Abstract
The aim of the present study was to clarify the influence of inulin and lactulose-fortified prebiotic food intakes on bone metabolism turnover among Japanese female athletes. The participants included 29 female athletes aged 18–25 years. They were requested to consume their habitual foods or drinks with one pack of prebiotic food every day for 12 weeks. Dietary intake, training time, body composition, blood sample, and fecal microbiota were assessed during this intervention period. Body composition, total energy intake, and training time of the participants revealed no significant changes during the intervention period. The occupation ratio of Bifidobacterium spp. was significantly increased at 3 and 4 weeks (18.0 ± 8.3% and 17.6 ± 8.5%, respectively) compared to that of pre-intervention (11.7 ± 7.3%) (p = 0.019 and p = 0.035, respectively). The serum TRACP-5b level was significantly decreased at 12 weeks (363 ± 112 mU/dL) compared to that at baseline (430 ± 154 mU/dL) (p = 0.018). These results suggest that the prebiotic food used in this study might have beneficial effects on bone health and gut microbial environment among female athletes. Further studies are warranted to identify the mechanism of the prebiotics–gut–bone axis.
Keywords: female athletes, prebiotics, gut microbiota, bone health, bone resorption
1. Introduction
Inadequate energy intake can cause a variety of health problems in female athletes. The American College of Sports Medicine defined the concept of the Female Athlete Triad model in 1997 [1] and renewed it in 2007 [2]. This concept highlights the importance of adequate energy intake to assist the athletes’ health, such as bone health, reproductive function, and sport performance.
Poor bone health in female athletes occurs when athletes consume inadequate energy with or without disordered eating. Bone health can be assessed indirectly by assessing the bone metabolism turnover markers, which indicate bone formation or bone resorption [3], to provide useful information on dietary interventions [4,5,6]. For example, inadequate energy intake negatively affects bone metabolism turnover, leading to rapid increases in bone resorption and decreased bone formation [6,7]. Similarly, this modification contributes to reduced bone mineral density and increased risk of stress fractures [8,9]. Female athletes may be at an increased risk of bone disorders, including osteoporosis during or after retiring from their athletic careers [10]. Thus, adequate energy intake is essential for these athletes to prevent impaired bone health.
Nutritional intervention to improve bone health in female athletes addresses chronic energy deficiency and improves energy intake to meet the energy demands. A previous study reported that an increase in habitual energy intake is a useful nutritional strategy for solving women’s health problems [11]. However, many female athletes control their body weight on a daily basis because they believe that the lighter body weight, the better performance [12]. This idea is considered to cause energy intake deficiency. Nevertheless, it may be practically challenging to increase energy intake largely from the habitual diet for maintaining their body weight and sports performance. Calcium and vitamin D are also essential nutrients with a major role in bone health. Calcium is needed for bone mineralization, and vitamin D is needed for maintaining calcium homeostasis and bone remodeling, which have been suggested as protective against bone health among athletes [13]. However, the general incidence of low habitual dietary calcium and vitamin D intakes indicates [14] that some Japanese female athletes are likely to experience high bone metabolism turnover with a concomitant increase in the risk of bone disorders.
In recent years, the important function of prebiotics in the treatment of various diseases, including bone health, was studied. Inulin and lactulose are prebiotics, which are natural ingredients found in many foods. Inulin is generally found in root vegetables such as chrysanthemum, chicory, onion, garlic, and sweet potato [15], whereas lactulose is detected in heated milk [16]. Humans have consumed them for a long time. Inulin and lactulose have an energy content of 2.0 kcal per gram, which is half the calories of carbohydrates [17]. Prebiotics such as inulin and lactulose are selectively metabolized by microbes inside and on the body surface, and several studies have reported that both inulin and lactulose have prebiotic effects [18,19,20]. A prebiotic is defined as a substrate that is selectively utilized by host microorganisms, thus conferring health benefits [21]. These effects occur via prebiotic–microbial interactions in the large intestine. Moreover, a recent review summarized the gut microbiota involved in bone remodeling [22]. Furthermore, a previous study revealed that the combination of different types of prebiotics may have synergistic effects on health by allowing prolonged fermentation throughout the large intestine [23]. In animal studies, the combined intake of different types of prebiotics has shown to improve bone health [24,25]. Therefore, the combined intake of different types of prebiotics may provide nutritional benefits in female athletes. From these contexts, we have developed a new food with low energy including prebiotics such as inulin and lactulose. However, no studies have assessed whether the combination of different types of prebiotics affects bone health and gut microbiota among female athletes. Therefore, a better understanding of the combination effect of prebiotics among female athletes is essential.
The aim of the present study was to clarify the influence of inulin and lactulose-fortified prebiotic food intakes on bone metabolism turnover among Japanese female athletes.
2. Materials and Methods
2.1. Participants
Thirty female athletes aged 18–30 years were recruited in this study. One female athlete (long-distance running) was excluded due to personal reasons. In total, 29 of the 30 female athletes were intervened. The sport events were as follows: rhythmic gymnastics (n = 13), soccer (n = 7), middle- and long-distance running (n = 7) and figure skating (n = 2). Exclusion criteria were as follows: (1) regular use of medication that may influence metabolism or hormones; (2) injury; (3) smoking; (4) history of fractures in the last 6 months; (5) pregnancy; (6) milk allergy, and (7) use of oral contraceptives and antibiotics. All athletes were adequately informed about the study by verbal and written descriptions, and written informed consent was obtained before commencing the study. All procedures were approved by the Ethics Review Committee of Waseda University on Research with Human Subjects (approval number: 2017-096) following the Declaration of Helsinki. This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000029589).
