Abstract
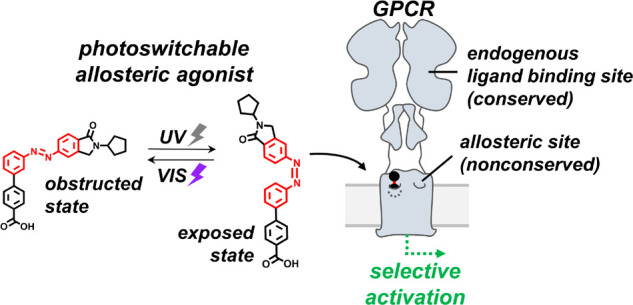
G protein-coupled receptors (GPCRs) are the most common targets of drug discovery. However, the similarity between related GPCRs combined with the complex spatiotemporal dynamics of receptor activation in vivo has hindered drug development. Photopharmacology offers the possibility of using light to control the location and timing of drug action by incorporating a photoisomerizable azobenzene into a GPCR ligand, enabling rapid and reversible switching between an inactive and active configuration. Recent advances in this area include (i) photoagonists and photoantagonists that directly control receptor activity but are nonselective because they bind conserved sites, and (ii) photoallosteric modulators that bind selectively to nonconserved sites but indirectly control receptor activity by modulating the response to endogenous ligand. In this study, we designed a photoswitchable allosteric agonist that targets a nonconserved allosteric site for selectivity and activates the receptor on its own to provide direct control. This work culminated in the development of aBINA, a photoswitchable allosteric agonist that selectively activates the Gi/o-coupled metabotropic glutamate receptor 2 (mGluR2). aBINA is the first example of a new class of precision drugs for GPCRs and other clinically important signaling proteins.
G protein-coupled receptors (GPCRs) are membrane proteins that play important roles in health and disease.1 They are expressed in virtually every cell, are activated by diverse stimuli (chemicals, peptides, light), and initiate pleiotropic signaling via canonical G protein- and noncanonical arrestin-dependent pathways.1 GPCRs are the most common targets of drug discovery efforts, constituting 36% of approved drugs (∼700 compounds).2 The development of therapeutically efficacious drugs, however, is hindered by the complex nature of GPCR function. First, the endogenous ligand binding site (orthosteric site) to which most drugs bind can be highly similar in related GPCRs, making it difficult to target specific receptor subtypes.3,4 Second, each GPCR can be expressed and have distinct functions in more than one location. Third, the timing by which GPCRs are turned on and off by their endogenous ligands (milliseconds to tens of seconds5−7) is a major determinant of downstream signaling and physiological outcome.8,9 These factors compound the challenge of developing drugs that can target specific receptor subtypes with spatiotemporal precision.
The advent of photopharmacology has opened the door to using patterned light to control drug action with spatial and temporal specificity.10−13 GPCR ligands are transformed into rapid and reversible optical photoswitches by employing azobenzene, a chemical moiety that isomerizes between its trans and cis configurations within milliseconds in response to specific wavelengths of light.14 Azobenzene is placed close to or within a receptor ligand,15 allowing it to switch between two states: one that is exposed and can bind the receptor and one that is obstructed and cannot bind the receptor. Nonetheless, most photoswitchable GPCR ligands are not absolutely selective for specific receptor subtypes because they contain inherently nonselective agonists or antagonists that bind the orthosteric site.11,16−23
To increase the specificity, a class of receptor ligands called allosteric modulators24−26 was recently converted into photopharmaceuticals. Allosteric modulators bind nonconserved receptor binding sites (allosteric sites), increasing the likelihood of establishing a selective interaction with a specific receptor subtype. Unlike agonists and antagonists, allosteric modulators have no effect on receptor function in the absence of endogenous ligand binding. Instead, they potentiate (as positive allosteric modulators; PAMs) or decrease (as negative allosteric modulators; NAMs) the actions of the endogenous ligand.27 This feature would allow photoswitchable allosteric modulators to control signaling without disrupting the natural temporal dynamics of receptor activation, which may be therapeutically beneficial for certain disease states but less effective when the endogenous ligand is dysregulated (Figure S1). For example, the precise millisecond to second-time scale release dynamics of the major excitatory neurotransmitter glutamate in the brain is disrupted in neurological disorders such as epilepsy, schizophrenia, bipolar disorder, and depression.28
Whereas allosteric modulators have no effect on their own, allosteric agonists bind to the allosteric site and activate the receptor independently of the endogenous ligand.29 In this study, we sought to combine the receptor subtype selective and agonistic properties of allosteric agonists with the rapid and reversible photoswitching properties of azobenzene. The development of photoswitchable allosteric agonists would represent a considerable advance in drug design, resulting in compounds that, by not relying on the endogenous ligand, have the potential to override aberrant receptor signaling with subtype-selectivity and spatiotemporal precision (Figure S1).
