Key Points
Question
Does extended adjuvant treatment with zoledronate for 5 years provide a survival benefit for patients with early breast cancer compared with 2 years of zoledronate treatment?
Findings
In the randomized phase 3 SUCCESS A clinical trial, no statistically significant difference in survival between 5 and 2 years of adjuvant zoledronate treatment could be shown in 2987 patients with early breast cancer, irrespective of menopausal status. The frequency of adverse events was higher with 5 years of zoledronate treatment—both all grades and grades 3 or 4 only.
Meaning
The results of this randomized clinical trial suggest that the recommended 3 to 5 years of adjuvant bisphosphonate treatment for patients with high-risk early breast cancer as published in current clinical guidelines could be reduced.
This phase 3 randomized clinical trial compares survival outcomes and the incidence of adverse events in patients with breast cancer treated with zoledronate for 5 vs 2 years.
Abstract
Importance
Bisphosphonate treatment in patients with early breast cancer has become part of care, but the optimal treatment duration is still unclear.
Objective
To compare 2 vs 5 years of zoledronate treatment following adjuvant chemotherapy in patients with early breast cancer.
Design, Setting, and Participants
The SUCCESS A phase 3 multicenter randomized open-label clinical trial with a 2 × 2 factorial design enrolled 3754 patients from September 21, 2005, to March 12, 2007 (last patient out, May 7, 2014). Final data analysis was conducted from September 2019 to October 2020. In 250 German study centers, patients were eligible for participation in the SUCCESS A trial if they had either node-positive or high-risk node-negative (defined as at least 1 of the following: tumor size ≥ pT2, histologic grade 3, negative hormone receptor status, or age ≤35 years) primary invasive breast cancer.
Interventions
Patients were first randomized to adjuvant chemotherapy with 3 cycles of fluorouracil, epirubicin, and cyclophosphamide followed by 3 cycles of docetaxel with or without gemcitabine (not presented in this report). After chemotherapy, patients underwent a second randomization of 5 years of zoledronate treatment (4 mg intravenously every 3 months for 2 years, followed by 4 mg intravenously every 6 months for 3 years) vs 2 years of zoledronate treatment (4 mg intravenously every 3 months for 2 years).
Main Outcomes and Measures
The primary end point of the study was disease-free survival; secondary end points were overall survival, distant disease-free survival, and the incidence of skeletal-related adverse events. Survival times were measured from 2 years after the start of zoledronate treatment (landmark analysis).
Results
Overall, data on 2987 patients were available for analysis; median age was 53 (range, 21-86) years. Disease-free survival, overall survival, and distant disease-free survival did not differ significantly between the 2 treatment arms (5 vs 2 years) as shown by adjusted multivariable Cox proportional hazards regression models (disease-free survival: hazard ratio [HR], 0.97; 95% CI, 0.75-1.25; P = .81; overall survival: HR, 0.98; 95% CI, 0.67-1.42; P = .90; distant disease-free survival: HR, 0.87; 95% CI, 0.65-1.18; P = .38). Adverse events were observed more often in the 5-year (46.2%) vs 2-year (27.2%) zoledronate treatment arm, which was particularly true for the skeletal-related events bone pain (5 years, 8.3% vs 2 years, 3.7%) and arthralgia (5 years, 5.1% vs 2 years, 3.1%).
Conclusions and Relevance
The results of this phase 3 randomized clinical trial indicate that extending the zoledronate treatment beyond 2 years does not improve the prognosis of high-risk patients with early breast cancer receiving chemotherapy, suggesting that the currently recommended bisphosphonate treatment duration of 3 to 5 years could be reduced.
Trial Registration
ClinicalTrials.gov Identifier: NCT02181101
Introduction
Bisphosphonates and the human monoclonal antibody denosumab play an important role in the adjuvant treatment of patients with cancer. Both agents can be added to systemic therapy to prevent accelerated bone loss and reduce the risk of skeletal-related events in cases in which cancer treatment leads to decreased bone density and/or estrogen levels.1,2 Several clinical trials have shown that bisphosphonates maintain bone mass and prevent skeletal-related adverse events in women with breast cancer (BC) receiving adjuvant therapy.3,4,5,6,7
Preclinical and clinical studies indicated that bisphosphonates may also exert a more direct antitumoral activity,8 with one of the postulated mechanisms so far not suggested for denosumab having a positive influence on the bone marrow microenvironment that negatively affects cancer spread through dormant tumor cells.9,10 Given that recent data have demonstrated the independent prognostic role of circulating tumor cells (CTCs) in the blood assessed during routine follow-up of patients with BC 2 or 5 years after adjuvant chemotherapy,11,12 the effect of bisphosphonates on the BC outcome might at least partly be due to a direct adverse effect on these tumor cells.
Several clinical trials evaluating the effect of adding bisphosphonates to standard adjuvant BC treatment on survival yielded somewhat inconsistent and controversial results.3,13,14,15,16 However, 2 meta-analyses with data on 18 766 (Early Breast Cancer Trialists’ Collaborative Group)17 and 13 949 (Cochrane) women with early BC (EBC)18 provided good data for a survival benefit of adjuvant bisphosphonate treatment, although this benefit was evident only in postmenopausal women.
