Key Points
Question
Can consensus classification for complication severity be developed to be used in clinical trials of rhegmatogenous retinal detachment (RRD) surgery?
Findings
In this study, following the development of a comprehensive list of complications of RRD surgery, a Delphi survey among retinal surgeons was undertaken (96% response rate). Consensus was achieved in grading severity of complications of RRD surgery.
Meaning
The proposed severity classification for complications of RRD surgery may facilitate quantification and comparison of harm of different RRD surgical interventions.
Abstract
Importance
Quantifying severity of complications in rhegmatogenous retinal detachment (RRD) surgical trials is needed. A consensus classification system will help surgeons to more effectively compare harms of different surgical techniques.
Objective
To develop a new consensus-based classification to quantify severity of complications of RRD surgery.
Design, Setting, and Participants
A comprehensive list of complications was developed followed by a Delphi consensus survey of international vitreoretinal surgeons. The survey was conducted in 17 countries in mainland Europe, the United Kingdom, the United States, Asia, South Africa, and Australia. Seventy vitreoretinal surgeons were invited to take part in the Delphi survey; 45 agreed to participate. Participants were selected through boards/members lists of retinal societies. Data were analyzed between April 2019 and August 2019.
Main Outcomes and Measures
Consensus-derived classification of complications of RRD surgery, according to their severity.
Results
Forty-three of 45 vitreoretinal surgeons who agreed to participate in the Delphi survey completed round 1 (96%); all but 1 (98%) completed round 2. Consensus was reached for 96% of the 84 complications assessed. Examples of complications classified as least severe (graded 1) included subconjunctival hemorrhage and chemosis while those classified as most severe included endophthalmitis and sympathetic ophthalmia (graded 9) and phthisis (graded 10).
Conclusions and Relevance
We propose a new classification for quantifying severity of surgical complications based on an international consensus of vitreoretinal surgeons to quantify harm and improve the reporting of complications of RRD surgery.
This study analyzes a survey of international vitreoretinal surgeons to develop a new consensus-based classification to quantify severity of complications of rhegmatogenous retinal detachment surgery.
Introduction
Rhegmatogenous retinal detachment (RRD) is the most common retinal emergency, carrying an annual incidence of 6.3 to 17.9 per 100 000 population, and is one of the most common indications for vitreoretinal intervention.1,2 Surgical techniques and maneuvers used to repair RRD have evolved over decades such that high rates of retinal reattachment are currently achieved with pars plana vitrectomy (PPV), scleral buckle (SB), pneumatic retinopexy (PR), or combinations thereof.3,4,5
Randomized clinical trials (RCTs) provide the best evidence to guide surgeons in the selection of interventions to optimize success for patients.6,7,8,9 When comparing surgical procedures, it is important that these interventions are evaluated comprehensively with regard to their potential harms as well as their effectiveness.10 There is evidence that the reporting of complications in clinical trials across many surgical and medical specialties lacks sufficient quality and consistency.11,12,13,14,15 For example, although RCTs commonly report complication frequency, data on severity of complications are often lacking.16,17,18 While classification systems for severity of surgical complications have been proposed and used in fields such as general surgery, neurosurgery, and urology,19,20,21,22,23 to our knowledge, these have not been incorporated into ophthalmic RCTs. In response to this need, in 2018, Sii et al24 published a grading system for complications of glaucoma surgery.
With this in mind, the purpose of the study was to reach consensus among an international group of vitreoretinal surgeons on severity scores for complications of RRD surgery and to generate a classification system for quantifying and reporting severity of complications of retinal detachment surgery (CORDS).
Methods
Three authors (Z.Y.X., N.L., and A.A.B.) generated first a preliminary list of complications of RRD surgery using a textbook on complications of vitreoretinal surgery.25 This pilot list was then distributed to a small group of vitreoretinal surgeons (authors K.K., T.M., S.N., D.S., W.S., and T.W.) who provided feedback and guided the establishment of a final list. This was developed into a survey to determine the severity of each of the complications in the list; the survey was prepared for distribution using the software platform Enalyzer PRO (Enalyzer).26 A pilot phase was run among 7 vitreoretinal surgeons (K.K., N.L., T.M., S.N., D.S., W.S., and T.W.) to refine the survey questions and test the user interface.
A consensus on the grading of complications of RRD surgery was pursued using the Delphi consensus method. The Delphi method comprises 2 or more rounds of anonymous surveys where responses are summated after each round and presented to the participants in the subsequent round. At each stage, respondents may choose to use the summary information to modify their responses from the previous round or opt to maintain their previous answers. The range of responses for each survey item tends to decrease with each cycle such that the group converges toward a consensus response.27
Members of several retinal societies including Macula Society, the Club Jules Gonin, and the British and Eire Association of Vitreoretinal Surgeons (BEAVRS) were invited to take part in the survey to gather an international and representative group of vitreoretinal surgeons (n = 70 from 17 countries). Members were selected from societies’ member lists to ensure there would be international representation from all continents. Institutional ethical review approval was obtained (Queen’s University Belfast Faculty research ethics committee ). All survey participants provided written informed consent; none received a stipend for their participation.