2.2. Dietary Intervention
All participants were requested to take one pack of prebiotic food every day for 12 weeks. The participants were instructed to mix it with their daily diet according to their preference. This milk-flavored prebiotic food was manufactured by Morinaga Milk Industry Co., Ltd. (Tokyo, Japan) as per the request from the research team based on previous studies [26,27]. One pack (25 g) provided 100 kcal of energy, 3.5 g of protein, 2.8 g of fat, 15.0 g of carbohydrate, 2.5 g of inulin, 1.0 g of lactulose, 100 mg of calcium, and 0.5 µg of vitamin D. Prebiotic food was dispensed to each participant at baseline and on a regular basis thereafter. Participants were instructed to record their status of food intake during the intervention period. Moreover, these consumption records were cross-checked between each participant and research team. Empty packs and uneaten packs were collected from each participant during the regular food deliveries in order to monitor their compliance.
2.3. Anthropometric and Body Composition Measurements
Height was measured to 0.1 cm using a stadiometer (YG-200, Yagami, Aichi, Japan). Body weight (BW) was measured by the participants themselves to the nearest 0.05 kg using an electronic scale (UC-321, A&D, Tokyo, Japan) after urination and under morning fasting conditions during the intervention period. Body mass index (BMI) was calculated by dividing the body weight by height squared. Fat mass (FM), fat-free mass (FFM), bone area, bone mineral content (BMC), bone mineral density (BMD), and z-score of the whole body were measured using dual-energy X-ray absorptiometry (DXA) (QDR-4500DXA Scanner, Hologic; Marlborough, MA, USA) during the pre- and post-intervention periods. All scans were performed and analyzed using Hologic software (version 12.4.3, Hologic, MA, USA) by an orthopedic surgeon, who is one of the co-authors of this study.
2.4. Dietary Intake
The total energy intake (TEI) and macro- and micronutrient intake were investigated using a 3-day consecutive weighed food record with a food scale at pre-, mid-, and post-intervention periods. Participants were instructed to record all consumed food and beverages using the provided cooking scale (SD-004, Tanita Co., Ltd., Tokyo, Japan). Additionally, participants were also instructed to record their daily consumption and to take photos of all foods and beverages, including their labels. After completing the dietary record forms, the participants were interviewed by a well-trained dietitian, and their nutritional status was analyzed using the nutritional analysis software (version 2.85, Wellness 21, Top Business System, Okayama, Japan). Energy, macronutrient, and micronutrient intakes were calculated based on the Japanese Standard Food Composition Table 2015 published by the Ministry of Education, Culture, Sports, Science and Technology.
2.5. Training Time
Training time (hours/week) was assessed at pre- and post-intervention periods. All participants were requested to record their weekly training time to their questionnaires.
2.6. Analysis of Fecal Microbiota
Fecal microbiota samples were collected pre-intervention, at 1, 2, 3, 4, and 8 weeks, and post-intervention. These samples were collected by the participants themselves in collection tubes with guanidine thiocyanate solution (Techno Suruga Laboratory Co., Ltd., Shizuoka, Japan). The participants were trained to collect fecal collection before starting this intervention. In addition, the research team distributed stool collection cups to the participants to avoid contamination during the fecal collection. DNA was extracted from these samples, and the samples were analyzed by the terminal restriction fragment length polymorphism (T-RFLP) method. All fecal microbiota samples were analyzed using T-RFLP by Techno Suruga Laboratory Co., Ltd. (Shizuoka, Japan). The abundance of each terminal restriction fragment (T-RF) was calculated by first dividing them into 29 operational taxonomic units (OTUs) by the previous researches [28,29]. Each OTU was quantified as a percentage of the total OTU area, expressed as a percentage of the area under the curve (%AUC). The reference database, human fecal microbiota T-RFLP profiling (www.tecsrg.co.jp/t-rflp/t_rflp_hito_OTU.html, accessed on 3 June 2021) was used to putatively identify the bacteria in each classification unit and the corresponding OTU.
2.7. Blood Samples
Blood samples at pre-intervention, at 4 and 8 weeks, and post-intervention were collected in the morning after overnight fasting for bone metabolism turnover marker, bone-specific alkaline phosphatase (BAP), tartrate-resistant acid phosphatase 5b (TRACP-5b), 25-hydroxyvitamin D3 (25(OH)D3), and estradiol (E2). After clotting, the blood samples were separated via centrifugation at 3000 rpm for 15 min (Spectrafuge™ 6C, Labnet International Inc., Edison, NJ, USA), and the remaining serum was separated into aliquots and frozen at −80 °C until further analysis. TRACP-5b was analyzed using the enzyme immunoassay method. BAP was analyzed using an electro chemiluminescent immunoassay method. 25(OH)D3 was analyzed using the liquid chromatography–tandem mass spectrometry method. E2 was determined using a chemiluminescent immunoassay. All blood samples were analyzed by the LSI Medience Corporation (Tokyo, Japan).
2.8. Statistical Analyses
Data are presented as means ± standard deviations (SD) or medians with interquartile range. The Kolmogorov–Smirnov test was used to check the non-normal data. In order to achieve homogeneity, data were log transformed. Statistical analyses were performed using statistical software (SPSS ver. 25.0, IBM Corporation, Armonk, NY, USA). Repeated-measures one-way analysis of variance (ANOVA) was used to assess the differences in nutritional status, exercise status, fecal microbiota analysis, and blood sample analysis. Bonferroni post hoc corrections for equal variance and the Games–Howell procedure for unequal variance were used to identify significantly different measurement points. The paired t-test or Mann–Whitney test were used to compare between the physical characteristics data during the pre- and post-intervention period. Statistical significance was set at p < 0.05 in two-sided tests for all analyses.