Guidelines and reviews have been published to aid clinicians on the use of bisphosphonates for patients with EBC in clinical practice.19,20 Currently, there is general consensus that adjuvant bisphosphonates should be given to postmenopausal patients with EBC who have any indication for systemic therapy. However, although guidelines recommend that bisphosphonates should be administered for 3 to 5 years, it is acknowledged that the optimal duration of adjuvant bisphosphonate treatment has not yet been determined.19,20
Herein, we report on survival of patients with EBC who received 5 compared with 2 years of adjuvant zoledronate treatment in the large randomized phase 3 trial, SUCCESS A. In addition, we compared the presence of CTCs in the blood 5 years after adjuvant chemotherapy between the 2 arms and assessed the frequency of adverse events related to the bisphosphonate treatment with emphasis on skeletal-related events.
Methods
Study Design
The SUCCESS A trial was an open-label, multicenter, prospective randomized phase 3 trial with a 2 × 2 factorial design conducted from September 21, 2005, to March 12, 2007 (last patient out, May 7, 2014). Final data analysis was conducted from September 2019 to October 2020. The trial protocol is available in Supplement 1. The complete study design is depicted in eFigure 1 in Supplement 2. The study included 3754 patients who were first randomized to 3 cycles of combined fluorouracil, 500 mg/m2; epirubicin, 100 mg/m2; and cyclophosphamide, 500 mg/m2 (FEC) every 3 weeks, followed by either 3 cycles of docetaxel, 100 mg/m2, every 3 weeks or 3 cycles of docetaxel, 75 mg/m2, plus gemcitabine, 1000 mg/m2 every 3 weeks (gemcitabine was given on days 1 and 8 of the 3-week cycle) . Adding gemcitabine to the standard chemotherapy with FEC-docetaxel did not improve the outcomes, indicating that gemcitabine should not be included in the adjuvant treatment setting in patients with high-risk EBC.21 After completion of chemotherapy, patients were randomized again to compare 5 years of zoledronate treatment (4 mg intravenously every 3 months for 2 years, followed by 4 mg intravenously every 6 months for 3 years) and 2 years of zoledronate treatment (4 mg intravenously every 3 months for 2 years). More details regarding the randomization procedure are available in eMethods 1 in Supplement 2. All patients were treated in accordance with national German guidelines regarding endocrine therapy (eMethods 2 in Supplement 2), radiotherapy, and anti-ERBB2 (formerly HER2) treatment. The study was written and conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki22 and was approved by the leading ethical board (Ludwig-Maximilian-University, Munich, Germany) and all other responsible ethical boards of the participating study centers. All patients provided written informed consent; the participants did not receive financial compensation. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients and Procedures
Patients were eligible for participation in the SUCCESS A trial if they had either node-positive or high-risk node-negative (defined as at least 1 of the following: tumor size ≥ pT2, histologic grade 3, negative hormone receptor status, or age ≤35 years) primary invasive BC. All patients underwent primary breast-conserving surgery or modified radical mastectomy with R0 resection. Follow-up visits at the study sites took place at 3-month intervals for the first 2 years, every 6 months during the subsequent 3 years, and yearly thereafter; the follow-up visits included clinical examination at each visit, mammography every 12 months, and appropriate examinations to evaluate symptoms if necessary. Details of CTC assessments are available in the eMethods 3 in Supplement 2.
Statistical Analysis
The primary objective with regard to the second randomization of the SUCCESS A trial was to compare disease-free survival (DFS) between patients randomized to 5 vs 2 years of zoledronate treatment. According to the original sample size calculations as stated in the study protocol, 3658 patients were required to detect an absolute increase of DFS rates at 5 years by 4%, from 78.3% to 82.3%, using a 2-sided log-rank test with a test power of 80% and the significance level α set to 0.05 (eMethods 4 in Supplement 2). The original sample size calculation was not specifically powered for the landmark-approach–based comparison of DFS in patients receiving 5 vs 2 years of zoledronate treatment presented in this article because both the sample size available for the landmark analysis and the median follow-up duration were reduced compared with an analysis of the full data set of randomized patients with survival times as from randomization, leading to a reduced statistical power (eMethods 4 in Supplement 2).
Secondary objectives included the comparison of overall survival (OS) and distant DFS (DDFS) between the 2 treatment arms and the analysis of the incidence of skeletal-related adverse events. Additional exploratory analyses were conducted to estimate differences in survival between patients who received 5 vs 2 years of zoledronate treatment in premenopausal and postmenopausal women and other patient subgroups.
Patient outcomes in terms of DFS, OS, and DDFS were analyzed as specified by the standardized definitions for efficacy end points criteria.23 We used a landmarking approach with all time-to-event intervals being measured starting 2 years after the start of zoledronate treatment (ie, the time at which the 2 treatments arms of interest separated into no further zoledronate treatment and 3 additional years of zoledronate treatment). The maximum observation time was set to 4 years to reduce a possible bias in follow-up times between the 2 arms caused by the fact that the trial was open-label and the patients thus were aware of whether they received 2 or 5 years of zoledronate treatment. The eMethods 5 in Supplement 2 provides exact definitions of the survival end points DFS, OS, DDFS, and bone-recurrence–free survival.
Survival rates based on time-to-event data were estimated by the Kaplan-Meier product limit method and survival curves were compared using log-rank tests. Hazard ratios (HRs) with 95% CIs and corresponding P values were estimated using univariable Cox proportional hazards regression models with the zoledronate treatment arm (5 vs 2 years) as the predictor variable. We used multivariable Cox proportional hazards regression models adjusted for age (continuous), body mass index (continuous), menopausal status (premenopausal, postmenopausal), type of surgery (breast conserving, mastectomy), tumor size (pT1, pT2, pT3, pT4), nodal category (pN0, pN1, pN2, pN3), histologic grade (G1, G2, G3), histologic type (ductal, lobular, other), hormone receptor status (positive, negative), ERBB2 status (positive, negative), and adjuvant chemotherapy (FEC-docetaxel, FEC-docetaxel, gemcitabine) to evaluate whether the duration of zoledronate treatment (5 vs 2 years) constitutes an independent prognostic factor for DFS, DDFS, and/or OS.