Survey participants were presented with complications of RRD surgery divided into general, common complications, and complications specific to SB, PPV, and PR. Each category was subdivided into intraoperative and postoperative complications (eTable 1 in the Supplement). Participants were asked to rank each complication from 1 to 10, with 1 representing “no harm to patient or vision” and 10 “worst possible harm to the patient or vision (eg, permanent total loss of vision or painful eye).” Unlike many other surgical specialties, mortality is a rare complication of ophthalmic surgery and was excluded along with complications from anesthesia. Aside from anchor statements for the least and worst grades of the scale (1 and 10), no additional guidance was provided. In addition, space was provided to allow vitreoretinal surgeons to justify their responses or provide feedback on the questions. The questions contained in the round 1 of the survey can be accessed on https://surveys.enalyzer.com?pid=rab5r4tg.
Round 1 responses were extracted from the software and analyzed in Excel (Microsoft), and the median severity grade and interquartile range (IQR) for each complication was calculated. In round 2, participants were presented with both median scores from the previous round and their own previous scores. Vitreoretinal surgeons then assigned their scores to the complications presented in the second round. Data were then reanalyzed and the survey cycle was to be repeated until consensus on more than 90% of the complications had been achieved. For the purposes of the survey, an IQR of 2 or less was required for a grade on the severity of the complication to be judged to have reached consensus. Items that reached consensus in the first round of the Delphi were removed from the second round of questioning. Comments made in round 1 were taken into consideration for the preparation of the second round, resulting in minor modifications to the survey items. The specific changes that were made allowed us to capture how severity