3. Results
3.1. Participants Characteristics
The intake rate of prebiotic food during intervention was 76 ± 21%. The comparison of body composition and bone parameter variables at pre- and post-intervention is presented in Table 1. No significant changes in any parameter, including body weight, were observed after the intervention.
Table 1.
Physical characteristics of the participants during the interventions.
| Pre | Post | p-Value | |
|---|---|---|---|
| Age (y) | 20 ± 1 | - | - |
| Height (cm) | 160.2 ± 5.3 | - | - |
| Body weight (kg) | 51.2 ± 5.5 | 51.3 ± 5.3 | 0.620 |
| BMI (kg/m2) | 19.9 ± 1.7 | 20.0 ± 1.8 | 0.557 |
| Fat mass (kg) | 9.8 (7.0, 10.6) | 9.3 (7.4, 10.9) | 0.184 |
| Fat-free mass (kg) | 40.6 ± 4.2 | 40.7 ± 4.4 | 0.902 |
| Bone area (cm2) | 1845 ± 118 | 1842 ± 125 | 0.620 |
| BMC (g) | 2109 ± 189 | 2096 ± 215 | 0.557 |
| BMD (g/cm2) | 1.143 ± 0.069 | 1.136 ± 0.066 | 0.184 |
| Whole body z-score | 1.3 ± 1.2 | 1.2 ± 1.2 | 0.242 |
| Training time (hours/week) | 25.6 ± 10.0 | 27.3 ± 13.5 | 0.474 |
Data were expressed as mean ± SD or median (inter quartile range); BMI, body mass index; BMC, bone mineral contents; BMD, bone mineral density. p values < 0.05 represent significantly different mean.
3.2. Dietary Status
The energy, and macro- and micronutrient intakes of the participants during intervention are presented in Table 2. TEI, protein, fat, carbohydrate, calcium, vitamin D, vitamin K and dietary fiber intakes at mid- and post-intervention were not significantly different compared with that at pre-intervention. Only iron intake was significantly different in this intervention.
Table 2.
Energy, macro, and micro-nutrient intakes of the participants.
| Pre | Mid | Post | p-Value | |
|---|---|---|---|---|
| TEI (kcal) | 1724 ± 441 | 1672 ± 524 | 1603 ± 575 | 0.295 |
| Protein (g) | 63.7 ± 24.1 | 65.8 ± 28.5 | 63.8 ± 30.4 | 0.774 |
| Fat (g) | 59.1 ± 18.1 | 60.8 ± 22.1 | 57.3 ± 26.2 | 0.688 |
| Carbohydrate (g) | 228.4 ± 62.0 | 209.2 ± 66.8 | 203.4 ± 67.1 | 0.069 |
| Calcium (mg) a | 418 (316, 713) | 480 (290, 765) | 364 (231, 694) | 0.088 |
| Iron (mg) a | 5.4 (3.7, 10.3) | 10.6 (5.9, 13.1) | 5.2 (3.6, 10.4) | <0.001 |
| Vitamin D (μg) a | 4.4 (2.4, 7.9) | 3.3 (2.3, 7.6) | 3.6 (1.7, 9.6) | 0.998 |
| Vitamin K (μg) a | 146 (68, 326) | 127 (58, 382) | 171 (68, 314) | 0.787 |
| Dietary fiber (g) | 11.5 ± 6.6 | 12.7 ± 6.0 | 10.7 ± 6.3 | 0.086 |
Data were expressed as mean ± SD or median (inter quartile range). TEI, total energy intake. a Log transformed variables for non-normally distributed variables were used for analysis. Mid date revealed the nutritional composition of prebiotic food intake.
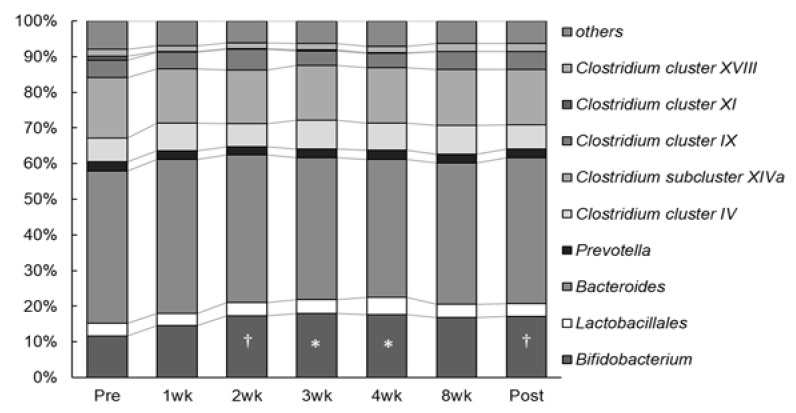
3.3. Fecal Microbiota Analyses
Human gut microbiota composition mainly comprises members of approximately 10 bacterial flora groups (Figure 1). As illustrated in Figure 1, one of the fecal microbiotas significantly changed during the intervention. The occupation ratio of Bifidobacterium spp. was significantly increased at 3 and 4 weeks (18.0 ± 8.3% and 17.6 ± 8.5%, respectively) compared to that during pre-intervention (11.7 ± 7.3%) (p = 0.019 and p = 0.035, respectively), and further, an increasing trend was observed at 2 week and post-intervention (17.3 ± 7.0% and 17.1 ± 7.6%, respectively) compared to pre-intervention (p = 0.057 and p = 0.073, respectively). No statistically significant changes were observed in the composition of other fecal microbiota during the intervention.