To develop a photoswitchable allosteric agonist, we targeted metabotropic glutamate receptor 2 (mGluR2), a Gi/o-coupled family C GPCR dimer (Figure 1), which has been proposed as a target for the treatment of schizophrenia and anxiety.30 Conventional mGluR2 agonists or antagonists are nonselective, as they bind a large extracellular glutamate-binding ligand binding domain (LBD) that is highly conserved with mGluR3 (Figure 1A).30 Several mGluR2-selective allosteric ligands have recently emerged that bind in the receptor’s transmembrane domain (TMD; Figure 1B,C),31 which is less conserved across mGluRs (Figure 1B). Among them, we chose biphenyl-indanone A (BINA) as a parent compound for the development of a photoswitchable allosteric agonist because it is highly mGluR2-selective and displays robust agonist activity.32−35
Figure 1.
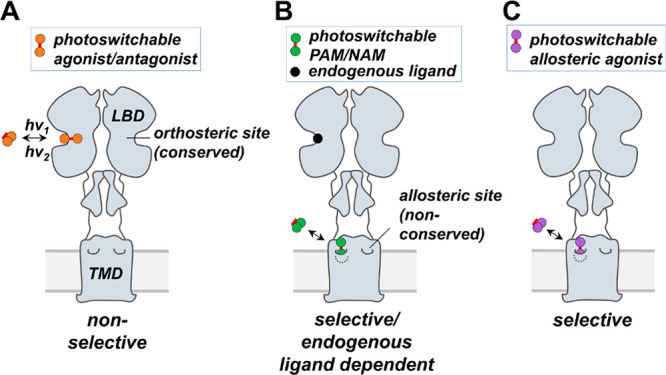
Photoswitchable allosteric agonists are receptor subtype selective and operate independently of endogenous ligands. (A) mGluR2 (gray) is an obligate dimer that binds its endogenous ligand glutamate in its orthosteric site formed by a large extracellular ligand binding domain (LBD). A photoswitchable agonist or antagonist binds to the orthosteric site in only one photoisomeric configuration. Because the orthosteric site of mGluR2 is conserved in mGluR3, photoswitchable agonists and antagonists are not likely to be receptor subtype selective. (B) A photoswitchable allosteric modulator (PAM or NAM) binds selectively to mGluR2 in a nonconserved allosteric site formed by the receptor transmembrane domain (TMD) but depends on glutamate binding to affect receptor function. (C) A photoswitchable allosteric agonist binds selectively to the allosteric site of mGluR2 and activates the receptor independently of glutamate.
To develop a photoswitchable analog of BINA, we used molecular docking analyses to explore the effect of exchanging its benzyloxy-dimethylbenzene group with azobenzene (azobenzene-BINA or aBINA; Figure 2A,B). As previously observed,33,36 our docking showed that BINA can bind in the mGluR2 TMD, forming hydrophobic contacts with residues L639 and F643 and hydrogen bonding with R635 (Figure 2C). Whereas the trans configuration of aBINA bound mGluR2 in a pose similar to that observed for BINA (Figure 2D), the cis configuration did not interact with residues deeper in the binding site or hydrogen bond with R635 (Figure 2E). These results suggested that aBINA can adopt two functionally distinct configurations, an extended pose that mimics the binding mode of BINA (trans) and a constrained pose that is unable to form critical contacts with the receptor (cis).
Figure 2.
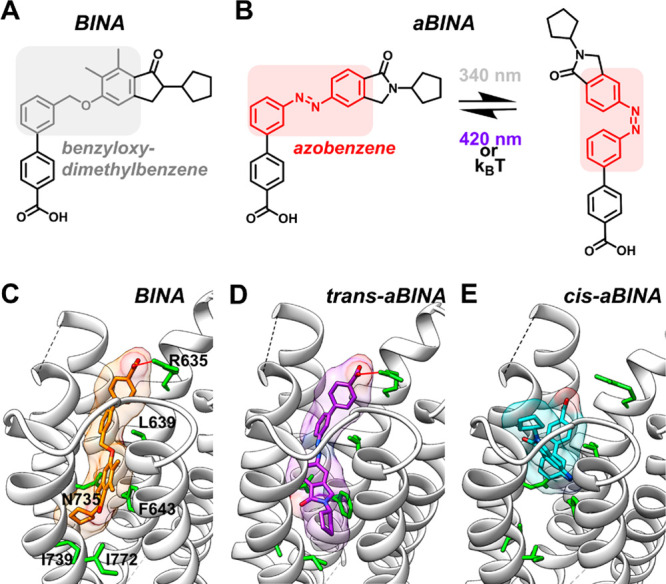
Design of aBINA. (A) Chemical structure of BINA, which contains a benzyloxy-dimethylbenzene group that is suitable for an exchange with azobenzene. (B) Chemical structure of aBINA, which interconverts between its trans and cis configurations with light. (C) According to molecular docking analyses, BINA binds mGluR2 at an allosteric site formed within the receptor transmembrane domain (TMD). The carboxyl group of BINA hydrogen bonds with R635 of mGluR2 (red line) and forms hydrophobic contacts with residues throughout the binding site (green side chains). (D) The trans configuration of aBINA binds in the TMD of mGluR2 in a manner similar to BINA. (E) Unlike BINA or the trans configuration of aBINA, the cis configuration of aBINA does not hydrogen bond with R635 or form hydrophobic contacts with residues deep within the allosteric binding pocket.