scores differed depending on the size of suprachoroidal and subretinal hemorrhage, whether the macula was involved, whether visual field loss affects driving, and whether fish-egg gas bubble formation affects the view of the retina. The round 2 questions can be accessed in https://surveys.enalyzer.com?pid=t7h4gaqb.
Results
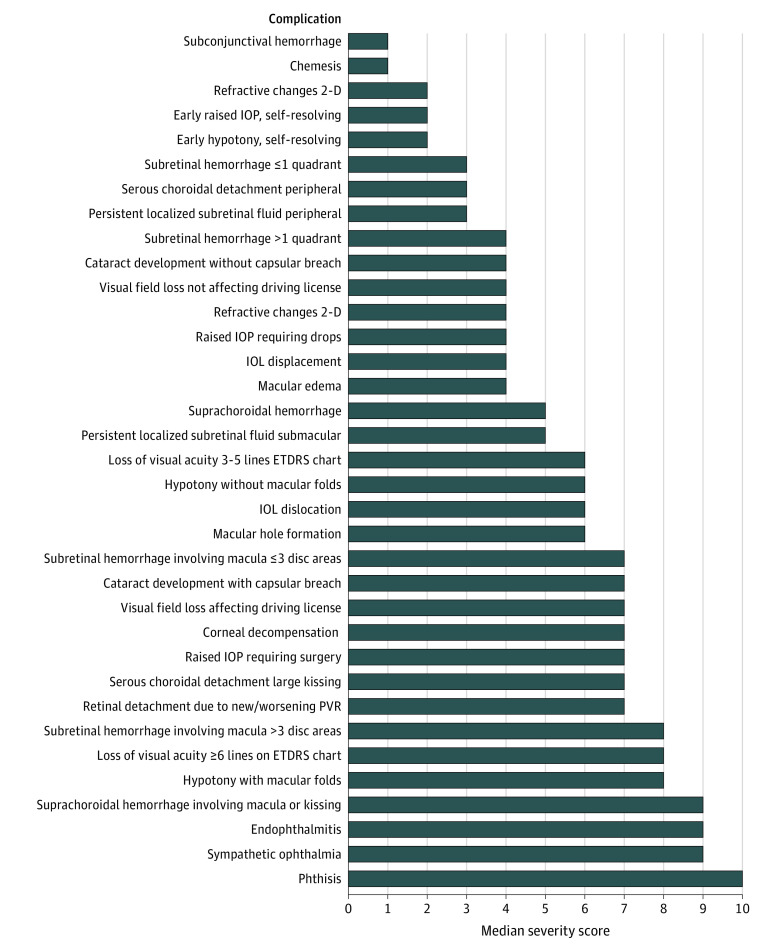
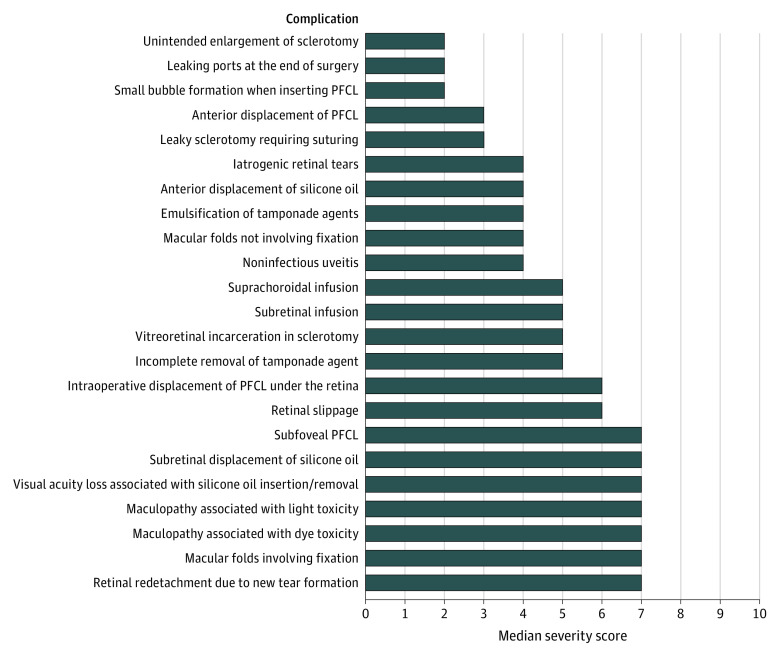
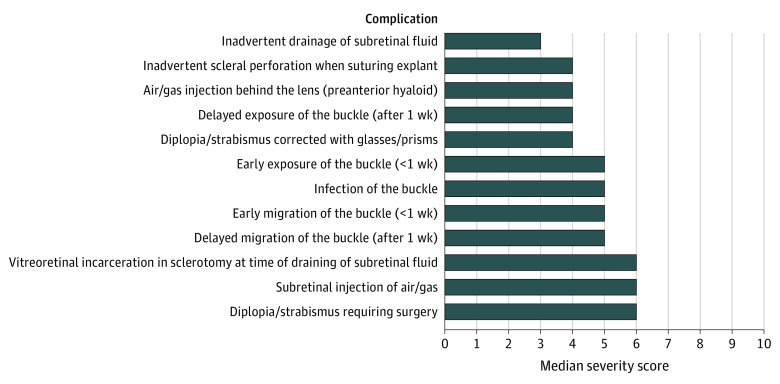
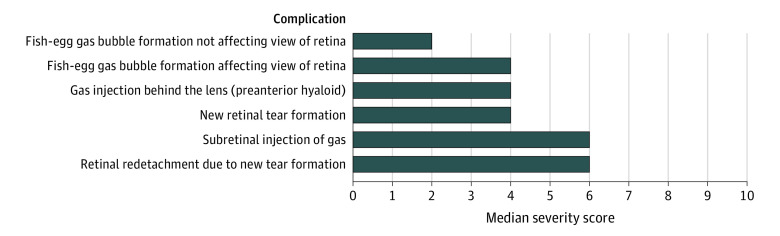
Forty-five of the 70 vitreoretinal surgeons approached (64%) replied to the initial contact and agreed to participate in the Delphi survey; 43 (96%) of these completed round 1 in full. Participants in the first round of the Delphi were based in mainland Europe (n = 14; 33%), United Kingdom (n = 10; 23%), United States (n = 10; 23%), Asia (n = 5; 12%), South Africa (n = 3; 7%), and Australia (n = 1; 2%). A consensus was reached for 32 of 78 complications (41%) in round 1 (IQR≤2); consensus items were removed from the round 2 question list. The remaining list of nonconsensus items comprised 46 items, which, taking into account feedback from round 1, was expanded to 55 items. The completion rate for round 2 of the survey was 98%; and 52 of 55 items (95%) achieved a consensus severity grading. Combining the results from round 1 and 2, the final list comprises 87 complications, of which 84 (97%) reached consensus. The 3 complications for which consensus was not reached were “suprachoroidal hemorrhage, not kissing and not involving the macula”; “subretinal infusion” in the context of PPV; and “early migration of the scleral buckle”; these achieved an IQR of 2.75. Full round 1 and 2 results can be accessed online (eTable 2 in the Supplement). The final list of complications with their assigned median severity gradings is shown in the Table. Figure 1, Figure 2, Figure 3, and Figure 4 present all complications ranked based on their scores; eTable 3 in the Supplement presents complications ranked and classified as mild, moderate, or severe.