Figure 1.
Change in gut microbiota composition at 12 weeks. * p < 0.05 versus pre-intervention at the same genus. † p < 0.10 versus pre-intervention at the same genus. No significant differences were found in the gut microbiota without Bifidobacterium spp.
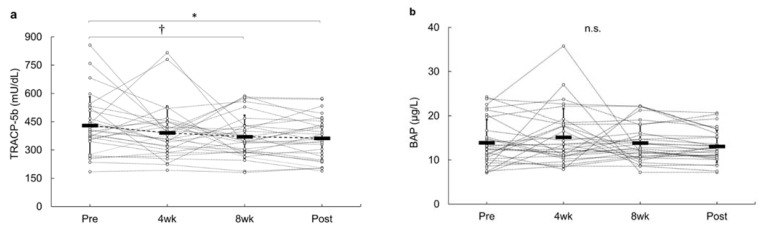
3.4. Changes in Bone Markers
Figure 2a,b depict the changes in bone metabolism turnover markers at pre-intervention, weekly, and post-intervention periods. The serum TRACP-5b level decreased significantly at 8 weeks and post-intervention in this study. Post hoc testing revealed that TRACP-5b at post-intervention (363 ± 112 mU/dL) was lower than that at pre-intervention (430 ± 154 mU/dL) (p = 0.018), and TRACP-5b at 8 weeks (370 ± 114 mU/dL) revealed a decreasing trend compared to that with the pre-intervention (p = 0.059). No significant changes were observed in BAP during the intervention. E2 and 25(OH)D3 levels from pre- to post-intervention did not reveal any significant change during the intervention.
Figure 2.
Change from pre-intervention for bone-related markers during 12 weeks of the intervention. (a) Change in TRACP-5b for 12 weeks. (b) Change in BAP for 12 weeks. * p < 0.05 was considered as statistically significant. † p < 0.10 was considered as trend toward a significant.
4. Discussion
The main finding of this study revealed that inulin and lactulose-fortified prebiotic food intakes for 12 weeks suppressed bone resorption marker and increased the occupation ratio of Bifidobacterium spp. in the gut microbiota without body weight gain among the Japanese female athletes. This finding provides novel insights into the existing literature, indicating that the combined intake of different types of prebiotics may play a pivotal role in improving bone health among Japanese female athletes.
Inulin is an indigestible, water-soluble dietary fiber, that is, a polysaccharide with a glucose molecule attached to the reducing end of the fructose chain [30], whereas lactulose is a synthetic disaccharide comprising fructose and galactose that cannot be digested or absorbed by humans [16]. These prebiotics are functional food ingredients and have been used in human studies. Previous studies have reported that the short-term administration of inulin or lactulose increased the occupation ratio of Bifidobacterium spp. in the gut microbiota [18,31,32], which is responsible for the growth of health-promoting bacterial species. Those observations are in agreement with the finding of our study (Figure 1). According to a consensus statement [21], prebiotics were recognized to particularly stimulate Bifidobacterium spp. (bifidogenesis). Thus, the combination of inulin and lactulose in this study might contribute to the “bifidogenic effect”. Moreover, the administration of inulin or lactulose reduced bone resorption markers in postmenopausal women and healthy young men [33,34,35]; however, only limited studies have demonstrated that supplementation with these prebiotics change the gut microbiota and bone metabolism turnover markers in humans [35]. In our study, the changes in bone metabolism turnover were identified after the changes Bifidobacterium spp. in the gut microbiota. A recent review summarized that prebiotics may indirectly or directly manipulate the gut–bone axis to improve bone health [22]. Increasing the occupation ratio of Bifidobacterium spp., short-chain fatty acids (SCFAs) levels [35], and suppression of pro-inflammatory cytokines [36] are possible pathways for the microbial manipulations to the improvement of bone health. Prebiotic intakes affected the gut microbiota and promoted SCFAs production [37]. In particular, Bifidobacterium species stimulate SCFAs productions by the intestinal microbiome [38], and associated with an increased production of SCFAs [39]. SCFAs are metabolites generated by gut microbial fermentation from prebiotics [40]. Acetic acid, butyric acid, and propionic acid are the main SCFAs produced by microorganisms in the intestine [41]. A previous study reported that SCFAs regulate the direct suppression of osteoclast synthesis and decrease bone resorption [42]. Mechanistically, this is thought to be because propionic acid and butyric acid inhibit osteoclast signal transduction substances and prevent osteoclast differentiation. After ingesting prebiotic food for 2 weeks, the occupation of Bifidobacterium spp. was higher than that before intervention and was maintained at a higher level until post-intervention in this study. Therefore, it is inferred that increases in the Bifidobacterium spp. induced the inhibition of bone resorption in this study as well.