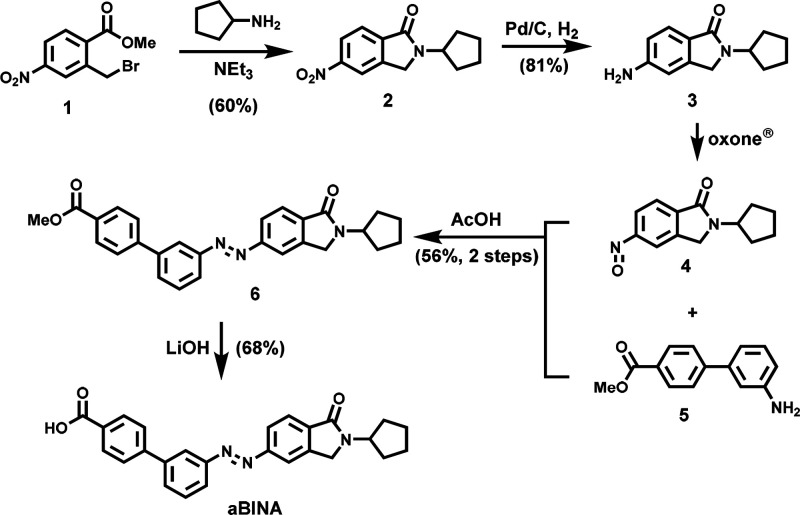
To synthesize aBINA (Figure 3), the commercial benzyl bromide 1 was substituted with cyclopentylamine. The resulting nitroarene 2 was hydrogenatively reduced to aniline 3 and selectively oxidized to the nitrosoarene 4. This nitrosoarene 4 was coupled with the biaryl aniline 5 in a Baeyer–Mills reaction and gave the azobenzene derivative 6. Hydrolysis of the methyl ester (6) with LiOH provided aBINA in good yields.
Figure 3.
Chemical synthesis of aBINA.
We next characterized the photophysical properties of aBINA. aBINA efficiently converted to its cis configuration with wavelengths ranging from 340 and 380 nm (UV) light and to its trans configuration with wavelengths ranging from 400 to 600 nm (visible) light, according to UV/vis spectroscopy (Figures 4A and S2A–D) and LCMS (Figure S2E,F). These photoswitching wavelengths are slightly hypsochromic relative to that of azobenzene,14 which is likely due to electron-withdrawing substituents in the aromatic system. In most subsequent studies, 420 nm light and 340 nm light were used. aBINA switched between its trans and cis configuration over multiple cycles without any loss of activity (Figure 4B). Furthermore, both configurations of aBINA were sufficiently stable toward thermal relaxation (Figure S2G–I), and thus the compound is considered bistable.
Figure 4.
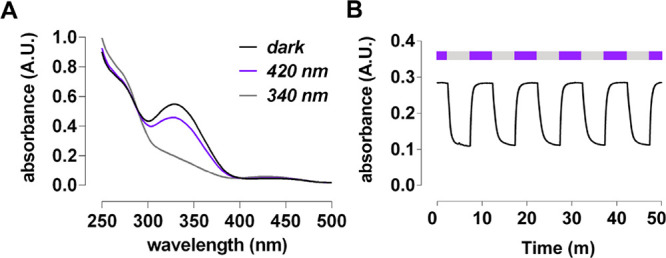
Photophysical properties of aBINA. (A) Absorbance spectra of aBINA (20 μM in 10% DMSO) in the dark or under 340 or 420 nm light. (B) aBINA reversibly and repeatedly switched to its trans configuration with 420 nm light and to its cis configuration with 340 nm light.
To evaluate the effect of aBINA on mGluR2, we used a mGluR2-mediated G protein-inwardly rectifying potassium channel (GIRK) activation assay (Figure S3).37 mGluR2 and a homotetramerizing mutant of GIRK1 (F137S) were transiently transfected into HEK293T cells, whereby receptor activation results in Gi/o-dependent GIRK activation and enhanced inward-current. Transfected cells were patch clamped in whole-cell configuration and exposed to alternating 420 nm light and 340 nm light to interconvert aBINA between its trans and cis configuration, respectively. The application of aBINA under 420 nm light (trans; 100 nM) robustly activated mGluR2 relative to a saturating concentration (1 mM) of glutamate (70 ± 4%, n = 8 cells; Figure 5A,C). This effect was not observed in cells expressing GIRK alone or in cells expressing a mutant of mGluR2 (N735D) in which the allosteric binding site in the receptor TMD is disabled (Figure S4),33 indicating that aBINA operates through the known allosteric mechanism. The effect of the trans configuration of aBINA was largely reversed by switching the compound to the cis configuration with 340 nm light (Figure 5A,C), a photoeffect not observed in the absence of aBINA (0 ± 1% of 1 mM glutamate, n = 4 cells; Figure 5A). mGluR2 activation in response to aBINA under 420 nm light could also be reversed with 360 nm light (Figure S5), which has greater biocompatibility than 340 nm light. Photoactivation of mGluR2 with aBINA was rapid and repeatable (Figure 5A), consistent with its photophysical properties.