Table. Median Scores for General and Specific Complications by Surgical Procedure Using the Complications of Retinal Detachment Surgery (CORDS) Severity Classification .
| Complication | Median |
|---|---|
| General intraoperative | |
| Subconjunctival hemorrhage | 1 |
| Chemosis | 1 |
| Subretinal hemorrhage not involving macula, ≤1 quadrant | 3 |
| Subretinal hemorrhage not involving macula, >1 quadrant | 4 |
| Suprachoroidal hemorrhage not involving macula and no kissinga | 5 |
| Subretinal hemorrhage involving macula, ≤3 disc areas | 7 |
| Subretinal hemorrhage involving macula, >3 disc areas | 8 |
| Suprachoroidal hemorrhage involving macula or kissing | 9 |
| General postoperative | |
| Subconjunctival hemorrhage | 1 |
| Chemosis | 1 |
| Refractive changes: <2-dimensional | 2 |
| Early raised IOP, self-resolving | 2 |
| Early hypotony, self-resolving | 2 |
| Serous choroidal detachment: peripheral | 3 |
| Persistent localized subretinal fluid: peripheral, nonprogressive | 3 |
| Visual field loss not related to retinal detachment but attributable to surgical procedure: not affecting driving license | 4 |
| Refractive changes: ≥2-dimensional | 4 |
| Persistently raised IOP manageable with drops | 4 |
| IOL displacement | 4 |
| Macular edema | 4 |
| Suprachoroidal hemorrhage: not involving macula and no kissing | 5 |
| Persistent localized subretinal fluid: submacular | 5 |
| Loss of visual acuity attributable to surgical procedure: moderate (3-5 lines ETDRS chart) | 6 |
| Persistent hypotony (IOP <5 mm Hg) without macular folds | 6 |
| IOL dislocation | 6 |
| Macular hole formation | 6 |
| Visual field loss not related to retinal detachment but attributable to surgical procedure: affecting driving license | 7 |
| Corneal decompensation/severe corneal edema | 7 |
| Persistently raised IOP requiring surgery | 7 |
| Serous choroidal detachment: large, kissing | 7 |
| Retinal redetachment owing to new or worsening PVR | 7 |
| Loss of visual acuity attributable to surgical procedure: severe (≥6 lines on ETDRS chart) | 8 |
| Persistent hypotony with macular folds | 8 |
| Suprachoroidal hemorrhage: involving macula or kissing | 9 |
| Endophthalmitis | 9 |
| Sympathetic ophthalmia | 9 |
| Phthisis | 10 |
| PPV intraoperative | |
| Unintended enlargement of sclerotomy | 2 |
| Leaking ports at the end of surgery requiring suturing | 2 |
| Small bubble formation when inserting PFCL | 2 |
| Cataract development intraoperatively (owing to lens touch): without capsular breach | 4 |
| Iatrogenic retinal tears | 4 |
| Suprachoroidal infusion | 5 |
| Subretinal infusiona | 5 |
| Cataract development intraoperatively (owing to lens touch): with capsular breach | 5 |
| Vitreoretinal incarceration in sclerotomy | 5 |
| Intraoperative displacement of PFCL under the retina | 6 |
| PPV postoperative | |
| Anterior displacement of tamponade agent: PFCL | 3 |
| Leaky sclerotomy requiring suturing | 3 |
| Anterior displacement of tamponade agent: silicone oil | 4 |
| Emulsification of tamponade agents | 4 |
| Macular folds: not involving fixation | 4 |
| Noninfectious uveitis | 4 |
| Incomplete removal of tamponade agent | 5 |
| Retinal slippage | 6 |
| Subfoveal PFCL | 7 |
| Subretinal displacement of silicone oil and heavy silicone oils | 7 |
| Unexplained visual acuity loss associated with insertion/removal of silicone oil | 7 |
| Maculopathy related to light toxicity | 7 |
| Maculopathy related to dye toxicity | 7 |
| Macular folds: involving fixation | 7 |
| Retinal redetachment owing to new tear formation | 7 |
| SB intraoperative | |
| Inadvertent drainage of subretinal fluid | 3 |
| Inadvertent scleral perforation when suturing explant | 4 |
| Air/gas injection behind the lens (preanterior hyaloid) | 4 |
| Cataract development intraoperatively (due to lens touch, eg, at time of injection of air/gas): without capsular breach | 4 |
| Vitreoretinal incarceration in sclerotomy at time of draining of subretinal fluid | 6 |
| Subretinal injection of air/gas | 6 |
| Cataract development intraoperatively: with capsular breach | 7 |
| SB postoperative | |
| Delayed exposure of the buckle (after 1 wk) | 4 |
| Diplopia/strabismus: corrected with glasses/prisms | 4 |
| Early exposure of the buckle (<1 wk) | 5 |
| Infection of the buckle | 5 |
| Early migration of the buckle (<1 wk)a | 5 |
| Delayed migration of the buckle (after 1 wk) | 5 |
| Diplopia/strabismus: requiring surgery | 6 |
| PR intraoperative | |
| Fish-egg gas bubble formation not affecting view of retina | 2 |
| Fish-egg gas bubble formation affecting view of retina | 4 |
| Gas injection behind the lens (preanterior hyaloid) | 4 |
| Cataract development intraoperatively (owing to lens touch at the time of gas injection): without capsular breach | 4 |
| Subretinal injection of gas | 6 |
| Cataract development intraoperatively: with capsular breach | 7 |
| PR postoperative | |
| New retinal tear formation | 4 |
| Retinal redetachment owing to new tear formation | 6 |
Abbreviations: D, diopters; ETDRS, Early Treatment Diabetic Retinopathy Study; IOP, intraocular pressure; PFCL, perfluorocarbon liquid; PPV, pars plana vitrectomy; PR, pneumatic retinopexy; SB, scleral buckle.