Probably because of the open-label, non–placebo-controlled trial design, there was a slight bias with regard to loss of follow-up. The eMethods 6 in Supplement 2 presents a description of this bias and the results of a sensitivity analysis addressing this issue.
Adverse event rates and presence of CTCs after 5 years were compared between the 2 treatment arms using the χ2 test. All statistical tests were 2-sided, and P values <.05 were regarded as statistically significant. The statistical analyses were performed using the SPSS statistical software package, version 24 (IBM Corp).
Results
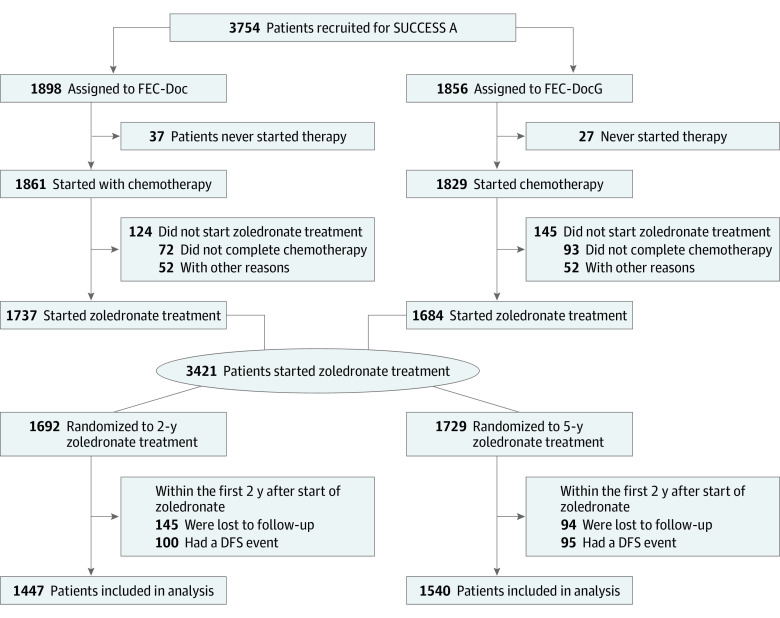
Of the 3754 patients who were randomized in the SUCCESS A trial, 3421 (91.1%) patients started with adjuvant zoledronate treatment following chemotherapy. Of these, 195 patients had a DFS event and 239 patients were lost to follow-up within the first 2 years of zoledronate treatment, resulting in 2987 patients (1447 patients in the 2-year zoledronate arm and 1540 patients in the 5-year zoledronate arm) being included in the final analysis (Figure 1). Median age was 53 (range, 21-86) years and 65.0% of the patients had node-positive disease. The 2 zoledronate treatment arms were well balanced with regard to baseline patient and tumor characteristics (Table).
Figure 1. CONSORT Patient Flow Diagram.
DFS indicates disease-free survival; FEC-Doc, 3 cycles of combined fluorouracil, epirubicin, and cyclophosphamide followed by 3 cycles of docetaxel; FEC DocG indicates 3 cycles of combined fluorouracil, epirubicin, and cyclophosphamide followed by 3 cycles of docetaxel and gemcitabine.
Table. Baseline Characteristics of Patients and Clinicopathological Variables According to Zoledronate Treatment Arm.
| Variable | Zoledronate treatment duration | Standardized mean difference (Cohen d)a | |
|---|---|---|---|
| 5 y (n = 1540) | 2 y (n = 1447) | ||
| Age, median (range), y | 53.0 (21-79) | 53.0 (22-86) | −0.010 |
| BMI, median (range) | 25.4 (15.8-53.4) | 25.3 (15.4-52.0) | 0.004 |
| Tumor category, No. (%) | |||
| pT1 | 647 (42.0) | 627 (43.3) | 0.001 |
| pT2 | 804 (52.2) | 724 (50.0) | |
| pT3 | 70 (4.5) | 77 (5.3) | |
| pT4 | 16 (1.0) | 18 (1.2) | |
| Unknown | 3 (0.2) | 1 (0.1) | |
| Nodal category, No. (%) | |||
| pN0 | 516 (33.5) | 520 (35.9) | 0.021 |
| pN1 | 741 (48.1) | 646 (44.6) | |
| pN2 | 194 (12.6) | 208 (14.4) | |
| pN3 | 83 (5.4) | 70 (4.8) | |
| Unknown | 6 (0.4) | 3 (0.2) | |
| Histologic grading, No. (%) | |||
| G1 | 82 (5.3) | 68 (4.7) | −0.022 |
| G2 | 752 (48.8) | 707 (48.9) | |
| G3 | 705 (45.8) | 672 (46.4) | |
| Unknown | 1 (0.1) | 0 | |
| Histologic type, No. (%) | |||
| Ductal | 1280 (83.1) | 1181 (81.6) | −0.033 |
| Lobular | 159 (10.3) | 169 (11.7) | |
| Other | 99 (6.4) | 97 (6.7) | |
| Unknown | 2 (0.1) | 0 | |
| Hormone receptor status, No. (%) | |||
| Negative | 406 (26.4) | 422 (29.2) | −0.077 |
| Positive | 1132 (73.5) | 1024 (70.8) | |