Figure 5.
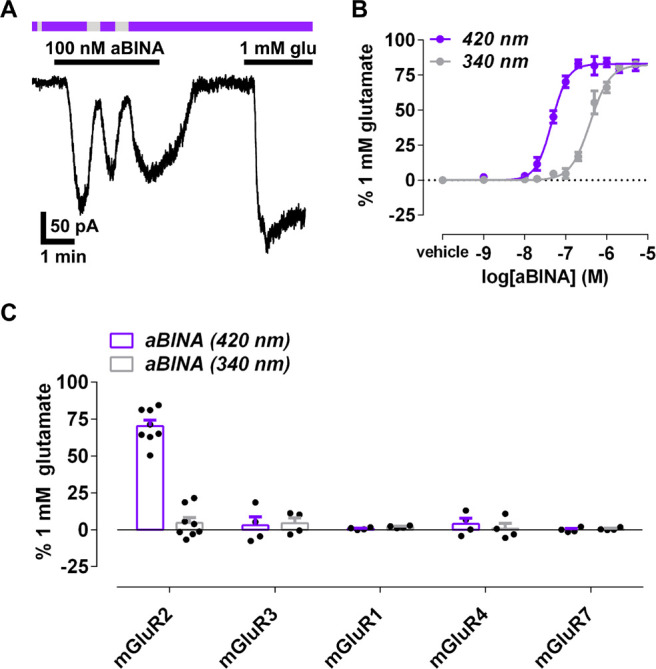
aBINA is a selective photoswitchable allosteric agonist of mGluR2. (A) mGluR2 is activated by the trans configuration of aBINA (420 nm light) in the GIRK assay. This effect is reversed by switching to cis configuration of aBINA (340 nm light) or washout. Photoactivation of mGluR2 with aBINA is rapid and repeatable. (B) Dose-dependent effect of aBINA on mGluR2 under 420 nm light and 340 nm light. n = 3–9 cells per concentration. (C) Summary of the effect of aBINA on various mGluRs. The effect of aBINA on mGluR2 under 420 nm light was significantly different from all other conditions. One-way ANOVA, F = 48.7, ****p < 0.0001 for each comparison.
The potency of the trans configuration of aBINA was ∼11-fold lower than that of the parent compound BINA32−34 (Figure S6), indicating that the replacement of benzyloxy-dimethylbenzene with the trans-isomer of azobenzene somewhat diminishes agonist affinity. aBINA activated mGluR2 at high concentrations under 340 nm light (Figure 5B). The degree of activation was consistent with the presence of a minor fraction of active trans configuration (Figures S2E,F and S7). Taken together, these results indicate that aBINA is a photoswitchable allosteric agonist of mGluR2 that activates the receptor in the trans configuration but not the cis configuration.
Whereas the effect of aBINA on mGluR2 was reversed after minutes of washout (Figures 5A and S8), the parent compound BINA is resistant to washout due to its high hydrophobicity (cLogP = 8.3) and ability to form a reservoir in the plasma membrane.35 The faster washout of aBINA is consistent with the reduction in hydrophobicity by ∼200-fold (cLogP = 6.0) due to the substitution of the indanone scaffold with isoindolinone and the replacement of the benzyloxy-dimethylbenzene with azobenzene.
The parent compound BINA is a PAM that potentiates the potency of glutamate at mGluR2 by ∼5–10 fold.32−34 To evaluate whether aBINA is also a PAM, we examined the activation induced by a low dose (100 nM) of glutamate in the presence of the active trans configuration of aBINA (Figure S9A,B). Low-dose glutamate activated mGluR2 under these conditions (Figure S9C–E), indicating that aBINA has PAM activity. However, because aBINA nearly fully activates mGluR2 on its own (Figure 5A,C), the effect of low-dose glutamate with aBINA was limited (Figure S9C–E). Thus, although aBINA acts as a PAM, its dominant effect on mGluR2 is allosteric agonism.
We next evaluated whether aBINA is selective for mGluR2. The eight mGluRs belong to three groups (I–III) based on sequence and function.30 We tested the effect of aBINA on the closest homologue of mGluR2, the single other group II member mGluR3, as well as one representative of the other groups: mGluR1 (Group I) and mGluR4 as well as mGluR7 (group III). Like BINA, aBINA had no effect on any of these receptors under 420 nm light or 340 nm light (Figures 5C and S10), indicating that aBINA is highly selective for mGluR2.