Interquartile range = 2.75 (ie, did not reach consensus).
Figure 1. Complications of Surgery for the Repair of Rhegmatogenous Retinal Detachment, Ranked by Median Severity Score.
General complications of surgery (pars plana vitrectomy, scleral buckling, and pneumatic retinopexy) for the repair of rhegmatogenous retinal detachment, ranked by median severity score. 2-D indicates 2-dimensional; IOP, intraocular pressure; IOL intraocular lens; ETDRS, Early Treatment Diabetic Retinopathy Study; PVR, proliferative vitreoretinopathy.
Figure 2. Complications of Pars Plana Vitrectomy.
PFCL indicates perfluorocarbon liquid.
Figure 3. Complications of Scleral Buckling.
Figure 4. Complications of Pneumatic Retinopexy.
Discussion
Using the Delphi method of consensus, we developed a comprehensive classification to quantify severity of complications of RRD surgery. Capturing data on severity of complications is important for comparing risks-benefits of new or emerging interventions. For example, a new surgical procedure found to be noninferior with regard to efficacy and to have equal frequency of complications to the standard surgery in an RCT may be considered a potential option for the management of a particular condition. However, this may not be the case if in the same scenario, complications observed following the new approach were identified to be more severe than those occurring following standard surgery.28
Several classifications of severity of surgical complications have been developed in other specialties. An example is the Clavien-Dindo classification, a 5-grade system for complications of general surgery that has been widely adopted in surgical trials since 2004.29,30,31,32 Others commonly used include the Accordion and Memorial Sloan Kettering Cancer Center classifications.33,34 There are few comparable grading systems adapted to the field of ophthalmology. A systematic review conducted by our group to inform this project found that RCTs on RRD surgery do not report well on harms associated with surgery nor did they quantify severity of complications. Recently, a grading system for complications of glaucoma surgery has been published and has shown potential for implementation in future trials in this area.24 These classifications should provide an additional tool in facilitating a more complete understanding, analysis, and interpretation of results in ophthalmic surgical RCTs.
We adhered as far as possible to the original “Clavien” definition of a surgical complication as “a deviation from the normal postoperative course, which cannot otherwise be classed as either a sequela (an ‘after effect’ that is inherent to the procedure) or a failure to cure” in developing our complication list.29 There are other important considerations that extend beyond the scope of the current study. Subjective patient experiences, for example, the reporting of pain, or patient’s opinion of complications severity could be explored in the future. Similarly, complications related to anesthesia were not investigated because they have dedicated representation in the literature.35,36,37,38
For patient and surgeon, ultimately a most important metric of complication severity is the visual outcome. Measuring relative effects of all possible surgical complications on vision would be daunting. Expert focus groups have been used in such circumstances in an attempt to resolve differences in opinion among surgeons.39,40,41 This is important because individual surgeon experiences may vary significantly, and the perceived severity of a surgical complication is likely to depend on the context in which it occurs. For instance, a complication may be perceived as being more severe if it occurs in an only eye or in an eye with significant visual potential. Human factors, such as prior experience of the operating surgeon in managing a particular complication, may also be relevant in this regard. These challenges make the development of a classification an excellent substrate for the Delphi method. Similar to an expert focus group, a Delphi survey assimilates information from a range of experts within a field. However, crucially, it carries the additional advantages of anonymity and equal weighting of all contributors, removing the potential influence of “strong personalities” and allowing controlled and structured feedback, guiding the group more systematically toward a convergence of opinion.27 The strengths of the method are supported by the high degree of consensus achieved in our study (97% of all items) among surgeons with a range of backgrounds and clinical experience.