Energy intake deficiency may exhibit uncoupled bone metabolism turnover, which decreases bone formation, increases bone resorption and/or a combination of the two, thereby consequently inducing unfavorable bone disorders. Subsequent reduction in the estrogen levels and associated reproductive dysfunction may also be indirectly influenced by bone health among the female athletes. Since serum E2 plays a crucial role in maintaining normal bone health in women, De Souza et al. [43] reported that low E2 levels were involved with suppressed bone formation and increased bone resorption. Since E2 did not show any significant changes during this intervention, it is inferred that E2 did not affect bone metabolism turnover. Participants in this study were in an energy intake deficient state, and the bone resorption marker during their pre-intervention was promoted. Indeed, the value of bone resorption marker was above the reference value [44]. These results were in accordance to those observed in previous studies [7,45]. In particular, energy intake deficiency elevates bone resorption markers in athletes. If the balance between bone resorption and bone formation is maintained (coupling), bone mass and bone mineral contents remain balanced; however, when uncoupling in bone metabolism turnover occurs and bone resorption becomes more dominant, bone mineral density decreases. TRACP-5b is recognized as a bone resorption marker with low intra- and inter-day variations [46]. Furthermore, this marker is a predictor of the risk of stress fractures in female athletes [47]. In this study, participants who had elevated bone resorption markers before the intervention had a positive effect from consuming the prebiotic foods (Figure 2a). The finding of reduced bone resorption marker in this study may prove beneficial for preventing issues related to bone health among female athletes.
As well as energy intake, key nutrients for bone health such as calcium, vitamin D and vitamin K intakes is also important in athletes. The adequate intake of these nutrients is one of the essential nutritional strategies that female athletes can utilize to reduce the risk of bone injuries [48]. Regarding these nutrient intakes, participants did not change their dietary intake throughout the study. However, calcium, vitamin D, and vitamin K intakes are lower than the dietary reference intakes (DRIs) in Japan. The previous studies suggested that an increase in certain micronutrients (i.e., calcium, vitamin D and vitamin K) has a favorable effect on bone health [49,50]. Although there was no significant change in these nutrient intakes during this intervention period, it should be noted that these nutrients were low. Since low intakes of these nutrients are a characteristic of the dietary habits among Japanese female athletes [14], there is a need to develop a nutritional approach to improve this problem. Iron intake also differed significantly during the intervention period in this study. Although iron is an essential micronutrient for humans, large amounts of iron intakes might cause gut inflammation, which might be due to irritation of the gut mucosa, adverse alterations in the gut microbiota, or both [51]. However, the significant changes in our study were within the DRIs in Japan and did not have adverse effects on the gut microbiota.
Body composition is related to athletic performance [52,53,54]. During the intervention, no changes were observed in BW, FFM, and FM. Cialdella-Kam et al. [55] found that a 6-month intervention provided extra energy (+360 kcal/day) that could successfully reverse the menstrual status, resulting in weight gain (+1.6 kg). In contrast, our study did not induce substantial weight gain (+0.1 kg). While habitual EI increment and body weight gain are useful strategies for solving women’s health problems [11,56], such strategies may not be practically feasible for long-distance runners and rhythmic gymnasts. Increased body fat reduces athletic performance [57]. FM did not change significantly in our study. Among the competing female athletes that require daily weight control, the prebiotic food used in this study may provide a “bone protection effect” without having a significant impact on body composition.
The advantage of this study is that it was conducted in multiple events where female athletes participated in intense training every day. Previous studies were conducted in postmenopausal women and adolescent girls. The rate of bone turnover varies with age; therefore, it was unclear whether prebiotic intake in female athletes would prove beneficial for bone health. Thus, the present findings can be used as a nutritional strategy in sports fields. Future athletes’ studies using probiotics or synbiotics are needed, as probiotic intake has reported to have a protective effect on bone parameter in rats [58]. In contrast, this study has certain limitations. First, our participants could not perform placebo trials. As our participants in this study had a high-performance level, it was difficult to get such athletes to commit to a control trial such as a long-term crossover intervention. Second, we did not measure SCFAs in fecal samples or serum proinflammatory cytokines. Thus, our study could not explicitly explain the prebiotics–gut–bone axis. Future studies need to re-evaluate the impact of prebiotic food on gut microbiota and bone metabolism turnover in other athletes.
In conclusion, intake of inulin and lactulose-fortified prebiotic food over 12 weeks might suppress bone resorption marker and increase the occupation ratio of Bifidobacterium spp. in feces without body weight gain among Japanese female athletes. The findings of this study can be applied to prevent bone health-related disorders among female athletes.
Acknowledgments
All authors thank the participants in our study. We are grateful to Hirohiko Nakamura and his team members from Morinaga Milk Industry Co., Ltd. for their kind support, and Rie Ishizawa, Hiroko Murata, and Mizuki Kato for their support with data collection.
Author Contributions
Conceptualization, M.T.; formal analysis, T.I.; investigation, T.I., E.T. and S.T.; writing—original draft preparation, T.I. and M.T.; writing—review and editing, T.I., E.T., S.T. and M.T.; supervision, M.T.; project administration, E.T.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by research funding for the female athletes 2017–2018, the Japan Sports Agency, grant number B2R400027601.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Review Committee of Waseda University on Research with Human Subjects (approval number: 2017-096; date of approval: 13 June 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analyzed during this study are not publicly available because our ethical approval did not include the use of these data by other researchers.