We next sought to determine whether aBINA can control the native mGluR2 of neurons. To accomplish this, we measured the effect of aBINA on the activity of primary cortical neurons (CNs) in culture using whole-cell current-clamp recordings. Consistent with the effects of the orthosteric mGluR2 agonist LY379268,37 the active trans configuration of aBINA (100 nM; 420 nm light) suppressed spontaneous CN firing (Figure 6A,B). This effect was reversed by switching aBINA to its inactive cis configuration with 340 nm light (Figure 6A,B). Photoinhibition was rapid and repeatable (Figure 6A,B). aBINA had no effect on spontaneous firing in the presence of a saturating concentration of the mGluR2-selective NAM MNI137 (10 μM; Figure 6C,D), which competes with aBINA for the allosteric binding site. Taken together, aBINA selectively photoactivates endogenous mGluR2 in CNs.
Figure 6.
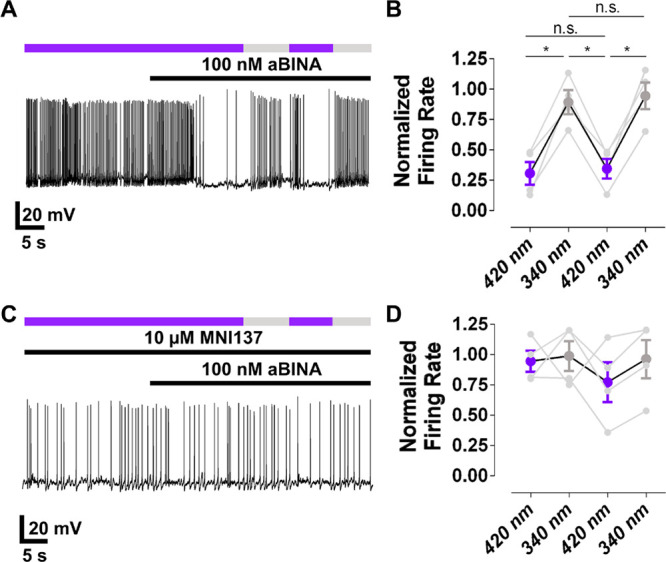
aBINA photoactivates endogenous mGluR2 in primary cortical neurons. (A) aBINA decreases spontaneous firing of primary cortical neurons (CNs) under 420 nm light (trans), which is reversed by switching to 340 nm light (cis). (B) Summary of the effect of aBINA on CNs. n = 4 neurons. RM one-way ANOVA, F = 31.6, *p < 0.05. (C) aBINA has no effect on CNs in the presence of a saturating concentration of the mGluR2-selective NAM MNI137. Summary of the effect of aBINA on CNs in the presence of MNI137. n = 4 neurons. RM one-way ANOVA, F = 0.9; there were no significant differences between any condition.
Although significant progress has recently been made toward applying photopharmacology in intact organisms,11 the success of photopharmaceuticals as therapeutics will depend on ligand efficacy, affinity, and target specificity. While orthosteric agonists can combine high efficacy and affinity, conservation of the orthosteric binding pocket makes it difficult to create completely selective ligands.4 In contrast, allosteric ligands usually bind to much less conserved pockets and can more readily be imparted with selectivity. However, these allosteric ligands typically have no direct activity and instead modulate the response to the endogenous orthosteric ligand. Our example of a photoswitchable allosteric agonist combines target specificity with high efficacy and affinity while also bypassing the endogenous ligand. In addition, the installation of the azobenzene moiety into the parent compound BINA resulted in a reduction in hydrophobicity without substantially sacrificing activity, increasing its therapeutic potential.
The development of photoswitchable allosteric agonists requires suitable parent molecules for incorporation with azobenzene. Like aBINA, some allosteric agonists are partial agonists and/or have PAM activity (so-called ago-PAMs). Therefore, further efforts could result in new high efficacy, pure allosteric agonists of GPCRs. Nevertheless, allosteric agonists are available for diverse and clinically important GPCRs such as other mGluRs,38 cannabinoid receptors,39 free fatty acid receptors,40 GABAB receptors,41 muscarinic receptors,42 and serotonin receptors.43 Moreover, small molecules that harness the same mechanism have been identified for ligand-gated ion channels such as GABAA receptors,44 suggesting that the photoswitchable allosteric agonist approach is broadly applicable. As such, our study sets the stage for the development of photoswitchable allosteric agonists that enable the control of GPCRs and ligand-gated ion channels with high spatiotemporal control and subtype specificity. We are also intrigued by the potential of combining this new class of drug with azobenzene derivatives that photoisomerize in response to wavelengths of light that penetrate biological tissue more efficiently (650–950 nm)45,46 and with membrane anchors that are introduced virally to provide photocontrol in genetically selected cells.37
Acknowledgments
We thank the members of the Isacoff lab for helpful discussion. This work was supported by the National Institutes of Health (DA044696, P.D.; 1RF1MH123246-01, E.Y.I. and D.T..; R01NS108151, D.T) the European Research Council (268795; D.T.), the Weill Neurohub (E.Y.I.), and the Friedrich-Ebert-Foundation doctoral scholarship (D.B.K.).