For the classification to be used widely by vitreoretinal surgeons, it is essential for it to be made accessible in a user-friendly manner to all. For this reason, we are in the process of creating a free app, expected to be available in autumn of 2021, through smartphones and computers, which will contain all complications with the corresponding severity scores. Thus, vitreoretinal surgeons will be able to incorporate the list of expected complications and their severity scoring to the protocol of clinical trials so that surgical procedures could be compared not only with regard to efficacy but also harms. It will be possible not only to report homogeneously intraoperative and postoperative complications in future RTCs, based on the comprehensive list proposed, but also to quantify harm by providing frequency of complications based on their scores or severity (mild, moderate, or severe) and potentially use a harm score (eg, score of each severe complication multiplied by the number of cases with the complication). The severity classification could be used also for the purpose of clinical auditing of surgical results and surgical registries.
Strengths and Limitations
This study has strengths and limitations. We used the Delphi approach, which is a robust and well-established method of generating consensus from a group of experts regarding a highly specialized topic.27 The reproducibility of the Delphi method among panels of experts in a particular area of interest has been confirmed in several studies.42,43 The sample size of our study is adequate as the survey targeted experts who have similar training and general understanding in the field of interest.27 We were unable to reach consensus in all complications. In Delphi studies, it is important to determine an appropriate a priori definition of consensus as well as an appropriate end point of the study.44,45 In quantitative Delphi studies, according to 1 systematic review, a commonly reported definition of consensus has been an IQR of 3 or less on a 9-point scale.46 Thus, under these criteria, our study would have achieved 100% consensus. However, we opted to use instead for a more stringent definition (IQR ≤2 on a 10-point scale), and we were able to reach consensus in 97% of the complications in 2 rounds of surveying. Future ways of validating CORDS may involve testing the classification against the patient’s perspective using patient-reported outcome measures.47
Conclusions
We believe having a comprehensive list of complications of RRD surgery with quantitative severity scores for each will improve the quality of the design and reporting of surgical RCTs, conforming with CONSORT recommendations for harms reporting, and ultimately will allow vitreoretinal surgeons to better select surgical procedures for their patients, based not only on their comparative effectiveness but also on their harms. The upcoming free app will facilitate its implementation.
eTable 1. Preliminary list of complications of retinal detachment surgery used to develop the Delphi survey
eTable 2. Delphi Survey – Full round 1 and 2 results
eTable 3. Complications ranked by severity scores and classified as mild (median scores 1-4), moderate (median scores 5-7) and severe (median scores 8-10)
References
- 1.Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94(6):678-684. doi: 10.1136/bjo.2009.157727 [DOI] [PubMed] [Google Scholar]
- 2.Jackson TL, Donachie PHJ, Sparrow JM, Johnston RL. United Kingdom National Ophthalmology Database Study of Vitreoretinal Surgery: report 1; case mix, complications, and cataract. Eye (Lond). 2013;27(5):644-651. doi: 10.1038/eye.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wykoff CC, Schwartz SG, Adelman RA, Brucker AJ, Flynn HW Jr. Primary rhegmatogenous retinal detachment repair: evidence supports an individualised approach. Br J Ophthalmol. 2015;99(11):1451-1453. doi: 10.1136/bjophthalmol-2015-307651 [DOI] [PubMed] [Google Scholar]
- 4.Haugstad M, Moosmayer S, Bragadόttir R. Primary rhegmatogenous retinal detachment: surgical methods and anatomical outcome. Acta Ophthalmol. 2017;95(3):247-251. doi: 10.1111/aos.13295 [DOI] [PubMed] [Google Scholar]
- 5.Kunikata H, Abe T, Nakazawa T. Historical, current and future approaches to surgery for rhegmatogenous retinal detachment. Tohoku J Exp Med. 2019;248(3):159-168. doi: 10.1620/tjem.248.159 [DOI] [PubMed] [Google Scholar]