Conflicts of Interest
All authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Otis C.L., Drinkwater B., Johnson M., Loucks A., Wilmore J. ACSM Position Stand: The Female Athlete Triad. Med. Sci. Sports Exerc. 1997;29:i–ix. doi: 10.1097/00005768-199705000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Nattiv A., Loucks A.B., Manore M.M., Sanborn C.F., Sundgot-Borgen J., Warren M.P. American College of Sports Medicine American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007;39:1867–1882. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 3.Vasikaran S., Eastell R., Bruyère O., Foldes A.J., Garnero P., Griesmacher A., McClung M., Morris H.A., Silverman S., Trenti T., et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 4.Koitaya N., Ezaki J., Nishimuta M., Yamauchi J., Hashizume E., Morishita K., Miyachi M., Sasaki S., Ishimi Y. Effect of low dose vitamin K2 (MK-4) supplementation on bio-indices in postmenopausal Japanese women. J. Nutr. Sci. Vitaminol. 2009;55:15–21. doi: 10.3177/jnsv.55.15. [DOI] [PubMed] [Google Scholar]
- 5.Villareal D.T., Fontana L., Das S.K., Redman L., Smith S.R., Saltzman E., Bales C., Rochon J., Pieper C., Huang M., et al. Effect of Two-Year Caloric Restriction on Bone Metabolism and Bone Mineral Density in Non-Obese Younger Adults: A Randomized Clinical Trial. J. Bone Miner. Res. 2016;31:40–51. doi: 10.1002/jbmr.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ihle R., Loucks A.B. Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Miner. Res. 2004;19:1231–1240. doi: 10.1359/JBMR.040410. [DOI] [PubMed] [Google Scholar]
- 7.Barrack M.T., Van Loan M.D., Rauh M.J., Nichols J.F. Physiologic and behavioral indicators of energy deficiency in female adolescent runners with elevated bone turnover. Am. J. Clin. Nutr. 2010;92:652–659. doi: 10.3945/ajcn.2009.28926. [DOI] [PubMed] [Google Scholar]
- 8.Lambrinoudaki I., Papadimitriou D. Pathophysiology of bone loss in the female athlete. Ann. N. Y. Acad. Sci. 2010;1205:45–50. doi: 10.1111/j.1749-6632.2010.05681.x. [DOI] [PubMed] [Google Scholar]
- 9.Wentz L., Liu P.-Y., Ilich J.Z., Haymes E.M. Dietary and training predictors of stress fractures in female runners. Int. J. Sport Nutr. Exerc. Metab. 2012;22:374–382. doi: 10.1123/ijsnem.22.5.374. [DOI] [PubMed] [Google Scholar]
- 10.Barrack M.T., Gibbs J.C., De Souza M.J., Williams N.I., Nichols J.F., Rauh M.J., Nattiv A. Higher Incidence of Bone Stress Injuries With Increasing Female Athlete Triad–Related Risk Factors: A Prospective Multisite Study of Exercising Girls and Women. Am. J. Sports Med. 2014;42:949–958. doi: 10.1177/0363546513520295. [DOI] [PubMed] [Google Scholar]
- 11.Compston J.E., McConachie C., Stott C., Hannon R.A., Kaptoge S., Debiram I., Love S., Jaffa A. Changes in bone mineral density, body composition and biochemical markers of bone turnover during weight gain in adolescents with severe anorexia nervosa: A 1-year prospective study. Osteoporos. Int. 2006;17:77–84. doi: 10.1007/s00198-005-1904-6. [DOI] [PubMed] [Google Scholar]
- 12.Loucks A.B. Low Energy Availability in the Marathon and Other Endurance Sports. Sports Med. 2007;37:348–352. doi: 10.2165/00007256-200737040-00019. [DOI] [PubMed] [Google Scholar]
- 13.Pepper M., Akuthota V., McCarty E.C. The pathophysiology of stress fractures. Clin. Sports Med. 2006;25:1–16. doi: 10.1016/j.csm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Ishizu T., Torii S., Taguchi M. Habitual Dietary Status and Stress Fracture Risk Among Japanese Female Collegiate Athletes. J. Am. Coll. Nutr. 2021 doi: 10.1080/07315724.2021.1920068. in press. [DOI] [PubMed] [Google Scholar]
- 15.van Loo J., Coussement P., de Leenheer L., Hoebregs H., Smits G. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit. Rev. Food Sci. Nutr. 1995;35:525–552. doi: 10.1080/10408399509527714. [DOI] [PubMed] [Google Scholar]
- 16.Seki N., Saito H. Lactose as a source for lactulose and other functional lactose derivatives. Int. Dairy J. 2012;22:110–115. doi: 10.1016/j.idairyj.2011.09.016. [DOI] [Google Scholar]
- 17.Oku T. Available Energy of Nondigestible and/or Nonabsorbable Saccharides. Jpn. J. Nutr. Diet. 1996;54:143–150. doi: 10.5264/eiyogakuzashi.54.143. [DOI] [Google Scholar]
- 18.Gibson G.R., Beatty E.R., Wang X., Cummings J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 19.Terada A., Hara H., Kataoka M., Mitsuoka T. Effect of Lactulose on the Composition and Metabolic Activity of the Human Faecal Flora. Microb. Ecol. Health Dis. 1992;5:43–50. doi: 10.3109/08910609209141303. [DOI] [Google Scholar]
- 20.Micka A., Siepelmeyer A., Holz A., Theis S., Schön C. Effect of consumption of chicory inulin on bowel function in healthy subjects with constipation: A randomized, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2017;68:82–89. doi: 10.1080/09637486.2016.1212819. [DOI] [PubMed] [Google Scholar]
- 21.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 22.Whisner C.M., Castillo L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018;102:443–479. doi: 10.1007/s00223-017-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coudray C., Tressol J.C., Gueux E., Rayssiguier Y. Effects of inulin-type fructans of different chain length and type of branching on intestinal absorption and balance of calcium and magnesium in rats. Eur. J. Nutr. 2003;42:91–98. doi: 10.1007/s00394-003-0390-x. [DOI] [PubMed] [Google Scholar]