Glossary
Abbreviations
- GPCR
G protein-coupled receptor
- mGluR
metabotropic glutamate receptor
- PAM
positive allosteric modulator
- NAM
negative allosteric modulator
- GIRK
G protein-inwardly rectifying potassium channel
- BINA
biphenyl-indanone A
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c02586.
Details of chemical synthesis and methods; comparison of photoswitchable allosteric modulators and photoswitchable allosteric agonists; extended photophysical characterization of aBINA; schematic of the mGluR2-dependent GIRK activation assay; controls for the photoeffect of aBINA on mGluR2; comparison of aBINA and its parent compound BINA; effect of the cis configuration of aBINA on mGluR2; kinetics of the effect of aBINA on mGluR2; effect of aBINA as a positive allosteric modulator; effect of aBINA on different mGluRs (PDF)
Author Contributions
# P.D. and D.B.K. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Katritch V.; Cherezov V.; Stevens R. C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531. 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K.; Insel P. A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs?. Mol. Pharmacol. 2018, 93, 251. 10.1124/mol.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A. S.; Attwood M. M.; Rask-Andersen M.; Schioth H. B.; Gloriam D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discovery 2017, 16, 829. 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michino M.; Beuming T.; Donthamsetti P.; Newman A. H.; Javitch J. A.; Shi L. What can crystal structures of aminergic receptors tell us about designing subtype-selective ligands?. Pharmacol. Rev. 2015, 67, 198. 10.1124/pr.114.009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F.; Zeng J.; Jing M.; Zhou J.; Feng J.; Owen S. F.; Luo Y.; Li F.; Wang H.; Yamaguchi T.; Yong Z.; Gao Y.; Peng W.; Wang L.; Zhang S.; Du J.; Lin D.; Xu M.; Kreitzer A. C.; Cui G.; Li Y. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 2018, 174, 481. 10.1016/j.cell.2018.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T.; Cho J. R.; Merten K.; Howe M. W.; Marley A.; Xiong W. H.; Folk R. W.; Broussard G. J.; Liang R.; Jang M. J.; Zhong H.; Dombeck D.; von Zastrow M.; Nimmerjahn A.; Gradinaru V.; Williams J. T.; Tian L. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 2018, 360, eaat4422. 10.1126/science.aat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R.; Brown D. A. Synaptic inhibition of the M-current: slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurones. J. Physiol. 1982, 332, 263. 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumit M.; Neubig R. R.; Takayama S.; Linderman J. J. Band-pass processing in a GPCR signaling pathway selects for NFAT transcription factor activation. Integrative biology: quantitative biosciences from nano to macro 2015, 7, 1378. 10.1039/C5IB00181A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann M.; Kostenis E. Temporal Bias: Time-Encoded Dynamic GPCR Signaling. Trends Pharmacol. Sci. 2017, 38, 1110. 10.1016/j.tips.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Lester H. A.; Chang H. W. Response of acetylcholine receptors to rapid photochemically produced increases in agonist concentration. Nature 1977, 266, 373. 10.1038/266373a0. [DOI] [PubMed] [Google Scholar]
- Hull K.; Morstein J.; Trauner D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710. 10.1021/acs.chemrev.8b00037. [DOI] [PubMed] [Google Scholar]
- Lester H. A.; Nass M. M.; Krouse M. E.; Nerbonne J. M.; Wassermann N. H.; Erlanger B. F. Electrophysiological experiments with photoisomerizable cholinergic compounds: review and progress report. Ann. N. Y. Acad. Sci. 1980, 346, 475. 10.1111/j.1749-6632.1980.tb22118.x. [DOI] [PubMed] [Google Scholar]