- 6.Heimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH; Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment Study Group . Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114(12):2142-2154. doi: 10.1016/j.ophtha.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 7.Jackson TL, Donachie PHJ, Sallam A, Sparrow JM, Johnston RL. United Kingdom National Ophthalmology Database study of vitreoretinal surgery: report 3, retinal detachment. Ophthalmology. 2014;121(3):643-648. doi: 10.1016/j.ophtha.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 8.Tornambe PE, Hilton GF; The Retinal Detachment Study Group . Pneumatic retinopexy: a multicenter randomized controlled clinical trial comparing pneumatic retinopexy with scleral buckling. Ophthalmology. 1989;96(6):772-783. doi: 10.1016/S0161-6420(89)32820-X [DOI] [PubMed] [Google Scholar]
- 9.Kreissig I. Primary retinal detachment: a review of the development of techniques for repair in the past 80 years. Taiwan J Ophthalmol. 2016;6(4):161-169. doi: 10.1016/j.tjo.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuervo LG, Clarke M. Balancing benefits and harms in health care. BMJ. 2003;327(7406):65-66. doi: 10.1136/bmj.327.7406.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin RCG II, Brennan MF, Jaques DP. Quality of complication reporting in the surgical literature. Ann Surg. 2002;235(6):803-813. doi: 10.1097/00000658-200206000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannidis JP, Contopoulos-Ioannidis DG. Reporting of safety data from randomised trials. Lancet. 1998;352(9142):1752-1753. doi: 10.1016/S0140-6736(05)79825-1 [DOI] [PubMed] [Google Scholar]
- 13.Zorzela L, Golder S, Liu Y, et al. Quality of reporting in systematic reviews of adverse events: systematic review. BMJ. 2014;348:f7668. doi: 10.1136/bmj.f7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breau RH, Gaboury I, Scales CD Jr, Fesperman SF, Watterson JD, Dahm P. Reporting of harm in randomized controlled trials published in the urological literature. J Urol. 2010;183(5):1693-1697. doi: 10.1016/j.juro.2010.01.030 [DOI] [PubMed] [Google Scholar]
- 15.Haidich A-B, Birtsou C, Dardavessis T, Tirodimos I, Arvanitidou M. The quality of safety reporting in trials is still suboptimal: survey of major general medical journals. J Clin Epidemiol. 2011;64(2):124-135. doi: 10.1016/j.jclinepi.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 16.Ioannidis JPA, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285(4):437-443. doi: 10.1001/jama.285.4.437 [DOI] [PubMed] [Google Scholar]
- 17.Parikh RP, Sharma K, Qureshi AA, Franco MJ, Myckatyn TM. Quality of surgical outcomes reporting in plastic surgery: a 15-year analysis of complication data. Plast Reconstr Surg. 2018;141(6):1332-1340. doi: 10.1097/PRS.0000000000004362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddad C, Sigha OB, Lebrun-Vignes B, Chosidow O, Fardet L. Reporting of harm and safety results in randomized controlled trials published in 5 dermatology journals. J Am Acad Dermatol. 2017;77(1):98-104.e1. doi: 10.1016/j.jaad.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 19.Kadlec AO, Greco KA, Fridirici ZC, Hart ST, Vellos TG, Turk TM. Comparison of complication rates for unilateral and bilateral percutaneous nephrolithotomy (PCNL) using a modified Clavien grading system. BJU Int. 2013;111(4 Pt B):E243-E248. doi: 10.1111/j.1464-410X.2012.11589.x [DOI] [PubMed] [Google Scholar]
- 20.Shafi S, Priest EL, Crandall ML, et al. ; American Association for the Surgery of Trauma Patient Assessment Committee . Multicenter validation of American Association for the Surgery of Trauma grading system for acute colonic diverticulitis and its use for emergency general surgery quality improvement program. J Trauma Acute Care Surg. 2016;80(3):405-410. doi: 10.1097/TA.0000000000000943 [DOI] [PubMed] [Google Scholar]
- 21.Smith JT, Johnston C, Skaggs D, Flynn J, Vitale M. A new classification system to report complications in growing spine surgery: a multicenter consensus study. J Pediatr Orthop. 2015;35(8):798-803. doi: 10.1097/BPO.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 22.Slaman AE, Lagarde SM, Gisbertz SS, van Berge Henegouwen MI. A quantified scoring system for postoperative complication severity compared to the Clavien-Dindo classification. Dig Surg. 2015;32(5):361-366. doi: 10.1159/000433608 [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Yu P, Shi Y, et al. Evaluation of Clavien-Dindo classification in patients undergoing total gastrectomy for gastric cancer. Med Oncol. 2015;32(4):120. doi: 10.1007/s12032-015-0573-3 [DOI] [PubMed] [Google Scholar]
- 24.Sii S, Barton K, Pasquale LR, Yamamoto T, King AJ, Azuara-Blanco A. Reporting harm in glaucoma surgical trials: systematic review and a consensus-derived new classification system. Am J Ophthalmol. 2018;194:153-162. doi: 10.1016/j.ajo.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 25.Lois N, Wong D.. Complications of Vitreo-retinal surgery. Lippincott, Williams, and Wilkins; 2013. [Google Scholar]
