Avery R., Friedman M., Oliva I., Jokerst C., Schipper D., Khalpey Z. Anti-inflammatory properties of amniotic membrane patch following pericardiectomy for constrictive pericarditis. J. Cardiothorac. Surg. 2017;12:6. doi: 10.1186/s13019-017-0567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Becker M., Maring J.A., Schneider M., Martin A.X.H., Seifert M., Klein O., Braun T., Falk V., Stamm C. Towards a Novel Patch Material for Cardiac Applications: Tissue-Specific Extracellular Matrix Introduces Essential Key Features to Decellularized Amniotic Membrane. Int. J. Mol. Sci. 2018;19:1032. doi: 10.3390/ijms19041032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Parveen S., Singh S.P., Panicker M.M., Gupta P.K. Amniotic membrane as novel scaffold for human iPSC-derived cardiomyogenesis. Vitr. Cell. Dev. Biol. Anim. 2019;55:272–284. doi: 10.1007/s11626-019-00321-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.