- 24.Bryk G., Coronel M.Z., Pellegrini G., Mandalunis P., Rio M.E., de Portela M.L.P.M., Zeni S.N. Effect of a combination GOS/FOS® prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur. J. Nutr. 2015;54:913–923. doi: 10.1007/s00394-014-0768-y. [DOI] [PubMed] [Google Scholar]
- 25.Seijo M., Bryk G., Zeni Coronel M., Bonanno M., Río M.E., Pita Martín de Portela M.L., Zeni S.N. Effect of Adding a Galacto-Oligosaccharides/Fructo-Oligosaccharides (GOS/FOS®) Mixture to a Normal and Low Calcium Diet, on Calcium Absorption and Bone Health in Ovariectomy-Induced Osteopenic Rats. Calcif. Tissue Int. 2019;104:301–312. doi: 10.1007/s00223-018-0490-5. [DOI] [PubMed] [Google Scholar]
- 26.Sakai Y., Seki N., Hamano H., Ochi H., Abe F., Shimizu F., Masuda K., Iino H. A study of the prebiotic effect of lactulose at low dosages in healthy Japanese women. Biosci. Microbiota Food Health. 2019;38:69–72. doi: 10.12938/bmfh.18-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouhnik Y., Raskine L., Champion K., Andrieux C., Penven S., Jacobs H., Simoneau G. Prolonged administration of low-dose inulin stimulates the growth of bifidobacteria in humans. Nutr. Res. 2007;27:187–193. doi: 10.1016/j.nutres.2007.01.013. [DOI] [Google Scholar]
- 28.Nagashima K., Hisada T., Sato M., Mochizuki J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl. Environ. Microbiol. 2003;69:1251–1262. doi: 10.1128/AEM.69.2.1251-1262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagashima K., Mochizuki J., Hisada T., Suzuki S., Shimomura K. Phylogenetic Analysis of 16S Ribosomal RNA Gene Sequences from Human Fecal Microbiota and Improved Utility of Terminal Restriction Fragment Length Polymorphism Profiling. Biosci. Microflora. 2006;25:99–107. doi: 10.12938/bifidus.25.99. [DOI] [Google Scholar]
- 30.Kalyani Nair K., Kharb S., Thompkinson D.K. Inulin Dietary Fiber with Functional and Health Attributes—A Review. Food Rev. Int. 2010;26:189–203. doi: 10.1080/87559121003590664. [DOI] [Google Scholar]
- 31.Tuohy K.M., Ziemer C.J., Klinder A., Knöbel Y., Pool-Zobel B.L., Gibson G.R. A Human Volunteer Study to Determine the Prebiotic Effects of Lactulose Powder on Human Colonic Microbiota. Microb. Ecol. Health Dis. 2002;14:165–173. doi: 10.1080/089106002320644357. [DOI] [Google Scholar]
- 32.Sakai Y., Seki N., Hamano K., Ochi H., Abe F., Masuda K., Iino H. Prebiotic effect of two grams of lactulose in healthy Japanese women: A randomised, double-blind, placebo-controlled crossover trial. Benef. Microbes. 2019;10:629–639. doi: 10.3920/BM2018.0174. [DOI] [PubMed] [Google Scholar]
- 33.Slevin M.M., Allsopp P.J., Magee P.J., Bonham M.P., Naughton V.R., Strain J.J., Duffy M.E., Wallace J.M., Mc Sorley E.M. Supplementation with Calcium and Short-Chain Fructo-Oligosaccharides Affects Markers of Bone Turnover but Not Bone Mineral Density in Postmenopausal Women. J. Nutr. 2014;144:297–304. doi: 10.3945/jn.113.188144. [DOI] [PubMed] [Google Scholar]
- 34.Kruger M.C., Chan Y.M., Kuhn-Sherlock B., Lau L.T., Lau C.C., Chin Y.S., Todd J.M., Schollum L.M. Differential effects of calcium- and vitamin D-fortified milk with FOS-inulin compared to regular milk, on bone biomarkers in Chinese pre- and postmenopausal women. Eur. J. Nutr. 2016;55:1911–1921. doi: 10.1007/s00394-015-1007-x. [DOI] [PubMed] [Google Scholar]
- 35.Su J., Changhai S., Chen X., Zhang Z., Hu Y., Cui J., Zhi X., Li X., Jiang H., Wang Y., et al. Lactulose Suppresses Osteoclastogenesis and Ameliorates Estrogen Deficiency-Induced Bone Loss in Mice. Aging Dis. 2019 doi: 10.14336/AD.2019.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tousen Y., Matsumoto Y., Nagahata Y., Kobayashi I., Inoue M., Ishimi Y. Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation. Nutrients. 2019;11:297. doi: 10.3390/nu11020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki H., Miyakawa H., Watanabe A., Nakayama Y., Lyu Y., Hama K., Shibata S. Mice Microbiota Composition Changes by Inulin Feeding with a Long Fasting Period under a Two-Meals-Per-Day Schedule. Nutrients. 2019;11:2802. doi: 10.3390/nu11112802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markowiak-Kopeć P., Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12:1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beards E., Tuohy K., Gibson G. Bacterial, SCFA and gas profiles of a range of food ingredients following in vitro fermentation by human colonic microbiota. Anaerobe. 2010;16:420–425. doi: 10.1016/j.anaerobe.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Scholz-Ahrens K.E., Schrezenmeir J. Inulin, oligofructose and mineral metabolism—experimental data and mechanism. Br. J. Nutr. 2002;87:S179. doi: 10.1079/BJN/2002535. [DOI] [PubMed] [Google Scholar]