| Unknown | 2 (0.1) | 1 (0.1) | |
| ERBB2 status, No. (%) | |||
| Negative | 1151 (74.7) | 1083 (74.8) | 0.006 |
| Positive | 357 (23.2) | 341 (23.6) | |
| Unknown | 32 (2.1) | 23 (1.6) | |
| Subtype, No. (%)b | |||
| Luminal A-like | 615 (39.9) | 567 (39.2) | −0.053 |
| Luminal B-like | 268 (17.4) | 228 (15.8) | |
| ERBB2 type | 357 (23.2) | 341 (23.6) | |
| Triple negative | 268 (17.4) | 288 (19.9) | |
| Unknown | 32 (2.1) | 23 (1.6) | |
| Menopausal status, No. (%) | |||
| Premenopausal | 649 (42.1) | 614 (42.4) | −0.007 |
| Postmenopausal | 891 (57.9) | 833 (57.6) | |
| Type of surgery, No. (%) | |||
| Breast conserving | 1090 (70.8) | 1054 (72.8) | −0.055 |
| Mastectomy | 449 (29.2) | 393 (27.2) | |
| Unknown | 1 (0.1) | 0 | |
| Adjuvant chemotherapy, No. (%) | |||
| FEC-DocG | 744 (48.3) | 732 (50.6) | −0.051 |
| FEC-Doc | 796 (51.7) | 715 (49.4) | |
| Radiotherapy, No. (%) | |||
| No | 180 (11.7) | 162 (11.2) | 0.027 |
| Yes | 1360 (88.3) | 1285 (88.8) | |
| Endocrine therapy, No. (%) | |||
| No | 346 (22.5) | 355 (24.5) | −0.061 |
| Yes | 1194 (77.5) | 1092 (75.5) | |
| ERBB2-targeted therapy, No. (%) | |||
| No | 1227 (79.7) | 1131 (78.2) | 0.050 |
| Yes | 313 (20.3) | 316 (21.8) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FEC-Doc, 3 cycles of combined fluorouracil, epirubicin, and cyclophosphamide followed by 3 cycles of docetaxel; FEC DocG indicates 3 cycles of combined fluorouracil, epirubicin, and cyclophosphamide followed by 3 cycles of docetaxel and gemcitabine.
Calculated without unknown variables.
Luminal-A-like: hormone receptor positive; ERBB2 negative, grading G1/G2. Luminal-B-like: hormone receptor positive, ERBB2 negative, grading G3. ERBB2 type: all ERBB2 positives. Triple negative: hormone receptor negative, ERBB2 negative.
Survival According to Zoledronate Treatment Duration
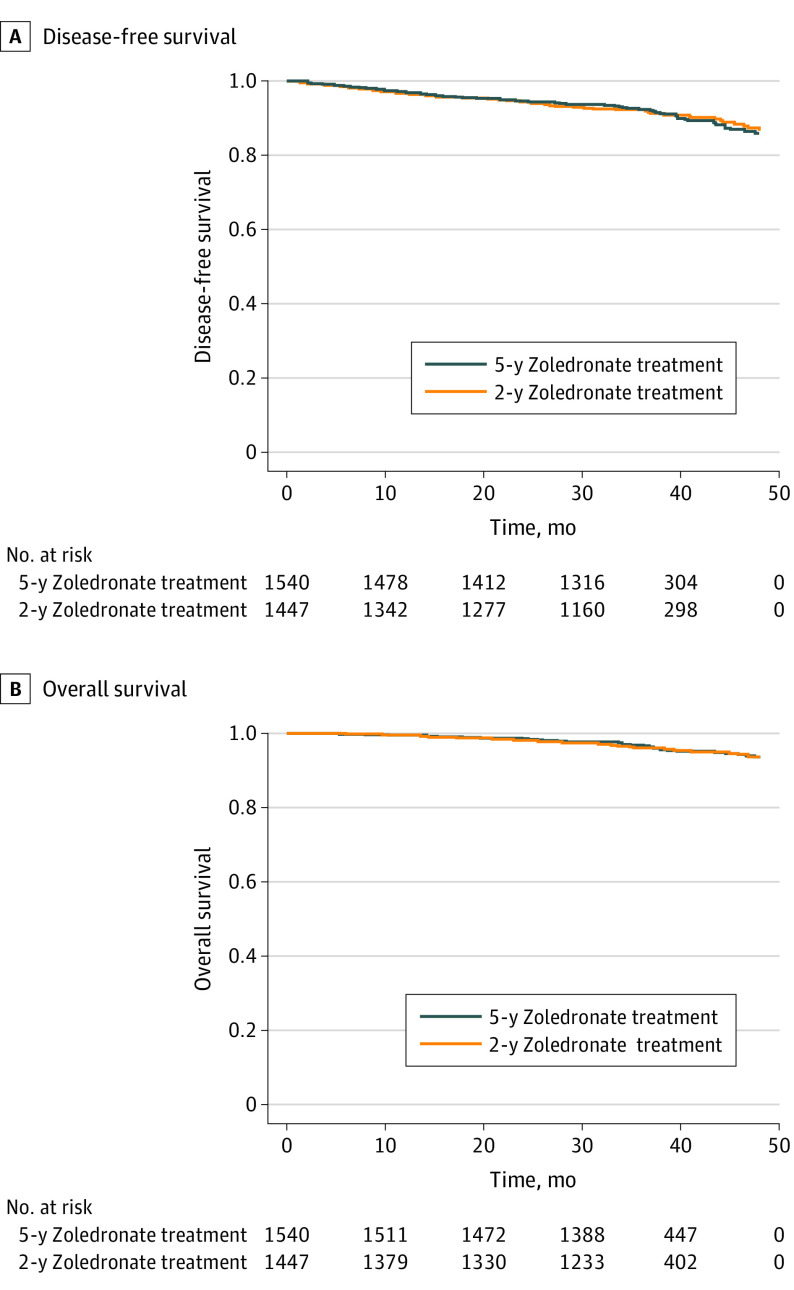
Median follow-up duration from 2 years after the start of zoledronate treatment was 35.4 months for both 2-year landmark DFS and DDFS and 36.0 months for 2-year landmark OS. Overall, 250 DFS events, including 116 deaths, were observed during the follow-up period. Univariable survival analyses revealed no statistically significant difference between patients receiving 5 vs 2 years of zoledronate treatment both with regard to the 2-year landmark DFS (HR, 0.97; 95% CI, 0.76-1.25; P = .83) (Figure 2A) and 2-year landmark OS (HR, 0.93; 95% CI, 0.65-1.34; P = .71) (Figure 2B). These results were confirmed by adjusted multivariable Cox proportional hazards regression models for DFS (HR, 0.97; 95% CI, 0.75-1.25; P = .81) and OS (HR, 0.98; 95% CI, 0.67-1.42; P = .90). Similarly, no statistically significant differences between patients receiving 5 vs 2 years of zoledronate treatment were found for 2-year landmark DDFS (177 events) in both univariable (HR, 0.86; 95% CI, 0.64-1.16; P = .32) and adjusted multivariable (HR, 0.87; 95% CI, 0.65-1.18; P = .38) survival analyses.