- Paoletti P.; Ellis-Davies G. C. R.; Mourot A. Optical control of neuronal ion channels and receptors. Nat. Rev. Neurosci. 2019, 20, 514. 10.1038/s41583-019-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharry A. A.; Woolley G. A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422. 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- Morstein J.; Awale M.; Reymond J. L.; Trauner D. Mapping the Azolog Space Enables the Optical Control of New Biological Targets. ACS Cent. Sci. 2019, 5, 607. 10.1021/acscentsci.8b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahamonde M. I.; Taura J.; Paoletta S.; Gakh A. A.; Chakraborty S.; Hernando J.; Fernandez-Duenas V.; Jacobson K. A.; Gorostiza P.; Ciruela F. Photomodulation of G protein-coupled adenosine receptors by a novel light-switchable ligand. Bioconjugate Chem. 2014, 25, 1847. 10.1021/bc5003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot J.; Lester H. A.; Birdsall N. J.; Stockton J.; Wassermann N. H.; Erlanger B. F. A photoisomerizable muscarinic antagonist. Studies of binding and of conductance relaxations in frog heart. J. Gen. Physiol. 1982, 79, 657. 10.1085/jgp.79.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donthamsetti P. C.; Winter N.; Schonberger M.; Levitz J.; Stanley C.; Javitch J. A.; Isacoff E. Y.; Trauner D. Optical Control of Dopamine Receptors Using a Photoswitchable Tethered Inverse Agonist. J. Am. Chem. Soc. 2017, 139, 18522. 10.1021/jacs.7b07659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberger M.; Trauner D. A photochromic agonist for mu-opioid receptors. Angew. Chem., Int. Ed. 2014, 53, 3264. 10.1002/anie.201309633. [DOI] [PubMed] [Google Scholar]
- Hauwert N. J.; Mocking T. A. M.; Da Costa Pereira D.; Kooistra A. J.; Wijnen L. M.; Vreeker G. C. M.; Verweij E. W. E.; De Boer A. H.; Smit M. J.; De Graaf C.; Vischer H. F.; de Esch I. J. P.; Wijtmans M.; Leurs R. Synthesis and Characterization of a Bidirectional Photoswitchable Antagonist Toolbox for Real-Time GPCR Photopharmacology. J. Am. Chem. Soc. 2018, 140, 4232. 10.1021/jacs.7b11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J. A.; Yushchenko D. A.; Fine N. H. F.; Duca M.; Citir M.; Broichhagen J.; Hodson D. J.; Schultz C.; Trauner D. Optical control of GPR40 signalling in pancreatic beta-cells. Chemical science 2017, 8, 7604. 10.1039/C7SC01475A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M. V.; Schafroth M. A.; Sarott R. C.; Imhof M. A.; Bold C. P.; Leippe P.; Dhopeshwarkar A.; Grandner J. M.; Katritch V.; Mackie K.; Trauner D.; Carreira E. M.; Frank J. A. Synthesis of Photoswitchable Delta(9)-Tetrahydrocannabinol Derivatives Enables Optical Control of Cannabinoid Receptor 1 Signaling. J. Am. Chem. Soc. 2017, 139, 18206. 10.1021/jacs.7b06456. [DOI] [PubMed] [Google Scholar]
- Duran-Corbera A.; Catena J.; Otero-Vinas M.; Llebaria A.; Rovira X. Photoswitchable Antagonists for a Precise Spatiotemporal Control of beta2-Adrenoceptors. J. Med. Chem. 2020, 63, 8458. 10.1021/acs.jmedchem.0c00831. [DOI] [PubMed] [Google Scholar]
- Pittolo S.; Gomez-Santacana X.; Eckelt K.; Rovira X.; Dalton J.; Goudet C.; Pin J. P.; Llobet A.; Giraldo J.; Llebaria A.; Gorostiza P. An allosteric modulator to control endogenous G protein-coupled receptors with light. Nat. Chem. Biol. 2014, 10, 813. 10.1038/nchembio.1612. [DOI] [PubMed] [Google Scholar]
- Rovira X.; Trapero A.; Pittolo S.; Zussy C.; Faucherre A.; Jopling C.; Giraldo J.; Pin J. P.; Gorostiza P.; Goudet C.; Llebaria A. OptoGluNAM4.1, a Photoswitchable Allosteric Antagonist for Real-Time Control of mGlu4 Receptor Activity. Cell chemical biology 2016, 23, 929. 10.1016/j.chembiol.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Font J.; Lopez-Cano M.; Notartomaso S.; Scarselli P.; Di Pietro P.; Bresoli-Obach R.; Battaglia G.; Malhaire F.; Rovira X.; Catena J.; Giraldo J.; Pin J. P.; Fernandez-Duenas V.; Goudet C.; Nonell S.; Nicoletti F.; Llebaria A.; Ciruela F. Optical control of pain in vivo with a photoactive mGlu5 receptor negative allosteric modulator. eLife 2017, 6, e23545. 10.7554/eLife.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey Conn P.; Christopoulos A.; Lindsley C. W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discovery 2009, 8, 41. 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. T.; Yang K. C.; Lin W. C. Glutamatergic Dysfunction and Glutamatergic Compounds for Major Psychiatric Disorders: Evidence From Clinical Neuroimaging Studies. Front. Psychiatry 2019, 9, 767. 10.3389/fpsyt.2018.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T. W.; Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act?. Trends Pharmacol. Sci. 2007, 28, 366. 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Niswender C. M.; Conn P. J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295. 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabanco A. A.; Bartolome J. M.; Cid J. M. mGluR2 positive allosteric modulators: an updated patent review (2013–2018). Expert Opin. Ther. Pat. 2019, 29, 497. 10.1080/13543776.2019.1637421. [DOI] [PubMed] [Google Scholar]