- 41.Cummings J.H., Macfarlane G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991;70:443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 42.Lucas S., Omata Y., Hofmann J., Böttcher M., Iljazovic A., Sarter K., Albrecht O., Schulz O., Krishnacoumar B., Krönke G., et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018;9:55. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Souza M.J., West S.L., Jamal S.A., Hawker G.A., Gundberg C.M., Williams N.I. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone. 2008;43:140–148. doi: 10.1016/j.bone.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Nishizawa Y., Miura M., Ichimura S., Inaba M., Imanishi Y., Shiraki M., Takada J., Chaki O., Hagino H., Fukunaga M., et al. Executive summary of the Japan Osteoporosis Society Guide for the Use of Bone Turnover Markers in the Diagnosis and Treatment of Osteoporosis (2018 Edition) Clin. Chim. Acta. 2019;498:101–107. doi: 10.1016/j.cca.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Taguchi M., Moto K., Lee S., Torii S., Hongu N. Energy intake deficiency promotes bone resorption and energy metabolism suppression in Japanese male endurance runners: A pilot study. Am. J. Mens. Health. 2020;14:1557988320905251. doi: 10.1177/1557988320905251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada S., Inaba M., Kurajoh M., Shidara K., Imanishi Y., Ishimura E., Nishizawa Y. Utility of serum tartrate-resistant acid phosphatase (TRACP5b) as a bone resorption marker in patients with chronic kidney disease: Independence from renal dysfunction. Clin. Endocrinol. 2008;69:189–196. doi: 10.1111/j.1365-2265.2008.03187.x. [DOI] [PubMed] [Google Scholar]
- 47.Nose-Ogura S., Yoshino O., Dohi M., Torii S., Kigawa M., Harada M., Hiraike O., Kawahara T., Osuga Y., Fujii T., et al. Relationship between tartrate-resistant acid phosphatase 5b and stress fractures in female athletes. J. Obstet. Gynaecol. Res. 2020;46:1436–1442. doi: 10.1111/jog.14256. [DOI] [PubMed] [Google Scholar]
- 48.Sale C., Elliott-Sale K.J. Nutrition and Athlete Bone Health. Sports Med. 2019;49:139–151. doi: 10.1007/s40279-019-01161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenforde A.S., Sayres L.C., Sainani K.L., Fredericson M. Evaluating the relationship of calcium and vitamin D in the prevention of stress fracture injuries in the young athlete: A review of the literature. Phys. Med. Rehabil. 2010;2:945–949. doi: 10.1016/j.pmrj.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Karpiński M., Popko J., Maresz K., Badmaev V., Stohs S.J. Roles of Vitamins D and K, Nutrition, and Lifestyle in Low-Energy Bone Fractures in Children and Young Adults. J. Am. Coll. Nutr. 2017;36:399–412. doi: 10.1080/07315724.2017.1307791. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann M.B., Chassard C., Rohner F., N’Goran E.K., Nindjin C., Dostal A., Utzinger J., Ghattas H., Lacroix C., Hurrell R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010;92:1406–1415. doi: 10.3945/ajcn.110.004564. [DOI] [PubMed] [Google Scholar]
- 52.Michopoulou E., Avloniti A., Kambas A., Leontsini D., Michalopoulou M., Tournis S., Fatouros I.G. Elite premenarcheal rhythmic gymnasts demonstrate energy and dietary intake deficiencies during periods of intense training. Pediatr. Exerc. Sci. 2011;23:560–572. doi: 10.1123/pes.23.4.560. [DOI] [PubMed] [Google Scholar]
- 53.Högström G.M., Pietilä T., Nordström P., Nordström A. Body composition and performance: Influence of sport and gender among adolescents. J. Strength Cond. Res. 2012;26:1799–1804. doi: 10.1519/JSC.0b013e318237e8da. [DOI] [PubMed] [Google Scholar]
- 54.Sundgot-Borgen J., Meyer N.L., Lohman T.G., Ackland T.R., Maughan R.J., Stewart A.D., Müller W. How to minimise the health risks to athletes who compete in weight-sensitive sports review and position statement on behalf of the Ad Hoc Research Working Group on Body Composition, Health and Performance, under the auspices of the IOC Medical Commission. Br. J. Sports Med. 2013;47:1012–1022. doi: 10.1136/bjsports-2013-092966. [DOI] [PubMed] [Google Scholar]
- 55.Cialdella-Kam L., Guebels C.P., Maddalozzo G.F., Manore M.M. Dietary intervention restored menses in female athletes with exercise-associated menstrual dysfunction with limited impact on bone and muscle health. Nutrients. 2014;6:3018–3039. doi: 10.3390/nu6083018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mallinson R.J., Williams N.I., Olmsted M.P., Scheid J.L., Riddle E.S., De Souza M.J. A case report of recovery of menstrual function following a nutritional intervention in two exercising women with amenorrhea of varying duration. J. Int. Soc. Sports Nutr. 2013;10:34. doi: 10.1186/1550-2783-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legaz A., Eston R. Changes in performance, skinfold thicknesses, and fat patterning after three years of intense athletic conditioning in high level runners. Br. J. Sports Med. 2005;39:851–856. doi: 10.1136/bjsm.2005.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parvaneh K., Ebrahimi M., Sabran M.R., Karimi G., Hwei A.N.M., Abdul-Majeed S., Ahmad Z., Ibrahim Z., Jamaluddin R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. BioMed Res. Int. 2015;2015:897639. doi: 10.1155/2015/897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during this study are not publicly available because our ethical approval did not include the use of these data by other researchers.