Figure 2. Disease-Free Survival and Overall Survival According to Zoledronate Treatment Duration.
A, Disease-free survival: 5-year zoledronate treatment, 129 events; 2-year zoledronate treatment, 121 events; P = .83, log-rank test. B, Overall survival: 5-year zoledronate treatment, 59 events; 2-year zoledronate treatment, 57 events; P = .71, log-rank test.
Survival According to Zoledronate Treatment Duration
We performed explorative subgroup analyses to evaluate whether there are patient subcohorts that might benefit from an extended zoledronate treatment duration. Subgroup analyses according to menopausal status revealed no statistically significant difference in DFS, OS, or DDFS between the treatment arms in premenopausal women (DFS: HR, 1.21; 95% CI, 0.81-1.81; P = .35; OS: HR, 0.93; 95% CI, 0.57-1.53; P = .78; DDFS: HR, 1.03; 95% CI, 0.65-1.63; P = .90) or postmenopausal women (DFS: HR, 0.85; 95% CI, 0.62-1.16; P = .30; OS: HR, 0.96; 95% CI, 0.67-1.39; P = .84; DDFS: HR, 0.76; 95% CI, 0.52-1.12; P = .16). In addition, there was no significant 2-way interaction between zoledronate treatment duration and menopausal status for adapted DFS (P = 0.17), adapted OS (P = 0.74), or adapted DDFS (P = 0.33), indicating that there is no evidence for a survival benefit of extended zoledronate treatment duration in either premenopausal or postmenopausal patients.
Figure 3 shows the forest plot for the comparison of 2-year landmark DFS between patients receiving 5 or 2 years of adjuvant zoledronate treatment for all subgroups tested. A significant 2-way interaction with zoledronate treatment duration was found only for tumor size (P = 0.02); however, the apparent DFS benefit of 2- vs 5-year zoledronate treatment observed in patients with pT3 tumors should be interpreted with care because it is based on a small subgroup with a low number of events and could be a chance finding. The comparison of 2-year landmark OS between patients receiving 5 or 2 years of adjuvant zoledronate treatment in the subgroups showed a similar overall pattern; however, there were no significant 2-way interactions (eFigure 2 in Supplement 2).