- 26.Anon. Home | Enalyzer. Accessed January 25, 2019. https://www.enalyzer.com/
- 27.Hsu C-C, Sandford BA. The Delphi Technique: making sense of consensus. Practical Assessment, Research, and Evaluation. 2019;12(1). doi:10.7275/pdz9-th90 [Google Scholar]
- 28.Majoko F, Nystrom L, Lindmark G. No benefit, but increased harm from high dose (100 microg) misoprostol for induction of labour: a randomised trial of high vs. low (50 microg) dose misoprostol. J Obstet Gynaecol. 2002;22(6):614-617. doi: 10.1080/0144361021000020376 [DOI] [PubMed] [Google Scholar]
- 29.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 31.Mitropoulos D, Artibani W, Graefen M, Remzi M, Rouprêt M, Truss M; European Association of Urology Guidelines Panel . Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol. 2012;61(2):341-349. doi: 10.1016/j.eururo.2011.10.033 [DOI] [PubMed] [Google Scholar]
- 32.Téoule P, Bartel F, Birgin E, Rückert F, Wilhelm TJ. The Clavien-Dindo classification in pancreatic surgery: a clinical and economic validation. J Invest Surg. 2019;32(4):314-320. doi: 10.1080/08941939.2017.1420837 [DOI] [PubMed] [Google Scholar]
- 33.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250(2):177-186. doi: 10.1097/SLA.0b013e3181afde41 [DOI] [PubMed] [Google Scholar]
- 34.Strong VE, Selby LV, Sovel M, et al. Development and assessment of Memorial Sloan Kettering Cancer Center’s Surgical Secondary Events grading system. Ann Surg Oncol. 2015;22(4):1061-1067. doi: 10.1245/s10434-014-4141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short TG, Campbell D, Frampton C, et al. ; Australian and New Zealand College of Anaesthetists Clinical Trials Network; Balanced Anaesthesia Study Group . Anaesthetic depth and complications after major surgery: an international, randomised controlled trial. Lancet. 2019;394(10212):1907-1914. doi: 10.1016/S0140-6736(19)32315-3 [DOI] [PubMed] [Google Scholar]
- 36.Vadivelu N, Huang Y, Kaye AD, Kodumudi V, Kai A, Adelman RA. Prevention and management of complications of regional orbital anesthesia. Middle East J Anaesthesiol. 2012;21(6):775-784. [PubMed] [Google Scholar]
- 37.Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. J Pain Symptom Manage . 1999;18(6):427-437. doi: 10.1016/s0885-3924(99)00093-7 [DOI] [PubMed] [Google Scholar]
- 38.Sarkar S, Maiti P, Nag S, Sasmal NK, Biswas M. Changing trends of ocular anaesthesia in phaco-emulsification surgery. J Indian Med Assoc. 2010;108(12):823-825, 828. [PubMed] [Google Scholar]
- 39.Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA) / International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) & grafts in female pelvic floor surgery. Int Urogynecol J. 2011;22(1):3-15. doi: 10.1007/s00192-010-1324-9 [DOI] [PubMed] [Google Scholar]
- 40.Śmietański M, Szczepkowski M, Alexandre JA, et al. European Hernia Society classification of parastomal hernias. Hernia. 2014;18(1):1-6. doi: 10.1007/s10029-013-1162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchings A, Raine R, Sanderson C, Black N. A comparison of formal consensus methods used for developing clinical guidelines. J Health Serv Res Policy. 2006;11(4):218-224. doi: 10.1258/135581906778476553 [DOI] [PubMed] [Google Scholar]
- 42.Duffield C. The Delphi technique: a comparison of results obtained using two expert panels. Int J Nurs Stud. 1993;30(3):227-237. doi: 10.1016/0020-7489(93)90033-Q [DOI] [PubMed] [Google Scholar]
- 43.Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2(3):i-iv, 1-88. doi: 10.3310/hta2030 [DOI] [PubMed] [Google Scholar]
- 44.Keeney S, Hasson F, McKenna H. Consulting the oracle: ten lessons from using the Delphi technique in nursing research. J Adv Nurs. 2006;53(2):205-212. doi: 10.1111/j.1365-2648.2006.03716.x [DOI] [PubMed] [Google Scholar]
- 45.Black N, Murphy M, Lamping D, et al Consensus development methods: a review of best practice in creating clinical guidelines Published online 1999. Accessed September 8, 2020. https://journals.sagepub.com/doi/10.1177/135581969900400410 [DOI] [PubMed]
- 46.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401-409. doi: 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 47.Braithwaite T, Calvert M, Gray A, Pesudovs K, Denniston AK. The use of patient-reported outcome research in modern ophthalmology: impact on clinical trials and routine clinical practice. Patient Relat Outcome Meas. 2019;10:9-24. doi: 10.2147/PROM.S162802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Preliminary list of complications of retinal detachment surgery used to develop the Delphi survey
eTable 2. Delphi Survey – Full round 1 and 2 results
eTable 3. Complications ranked by severity scores and classified as mild (median scores 1-4), moderate (median scores 5-7) and severe (median scores 8-10)