- Galici R.; Jones C. K.; Hemstapat K.; Nong Y.; Echemendia N. G.; Williams L. C.; de Paulis T.; Conn P. J. Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J. Pharmacol. Exp. Ther. 2006, 318, 173. 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- Farinha A.; Lavreysen H.; Peeters L.; Russo B.; Masure S.; Trabanco A. A.; Cid J.; Tresadern G. Molecular determinants of positive allosteric modulation of the human metabotropic glutamate receptor 2. British journal of pharmacology 2015, 172, 2383. 10.1111/bph.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien D. E.; Shaw D. M.; Cho H. P.; Cross A. J.; Wesolowski S. S.; Felts A. S.; Bergare J.; Elmore C. S.; Lindsley C. W.; Niswender C. M.; Conn P. J. Differential Pharmacology and Binding of mGlu2 Receptor Allosteric Modulators. Mol. Pharmacol. 2018, 93, 526. 10.1124/mol.117.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit V. A.; Thibado J.; Stor D. S.; Zhou Z.; Blanchard S. C.; Andersen O. S.; Levitz J. Conformational dynamics between transmembrane domains and allosteric modulation of a metabotropic glutamate receptor. eLife 2019, 8, e45116. 10.7554/eLife.45116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z.; Ma S.; Hu G.; Xie X. Q. Allosteric Binding Site and Activation Mechanism of Class C G-Protein Coupled Receptors: Metabotropic Glutamate Receptor Family. AAPS J. 2015, 17, 737. 10.1208/s12248-015-9742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donthamsetti P. C.; Broichhagen J.; Vyklicky V.; Stanley C.; Fu Z.; Visel M.; Levitz J. L.; Javitch J. A.; Trauner D.; Isacoff E. Y. Genetically Targeted Optical Control of an Endogenous G Protein-Coupled Receptor. J. Am. Chem. Soc. 2019, 141, 11522. 10.1021/jacs.9b02895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzel M. J.; Rook J. M.; Vinson P. N.; Cho H. P.; Days E.; Zhou Y.; Rodriguez A. L.; Lavreysen H.; Stauffer S. R.; Niswender C. M.; Xiang Z.; Daniels J. S.; Jones C. K.; Lindsley C. W.; Weaver C. D.; Conn P. J. Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol. Pharmacol. 2012, 81, 120. 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie R. B.; Kulkarni P. M.; Deschamps J. R.; Kelly M. E. M.; Janero D. R.; Cascio M. G.; Stevenson L. A.; Pertwee R. G.; Kenakin T. P.; Denovan-Wright E. M.; Thakur G. A. Enantiospecific Allosteric Modulation of Cannabinoid 1 Receptor. ACS Chem. Neurosci. 2017, 8, 1188. 10.1021/acschemneuro.6b00310. [DOI] [PubMed] [Google Scholar]
- Yabuki C.; Komatsu H.; Tsujihata Y.; Maeda R.; Ito R.; Matsuda-Nagasumi K.; Sakuma K.; Miyawaki K.; Kikuchi N.; Takeuchi K.; Habata Y.; Mori M. A novel antidiabetic drug, fasiglifam/TAK-875, acts as an ago-allosteric modulator of FFAR1. PLoS One 2013, 8, e76280 10.1371/journal.pone.0076280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet V.; Brajon C.; Le Corre L.; Acher F.; Pin J. P.; Prezeau L. The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J. Biol. Chem. 2004, 279, 29085. 10.1074/jbc.M400930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding T. A.; Trotter C.; Skjaerbaek N.; Messier T. L.; Currier E. A.; Burstein E. S.; Li D.; Hacksell U.; Brann M. R. Discovery of an ectopic activation site on the M(1) muscarinic receptor. Mol. Pharmacol. 2002, 61, 1297. 10.1124/mol.61.6.1297. [DOI] [PubMed] [Google Scholar]
- Im W. B.; Chio C. L.; Alberts G. L.; Dinh D. M. Positive allosteric modulator of the human 5-HT2C receptor. Mol. Pharmacol. 2003, 64, 78. 10.1124/mol.64.1.78. [DOI] [PubMed] [Google Scholar]
- Drasbek K. R.; Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex 2006, 16, 1134. 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Frank J. A.; Antonini M. J.; Chiang P. H.; Canales A.; Konrad D. B.; Garwood I. C.; Rajic G.; Koehler F.; Fink Y.; Anikeeva P. In Vivo Photopharmacology Enabled by Multifunctional Fibers. ACS Chem. Neurosci. 2020, 11, 3802. 10.1021/acschemneuro.0c00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad D. B.; Savasci G.; Allmendinger L.; Trauner D.; Ochsenfeld C.; Ali A. M. Computational Design and Synthesis of a Deeply Red-Shifted and Bistable Azobenzene. J. Am. Chem. Soc. 2020, 142, 6538. 10.1021/jacs.9b10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.