Figure 3. Results of Explorative Subgroup Analyses in Terms of the Comparison of Disease-Free Survival Between Patients With 5 or 2 Years of Zoledronate Treatment Duration.
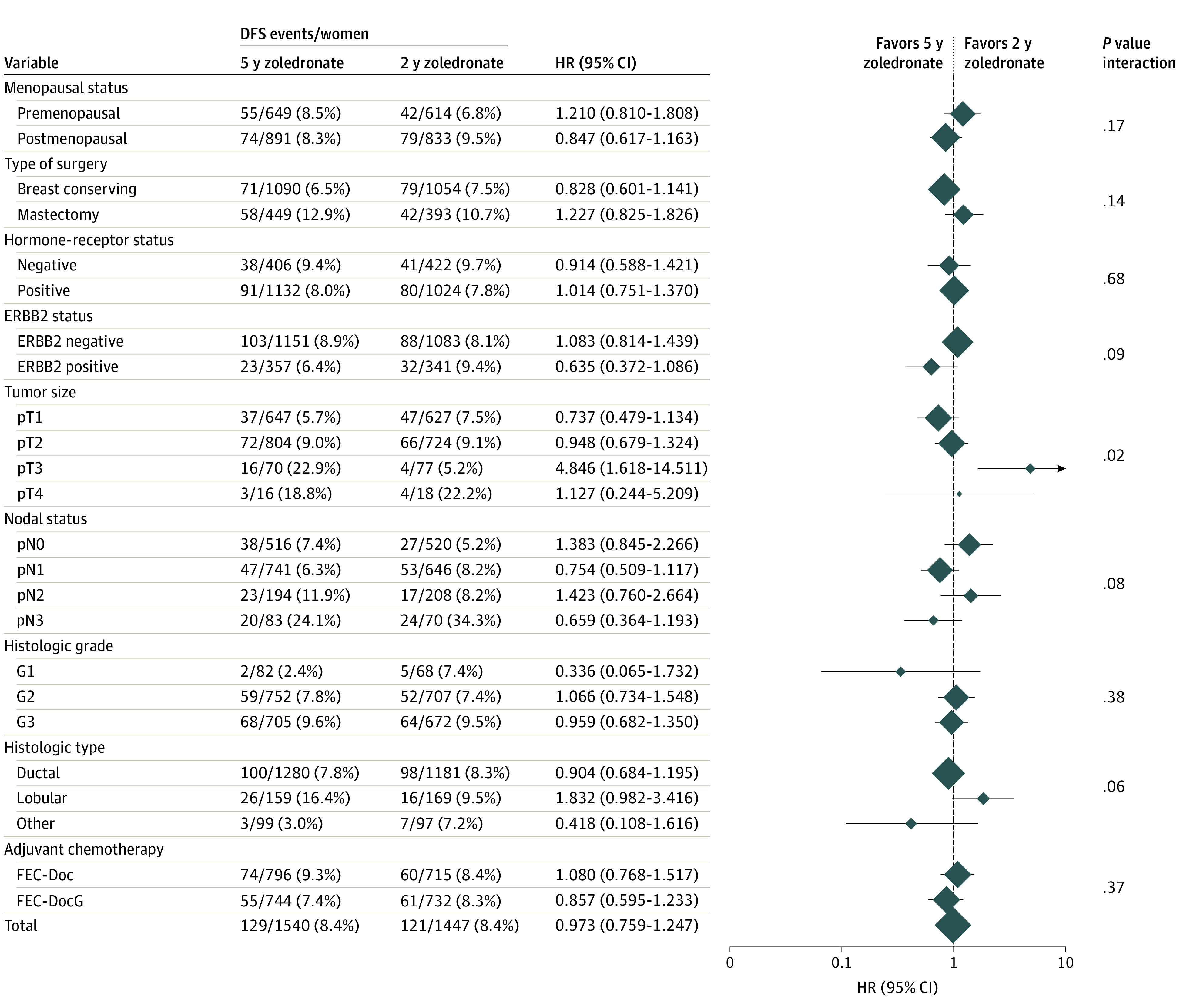
The diamonds indicate the hazard ratios (5 vs 2 years of zoledronate treatment), and diamond size is proportional to the number of patients per subgroup. The horizontal lines indicate the corresponding 95% CIs for the hazard ratios. The solid vertical line represents a hazard ratio of 1.0 (ie, no difference in survival between 5 and 2 years of zoledronate treatment). DFS indicates disease-free survival; FEC-Doc, 3 cycles of combined fluorouracil, epirubicin, and cyclophosphamide followed by 3 cycles of docetaxel; FEC DocG indicates 3 cycles of combined fluorouracil, epirubicin, and cyclophosphamide followed by 3 cycles of docetaxel and gemcitabine.
CTC Status 5 Years After Chemotherapy
The status of CTCs after 5 years was assessed for 714 patients. At least 1 CTC was detected in 43 of 410 (10.5%) patients in the 5-year zoledronate arm and in 22 of 304 (7.2%) patients in the 2-year zoledronate arm; the difference was not significant (χ2 test, P = .14).
Bone Recurrences and Adverse Events According to Treatment Duration
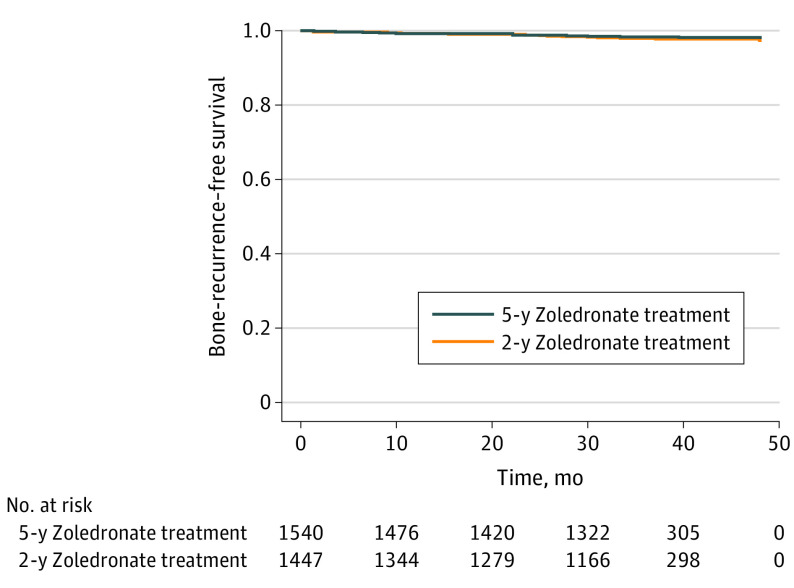
In total, 53 bone recurrences as the first distant recurrence, with or without other concurrent recurrences, were observed, with 25 events in the 5-year zoledronate arm and 28 events in the 2-year zoledronate arm. No statistically significant difference was noted between the 2 zoledronate treatment arms in bone-recurrence–free survival (HR, 0.80; 95% CI, 0.47- 1.38; P = .43) (Figure 4).
Figure 4. Bone-Recurrence–Free Survival According to Zoledronate Treatment Duration.
Bone-recurrence–free survival includes any first distant recurrence involving bone metastases as event (with or without recurrences at other distant sites): 5-year zoledronate treatment, 25 events; 2-year zoledronate treatment, 28 events; P = .43, log-rank test.
Overall, 2845 adverse events (all grades) were observed from 2 years after the start of zoledronate treatment; 1954 and 891 adverse events were observed in the 5-year zoledronate arm, respectively, affecting 46.2% and 27.2% of patients (χ2 test, P < .001). A similar pattern was observed with regard to grade 3 or 4 adverse events only: 159 grade 3 or 4 adverse events were observed in 7.6% of patients in the 5-year zoledronate arm, and 98 grade 3 or 4 adverse events affecting 5.1% of patients were seen in the 2-year zoledronate arm (χ2 test, P = .006). The eTable in Supplement 2 presents the observed frequencies of the 10 most common adverse events. In both zoledronate treatment arms, the skeletal-related events bone pain and arthralgia were the 2 most common adverse events, and both were seen in more patients in the 5-year zoledronate arm (bone pain: 5 years, 8.3% vs 2 years, 3.7%; arthralgia: 5 years, 5.1% vs 2 years, 3.1%). Fractures were observed in 14 patients who received 5-year treatment and 3 patients who received 2-year treatment (not distinguished between atypical and ordinary fractures). Osteonecrosis of the jaw occurred in 11 patients in the 5-year and 5 patients in the 2-year zoledronate arms.
Discussion
Current recommendations suggesting 3 to 5 years of adjuvant treatment with bisphosphonates for patients with EBC are based only on indirect evidence19,20; to our knowledge, trials directly comparing different bisphosphonate treatment durations have not been published. Subgroup analyses of the Early Breast Cancer Trialists’ Collaborative Group meta-analysis reported that trials studying 2 years or more than 2 years of bisphosphonate treatment revealed similar HRs for bisphosphonate treatment vs no bisphosphonate treatment regarding any recurrence and distant recurrences.17 Although the data suggest that a longer duration (ie, >2 years) of bisphosphonate treatment might not provide additional benefits in terms of survival, these results are from exploratory subgroup analyses. Concerns about adverse effects of prolonged bisphosphonate treatments, such as atypical bone fractures and osteonecrosis of the jaw, as well as results from both preclinical and clinical studies indicating that the benefit of bisphosphonates continues after termination of treatment,24 are additional reasons to question the necessity of extended bisphosphonate therapy.
There is clearly a lack of data regarding the optimal adjuvant treatment duration of bisphosphonates. To our knowledge, the SUCCESS A trial reported herein is the first randomized clinical trial filling this gap. The results of this large, multicenter phase 3 trial indicate that there is no benefit with regard to DFS, OS, and DDFS for extending adjuvant zoledronate treatment beyond 2 years in patients with high-risk EBC receiving chemotherapy, independent of their menopausal status. Furthermore, there was no statistically significant difference between 5 and 2 years of zoledronate treatment with respect to bone recurrences as first distant recurrence. These results are corroborated by the fact that we found no statistically significant differences between the 2 treatment arms with regard to the presence of CTCs 5 years after adjuvant chemotherapy, which is a prognostic factor for late recurrences in hormone receptor–positive disease.12,25 In addition, 5 years of zoledronate treatment was associated with a higher frequency of adverse events compared with 2 years of zoledronate treatment; this was true for all adverse events, grade 3 or 4 adverse events only, and for the skeletal-related events bone pain, arthralgia, fractures, and osteonecrosis of the jaw.
Limitations
This trial had limitations. Although the SUCCESS A trial provides some evidence for the lack of a survival benefit of 5 vs 2 years of adjuvant zoledronate treatment in patients with EBC obtained in a large randomized clinical phase 3 trial, there are some issues that need consideration as well as some limitations. First, the SUCCESS A trial included only patients with high-risk EBC who received adjuvant chemotherapy. Thus, the results cannot be generalized to all patients with EBC. Second, different dosing schedules of zoledronate are used in the adjuvant setting, and results might differ for dosing schedules other than the one used in the SUCCESS A trial. Because the median observation time was limited, long-term outcomes representing possible carryover effects could not be fully evaluated. Third, the study was not specifically powered for the landmark-approach–based analysis for the comparison of DFS in patients receiving 5 vs 2 years of zoledronate treatment (eMethods 4 in Supplement 2). Fourth, there was a relatively low number of adverse events (particularly skeletal-related events), which limits the power of the subgroup analyses, especially the power for the statistical analyses regarding bisphosphonate key events. Fifth, there was a slight bias with regard to loss of follow-up between the 2 treatment arms, because more patients were lost to follow-up during the first 200 days of the follow-up period in the 2-year zoledronate arm, possibly because of the open-label, non–placebo-controlled study design. However, as shown by sensitivity analyses, this bias is unlikely to affect the main results of the study (eMethods 6 in Supplement 2).
Conclusions
The results of the SUCCESS A randomized clinical trial showed no statistically significant difference in DFS, OS, or DDFS between 5 years and 2 years of adjuvant zoledronate treatment following chemotherapy in patients with high-risk EBC, regardless of menopausal status. Our results suggest that extended adjuvant bisphosphonate treatment with zoledronate for 5 years should not be considered in patients with EBC in the absence of decreased bone density. Based on our results, the recommended 3 to 5 years of adjuvant bisphosphonate treatment for patients with high-risk EBC as published in current clinical guidelines could be reduced.
Trial Protocol
eMethods 1. Randomization Procedures
eMethods 2. Endocrine Treatment
eMethods 3. CTC Detection
eMethods 4. Power Analysis and Sample Size Calculations
eMethods 5. Definitions of Survival Endpoints
eMethods 6. Potential Bias and Sensitivity Analysis
eTable. Observed Frequencies of the Ten Most Common Adverse Events
eFigure 1. Study Design of the SUCCESS A Trial
eFigure 2. Forest Plot Showing Results of Explorative Subgroup Analyses in Terms of the Comparison of Overall Survival Between Patients With Five or Two Years of Zoledronate Treatment Duration According to Different Patient and Tumor Characteristic Subgroups
Data Sharing Statement
References
- 1.Clemons M, Gelmon KA, Pritchard KI, Paterson AHG. Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: the state of the art. Curr Oncol. 2012;19(5):259-268. doi: 10.3747/co.19.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lüftner D, Niepel D, Steger GG. Therapeutic approaches for protecting bone health in patients with breast cancer. Breast. 2018;37:28-35. doi: 10.1016/j.breast.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 3.Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013;24(2):398-405. doi: 10.1093/annonc/mds277 [DOI] [PubMed] [Google Scholar]
- 4.Brufsky AM, Harker WG, Beck JT, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192-1201. doi: 10.1002/cncr.26313 [DOI] [PubMed] [Google Scholar]
- 5.Strobl S, Wimmer K, Exner R, et al. Adjuvant bisphosphonate therapy in postmenopausal breast cancer. Curr Treat Options Oncol. 2018;19(4):18. doi: 10.1007/s11864-018-0535-z [DOI] [PubMed] [Google Scholar]
- 6.Gnant MFX, Mlineritsch B, Luschin-Ebengreuth G, et al. ; Austrian Breast and Colorectal Cancer Study Group . Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25(7):820-828. doi: 10.1200/JCO.2005.02.7102 [DOI] [PubMed] [Google Scholar]
- 7.Shapiro CL, Halabi S, Hars V, et al. Zoledronic acid preserves bone mineral density in premenopausal women who develop ovarian failure due to adjuvant chemotherapy: final results from CALGB trial 79809. Eur J Cancer. 2011;47(5):683-689. doi: 10.1016/j.ejca.2010.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34(5):453-475. doi: 10.1016/j.ctrv.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Wang S-H, Chen S-C, Chen C-Y, Lin T-M. Zoledronic acid blocks the interaction between breast cancer cells and regulatory T-cells. BMC Cancer. 2019;19(1):176. doi: 10.1186/s12885-019-5379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ubellacker JM, Haider M-T, DeCristo MJ, et al. Zoledronic acid alters hematopoiesis and generates breast tumor-suppressive bone marrow cells. Breast Cancer Res. 2017;19(1):23. doi: 10.1186/s13058-017-0815-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trapp E, Janni W, Schindlbeck C, et al. ; SUCCESS Study Group . Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J Natl Cancer Inst. 2019;111(4):380-387. doi: 10.1093/jnci/djy152 [DOI] [PubMed] [Google Scholar]
- 12.Sparano J, O’Neill A, Alpaugh K, et al. Association of circulating tumor cells with late recurrence of estrogen receptor–positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(12):1700-1706. doi: 10.1001/jamaoncol.2018.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnant M, Mlineritsch B, Stoeger H, et al. ; Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria . Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015;26(2):313-320. doi: 10.1093/annonc/mdu544 [DOI] [PubMed] [Google Scholar]
- 14.Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol. 2008;19(12):2007-2011. doi: 10.1093/annonc/mdn429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman R, Cameron D, Dodwell D, et al. ; AZURE investigators . Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15(9):997-1006. doi: 10.1016/S1470-2045(14)70302-X [DOI] [PubMed] [Google Scholar]
- 16.Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006;8(2):R13. doi: 10.1186/bcr1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353-1361. doi: 10.1016/S0140-6736(15)60908-4 [DOI] [PubMed] [Google Scholar]
- 18.O’Carrigan B, Wong MHF, Willson ML, Stockler MR, Pavlakis N, Goodwin A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2017;10(10):CD003474. doi: 10.1002/14651858.CD003474.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman R, Hadji P, Body JJ, et al. ; ESMO Guidelines Committee . Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31(12):1650-1663. doi: 10.1016/j.annonc.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 20.Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(18):2062-2081. doi: 10.1200/JCO.2016.70.7257 [DOI] [PubMed] [Google Scholar]
- 21.de Gregorio A, Häberle L, Fasching PA, et al. Gemcitabine as adjuvant chemotherapy in patients with high-risk early breast cancer—results from the randomized phase III SUCCESS-A trial. Breast Cancer Res. 2020;22(1):111. doi: 10.1186/s13058-020-01348-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127-2132. doi: 10.1200/JCO.2006.10.3523 [DOI] [PubMed] [Google Scholar]
- 24.Black DM, Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2016;374(3):254-262. doi: 10.1056/NEJMcp1513724 [DOI] [PubMed] [Google Scholar]
- 25.Janni W, Rack BK, Fasching PA, et al. Persistence of circulating tumor cells in high risk early breast cancer patients five years after adjuvant chemotherapy and late recurrence: results from the adjuvant SUCCESS A trial. J Clin Oncol. 2018;36(15 suppl):515. doi: 10.1200/JCO.2018.36.15_suppl.51529267131 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Randomization Procedures
eMethods 2. Endocrine Treatment
eMethods 3. CTC Detection
eMethods 4. Power Analysis and Sample Size Calculations
eMethods 5. Definitions of Survival Endpoints
eMethods 6. Potential Bias and Sensitivity Analysis
eTable. Observed Frequencies of the Ten Most Common Adverse Events
eFigure 1. Study Design of the SUCCESS A Trial
eFigure 2. Forest Plot Showing Results of Explorative Subgroup Analyses in Terms of the Comparison of Overall Survival Between Patients With Five or Two Years of Zoledronate Treatment Duration According to Different Patient and Tumor Characteristic Subgroups
Data Sharing Statement